Abstract
Several clinical, electrocardiographic (ECG) and electrophysiological markers have been proposed to provide optimal risk stratification in patients with Brugada syndrome (BrS). Of the different markers, only a spontaneous type 1 ECG pattern has clearly shown a sufficiently high predictive value. This review article highlights specific ECG markers based on depolarization and/or repolarization that have been associated with an increased risk of arrhythmic events in patients with BrS.
Keywords: Brugada syndrome, Electrocardiogram, Sudden cardiac death
1. Introduction
The Brugada syndrome (BrS) is considered a primary arrhythmogenic disorder associated with increased risk of sudden cardiac death due to polymorphic ventricular arrhythmias in patients without overt structural cardiac abnormalities [1,2]. The syndrome is responsible for 4–12% of all sudden deaths and at least 20% of deaths in patients with structurally normal hearts [3].
There are two main, not necessarily mutually exclusive, mechanisms on the pathophysiologic basis of BrS: the depolarization and the repolarization hypotheses [4,5], with much insights derived from pre-clinical animal models [[6], [7], [8], [9], [10]]. According to the depolarization hypothesis, the delayed depolarization of the right ventricular outflow tract (RVOT) creates a potential difference between it and the right ventricle. The repolarization model is related to the higher level of transmural dispersion of repolarization, driven by the loss of the spike and dome action potential morphology at right ventricular epicardium, involving in local and transmural repolarization alterations leading to phase 2 re-entry [2,11].
Although the diagnosis of asymptomatic BrS patients may be achieved relatively easily through ECG, the risk stratification of these patients has still been one of the most challenging and - up to now - unresolved clinical problems. Currently, guidelines provide clear recommendations for the management of symptomatic patients [1]. On the contrary, there is no consensus for the asymptomatic patients and the management depends on evaluation of different parameters. Several clinical, ECG and electrophysiological markers have been proposed to provide optimal risk stratification [1]. This review article briefly describes current knowledge on the assessment of the risk of arrhythmic events in patients with BrS based on ECG markers.
2. Brugada syndrome diagnosis and ECG pattern
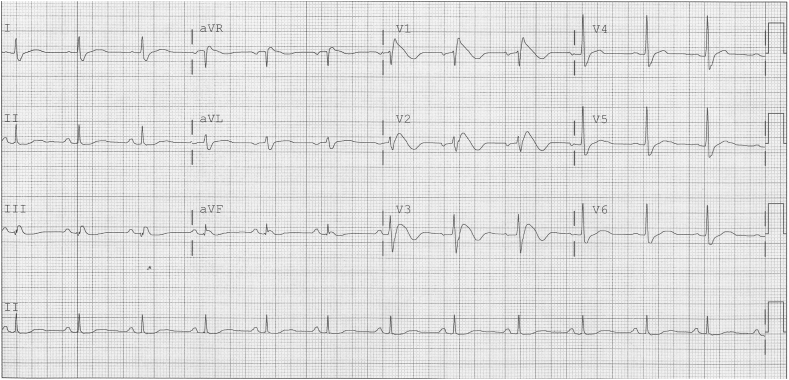
The diagnosis of BrS is based on the characteristic coved-type ST-segment elevation in at least one of the right precordial leads V1 and V2 positioned in the 2nd, 3rd or 4th intercostal space (Fig. 1). The diagnostic type 1 electrocardiogram (ECG) may occur spontaneously or after drug challenge with a sodium channel blocker (ajmaline, flecainide, procainamide or pilsicainide) which can convert type 2 or type 3 to type 1 ECG pattern [1]. Type 2 ECG pattern shows a high take-off ≥2 mm and a saddleback ST-configuration ≥1 mm, while type 3 pattern is characterized by J-point elevation of <2 mm and either a saddleback or coved-type ST-segment elevation of ≤1 mm.
Fig. 1.
Spontaneous type 1 ECG pattern of BrS in lead V1.
Reproduced from [69] with permission.
