Abstract
Marfan syndrome is consequent upon mutations in FBN1, which encodes the extracellular matrix microfibrillar protein fibrillin-1. The phenotype is characterised by development of thoracic aortic aneurysm. Current understanding of the pathogenesis of aneurysms in Marfan syndrome focuses upon abnormal vascular smooth muscle cell signalling through the transforming growth factor beta (TGFβ) pathway. Angiotensin II (Ang II) can directly induce aortic dilatation and also influence TGFβ synthesis and signalling. It has been hypothesised that antagonism of Ang II signalling may protect against aortic dilatation in Marfan syndrome. Experimental studies have been supportive of this hypothesis, however results from multiple clinical trials are conflicting. This paper examines current knowledge about the interactions of Ang II and TGFβ signalling in the vasculature, and critically interprets the experimental and clinical findings against these signalling interactions.
Keywords: Aneurysm, Angiotensin blocker, Cell Signalling, Clinical trial
1. Introduction
Marfan syndrome (MFS), consequent upon mutations in FBN1, is characterised by development of thoracic aortic aneurysm (TAA) in early adult life. The FBN1 mutations may result in haploinsufficiency (HI) or dysfunctional dominant-negative (DN) fibrillin-1 in the extracellular matrix (ECM) microfibrils. It has been proposed that abnormal transforming growth factor beta (TGFβ) signalling drives TAA formation in MFS [[1], [2], [3]], possibly as a result of impaired sequestration of TGFβ by mutant fibrillin-1 [1,4]. At the same time, angiotensin II (Ang II) can directly induce adverse vascular remodelling with aortic dilatation and can also influence TGFβ synthesis and receptor expression, as well as interact with TGFβ signalling [5,6]. It has therefore been proposed that Ang II and TGFβ may be synergistic in ECM remodelling and thus antagonism of Ang II signalling may protect against TAA in MFS.
Studies in murine models of MFS, using the angiotensin receptor blocker (ARB) losartan, observed reduced TAA formation [7]. These encouraging experimental studies stimulated clinical trials of ARBs in patients with Marfan syndrome. Although the first major clinical trial of losartan in MFS did describe reduced TAA progression, multiple subsequent studies have found no apparent benefit of ARBs over conventional treatment [[8], [9], [10], [11], [12]]. The discrepancy between the experimental findings and the clinical trial data remains a dilemma for clinicians.
At the same time, our understanding of the roles of TGFβ and Ang II in the pathogenesis of TAA has evolved considerably. The role of TGFβ as a driver of TAA formation has been challenged [13,14], whilst the triggers of TAA are more complex than simple dysregulation of latent TGFβ binding and appear to hinge upon abnormal mechano-transduction responses to hemodynamic stress upon the aortic wall [15]. The interplay between TGFβ and Ang II in determining VSMC phenotype and structural change in the ECM is better understood and appears to involve both synergistic and antagonistic interactions, which may be age-related [16].
This paper examines the current understanding of the relationships between TGFβ and Ang II signalling in vascular smooth muscle and reviews the experimental evidence for a protective effect of ARBs upon TAA in MFS. The clinical trial evidence is then interpreted with reference to the experimental data, in order to more clearly define the potential therapeutic benefit of ARBs.
2. Vascular smooth muscle cells and the aorta in Marfan syndrome
Although the association between mutations in the FBN1 gene and MFS is well established, there remain questions about how the mutations result in aortic aneurysm formation. The autosomal dominant mutations in FBN1 have been classified as either haploinsufficient (HI) resulting in absolute deficiency of fibrillin-1 in the microfibrils or dominant negative (DN) resulting in incorporation of dysfunctional fibrillin-1 within the microfibrils. Some clinical studies have described a more severe cardiovascular phenotype for MFS patients with HI mutations than for those with DN mutations [17], whilst those with HI mutations may be more likely to suffer aortic dissection and have worse survival [18]. The opposite result of HI mutations (i.e. premature termination codons) has however been demonstrated by others [19], and the picture is further confounded by observations that incorporation of fibrillin-1 DN mutants into microfibrils appears to cause microfibrillar dysfunction through haploinsufficiency of wild-type (WT) fibrillin-1. Indeed, the presence of the DN fibrillin-1 mutant appeared irrelevant when there was adequate WT fibrillin-1 [20]. Thus, a key determinant of the severity of phenotype in MFS appears to be the absolute amount of normal fibrillin-1 present in the microfibrils, which itself will reflect the degree of expression of the normal FBN1 allele and the degree of incorporation or otherwise of mutant fibrillin-1 into the microfibrils.
The vascular smooth muscle cells (VSMC) play a key role in aortic development through synthesis of proteins and glycosaminoglycans necessary for normal structure of the ECM and this synthetic phenotype (s-VSMC) is most active in prenatal and early postnatal life [21] before subsequent switch to the more quiescent contractile phenotype (c-VSMC) characteristic of the mature aorta. Stress injury to the aorta can result in de-differentiation of the VSMC towards a deleterious and pro-inflammatory phenotype (i-VSMC) [22].
The aorta in patients with MFS is characterised by fragmentation and thinning of elastin fibres in the media, increased extracellular matrix and increased collagen deposition in the adventitia, with evidence of abnormal VSMC phenotypic transition from contractile to pro-inflammatory form [23]. The switching in phenotype of VSMC appears to be dependent upon age and disease state. Premature switching from s-VSMC to c-VSMC may occur in MFS patients, with resultant impairment of tropoelastin deposition in the media [24]. Experimental data shows that such phenotypic shift occurs in early life. In Fbn1mgR/mgR mice, elastin fragmentation is evident by D7, with TAA by D35. At the same time, the Fbn1mgR/mgR mice appeared to have a premature shift in VSMC phenotype from s-VSMC to c-VSMC, with reduced expression of tropoelastin and increased expression of α-actin and myosin heavy chain, as well as matrix metalloproteinases 2 and 9 [25]. In contrast to the histological changes, TGFβ was initially normal and only began to rise after D14. The later increase in TGFβ may result from impaired sequestration of LTBP-TGFβ complexes in the mutant fibrillin, microfibrillar damage secondary to MMP2 and MMP9 release from the VSMC, or increased TGFβ synthesis. Increased TGFβ signalling may then drive a further switch in VSMC phenotype to the i-VSMC form, leading to further degradation of the ECM and the elastin lamellae. The foundations of TAA formation, through inappropriate VSMC phenotype switches, appear to be laid in early postnatal life and therefore treatment interventions are more likely to be beneficial when instituted in infancy or childhood.
3. Angiotensin signalling in vascular smooth muscle cells
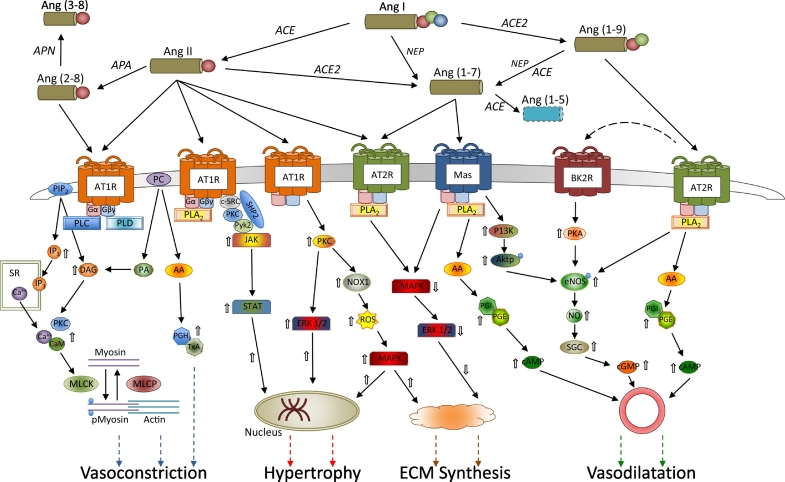
The renin-angiotensin system has a key role in circulatory homeostasis, including regulation of systemic vascular resistance, intravascular volume and arterial structure. The effects of angiotensin in the vasculature are bi-functional, depending upon the cellular environment. Angiotensin I is metabolised by angiotensin converting enzyme (ACE) to the active octapeptide Ang II, which signals VSMC contraction and vascular remodelling. In contrast, the carboxypeptidase ACE2, expressed in heart and vasculature, can cleave Ang I to Ang(1–9) and Ang II to Ang(1–7) (Fig. 1) and these latter peptides are associated with effects on VSMC synthetic activity and vascular structure opposite to those of Ang II [6].
Fig. 1.
Counter-regulatory signalling mechanisms within the angiotensin system in vascular smooth muscle. Extracellular metabolism of angiotensin 1 yields a variety of vasoactive peptides, with differing receptor affinities. Activation of Type 1 receptors (AT1R) results in vasconstriction, smooth muscle cell hypertrophy and increased extracellular matrix synthetic activity. Multiple intracellular signalling pathways mediate these effects including increased intracellular calcium release from the sarcoplasmic reticulum (SR), and increased growth factor signalling as well as activation of MAP kinases. In contrast, activation of Type 2 receptors (AT2R) and affiliated MASRD receptors results in opposite effects, including vasodilatation and reduced extracellular matrix synthetic activity, via inhibition of MAP kinases and increased nitric oxide and prostacyclin synthesis.
