Abstract
Estrogen has a crucial role in the regulation of reproductive and neuroendocrine function and exerts its effects through two classes of receptors, nuclear and membrane estrogen receptors (mERs). G protein-coupled estrogen receptor 1 (GPER) is a member of mERs, and despite limited research on the levels of GPER in patients with psychiatric diseases, a role of GPER in such conditions has been suggested. Here we evaluated serum estrogen and GPER levels in children with attention deficit hyperactivity disorder (ADHD) in relation to their age- and gender-matched healthy controls. A total of 82 children were included in the study, 47 drug- naïve patients with ADHD (age: 6–12 years; male/female: 34/13) and 35 healthy controls (age: 6–12 years; male/female: 19/16). The subgroups according to ADHD types were inattentive, hyperactive/impulsive, and combined. Serum estrogen was measured using an immunoassay system, while serum GPER was determined using a commercial sandwich enzyme-linked immunosorbent assay kit. Estrogen levels in children with ADHD were similar as in control group, while GPER levels were significantly lower in ADHD group compared to controls (p < 0.05). Logistic regression analysis showed a significant association between GPER levels and ADHD (p < 0.05), and no association between estrogen levels and ADHD (p > 0.05). No significant differences were found in GPER and estrogen levels between ADHD subgroups (p > 0.05). To the best of our knowledge, this study is the first to investigate estrogen and GPER levels in ADHD. Our preliminary findings suggest a relationship between serum GPER levels and ADHD, and this should be further investigated.
Keywords: Attention deficit hyperactivity disorder, ADHD, estrogen, estrogen receptors, GPER, G protein-coupled estrogen receptor 1
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is a condition characterized by hyperactivity, impulsivity, and lack of attention. ADHD is one of the most common neuropsychiatric disorders in childhood, and current estimates indicate it affects 3% to 10% of school-aged children worldwide. A higher prevalence of ADHD has been traditionally observed among boys, with the male/female ratio ranging from 4:1 to 9:1, depending on the ADHD type and the setting [1]. In addition, observed gender differences in the behavioral symptoms and neuropsychological profiles of ADHD patients [2,3] indicated an important role of the neuroendocrine system in the etiology of the disease [4,5]. For example, the risk of developing ADHD appears to be higher in male children with genetic causes of hyperandrogenism compared to general population [6], and a positive relationship between salivary testosterone and aggression was reported in disruptive children [7].
Estrogen is the primary female sex hormone with a crucial role in the regulation of reproductive and neuroendocrine function. Different studies reported that estrogen also modulates synaptic plasticity in the hippocampus, regulates the formation of amyloid-β peptide, inactivates the expression of proapoptotic factors, and demonstrates antioxidant activity [8].
Estrogen exerts its effects through two different classes of receptors. The class of nuclear receptors includes estrogen receptors alpha (ERα) and beta (ERβ), which are transcription factors and regulate the activity of different genes at the transcriptional level. The second class comprises membrane estrogen receptors (mERs) and also includes G protein-coupled estrogen receptor 1 (GPER) [9]. GPER, which is structurally different from ERα and ERβ receptors, is a transmembrane protein that associates with G proteins to initiate signaling pathways involved in non-genomic actions of estrogen. GPER has been detected in several subcellular locations, including the endoplasmic reticulum, nuclear envelope and plasma membrane, and it may bind different agonists, such as chemokines, vasoactive substances and neurotransmitters [10]. In experimental studies on animals, GPER was found throughout the central and peripheral nervous system (CNS and PNS) of male and female rodents, i.e., in the hypothalamus, hippocampus, midbrain, spinal cord and dorsal root ganglia [11,12]. Moreover, GPER was localized in different tissues and organs of the human body, including adipose tissue, the kidneys, CNS, immune, reproductive, and cardiovascular systems [13,14].
