Abstract
The data presented in this article was related to the research article entitled, “The use of Cerastoderma Lamarcki shell for Acid Black 1 adsorption from aqueous solutions.” The characterization data of Cerastoderma Lamarcki shell was analyzed using various instrumental techniques (X-ray diffraction and SEM). The kinetic and isotherm data of pH, initial AB1 concentration, contact time, and CLS dosage were investigated. The optimum conditions for AB1 adsorption using CLS adsorbent were found to be 2 g of adsorbent, pH 2, and a contact time of 60 min. The adsorption data of CLS fit well with the Langmuir model and pseudo-second order model. Finally, the experimental data showed that CLS is a suitable and low-cost adsorbent for the removal of AB1 from aqueous solutions.
Keywords: Acid black1, Adsorption, Dye, Cerastoderma Lamarcki, Low-cos adsorption
| Subject area | Environmental Engineering |
| More specific subject area | Adsorption |
| Type of data | Table, image, figure |
| How data was acquired | Characteristics of the CLS adsorbent were identified with X-ray diffraction and Field Emission Scanning Electron Microscopy. Adsorption of acid black 1 (AB1) by low-cost adsorbent of CLS was examined using batch studies. The effect of different variables such as solution pH (2–11), initial AB1 concentration (50–250 mg l-1), contact time (5–240 min), and CLS dosage (2–20 g l-1) was investigated. To describe AB1 adsorption on the CLS adsorbent, four types of kinetic models, pseudo-first-order and pseudo-second-order, Elovich and intraparticle diffusion model, were used. The AB1concentration measurement was performed by an atomic absorption spectroscopy (AAnalyst 200 Perkin-Elmer). |
| Data format | Raw, analyzed |
| Experimental factors | Shell samples of Cerastoderma lamarcki were collected from the coast of Caspian Sea in Mazandaran province, Iran. CLS were dried in the oven at 85 °C for 12 h. CLS using hammer mill were crushed into the smaller size and it was sieved to 70– 250 μm. |