(AA = arachidonic acid; ACE = angiotensin converting enzyme; ACE2 = angiotensin converting enzyme type 2; Akt = protein kinase B; APA = aminopeptidase A; APN = aminopeptidase N; BK2R = bradykinin receptor type 2; CaM = calmodulin; c-Src = proto-oncogene tyrosine-protein kinase Src; DAG = diacylglycerol; ERK = extracellular signal-related kinases; IP3 = inositol triphosphate; JAK = Janus kinase; MAPK = mitogen-activated protein kinases; MASRD = Mas-related G-protein coupled receptor type D; MLCK = myosin light chain kinase; MLCP = myosin light chain phosphatase; NEP = neutral endopeptidase; NO = nitric oxide; NOS = nitric oxide synthase; NOX1 = NADPH oxidase 1; PA = phosphatidic acid; PC = phosphatidylcholine; PGE2/H2/I2 = prostaglandin E2/H2/I2; PIP2 = phosphatidylinositol bisphosphate; PI3K = phosphatidylinositide 3-kinases, PKA/C = protein kinase A/C; PLA2/C/D = phospholipase A2/C/D; Pyk2 = proline-rich tyrosine kinase 2; ROS = reactive oxygen species; SGC = soluble guanylate cyclase; SHP2 = tyrosine phosphatase non-receptor type II; STAT = signal transducer and activator of transcription; TXA2 = thromboxane A2).
Cell surface receptors for Ang II are Type 1 (AT1R) and Type 2 (AT2R) G-protein-coupled receptors. Activation of ATR2 results in effects opposite to those upon AT1R activation. In adults, Ang II signalling is almost entirely via AT1R in VSMC and heart. The AT2R is predominantly expressed in foetal life but may be re-expressed in adult life in response to pathological states associated with tissue remodelling or inflammation such as hypertension, atherosclerosis, and myocardial infarction [26]. The AT1R are subject to multiple regulatory influences, which can alter receptor density and thus VSMC responsiveness to Ang II. An acute increase in circulating Ang II results in increased AT1R activation, however chronic exposure to elevated Ang II leads to down-regulation of AT1R in a negative feedback pattern [27]. Other up-regulators of AT1Rs in VSMC include insulin and erythropoietin [28,29]; down-regulators include nitric oxide and platelet derived growth factor (PDGF) [30,31].
Binding of Ang II to AT1R can activate multiple signalling pathways. The G-protein coupled pathway is considered to be the main driver of VSMC contraction (see Fig. 1). Activation of the AT1R, results in coupling to Gαq/11, Gα12/13, and Gβy, which activate phospholipases C (PLC), A2 (PLA2) and D (PLD), leading to release of inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG) [6]. Binding of IP3 to its receptor on the sarcoplasmic reticulum, results in calcium efflux into the cytoplasm, with calcium binding to calmodulin leading to activation of myosin light chain kinase (MLCK), phosphorylation of the myosin light chain, enhanced interaction between actin and myosin, and increased force of VSMC contraction. At the same time, DAG is associated with protein kinase C (PKC) activation and inhibition of myosin light chain phosphatase, prolonging VSMC contraction [6].
The non G-protein pathways associated with the AT1R include the receptor-linked tyrosine kinase (PDGF, epidermal growth factor receptor EGFR, insulin receptor) and non-receptor tyrosine kinase pathways (Src, JAK/STAT, focal adhesion kinase (FAK)) (See Fig. 1). Mitogen–activated protein kinase (MAPK) is an important mediator of the tyrosine kinase receptor pathway downstream of AT1R, influencing protein synthesis, gene expression and growth [6]. Extracellular signal regulated kinase (ERK), p38MAPK and JNK are elements of the MAPK pathway and appear to stimulate vascular fibrosis [32].
The phosphorylation of ERK is activated by AngII binding to AT1R, but blocked by inhibition of PLC, suggesting that ERK activation is calcium dependent, given calcium's interaction with PLC [33]. Alternately, the signalling molecule Src and calcium-dependent kinase Pyk2 can induce phosphorylation of EGFR, leading to formation of the Shc/Grb2 complex, which in turn, activates Raf, and thence phosphorylation of the MAPK/ERK kinase (MEK). The activated MAPK pathway results in increased c-fos (activated by ERK), c-jun (activated by JNK) and AP-1 transcription factor, thereby promoting shift in VSMC phenotype and synthetic activity [6]. AT1R activation can also stimulate the JAK/STAT pathway, mediating transcription of early growth response genes in VSMC. In VSMCs PLC and its downstream second messengers, IP3/Ca2+ and DAG/PKC, are required for JAK activation via Ang II-induced AT1R [34].
Oxidative stress is involved in the regulation of tyrosine kinases, the expression of inflammatory genes, endothelial function, VSMC growth, and extracellular matrix formation [35]. AngII is a potent mediator of oxidative stress. In VSMC AngII activates membrane NAD(P)H oxidases to produce superoxide and hydrogen peroxide (H2O2), which in turn promote VSMC fibrosis [35]. Additionally, Ang II activates nuclear factor κB (NF-κB) in monocytes, macrophages, VSMCs, and endothelial cells. This commences a cascade with the production of the cell adhesion molecules, VCAM-1 and ICAM-1, as well as chemokines, monocyte chemoattractant protein-1 (MCP-1), IL-6, and IL-8 [6]. Intracellular ROS activates transcription of NF-κB and its degradation inhibitor, IκB, creating a pro-inflammatory environment. MCP-1 and IL-6 activation by Ang II is dependent on the activation of NAD(P)H oxidase [6]. Thus, Ang II plays a key role in pro-inflammatory environment by ROS production, leading to further inflammation and subsequently fibrosis through NF-κB, IL-6 and VCAM-1.
There are multiple other metabolites of angiotensin, most of which have biological actions opposite to those of ATR1 mediated Ang II signalling (Fig. 1). Thus, Ang(1–7) inhibits the actions of Ang II initiated cell growth, migration and inflammation. Ang(1–7) is an endogenous ligand for the Mas receptor [36] and has been shown to inhibit MAPK signalling, via activation of phosphatases, including DUSP-1, thereby reducing ERK1/2 phosphorylation, resulting in reduced VSMC proliferation and migration [37,38]. It appears that Ang(1–7) acts via Mas to increase activity of endothelial NO synthase and thus via cGMP to increase DUSP-1 expression. Ang(1–7) signalling via AT2R is also associated with release of PGI2 and PGE2 with increase in cAMP and inhibition of cyclooxygenases [39,40]. The net result of Ang(1–7) action via AT2R, in presence of AT1R blockade, is vasodilatation.
Treatment with the ACE inhibitor Lisinopril is associated with increased plasma Ang-(1–7), decreased Ang II, and increased cardiac ACE2 expression. In contrast, the ARB Losartan increased both plasma Ang II and Ang-(1–7), as well as cardiac ACE2 expression and activity. Thus, the actual mechanisms of antihypertensive and antifibrotic effects of ACE inhibitors and ARBs appear to differ [41].
The role of Ang(1–9) is less well known. Inhibition of ROCK (RhoA/Rho-associated kinase) leads to increased ACE2 in aorta and increased Ang(1–9) and at the same time, eNOS is increased, ACE is decreased and AngII is decreased [40]. Infusion of Ang(1–9) is associated with increased NO (possibly via bradykinin) and aortic VSMC relaxation [42].
4. Transforming growth factor β signalling in vascular smooth muscle cells
The TGFβ superfamily includes multiple ligands (3 TGFβ isoforms, 10 bone morphogenic proteins (BMP)) and multiple receptor types (7 Type 1 receptors and 5 Type 2 receptors) with capacity for both cross-signalling and adaptive signalling in a context-dependent manner [43], through which TGFβ can both positively and negatively regulate cell proliferation and vascular remodelling.
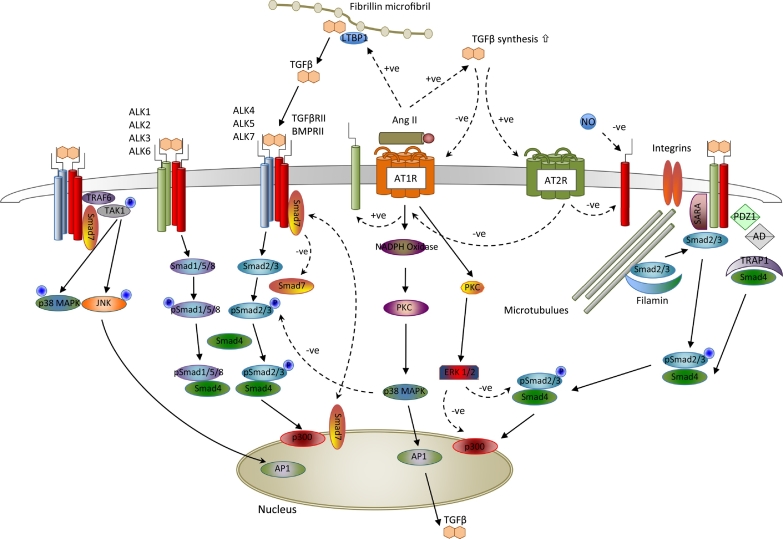
The trans-membrane TGFβ receptors (TGFβR1 and TGFβR2), characterised by intracellular serine-threonine kinase moieties, exist as monomers in the absence of ligand binding. Upon ligand binding TGFβR1 and TGFβR2 monomers are apposed to form the activated receptor complex [44]. Eight SMAD proteins are involved in signal transduction from receptor to nucleus, including receptor-regulated R-SMADs (SMADS 1,2,3,5,8); inhibitory I-SMADs (SMADS 6,7) and the co-operative SMAD4. In VSMC, TGFβR1 subtype ALK5 (TGFβ, activin) phosphorylates SMAD2 and SMAD3, through cooperative binding with the cofactor SARA. The subtype ALK1 (BMP) phosphorylates SMAD1, SMAD5 and SMAD8 [45]. Whilst SMAD6 appears to selectively inhibit BMP/ALK1 signalling, SMAD7 inhibits both BMP/ALK1 and TGFβ/ALK5 signalling. The I-SMADs appear to have multiple modes of action, including competitive inhibition of R-SMAD binding to TGFβR1, interaction with ligases SMURF1/2 leading to degradation of TGFβR1 and inactivation of the receptors via dephosphorylation [46]. The mode of internalization of the TGFβ/receptor complex appears to determine subsequent SMAD signalling. Internalization of the complex via clathrin pits results in either SMAD2 and SMAD 3 phosphorylation (ALK1 with SARA) or SMAD1 and SMAD5 (ALK5). In contrast, internalization via caveolin-1 vesicles is associated with SMAD7 activation and signal degradation [47,48]. Both types of internalization occur in MFS, however SMA2 and SARA expression are increased, supporting increased TGFβ signalling [48].