A series of clinical and preclinical studies suggested a protective role of estrogen in neurodevelopmental disorders, such as autism spectrum disorder (ASD) and schizophrenia. They observed changes in the level of estrogen receptors in the patients with ASD and schizophrenia, and demonstrated that estrogen replacement therapy is effective in the treatment of schizophrenia [15,16]. Other studies on patients with ASD identified a significant relationship between decreased expression of the ERβ gene (ESR2) and autism [17,18]. Furthermore, in our previous study, we determined significantly lower serum GPER levels in patients with ASD compared to controls [19]. To the best of our knowledge, serum estrogen and GPER levels have not been investigated in ADHD patients up until now. To better understand the role of estrogen and GPER in ADHD we evaluated serum estrogen and GPER levels in children with ADHD in relation to their age- and gender-matched healthy controls.
MATERIALS AND METHODS
A total of 82 children were enrolled in this study. The ADHD group included 47/82 preadolescent children presented at the Child and Adolescent Psychiatry Unit of the School of Medicine at Mugla Sitki Kocman University and diagnosed with ADHD (age: 6–12 years; 34 males and 13 females). The control group included 35 preadolescent children (age: 6–12 years; 19 males and 16 females).
The diagnosis of ADHD was reached based on a clinical interview and using the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) [20]. To support the diagnosis of ADHD and exclude comorbid psychiatric disorders, the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) was applied [21]. The K-SADS-PL is a semi-structured interview, and the version adapted for the Turkish population [22] was used in this study. Patients in ADHD group did not take any medication 6 weeks prior to the study. Patients with comorbid psychiatric disorders, genetic syndromes, metabolic disorders, or neurological disease were excluded from the study. Children without known neurodevelopmental/neurological disorders, without physical and psychiatric disorders, and who had not been on any medication were selected for the control group. Medical illnesses in both groups were excluded based on the patient medical history, clinical examinations, and routine laboratory tests (biochemical, hematological, and thyroid function tests). The intelligence quotient (IQ) was determined using Wechsler Intelligence Scale for Children-Revised (WISC-R), and children with an IQ score greater than 80 were included in the study. The parents of the children were given the Conners’ Parent Rating Scale-Revised Long Form [23,24], and the teachers of children from both groups completed the Conners’ Teacher Rating Scale [25-27].
This study was approved by the local Ethics Committee of Sutcu Imam University, Medical Faculty, Kahramanmaras, Turkey (approval date: 24.08.2015; no: 2015/12-04) and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients and parents involved in the study.
Biochemistry assays
Blood samples of all children were collected between 9:00 a.m. and 11:00 a.m., centrifuged, and stored at -20 °C until the analysis. The serum estrogen levels were measured using ADVIA Centaur XP immunoassay system (Siemens Healthcare Diagnostic Inc., Ireland). The serum GPER levels were determined using a commercial sandwich enzyme-linked immunosorbent assay (ELISA) kit (SEG045Hu, Cloud-Clone Corp., Houston, TX, United States), according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 22.0. (IBM Corp., Armonk, NY, USA). Descriptive data were expressed as mean ± standard deviation (SD) and numbers and frequencies (%). The results were compared between the two groups using the Chi-squared test (categorical variables) or independent sample t-test (parametric variables). A correlation between the serum GPER/estrogen levels and clinical variables in ADHD group, including ADHD scores, age, and estradiol/GPER levels, were analyzed with the Pearson correlation coefficient. One-way ANOVA test was used to compare GPER and estrogen levels between ADHD subgroups (inattentive, hyperactive/impulsive, and combined subgroup). A logistic regression analysis was used to determine the association between serum GPER and estrogen levels and ADHD. A p value <0.05 was considered statistically significant.
