Abstract
Studies on the effects of third-line chemotherapy (CT) in advanced gastric cancer (GC) patients are still scarce. The aim of this study was to evaluate the efficacy and safety of the modified 5-fluorouracil, leucovorin, and irinotecan (mFOLFIRI) regimen as a third-line CT in metastatic GC patients, after failure of fluoropyrimidine, platinum, anthracycline, and taxane. After failure of first- and second-line therapies, 42 patients received third-line FOLFIRI (180 mg/m² irinotecan and 400 mg/m² leucovorin administered concomitantly as a 90-minute intravenous (IV) infusion on day 1, followed by a 400 mg/m² 5-fluorouracil IV bolus then 2600 mg/m² continuous infusion over 46 hours), between January 2009 and December 2015. FOLFIRI was administered for a median of 6 cycles (range 4-12 cycles). Eight patients achieved partial response, while 13 patients showed stable disease, resulting in the overall response rate (ORR) of 19% and disease control rate (DCR) of 50%. The most frequent grade 3-4 hematological and non-hematological toxicities were neutropenia (14.2%) and diarrhea (7.1%). The median progression-free survival (PFS) and overall survival (OS) from the start of third-line CT were 3.8 months (95% confidence interval [CI], 3.0-4.5) and 6.8 months (95% CI, 5.6-7.9), respectively. According to the multivariate analysis, two factors were independently predictive of the poor OS: >2 regions of metastasis (relative risk [RR], 2.6; 95% CI, 1.3-5.4) and a high level of carcinoembryonic antigen [CEA] (RR, 3.4; 95% CI, 1.6-7.4). In conclusion, FOLFIRI was well tolerated as third-line CT and showed promising PFS and OS in advanced GC patients, after failure of fluoropyrimidine, platinum, anthracycline, and taxane.
Keywords: Chemotherapy, metastatic gastric cancer, modified FOLFIRI, third-line therapy, prognosis, 5-fluorouracil, leucovorin, irinotecan
INTRODUCTION
Although the incidence of gastric cancer (GC) has substantially decreased over the years, gastric malignancies still represent the fourth most common cause of cancer-related deaths worldwide [1]. A typical GC case is usually in the advanced stage at admission, and chemotherapy (CT) is considered the standard treatment. Compared with the best supportive care (BSC), systemic CT in metastatic GC improves patient survival and contributes to a better quality of life [2,3]. In previous studies, when used as a first-line option, combination regimens were reported to be better than single-agent treatments [2,3]. In accordance with existing evidence, contemporary approaches to patients in a stable condition generally involve a combination of platinum-based agents and fluoropyrimidine drugs, which is considered by many as a standard therapy. The Tax 325 study showed that the addition of docetaxel to platinum and fluoropyrimidine (i.e. docetaxel, cisplatin, and 5-fluorouracil [DCF]) in the first-line treatment of advanced GC contributed to better outcomes [4]. However, DCF treatment was also associated with a significantly higher rate of adverse events, supporting the use of the current regimen with modified doses [5,6].
In metastatic GC cases that were treated with a first-line therapy but have progressed during the follow-up, those with better performance status and adequate organ function could be considered candidates for a second-line therapy. In Phase 3 studies, irinotecan and docetaxel CT resulted in a significantly higher improvement in the overall survival (OS) rate compared with BSC [7-9]. Another study reported similar efficacy of paclitaxel and irinotecan, but paclitaxel had a better adverse-event profile [10]. Recently, the efficacy of paclitaxel in combination with ramucirumab was shown in second-line treatment of patients with advanced GC [11].
