Highlights
-
•
First multicenter program evaluation of influenza vaccine effectiveness in Latin America.
-
•
Successful integration of influenza surveillance and vaccination platforms.
-
•
Influenza vaccines provided moderate protection among young children and older adults.
Keywords: Influenza, Vaccine effectiveness, Influenza vaccines, Children, Adults, International
Abstract
Background
Despite widespread utilization of influenza vaccines, effectiveness (VE) has not been routinely measured in Latin America.
Methods
We used a case test-negative control design to estimate trivalent inactivated influenza VE against laboratory-confirmed influenza among hospitalized children aged 6 months-5 years and adults aged ≥60 years which are age-groups targeted for vaccination. We sought persons with severe acute respiratory infections (SARI), hospitalized at 71 sentinel hospitals in Argentina, Brazil, Chile, Colombia, Costa Rica, El Salvador, Honduras, Panama, and Paraguay during January–December 2013. Cases had an influenza virus infection confirmed by real-time reverse transcription PCR (rRT-PCR); controls had a negative rRT-PCR result for influenza viruses. We used a two-stage random effects model to estimate pooled VE per target age-group, adjusting for the month of illness onset, age and preexisting medical conditions.
Results
We identified 2620 SARI patients across sites: 246 influenza cases and 720 influenza-negative controls aged ≤5 years and 448 cases and 1206 controls aged ≥60 years. The most commonly identified subtype among participants (48%) was the influenza A(H1N1)pdm09 virus followed by influenza A(H3N2) (34%) and influenza B (18%) viruses. Among children, the adjusted VE of full vaccination (one dose for previously vaccinated or two if vaccine naïve) against any influenza virus SARI was 47% (95% confidence interval [CI]: 14–71%); VE was 58% (95% CI: 16–79%) against influenza A(H1N1)pdm09, and 65% (95% CI: −9; 89%) against influenza A(H3N2) viruses associated SARI. Crude VE of full vaccination against influenza B viruses associated SARI among children was 3% (95% CI: −150; 63). Among adults aged ≥60 years, adjusted VE against any influenza SARI was 48% (95% CI: 34–60%); VE was 54% (95% CI: 37–69%) against influenza A(H1N1)pdm09, 43% (95% CI: 18–61%) against influenza A(H3N2) and 34% (95% CI: −4; 58%) against B viruses associated SARI.
Conclusion
Influenza vaccine provided moderate protection against severe influenza illness among fully vaccinated young children and older adults, supporting current vaccination strategies.
1. Introduction
There is substantial use of influenza vaccines in the Latin American and the Caribbean (LAC) region as compared to other regions globally. As of 2014, 38 out of 43 LAC countries/territories offered influenza vaccines free of charge through their national expanded programs on immunization (EPI) [1]. The LAC EPIs target individuals at increased risk of exposure to influenza virus such as healthcare workers as well as those at heightened risk of developing secondary complications from influenza infections (e.g., pregnant women, children aged <5 years, older adults and individuals with underlying medical conditions (Table 1)) [1], [2], [3], [4], [5].
Table 1.
Overview of vaccination target groups, timing of vaccination campaigns, and sentinel hospitals among countries participating in the multicenter case-control study in nine Latin American countries, 2013.
| Country | Year of vaccine introduction | Target groups for vaccination | Definition of chronic conditions used for vaccination | Timing and duration of vaccination campaigns | Start date of vaccination campaign in 2013 | Vaccine formulation used | Number of participating severe acute respiratory infections (SARI) sentinel surveillance hospitals | Types of respiratory specimens collected in SARI surveillance |
|---|---|---|---|---|---|---|---|---|
| Argentina | 1993 | 6–23 monthsa, ≥65 yearsa, pregnant and postpartum women, healthcare workers, 2–64 years with chronic conditions, security and essential services personnel | Respiratory diseases, heart diseases, congenital or acquired immunodeficiencies, transplanted oncohematologic patients, morbid obesity, diabetes, chronic renal failure on dialysis, severe cognitive impairment, genetic or neuromuscular respiratory distress syndromes, chronic treatment with Acetylsalicylic Acid in 18 years, partners of oncohematologic patients, contacts of infants <1500 g of weight | End of February or mid-March and vaccine offered throughout the season through routine services | 1 March 2013 | Southern Hemisphere. | 4 hospitals (50%)selected out of 8 SARI sentinel surveillance hospitals | Nasopharyngeal aspirates |
| Brazil | 1999 | 6–23 monthsa, ≥60 yearsa, pregnant and postpartum women, healthcare workers, indigenous populations, persons with chronic diseases | Diabetes, chronic respiratory diseases, chronic hepatic diseases, chronic cardiac diseases, chronic renal diseases, chronic neurologic diseases, obesity, immuno suppressed individuals, transplanted individuals, Down syndrome | End of April to mid-may | 17 April 2013 | Southern Hemisphere | 29 hospitals selected from the South and South East regions of Brazil (SARI reporting is universal in Brazil) | Nasopharyngeal aspirates; nasopharyngeal swabs in Brazil, Panama, and three hospitals in Chile. |
| Chile | 1975 | 6–23 monthsa, ≥65 yearsa, pregnant women, healthcare workers, persons with chronic diseases, poultry workers | Diabetes, chronic pulmonary disease specifically bronchial asthma, COPD, Pulmonary fibrosis, cardiopathy: congenital, rheumatic ischemic, and cardiomyopathies, neuromuscular disease, (congenital or acquired), Morbid obesity, chronic renal insufficiency in phase ≥4, chronic renal insufficiency in dialysis, chronic hepatic insufficiency, autoimmune disorders (LES, scleroderma; AR, Crohn’s disease etc.), Cancer under radiotherapy treatment, chemotherapy, hormonal therapy or palliative measures of any kind. HIV, congenital or acquired immunodeficiency, healthcare personnel (private or public) | End of March | 18 March 2013 | Southern Hemisphere | 6 (all SARI sentinel surveillance hospitals) | Nasopharyngeal aspirates in 5 hospitals; nasopharyngeal swabs in 3 hospitals |
| Colombia | 2005 | 6–23 monthsa, ≥60 yearsa, healthcare workers, persons with chronic diseases or cohabiting with cancer patients, pregnant women | Chronic renal disease or other conditions as recommended by the physician | Beginning of April | 17 April 2013 | Southern Hemisphere in 2013. Changed from Northern to Southern Hemisphere vaccine in 2007 | 7 hospitals (50%) selected out of 15 SARI sentinel surveillance hospitals | Nasopharyngeal aspirates |
| Costa-Rica | 2004 | 6 months–11 years with chronic diseasesa, ≥65 yearsa, pregnant women, individuals with chronic diseases, and health care workers | Diabetes, morbid obesity, respiratory illnesses, and cardiovascular disease | Mid February, lasts for 6–8 weeks | 1 February 2013 | Northern Hemisphere in 2013. Changed from Northern to Southern Hemisphere vaccine in 2015 | 6 (all SARI sentinel surveillance hospitals) | Nasopharyngeal aspirates |
| El Salvador | 2004 | 6–59 monthsa, ≥60 yearsa, pregnant women, healthcare workers, persons with chronic conditions | Cardiovascular disease, renal disease and chronic respiratory diseases | 6 weeks | 30 April -June 30 | Southern Hemisphere. Changed from Northern Hemisphere to Southern Hemisphere vaccine in 2011 | 4 (all SARI sentinel surveillance hospitals) | Combined mid-turbinate nasal and oropharyngeal swabs |
| Honduras | 2003 | ≥60 yearsa, 6–35 months with chronic diseasesa, 3–64 years with chronic conditions, health care workers, and poultry workers | Respiratory diseases specified as asthma, bronchitis, chronic obstructive pulmonary disease, emphysema, renal disease, cardiovascular disease, metabolic disease specified as diabetes, immunodeficiency, obesity, and patients with long-term aspirin treatment | Mid-November, lasts for 6 weeks. | 19–30 November 2013 | Northern Hemisphere. Changed to Southern Hemisphere in 2015 | 3 (all SARI sentinel surveillance hospitals) | Combined mid-turbinate nasal and oropharyngeal swabs |
| Panama | 2005 | 6–59 monthsa, ≥60 yearsa, pregnant women, individuals with chronic diseases, healthcare workers, and poultry workers | Metabolic diseases, cardiovascular diseases, chronic respiratory diseases, morbid obesity | Mid-April, vaccination concentrated during the “vaccination week of the Americas” and offered throughout the season through routine services | April 1 2013 | Southern Hemisphere | 10 (all SARI sentinel surveillance hospitals) | Nasopharyngeal swabs |
| Paraguay | 2005 | 6–35 monthsa, ≥60 yearsa, healthcare workers, pregnant women, persons with chronic conditions, poultry workers | Chronic diseases (unspecified), morbid obesity, immunocompromised | Mid-April | April 11 2013 | Southern Hemisphere | 2 hospitals (29%) selected out of 7 SARI sentinel surveillance hospitals | Combined mid-turbinate nasal and oropharyngeal swabs; nasopharyngeal aspirates |
Included in multicenter case-control study, 2013.