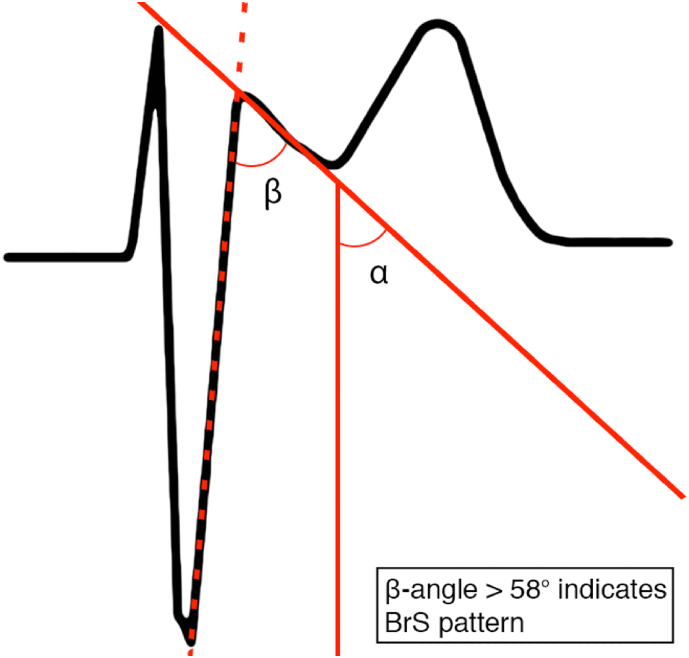
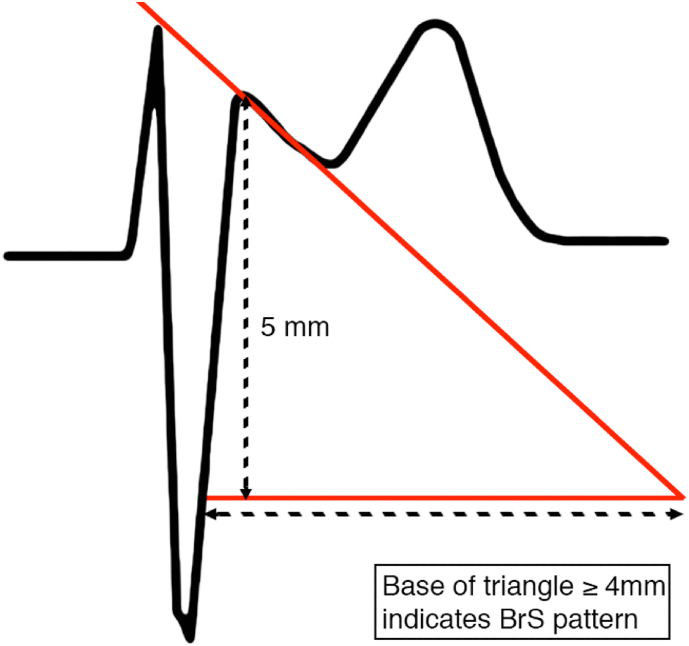
Although the type 1 ECG pattern is diagnostic for BrS, the type 2 pattern requires diagnostic distinction between true BrS and different clinical entities (myocardial ischemia, pulmonary embolism, electrolyte abnormalities), the so called Brugada phenocopies [12], which may develop a similar ECG pattern in mimicking conditions such as coronary artery dissection [13], myocardial infarction [14], hyperkalaemia [15], or pulmonary embolism [16]. Therefore, a careful assessment of the ECG is required in order to make an accurate diagnosis. The use of the β-angle (≥58°) (Fig. 2) and base of the triangle (Fig. 3) in type 2 Brugada ECG pattern may distinct a true Brugada ECG from other conditions with high sensitivity and specificity [17,18].
Fig. 2.
Beta angle helps distinguish type 2 Brugada pattern from Brugada phenocopies.
Fig. 3.
Base of triangle helps distinguish type 2 Brugada pattern from Brugada phenocopies.
3. High risk ECG markers in Brugada syndrome
There has been evidence that subjects with spontaneous coved type ECG pattern are at higher risk than those with drug-induced ECG for arrhythmic events [19,20]. A meta-analysis showed that the presence of a spontaneous type 1 Brugada ECG predicts a more malignant natural history exhibiting a 3-fold to 4-fold increased risk of adverse events compared to those with a drug-induced Brugada ECG pattern [21]. A recent meta-analysis reported that the prevalence of a type 1 pattern was higher in male, Asians, adults, and fever subjects [22]. Of the different markers, only the presence of a spontaneous type 1 ECG pattern has clearly shown sufficiently high risk predictive value. Nevertheless, as reviewed previously [23], fragmented QRS complexes and early repolarization are associated with a higher risk of adverse outcomes in several studies. Additional ECG parameters based on depolarization and/or repolarization may provide incremental value for risk stratification of subjects with BrS phenotype [24].
4. Depolarization ECG markers
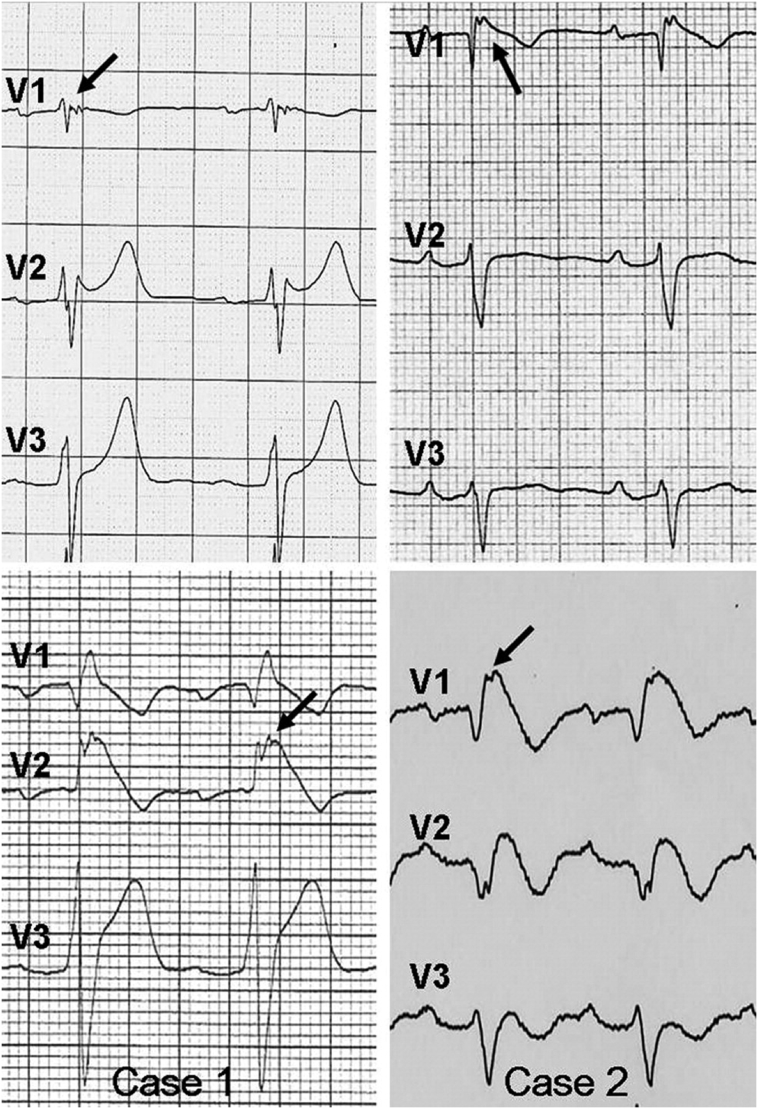
Among the depolarization ECG markers, the PRELUDE study confirmed that QRS-fragmentation (f-QRS), defined as the presence of multiple spikes (fragmented components) within the QRS in precordial leads V1 and/or V2–V3, is an independent predictor for future arrhythmias in BrS (Fig. 4) [19]. Morita et al. reported that fQRS is more frequently observed in BrS patients with VF (85%) and syncope (50%) compared to asymptomatic patients (34%) [25]. Indeed, a meta-analysis conducted by our group reported that the presence of fQRS was associated with a 3.9-fold increase in the risk of future arrhythmic events in BrS [26]. Tokioka et al. have also found that f-QRS was associated with PQ and QRS prolongation, demonstrating that depolarization defects in the atria and/or specialized conduction system, and ventricles are important factors for ventricular fibrillation (VF) development [27].