In metastatic GC, 20% of patients are candidates for a third-line therapy, based on their performance or as a result of their preferences or expectations [12]. However, the related information is scarce and is mostly based on retrospective studies in which taxane and irinotecan were used either in combination or as monotherapies [13,14]. The FOLFIRI regimen is a combination of 5-fluorouracil, leucovorin and irinotecan, and can be used as a salvage CT regimen in metastatic GC, usually during the second-line treatment. The response rate to the second-line treatments in GC ranges between 10% and 29%, while the OS time ranges from 6.2 to 10.9 months [15-18]. Currently, there is no standardized third-line treatment for metastatic GC; therefore, FOLFIRI may be used as a third-line therapy in patients who have progressed after fluoropyrimidine, platinum, anthracycline, or taxane treatments. This study aimed to evaluate the efficacy and safety of the modified FOLFIRI (mFOLFIRI) regimen as a third-line CT in advanced GC.
MATERIALS AND METHODS
Patient selection
In this study we retrospectively evaluated patients diagnosed with metastatic GC at the Ankara Numune Training and Research Hospital, who had been treated with first- and second-line therapies of fluorouracil, platinum, anthracycline or taxane, and after that with mFOLFIRI as a third-line treatment, between January 2009 and December 2015. Patients with GC or gastroesophageal junction adenocarcinoma, older than 18 years, who progressed after the second-line therapy as determined by radiology, had a performance status (PS) of 0-2 according to the Eastern Cooperative Oncology Group (ECOG) scale, showed no sign of metastasis in the central nervous system or any serious and uncontrolled concomitant medical condition, and who had adequate organ function were included in this retrospective analysis. The patients who were not treated with fluorouracil, platinum, anthracycline, or taxane as the first-line therapy or who were previously treated with irinotecan were excluded.
Treatment and toxicity assessment
The chemotherapeutics were given through ports placed into either the right or the left subclavian vein. Patients were treated with mFOLFIRI consisting of 180 mg/m2 irinotecan and 400 mg/m2 leucovorin (administered concomitantly as a 90-minute intravenous [IV] infusion on day 1), followed by a 400 mg/m² 5-fluorouracil IV bolus then 2600 mg/m² continuous infusion over 46 hours. Considering multiple previous CT regimens in the patients and possible low tolerance, the doses were reduced by up to 25%. The treatment was repeated every 2 weeks and continued until documented disease progression, unacceptable toxicity, or patient refusal. Modification and necessary postponement of drug administration were applied in accordance with hematological or non-hematological toxic effects during the CT cycles.
Evaluation of response and follow-up
Tolerability to treatment was evaluated by general examination, routine biochemistry, and hematologic workup at the beginning of each cycle. The response to therapy was determined by analyzing computerized tomography images after the second or third CT cycle. Tumor response was classified using the standard Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 [19]. Toxicity evaluations were performed based on the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 of the National Cancer Institute. The survival status of the patients was assessed from the hospital files and records of the Central Civil Registration System using the Turkish Republic Registration Number.
Statistical analysis
PASW Statistics for Windows, Version 18.0. (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Descriptive statistics were reported as percentage and median. Categorical variables were analyzed using the Chi-squared test or Fisher’s exact test. A value of p < 0.05 was considered statistically significant. Survival analysis was performed according to the Kaplan-Meier method. The factors identified in the univariate analysis were entered into the Cox regression analysis with backward selection to determine independent predictors of survival. Progression-free survival (PFS) was defined as the time interval from the 1st day of FOLFIRI therapy to the date of objective tumor progression or death due to any cause, whichever occurred first. OS was defined as the time interval from the 1st day of FOLFIRI therapy to the time of death for any reason or the date of the last follow-up.
RESULTS
Patient characteristics
A total of 42 patients were included in this study. Their baseline characteristics are shown in Table 1. The median age of patients was 54 years (range 27-70 years), and the majority of the patients were male (76.2%). With regard to histological types and tumor localization, 66.7% of patients had poorly differentiated/signet ring cell type of GC and in 52.4% of patients the cancer was localized at the level of the cardia or fundus. The ECOG PS was 0 in 6/42 patients (14.3%), 1 in 28/42 (66.7%), and 2 in 8/42 patients (19.0%). Out of 9 patients that underwent curative surgery, 6 received adjuvant CT or chemoradiotherapy. The most common site of metastasis were the intra-abdominal distant lymph nodes (66.7%), followed by the liver (52.4%) and the peritoneum (45.2%). In addition, 35.7% of patients had metastasis in more than two sites.