In South America, influenza virus activity typically peaks between April and September [6], [7]. Thus, these countries administer the Southern Hemisphere influenza vaccine, which becomes available during March–April, the beginning of the austral winter. In Central America, situated in the American Tropics, influenza seasonality has been more difficult to define. However, recent epidemiological analyses suggest that epidemics start in May (±two months) when the Southern Hemisphere vaccine is also the most up-to-date formulation available [6], [7].
High income countries that annually use influenza vaccines monitor their effectiveness in order to evaluate vaccination programs’ investments and guide risk communication messages to the public and health professionals [8], [9], [10], [11], [12], [13], [14]. In these countries, the use of the test-negative design (TND) (which compares the odds of vaccination among influenza positive patients and influenza negative “controls”) coupled with highly sensitive and specific molecular diagnostic has been established as an efficient evaluation method that minimizes bias due to health-seeking behavior while providing robust estimates [15], [16], [17], [18], [19].
Since 2013, the network for influenza VE in LAC denominated as REVELAC-i for its acronym in Spanish (Red para la Evaluación de Vacunas En Latino América y el Caribe–influenza) has been estimating influenza VE as an integral component of evaluating influenza prevention in the region [20], [21]. We present the results of the REVELAC-i multicenter VE evaluation in nine Latin American countries during the 2013 influenza season.
2. Methods
The populations targeted for government-sponsored influenza vaccination varied among REVELAC-i countries (Table 1). All countries targeted young children but differed in age cut-offs and their focus on preexisting conditions, including the following groups: all children aged 6–59 months (El Salvador and Panama), all aged 6–23 months (Argentina, Brazil, Chile, and Colombia), all aged 6–35 months (Paraguay), children aged 6–35 months with preexisting conditions (Honduras), and those aged 6 months–11 years with preexisting conditions (Costa-Rica). All countries also targeted older adults aged ≥60 years (Brazil, Colombia, El Salvador, Honduras, Panama, and Paraguay) or ≥65 years (Argentina, Chile, and Costa-Rica), regardless of preexisting conditions. For this study, each country contributed children and older adults within their local targeted age ranges which resulted in a combined sample that included children aged 6 months–5 years and older adults aged ≥60 years.
We used a common protocol, as previously described [20], [21], [22], to conduct the study at 71 severe acute respiratory infections (SARI) sentinel surveillance hospitals (4 in Argentina, 29 in Brazil, 6 in Chile, 7 in Colombia, 6 in Costa-Rica, 4 in El Salvador, 3 in Honduras, 10 in Panama and 2 in Paraguay) during 2013 (Fig. 1.) Surveillance staff identified SARI patients as persons presenting with fever (i.e., measured temperature ≥38 °C or parental- or self-reported history of fever), cough, and difficulty breathing who were hospitalized [22] and collected a respiratory specimen, using one or more standard collection methods (i.e., combined mid-turbinate nasal and oropharyngeal swabs, nasopharyngeal aspirates, nasopharyngeal swabs, or pharyngeal washes). Hospitals aimed to collect specimens from all SARI patients in Argentina, Brazil, Chile, Costa-Rica, Honduras and Paraguay and from a convenience sample of five weekly SARI patients in Colombia, El Salvador and Panama. Specimens were tested for influenza through real-time reverse transcription polymerase chain reaction (rRT-PCR) at national reference laboratories, using US-CDC protocols, primers, and probes [23], [24].
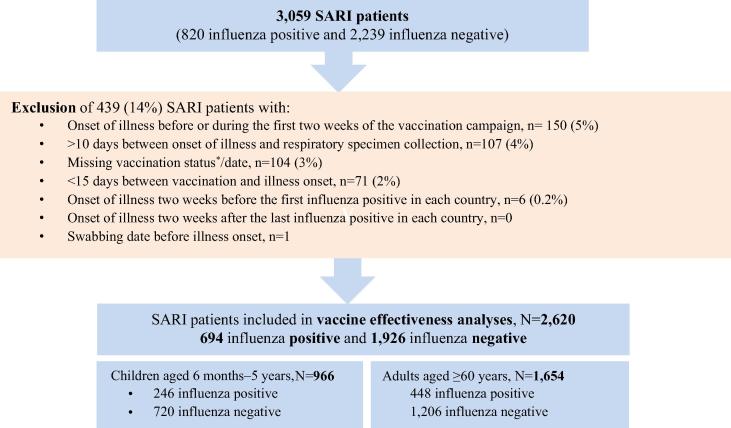
Fig. 1.
Selection of SARI patients included in influenza vaccine effectiveness analyses, REVELAC-i multicenter case-control study in nine Latin American countries, 2013. aBased on the receipt of any dose of influenza vaccine during 2013. Among 734 (76%) children who provided information on the data source used for vaccination status ascertainment, 408 (56%) had vaccination cards reviewed, 317 (43%) had electronic vaccination registers reviewed and 9 (1%) had EPI paper records reviewed. Among older adults, electronic registers were also the main data source used: 948 of 1292 (73%) followed by vaccination cards (338 [26%]) and EPI paper records (6[0.5%]).
The study start date for each country was (1) after the start of the country’s 2013 national influenza vaccination campaign, (2) after confirmation of the start of local influenza circulation by study leads, and (3) after the identification of the first SARI patient with rRT-PCR confirmed influenza. The study period ended on the last day of local influenza circulation in 2013 as determined by study leads. A case was defined as a SARI patient with rRT-PCR confirmed infection with influenza A or B viruses. A “control” was a SARI patient who tested negative for influenza by rRT-PCR. Argentina, Chile, Honduras, and Panama contributed all their available influenza-negative controls. Due to limitations in resources to review vaccination cards records, Brazil, Colombia, Costa-Rica, El Salvador, and Paraguay randomly selected three influenza-negative controls per case (frequency-matched by children versus adults and by month of illness onset).
We extracted information from the SARI case report forms, including age, sex, date of illness onset, date of respiratory specimen collection, diagnosed preexisting medical conditions, dates of admission and discharge, receipt of antiviral treatment, intensive care unit admission, death, influenza vaccination status and dates in the current and prior seasons, number of doses of influenza vaccine among children. Preexisting conditions common to all SARI case-report forms included diabetes, cardiovascular disease, chronic respiratory diseases (including chronic pulmonary disease), renal diseases, obesity, hepatic diseases, immunosuppression, and immunodeficiency. Reference laboratories for influenza provided information on influenza rRT-PCR results, influenza type/subtype, and positivity for other respiratory viruses (using indirect immunofluorescence assay).
Influenza vaccination status was documented in three ways. The SARI case report form at all study hospitals required staff to document influenza vaccination status (including the number and dates of vaccine doses) by reviewing vaccination cards brought in by patients or family members during hospitalization. For SARI patients without vaccination cards, surveillance staff liaised with EPI local/regional teams to obtain information from local sources of vaccination records, including EPI records. In Chile, Colombia (Bogota), Costa-Rica and Panama where a national electronic immunization registry was available, the national surveillance team retrieved the vaccination status by linking the SARI surveillance database and the vaccination registry through a unique citizen or national identifiers. Patients without documentation through any of these methods were considered unvaccinated. Investigations to document status took place for all patients regardless of self-reported vaccination status.