The phosphorylated R-SMADs bind cooperatively to SMAD4, which complex can then regulate transcription of target genes (Fig. 2). This regulation appears to be via cofactors, leading to chromatin remodelling, rather than direct binding to transcriptional promoters. The actual signalling outcome of the R-SMAD/SMAD4 binding is thus heavily dependent upon cooperative activators and repressors and is also subject to epigenetic influence [49]. The R-SMAD/SMAD4 complexes are themselves subject to further regulation through phosphorylation by MAPKs and other kinases [50]. In addition, the expression of I-SMADs is promoted by the R-SMAD/SMAD4 complexes, yielding potential negative feedback regulation of TGFβ and BMP signalling.
Fig. 2.
Illustration of regulatory interactions between TGFβ and Ang II in vascular smooth muscle. Binding of TGFβ to Alk1 or Alk5 Type I receptors results in dimerization with TGFβRII Type 2 receptors and activation of multiple intracellular signalling pathways. Binding of Ang II to AT1R is associated with up-regulation of Alk1 receptors and increased transcription of TGFβ mRNA, as well as increased TGFβ cleavage from binding proteins in the extracellular matrix. Conversely, Ang II binding to AT2R results in down-regulation of both AT1R and TGFβRII Type 2 receptors. Canonical TGFβ signalling via the Smad system results in altered nuclear chromatin patterning and activation of transcription of multiple gene targets, with internal inhibitory regulation by Smad7. Presentation of Smads 2/3 to the Alk1/TGFβRII heterodimer appears dependent upon integrin-linked mechanotransduction of external forces to intracellular microtubules. Additionally, TGFβ can signal via p38MAPK and regulate gene transcription independently of the Smad system. Ang II binding to AT1R also activates the MAPK/ERK system, and is associated with inhibition of Smad2/3 binding to Smad4.
(AD = adaptor proteins; AP1 = activator protein 1 transcriptional regulator; LTBP1 = latent transforming growth factor binding protein 1; PDZ1 = scaffolding protein with PDZ domain; SARA = Smad anchor for receptor activation; TAK-1 = transforming growth factor β activated kinase; TRAF-6 = Tissue Necrosis Factor receptor associated factor 6; TRAP-1 = Tissue Necrosis Factor receptor associated protein 1).
The complexity of TGFβ signal transduction is underscored by the existence of multiple non-SMAD signalling pathways, which results from the dual functionality of activated TGFβR1as serine/threonine kinases and also as tyrosine kinases.
Thus, TGFβ can act via a SMAD independent pathway, through MAPKs such as ERK, p38-MAPK, and c-Jun-N-terminal kinase (JNK). These signalling cascades can, in turn, influence SMAD signalling, cell proliferation and apoptosis, as p38-MAPK and JNK potentiate TGF-β/SMAD effects, whereas ERK can also antagonise SMAD signalling [51].
Bone morphogenetic proteins (BMP) may also play a role in VSMC differentiation and function. Thus, BMP7 inhibits VSMC growth induced by TGFβ1R, whilst BMP2 can induce VSMC migration and inhibit PDGF-induced proliferation of VSMC [52]. In patients with idiopathic pulmonary hypertension, loss of BMPR2 may lead to unregulated TGF-β/ALK5 activity in VSMC, highlighting its importance in disease pathophysiology [53]. Like TGFβ, BMPs can be sequestered within the extracellular microfibril network and mice with Fbn1 mutations exhibit disturbed BMP signalling [54].
A key question is how regulation of SMAD vs non-SMAD signalling is achieved. There is evidence that the expression levels of Type I vs Type II receptors play a role [55]. It appears that TGFβ signalling through the Type I receptor ALK-5 activates the SMAD2/3 pathway resulting in cellular proliferation and migration, whereas signalling through ALK-1 activates SMAD1/5 inhibiting these processes [56]. Activation of ERK-MAPK antagonises the cofactors required for R-SMAD/SMAD4 nuclear signalling and inhibits nuclear translocation of the complex [44].
Most recent evidence suggests that TGFβ signalling may limit TAA formation in early life for MFS [13]. This is supported by studies using TGFβ neutralizing antibody, which show that antibody treatment at D16 made TAA worse [57]. In contrast, antibody treatment at D45 ameliorated TAA formation. Thus, the role of TGFβ in TAA formation in MFS is multifunctional and appears dependent upon age and disease state.
5. Interactions of angiotensin and transforming growth factor β signalling
There are multiple levels of interaction between the Ang II and TGFβ pathways, including influencing ligand and/or receptor expression; synergy or antagonism of intracellular signalling; cross-activation of common effector pathways and regulatory feedback upon each pathway.
There appears to be cross-regulation of ligand and receptor expression between the Ang II and TGFβ pathways. Ang II signalling via ATR1 leads to increased TGFβ mRNA expression and protein synthesis, which can be abolished by pretreatment with an ARB [58]. In addition, Ang II stimulation is associated with increased release of active TGFβ [59]. Conversely TGFβ signalling via TGFβR1/2 is associated with upregulation of ACE synthesis in fibroblasts and downregulation of AT1R in VSMC [60]. Both ACE inhibitors and ARBs reduce tissue expression of TGFβ and reduce vascular fibrosis, whilst blockade of TGFβ decreases Ang II–induced synthesis of extracellular matrix glycoproteins [5]. Ang II concentration is increased in aortas of mice with fibrillin mutations and Ang II also induces the production of a potent activator of TGF-β, thrombospondin-1 (TSP1). The increase in circulating and tissue levels of TGFβ observed in murine and human MFS may therefore represent an effect of Ang II signalling via AT1R, rather than excess release of TGFβ from storage on mutant fibrillin in the extracellular matrix.
The interaction between SMADs and nuclear transcription factors is partly regulated by the p38 MAPK pathway, which is in turn influenced by Ang II signalling via ATR1. Although many studies have employed measurement of p-SMAD2 as a marker of TGFβ signalling, it is now known that Ang II also directly activates SMAD2/SMAD4, with similar kinetics between TGFβ and Ang II stimulated responses [5]. Ang II infusion in rats leads to increased SMAD2 expression and phosphorylation, and also increased SMAD4 and CTGF expression, CTGF being a mediator of both TGFβ and Ang II–induced vascular fibrosis [61]. These effects are blocked by Losartan, but blockade of endogenous TGFβ had no effect on AngII stimulation of SMAD2. This independent Ang II influence on SMAD signalling appears dependent on p38 MAPK activation [5].
The data on changes in TGFβ expression induced by Ang II and the increase in ACE expression following TGFβ administration support a regulatory or feedback mechanism between the two pathways. The observation that administration of a TGFβ neutralizing antibody in Ang II infused mice results in worse aortic aneurysm formation and rupture suggests a protective effect of TGFβ signalling in the setting of Ang II stimulation [62].
6. Angiotensin II and aortic aneurysm in Marfan syndrome - experimental evidence
Amongst patients with MFS, arterial function is abnormal, with increased vascular stiffness, increased systemic pulse wave velocity and abnormal ventricular-vascular coupling [63]. The role of AngII in mediating these changes is now supported by experimental evidence. In Fbn1mgR/mgR mice, with severe fibrillin deficiency, age-related medial thickening and elastin fragmentation is observed, accompanied by softening of the media [64]. The observed softening of the media is opposite to observations of increased vascular stiffness in vivo, however the latter may well reflect adventitial collagen deposition. Thus, disparate changes in the mechanical properties of the media and adventitia may predispose towards aortic dissection in MFS. Both the histological and micromechanical changes in the media were attenuated by Losartan treatment and Losartan may prolong survival in this model of severe MFS [57].
A primary research objective has been to determine whether or not ARBs can prevent aortic aneurysm formation in MFS. The key experimental studies examining this question summarised in Table 1.
Table 1.