TABLE 1.
Demographic characteristics of patients with metastatic gastric cancer

As the first-line therapy, 40/42 patients (95%) were treated with modified DCF, 1 (2.5%) was treated with capecitabine, and 1 (2.5%) with 5-fluorouracil and folinic acid (FUFA). For the second-line therapy, 34/42 patients (81%) were treated with epirubicin, oxaliplatin, and capecitabine (EOX), 6 (14%) were treated with capecitabine/5-fluorouracil and oxaliplatin (CAPOX/FOLFOX), and 2 (5%) were treated with modified DCF. The overall response rate (ORR) and disease control rate (DCR) for the first-line therapy were 45% and 83%, respectively. For the second-line therapy, the ORR and DCR were 43% and 62%, respectively. The median PFS was 6.7 months (95% confidence interval [CI]: 5.1-8.3 months) during the first-line CT and 5.6 months (95% CI: 4.8-6.4 months) during the second-line CT. The median duration from the first- to third-line CT was 11.8 months (range 4.5-33.8 months).
Treatment administration and toxicity
Three hundred and twenty cycles of mFOLFIRI were administered with a median of 6 doses (range 4-12 doses). Grade 3-4 hematologic and non-hematologic toxicities are summarized in Table 2. Treatment delay was recorded in 10/42 patients (23.8%) due to toxicity, and 6/10 (14.3%) required a dose reduction of either irinotecan or 5-fluorouracil. The most common grade 3-4 hematologic and non-hematologic toxicities were neutropenia (14.2%) and diarrhea (7.1%). One patient (2.4%) had febrile neutropenia. A total of 4 patients (9.5%) required secondary granulocyte colony-stimulating factor prophylaxis.
TABLE 2.
Grade 3 or 4 toxicity in patients with metastatic gastric cancer treated with modified 5-fluorouracil, leucovorin, and irinotecan (mFOLFIRI)

Response and survival
Out of 42 patients, 8 responded partially while 13 remained stable, resulting in an ORR of 19% and a DCR of 50%. At the time of the analysis, all patients had progressive disease and 38 patients (90.5%) had died. The median duration of follow-up was 6.6 months (range: 2.1-18.1 months). The median PFS and OS from the start of the third-line CT were 3.8 months (95 % CI: 3.0-4.5 months) and 6.8 months (95 % CI: 5.6-7.9 months), respectively (Figures 1 and 2).
FIGURE 1.
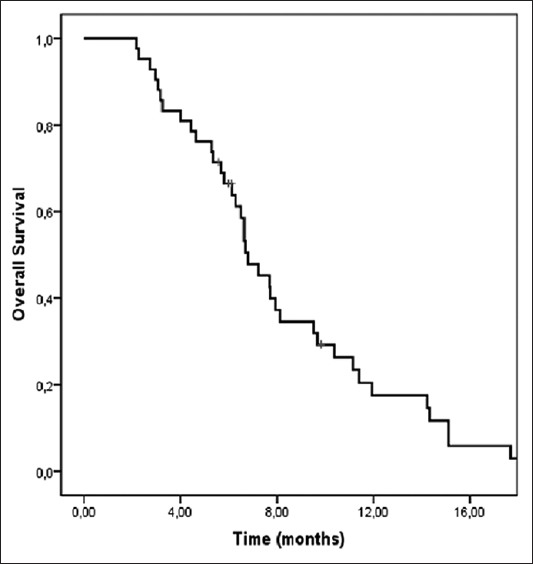
Kaplan-Meier curve of overall survival (OS) in patients with metastatic gastric cancer. The median OS from the start of the third-line chemotherapy was 6.8 months (95 % CI: 5.6-7.9 months).