We calculated the minimum sample size needed to estimate regional, age-group specific VEs. To detect a hypothetical VE of 50% with 80% power, an alpha-type error of 5%, and an estimated vaccine coverage of 30% among the controls, we anticipated needing at least 138 influenza cases and 414 controls per age-group from the region [20]. Assuming that approximately 13% of SARI patients would test positive for influenza in the participating countries [CDC's World Health Organization (WHO) Collaborating Center for Surveillance, Epidemiology and Control of Influenza and National Influenza Centers in participating countries, unpublished data], we would need ≥1061 SARI patients per age group to reach sample size. Unadjuvanted trivalent inactivated influenza vaccines (IIV3) using the 2012–13 Northern Hemisphere formulation were available in Honduras for its November–December 2012 vaccination campaign. All other participating countries used the Southern Hemisphere formulation during April–May, 2013 campaigns. Both IIV3 vaccines contained the same components (A/California/7/2009 (H1N1)-like virus, A/Victoria/361/2011 (H3N2)-like virus, and B/Wisconsin/1/2010-like Yamagata B virus) [25]. We considered a person vaccinated if he/she received the vaccine between 14 days–12 months before the onset of SARI symptoms [26]. Among the subset of children with complete vaccination histories, specifically information about the receipt of a second dose and information about prior year vaccination, we considered a child aged ≤5 years fully vaccinated if he/she received two doses of vaccine if vaccine-naïve or one dose of vaccine if previously vaccinated (one dose in 2013 and one or two doses in 2012) [2]; a vaccine-naïve child who received only one dose in 2013 was considered partially vaccinated. Thus, for the analysis of full vaccination VE, we excluded children who had received one dose in 2013 but no vaccination in 2012 (i.e. partially vaccinated) and children who received one dose in 2013 but had no information about vaccination in 2012 (not classifiable). For the analysis of partial vaccination VE, we only included children that had evidence of no prior vaccination in 2012 and only one dose in 2013 (supplemental Table 1).
We excluded SARI patients with an onset of illness ≤2 weeks after the start of the national influenza vaccination campaign in each country. We also excluded SARI patients whose illness onset was outside the study period, defined as ≤2 weeks before the illness onset date of the first laboratory-confirmed influenza case in the country or ≥2 weeks after the illness onset of the last influenza case. Patients vaccinated after the onset of illness were classified as unvaccinated; those vaccinated <2 weeks before the onset of illness were excluded from the analysis. We also excluded SARI patients with >10 days between illness onset and specimen collection and those for whom illness onset date was unavailable. Only the availability of information about the receipt of at least one dose of IIV3 and its date of administration was considered a prerequisite for inclusion in VE analyses (complete cases analysis for VE of any dose of influenza vaccine in 2013) (Fig. 1.). At a later stage, children with a missing vaccination status for the second dose of IIV3 and children or adults with no information about the prior year vaccination were excluded to carry out a complete cases analysis of full/partial vaccination VE.
We calculated the odds ratio of vaccination in cases versus controls in Argentina, Chile, Colombia, Brazil, Paraguay and Central America, whereby Costa-Rica, El Salvador, Honduras and Panama were pooled into one geographical unit. We estimated pooled VE for each age group as (1- odds ratio of vaccination in cases versus controls)∗100 using a two-stage model with random effects for the country/geographical unit [27]. In logistic regression models for each country/geographical unit, we adjusted VE for age (years), presence of chronic medical conditions, and month of illness onset, which are common adjustment covariates in TND studies [15]. We did not identify additional empirical indicators of possible confounding (characteristics associated with both vaccination and influenza case-status). We then further estimated VE per influenza type/subtype for each age group. In a meta-analysis, we explored the heterogeneity between countries’ VE estimates, using Cochrańs Q-test and the I2 index that quantifies the percentage of the variance attributable to significant differences between estimates rather than due to chance alone (shown in supplemental Figs. 2–4). We considered an I2 of 25% as low, 50% as medium and 75% high heterogeneity [28].
As a secondary analysis, we estimated VE for combinations of current and prior season vaccination among older adults [29] given recent reports that receipt of influenza vaccine in previous seasons can modify current season VE among adults [29], [30], [31].
2.1. Ethical considerations
Costa Rica’s Ethics committee approved the protocol and Argentina, Brazil, Colombia, Chile, El Salvador, Honduras, Panama, Paraguay and CDC waived its review because it was considered a vaccine program evaluation using routinely collected surveillance data. We did not collect personal identifiers.
3. Results
3.1. SARI patients included in VE analyses
Summing across sites and age groups, we identified 3059 SARI patients: 289 (9%) from Central America and 2770 (91%) from South America. The majority of SARI patients were from Brazil (34%), Chile (18%), Colombia (13%) and Paraguay (10%). Across sites, 1192 (39%) were children and 1867 (61%) were older adults. Of 3059 SARI patients, we excluded 439 (14%) enrolled outside the study period or meeting other exclusion criteria as summarized in Fig. 1. Among the remaining 2620 SARI patients, 694 (27%) were influenza-positive, and of these, 583 (84%) of them completed type/subtype testing: 282 (48%) were influenza A(H1N1)pdm09, 197 (34%) influenza A(H3N2), and 104 (18%) influenza B viruses. The majority (74%) of influenza cases had illness onset during May-October, with 49% of cases occurring during a peak period of June and July (Fig. 2).
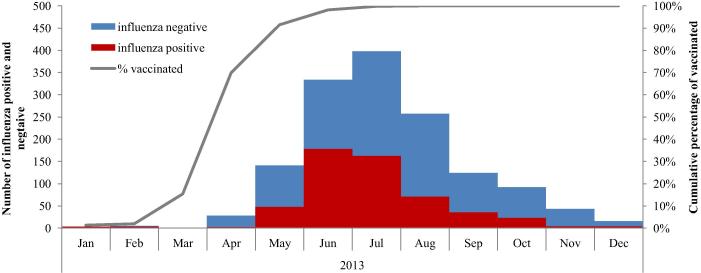
Fig. 2.
Distribution of patients with influenza positive and negative severe acute respiratory infections per month of illness onset and distribution of vaccinees per month of vaccination, multicenter case-control study in nine Latin American countries, 2013 (n = 2620; 90 were enrolled in Argentina; 1005 in Brazil, 749 in Chile, 234 in Colombia, 245 in Central America, and 297 in Paraguay). The line chart is limited to vaccinated patients hence the cumulative 100%.
3.2. Vaccination among SARI patients
All children had information about whether they had received at least one dose of IIV3 (i.e. 100% completeness) (n = 966), and 823 of them (85%) had information that allowed us to further classify them into fully, partially vaccinated or unvaccinated in both years. Out of 966 children, 488 (50%) received at least one IIV3 dose. Out of 823 children that had the necessary information to be classified into fully, partially vaccinated or unvaccinated in both years: 228 were fully vaccinated (28%) (205 (25%) received two doses of IIV3 and 23 (2.7%) received one dose in 2013 and at least one dose in 2012) and 189 (23%) were partially vaccinated. Influenza vaccine uptake was overall higher in South America, where 450 children (56%) received at least one dose of influenza vaccine in 2013 compared to 38 children (23%) in Central America (p < 0.01). This observation was also true for full vaccination whereby 236 (44%) children were fully vaccinated in South America compared to 19 (16%) in Central America (p < 0.01).
Among older adults, 859 of 1654 (52%) had received influenza vaccine in 2013. The majority (65%) were vaccinated in both the study year (2013) and the prior year (2012), while 16% were vaccinated in the study year only (2013) and 19% in the prior year only (2012). Influenza vaccine uptake was also higher in South America, where 794 older adults (50%) received at least one dose of influenza vaccine in 2013 compared to 16 older adults (20%) in Central America (p < 0.01).
Vaccination campaigns largely preceded the influenza season; 92% of all vaccinees were vaccinated by May 31 when only 56/533 [11%] influenza cases had been identified (Fig. 2).