Fig. 4.
Fragmented QRS complex.
Reproduced from [27] with permission.
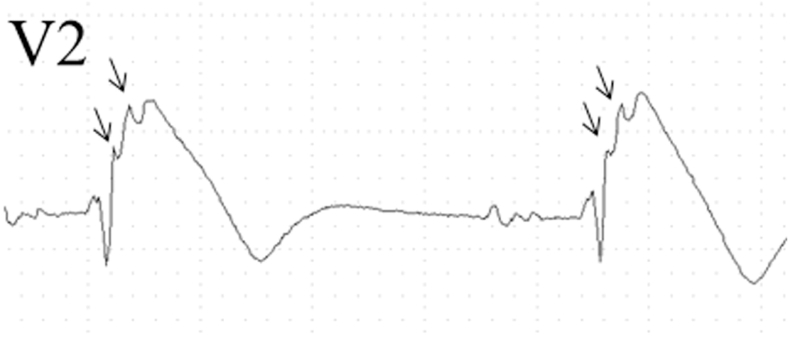
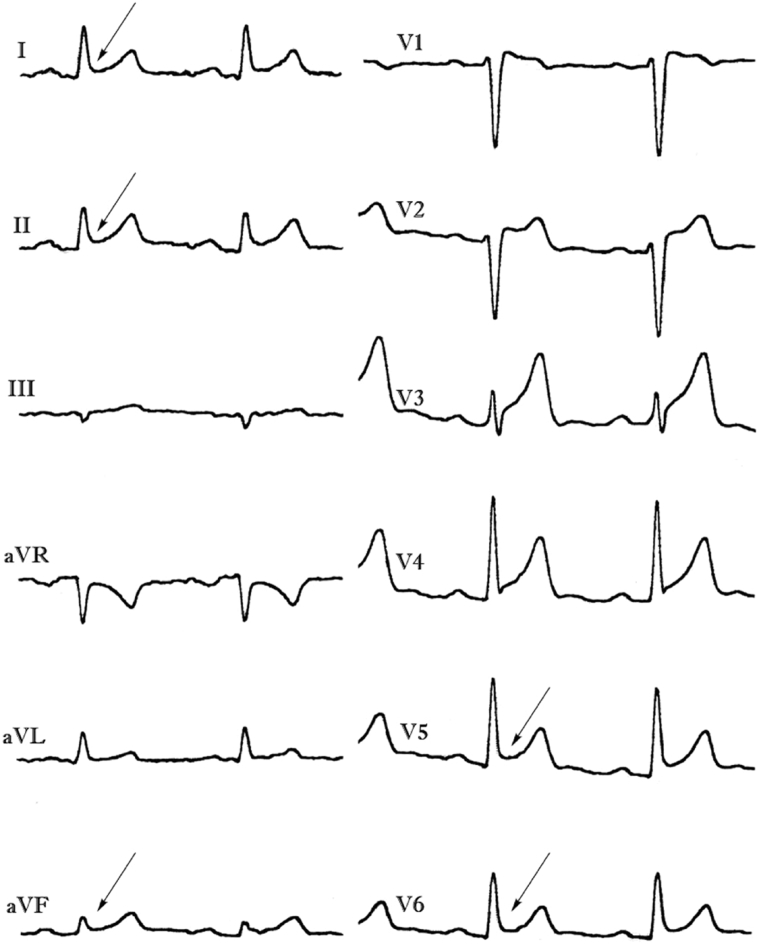
Low-amplitude signals in right precordial leads, the so-called epsilon-like waves have been reported as additional ECG findings of BrS, and possibly reflect slow conduction in the right ventricle. In particular, we found that epsilon-like waves in leads V1–V3 were observed in 12.7% of patients with spontaneous or drug-induced type 1 Brugada ECG pattern, while localized prolongation (>110 ms) of the QRS complex in leads V1–V3, QRS duration ratio in (V1 + V2 + V3)/(V4 + V5 + V6) ≥ 1.2, and prolonged S wave upstroke (>55 ms) in leads V1–V3 were seen in 48.8%, 29.8%, and 40.4% of subjects, respectively (Fig. 5) [28].