FIGURE 2.
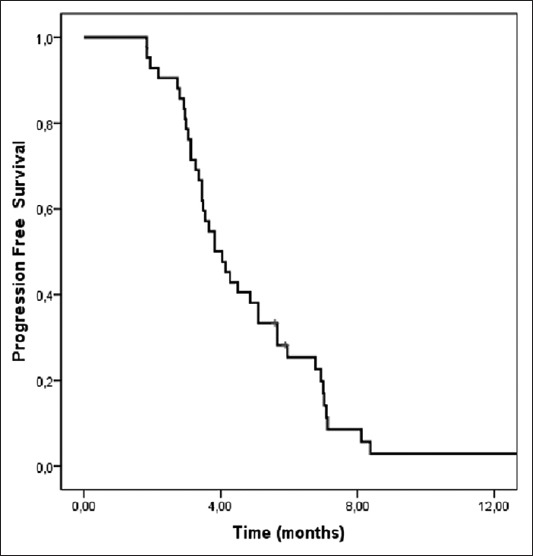
Kaplan-Meier curve of progression-free survival (PFS) in patients with metastatic gastric cancer. The median PFS from the start of the third-line chemotherapy was 3.8 months (95 % CI: 3.0-4.5 months).
Prognostic factors
Prognostic factors that affect OS and PFS were determined using univariate and multivariate analyses. The factors associated significantly with a better OS in the univariate analysis were: hemoglobin level >10 g/dL, number of metastatic sites ≤2, serum carcinoembryonic antigen (CEA) level within normal limits (0-5 ng/mL), ECOG performance score between 0 and 1, and age <60 years. However, in the multivariate analysis, only two parameters were associated with a significantly higher OS: number of metastatic sites ≤2 and serum CEA level within normal limits [0-5 ng/mL] (Table 3). The univariate analysis revealed that factors associated with a significantly better PFS were: age <60 years, number of metastatic sites ≤2, and serum CEA level within normal limits (0-5 ng/mL). As a prognostic factor, female sex showed a tendency toward statistical significance (p = 0.09). The multivariate analysis revealed that the following 3 factors were independently associated with a higher PFS: number of metastatic sites ≤2, serum CEA level within normal limits (0-5 ng/mL), and female gender (Table 4).
TABLE 3.
Univariate and multivariate analysis of OS in patients with metastatic gastric cancer
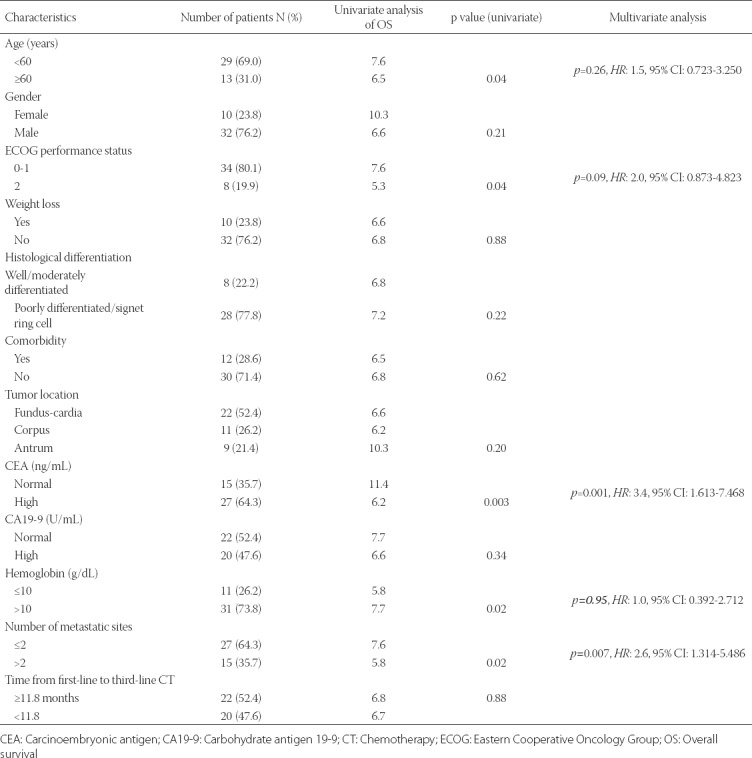
TABLE 4.