RESULTS
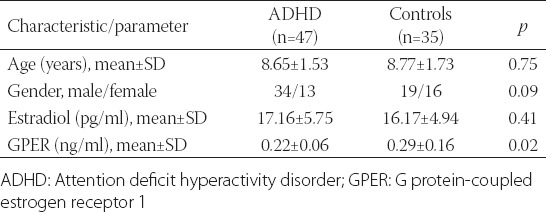
In this study, we included 47 patients with ADHD 6–12 years old, and 35 age- and gender-matched healthy controls. No significant difference was found between the groups regarding the age (ADHD group: 8.65 years ± 1.53, controls: 8.77 years ± 1.73; p > 0.05) and gender (ADHD male/female ratio: 34/13, controls male/female: 19/16; p > 0.05). The mean levels of estradiol were similar in both groups (ADHD group: 17.16 ± 5.75 pg/mL, controls: 16.17 ± 4.94 pg/mL; p > 0.05), while the mean serum GPER levels were significantly lower in children with ADHD compared to controls [0.22 ± 0.06 ng/mL and 0.29 ± 0.16 ng/mL, respectively; p < 0.05] (Table 1). The logistic regression analysis showed no association between the serum estradiol levels and ADHD. On the contrary, a statistically significant association was found between the serum GPER levels and ADHD (p = 0.017, odds ratio [OR] = 0.006).
TABLE 1.
General characteristics and laboratory results of children with ADHD and healthy controls
No significant difference was observed in the GPER and estradiol levels between males and females in both groups (Table 2) nor between ADHD subgroups [p > 0.05] (Table 3).
TABLE 2.
GPER and estradiol levels in relation to gender in ADHD and control groups
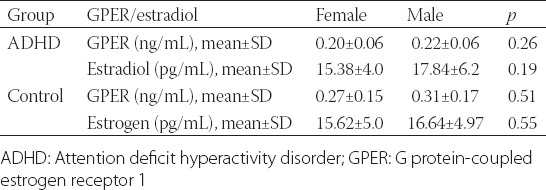
TABLE 3.
GPER and estrogen levels according to ADHD subgroups

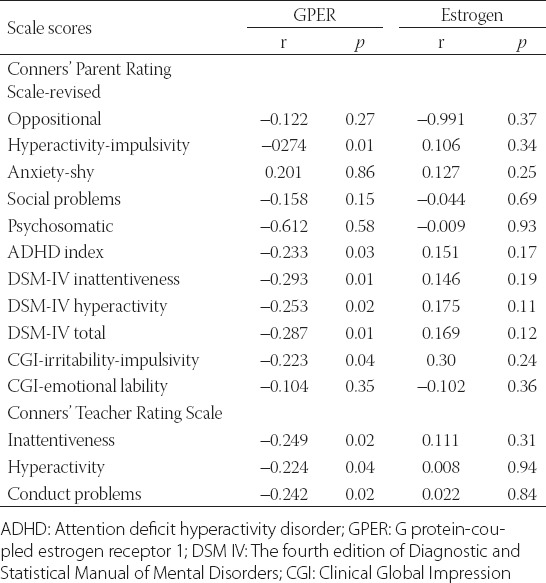
The lower serum GPER levels corresponded to higher ADHD scores. The GPER levels were negatively correlated with the hyperactivity, Clinical Global Impression (CGI)-irritability-impulsivity, ADHD index, DSM-IV inattentiveness and hyperactivity, and the total scores of the Conners’ Parent Rating Scale-Revised Long Form, as well as with the inattentiveness, hyperactivity and conduct sub-scores of the Conners’ Teacher Rating Scale (p < 0.05). On the contrary, no significant correlation was observed between estrogen levels and any of the scales [p > 0.05] (Table 4). Similarly, there was no significant relationship between GPER and estradiol levels, nor between GPER and age (p > 0.05).
TABLE 4.
Relationship of GPER and estrogen levels with the Conners’ Parent and Teacher Rating Scale Scores
DISCUSSION
The aim of this study was to determine if serum estrogen and GPER levels are altered in children with ADHD, compared to healthy controls. We showed that serum estrogen levels in children with ADHD were similar as in control group, while the serum GPER levels were significantly lower in ADHD group compared to controls. The logistic regression analysis indicated a significant association between serum GPER levels and ADHD, and no association between estrogen and ADHD. Also, the lower serum GPER levels corresponded to higher ADHD scores. In addition, we observed no difference in the GPER decrease between males and females. To the best of our knowledge, this study is the first to evaluate serum estrogen and GPER levels in children with ADHD.
