Abstract
Background and study aims
Complex colorectal polyps or those positioned in difficult anatomic locations are an endoscopic therapeutic challenge. Underwater endoscopic submucosal dissection (UESD) is a potential technical solution to facilitate efficient polyp removal. In addition, endoscopic tissue retraction has been confined to limited methods of varying efficacy and complexity. The aim of this study was to evaluate the efficiency of a unique UESD technique for removing complex polyps using double-balloon-assisted retraction (R).
Materials and methods
Using fresh ex-vivo porcine rectum, 4-cm polyps were created using electrosurgery and positioned at “6 o’clock” within an established ESD model. Six resections were performed in each group. Underwater techniques were facilitated using a novel double-balloon platform (Dilumen, Lumendi, Westport, Connecticut, United States).
Results
UESD-R had a significantly shorter total procedural time than cap-assisted ESD and UESD alone (24 vs. 58 vs. 56 mins). UESD-R produced a dissection time on average of 5 minutes, attributed to the retraction provided. There was also a subjective significant reduction in electrosurgical smoke with the underwater techniques contributing to improved visualization.
Conclusions
Here we report the first ex-vivo experience of a unique double-balloon endoscopic platform optimized for UESD with tissue traction capability. UESD-R removed complex lesions in significantly shorter time than conventional means. The combined benefits of UESD and retraction appeared to be additive when tackling complex polyps and should be studied further.
Introduction
Complex colorectal polyps defined as those greater than 2 cm or those positioned in difficult anatomic locations can be a major challenge for endoscopic therapy 1 2 3 . Underwater endoscopic submucosal dissection (UESD) has recently emerged as a potential technical solution to facilitate complex polyp removal 4 . The major benefits of UESD are gravity-assistance independence, magnification, hydro-dissection, reduced tissue burn artifact and electrosurgical smoke reduction 4 5 6 . In addition, tissue traction and counter-traction, cornerstone principles of effective dissection, have been confined to limited methods ranging from clipping/retraction to using dual endoscopes 7 8 9 . Despite the clear benefits of endoscopic submucosal dissection (ESD) (en-bloc resection and low recurrence rate), it remains a technically challenging procedure with higher perforation rates compared to endoscopic mucosal resection (EMR) 10 . This difficulty can be particularly amplified when the lesion is unamenable to gravity assistance, i. e. located in the inferior portion of the clock face. Double- and single-balloon systems have demonstrated some utility within the endolumenal space, particularly in small bowel locomotion and stabilization of the scope within the lumen when performing advanced endoscopic procedures 11 12 .
Using an ex vivo tissue model, we explored a hybrid approach using UESD facilitated by a unique endoscopic double-balloon system that also permits tissue retraction, to decrease technical difficulty and increase safety. The Dilumen double-balloon (DB) platform is a US Food and Drug Administration – approved commercially available double balloon oversheath device (Dilumen; Lumendi, Westport, Connecticut, United States) mounted onto adult and pediatric colonoscopes. The device allows inflation of two independently controlled balloons, enhancing endoscopic stability and visualization. In addition, the device provides tissue traction through clipping of tissue to the balloon ( Fig. 1 ).
Fig. 1.
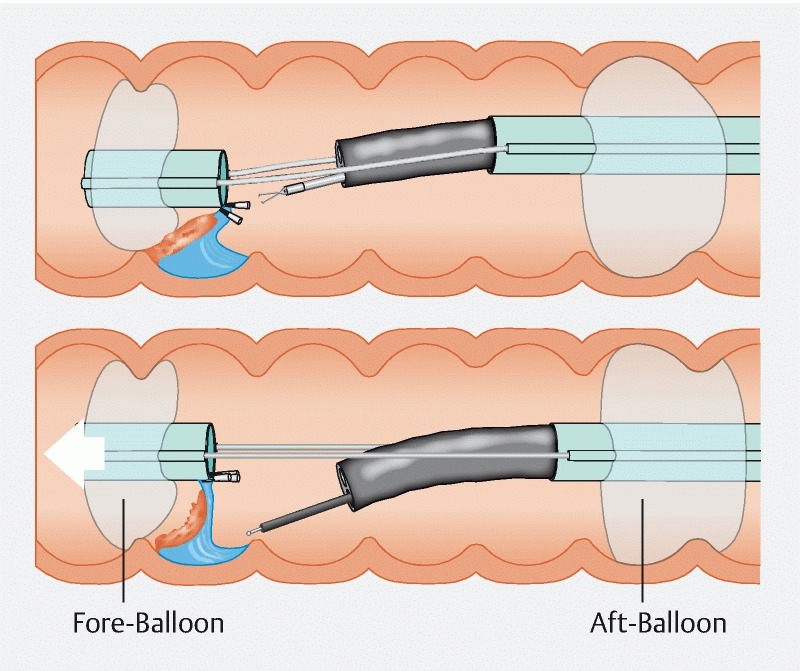
Device set-up.