Univariate and multivariate analysis of PFS in patients with metastatic gastric cancer
DISCUSSION
In metastatic GC cases, first- and second-line CT were associated with a better OS compared to BSC [7]. However, more data is required to conclude that third-line CT is also superior to BSC in terms of OS. Despite this gray area, in previous studies, 18.1-34.6% of patients with GC were introduced to a third-line CT regimen [12,20,21]. Those studies were mostly conducted in the countries of the Far East, in which gastric malignancies are more common. Monotherapies of fluorouracil, platinum agents, taxanes, irinotecan, and anthracyclines or various combination regimens were reported to be efficient in cases of advanced-stage GC [22]. Since previous studies did not identify a specific treatment to be better than any other, currently, there is no standardized first-, second- or third-line regimen for GC. In previous studies, FOLFIRI was usually started as the first- or second-line CT [15,16,23,24], and only a handful of studies used FOLFIRI as the third-line regimen [13,25]. In those studies, fluorouracil, platinum agents, taxanes, and capecitabine were used in the first- and second-line treatments. Unique to our study is that we included anthracycline in the combination therapies during the second-line treatment.
With respect to the third-line therapies, monotherapies of paclitaxel, docetaxel, and irinotecan and FOLFIRI were the regimens of choice in previous studies. The PFS ranged between 1.9 and 3.5 months, whereas the OS ranged between 3.6 and 7.5 months. The ORR and DRR were 3.0-23.2% and 22.0-65.9%, respectively [13,21,25-30]. The most comprehensive study in which FOLFIRI was used as a third-line treatment in 158 patients with GC, was described by Kang et al. [13]. In that study, patients of Asian origin had a median PFS and OS of 2.1 and 5.6 months, respectively, and the ORR was 9.6%. In an Italian study, using FOLFIRI as a third-line treatment Pasquini et al. reported PFS and OS of 3.3 and 7.5 months, respectively, whereas the ORR was 6% [25]. Similarly as in previous studies, here we reported a median PFS of 3.8 months, median OS of 6.8 months, and ORR of 19%. The fact that our PFS and OS were higher than those described by Kang et al. [13] may be due to racial differences or because two different FOLFIRI regimens were used in their study (FOLFIRI-1 regimen [irinotecan (180 mg/m2 in a 2-hour infusion) on day 1, and then leucovorin (200 mg/m2 in a 2-hour infusion) and 5-FU (a 400 mg/m2 bolus, followed by 600 mg/m2 in a 22-hour continuous infusion) on days 1 and 2] was used in 70% of patients; FOLFIRI-2 regimen [irinotecan (180 mg/m2 in a 2-hour infusion) on day 1, and then leucovorin (400 mg/m2 in a 2-hour infusion) and 5-FU (a 400 mg/m2 bolus, followed by 2400 mg/m2 in a 46-hour continuous infusion) on day 1] was used in 30% of patients).
In this study, the most common grade 3-4 hematologic and non-hematologic toxicities associated with the therapy were neutropenia (14.2%) and diarrhea (7.1%), respectively. There was only one patient who had febrile neutropenia (2.4%). The incidence of adverse events was consistent with the previous studies (neutropenia 6.0-36.8% and diarrhea 1.3-9.0%). Moreover, the rate of febrile neutropenia reported here was also similar as in the previous studies (1.3-12%) [13,25,30].