Fig. 5.
Baseline (top) and after drug challenge (bottom) electrocardiographic tracings showing spontaneous epsilon-like waves (arrows) in subjects with Brugada phenotype.
Reproduced from [28] with permission.
A prolonged QRS duration in leads II, V2 and V6 has been associated with an increased risk for arrhythmic events [29,30]. Ohkubo et al. have demonstrated that a prolonged QRS duration >120 ms in lead V2 of predicts ventricular arrhythmia and/or syncope in BrS patients [31].
Recently, Calo et al. confirmed the presence of a wide (≥0.1 mV) and/or large S-wave (≥40 ms) in lead I as a powerful predictor of ventricular arrhythmias. The authors demonstrated that the presence of a wide S-wave in lead I is related to the delayed activation in the RVOT [32]. Babai et al., have shown that he presence aVR sign which is defined as R wave ≥0.3 mV or R/q ≥ 0.75 in lead aVR, may reflect more right ventricular conduction delay and consequently increased risk for development of arrhythmic events in BrS patient [33].
Additional studies showed that the presence of late potentials (LPs) detected by signal-averaged ECG is associated with increased risk of arrhythmic events. Huang et al. reported more frequent detection of LPs in symptomatic than asymptomatic patients with BrS (91.7% vs 36.8% respectively) and more arrhythmic events in patients with positive LPs (72.4%) than in those without LPs (14.3%) during a mean follow-up of 33.8 months [34]. Moreover, a prospective study of 124 patients from Japan found that LPs by signal-averaged ECGs had a 92% sensitivity but only 46% specificity for the endpoint of sudden death or ventricular tachyarrhythmias [35]. Nevertheless, LPs alone in both symptomatic and asymptomatic patients show limited prognostic value. We believe that LP could be a powerful risk stratification tool when used with other specific ECG markers. For example, the same prospective study found that when LPs were combined with spontaneous change in the ST segment, the specificity increased to 72% with a slight decrease in sensitivity to 82% [35].
5. Repolarization ECG markers
Among the repolarization ECG markers, the presence of inferolateral early repolarization (ER) pattern triplicates the risk of future arrhythmic events in BrS patients (Fig. 6) [27,36]. BrS and ER syndrome display several clinical and pathophysiological similarities [37,38]. The ER pattern in inferior and/or lateral leads is frequently seen in BrS (12–15%) and several studies demonstrated the association between the presence of ER pattern and the risk of malignant arrhythmic events [[39], [40], [41]]. In a multicentre study, Takagi et al. confirmed a higher incidence of cardiac events in BrS patients with J wave in multiple leads and horizontal ST-segment morphology after J wave may indicate a highly arrhythmogenic substrate in patients with BS than those without ER pattern [40]. Our group has recently conducted a meta-analysis on the prognostic significance of ER pattern in BrS. Overall, BrS patients with ER pattern displayed an increased risk of arrhythmic events compared to patients without ER (odds ratio [OR]: 3.29) [36].
Fig. 6.
Inferolateral early repolarization pattern.
Reproduced from [36] with permission.
A prolonged QTc interval, which reflects delayed cellular repolarization, has been associated with an increased risk for VF/sudden cardiac death [27,42,43]. Moreover, an exacerbated transmural dispersion of repolarization across the ventricular myocardium has been suggested to underlie arrhythmogenesis in BrS [44,45]. These are reflected by a longer interval between the peak and the end of the T wave (Tpeak-Tend interval) [46], a higher Tpeak-Tend/QT ratio, a higher Tpeak-Tend dispersion in precordial leads, all of which are observed in BrS patients with recurrent arrhythmic events [43]. Similarly, Maury et al. showed that the Tpeak-Tend interval is highly related to arrhythmic events of the BrS patients; they reported that an increased Tpeak-Tend interval and dispersion was significantly higher in patients with sudden death and appropriate ICD therapies [47]. Additionally, Zumhagen et al. reported that Tpeak-Tend interval and Tpeak-Tend/QT ratio in V1 were higher in high-risk BrS patients than in other BrS patients [48]. However, the largest retrospective study to date did not demonstrate significant differences in Tpeak-Tend, Tpeak-Tend/QT, maximum Tpeak-Tend, and Tpeak-Tend dispersion between asymptomatic patients and those with syncope and malignant arrhythmias [49]. Nevertheless, a recent meta-analysis conducted by our group found that prolonged Tpeak-Tend was associated with a 5.6-fold increase in the risk of ventricular tachycardia, ventricular fibrillation and/or sudden cardiac death in BrS [50]. Although Tpeak-Tend/QT ratio, Tpeak-Tend dispersion and more complex derivations of Tpeak-Tend interval are also predictive of arrhythmogenesis, it is unclear whether these have better sensitivity or specificity when compared to Tpeak-Tend interval alone [49,[51], [52], [53]]. Since Tpeak-Tend interval is simpler and less difficult to calculate, we would recommend its use as a surrogate marker for higher dispersion of repolarization [54]. It should be noted that although Tpeak-Tend interval correlates with transmural dispersion of repolarization, experimental studies suggest that is a marker of global dispersion, because Tpeak and Tend coincided with earliest and latest end of repolarization, respectively [46,[54], [55], [56]].