3.3. Characteristics by vaccination status and by influenza positivity
Among children, preexisting conditions were more frequent among influenza positive cases (39%) than among influenza negative controls (30%) (p = 0.02). The proportion of vaccinated among children with chronic conditions (42%) was lower than among healthy children (53%) (p < 0.01), and it was lower among children admitted to intensive care (39%) compared to those who were not (51%) (p = 0.015). No other differences in characteristics were noted among the influenza positive and the IIV3 vaccinated children (Table 2).
Table 2.
Characteristics of enrolled patients aged 6 months-5 years with severe acute respiratory infections (SARI) among those influenza virus positive and among those vaccinated with at least one current season dose of trivalent inactivated influenza vaccine (IIV3), multicenter case-control study in nine Latin American countries, 2013 (n = 966).
| Characteristic | Test result status |
Vaccination status |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Influenza positive |
Influenza negative |
||||||||
| n | (%) | n | (%) | p-valuea | n vaccinatedb | Total | (%) | p-valuea | |
| Overall | 246 | (25) | 720 | (75) | 458 | 966 | (47) | ||
| Country | <0.001 | <0.001 | |||||||
| Argentina | 13 | (5.3) | 33 | (4.6) | 22 | 46 | (48) | ||
| Brazil | 92 | (37) | 244 | (34) | 199 | 336 | (59) | ||
| Central America | 62 | (25) | 104 | (14) | 27 | 166 | (16) | ||
| Chile | 38 | (15) | 176 | (24) | 122 | 214 | (57) | ||
| Colombia | 18 | (7.3) | 43 | (6.0) | 26 | 61 | (43) | ||
| Paraguay | 23 | (9.3) | 120 | (17) | 62 | 143 | (43) | ||
| Demographics | |||||||||
| Gender | 0.24 | 0.37 | |||||||
| Male | 147 | (60) | 399 | (55) | 252 | 546 | (46) | ||
| Female | 99 | (40) | 321 | (45) | 206 | 420 | (49) | ||
| Age group (months) | 0.26 | <0.001 | |||||||
| 6–11 | 115 | (47) | 334 | (46) | 21 | 90 | (4.6) | ||
| 12–23 | 102 | (41) | 325 | (45) | 264 | 427 | (58) | ||
| 24–59 | 29 | (18) | 61 | (8.5) | 173 | 449 | (38) | ||
| Clinical characteristics | |||||||||
| At least one preexisting condition | 0.02 | <0.001 | |||||||
| Yes | 89 | (39) | 211 | (30) | 114 | 300 | (38) | ||
| No | 141 | (61) | 485 | (70) | 322 | 626 | (51) | ||
| Admitted to intensive care | 0.31 | 0.014 | |||||||
| Yes | 33 | (15) | 80 | (12) | 41 | 113 | (36) | ||
| No | 191 | (85) | 581 | (88) | 375 | 772 | (49) | ||
| Deceased | 0.21 | 0.82 | |||||||
| Yes | 6 | (2.9) | 10 | (1.5) | 8 | 16 | (50) | ||
| No | 204 | (97) | 646 | (98) | 401 | 850 | (47) | ||
| Days from illness onset to specimen collection | 0.18 | 0.12 | |||||||
| 0–4 | 170 | (69) | 539 | (75) | 325 | 709 | (46) | ||
| 5–7 | 68 | (28) | 166 | (23) | 124 | 234 | (53) | ||
| 8–10 | 8 | (3.2) | 15 | (2.1) | 9 | 23 | (39) | ||
| Influenza RT-PCR result | |||||||||
| Negative | 384 | 720 | (53) | ||||||
| Positive | 104 | 246 | (42) | 0.003 | |||||
| Influenza A positive | 83 | 210 | (40) | <0.001 | |||||
| A (H1N1)pdm09 | 50 | 115 | (43) | 0.049 | |||||
| A(H3N2) | 22 | 56 | (39) | 0.043 | |||||
| Influenza B positive | 21 | 36 | (58) | 0.557 | |||||
We compared the proportion of patients with selected characteristics between influenza positive and influenza negative patients and the proportion of vaccinated between strata of each characteristic using the chi-square statistic test or Fisher’s exact test when sample size was small.
Defined as having received ≥1 dose of influenza vaccine ≥14 days before illness onset.
Among older adults, influenza positives were younger (median = 73 years) than influenza negatives (median = 76 years) (p < 0.001). The proportion of vaccination among older adults who died in hospital was lower (44%; 135/307) than among discharged adults (50%; 638/1267) (p = 0.045) (Table 3).
Table 3.
Characteristics of enrolled patients aged ≥ 60 years with severe acute respiratory infections (SARI) among those influenza virus positive and among those vaccinated with at least one current season dose of trivalent inactivated influenza vaccine (IIV3), multicenter case-control study in nine Latin American countries, 2013 (n = 1654).
| Characteristic | Test result status |
Vaccination status (IIV3b) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Influenza positive |
Influenza negative |
p-valuea | |||||||
| n | (%) | n | (%) | n vaccinated | Total | (%) | p-valuea | ||
| Overall | 448 | (27) | 1206 | (73) | 859 | 1654 | (52) | ||
| Country | <0.001 | <0.001 | |||||||
| Argentina | 23 | (5.1) | 21 | (1.7) | 19 | 44 | (43) | ||
| Brazil | 185 | (41) | 484 | (40) | 419 | 669 | (63) | ||
| Central America | 42 | (9.4) | 37 | (3.1) | 16 | 79 | (20) | ||
| Chile | 95 | (21) | 440 | (36) | 294 | 535 | (55) | ||
| Colombia | 51 | (11) | 122 | (10) | 24 | 173 | (14) | ||
| Paraguay | 52 | (12) | 102 | (8.5) | 38 | 154 | (25) | ||
| Demographics | |||||||||
| Gender | 0.91 | 0.016 | |||||||
| Male | 212 | (47) | 567 | (47) | 406 | 779 | (52) | ||
| Female | 236 | (53) | 639 | (53) | 404 | 875 | (46) | ||
| Age | <0.001 | 0.005 | |||||||
| 60–69 years | 170 | (38) | 317 | (26) | 217 | 487 | (45) | ||
| 70–79 years | 151 | (34) | 437 | (36) | 279 | 588 | (47) | ||
| ≥80 years | 127 | (28) | 452 | (38) | 314 | 579 | (54) | ||
| Clinical characteristics | |||||||||
| At least one preexisting condition | 0.78 | 0.024 | |||||||
| Yes | 335 | (76) | 891 | (75) | 616 | 1226 | (50) | ||
| No | 107 | (24) | 295 | (25) | 176 | 402 | (44) | ||
| Diabetes | 0.24 | 0.039 | |||||||
| Yes | 103 | (26) | 251 | (23) | 166 | 354 | (47) | ||
| No | 294 | (74) | 840 | (77) | 603 | 1134 | (53) | ||
| Cardiovascular disease | 0.39 | 0.92 | |||||||
| Yes | 138 | (34) | 399 | (36) | 276 | 537 | (51) | ||
| No | 271 | (66) | 705 | (64) | 499 | 976 | (51) | ||
| Respiratory disease | 0.88 | 0.607 | |||||||
| Yes | 144 | (36) | 388 | (35) | 278 | 532 | (52) | ||
| No | 261 | (64) | 716 | (65) | 497 | 977 | (51) | ||
| Liver disease | 0.57 | 0.703 | |||||||
| Yes | 4 | (1.0) | 15 | (1.4) | 9 | 19 | (47) | ||
| No | 387 | (99) | 1060 | (99) | 749 | 1447 | (52) | ||
| Renal disease | 0.67 | 0.67 | |||||||
| Yes | 33 | (8.4) | 99 | (9.1) | 66 | 132 | (50) | ||
| No | 359 | (92) | 984 | (914) | 698 | 1343 | (52) | ||
| Obesity | 0.86 | 0.326 | |||||||
| Yes | 28 | (7.1) | 80 | (7.4) | 51 | 108 | (47) | ||
| No | 363 | (93) | 997 | (93) | 709 | 1360 | (52) | ||
| Immunocompromised | 0.57 | 0.58 | |||||||
| Yes | 17 | (4.3) | 55 | (5.1) | 35 | 72 | (49) | ||
| No | 375 | (96) | 1033 | (95) | 732 | 1408 | (52) | ||
| Admitted to intensive care | 0.12 | 0.95 | |||||||
| Yes | 81 | (21) | 264 | (25) | 183 | 345 | (53) | ||
| No | 307 | (79) | 801 | (75) | 590 | 1108 | (53) | ||
| Deceased | 0.14 | 0.045 | |||||||
| Yes | 74 | (17) | 233 | (20) | 135 | 307 | (44) | ||
| No | 358 | (83) | 909 | (80) | 638 | 1267 | (50) | ||
| Days from illness onset to specimen collection | 0.83 | <0.001 | |||||||
| 0–4 | 285 | (73) | 712 | (73) | 631 | 1214 | (52) | ||
| 5–7 | 87 | (22) | 227 | (23) | 156 | 386 | (40) | ||
| 8–10 | 16 | (4.1) | 37 | (3.8) | 23 | 54 | (43) | ||
| Influenza RT-PCR result | <0.001 | ||||||||
| Negative | 648 | 1206 | (54) | ||||||
| Positive | 162 | 448 | (36) | ||||||
| Influenza A positive | 126 | 352 | (36) | ||||||
| A (H1N1)pdm09 | 65 | 167 | (39) | ||||||
| A(H3N2) | 55 | 141 | (39) | ||||||
| Influenza B positive | 29 | 68 | (43) | ||||||
We compared the proportion of patients with selected characteristics between influenza positive and influenza negative patients and the proportion of vaccinated between strata of each characteristic using the chi-square statistic test or Fisher’s exact test when sample size was small.