Estrogen is considered to be neuroprotective in a number of neurodegenerative disorders, and chronic estrogen deprivation was associated with an increased risk of stroke, Alzheimer’s disease and Parkinson’s disease among other brain conditions. Animal studies on neuroprotective effects of estrogen, showed that exogenous 17β-estradiol (E2) replacement prior to stroke decreases behavioral deficits in ovariectomized female rats subjected to global cerebral ischemia (GCI), and that exogenous E2 facilitates post-stroke recovery in mice [28]. Moreover, estrogen is known to contribute to neurotrophin synthesis and to protect the brain against inflammation and stress. Numerous studies showed that estrogen can regulate the expression of brain-derived neurotrophic factor (BDNF), a key molecule involved in cell survival, cell differentiation, and synaptic plasticity in the CNS [29,30]. For example, Yi et al. [31] showed that estrogen treatment increased the expression of BDNF mRNA in the midbrain region of ovariectomized rats [31]. Preclinical evidence suggests that BDNF knockout mice are hyperactive, and cerebral levels of BDNF were decreased in an ADHD rodent model. Moreover, clinical trials including blood-level and genetic studies, suggest that neurotrophins are involved in the pathogenesis of ADHD as well as in the mechanism of therapeutic agents for ADHD [32]. However, as far as we know, there has been no study investigating the association between estrogen and neurotrophins in ADHD patients, to date. Despite the lack of difference in the estrogen levels between the two groups in our study, the decreased GPER levels might have affected neurotrophin expression in ADHD patients.
A significant role of estrogen has also been suggested in neuropsychiatric disorders. In the recent decades, the role of estradiol and the activation of estrogen receptors has been investigated in animal models of neurodegeneration, cognitive impairment and affective disorders [33]. Such studies showed that estrogen affects various neurotransmitter systems, including dopamine, serotonin and norepinephrine, which have a role in ADHD and mood disorders [34].
Similarly, a protective role of estrogen has been indicated in psychiatric disorders, such as schizophrenia, anxiety and depression [35]; for example, a correlation between decreased plasma estrogen levels and an increased risk of schizophrenia symptoms in women was reported in clinical trials [36]. Conversely, in two independent studies, a positive correlation was described between the cognitive performance and estrogen levels in women with schizophrenia [37,38]. Other studies showed reductions in total, positive symptom, negative symptom and general psychopathology Positive and Negative Syndrome Scale (PANSS) scores in women with schizophrenia treated with estradiol alone or in combination with standard antipsychotic treatment [39]. Anxiety and depression have been reported to be more prevalent in women, and there is growing evidence that hormonal fluctuations and rapid decreases in estrogen levels during specific periods of a woman’s life increase the risk of anxiety and depressive symptoms [35].
Despite limited research on the levels of GPER in patients with psychiatric diseases, a role of GPER in mood disorders, anxiety and ASD has been suggested [40,41]. Recently, it was demonstrated that patients with generalized anxiety disorder and depression had higher serum GPER levels compared to healthy controls, and the increase in GPER levels was not gender-dependent [42,43].
ASD appears to be gender-specific, where it affects five times more males than females, and in Asperger syndrome (a highly functional form of ASD) males are affected ten times more than females. Similarly, ADHD is a gender-specific disorder, although clinical appearance of the disorder may vary between genders. However, the etiologic factors of these differences have yet to be clarified and the role of genetic risks and hormones is emphasized. So far, the focus has been mainly on the relationship between testosterone and ADHD. Candidate gene studies have shown a significant relationship between neurodevelopmental disorders and genes involved in androgen biosynthesis or action [5]. Nevertheless, the molecular mechanisms by which sex hormones may play a role in susceptibility to ADHD are still unclear. In our previous study on children with ASD, we found a negative correlation between serum GPER levels and ASD severity, and contrary to the estrogen levels, the decrease in serum GPER levels in ASD patients was gender-independent [19]. In the current study, estrogen levels were similar in ADHD and control group, while the serum GPER levels were lower in ADHD group, although again this decrease was not associated with gender. Comparable to our previous study [19], we found that higher ADHD scores correlated with lower GPER levels.