The reason why toxic events were somewhat rare despite the use of the third-line regimens may be due to the retrospective nature of our and previous studies. We also reduced the dose by up to 25%. Patients with ECOG scores of 0-1 and with higher tolerance levels may experience less toxicity. Considering that aggressive chemotherapies had previously been used for this purpose, mFOLFIRI with relatively tolerable toxicity, can be used safely and efficiently as a third-line treatment in a selective population.
Prognostic factors associated with worse survival rates were reported for third-line CT. Kang et al. [13] showed that poor performance scores (ECOG ≥2), an increased number of metastatic organs (≥3), and a short duration between first- and third-line CT (10.9 months) were negative prognostic factors of OS [13]. Another clinical trial demonstrated that a poor performance score (ECOG ≥2), low serum albumin (<4.0 g/dL), poor histologic type, and a shorter duration of second-line CT (<2.7 months) were related to poor survival outcomes [21]. In a similar manner, we reported that the number of metastatic sites ≥3 was an independent prognostic factor of PFS and OS in both univariate and multivariate analysis. Although the ECOG performance score of 2 was predictive of OS in the univariate analysis, the correlation was not significant in the multivariate analysis. This association may not have reached statistical significance due to the limited number of patients we included. The serum CEA level, as a prognostic factor, has been assessed in first- and second-line therapies [31,32]. Our univariate and multivariate analyses of patients with GC treated with FOLFIRI as the third-line regimen showed that a higher-than-normal serum CEA level was associated with poor PFS and OS. To the best of our knowledge, no previous study has evaluated the prognostic value of serum CEA level in GC cases treated with third-line FOLFIRI. Moreover considering the related data obtained with patients receiving first- and second-line therapies, the CEA level may be of prognostic value in patients receiving FOLFIRI as the third-line treatment. This finding also suggests that patients with metastatic GC should be carefully selected for third-line CT.
The limitations of our study include the small number of cases and retrospective design; thus, we could not perform comprehensive evaluation of toxicities.
CONCLUSION
The mFOLFIRI CT regimen used as third-line therapy in metastatic GC patients who progressed after fluorouracil, platinum agents, taxanes, or anthracyclines, appears to be safe, well tolerated, and with modest activity in patients who have adequate organ function, acceptable ECOG performance scores, and a relatively low number of metastatic sites involved.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. https://doi.org/10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24(18):2903–9. doi: 10.1200/JCO.2005.05.0245. https://doi.org/10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 3.Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;3:CD004064. doi: 10.1002/14651858.CD004064.pub3. https://doi.org/10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol. 2006;24(31):4991–7. doi: 10.1200/JCO.2006.06.8429. https://doi.org/10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 5.Chi Y, Ren JH, Yang L, Cui CX, Li JL, Wang JW. Phase II clinical study on the modified DCF regimen for treatment of advanced gastric carcinoma. Chin Med J (Engl) 2011;124(19):2997–3002. [PubMed] [Google Scholar]
- 6.Keskin S, Yildiz I, Sen F, Aydogan F, Kilic L, Ekenel M, et al. Modified DCF (mDCF) regimen seems to be as effective as original DCF in advanced gastric cancer (AGC) Clin Transl Oncol. 2013;15(5):403–8. doi: 10.1007/s12094-012-0942-8. https://doi.org/10.1007/s12094-012-0942-8. [DOI] [PubMed] [Google Scholar]