IIV3: Trivalent Inactivated Influenza Virus Vaccine.
3.4. VE estimates for children
The proportion of children vaccinated with at least one dose during 2013 was lower among influenza-positives (42%) than among influenza-negatives (54%) (p = 0.03) (Table 4). VE of at least one dose of IIV3 against SARI hospitalization associated with any influenza virus (adjusted for age, preexisting conditions, and the month of illness onset) among children was 30% [95%CI: 1–51%]. Virus-specific adjusted VE estimates were 31% [−15; 59%] against A(H1N1)pdm09 virus SARI, and 27% [95%CI: −19; 67%] against A(H3N2) SARI, both had wide confidence intervals that overlapped with zero. An adjusted model could not be estimated for VE against influenza B viruses because of the small number of positives, however the unadjusted VE against influenza B SARI was −27% [95%CI: −193; 45%] among children (Table 4).
Table 4.
Number of patients with severe acute respiratory infections per influenza test result status, crude and adjusted influenza vaccine effectiveness among children aged 6 months-5 years, REVELAC-i multicenter case-control in nine Latin American countries, 2013 (n = 966).
| Influenza type | Vaccine effectiveness |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Influenza positive |
Influenza negative |
Unadjusted |
Adjustede |
|||||||
| n vaccinated | Total | (%) | n vaccinated | total | (%) | (%) | (95%CI) | (%) | (95%CI) | |
| Influenza A and B | ||||||||||
| Any vaccine dose in 2013a | 104 | 246 | (42) | 391 | 720 | (54) | 30 | 4–49 | 30 | 1–51 |
| Unvaccinated in 2013 | Reference | Reference | ||||||||
| At least one dose in 2013b | 91 | 208 | (44) | 326 | 615 | (53) | 30 | 1–50 | 32 | −14; 47 |
| Partially vaccinatedc | 51 | 168 | (30) | 138 | 427 | (32) | 6 | −43; 39 | 0 | −68; 35 |
| Fully vaccinatedd | 45 | 162 | (28) | 210 | 499 | (42) | 46 | 17–65 | 47 | 14–71 |
| Unvaccinated in 2012 and 2013 | Reference | Reference | ||||||||
| Influenza A(H1N1)pdm09 | ||||||||||
| Any vaccine dose in 2013a | 55 | 115 | (48) | 391 | 720 | (54) | 31 | −8; 56 | 31 | −15; 59 |
| Unvaccinated in 2013 | Reference | Reference | ||||||||
| At least one dose in 2013b | 46 | 101 | (45) | 326 | 615 | (53) | 35 | −7; 60 | 35 | −30; 57 |
| Partially vaccinatedc | 23 | 78 | (29) | 138 | 427 | (32) | 3 | −76; 47 | −13 | −121; 43 |
| Fully vaccinatedd | 24 | 79 | (30) | 210 | 499 | (42) | 60 | 27–79 | 58 | 16–79 |
| Unvaccinated in 2012 and 2013 | Reference | Reference | ||||||||
| Influenza A(H3N2) | ||||||||||
| Any vaccine dose in 2013a | 21 | 56 | (37) | 391 | 720 | (54) | 20 | −46; 56 | 27 | −19; 67 |
| Unvaccinated in 2013 | Reference | Reference | ||||||||
| At least one dose in 2013b | 21 | 50 | −42 | 326 | 615 | −53 | 10 | −70; 52 | 23 | −51; 61 |
| Partially vaccinatedc | 16 | 45 | (36) | 138 | 427 | (32) | −21 | −143; 40 | 0 | −128; 52 |
| Fully vaccinatedd | 5 | 34 | (15) | 210 | 499 | (42) | 52 | −36; 83 | 65 | −9; 89 |
| Unvaccinated in 2012 and 2013 | Reference | Reference | ||||||||
| Influenza B | ||||||||||
| Any vaccine dose in 2013a | 21 | 36 | (58) | 391 | 720 | (54) | −27 | −193; 45 | ||
| Unvaccinated in 2013 | Reference | NA | ||||||||
| At least one dose in 2013b | 18 | 33 | (54) | 326 | 615 | (53) | −18 | −178; 50 | ||
| Partially vaccinatedc | 11 | 26 | (42) | 138 | 445 | (31) | NA | NA | ||
| Fully vaccinatedd | 10 | 25 | (40) | 210 | 499 | (42) | 3 | −150; 63 | NA | |
| Unvaccinated in 2012 and 2013 | Reference | Reference | ||||||||
Abbreviations: 95%CI=95% confidence interval. NA: not available, VE could not be estimated due to low sample size.
Defined as having received ≥1 dose of influenza vaccine ≥14 days before illness onset. The vaccinated group may include fully and partially vaccinated children. The reference group includes only unvaccinated in 2013 and thus may include children vaccinated in 2012. This VE estimate corresponds to the complete cases analysis based on the availability of current vaccination status and date, not on the availability of the number of doses for the current vaccination or on the prior vaccination status).
Defined as having received ≥1 dose of influenza vaccine ≥14 days before illness onset. The reference group includes only children unvaccinated in both years. This category sums up the two categories below: partially and fully vaccinated. For this complete cases analysis, subjects with missing information on vaccine doses and prior vaccination were excluded.
Defined as having received “no doses in 2012 and 1 dose in 2013”.
Defined as having received 2 doses in 2013 or 1 dose in 2013 and ≥1 dose in 2012.
Adjusted for the month of illness onset, presence of at least one preexisting condition, and age (years).
When considering full vaccination vs. no vaccination (and thus excluding partially vaccinated children), the adjusted VE was 47% [95%CI: 14–71%] against SARI hospitalization associated with any influenza virus. When considering partial vaccination (received only one dose in 2013 and were certainly not vaccinated in 2012) vs. no vaccination, the adjusted VE was 0% [95%CI: −68; 35%] against SARI hospitalization associated with any influenza virus. When using partial vaccination as the reference group, the odds ratio of influenza positivity comparing full versus partial vaccination was 0.67 [95%CI: 0.48–0.94]; p = 0.015), adjusted for age, onset month and preexisting conditions; suggesting that full vaccination was 33% more effective in preventing influenza than partial vaccination (Table 4).
3.5. VE estimates for older adults
Among older adults, 36% of influenza positives were vaccinated compared to 54% of influenza negatives (p < 0.001). The adjusted VE against SARI hospitalization associated with any influenza virus was 48% [95%CI: 34–60%] (Table 5). We also observed statistically significant adjusted VE of 54% [95%CI: 37–69%] against A(H1N1)pdm09 SARI and 42% [95%CI: 17–60%] against A(H3N2) SARI (Table 5).
Table 5.