In addition to the static substrates mentioned above, dynamic factors are also important for initiation or maintenance of ventricular arrhythmias [57]. Firstly, T-wave alternans (TWA) has been reported in patients with BrS after sodium channel blocker administration. The investigators found that patients with TWA had a significantly higher incidence of spontaneous VF and syncope than patients without TWA [58]. Furthermore, ECG changes in patients with BrS during the exercise testing are of great prognostic importance. Makimoto et al. showed that augmentation of ST-segment elevation during recovery time of the exercise testing can be observed in patients BrS with high specificity and can be associated with a higher cardiac event rate particularly for patients with syncope alone and for asymptomatic patients [59].
6. Atrial and atrioventricular conduction abnormalities
First degree atrioventricular block (AVB) is frequent in patients with BrS and independently associated with sudden death or appropriate implantable cardioverter-defibrillator therapies [70]. Miyamoto et al. showed that a PQ ≥ 170 ms in lead V1 wan an independent risk factor of arrhythmic events associated with poor prognosis [60]. Finally, atrial arrhythmias and mainly atrial fibrillation often occurred in subjects with BrS [61]. Spontaneous atrial fibrillation has been associated with a high incidence of syncopal episodes and documented ventricular fibrillation in BrS patients [42].
7. Concluding remarks
Although several ECG findings were found to correlate with an increased risk for malignant arrhythmic events, their sensitivity, specificity and accuracy are relatively low with limited prognostic value. Moreover, due to the relative scarcity of prospective studies, much of the evidence came from retrospective data, which may have higher degrees of bias. However, the combination of specific depolarization and repolarization abnormalities seems to provide more reliable risk stratification in asymptomatic BrS patients. The development of multiparametric risk scores assessing different clinical factors, genetic status, standard ECG parameters, ECG imaging and invasive electrophysiological mapping may provide more accurate risk stratification [[62], [63], [64], [65], [66], [67], [68]]. Further prospective studies may be needed to establish a more definite diagnostic model in order to identify high risk patients with BrS.
Conflicts of interest
The authors report no relationships that could be construed as a conflict of interest.
Contributor Information
Gary Tse, Email: tseg@cuhk.edu.hk.
Konstantinos P. Letsas, Email: k.letsas@gmail.com.
References
- 1.Antzelevitch C., Yan G.X., Ackerman M.J., Borggrefe M., Corrado D., Guo J. J-wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Heart Rhythm. 2016;13:e295–324. doi: 10.1016/j.hrthm.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antzelevitch C., Patocskai B. Brugada syndrome: clinical, genetic, molecular, cellular, and ionic aspects. Curr. Probl. Cardiol. 2016;41:7–57. doi: 10.1016/j.cpcardiol.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antzelevitch C., Brugada P., Borggrefe M., Brugada J., Brugada R., Corrado D. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 4.Tse G., Liu T., Li K.H., Laxton V., Chan Y.W., Keung W. Electrophysiological mechanisms of Brugada syndrome: insights from pre-clinical and clinical studies. Front. Physiol. 2016;7:467. doi: 10.3389/fphys.2016.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilde A.A., Postema P.G., Di Diego J.M., Viskin S., Morita H., Fish J.M. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J. Mol. Cell. Cardiol. 2010;49:543–553. doi: 10.1016/j.yjmcc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tse G., Wong S.T., Tse V., Yeo J.M. Depolarization vs. repolarization: what is the mechanism of ventricular arrhythmogenesis underlying sodium channel haploinsufficiency in mouse hearts? Acta Physiol (Oxford) 2016;218:234–235. doi: 10.1111/apha.12694. [DOI] [PubMed] [Google Scholar]
- 7.Choy L., Yeo J.M., Tse V., Chan S.P., Tse G. Cardiac disease and arrhythmogenesis: mechanistic insights from mouse models. Int. J. Cardiol. Heart Vasc. 2016;12:1–10. doi: 10.1016/j.ijcha.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerrone M., Lin X., Zhang M., Agullo-Pascual E., Pfenniger A., Chkourko Gusky H. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation. 2014;129:1092–1103. doi: 10.1161/CIRCULATIONAHA.113.003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szel T., Koncz I., Antzelevitch C. Cellular mechanisms underlying the effects of milrinone and cilostazol to suppress arrhythmogenesis associated with Brugada syndrome. Heart Rhythm. 2013;10:1720–1727. doi: 10.1016/j.hrthm.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minoura Y., Panama B.K., Nesterenko V.V., Betzenhauser M., Barajas-Martinez H., Hu D. Effect of Wenxin Keli and quinidine to suppress arrhythmogenesis in an experimental model of Brugada syndrome. Heart Rhythm. 2013;10:1054–1062. doi: 10.1016/j.hrthm.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meregalli P.G., Wilde A.A., Tan H.L. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc. Res. 2005;67:367–378. doi: 10.1016/j.cardiores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Baranchuk A., Nguyen T., Ryu M.H., Femenia F., Zareba W., Wilde A.A. Brugada phenocopy: new terminology and proposed classification. Ann. Noninvasive Electrocardiol. 2012;17:299–314. doi: 10.1111/j.1542-474X.2012.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrizo A.G., Goransky A., Baranchuk A. Brugada phenocopy during right coronary artery dissection. J. Electrocardiol. 2017;50:969–971. doi: 10.1016/j.jelectrocard.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Alper A.T., Tekkesin A.I., Cinier G., Turkkan C., Baranchuk A. First description of a Brugada phenocopy in the inferior leads in the context of an acute inferior myocardial infarction. Europace. 2017;19:1219. doi: 10.1093/europace/eux182. [DOI] [PubMed] [Google Scholar]