The aim of this study was to evaluate the efficiency of a unique UESD technique for removing complex polyps using double balloon-assisted retraction (R).
Materials and methods
Using fresh ex-vivo porcine rectum, 4-cm polyps were created using electrosurgery and positioned at “6 o’clock” (4-cm polyp with 5-mm margin) within an established ESD model. The position of the lesion was intentionally placed at 6 o’clock to increase the difficulty with negative gravity effect. Two lesions were placed on each animal specimen in diametrically opposite positions so as to utilize the animal specimen to its maximum. Six resections were performed in each group.
Three different polypectomy methods (equipment listed below) were compared:
UESD with retraction (UESD-R)
UESD no retraction
Traditional cap-assisted ESD technique.
Dilumen use
The Dilumen device was mounted onto a colonoscope (Olympus PCF-H180AL; Olympus Corporation, Japan). Upon insertion into the model and at an appropriate section related to the lesion, the Aft-Balloon (AB) inflation selector was selected via the inflation handle control knob. The inflation/deflation bulb was depressed until the desired pressure was reached (indicated by the indicator turning green). Upon confirmation of scope stability using slight longitudinal movements on the scope shaft, the Fore-Balloon (FB) was extended beyond the endoscope tip using the FB slider located on the handle of the device. Upon extension to the desired distance the FB inflation position was selected and the inflation bulb was again squeezed to inflate the FB, the degree of which was confirmed using the indicator as well as visual representation on the endoscopic view. After confirmation of mucosal gripping, the FB was further extended using the handle knob to provide mucosal traction.
UESD technique
A circumferential mucosal incision was made at the lesion margin with the Dualknife (see below for equipment used). Following this, the balloons were deployed behind and in front of the lesion. After the balloons were inflated, a sealed “therapeutic zone” was created and saline solution instilled. The assistant activated the water injector pump at the same time the operator activated the electrosurgery. Once dissection was completed with the IT-nano and cap on the endoscope tip, the fluid was suctioned out and specimen removed.
UESD-R technique
A circumferential mucosal incision was made at the lesion margin with the Dualknife ( Video 1 ). Following this, the balloons were deployed behind and in front of the lesion ( Fig. 1 ) as described above. After the balloons were inflated, a sealed “therapeutic zone” was created and saline solution instilled. The incision leading edge was dissected further using underwater dissection. The assistant activated the water injector pump at the same time the operator activated the electrosurgery. Once completed, the fluid was suctioned out and the mucosal edge clipped to the base of the fore balloon ( Fig. 2 ). Using variable tension on the fore balloon, tissue dissection continued until resection was completed (using the IT-nano) ( Fig. 3 ).
Fig. 2.
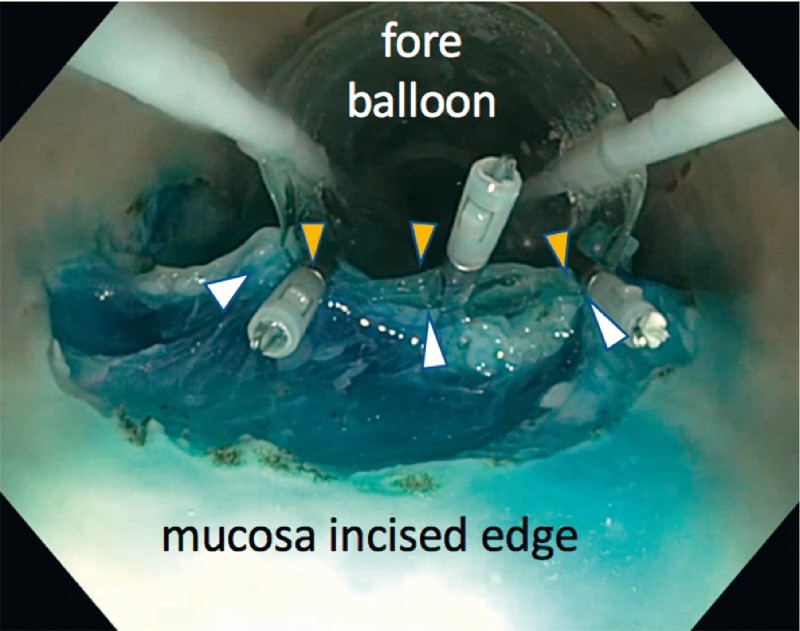
Mucosal (white arrows) clipping to balloon (yellow arrows).