Experimental Studies of AngII Antagonism and TGFβ signalling in TAA.
| Study | Model | Intervention | n | Outcome relative to WT controls |
|||
|---|---|---|---|---|---|---|---|
| TGFβ1 | pSmad2 | pERK | Aortic dilatation | ||||
| Habashi [7] | Fbn1C1039G/+ | Nil | 12 | +++ | +++ | ||
| 2006 | TGFβ NAb postnatal | 6 | + | + | |||
| Propranolol prenatal | 6 | ++ | |||||
| Losartan prenatal | 10 | − | |||||
| Propranolol postnatal | 7 | ++ | + | ||||
| Losartan postnatal | 5 | = | = | ||||
| Yang [66] | Fbn1C1039G/+ | Nil | 30 | ++ | ++ | +++ | |
| 2010 | Losartan postnatal | 30 | = | = | ++ | ||
| Nistala [67] | Fbn1mgR/mgR | Nil | 7 | +++ | |||
| 2010 | Losartan postnatal | 8 | + | ||||
| Habashi [68] | Fbn1C1039G/+ | Nil | 10 | ++ | ++ | ++ | |
| 2011 | Losartan postnatal | 5 | = | = | − | ||
| AT2KO | Nil | 17 | = | = | = | ||
| AT2KO·Fbn1C1039G/+ | Nil | 19 | +++ | +++ | +++ | ||
| Losartan postnatal | 6 | +++ | +++ | ++ | |||
| Holm [64] | Fbn1C1039G/+ | Nil | 8 | ++ | ++ | ++ | |
| 2011 | TGFβ NAb postnatal | 4 | = | + | |||
| Losartan postnatal | 3 | − | + | ||||
| RDEA 119 postnatal | 7 | ++ | − | − | |||
| S4+/− | Nil | 11 | = | = | = | ||
| S4+/−/Fbn1C1039G/+ | Nil | 26 | +++ | ++ | +++ | ||
| Merk [65] | Fbn1C1039G/+ | Nil | 5 | ++ | |||
| 2012 | TGFβ NAb Postnatal | 5 | = | ||||
| Losartan prenatal | 5 | = | |||||
| Xiong [83] | Fbn1mgR/mgR | Nil | 9 | +++ | +++ | +++ | +++ |
| 2012 | Losartan postnatal | 6 | ++ | ++ | ++ | ++ | |
| MMP2−/−/Fbn1mgR/mgR | 5 | ++ | ++ | +++ | |||
| Gallo [57] | Tgfbr1+/− | Nil | ≥9 | = | |||
| 2014 | Tgfbr1M318R/− | Nil | ≥9 | ++ | + | +++ | |
| Tgfbr2+/− | Nil | ≥9 | = | = | |||
| TGFβ | 3 | = | |||||
| Tgfbr2G357W/− | Nil | ≥9 | + | ++ | + | +++ | |
| TGFβ | 3 | − | |||||
| Propranolol postnatal | ≥8 | ++ | + | ++ | |||
| Losartan postnatal | ≥8 | = | = | = | = | ||
| Cook [56] | Fbn1mgR/mgR | Nil | 5 | +++ | +++ | +++ | |
| 2015 | TGFβ NAb Early postnatal | +++ | |||||
| TGFβ NAb Late postnatal | 5 | ++ | ++ | ++ | |||
| Losartan postnatal | 5 | + | = | = | |||
| TGFβ NAb + Losartan | 5 | ++ | ++++ | + | |||
| Lee [63] | Fbn1mgR/mgR | Nil | 9 | +++ | |||
| 2016 | Losartan postnatal | 8 | + | ||||
The hypothesis that excessive TGFβ signalling underlies aortic dilatation in MFS is not well supported by the experimental evidence. The initial study of Habashi et al. [7] employed Fbn1C1039G/+ mice, with the missense mutation affecting a cysteine residue as is commonly observed in human MFS. The Fbn1C1039G/+ aortas exhibit elastin disarray and increased collagen. Antagonism of Tgfβ signalling with a neutralizing antibody limited, but did not abolish, aortic dilatation and residual pSmad2 signalling was observed. Similar findings were reported by others [65,66]. In Fbn1mgR/mgR mice, Cook et al. observed little benefit of the Tgfβ neutralizing antibody upon aortic dilatation, and then only if given in later postnatal life [57].
The study of Tgfbr knock-out mice is informative [58]. Mice with Tgfbr1 or Tgfbr2 heterozygous knockout had 50% reduction in receptor density but no abnormal morphology and normal aortic size. In contrast, mice with heterozygous missense knock-in in Tgfbr1 or Tgfbr2 had the phenotype of Loeys-Dietz syndrome and increased aorta size, although interestingly some still had normal aortic size. Although cell surface expression and presentation of Tgfbr2 was normal in Tgfbr2G357W/− mutants, these mutants had reduced acute response to Tgfβ signalling (ie less pSmad2), however the Tgfbr2+/− haploinsufficient mutants had normal signalling and pSmad2 levels. In contrast, under steady-state conditions, the Tgfbr2G357W/− mutants actually had normal pSmad2 and Tgfβ signalling appeared normal in Tgfbr2G357W/− mice up to 8 weeks of age. From 12 to 24 weeks the pSmad2 and pERK levels in aorta increased in Tgfbr2G357W/− mice. Losartan, but not propranolol, reduced aortic growth and reduced pSmad2 and pERK. Treatment with the Tgfβ neutralizing antibody had no effect on aortic dilatation and did not normalize pSmad2 in Tgfbr2G357W/− mice. In AngII-infused mice, administration of Tgfβ neutralizing antibody is actually detrimental, leading to aortic rupture [62].
These studies indicate only a limited direct role for TGFβ in the pathogenesis of TAA and also suggest that the effect of TGFβ may be influenced by age and by the underlying FBN1 mutation.
In contrast, the experimental evidence does support a key role for AngII in the pathogenesis of TAA in MFS. Prenatal treatment with Losartan can prevent aortic dilatation but prenatal treatment with propranolol has little effect [7]. Concordant findings using postnatal Losartan treatment in the same Fbn1C1039G/+ murine model were reported by others [65,67] although the reduction in severity of aortic dilatation was not as great as that originally observed by Habashi et al. [7]. In the more severely affected Fbn1mgR/mgR model, both Nistala [68] and Lee [6] also reported that pos4tnatal treatment with Losartan reduced, but did not abolish, aortic dilatation. These studies indicate that Ang II signalling is an important contributor to the pathogenesis of aortic dilatation in MFS.
The apparent benefit of ARBs in attenuating aortic dilatation appear to be at least partly mediated by alternate Ang II signalling via AT2R [69]. In Fbn1C1039G/+ mice with knockout of the AT2R, Losartan only partly attenuates aortic dilatation and fails to normalize increased pSmad2 and pERK levels. Thus, both reduced AT1R and increased AT2R signalling are likely to underly the observed benefit of ARBs in limiting aortic dilatation.
The effects of beta-adrenergic blockers have been compared with ARBs in several studies. TGFβ can stimulate its own expression and activation and can increase beta-adrenergic receptor density and signalling. Beta-blockers can reduce TGFβ expression and ARBs can reduce both TGFβ expression and activation [70]. In the Fbn1C1039G/+ model, postnatal treatment with propranolol limited aortic dilatation and reduced pSmad2 levels, albeit to lesser extent than did Losartan [7]. In Tgfbr2G357W/− mice, propranol had similar effects [58]. These findings indicate that hemodynamics are likely to play a role in stimulating Ang II signalling in TAA pathogenesis.
7. Angiotensin II and aortic aneurysm in Marfan syndrome - clinical evidence
As with murine models, circulating TGFβ levels are increased in humans [3]. This observation would be consistent with a pathogenic model of impaired TGFβ sequestration in MFS. A short clinical study found that treatment with Losartan reduced the TGFβ levels, but of the 55 patients treated with Losartan, 45 also received beta-blockers and treatment with beta-blockers alone can reduce circulating TGFβ in humans [3]. As the degree of reduction in TGFβ was similar for beta-blockers alone and beta-blockers plus Losartan, and as the TGFβ levels remained much higher than controls, the clinical import of these intriguing findings remains uncertain.
It is also necessary to discriminate between total TGFβ and free TGFβ levels, as the latter are tightly regulated and are very low and indistinguishable between controls and MFS patients [3]. Thus, the elevated circulating TGFβ levels observed in some clinical studies may not reflect actual TGFβ signalling in the aortic wall.
In concert with experimental findings, it appears that ARBs may help preserve arterial function in patients with MFS. Six months treatment with losartan reduced central aortic systolic pressure augmentation, but had no effect on pulse wave velocity [71]. Beta-blockers had the opposite effect, suggesting that combination therapy may be more efficacious. If ARBs can ameliorate TAA in experimental models of MFS and can improve arterial function in patients with MFS, can these drugs prevent TAA in the patients with MFS?
An initial retrospective study reported that ARBs slowed aortic dilatation in MFS patients refractory to other medical therapy [10], but subsequent clinical studies have yielded discordant results. Three small studies and four large clinical trials are available for analysis (Table 2). Chiu et al. [72] reported that addition of Losartan to beta-blockers in a young patient group did result in reduced rate of aortic dilatation, although the annual rate of aortic dilatation amongst those receiving only beta-blockers was greater than that reported in any other clinical study. In contrast, neither the Ghent [73] nor the Vancouver [74] studies observed any apparent benefit from Losartan treatment, with the latter study conducted in a similar age group to the Taiwan study.