- 15.Abu Shama R., Bayes de Luna A., Baranchuk A. Tachycardia-dependent Brugada phenocopy due to hyperkalemia. J. Cardiovasc. Electrophysiol. 2017;28:1084–1085. doi: 10.1111/jce.13269. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N., Liu T., Tse G., Yu S., Fu H., Xu G. Brugada phenocopy in a patient with acute pulmonary embolism presenting with recurrent syncope. Oxf. Med. Case Reports. 2017;2017 doi: 10.1093/omcr/omx014. (omx014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottschalk B.H., Garcia-Niebla J., Anselm D.D., Glover B., Baranchuk A. Methods for improving the diagnosis of a Brugada ECG pattern. Ann. Noninvasive Electrocardiol. 2016;21:210–213. doi: 10.1111/anec.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottschalk B.H., Garcia-Niebla J., Anselm D.D., Jaidka A., De Luna A.B., Baranchuk A. New methodologies for measuring Brugada ECG patterns cannot differentiate the ECG pattern of Brugada syndrome from Brugada phenocopy. J. Electrocardiol. 2016;49:187–191. doi: 10.1016/j.jelectrocard.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Priori S.G., Gasparini M., Napolitano C., Della Bella P., Ottonelli A.G., Sassone B. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J. Am. Coll. Cardiol. 2012;59:37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 20.Bayoumy A., Gong M.Q., Christien Li K.H., Wong S.H., Wu W.K., Li G.P. Spontaneous type 1 pattern, ventricular arrhythmias and sudden cardiac death in Brugada syndrome: an updated systematic review and meta-analysis. J. Geriatr. Cardiol. 2017;14:639–643. doi: 10.11909/j.issn.1671-5411.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gehi A.K., Duong T.D., Metz L.D., Gomes J.A., Mehta D. Risk stratification of individuals with the Brugada electrocardiogram: a meta-analysis. J. Cardiovasc. Electrophysiol. 2006;17:577–583. doi: 10.1111/j.1540-8167.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 22.Shi S., Barajas-Martinez H., Liu T., Sun Y., Yang B., Huang C. Prevalence of spontaneous Brugada ECG pattern recorded at standard intercostal leads: a meta-analysis. Int. J. Cardiol. 2017;1(254):151–156. doi: 10.1016/j.ijcard.2017.11.113. [DOI] [PubMed] [Google Scholar]
- 23.Adler A., Rosso R., Chorin E., Havakuk O., Antzelevitch C., Viskin S. Risk stratification in Brugada syndrome: clinical characteristics, electrocardiographic parameters, and auxiliary testing. Heart Rhythm. 2016;13:299–310. doi: 10.1016/j.hrthm.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 24.Tse G., Yan B.P. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace. 2017;19:712–721. doi: 10.1093/europace/euw280. [DOI] [PubMed] [Google Scholar]
- 25.Morita H., Kusano K.F., Miura D., Nagase S., Nakamura K., Morita S.T. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 26.Meng L., Letsas K.P., Baranchuk A., Shao Q., Tse G., Zhang N. Meta-analysis of fragmented QRS as an electrocardiographic predictor for arrhythmic events in patients with Brugada syndrome. Front. Physiol. 2017;8:678. doi: 10.3389/fphys.2017.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tokioka K., Kusano K.F., Morita H., Miura D., Nishii N., Nagase S. Electrocardiographic parameters and fatal arrhythmic events in patients with Brugada syndrome: combination of depolarization and repolarization abnormalities. J. Am. Coll. Cardiol. 2014;63:2131–2138. doi: 10.1016/j.jacc.2014.01.072. [DOI] [PubMed] [Google Scholar]
- 28.Letsas K.P., Efremidis M., Weber R., Korantzopoulos P., Protonotarios N., Prappa E. Epsilon-like waves and ventricular conduction abnormalities in subjects with type 1 ECG pattern of Brugada syndrome. Heart Rhythm. 2011;8:874–878. doi: 10.1016/j.hrthm.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 29.Letsas K.P., Weber R., Efremidis M., Korantzopoulos P., Astheimer K., Charalampous C. Long-term prognosis of asymptomatic individuals with spontaneous or drug-induced type 1 electrocardiographic phenotype of Brugada syndrome. J. Electrocardiol. 2011;44:346–349. doi: 10.1016/j.jelectrocard.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Junttila M.J., Brugada P., Hong K., Lizotte E., DEZ M., Sarkozy A. Differences in 12-lead electrocardiogram between symptomatic and asymptomatic Brugada syndrome patients. J. Cardiovasc. Electrophysiol. 2008;19:380–383. doi: 10.1111/j.1540-8167.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 31.Ohkubo K., Watanabe I., Okumura Y., Ashino S., Kofune M., Nagashima K. Prolonged QRS duration in lead V2 and risk of life-threatening ventricular arrhythmia in patients with Brugada syndrome. Int. Heart J. 2011;52:98–102. doi: 10.1536/ihj.52.98. [DOI] [PubMed] [Google Scholar]