The importance of estrogen and estrogen receptors in cognition is well-recognized today. Different estrogen receptors may be involved in the mechanisms of E2 treatments and their time- and dose-dependent effects on cognition. For instance, E2 is thought to affect memory through the estrogen receptors on the membrane that activate and mediate rapid signaling involved in the processes of synaptic plasticity that are required for learning and memory [44]. All estrogen receptors, including ERα, ERβ and GPER, are expressed in the hippocampus, but have different distributions and densities. Current evidence suggests that GPER has a role in regulation of memory function. Hippocampal GPER affects cell proliferation and regulates its own expression differently within the dorsal and ventral regions of the hippocampus. In female rats, after tasks that involve the hippocampus, binding of an agonist to GPER strengthen their reference memory, while the binding of an antagonist inhibits the reference memory. The activation of GPER affects cognitive function, in a time- and dose-dependent manner [45]. Another study investigated the effect of E2 on social transmission of food preferences (STFP) task in ovariectomized mice and the role of estrogen receptors in this process. Overall, they showed that E2 rapidly enhances social learning in the STFP and that the GPER activation further facilitates this process [46].
ADHD is associated with a significant deterioration in various areas of functioning, including social and peer functioning, academic achievement, as well as emotional and cognitive functions. In a meta-analysis of 83 studies, Willcutt et al. [47] showed that, compared to children without ADHD, children/adolescents with ADHD demonstrate significant deficits in measures of executive functions (EF), such as response inhibition, vigilance, working memory and planning. Low serum GPER levels observed in our ADHD group indicate that the role of GPER in the development of neurocognitive deficits in ADHD should be further investigated.
The limitations of this study include the small sample size and its cross-sectional design. Also, the results obtained with serum GPER levels may not completely reflect the brain levels of GPER in children with ADHD. Thus, experimental animal studies are required to gain more insight into the brain levels of GPER. On the other hand, the major strength of our study is that, as far as we know, this is the first report on serum estrogen and GPER levels in children with ADHD. Additional large-scale studies are needed to clarify the regulatory mechanisms of estrogen and GPER in ADHD.
Increasing understanding of the GPER cellular and tissue distribution, as well as its function in various diseases, may contribute to the development of new diagnostic and prognostic approaches and customized treatment modalities, including the use of GPER agonists and antagonists [48]. For example, previous studies demonstrated anxiolytic effects of GPER agonists in ovariectomized female rats [49] and male gonadectomized rats [50], and understanding the exact mechanisms underlying those effects will lead to successful therapeutic strategies in humans.