Number of patients with severe acute respiratory infections per influenza test result status, crude and adjusted influenza vaccine effectiveness among adults aged ≥ 60 years, REVELAC-i multicenter case-control in nine Latin American countries, 2013 (n = 1654).
| Influenza type | Vaccine effectiveness |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Influenza positive |
Influenza negative |
Unadjusted |
Adjusteda |
|||||||
| n vaccinated | Total | (%) | n vaccinated | Total | (%) | % | 95%CI | % | 95%CI | |
| Influenza A and B | ||||||||||
| Vaccinated in 2013 | 162 | 448 | (36) | 648 | 1206 | (54) | 50 | 21–68 | 48 | 34–60 |
| Unvaccinated in 2013b | Reference | Reference | ||||||||
| Influenza A and B | ||||||||||
| Current and prior vaccination | ||||||||||
| Vaccinated in 2012 and 2013 | 114 | 295 | (39) | 487 | 818 | (60) | 56 | 41; 68 | 56 | 40; 68 |
| Vaccinated in 2013 | 33 | 214 | (15) | 125 | 456 | (27) | 43 | 18–66 | 50 | 19; 68 |
| Vaccinated in 2012c | 52 | 233 | (22) | 134 | 465 | (29) | 22 | −17; 47 | 28 | −10; 53 |
| Unvaccinated in both years | Reference | Reference | ||||||||
| Influenza A and B | ||||||||||
| Comparison of current vaccination only versus vaccination in both years | ||||||||||
| Vaccinated in 2013 | 33 | 147 | (22) | 125 | 612 | (20) | −7 | −72; 23 | 8 | −54; 44 |
| Vaccinated in 2012 and 2013 | 114 | 147 | (78) | 487 | 612 | (80) | Reference | Reference | ||
| Influenza A(H1N1)pdm09 | 78 | 239 | (33) | 648 | 1206 | (54) | 56 | 39–68 | 54 | 37–69 |
| Unvaccinated in 2013b | Reference | Reference | ||||||||
| Influenza A(H3N2) | 68 | 213 | (32) | 648 | 1206 | (54) | 43 | 18–60 | 43 | 18–61 |
| Unvaccinated in 2013b | Reference | Reference | ||||||||
| Influenza B | 42 | 140 | (30) | 648 | 1206 | (54) | 33 | −4; 57 | 34 | −4; 58 |
| Unvaccinated in 2013b | Reference | Reference | ||||||||
Abbreviations: 95%CI = 95% confidence interval.
Adjusted for the month of illness onset, presence of at least one preexisting condition, and age (years).
The reference group only includes unvaccinated in 2013 regardless of prior vaccination status.
Reference group = unvaccinated in 2012 and 2013.
We did not observe indications of effect modification by prior vaccination among older adults (p = 0.38 for the interaction term between prior and current vaccination). The adjusted VE against SARI associated with any influenza virus was 56% [95%CI: 40–68%] for those who received vaccine in both the current and prior year, 50% [95%CI: 19–68%] for those vaccinated the current year only, and 28% [95%CI: −10; 68%] for those vaccinated in the prior year only. We observed a similar pattern when analyses were restricted to A(H3N2) (data not shown). We noted a somewhat different trend for A(H1N1)pdm09 SARI, in that all combinations of vaccine exposure had significant VE (p = 0.27 for the interaction term between prior and current vaccination); adjusted VE was 64% [95%CI: 44–77%] for those who received vaccine in both the current and prior year, 60% [95%CI: 23–80%] for those vaccinated the current year only, and 52% [95%CI: 13; 74%] for those vaccinated in the prior year only.
4. Discussion
The results of the first season of the multicenter VE case-control study in nine Latin American countries suggest moderate VE against influenza-associated SARI among fully vaccinated young children (VE of 52%) and older adults (VE of 48%) during 2013. The lower VE point estimates of 0% for partial vaccination and of 36% for any IIV3 dose suggests that fully vaccinated children may have enjoyed better protection than partially vaccinated children, as recommended [2], [3] and recently described in the literature [32]. Indeed, we found the odds of being influenza positive were about one-third lower among fully vaccinated compared to partially vaccinated children.
The 2013 influenza season in Latin America was characterized by mid-year predominant circulation of influenza A(H1N1)2009, followed by moderate circulation of A(H3N2) and low circulation of influenza B viruses. The pattern of viruses we observed was similar to surveillance reports for the region overall (supplemental Fig. 1) [33]. A limitation of our study was that we did not have antigenic or genetic characterization information on the infecting viruses among the patients we enrolled. WHO and US CDC influenza surveillance indicated that the A(H1N1)pdm09 viruses were antigenically well-matched to the influenza vaccine strains (A/California/7/2009(H1N1)pdm09-like viruses during the study season, but that about one-third of A(H3N2) viruses in the region were not well-matched to the vaccine component. Of n = 150 samples obtained from 11 LAC countries during 2013 and characterized by CDC as H3 viruses, 28.6% (n = 43) were A/Texas and 71.3% (n = 107) were A/Victoria [CDC's WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza and National Influenza Centers in participating countries, unpublished data]. This suboptimal match may have contributed to the observed lower VE against influenza A(H3N2) viruses associated SARI, which is consistent with the low VE reported during subsequent seasons with predominant circulation of drifted influenza A(H3N2) viruses in the Northern and Southern Hemispheres [34], [35], [36], [37], [38], [39]. With regards to influenza B viruses’ match between vaccine and circulating strains, it is noteworthy that Latin American countries have started to determine and report the influenza B viruses lineage fairly recently, following the US CDC’s support in the procurement of the necessary reagents. The limited available information we have compiled from national influenza centers suggested co-circulation of both influenza B lineages among all participating sites with varying predominance. Argentina, Costa Rica and Honduras reported the predominance of the vaccine-lineage Yamagata; Brazil, Colombia and El Salvador reported a majority of Victoria influenza B viruses and Chile and Paraguay reported no clear influenza B viruses lineage predominance (supplemental Table 2).
Our VE estimates were similar to others reported for the Northern Hemisphere (NH) seasons immediately before and after our Southern Hemisphere (SH) season by platforms using test-negative designs [8], [9], [10], [11], [12], [13], [14], [40]. Our estimates were consistent with the adjusted VE of 58% against any influenza virus and 63% against influenza A(H1N1)pdm09 associated hospitalizations among adults ≥65 years in Canada during 2013–14 [10]. Similarly, VE was 44% [95%CI: −11; 72%] against influenza A(H1N1)pdm09 hospitalizations among adults aged 65–79 years in four European countries during the previous NH season (2012–13) [11]. Our findings were also similar to cross-season VE trends reported in a recent meta-analysis of test-negative design VE studies [40], including a pooled VE among adults aged >60 years of 62% [95%CI: 36–78] against H1N1pdm09 illness, a VE of 63% [95%CI: 33–79] for influenza B illness, and a lower VE of 24% [95%CI: −6 to 45%] against H3N2 illness; indeed, our parallel VE point estimates were 54%, 34%, and 43% respectively. A more recent meta-analysis of test-negative design case-control studies among community-dwelling elderly, found a similarly higher VE against H1N1 pdm09 influenza illnesses (53.2% [10·3–75.6]) but lowest VE was estimated against influenza B virus influenza illnesses (–1.5% [–39.6; 26.2]) [41].
We explored the effects of prior vaccination on VE among older adults. We did not observe significant differences in current season only vs. current and prior season vaccinees as has been noted in some prior TND VE studies [29], [30], [31]. Interestingly, we observed a statistically significant VE of 52% against A(H1N1)pdm09 SARI among those who had missed the current season vaccination but were vaccinated the prior season. This evidence of residual protections against A(H1N1)pdm09 illness is consistent with recent VE observations among adults [40] and children [42], [43].
Among the study’s strengths is our use of a common protocol across our regional network of sentinel hospitals for SARI surveillance, including a common SARI case definition and standard procedures for laboratory testing for influenza viruses. We used the test-negative case-control design that has been widely validated in outpatient settings and that has been recently shown to provide similar results in inpatient settings [44]. Specimens were collected from all SARI patients at most surveillance sites (and from sites contributing the majority of data); quota sampling which may have been biased by clinical selection only contributed 12% of the analysis sample size. In order to minimize the misclassification of the disease status, we used highly specific and sensitive rRT-PCR assays to confirm influenza-associated illness [23], [24]. We aimed to avoid the potential misclassification of exposure by using only documented vaccination (vaccination cards or EPI records). Additionally, EPI staff did not have access to rRT-PCR results when collecting patients’ vaccination history, thus preventing biases in exposure classification based on the outcome.