- 32.Calo L., Giustetto C., Martino A., Sciarra L., Cerrato N., Marziali M. A new electrocardiographic marker of sudden death in Brugada syndrome: the S-wave in lead I. J. Am. Coll. Cardiol. 2016;67:1427–1440. doi: 10.1016/j.jacc.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Babai Bigi M.A., Aslani A., Shahrzad S. aVR sign as a risk factor for life-threatening arrhythmic events in patients with Brugada syndrome. Heart Rhythm. 2007;4:1009–1012. doi: 10.1016/j.hrthm.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Huang Z., Patel C., Li W., Xie Q., Wu R., Zhang L. Role of signal-averaged electrocardiograms in arrhythmic risk stratification of patients with Brugada syndrome: a prospective study. Heart Rhythm. 2009;6:1156–1162. doi: 10.1016/j.hrthm.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda T., Takami M., Sugi K., Mizusawa Y., Sakurada H., Yoshino H. Noninvasive risk stratification of subjects with a Brugada-type electrocardiogram and no history of cardiac arrest. Ann. Noninvasive Electrocardiol. 2005;10:396–403. doi: 10.1111/j.1542-474X.2005.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgopoulos S., Letsas K.P., Liu T., Kalafateli M., Korantzopoulos P., Burkle G. A meta-analysis on the prognostic significance of inferolateral early repolarization pattern in Brugada syndrome. Europace. 2018;20:134–139. doi: 10.1093/europace/euw394. [DOI] [PubMed] [Google Scholar]
- 37.Conte G., Caputo M.L., Regoli F., Moccetti T., Brugada P., Auricchio A. Brugada syndrome and early repolarisation: distinct clinical entities or different phenotypes of the same genetic disease? Arrhythmia Electrophysiol. Rev. 2016;5:84–89. doi: 10.15420/AER.2016.23.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu D., Barajas-Martinez H., Terzic A., Park S., Pfeiffer R., Burashnikov E. ABCC9 is a novel Brugada and early repolarization syndrome susceptibility gene. Int. J. Cardiol. 2014;171:431–442. doi: 10.1016/j.ijcard.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawata H., Morita H., Yamada Y., Noda T., Satomi K., Aiba T. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: a novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm. 2013;10(8):1161. doi: 10.1016/j.hrthm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Takagi M., Aonuma K., Sekiguchi Y., Yokoyama Y., Aihara N., Hiraoka M. The prognostic value of early repolarization (J wave) and ST-segment morphology after J wave in Brugada syndrome: multicenter study in Japan. Heart Rhythm. 2013;10:533–539. doi: 10.1016/j.hrthm.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Sarkozy A., Chierchia G.B., Paparella G., Boussy T., De Asmundis C., Roos M. Inferior and lateral electrocardiographic repolarization abnormalities in Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2009;2:154–161. doi: 10.1161/CIRCEP.108.795153. [DOI] [PubMed] [Google Scholar]
- 42.Hiraoka M., Takagi M., Yokoyama Y., Sekiguchi Y., Aihara N., Aonuma K. Prognosis and risk stratification of young adults with Brugada syndrome. J. Electrocardiol. 2013;46:279–283. doi: 10.1016/j.jelectrocard.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Castro Hevia J., Antzelevitch C., Tornes Barzaga F., Dorantes Sanchez M., Dorticos Balea F., Zayas Molina R. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J. Am. Coll. Cardiol. 2006;47:1828–1834. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan G.X., Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 45.Antzelevitch C. Ion channels and ventricular arrhythmias: cellular and ionic mechanisms underlying the Brugada syndrome. Curr. Opin. Cardiol. 1999;14:274–279. doi: 10.1097/00001573-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Antzelevitch C., Sicouri S., Di Diego J.M., Burashnikov A., Viskin S., Shimizu W. Does Tpeak-Tend provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4:1114–1116. doi: 10.1016/j.hrthm.2007.05.028. (author reply 6-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maury P., Sacher F., Gourraud J.B., Pasquie J.L., Raczka F., Bongard V. Increased Tpeak-Tend interval is highly and independently related to arrhythmic events in Brugada syndrome. Heart Rhythm. 2015;12:2469–2476. doi: 10.1016/j.hrthm.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 48.Zumhagen S., Zeidler E.M., Stallmeyer B., Ernsting M., Eckardt L., Schulze-Bahr E. Tpeak-Tend interval and Tpeak-Tend/QT ratio in patients with Brugada syndrome. Europace. 2016;18:1866–1872. doi: 10.1093/europace/euw033. [DOI] [PubMed] [Google Scholar]
- 49.Mugnai G., Hunuk B., Hernandez-Ojeda J., Stroker E., Velagic V., Ciconte G. Role of electrocardiographic Tpeak-Tend for the prediction of ventricular arrhythmic events in the Brugada syndrome. Am. J. Cardiol. 2017;120:1332–1337. doi: 10.1016/j.amjcard.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 50.Tse G., Gong M., Wong W.T., Georgopoulos S., Letsas K.P., Vassiliou V.S. The Tpeak - Tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: a systematic review and meta-analysis. Heart Rhythm. 2017;14:1131–1137. doi: 10.1016/j.hrthm.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 51.Tse G. (Tpeak − Tend)/QRS and (Tpeak − Tend)/(QT × QRS): novel markers for predicting arrhythmic risk in the Brugada syndrome. Europace. 2017;19:696. doi: 10.1093/europace/euw194. [DOI] [PubMed] [Google Scholar]