Fig. 3.
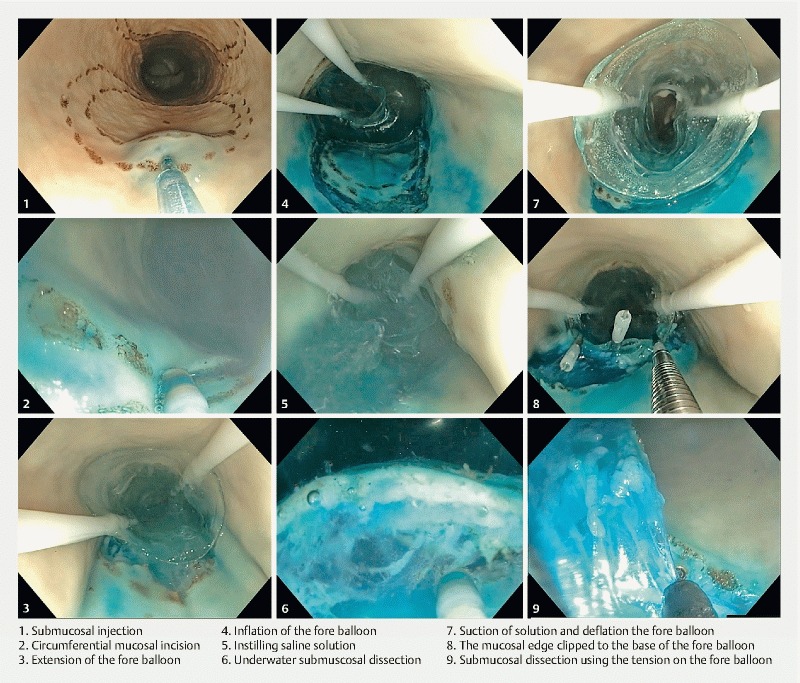
Stages of UESD-R procedure.
Cap technique
A circumferential mucosal incision was made at the lesion margin with the DualKnife. Dissection progressed by pressing the endoscope cap into the submucosal plane and progressing with dissection using the IT-nano.
Variables measured
Variables measured were total procedure time (time from first submucosal injection to complete removal of specimen), dissection time (non-marking electrosurgery activation time), specimen size and perforations. Data were recorded and analyzed using Graphpad Prism software (Graphpad, UKR). All procedures were video recorded and resected specimens photographed. Data were quoted as mean +/– standard deviation and statistical comparisons were made using a one-way ANOVA. A P value < 0.05 was considered significant.
Equipment
UESD, UESD-R (DiLumen, Lumendi, LLC) or traditional cap-assisted ESD method (Olympus cap D-201-12704) were performed using a pediatric colonoscope (Olympus PCF-H180AL). Monopolar electrosurgery was performed using ERBE electrosurgical generator with Olympus Dualknife (KD-650U) and IT nano (KD-612U), 80w Cut 40w Coagulation. Submucosal injection was used in all cases (0.04 % methylene blue, normal saline solution) through a Boston Scientific 25G endoscopic needle injector. Clips – Olympus EZ clip – Long clip.
Operator experience
One experienced endoscopist (SS) performed all procedures in a sequential fashion i. e. cap, UESD, UESD-R and repeat. The endoscopist had 3 years’ experience using the model.
Results
UESD-R had a significantly shorter total procedural time than cap-assisted ESD and UESD ( Table 1 and Fig. 4 , CAP 58 vs. UESD 56 vs. UESDR 24 mins). The same trend was seen with dissection time (CAP 52 vs. UESD 45 vs. UESDR 5 mins). Surprisingly, UESDR produced a dissection time on average of 5 minutes ( Video 1 ), attributed to the retraction provided. The observation that the procedural time did not differ significantly from the UESD group and the standard cap technique indicated the benefits of traction devices over the underwater approach. Excised specimen size did not vary significantly between the 3 groups (Cap 12.8 cm 2 vs. UESD 15.9 cm 2 vs. UESDR 16.7 cm 2 ). No perforations were observed.