| Data of CLS were acquired for AB1removal from aqueous solution | |
| Experimental features | CLS for dye adsorption from wastewater |
| Data source location | Neyshabour, University of Medical Sciences, Neyshabur, Iran |
| Data accessibility | Data are included in this article. |
Value of the data
-
•
Biochar from CLS was applied to remove Acid Black 1 from an aqueous solution.
-
•
Data in this article, including isotherm and kinetic parameters, is informative for modeling and predicting the adsorbtion capacity of CLS for Acid Black 1 removal.
-
•
The acquired data is advantageous for coastal areas wanting to scale up and design an adsorption column with Cerastoderma lamarcki shells as the medium for removing AB1 from wastewater.
1. Data
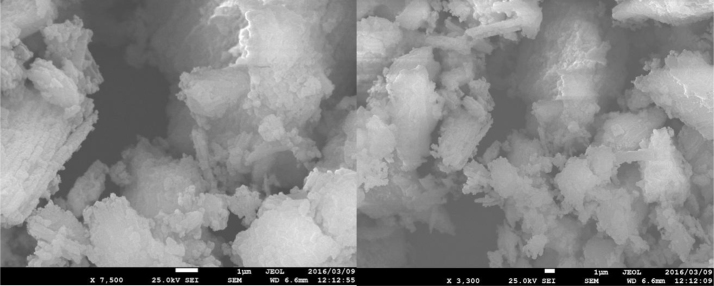
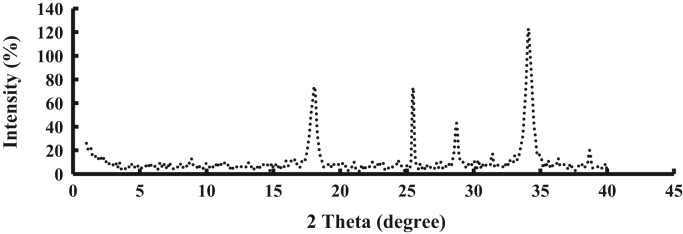
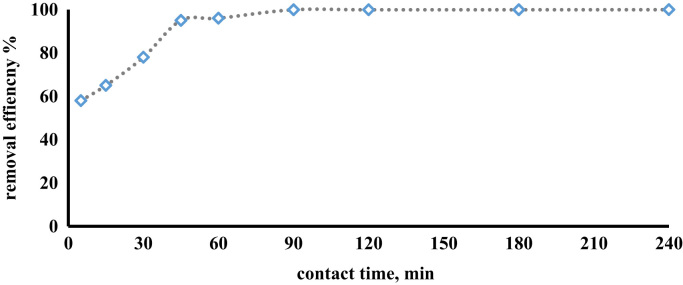
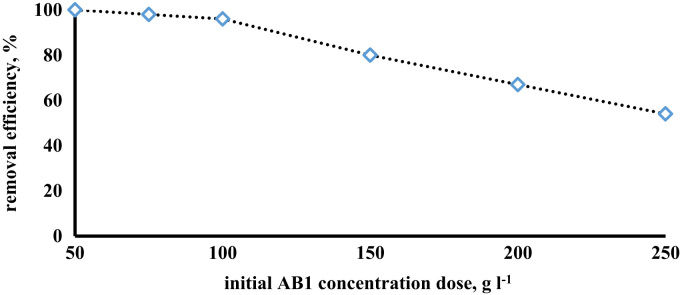
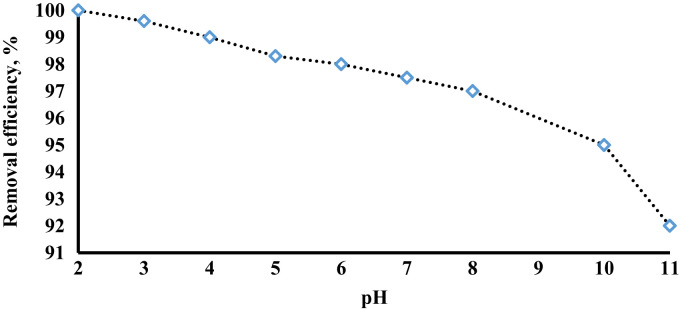
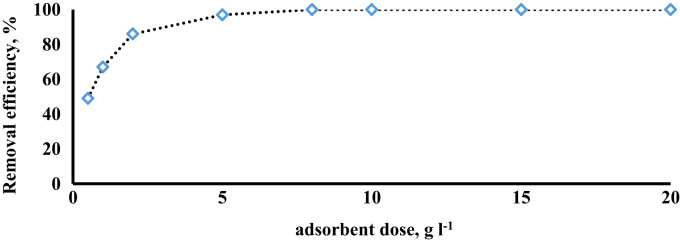
The prepared CLS adsorbent was in the form of a powder (Fig. 1). The morphology of the CLS adsorbent is shown in Fig. 2. The crystal structure of CLS was studied by x-ray diffraction (Fig. 3). The kinetic, isotherm, and thermodynamic parameters were estimated using models listed in Table 1. Data on the isotherms and kinetics for adsorption of chromium ions onto Cerastoderma lamarcki shell is presented in Table 2, Table 3. Fig. 4, Fig. 5, Fig. 6, Fig. 7 present the comparison data for AB1 adsorption by CLS for the parameters of contact time, initial AB1 concentration, pH, and CLS dosage, respectively.
Fig. 1.
Cerastoderma lamarcki shells and its powder.
Fig. 2.
FE-SEM image of low-cost CLS adsorbent.
Fig. 3.
X-ray diffraction spectra of low-cost CLS adsorbent.
Table 1.
Empirical formulas of the applied kinetic models used in this study [3].
| Models type | formula | plot |
|---|---|---|
| Pseudo first order | log (qe-qt) vs. t | |
| Pseudo second order | t/qt vs. t | |
| Elovich | In t vs. qt | |
| Intra-particle diffusion | qt vs. t1/2 |
Table 2.