CONCLUSION
Overall, serum levels of GPER were significantly decreased in our ADHD patients compared to controls, while estrogen levels were not different between the two groups. Our preliminary findings suggest a possible relationship between GPER levels and ADHD that is independent of serum estradiol levels, and this should be further investigated.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2010;33(2):357–73. doi: 10.1016/j.psc.2010.01.006. https://doi.org/10.1016/j.psc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Hasson R, Fine JG. Gender differences among children with ADHD on continuous performance tests: A meta-analytic review. J Atten Disord. 2012;16(3):190–8. doi: 10.1177/1087054711427398. https://doi.org/10.1177/1087054711427398. [DOI] [PubMed] [Google Scholar]
- 3.Wang LJ, Chen CK, Huang YS. Gender differences in the behavioral symptoms and neuropsychological performance of patients with attention-deficit/hyperactivity disorder treated with methylphenidate: A two-year follow-up study. J Child Adolesc Psychopharmacol. 2015;25(6):501–8. doi: 10.1089/cap.2014.0175. https://doi.org/10.1089/cap.2014.0175. [DOI] [PubMed] [Google Scholar]
- 4.Martel MM, Klump K, Nigg JT, Breedlove SM, Sisk CL. Potential hormonal mechanisms of attention-deficit/hyperactivity disorder and major depressive disorder: A new perspective. Horm Behav. 2009;55(4):465–79. doi: 10.1016/j.yhbeh.2009.02.004. https://doi.org/10.1016/j.yhbeh.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies W. Sex differences in attention deficit hyperactivity disorder: Candidate genetic and endocrine mechanisms. Front Neuroendocrinol. 2014;35(3):331–46. doi: 10.1016/j.yfrne.2014.03.003. https://doi.org/10.1016/j.yfrne.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Mueller SC, Ng P, Sinaii N, Leschek EW, Green-Golan L, VanRyzin C, et al. Psychiatric characterization of children with genetic causes of hyperandrogenism. Eur J Endocrinol. 2010;163:801–10. doi: 10.1530/EJE-10-0693. https://doi.org/10.1530/EJE-10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: Relationship to aggressive, hyperactive, and internalizing behaviors. J Am Acad Child Adolesc Psychiatry. 1994;33(8):1174–84. doi: 10.1097/00004583-199410000-00013. https://doi.org/10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Alexander A, Irving AJ, Harvey J. Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology. 2017;113(Pt B):652–60. doi: 10.1016/j.neuropharm.2016.07.003. https://doi.org/10.1016/j.neuropharm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Barton M. Position paper: The membrane estrogen receptor GPER – Clues and questions. Steroids. 2012;77(10):935–42. doi: 10.1016/j.steroids.2012.04.001. https://doi.org/10.1016/j.steroids.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–30. doi: 10.1126/science.1106943. https://doi.org/10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 11.Altun I, Kurutas EB. G protein-coupled estrogen receptor levels after peripheral nerve injury in an experimental rat model. World neurosurgery. 2015;84(6):1903–6. doi: 10.1016/j.wneu.2015.08.028. https://doi.org/10.1016/j.wneu.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Dun SL, Brailoiu GC, Gao X, Brailoiu E, Arterburn JB, Prossnitz ER, et al. Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J Neurosci Res. 2009;87(7):1610–9. doi: 10.1002/jnr.21980. https://doi.org/10.1002/jnr.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308(1-2):9–16. doi: 10.1016/j.mce.2009.03.009. https://doi.org/10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–26. doi: 10.1038/nrendo.2011.122. https://doi.org/10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crider A, Pillai A. Estrogen signaling as a therapeutic target in neurodevelopmental disorders. J Pharmacol Exp Ther. 2017;360(1):48–58. doi: 10.1124/jpet.116.237412. http://dx.doi.org/10.1124/jpet.116.237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heringa SM, Begemann MJ, Goverde AJ, Sommer IE. Sex hormones and oxytocin augmentation strategies in schizophrenia: A quantitative review. Schizophr Res. 2015;168(3):603–13. doi: 10.1016/j.schres.2015.04.002. http://dx.doi.org/10.1016/j.schres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarti B, Dudbridge F, Kent L, Wheelwright S, Hill-Cawthorne G, Allison C, et al. Genes related to sex steroids, neural growth, and social-emotional behavior are associated with autistic traits, empathy, and Asperger syndrome. Autism Res. 2009;2(3):157–77. doi: 10.1002/aur.80. https://doi.org/10.1002/aur.80. [DOI] [PubMed] [Google Scholar]