Table 1. Results with UESD versus UESD-R.
| ESD technique (n = 6/group) | Cap | UESD | UESD-R | Significance |
| Procedural time (mins +/– SD) | 58 (+/– 21) | 56 (+/– 10) | 25 (+/– 7) | Cap vs. UESD = 0.97 UESD vs. UESD-R = 0.005 Cap vs. UESD-R = 0.003 |
| Dissection time (mins +/– SD) | 52 (+/– 20) | 45 (+/– 6) | 5 (+/– 3) | Cap vs. UESD = 0.57 UESD vs. UESD-R = 0.0001 Cap vs. UESD-R = 0.0001 |
UESD, underwater endoscopic submucosal dissection; UESD-R, underwater endoscopic submucosal dissection with retraction; SD, standard deviation
Fig. 4.
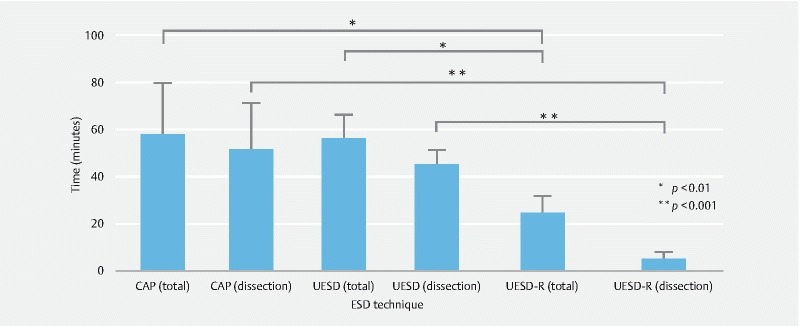
Procedural and dissection time for different dissection techniques.
There was a subjective significant electrosurgical smoke reduction with the UESD and UESD-R technique contributing to improved visualization ( Fig. 5 ). The combination of dissection and saline solution irrigation in UESD-R provided rapid dissection of the mucosal leading edge through elevation of the incised tissue.
Fig. 5.
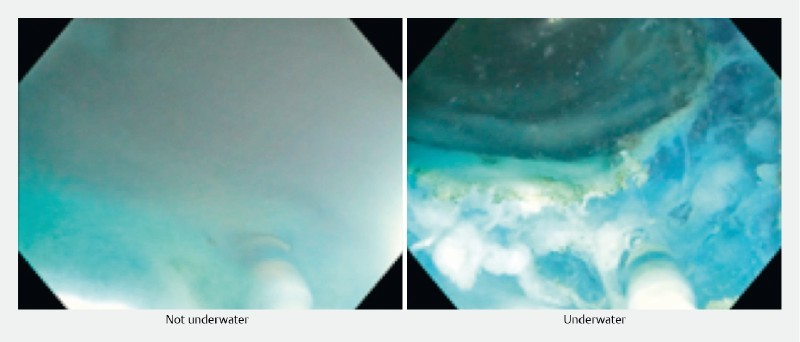
Electrosurgical smoke production non-underwater versus underwater.
Discussion
Although double-balloon endoscopic systems have been available for some time, their utility has been largely confined to small bowel enteroscopy 13 . However, recent reports have attempted to utilize single-balloon technology in performing large intestine therapeutic procedures through aiding cecal intubation 14 .
ESD and EMR remain the most widespread complex therapeutic procedures performed in the gastrointestinal tract. ESD can be considered more challenging due to the technical skills involved in the procedure. Factors making this more difficult are size, location, and lack of gravity assistance. Patient positioning can be employed to facilitate gravity assistance, but this is not always possible.
Another important factor affecting the long procedure times and technical difficulty associated with advanced endoscopic procedures is lack of robust and effective tissue traction. Tissue traction increases tension of tissues to facilitate efficient dissection. Traction not only aids in tissue dissection but also improves submucosal layer visualization by lifting away the mucosal flap from the field of view. This increases safety through anatomical layer and submucosal vessel identification. Traction is the most promising avenue for reducing endolumenal procedure times and complications.