Table 2.
Clinical Trials of AngII Antagonism and TAA in Marfan syndrome.
| Study | Design | Intervention | n | % on BBa | Age (years) | Follow-up (years) | Basal aorta diameter (mm) | Mean change in aorta (mm/year) |
|---|---|---|---|---|---|---|---|---|
| COMPARE [8] | MRI, R, MC, C | Losartan | 116 | 75% | 36.8 ± 12.3 | 3.1 | 44.8 ± 5.6 | +0.19 |
| Usual treatment | 117 | 70% | 38.3 ± 13.4 | 43.7 ± 4.8 | +0.45 | |||
| Marfan-Sartan [9] | Echo, R, DB, MC | Losartan | 153 | 86% | 30.9 ± 15.9 | 3.5 | 39.1 ± 5.8 | +0.44 |
| Usual treatment | 150 | 86% | 28.9 ± 13.6 | 39.2 ± 5.9 | +0.51 | |||
| Pediatric Heart Network [11] | Echo, R, MC | Losartan | 305 | 56% | 11.0 ± 6.2 | 3.0 | 34.0 ± 0.7 | +0.75 |
| Atenolol | 303 | 57% | 11.5 ± 6.5 | 34.0 ± 0.7 | +0.69 | |||
| Barcelona/Madrid [12] | MRI, R, DB, MC, MG | Losartan | 60 | 0% | 26.1 ± 13.6 | 3.0 | 35.8 ± 5.8 | +0.37 |
| Atenolol | 56 | 100% | 24.3 ± 13.9 | 36.3 ± 6.5 | +0.47 | |||
| Taiwan [71] | Echo, R | Losartan + BB | 15 | 100% | 12.5 ± 5.0 | 2.9 | 34.3 ± 6.9 | +0.10 |
| BB | 13 | 100% | 13.7 ± 7.5 | 31.4 ± 4.7 | +0.89 | |||
| Ghent [72] | Echo, R, DB | Losartan + BB | 10 | 100% | 36.8 ± 6.9 | 3.0 | 40.8 ± 3.9 | +0.33 |
| BB | 10 | 100% | 35.4 ± 5.6 | 42.0 ± 2.5 | +0.33 | |||
| Vancouver [73] | Echo, R, SB | Losartan | 8 | 0% | 17.0 ± 4.0 | 1.0 | +0.10 | |
| Atenolol | 9 | 100% | 17.6 ± 2.8 | +0.10 |
BB = beta blocker treatment; C = core laboratory analysis of aortic images; DB = double-blinded; MC = multicentre trial; MG = multi-group trial; MRI = magnetic resonance imaging; R = randomised trial; SB = single-blinded; TAA = thoracic aortic aneurysm.
Refers to proportion taking beta-blockers before enrolment in study for [8].
The first large clinical trial to report was the COMPARE trial, which examined addition of losartan to baseline therapy, including beta-blockers [8]. On intention-to-treat analysis, losartan was associated with lesser aortic dilatation vs controls after 3 years. There was no correlation between change in systemic blood pressure and rate of aortic dilatation and no significant differences in clinical endpoints were found. This study was not blinded and the analysis excluded patients who progressed to needing aortic surgery during the study. Many participants in both losartan and control arms had no change in aortic diameter over 3 years and most patients also received beta-blocker treatment (losartan 75%, controls 70%). Furthermore, 22% of the losartan group did not actually take losartan throughout due to adverse effects (e.g. hypotension).
The Marfan Sartan study also examined addition of losartan to basal therapy, which was predominately beta-adrenergic blockers (91% of cohort) [9]. The study was a randomized, double-blinded study of a larger, albeit younger, patient group than in COMPARE. The study showed no effect of adding losartan upon the rate of aortic dilatation, despite reduction in blood pressure. As the trial had no true placebo group (i.e. no therapy) the essential question of whether either beta-blockers or losartan had any benefit upon aortic dilatation rates compared to no therapy at all remains unanswered.
Two studies have compared aortic dilatation between ARB monotherapy and beta-blocker monotherapy [11,12]. The Pediatric Heart Network study is the largest to date and compared atenolol and losartan, but like COMPARE, was not double-blinded [11]. The patient cohort was relatively young with the rationale that older individuals with MFS, who have not yet required surgery, are biased towards a milder phenotype and are less likely to demonstrate a treatment effect within the study time-frame. There was no significant difference in the baseline-adjusted annual rate of change in aortic Z score between atenolol and losartan. Interestingly, both treatment groups showed a decrease in aortic Z score during the study, however as there was no control group without therapy, the question of whether either atenolol or losartan had any net effect on changes in aortic diameter is again unanswered.
A fourth study reported by Forteza et al. was a randomized, parallel, double-blind study comparing atenolol and losartan monotherapy [12]. Using magnetic resonance imaging, changes in aortic diameter, indexed by body surface area (BSA), were determined after 36 months treatment. Aortic diameters increased to similar degree in both groups. The clinical benefit of ARBs in limiting aortic dilatation in MFS remains doubtful.
Although a full meta-analysis of all published clinical studies is planned [75], the fundamental question of whether or not ARBs and or beta-blockers are any better than placebo may not be answerable in the near future. It is noteworthy that experimental studies have shown that beta-blockers can attenuate the rate of aortic dilatation in MFS mice, suggesting that haemodynamics, rather than specific signalling pathways, are the key to intervention in MFS.
Subsequent studies have sought to identify potential risk-stratifiers, which may influence response to ARBs. Males with FBN1 mutations appear to be at greater risk of aortic dilatation and dissection than females [76]. A substudy of COMPARE provides preliminary evidence that MFS patient response to ARBs may be dependent upon the type of FBN1 mutation, with haploinsufficient mutations being more responsive than dominant negative mutations [77]. There is also some evidence suggesting that plasma TGFβ levels may be a marker of patients more likely to respond to ARB treatment [78].
8. Discrepancies between experimental and clinical studies
The weight of evidence from experimental studies suggests that ARBs can reduce the rate of aortic dilatation in murine models of MFS, yet no consistent benefit is observed in humans. There are several factors likely to underly this discrepancy.
The first consideration is the actual design of the clinical trials, of which 3 are too small to be sufficiently powered to detect likely reduction in aortic diameter with ARBs. Of the 4 larger trials, only 3 have >100 patients per treatment arm. The average rate of dilatation in the non-Losartan arms of the 4 larger trials was +0.53 mm/year with a mean standard deviation of approximately 1.3 mm/year. The trials were designed based upon older estimates of annual aortic dilatation of 1.0–1.5 mm/year. In order to detect a reduction in rate of aortic dilatation of 0.5 mm/year, it has been estimated that approximately 300 patients are required [79], yet the observed rates of dilatation are half those predicted and therefore even larger patient groups would be required.
Another factor is that the majority of patients receiving ARB treatment (4 of the 7 trials in Table 2) were also receiving beta-blocker treatment. Whilst the largest trial had suspended beta-blockers in the Losartan arm, the majority of those patients had previously been taking beta-blockers. As the experimental studies show, beta-blockers can reduce the rate of aortic dilatation and reverse some markers of adverse cell signalling. Thus, the power of these trials to demonstrate incremental benefit from ARBs is further impeded.
A third consideration is genetic variability in the human populations. Most experimental studies have used mice heterozygous for an Fbn1 allele encoding a cysteine substitution (C1039G), in an epidermal growth factor–like domain of fibrillin-1 with the premise that this represented the commonest mutation class in MFS [7]. On the other hand, haploinsufficiency in FBN1 appears to be associated with a more aggressive vascular phenotype in human MFS [18]. Importantly, with thousands of pathogenic mutations described in the FBN1 gene, the genetic substrate for human MFS is different from that of a single gene mouse model. The varied genetic background of humans can also influence drug metabolism, interaction and receptor sensitivity. Thus, the 1166C polymorphism of the AT1R gene is associated with increased arterial stiffness and losartan causes greater reduction in blood pressure in 1166C carriers [80]. The response to ARBs may also be influenced by co-inheritance of the ACE polymorphisms common in the general population, which have been shown to be a risk factor for TAA formation [81], whilst epigenetic influences add yet a further layer of complexity [49]. The heterogenous genetic background of humans with MFS, compared to experimental models, will mandate large patient numbers and/or risk stratified randomization, taking account of age, gender and key genetic descriptors (haploinsufficiency vs dominant negative), in order to better define the impact of ARBs upon aortic dilatation.
Finally, dose and timing of treatment with ARBs may be important. The dose per body weight of Losartan used in the experimental studies was much greater that the dose used in the human studies, raising the question of whether or not such high doses are required for benefit in humans, and if so what the mechanism would be given known pharmacokinetics of ARBs in humans. The timing of treatment with ARBs may be relevant, particularly given the change in angiotensin receptor expression occurring in early life. Murine studies have involved pre-natal or early post-natal treatment, however the youngest clinical trial group had a mean age of 11 years. Further experimental and clinical studies of ARBs in MFS at different ages would be helpful.
9. Conclusions
Despite the findings from experimental studies, the clinical evidence base does not yet support the routine use of ARBs as initial monotherapy in MFS. Beta-blockers, notwithstanding the relatively limited evidence supporting their use, remain the standard of care. There may be benefit from using an ARB as monotherapy if a patient cannot take a beta-blocker, as some of the clinical studies suggest that ARBs may be equivalent to beta-blockers. The weight of current evidence does not, however, support adding an ARB to a beta-blocker, if the patient is able to tolerate adequate dosing of the beta-blocker.