- 18.Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERβ), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol Autism. 2014;5(1):46. doi: 10.1186/2040-2392-5-46. https://doi.org/10.1186/2040-2392-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altun H, Kurutaş EB, Şahin N, Sınır H, Fındıklı E. Decreased levels of G protein-coupled estrogen receptor in children with autism spectrum disorders. Psychiatry Res. 2017;257:67–71. doi: 10.1016/j.psychres.2017.06.008. https://doi.org/10.1016/j.psychres.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) USA: American Psychiatric Publishing; 2013. [Google Scholar]
- 21.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–8. doi: 10.1097/00004583-199707000-00021. https://doi.org/10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Gökler B, Ünal F, Pehlivantürk B, Kültür EÇ, Akdemir D, Taner Y. Reliability and validity of Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version-Turkish Version (K-SADS-PL-T) Turk J Child Adolesc Ment Health. 2004;11(3):109–16. [Google Scholar]
- 23.Conners CK. Manual for the Conners’ Rating Scales – revised. North Tonawanda, NY: Multi-Health Systems; 1997. [Google Scholar]
- 24.Kaner S, Büyüköztürk Ş, İşeri E, Ak A, Özaydın L. Validity and reliability study of the Conners’ Parent Rating Scale Revised Long Form. Antalya, XVI. National Child and Adolescent Psychiatry Meeting; 2006 (abstract) [Google Scholar]
- 25.Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners’ Parent and Teacher Rating Scales. J Abnorm Child Psychol. 1978;6(2):221–36. doi: 10.1007/BF00919127. https://doi.org/10.1007/BF00919127. [DOI] [PubMed] [Google Scholar]
- 26.Dereboy Ç, Şenol S, Şener Ş, Dereboy F. Validation of the Turkish versions of the short-form Conners’ Teacher and Parent Rating Scales. [Article in Turkish] Turk Psikiyatri Derg. 2007;18(1):48–58. [PubMed] [Google Scholar]
- 27.Şener S, Dereboy C, Dereboy IF, Sertcan Y. Conners’ Teacher Rating Scale Turkish version-I. Turk J Child Adolesc Ment Health. 1993;2(3):131–41. [Google Scholar]
- 28.Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D. Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol. 2012;33(1):85–104. doi: 10.1016/j.yfrne.2011.10.001. https://doi.org/10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22(7):2650–9. doi: 10.1523/JNEUROSCI.22-07-02650.2002. DOI: 20026251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Numakawa T, Yokomaku D, Richards M, Hori H, Adachi N, Kunugi H. Functional interactions between steroid hormones and neurotrophin BDNF. World J Biol Chem. 2010;1(5):133–43. doi: 10.4331/wjbc.v1.i5.133. https://doi.org/10.4331/wjbc.v1.i5.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi H, Bao X, Tang X, Fan X, Xu H. Estrogen modulation of calretinin and BDNF expression in midbrain dopaminergic neurons of ovariectomised mice. J Chem Neuroanat. 2016;77:60–7. doi: 10.1016/j.jchemneu.2016.05.005. https://doi.org/10.1016/j.jchemneu.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Tsai SJ. Role of neurotrophic factors in attention deficit hyperactivity disorder. Cytokine Growth Factor Rev. 2017;34:35–41. doi: 10.1016/j.cytogfr.2016.11.003. https://doi.org/10.1016/j.cytogfr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Segura LM, Balthazart J. Steroids and neuroprotection: New advances. Front Neuroendocrinol. 2009;30(2):v–ix. doi: 10.1016/j.yfrne.2009.04.006. https://doi.org/10.1016/j.yfrne.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58(3):415–26. doi: 10.1016/j.yhbeh.2010.05.013. https://doi.org/10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson CS, Alyea RA, Cunningham KA, Jeng YJ. Estrogens of multiple classes and their role in mental health disease mechanisms. Int J Womens Health. 