- 7.Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30(13):1513–8. doi: 10.1200/JCO.2011.39.4585. https://doi.org/10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW, Baek SK, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: a meta-analysis. Ann Oncol. 2013;24(11):2850–4. doi: 10.1093/annonc/mdt351. https://doi.org/10.1093/annonc/mdt351. [DOI] [PubMed] [Google Scholar]
- 9.Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer - a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47(15):2306–14. doi: 10.1016/j.ejca.2011.06.002. https://doi.org/10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31(35):4438–44. doi: 10.1200/JCO.2012.48.5805. https://doi.org/10.1200/JCO.2012.48.5805. [DOI] [PubMed] [Google Scholar]
- 11.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35. doi: 10.1016/S1470-2045(14)70420-6. https://doi.org/10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 12.Hess LM, Michael D, Mytelka DS, Beyrer J, Liepa AM, Nicol S. Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective analysis of electronic medical record (EMR) and administrative claims data. Gastric Cancer. 2016;19(2):607–15. doi: 10.1007/s10120-015-0486-z. https://doi.org/10.1007/s10120-015-0486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang EJ, Im SA, Oh DY, Han SW, Kim JS, Choi IS, et al. Irinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy after failure of fluoropyrimidine, platinum, and taxane in gastric cancer: treatment outcomes and a prognostic model to predict survival. Gastric Cancer. 2013;16(4):581–9. doi: 10.1007/s10120-012-0227-5. https://doi.org/10.1007/s10120-012-0227-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Kim SH, Oh SY, Lee S, Lee H, Lee HJ, et al. Third-line docetaxel chemotherapy for recurrent and metastatic gastric cancer. Korean J Intern Med. 2013;28(3):314–21. doi: 10.3904/kjim.2013.28.3.314. http://192.168.2.1:8090/httpclient.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assersohn L, Brown G, Cunningham D, Ward C, Oates J, Waters JS, et al. Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol. 2004;15(1):64–9. doi: 10.1093/annonc/mdh007. https://doi.org/10.1093/annonc/mdh007. [DOI] [PubMed] [Google Scholar]
- 16.Kim SG, Oh SY, Kwon HC, Lee S, Kim JH, Kim SH, et al. A phase II study of irinotecan with bi-weekly, low-dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFIRI) as salvage therapy for patients with advanced or metastatic gastric cancer. Jpn J Clin Oncol. 2007;37(10):744–9. doi: 10.1093/jjco/hym103. https://doi.org/10.1093/jjco/hym103. [DOI] [PubMed] [Google Scholar]
- 17.Seo MD, Lee KW, Lim JH, Yi HG, Kim DY, Oh DY, et al. Irinotecan combined with 5-fluorouracil and leucovorin as second-line chemotherapy for metastatic or relapsed gastric cancer. Jpn J Clin Oncol. 2008;38(9):589–95. doi: 10.1093/jjco/hyn078. https://doi.org/10.1093/jjco/hyn078. [DOI] [PubMed] [Google Scholar]
- 18.Sym SJ, Ryu MH, Lee JL, Chang HM, Kim TW, Lee SS, et al. Salvage chemotherapy with biweekly irinotecan, plus 5-fluorouracil and leucovorin in patients with advanced gastric cancer previously treated with fluoropyrimidine, platinum, and taxane. Am J Clin Oncol. 2008;31(2):151–6. doi: 10.1097/COC.0b013e31815878a2. https://doi.org/10.1097/COC.0b013e31815878a2. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. https://doi.org/10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Ji SH, Lim DH, Yi SY, Kim HS, Jun HJ, Kim KH, et al. A retrospective analysis of second-line chemotherapy in patients with advanced gastric cancer. BMC Cancer. 2009;9:110. doi: 10.1186/1471-2407-9-110. https://doi.org/10.1186/1471-2407-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shim HJ, Yun JY, Hwang JE, Bae WK, Cho SH, Chung IJ. Prognostic factor analysis of third-line chemotherapy in patients with advanced gastric cancer. Gastric Cancer. 2011;14(3):249–56. doi: 10.1007/s10120-011-0032-6. https://doi.org/10.1007/s10120-011-0032-6. [DOI] [PubMed] [Google Scholar]