The present experience with trials of ARBs in MFS highlights the difficulties of undertaking intervention studies in a genetic condition with variable phenotype and incompletely characterised pathophysiology. The challenges of recruiting a sufficiently large patient population and randomization accounting for key risk stratifiers will require larger collaborative studies and longer follow-up times. The challenge of trial design which may require withholding beta-blockers for some participants will need to be addressed.
As we continue to discover the pathophysiology of TAA in conditions such as MFS, so new therapeutic options will emerge. The mechanisms of aortic dilatation in MFS appear to include multiple pathways, with functional redundancy, so that potential benefits of AT1R blockade may be obviated by alternate signalling. Thus, pharmacological Alk5 inhibition might potentially be beneficial and Alk5 inhibitors are in development [82]. An alternative treatment target could also be cardiac microRNAs (miR) and miR-21, which is regulated by SMADs upon TGFβ activation, has been shown to have a role in cell proliferation and fibrosis [83]. Antisense oligonucleotides against specific mRNA may have a future role in MFS treatment.
Conflict of interest
No potential conflicts of interest.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Judge D.P., Dietz H.C. Marfan's syndrome. Lancet. 2005;366(9501):1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson P.N., Arteaga-Solis E., Baldock C., Collod-Béroud G., Booms P., Dietz H.C., De Paepe A., Guo G., Handford P.A., Judge D.P., Kielty C.M., Loeys B., Milewicz D.M., Ramirez F., Ney A., Reinhardt D.P., Tiedemann K., Whiteman P., Godfrey M. The molecular genetics of Marfan syndrome and related disorders. J. Med. Genet. 2006;43:769–787. doi: 10.1136/jmg.2005.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matt P., Schoenhoff F., Habashi J., Holm T., Van Erp C., Loch D., Carlson O.D., Griswold B.F., Fu Q., De Backer J., Loeys B., Huso D.L., McDonnell N.B., Van Eyk J.E., Dietz H.C., the GenTAC Consortium Circulating transforming growth factor-β in Marfan syndrome. Circulation. 2009;120:526–532. doi: 10.1161/CIRCULATIONAHA.108.841981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nataatmadja M., West J., West M. Overexpression of transforming growth factor-β Is associated with increased hyaluronan content and impairment of repair in Marfan syndrome aortic aneurysm. Circulation. 2006;114(Suppl. I):I-371–I-377. doi: 10.1161/CIRCULATIONAHA.105.000927. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Vita J., Sanchez-Lopez E., Esteban V., Ruperez M., Egido J., Ruiz-Ortega M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111:2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- 6.Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 7.Habashi J.P., Judge D.P., Holm T.M., Cohn R.D., Loeys B.L., Cooper T.K., Myers L., Klein E.C., Liu G., Calvi C., Podowski M., Neptune E.R., Halushka M.K., Bedja D., Gabrielson K., Rifkin D.B., Carta L., Ramirez F., Huso D.L., Dietz H.C. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312(5770):117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groenink M., den Hartog A.W., Franken R., Radonic T., de Waard V., Timmermans J., Scholte A.J., van den Berg M.P., Spijkerboer A.M., Marquering H.A., Zwinderman A.H., Mulder B.J.M. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur. Heart J. 2013;34:3491–3500. doi: 10.1093/eurheartj/eht334. [DOI] [PubMed] [Google Scholar]
- 9.Milleron O., Arnoult F., Ropers J., Aegerter P., Detaint D., Delorme G., Attias D., Tubach F., Dupuis-Girod S., Plauchu H., Barthelet M., Sassolas F., Pangaud N., Naudion S., Thomas-Chabaneix J., Dulac Y., Edouard T., Wolf J.-E., Faivre L., Odent S., Basquin A., Habib G., Collignon P., Boileau C., Jondeau G. Marfan Sartan: a randomized, double-blind, placebo-controlled trial. Eur. Heart J. 2015;36:2160–2166. doi: 10.1093/eurheartj/ehv151. [DOI] [PubMed] [Google Scholar]
- 10.Brooke B.S., Habashi J.P., Judge D.P., Patel N., Loeys B., Dietz H.C. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N. Engl. J. Med. 2008;358:2787–2795. doi: 10.1056/NEJMoa0706585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacro R.V., Dietz H.C., Sleeper L.A., Yetman A.T., Bradley T.J., Colan S.D., Pearson G.D., Selamet Tierney E.S., Levine J.C., Atz A.M., Benson D.W., Braverman A.C., Chen S., De Backer J., Gelb B.D., Grossfeld P.D., Klein G.L., Lai W.W., Liou A., Loeys B.L., Markham L.W., Olson A.K., Paridon S.M., Pemberton V.L., Pierpont M.E., Pyeritz R.E., Radojewski E., Roman M.J., Sharkey A.M., Stylianou M.P., Burns Wechsler S., Young L.T., Mahony L., for the Pediatric Heart Network Investigators Atenolol versus losartan in children and young adults with Marfan's syndrome. N. Engl. J. Med. 2014;371:2061–2071. doi: 10.1056/NEJMoa1404731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forteza A., Evangelista A., Sánchez V., Teixidó-Turà G., Sanz P., Gutiérrez L., Gracia T., Centeno J., Rodríguez-Palomares J., Rufilanchas J.J., Cortina J., Ferreira-González I., García-Dorado D. Efficacy of losartan vs. atenolol for the prevention of aortic dilation in Marfan syndrome: a randomized clinical trial. Eur. Heart J. 2016;37:978–985. doi: 10.1093/eurheartj/ehv575. [DOI] [PubMed] [Google Scholar]
- 13.Wei H., Hu J.H., Angelov S.N., Fox K., Yan J., Enstrom R., Smith A., Dichek D.A. Aortopathy in a mouse model of Marfan syndrome is not mediated by altered transforming growth factor-β signaling. J. Am. Heart Assoc. 2017;6(1) doi: 10.1161/JAHA.116.004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallat Z., Ait-Oufella H., Tedgui A. The pathogenic transforming growth factor-β overdrive hypothesis in aortic aneurysms and dissections: a mirage? Circ. Res. 2017;120:1718–1720. doi: 10.1161/CIRCRESAHA.116.310371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeremy R.W., Robertson E., Lu Y., Hambly B.D. Perturbations of mechanotransduction and aneurysm formation in heritable aortopathies. Int. J. Cardiol. 2013;169:7–16. doi: 10.1016/j.ijcard.2013.08.056. [DOI] [PubMed] [Google Scholar]
- 16.Bee K.J., Wilkes D.C., Devereux R.B., Basson C.T., Hatcher C.J. TGFβRII mutations trigger aortic aneurysm pathogenesis by altering TGFβ2 signal transduction. Circ. Cardiovasc. Genet. 2012;5:621–629. doi: 10.1161/CIRCGENETICS.112.964064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franken R., Teixido-Tura G., Brion M., Forteza A., Rodriguez-Palomares J., Gutierrez L., Dorado D.G., Pals G., Mulder B.J.M., Evangelista A. Relationship between fibrillin-1 genotype and severity of cardiovascular involvement in Marfan syndrome. Heart. 2017;103:1795–1799. doi: 10.1136/heartjnl-2016-310631. [DOI] [PubMed] [Google Scholar]
- 18.Franken R., Groenink M., de Waard V., Feenstra H.M., Scholte A.J., Berg M.P., Pals G., Zwinderman A.H., Timmermans J., Mulder B.J. Genotype impacts survival in Marfan syndrome. Eur. Heart J. 2016;37:3285–3290. doi: 10.1093/eurheartj/ehv739. [DOI] [PubMed] [Google Scholar]
- 19.Schrijver I., Liu W., Odom R., Brenn T., Oefner P., Furthmayr H., Francke U. Premature termination mutations in FBN1: distinct effects on differential allelic expression and on protein and clinical phenotypes. Am. J. Hum. Genet. 2002;71:223–237. doi: 10.1086/341581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Judge D.P., Biery N.J., Keene D.R., Geubtner J., Myers L., Huso D.L., Sakai L.Y., Dietz H.C. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J. Clin. Invest. 2004;114:172–181. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross R., Klebanoff S.J. The smooth muscle cell. I. In vivo synthesis of connective tissue proteins. J. Cell Biol. 1971;50(1):159–171. doi: 10.1083/jcb.50.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao G., Fu Y., Cai Z., Yu F., Gong Z., Dai R., Hu Y., Zeng L., Xu Q., Kong W. Unspliced XBP1 confers VSMC homeostasis and prevents aortic aneurysm formation via FoxO4 interaction. Circ. Res. 2017;121:1331–1345. doi: 10.1161/CIRCRESAHA.117.311450. [DOI] [PubMed] [Google Scholar]
- 23.Ailawadi G., Moehle C.W., Pei H., Walton S.P., Yang Z., Kron I.L., Lau C.L., Owens G.K. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2009;138:1392–1399. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crosas-Molist E., Meirelles T., López-Luque J., Serra-Peinado C., Selva J., Caja L., del Blanco D.G., Uriarte J.J., Bertran E., Mendizábal Y., Hernández V., García-Calero C., Busnadiego O., Condom E., Toral D., Castellà M., Forteza A., Navajas D., Sarri E., Rodríguez-Pascual F., Dietz H.C., Fabregat I., Egea G. Vascular smooth muscle cell phenotypic changes in patients with Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015;35(4):960–972. doi: 10.1161/ATVBAHA.114.304412. [DOI] [PubMed] [Google Scholar]