Multiple endolumenal tissue traction methods have been attempted with varying degrees of success. Internal traction uses rings or springs that are clipped to one edge of the incised mucosa and then clipped to the opposite facing mucosa 15 16 17 . This method provides continuous traction but cannot generally be repositioned. Suture and hemostatic clip placement uses a 3-0 silk suture tied to a hemostatic clip. Once a mucosal flap has been developed, the clip with the suture attached is deployed on the lesion edge. The free end of the suture outside the patient is pulled towards the endoscope tip, thereby providing tissue traction towards the operator 18 . Clip-and-snare pulling uses a hemostatic clip and a snare. After circumferential incision, the endoscope is externally looped into a snare. The scope and snare are reinserted into the patient. A working channel introduced hemostatic clip grasps the incised mucosal flap. The snare is pressed up to the clip. After tightening the snare to grasp the clip, it is deployed providing traction towards the operator 19 . With external forceps, an accessory working channel – an external channel placed outside the colonoscope – is used to deliver a second grasping forceps, which grasps the lesion edge. Traction direction can be either towards or away from the endoscope tip. This method has been reported to increase the risk of mucosal injury upon insertion 20 . With the double-scope method, a smaller-caliber endoscope is inserted alongside the main scope. Using grasping forceps, the smaller-caliber scope grasps the incised mucosa while the main scope dissects. Because this method requires two operators, it is not always feasible 21 .
There are multiple challenges associated with these methods. Most are technically difficult, require two operators in the case of the double endoscope method and pull the tissue towards the scope tip, which is not ideal in the case of tissue dissection. The ideal position is traction away from the perspective of the operator. In addition, most studies reporting traction methods are for gastric lesions.
Electrosurgical tool use is a necessity for complex therapeutic procedures. However, it can cause significant smoke production as well as fat deposition on the endoscope lens, sometimes requiring removal of the endoscope from the patient and cleaning of the lens, thus decreasing efficiency.
Here we report the first ex-vivo experience with a unique double-balloon endoscopic platform optimized for UESD with tissue traction capability. In lesions considered difficult (4 cm and at 6 o’clock), UESD-R removed lesions in significantly shorter time than conventional means. Our experience also subjectively showed a significant decrease in smoke obscuring the dissection capability of the dissection. Similar experiences have been quoted in the literature 4 5 6 . Employing double balloon technology to facilitate underwater dissection, to our knowledge, has never been described before in the literature.
This study has several limitations, including its ex-vivo nature. The porcine model, although well established as an ESD trainer, has several limitations that limit its accuracy including lack of contractions, respiration, submucosal fibrosis and bleeding. Six resections in each group is a small number of procedures, which prevented any learning curve calculations. Smoke production is difficult to measure, however, several studies have corroborated our findings and we included visual representation of the endoscopic view to demonstrate this to the viewer. Our dissection strategy may not be considered optimal, i. e. performing a circumferential incision. This point and the lesion position at 6 o’clock may not fully simulate the reality of ESD procedures currently.
Conclusion
In conclusion, the double-balloon system enabled UESD-R. This led to significantly shorter observed procedural and dissection times versus traditional ESD methods. The combined benefits of UESD and retraction appeared to be additive when tackling complex polyps unamenable to gravity assistance and should be studied further.
Footnotes
Competing interests Milsom: Clinical advisory board member lumendi.