- 22.Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015;33(16):1760–9. doi: 10.1200/JCO.2014.60.1799. https://doi.org/10.1200/JCO.2014.60.1799. [DOI] [PubMed] [Google Scholar]
- 23.Kim BG, Oh SY, Kwon HC, Lee S, Lee DM, Kim SG, et al. A phase II study of irinotecan with biweekly, low dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFIRI) as first line therapy for patients with recurrent or metastatic gastric cancer. Am J Clin Oncol. 2010;33(3):246–50. doi: 10.1097/COC.0b013e3181a650d4. DOI: 10.1097/COC.0b013e3181a650d4. [DOI] [PubMed] [Google Scholar]
- 24.Kwon HJ, Park MI, Park SJ, Moon W, Kim SE, Lee HW, et al. Efficacy and safety of FOLFIRI after failure of FOLFOX-4 in advanced gastric cancer. Korean J Gastroenterol. 2015;66(1):10–6. doi: 10.4166/kjg.2015.66.1.10. https://doi.org/10.4166/kjg.2015.66.1.10. [DOI] [PubMed] [Google Scholar]
- 25.Pasquini G, Vasile E, Caparello C, Vivaldi C, Musettini G, Lencioni M, et al. Third-line chemotherapy with irinotecan plus 5-fluorouracil in Caucasian metastatic gastric cancer patients. Oncology. 2016;91(6):311–6. doi: 10.1159/000443962. https://doi.org/10.1159/000443962. [DOI] [PubMed] [Google Scholar]
- 26.Godai TI, Oshima T, Numata M, Fukahori M, Sato T, Makino H, et al. Clinical efficacy and safety of CPT-11+CDDP therapy as third-line chemotherapy for advanced and recurrent gastric cancer. [Article in Japanese] Gan To Kagaku Ryoho. 2011;38(6):945–9. [PubMed] [Google Scholar]
- 27.Lee MJ, Hwang IG, Jang JS, Choi JH, Park BB, Chang MH, et al. Outcomes of third-line docetaxel-based chemotherapy in advanced gastric cancer who failed previous oxaliplatin-based and irinotecan-based chemotherapies. Cancer Res Treat. 2012;44(4):235–41. doi: 10.4143/crt.2012.44.4.235. https://doi.org/10.4143/crt.2012.44.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon YW, Rha SY, Jeung HC, Kim C, Hong MH, Chang H, et al. Outcomes of multiple salvage chemotherapy for advanced gastric cancer: implications for clinical practice and trial design. Cancer Chemother Pharmacol. 2010;66(4):797–805. doi: 10.1007/s00280-010-1295-z. https://doi.org/10.1007/s00280-010-1295-z. [DOI] [PubMed] [Google Scholar]
- 29.Shimoyama R, Yasui H, Boku N, Onozawa Y, Hironaka S, Fukutomi A, et al. Weekly paclitaxel for heavily treated advanced or recurrent gastric cancer refractory to fluorouracil, irinotecan, and cisplatin. Gastric Cancer. 2009;12(4):206–11. doi: 10.1007/s10120-009-0524-9. https://doi.org/10.1007/s10120-009-0524-9. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura T, Iwasa S, Nagashima K, Okita N, Takashima A, Honma Y, et al. Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer. 2017;20(4):655–62. doi: 10.1007/s10120-016-0670-9. https://doi.org/10.1007/s10120-016-0670-9. [DOI] [PubMed] [Google Scholar]
- 31.Catalano V, Graziano F, Santini D, D’Emidio S, Baldelli AM, Rossi D, et al. Second-line chemotherapy for patients with advanced gastric cancer: who may benefit? Br J Cancer. 2008;99(9):1402–7. doi: 10.1038/sj.bjc.6604732. https://doi.org/10.1038/sj.bjc.6604732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louvet C, Carrat F, Mal F, Mabro M, Beerblock K, Vaillant JC, et al. Prognostic factor analysis in advanced gastric cancer patients treated with hydroxyurea, leucovorin, 5-fluorouracil, and cisplatin (HLFP regimen) Cancer Invest. 2003;21(1):14–20. doi: 10.1081/cnv-120016399. https://doi.org/10.1081/CNV-120016399. [DOI] [PubMed] [Google Scholar]