- 25.Dale M., Fitzgerald M.P., Liu Z., Meisinger T., Karpisek A., Purcell L.N., Carson J.S., Harding P., Lang H., Koutakis P., Ri Batra, Mietus C.J., Casale G., Pipinos I., Baxter B.T., Xiong W. Premature aortic smooth muscle cell differentiation contributes to matrix dysregulation in Marfan syndrome. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemarie C.A., Schiffrin E.L. The angiotensin II type 2 receptor in cardiovascular disease. J. Renin-Angiotensin-Aldosterone Syst. 2010;11:19–31. doi: 10.1177/1470320309347785. [DOI] [PubMed] [Google Scholar]
- 27.Touyz R.M., He G., Deng L.-Y., Schiffrin E.L. Role of extracellular signal-regulated kinases in angiotensin II–stimulated contraction of smooth muscle cells from human resistance arteries. Circulation. 1999;99:392–399. doi: 10.1161/01.cir.99.3.392. [DOI] [PubMed] [Google Scholar]
- 28.Nickenig G., Röling J., Strehlow K., Schnabel P., Böhm M. Insulin induces upregulation of vascular AT1 receptor gene expression by posttranscriptional mechanisms. Circulation. 1998;98:2453–2460. doi: 10.1161/01.cir.98.22.2453. [DOI] [PubMed] [Google Scholar]
- 29.Barrett J.D., Zhang Z., Zhu J.H., Lee D.B., Ward H.J., Jamgotchian N., Hu M.S., Fredala A., Giordania M., Eggenaa P. Erythropoietin upregulates angiotensin receptors in cultured rat vascular smooth muscle cells. J. Hypertens. 1998;16:1749–1757. doi: 10.1097/00004872-199816120-00007. [DOI] [PubMed] [Google Scholar]
- 30.Ichiki T., Usui M., Kato M., Funakoshi Y., Ito K., Egashira K., Takeshita A. Downregulation of angiotensin II type 1 receptor gene transcription by nitric oxide. Hypertension. 1998;31(Suppl. 1):342–346. doi: 10.1161/01.hyp.31.1.342. [DOI] [PubMed] [Google Scholar]
- 31.Nickenig G., Sachinidis A., Ko Y., Vetter H. Regulation of angiotensin AT1 receptor gene expression during cell growth of vascular smooth muscle cells. Eur. J. Pharmacol. 1996;297:307–312. doi: 10.1016/0014-2999(95)00771-7. [DOI] [PubMed] [Google Scholar]
- 32.Taniyama Y., Ushio-Fukai M., Hitomi H., Rocic P., Kingsley M.J., Pfahnl C., Weber D.S., Alexander R.W., Griendling K.K. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am. J. Physiol. 2004;287 doi: 10.1152/ajpcell.00439.2003. [DOI] [PubMed] [Google Scholar]
- 33.Eguchi S., Matsumoto T., Motley E.D., Utsunomiya H., Inagami T. Identification of an essential signaling cascade for mitogen-activated protein kinase activation by angiotensin II in cultured rat vascular smooth muscle cells. Possible requirement of Gq-mediated p21ras activation coupled to a Ca2+/calmodulin-sensitive tyrosine kinase. J. Biol. Chem. 1996;271:14169–14175. doi: 10.1074/jbc.271.24.14169. [DOI] [PubMed] [Google Scholar]
- 34.Frank G.D., Saito S., Motley E.D., Sasaki T., Ohba M., Kuroki T., Inagami T., Eguchi S. Requirement of Ca(2+) and PKCδ for Janus kinase 2 activation by angiotensin II: involvement of PYK2. Mol. Endocrinol. 2002;16:367–377. doi: 10.1210/mend.16.2.0768. [DOI] [PubMed] [Google Scholar]
- 35.Griendling K.K., Sorescu D., Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 36.Santos R.A.S., Simoes e Silva A.C., Maric C., Silva D.M.R., Machado R.P., de Buhr I., Heringer-Walther S., Pinheiro S.V.B., Lopes M.T., Bader M., Mendes E.P., Lemos V.S., Campagnole-Santos M.J., Schultheiss H.-P., Speth R., Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. 2003;100(14):8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F., Hu Y., Xu Q., Ye S. Different effects of angiotensin II and angiotensin-(1-7) on vascular smooth muscle cell proliferation and migration. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCollum L.T., Gallagher P.E., Tallant E.A. Angiotensin-(1–7) attenuates angiotensin II-induced cardiac remodeling associated with upregulation of dual-specificity phosphatase 1. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H801–H810. doi: 10.1152/ajpheart.00908.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaiswal N., Tallant E.A., Jaiswal R.K., Diz D.I., Ferrario C.M. Differential regulation of prostaglandin synthesis by angiotensin peptides in porcine aortic smooth muscle cells: subtypes of angiotensin receptors involved. J. Pharmacol. Exp. Ther. 1993;265:664–673. [PubMed] [Google Scholar]
- 40.McKinney C.A., Fattah C., Loughrey C.M., Milligan G., Nicklin S.A. Angiotensin-(1–7) and angiotensin-(1–9): function in cardiac and vascular remodelling. Clin. Sci. 2014;126:815–827. doi: 10.1042/CS20130436. [DOI] [PubMed] [Google Scholar]
- 41.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 42.Flores-Munoz M., Work L.M., Douglas K., Denby L., Dominiczak A.F., Graham D., Nicklin S.A. Angiotensin-(1–9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin type 2 receptor. Hypertension. 2012;59:300–307. doi: 10.1161/HYPERTENSIONAHA.111.177485. [DOI] [PubMed] [Google Scholar]
- 43.Schmierer B., Hill C.S. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 44.Feng X.H., Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 45.Ruiz-Ortega M., Rodríguez-Vita J., Sanchez-Lopez E., Carvajal G., Egido J. TGF-β signaling in vascular fibrosis. Cardiovasc. Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Ebisawa T., Fukuchi M., Murakami G., Chiba T., Tanaka K., Imamura T., Miyazono K. Smurf1 interacts with transforming growth factor-β type I receptor through Smad7 and induces receptor degradation. J. Biol. Chem. 2001;276(16):12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- 47.Di Guglielmo G.M., Le Roy C., Goodfellow A.F., Wrana J.L. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat. Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 48.Siegerta A.-M., Serra-Peinadoa C., Gutiérrez-Martíneza E., Rodríguez-Pascualb F., Fabregatc I., Egeaa G. Altered TGF-β endocytic trafficking contributes to the increased signaling in Marfan syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:554–562. doi: 10.1016/j.bbadis.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Gomez D., Coyet A., Ollivier V., Jeunemaitre X., Jondeau G., Michel J.B., Vranckx R. Epigenetic control of vascular smooth muscle cells in Marfan and non-Marfan thoracic aortic aneurysms. Cardiovasc. Res. 2011;89:446–456. doi: 10.1093/cvr/cvq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu L. Regulation of Smad activities. Biochim. Biophys. Acta. 2006;1759:503–513. doi: 10.1016/j.bbaexp.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kretzschmar M., Doody J., Timokhina I., Massague J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pardali E., Goumans M.-J., ten Dijke P. Signaling by members of the TGF-β family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20:556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Thomas M., Docx C., Holmes A.M., Beach S., Duggan N., England K., Leblanc C., Lebret C., Schindler F., Raza F., Walker C., Crosby A., Davies R.J., Morrell N.W., Budd D.C. Activin-like kinase 5 (ALK5) mediates abnormal proliferation of vascular smooth muscle cells from patients with familial pulmonary arterial hypertension and is involved in the progression of experimental pulmonary arterial hypertension induced by monocrotaline. Am. J. Pathol. 2009;174(2):380–389. doi: 10.2353/ajpath.2009.080565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sengle G., Charbonneau N.L., Ono R.N., Sasaki T., Alvarez J., Keene D.R., Bachinger H.P., Sakai L.Y. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J. Biol. Chem. 2008;283(20):13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bandyopadhyay B., Han A., Dai J., Fan J., Li Y., Chen M., Woodley D.T., Li W. TβRI/Alk5-independent TβRII signaling to ERK1/2 in human skin cells according to distinct levels of TβRII expression. J. Cell Sci. 2011;124:19–24. doi: 10.1242/jcs.076505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wrighton K.H., Lin X., Feng X.-H. Phospho-control of TGF-β superfamily signaling. Cell Res. 2009;19:8–20. doi: 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cook J.R., Clayton N.P., Carta L., Galatioto J., Chiu E., Smaldone S., Nelson C.A., Cheng S., Wentworth B.M., Ramirez F. Dimorphic effects of transforming growth factor-β signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015;35:911–917. doi: 10.1161/ATVBAHA.114.305150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallo E.M., Loch D.C., Habashi J.P., Calderon J.F., Chen Y., Bedja D., van Erp C., Gerber E.E., Parker S.J., Sauls K., Judge D.P., Cooke S.K., Lindsay M.E., Rouf R., Myers L., ap Rhys C.M., Kent K.C., Norris R.A., Huso D.L., Dietz H.C. Angiotensin II–dependent TGF-β signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J. Clin. Invest. 2014;124(1):448–460. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kagami S., Border W.A., Miller D.E., Noble N.A. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J. Clin. Invest. 1994;93(6):2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrov V.V., Fagard R.H., Lijnen P.J. Transforming growth factor-β1 induces angiotensin-converting enzyme synthesis in rat cardiac fibroblasts during their differentiation to myofibroblasts. J. Renin-Angiotensin-Aldosterone Syst. 2000;1:342–352. doi: 10.3317/jraas.2000.064. [DOI] [PubMed] [Google Scholar]