- 52.Zumhagen S., Stallmeyer B., Eckardt L., Schulze-Bahr E. (Tpeak − Tend)/QRS and (Tpeak − Tend)/(QT × QRS) as risk markers in Brugada syndrome: authors' reply. Europace. 2017;19:696–697. doi: 10.1093/europace/euw210. [DOI] [PubMed] [Google Scholar]
- 53.Tse G., Yan B.P. Novel arrhythmic risk markers incorporating QRS dispersion: QRSd × (Tpeak − Tend)/QRS and QRSd × (Tpeak − Tend)/(QT × QRS) Ann. Noninvasive Electrocardiol. 2017;22 doi: 10.1111/anec.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antzelevitch C., Di Diego J.M., Argenziano M. Tpeak-Tend as a predictor of ventricular arrhythmogenesis. Int. J. Cardiol. 2017;249:75–76. doi: 10.1016/j.ijcard.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Opthof T., Coronel R., Wilms-Schopman F.J., Plotnikov A.N., Shlapakova I.N., Danilo P., Jr. Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm. 2007;4:341–348. doi: 10.1016/j.hrthm.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Xia Y., Liang Y., Kongstad O., Liao Q., Holm M., Olsson B. In vivo validation of the coincidence of the peak and end of the T wave with full repolarization of the epicardium and endocardium in swine. Heart Rhythm. 2005;2:162–169. doi: 10.1016/j.hrthm.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Tse G., Wong S.T., Tse V., Lee Y.T., Lin H.Y., Yeo J.M. Cardiac dynamics: alternans and arrhythmogenesis. J. Arrhythm. 2016;32:411–417. doi: 10.1016/j.joa.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tada T., Kusano K.F., Nagase S., Banba K., Miura D., Nishii N. Clinical significance of macroscopic T-wave alternans after sodium channel blocker administration in patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2008;19:56–61. doi: 10.1111/j.1540-8167.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 59.Makimoto H., Nakagawa E., Takaki H., Yamada Y., Okamura H., Noda T. Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J. Am. Coll. Cardiol. 2010;56:1576–1584. doi: 10.1016/j.jacc.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 60.Miyamoto A., Hayashi H., Makiyama T., Yoshino T., Mizusawa Y., Sugimoto Y. Risk determinants in individuals with a spontaneous type 1 Brugada ECG. Circ. J. 2011;75:844–851. doi: 10.1253/circj.cj-10-0903. [DOI] [PubMed] [Google Scholar]
- 61.Letsas K.P., Weber R., Astheimer K., Mihas C.C., Stockinger J., Blum T. Predictors of atrial tachyarrhythmias in subjects with type 1 ECG pattern of Brugada syndrome. Pacing Clin. Electrophysiol. 2009;32:500–505. doi: 10.1111/j.1540-8159.2009.02311.x. [DOI] [PubMed] [Google Scholar]
- 62.Ashino S., Watanabe I., Kofune M., Nagashima K., Ohkubo K., Okumura Y. Abnormal action potential duration restitution property in the right ventricular outflow tract in Brugada syndrome. Circ. J. 2010;74:664–670. doi: 10.1253/circj.cj-09-0872. [DOI] [PubMed] [Google Scholar]
- 63.Nishii N., Nagase S., Morita H., Kusano K.F., Namba T., Miura D. Abnormal restitution property of action potential duration and conduction delay in Brugada syndrome: both repolarization and depolarization abnormalities. Europace. 2010;12:544–552. doi: 10.1093/europace/eup432. [DOI] [PubMed] [Google Scholar]
- 64.Leong K.M.W., Ng F.S., Roney C., Cantwell C., Shun-Shin M.J., Linton N.W.F. Repolarization abnormalities unmasked with exercise in sudden cardiac death survivors with structurally normal hearts. J. Cardiovasc. Electrophysiol. 2017;29(1):115–126. doi: 10.1111/jce.13375. [DOI] [PubMed] [Google Scholar]
- 65.Letsas K.P., Efremidis M., Asvestas D., Vlachos K., Georgopoulos S., Tse G. Right ventricular outflow tract electroanatomical abnormalities predict ventricular fibrillation inducibility in Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2018;11(2) doi: 10.1161/CIRCEP.117.005928. [DOI] [PubMed] [Google Scholar]
- 66.Milman A., Andorin A., Gourraud J.B., Postema P.G., Sacher F., Mabo P. Heart Rhythm. 2018. Profile of Brugada syndrome patients presenting with their first documented arrhythmic event. Data from the Survey on Arrhythmic Events in BRUgada Syndrome (SABRUS) [DOI] [PubMed] [Google Scholar]
- 67.Makarawate P., Chaosuwannakit N., Vannaprasaht S., Sahasthas D., Koo S.H., Lee E.J.D. SCN5A genetic polymorphisms associated with increased defibrillator shocks in Brugada syndrome. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu D., Barajas-Martinez H., Pfeiffer R., Dezi F., Pfeiffer J., Buch T. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J. Am. Coll. Cardiol. 2014;64:66–79. doi: 10.1016/j.jacc.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li K.H.C., Liu T., To O.T.L., Chan Y.S., Tse G., Yan B.P. A1427S missense mutation in SCN5A causes type 1 Brugada pattern, recurrent ventricular tachyarrhythmias and right ventricular structural abnormalities. Res. Cardiovasc. Med. 2017;6 [Google Scholar]
- 70.Maury P., Rollin A., Sacher F. Prevalence and prognostic role of various conduction disturbances in patients with the Brugada syndrome. Am J Cardiol. 2013;112:1384–1389. doi: 10.1016/j.amjcard.2013.06.033. [DOI] [PubMed] [Google Scholar]