References
- 1.Voloyiannis T, Snyder M J, Bailey R R et al. Management of the difficult colon polyp referred for resection: resect or rescope? Dis Colon Rectum. 2008;51:292–295. doi: 10.1007/s10350-007-9175-2. [DOI] [PubMed] [Google Scholar]
- 2.Saunders B P, Tsiamoulos Z P. Endoscopic mucosal resection and endoscopic submucosal dissection of large colonic polyps. Nat Rev Gastroenterol Hepatol. 2016;13:486–496. doi: 10.1038/nrgastro.2016.96. [DOI] [PubMed] [Google Scholar]
- 3.Waye J D. Advanced polypectomy. Gastrointest Endosc Clin N Am. 2005;15:733–756. doi: 10.1016/j.giec.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Yoshii S, Hayashi Y, Matsui Tet al. “Underwater” endoscopic submucosal dissection: a novel technique for complete resection of a rectal neuroendocrine tumor Endoscopy 201648UCTN:E67–E68. [DOI] [PubMed] [Google Scholar]
- 5.Akasaka T, Takeuchi Y, Uedo N et al. “Underwater” endoscopic submucosal dissection for superficial esophageal neoplasms. Gastrointest Endosc. 2017;85:251–252. doi: 10.1016/j.gie.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Yoshii S, Hayashi Y, Tsujii Y et al. Underwater endoscopic submucosal dissection: a novel resection strategy for early gastric cancer located on the greater curvature of the gastric body. Ann Gastroenterol. 2017;30:364. doi: 10.20524/aog.2017.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Renteln D, Schmidt A, Vassiliou M C et al. Endoscopic mucosal resection using a grasp-and-snare technique. Endoscopy. 2010;42:475–480. doi: 10.1055/s-0029-1244121. [DOI] [PubMed] [Google Scholar]
- 8.Tsuji K, Yoshida N, Nakanishi H et al. Recent traction methods for endoscopic submucosal dissection. World J Gastroenterol. 2016;22:5917–5926. doi: 10.3748/wjg.v22.i26.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacques J, Legros R, Charissoux A et al. A combination of pocket, double-clip countertraction, and isolated HybridKnife as a quick and safe strategy for colonic endoscopic submucosal dissection. Endoscopy. 2017;49:E134–E135. doi: 10.1055/s-0043-104522. [DOI] [PubMed] [Google Scholar]
- 10.Niikura R, Yasunaga H, Yamada Aet al. Factors predicting adverse events associated with therapeutic colonoscopy for colorectal neoplasia: a retrospective nationwide study in Japan Gastrointest Endosc 201684971–982.e976 [DOI] [PubMed] [Google Scholar]
- 11.Messer I, May A, Manner H et al. Prospective, randomized, single-center trial comparing double-balloon enteroscopy and spiral enteroscopy in patients with suspected small-bowel disorders. Gastrointestinal endoscopy. 2013;77:241–249. doi: 10.1016/j.gie.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto H, Yano T, Ohmiya N et al. Double‐balloon endoscopy is safe and effective for the diagnosis and treatment of small‐bowel disorders: Prospective multicenter study carried out by expert and non‐expert endoscopists in Japan. Digestive Endoscopy. 2015;27:331–337. doi: 10.1111/den.12378. [DOI] [PubMed] [Google Scholar]
- 13.Yano T, Yamamoto H. Current state of double balloon endoscopy: the latest approach to small intestinal diseases. J Gastroenterol Hepatol. 2009;24:185–192. doi: 10.1111/j.1440-1746.2008.05773.x. [DOI] [PubMed] [Google Scholar]
- 14.Hotta K, Katsuki S, Ohata K et al. Efficacy and safety of endoscopic interventions using the short double-balloon endoscope in patients after incomplete colonoscopy. Dig Endosc. 2015;27:95–98. doi: 10.1111/den.12318. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto K, Nagahara A, Sakamoto N et al. A new traction device for facilitating endoscopic submucosal dissection (ESD) for early gastric cancer: the “medical ring”. Endoscopy. 2011;43:E67–E68. doi: 10.1055/s-0030-1255923. [DOI] [PubMed] [Google Scholar]
- 16.Parra-Blanco A, Nicolas D, Arnau M R et al. Gastric endoscopic submucosal dissection assisted by a new traction method: the clip-band technique. A feasibility study in a porcine model (with video) Gastrointestinal endoscopy. 2011;74:1137–1141. doi: 10.1016/j.gie.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 17.Chen P-J, Chu H-C, Chang W-K et al. Endoscopic submucosal dissection with internal traction for early gastric cancer (with video) Gastrointestinal endoscopy. 2008;67:128–132. doi: 10.1016/j.gie.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc. 2012;45:375. doi: 10.5946/ce.2012.45.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasuda M, Naito Y, Kokura S et al. Sa1687 Newly-developed ESD (CSL-ESD) for early gastric cancer using convenient and low-cost lifting method (lifting method using clips and snares) for lesions is clinically useful. Gastrointest Endosc. 2012;75:AB244. [Google Scholar]
- 20.Imaeda H, Hosoe N, Ida Y et al. Novel technique of endoscopic submucosal dissection using an external grasping forceps for superficial gastric neoplasia. Dig Endosc. 2009;21:122–127. doi: 10.1111/j.1443-1661.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi K, Tanabe S, Azuma M et al. Double-endoscope endoscopic submucosal dissection for the treatment of early gastric cancer accompanied by an ulcer scar (with video) Gastrointest Endosc. 2013;78:266–273. doi: 10.1016/j.gie.2013.01.010. [DOI] [PubMed] [Google Scholar]