There were several limitations to this analysis. Like all observational studies, our program evaluation may be subject to biases or residual confounding. Our sample size was limited for estimates of VE against influenza B SARI among both age groups and against influenza A(H3N2) SARI among children. Moreover, the small number of partially vaccinated children yielded VE estimates with low precision for this subgroup. In exploring the effect of full vaccination among children, we were unable to disentangle the effect of receiving only one IIV3 dose in the prior year and one IIV3 dose in the current year versus receiving two doses in 2012 and one in the current year. Finally, we did not have access to antigenic or genetic characterization data for the SARI patients enrolled in the VE analysis, but rather used virological surveillance data that might include specimens collected from populations and diagnoses that were not restricted to target groups and SARI. Confounders-adjusted influenza VE estimates per country are provided in supplemental Figs. 2–4.
5. Conclusion
In conclusion, our findings suggest a reduction by half of hospitalizations associated with influenza viruses among fully vaccinated children ≤5 years and adults aged ≥60 years during 2013. These results support the current vaccination strategies in place in the LAC region for both target groups. Our results also suggest that LAC countries should continue boosting the coverage of vaccination with the second dose among vaccine-naïve children ≤5 years to ensure their optimal immunization. The REVELAC-i network efforts highlight the utility of sharing information from influenza SARI surveillance and EPI nominal vaccination data in order to guide vaccination programs. We recommend that LAC countries attempt to pool data earlier during the influenza season to generate early season estimates and guide risk communication to the public and to health care providers. Evidence of influenza VE and impact among all currently targeted groups for vaccination in Latin America is crucial for a region with widespread use and substantial vaccine uptake.
Funding
This work was supported by a grant from the Centers for Disease Control and Prevention (CDC) through cooperative agreements with the Pan American Health Organization/World Health Organization and TEPHINET, a program of the Task Force for Global Health, Inc.
Conflicts of interest
None.
Acknowledgements
We thank the epidemiological and virological influenza surveillance and EPI staff at the local and regional levels in participating countries. We thank Dr Cuauhtémoc Ruiz-Matus and the PAHO immunization focal points for their support in the implementation of the REVELAC-i network: Samia Abdul Samad (Brazil), Odalys Garcia (Honduras), Mirta Magarinos (Argentina), Cristina Pedreira, Yenny Neira (Colombia), Rafael Baltrons (El Salvador), and Dilsa Lara (Panama). We thank those who supported surveillance activities: Leticia Lopez (El Salvador), Leandro Luciani Duque (Argentina), Ana Carolina de Lacerda Sousa, Daiana Araujo da Silva, Jeanine Rocha Woycicki (Ministry of Health, Brazil; Leticia Garay Martins, Rio Grande do Sul–Brasil, Janaina Almeida, Minas Gerais-Brasil, Patricia Marques Ferreira, São Paulo–Brasil, Laurina Tanabe, Paraná–Brasil, Eduardo Marques Macario, Santa Catarina–Brasil, staff of the Instituto Adolfo Lutz– National Influenza Center (NIC), Fundação Oswaldo Cruz (FIOCRUZ)–NIC, Brazil; Hilda Salazar, Xiomara Badilla (Costa-Rica); staff of the Clinical and biochemical laboratory of Hospital Nacional de Niños (Costa-Rica); Eduardo Suarez-Castañeda (El Salvador); Oscar Pacheco (Colombia); Antonio Pérez-Garcia (Costa-Rica); Daisy Moros, Danilo Franco, Marlene Castillo (Panama); Dulcelina Urbina (Honduras), José Sánchez (Paraguay); Jairo Méndez-Rico (PAHO); Daniel Otzoy, Naomi Iihoshi, Antonio Mendez (TEPHINET) and Rafael Chacón (UVG–Guatemala). We thank Miguel Descalzo (UVG–Guatemala) for his support in the data analysis and Alain Levêque, Olivier Vandenberg (Université libre de Bruxelles, Belgium) and Tessa Goetghebuer, Yves Van Laethem (St Pierre Hospital, Brussels, Belgium) for their critical review of this work. We also thank the I-MOVE team at Epiconcept (France): Marta Valenciano, Esther Kissling, Marc Rondy, Camelia Savulescu, and Alain Moren for their technical feedback and support throughout the implementation of REVELAC-i, as well as I-MOVE members Amparo Larrauri and Jesus Castilla (Spain).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Prevention and Control.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.06.036.
Appendix A. Supplementary material
References
- 1.Ropero-Alvarez A.M., El Omeiri N., Kurtis H.J., Danovaro-Holliday M.C., Ruiz-Matus C., Andrus J.K. Influenza vaccination in the Americas: Progress and challenges after the 2009 A(H1N1) influenza pandemic. Hum Vaccine Immunother. 2016;12(8):2206–2214. doi: 10.1080/21645515.2016.1157240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaccines against influenza WHO position paper -Weekly Epidemiological Record. No. 47, 2012, 87, 461–476. 23 November 2012. Available at: http://www.who.int/werhttp://www.who.int/influenza/vaccines/virus/recommendations/2013_south/en/.
- 3.World Health Organization (WHO) Strategic Advisory Group of Experts on immunization (SAGE). Background paper on influenza vaccines and immunization. SAGE Working Group. Geneva: WHO; 2012. [Accessed 19 Apr 2013]. Available from:http://www.who.int/immunization/sage/meetings/2012/april/1_Background_Paper_Mar26_v13_cleaned.pdf.