2010;2:153–66. doi: 10.2147/ijwh.s6907. https://doi.org/10.2147/IJWH.S6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahé V, Dumaine A. Oestrogen withdrawal associated psychoses. Acta Psychiatr Scand. 2001;104(5):323–31. doi: 10.1034/j.1600-0447.2001.00288.x. https://doi.org/10.1034/j.1600-0447.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 37.Ko YH, Joe SH, Cho W, Park JH, Lee JJ, Jung IK, et al. Estrogen, cognitive function and negative symptoms in female schizophrenia. Neuropsychobiology. 2006;53(4):169–75. doi: 10.1159/000093780. https://doi.org/10.1159/000093780. [DOI] [PubMed] [Google Scholar]
- 38.Hoff AL, Kremen WS, Wieneke MH, Lauriello J, Blankfeld HM, Faustman WO, et al. Association of estrogen levels with neuropsychological performance in women with schizophrenia. Am J Psychiatry. 2001;158(7):1134–9. doi: 10.1176/appi.ajp.158.7.1134. https://doi.org/10.1176/appi.ajp.158.7.1134. [DOI] [PubMed] [Google Scholar]
- 39.Kulkarni J, Gavrilidis E, Worsley R, Hayes E. Role of estrogen treatment in the management of schizophrenia. CNS Drugs. 2012;26(7):549–57. doi: 10.2165/11630660-000000000-00000. https://doi.org/10.2165/11630660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–26. doi: 10.1038/nrendo.2011.122. https://doi.org/10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prossnitz ER, Barton M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat. 2009;89(3-4):89–97. doi: 10.1016/j.prostaglandins.2009.05.001. https://doi.org/10.1016/j.prostaglandins.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fındıklı E, Camkurt MA, Karaaslan MF, Kurutas EB, Altun H, İzci F, et al. Serum levels of G protein-coupled estrogen receptor 1 (GPER1) in drug-naive patients with generalized anxiety disorder. Psychiatry Research. 2016;244:312–6. doi: 10.1016/j.psychres.2016.04.098. https://doi.org/10.1016/j.psychres.2016.04.098. [DOI] [PubMed] [Google Scholar]
- 43.Findikli E, Kurutas EB, Camkurt MA, Karaaslan MF, Izci F, Fındıklı HA, et al. Increased serum G protein-coupled estrogen receptor 1 levels and its diagnostic value in drug naïve patients with major depressive disorder. Clin Psychopharmacol Neurosci. 2017;15(4):337–42. doi: 10.9758/cpn.2017.15.4.337. https://doi.org/10.9758/cpn.2017.15.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20(5):534–45. doi: 10.1177/1073858413519865. https://doi.org/10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu CL, Herndon C. New roles for neuronal estrogen receptors. Neurogastroenterol Motil. 2017;29(7) doi: 10.1111/nmo.13121. https://doi.org/10.1111/nmo.13121. [DOI] [PubMed] [Google Scholar]
- 46.Ervin KS, Mulvale E, Gallagher N, Roussel V, Choleris E. Activation of the G protein-coupled estrogen receptor, but not estrogen receptor α or β, rapidly enhances social learning. Psychoneuroendocrinology. 2015;58:51–66. doi: 10.1016/j.psyneuen.2015.04.002. https://doi.org/10.1016/j.psyneuen.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57(11):1336–46. doi: 10.1016/j.biopsych.2005.02.006. https://doi.org/10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Prossnitz ER, Barton M. Estrogen biology: New insights into GPER function and clinical opportunities. Mol Cell Endocrinol. 2014;389(1-2):71–83. doi: 10.1016/j.mce.2014.02.002. https://doi.org/10.1016/j.mce.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anchan D, Clark S, Pollard K, Vasudevan N. GPR30 activation decreases anxiety in the open field test but not in the elevated plus maze test in female mice. Brain Behav. 2014;4(1):51–9. doi: 10.1002/brb3.197. https://doi.org/10.1002/brb3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hart D, Nilges M, Pollard K, Lynn T, Patsos O, Shiel C, et al. Activation of the G-protein coupled receptor 30 (GPR30) has different effects on anxiety in male and female mice. Steroids. 2014;81:49–56. doi: 10.1016/j.steroids.2013.11.004. https://doi.org/10.1016/j.steroids.2013.11.004. [DOI] [PubMed] [Google Scholar]