Kinetic constants for AB1 adsorption using CLS adsorbent.
| Isotherm type | Isotherm parameters |
AB1 Concentration (mg l-1) |
||
|---|---|---|---|---|
| 50 | 100 | 200 | ||
| Psudo first order model | K1 | 0.033 | 0.046 | 0.015 |
| R2 | 0.831 | 0.819 | 0.815 | |
| qcal | 1.619 | 3.474 | 2.339 | |
| Psudo second order model | K2 | 0.046 | 0.022 | 0.038 |
| R2 | 0.999 | 0.999 | 0.999 | |
| qm | 5.112 | 10.233 | 8.953 | |
| Elovich | α | 1.691 | 0.293 | 0.274 |
| β | 1.127 | 0.563 | 0.718 | |
| R2 | 0.903 | 0.904 | 0.787 | |
| Intraparticle diffusion | Kdif | 0.266 | 0.532 | 0.397 |
| R2 | 0.668 | 0.669 | 0.526 | |
| C | 1.953 | 3.905 | 4.349 | |
Table 3.
Isotherm model constants for AB1 adsorption onto CLS adsorbent.
| Isotherm type | Isotherm parameters | Value | |
|---|---|---|---|
| Fraundlich | n | 2.022 | |
| Kf | 1.473 | ||
| R2 | 0.889 | ||
| Langmuir | I type | KL | 0.039 |
| R2 | 0.983 | ||
| qm | 15.877 | ||
| II type | KL | 0.025 | |
| R2 | 0.991 | ||
| qm | 20.894 | ||
| III type | KL | 0.042 | |
| R2 | 0.75 | ||
| qm | 15.6 | ||
| IV type | KL | 0.031 | |
| R2 | 0.75 | ||
| qm | 18.133 |
Fig. 4.
Effect of contact time on AB1 adsorption on CSL adsorbent (pH = 2, adsorbent dosage = 7 g l-1).
Fig. 5.
Effect of initial AB1 concentration of on adsorption on CLS adsorbent (pH = 2, adsorbent dosage = 7 g l-1, contact time = 60 min).
Fig. 6.
Effect of pH variations on AB1 adsorption onto CLS (adsorbent dose = 7 g l-1, contact time = 60 min).
Fig. 7.
Effect of adsorbent dose on AB1 adsorption onto CLS adsorbent (initial AB1 concentration = 50 g l-1, pH = 2, contact time = 60 min).
2. Materials and methods
2.1. Materials
Acid black 1 (80% purity), HCl, and NaOH (to adjust pH) were supplied by Sigma-Aldrich. All chemical materials required in this study were purchased from Merck Co. Double-distilled water was used to prepare working solutions.
2.2. Preparation of biosorbent
Samples of Cerastoderma lamarcki shell (CLS) were collected from the coast of the Caspian Sea in Mazandaran province, Iran. After collection, the shells were washed with tap water to remove any dirt or other contaminant. After the initial wash, they were washed twice more with deionized water. Then, the shells were dried in an oven at 85 °C for 12 h. Next, they were crushed using a hammer mill and sieved to 70–250 μm. Finally, the end product was stored in a polyethylene container for later use. Fig. 1. shows the Cerastoderma Lamarcki shells [1], [2], [3], [4], [5], [6], [7].
2.3. Design of experiments
2.3. Experimental Design.
The adsorption of Acid Black 1 (AB1) by the low-cost adsorbent CLS was examined using batch studies. The effects of different variables, namely solution pH (2–11), initial AB1 concentration (50–250 mg l-1), contact time (5–240 min), and CLS dosage (2–20 g l-1) were investigated. Initially, the stock solution of AB1 (1000 mg l-1) was prepared with double-distilled water and stored under standard conditions [8]. AB1 concentrations were prepared by proper dilution (C1V1 = C2V2) using the stock solution. To start the tests, a 250-ml Erlenmeyer flask was employed. Then, certain amounts of the stock solution and CLS were added. To obtain optimum contact time, 25 ml of the stock solution prepared by dilution was poured into the flask; 0.7 gr (7 g l-1) of adsorbent was added at an adjusted pH of 3. The samples were placed in a shaker and shaken at a constant rate of 150 rpm for various time periods. Each CLS dosage was added to 100 ml of AB 1 solution. The solution pH was adjusted using 0.1 M HCl and NaOH. After experiments, the remaining adsorbent was separated from the solution by centrifugation (3500 rpm, 10 min). Then, the residual AB1 concentration was determined by spectrophotometry (UV-UVIS, 622 nm). The experiments were conducted at the constant temperature of 25 ± 1 °C [2], [8]. Finally, the amount of AB1 adsorbed onto the CLS adsorbent was calculated using Eq. 1 [8]:
| (1) |
Where, Co and Ce are the initial and final concentrations of AB1 in solution (mg l-1), respectively, V is the volume of AB1 solution (ml), and m is the weight of the CLS (g). The removal efficiency of AB1 was calculated using Eq. 2 [9]:
| (2) |
Where, Co and Ct represent the initial and t AB1 concentrations (mg l-1), respectively. All stages were repeated several times to determine optimum pH, CLS dosage, and AB1 concentration values.