- 4.Freitas F.T., Souza L.R., Azziz-Baumgartner E., Cheng P.Y., Zhou H., Widdowson M.A. Influenza-associated excess mortality in southern Brazil, 1980–2008. Epidemiol Infect. 2013;141:1731–1740. doi: 10.1017/S0950268812002221. 10.1017/S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcone D.N., Durand L., Azziz-Baumgartner E., Vidaurreta S., Ekstrom J., Carballal G. Incidence of viral respiratory infections in a prospective cohort of outpatient and hospitalized children aged ≤5 years and its associated cost in Buenos Aires Argentina. BMC Infect Dis. 2015;15:447. doi: 10.1186/s12879-015-1213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durand L.O., Cheng P.Y., Palekar R., Clara W., Jara J., Cerpa M. Timing of influenza epidemics and vaccines in the American tropics, 2002–2008, 2011–2014. Influenza Other Respir Viruses. 2016;10:170–175. doi: 10.1111/irv.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirve S, Newman LP, Paget J, Azziz-Baumgartner E, Fitzner J, Bhat N, et al. Influenza seasonality in the tropics and subtropics – when to vaccinate? PLOS One; 2016 [in press]. [DOI] [PMC free article] [PubMed]
- 8.Flannery B., Thaker S.N., Clippard J. Interim estimates of 2013–14 seasonal influenza vaccine effectiveness—United States, February 2014. MMWR Morb Mortal Wkly Rep. 2014;63:137–142. [PMC free article] [PubMed] [Google Scholar]
- 9.Skowronski D.M., Chambers C., Sabaiduc S., De Serres G., Dickinson J.A. Interim estimates of 2013/14 vaccine effectiveness against influenza A(H1N1)pdm09 from Canada’s sentinel surveillance network, January 2014. Euro Surveill. 2014;19(5) doi: 10.2807/1560-7917.es2014.19.5.20690. [DOI] [PubMed] [Google Scholar]
- 10.McNeil S., Shinde V., Andrew M. Interim estimates of 2013/14 influenza clinical severity and vaccine effectiveness in the prevention of laboratory-confirmed influenza-related hospitalisation, Canada, February 2014. Euro Surveill. 2014;19(9) doi: 10.2807/1560-7917.es2014.19.9.20729. [DOI] [PubMed] [Google Scholar]
- 11.Rondy M., Launay O., Puig-Barberà J., Gefenaite G., Castilla J., de Gaetano Donati K., Galtier F., Hak E., Guevara M., Costanzo S. European hospital IVE network, Moren A. 2012/13 influenza vaccine effectiveness against hospitalised influenza A(H1N1)pdm09, A(H3N2) and B: estimates from a European network of hospitals. Euro Surveill. 2015;20(2) doi: 10.2807/1560-7917.es2015.20.2.21011. [DOI] [PubMed] [Google Scholar]
- 12.Valenciano M., Kissling E., Reuss A. The European I-MOVE Multicentre 2013–2014 Case-Control Study. Homogeneous moderate influenza vaccine effectiveness against A(H1N1)pdm09 and heterogenous results by country against A(H3N2) Vaccine. 2015;33:2813–2822. doi: 10.1016/j.vaccine.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Turner N., Pierse N., Bissielo A., Huang Q.S., Radke S., Baker M.G., Widdowson M.A., Kelly H., on behalf of the SHIVERS investigation team Effectiveness of seasonal trivalent inactivated influenza vaccine in preventing influenza hospitalisations and primary care visits in Auckland, New Zealand, in 2013. Euro Surveill. 2014;19(34) pii=20884. [PMC free article] [PubMed] [Google Scholar]
- 14.Carville K.S. Understanding influenza vaccine protection in the community: An assessment of the 2013 influenza season in Victoria, Australia. Vaccine. 2015;33(2):341–345. doi: 10.1016/j.vaccine.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan S.G., Feng S., Cowling B.J. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert Rev Vaccines. 2014 Dec;13(12):1571–1591. doi: 10.1586/14760584.2014.966695. Epub 2014 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill 2013;18(37):pii=20585. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20585. [DOI] [PubMed]
- 17.Jackson M.L., Nelson J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165–2168. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 18.Valenciano M., Kissling E., Ciancio B.C., Moren A. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioner networks. Vaccine. 2010;28(46):7381–7388. doi: 10.1016/j.vaccine.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Foppa I.M., Haber M., Ferdinands J.M., Shay D.K. The case test-negative design for studies of the effectiveness of seasonal influenza vaccine. Vaccine. 2013;31:3104–3109. doi: 10.1016/j.vaccine.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 20.EL Omeiri N., Azziz-Baumgartner E., Clará W., Guzmán-Saborío G., Elas M., Mejía H. Pilot to evaluate the feasibility of measuring seasonal influenza vaccine effectiveness using surveillance platforms in Central-America, 2012. BMC Public Health. 2015;15:673. doi: 10.1186/s12889-015-2001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.REVELAC-i network webpage, available from http://www.paho.org/revelac-i/.
- 22.PAHO/WHO. Operational Guidelines for Sentinel Severe Acute Respiratory Infection (SARI) Surveillance (September 2014). Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=31162&lang=en.
- 23.WHO Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza. Available from http://www.who.int/influenza/gisrs_laboratory/manual_diagnosis_surveillance_influenza/en/.
- 24.US CDC protocol of real-time RTPCR for swine influenza A(H1N1). Available at http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf.
- 25.World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2013 southern hemisphere influenza season. September 2012. Geneva: WHO; 2012. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/201209_recommendation.pdf. [PubMed]
- 26.Gross P.A., Russo C., Dran S., Cataruozolo P., Munk G., Lancey S.C. Time to earliest peak serum antibody response to influenza vaccine in the elderly. Clin Diagnos Lab Immunol. 1997;4(4):491–492. doi: 10.1128/cdli.4.4.491-492.1997. PubMed PMID: 9220171. Pubmed Central PMCID: 170557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stukel T.A., Demidenko E., Dykes J., Karagas M.R. Two-stage methods for the analysis of pooled data. Stat Med. 2001;20(14):2115–2130. doi: 10.1002/sim.852. [DOI] [PubMed] [Google Scholar]
- 28.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Method. 2006;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 29.McLean H.Q., Thompson M.G., Sundaram M.E. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis. 2014;59:1375–1385. doi: 10.1093/cid/ciu680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohmit S.E., Thompson M.G., Petrie J.G., Thaker S.N., Jackson M.L., Belongia E.A. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58(3):319–327. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan S.G., Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clin Infect Dis. 2013;57:474–476. doi: 10.1093/cid/cit255. [DOI] [PubMed] [Google Scholar]
- 32.Jessie Thompson M.G., Clippard Joshua G., Petrie Michael L., Jackson Huong Q., McLean Manjusha Gaglani. Influenza vaccine effectiveness for fully and partially vaccinated children 6 months to 8 years old during 2011–2012 and 2012–2013. Pediatr Infect Dis J. 2016;35:299–308. doi: 10.1097/INF.0000000000001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.PAHO/WHO Influenza situation report, January 2014. Available from: http://www.paho.org/hq/index.php?option=com_content&view=article&id=3352%3A2010-influenza-situation-report&catid=2407%3Ainfluenza-respiratory-viruses&Itemid=2469&lang=en.
- 34.Pierse N., Kelly H., Thompson M.G., Bissielo A., Radke S., Huang Q.S. Influenza vaccine effectiveness for hospital and community patients using control groups with and without non-influenza respiratory viruses detected, Auckland, New Zealand 2014. Vaccine. 2016;34:503–509. doi: 10.1016/j.vaccine.2015.11.073. [DOI] [PubMed] [Google Scholar]
- 35.Mcneil S., Andrew M., Ye L., Haguinet F., Hatchette T., Elsherif M. Interim estimates of 2014/15 influenza vaccine effectiveness in preventing laboratory-confirmed influenza-related hospitalisation from the Serious Outcomes Surveillance Network of the Canadian Immunization Research Network, January 2015. Euro Surveill. 2015;20:21024. doi: 10.2807/1560-7917.es2015.20.5.21024. [DOI] [PubMed] [Google Scholar]
- 36.Skowronski D.M., Chambers C., Sabaiduc S., Serres G.D., Winter A.-L., Dickinson J.A. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis. 2016;63:21–32. doi: 10.1093/cid/ciw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrie J.G., Ohmit S.E., Cheng C.K., Martin E.T., Malosh R.E., Lauring A.S. Influenza vaccine effectiveness against antigenically drifted influenza higher than expected in hospitalized adults: 2014–2015. Clin Infect Dis. 2016;63:1017–1025. doi: 10.1093/cid/ciw432. [DOI] [PubMed] [Google Scholar]
- 38.Pebody R., Warburton F., Andrews N., Ellis J., Wissmann B.V., Robertson C. Effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 end of season results. Euro Surveill. 2015;20 [PubMed] [Google Scholar]
- 39.Kissling E., Valenciano M. Early influenza vaccine effectiveness results 2015–16: I-MOVE multicentre case-control study. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.6.30134. [DOI] [PubMed] [Google Scholar]
- 40.Belongia E.A., Simpson M.D., King J.P., Sundaram M.E., Kelley N.S., Osterholm M.T. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. August 2016;16(8):942–951. doi: 10.1016/S1473-3099(16)00129-8. [DOI] [PubMed] [Google Scholar]
- 41.Darvishian M., Heuvel E.R.V.D., Bissielo A., Castilla J., Cohen C., Englund H. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: an individual participant data meta-analysis of test-negative design case-control studies. Lancet Respir Med. 2017;5:200–211. doi: 10.1016/S2213-2600(17)30043-7. [DOI] [PubMed] [Google Scholar]
- 42.Gaglani M., Pruszynski J., Murthy K., Clipper L., Robertson A., Reis M. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013–2014 in the United States. J Infect Dis. 2016;213:1546–1556. doi: 10.1093/infdis/jiv577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu C., Xu J., Lin J. Concurrent and cross-season protection of inactivated influenza vaccine against A(H1N1)pdm09 illness among young children:2012–13 case-control evaluation of influenza vaccine effectiveness. Vaccine. 2015;33:2917–2921. doi: 10.1016/j.vaccine.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 44.Feng S., Cowling B.J., Sullivan S.G. Influenza vaccine effectiveness by test-negative design – Comparison of inpatient and outpatient settings. Vaccine. 2016;34(14):1672–1679. doi: 10.1016/j.vaccine.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.