- 61.Ruperez M., Lorenzo O., Blanco-Colio L.M., Esteban V., Egido J., Ruiz-Ortega M. Connective tissue growth factor is a mediator of angiotensin II–induced fibrosis. Circulation. 2003;108:1499–1505. doi: 10.1161/01.CIR.0000089129.51288.BA. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y., Ait-Oufella H., Herbin O., Bonnin P., Ramkhelawon B., Taleb S., Huang J., Offenstadt G., Combadière C., Rénia L., Johnson J.L., Tharaux P.-L., Tedgui A., Mallat Z. TGF-β activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Invest. 2010;120:422–432. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loeper F., Oosterhof J., van den Dorpel M., van der Linde D., Lu Y., Robertson E., Hambly B., Jeremy R. Ventricular-vascular coupling in Marfan and non-Marfan aortopathies. J. Am. Heart Assoc. 2016;5(11) doi: 10.1161/JAHA.116.003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee J.J., Galatioto J., Rao S., Ramirez F., Costa K.D. Losartan attenuates degradation of aorta and lung tissue micromechanics in a mouse model of severe Marfan syndrome. Ann. Biomed. Eng. 2016;44(10):2994–3006. doi: 10.1007/s10439-016-1616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holm T.M., Habashi J.P., Doyle J.J., Bedja D., Chen Y.-C., van Erp C., Lindsay M.E., Kim D., Schoenhoff F., Cohn R.D., Loeys B.L., Thomas C.J., Patnaik S., Marugan J.J., Judge D.P., Dietz H.C. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merk D.R., Chin J.T., Dake B.A., Maegdefessel L., Miller M.O., Kimura N., Tsao P.S., Iosef C., Berry G.J., Mohr F.W., Spin J.M., Alvira C.M., Robbins R.C., Fischbein M.P. miR-29b participates in early aneurysm development in Marfan syndrome. Circ. Res. 2012;110:312–324. doi: 10.1161/CIRCRESAHA.111.253740. [DOI] [PubMed] [Google Scholar]
- 67.Yang H.H.C., Kim J.M., Chum E., van Breemen C., Chung A.W.Y. Effectiveness of combination of losartan potassium and doxycycline versus single-drug treatments in the secondary prevention of thoracic aortic aneurysm in Marfan syndrome. J. Thorac. Cardiovasc. Surg. 2010;140:305–312. doi: 10.1016/j.jtcvs.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 68.Nistala H., Lee-Arteaga S., Carta L., Cook J.R., Smaldone S., Siciliano G., Rifkin A.N., Dietz H.C., Rifkin D.B., Ramirez F. Differential effects of alendronate and losartan therapy on osteopenia and aortic aneurysm in mice with severe Marfan syndrome. Hum. Mol. Genet. 2010;19:4790–4798. doi: 10.1093/hmg/ddq409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Habashi J.P., Doyle J.J., Holm T.M., Aziz H., Schoenhoff F., Bedja D., Chen Y.C., Modiri A.N., Judge D.P., Dietz H.C. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung A.W.Y., Yang H.H.C., Radomski M.W., van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in Marfan syndrome through the inhibition of matrix etalloproteinase-2 and -9. Circ. Res. 2008;102:e73–e85. doi: 10.1161/CIRCRESAHA.108.174367. [DOI] [PubMed] [Google Scholar]
- 71.Bhatt A.B., Buck J.S., Zuflacht J.P., Milian J., Kadivar S., Gauvreau K., Singh M.N., Creager M.A. Distinct effects of losartan and atenolol on vascular stiffness in Marfan syndrome. Vasc. Med. 2015;20(4):317–325. doi: 10.1177/1358863X15569868. [DOI] [PubMed] [Google Scholar]
- 72.Chiu H.-H., Wu M.-H., Wang J.-K., Lu C.-W., Chiu S.-N., Chen C.-A., Lin M.-T., Hu F.-C. Losartan added to β-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin. Proc. 2013;88(3):213–308. doi: 10.1016/j.mayocp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Muino-Mosquera L., De Nobele S., Devos D., Campens L., De Paepe A., De Backer J. Efficacy of losartan as add-on therapy to prevent aortic growth and ventricular dysfunction in patients with Marfan syndrome: a randomized, double-blind clinical trial. Acta Cardiol. 2017;72(6):616–624. doi: 10.1080/00015385.2017.1314134. [DOI] [PubMed] [Google Scholar]
- 74.Sandor G.G.S., Alghamdi M.H., Raffin L.A., Potts M.T., Williams L.D., Potts J.E., Kiess M., van Breemen C. A randomized, double blind pilot study to assess the effects of losartan vs. atenolol on the biophysical properties of the aorta in patients with Marfan and Loeys–Dietz syndromes. Int. J. Cardiol. 2015;179:470–475. doi: 10.1016/j.ijcard.2014.11.082. [DOI] [PubMed] [Google Scholar]
- 75.Pitcher A., Emberson J., Lacro R.V., Sleeper L.A., Stylianou M., Mahony L., Pearson G.D., Groenink M., Mulder B.J., Zwinderman A.H., De Backer J., De Paepe A.M., Arbustini E., Erdem G., Jin X.Y., Flather M.D., Mullen M.J., Child A.H., Forteza A., Evangelista A., Chiu H.-H., Wu M.-H., Sandor G., Bhatt A.B., Creager M.A., Devereux R.B., Loeys B., Forfar J.C., Neubauer S., Watkins H., Boileau C. Design and rationale of a prospective, collaborative meta-analysis of all randomized controlled trials of angiotensin receptor antagonists in Marfan syndrome, based on individual patient data: a report from the Marfan Treatment trialists' Collaboration. Am. Heart J. 2015;169:605–612. doi: 10.1016/j.ahj.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Detaint D., Faivre L., Collod-Beroud G., Child A.H., Loeys B.L., Binquet C., Gautier E., Arbustini E., Mayer K., Arslan-Kirchner M., Stheneur C., Halliday D., Beroud C., Bonithon-Kopp C., Claustres M., Plauchu H., Robinson P.N., Kiotsekoglou A., De Backer J., Ades L., Francke U., De Paepe A., Boileau C., Jondeau G. Cardiovascular manifestations in men and women carrying a FBN1 mutation. Eur. Heart J. 2010;31:2223–2229. doi: 10.1093/eurheartj/ehq258. [DOI] [PubMed] [Google Scholar]
- 77.Franken R., den Hartog A.W., Radonic T., Micha D., Maugeri A., van Dijk F.S., Meijers-Heijboer H.E., Timmermans J., Scholte A.J., van den Berg M.P., Groenink M., Mulder B.J.M., Zwinderman A.H., de Waard V., Pals G. Beneficial outcome of Losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ. Cardiovasc. Genet. 2015;8:383–388. doi: 10.1161/CIRCGENETICS.114.000950. [DOI] [PubMed] [Google Scholar]
- 78.Franken R., Radonic T., den Hartog A.W., Groenink M., Pals G., van Eijk M., Lutter R., Mulder B.J.M., Zwinderman A.H., de Waard V., The COMPARE study group The revised role of TGF-β in aortic aneurysms in Marfan syndrome. Neth. Hear. J. 2015;23:116–121. doi: 10.1007/s12471-014-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Radonic T., de Witte P., Baars M.J.H., Zwinderman A.H., Mulder B.J.M., Groenink M., COMPARE study group Losartan therapy in adults with Marfan syndrome: study protocol of the multi-center randomized controlled COMPARE trial. Trials. 2010;11:3. doi: 10.1186/1745-6215-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benetos A., Gautier S., Ricard S., Topouchian J., Asmar R., Poirier O., Larosa E., Guize L., Safar M., Soubrier F., Cambien F. Influence of angiotensin-converting enzyme and angiotensin II type 1 receptor gene polymorphisms on aortic stiffness in normotensive and hypertensive patients. Circulation. 1996;94:698–703. doi: 10.1161/01.cir.94.4.698. [DOI] [PubMed] [Google Scholar]
- 81.Foffa I., Murzi M., Mariani M., Mazzone A.M., Glauber M., Ali L.A., Andreassi M.G. Angiotensin-converting enzyme insertion/deletion polymorphism is a risk factor for thoracic aortic aneurysm in patients with bicuspid or tricuspid aortic valves. J. Thorac. Cardiovasc. Surg. 2012;144:390–395. doi: 10.1016/j.jtcvs.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 82.Akhurst R.J., Hata A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Rooij E., Olson E.N. Searching for miR-acles in cardiac fibrosis. Circ. Res. 2009;104:138–140. doi: 10.1161/CIRCRESAHA.108.192492. [DOI] [PMC free article] [PubMed] [Google Scholar]