2.4. Equilibrium adsorption modeling
Isotherm models such as Langmuir and Freundlich were applied to determine the relationship between equilibrium capacity (qe) and equilibrium concentration (Ce). Adsorption kinetic models were used to predict the rate of adsorption and adsorption mechanisms. To describe AB1 adsorption on the CLS adsorbent, four kinetic models (pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion) were used [9], [10].
Acknowledgements
This research was supported by Neyshabur University of Medical Science (NUMS), Iran. Also, the authors wish to acknowledge to the personnel of laboratory of the Health Department of Tehran University of Medical Sciences for their great help in the achievement of this project.
Acknowledgments
Funding sources
This work was a project with Grant Number 208 was funded by Nums.ac.ir.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2018.01.107.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Golmohammadi S., Ahmadpour M., Mohammadi A., Alinejad A., Mirzaei N., Ghaderpoori M., Ghaderpoori A. Removal of blue cat 41 dye from aqueous solutions with ZnO nanoparticles in combination with US and US-H2O2 advanced oxidation processes”. Environ. Health Eng. Manag. J. 2016;3:107–113. [Google Scholar]
- 2.A.D. Eaton, L.S. Clesceri, E.W. Rice, Standard Methods for the examination of water and wastewater (PAHA), ed. 21st Washington DC, USA: American Water Works Association, 2005.
- 3.Faraji H., Mohamadi A.A., Arezomand S., Reza H., Mahvi A.H. kinetics and equilibrium studies of the removal of blue basic 41 and methylene blue from aqueous solution using rice stems. Iran. J. Chem. Chem. Eng. 2015;34:33–42. [Google Scholar]
- 4.Azari A., Gholami M., Torkshavand Z., Yari A.R., Ahmadi E., Kakavandi B. evaluation of basic violet 16 adsorption from aqueous solution by magnetic zero valent iron-activated carbon nanocomposite using response surface method: isotherm and kinetic studies. J. Mazandaran Univ. Med. Sci. 2015;25(121):333–347. [Google Scholar]
- 5.Radhika M., Palanivelu K. Adsorptive removal of chlorophenols from aqueous solution by low-cost adsorbent-Kinetics and isotherm analysis. J. Hazard. Mater. 2006;138:116–124. doi: 10.1016/j.jhazmat.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 6.Smitha T., Santhi T., Makeswari M. Adsorption of acid black-7 from synthetic aqueous solution onto Cucumis sativus peel. J.. Chem. Pharm. Res. 2015;7:1617–1625. [Google Scholar]
- 7.Naghizadeh A., Ghafouri M., Jafari A. Investigation of equilibrium, kinetics and thermodynamics of extracted chitin from shrimp shell in reactive blue 29 (RB-29) removal from aqueous solutions. Desalination Water Treat. 2017;70:355–363. [Google Scholar]
- 8.Shahverdi M., Kouhgardi E., Ramavandi B. Characterization,kinetic, and isotherm data for Cr(VI) removal from aqueous solution by Populusalba biochar modified by acationic surfactant. Data Brief. 2016;9:163–168. doi: 10.1016/j.dib.2016.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozbaş S.K., Boz Y. Low-cost biosorbent: anadara inaequivalvis shells for removal of Pb (II) and Cu (II) from aqueous solution. Process Saf. Environ. Prot. 2016;103:144–152. [Google Scholar]
- 10.Mohammadi A.A., Alinejad A., Kamarehie B., Javan S., Ghaderpoury A., Ahmadpour M., Ghaderpoori M. Metal-organic framework Uio-66 for adsorption of methylene blue dye from aqueous solutions. Int. J. Environ. Sci. Technol. 2017;14:1959–1968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material