Graphical abstract
Keywords: Antibody Drug Conjugate (ADC), Native mass spectrometry, Drug-to-antibody ratio (DAR), Monoclonal antibodies (mAbs)
Highlights
-
•
The use of nESI-MS to examine intact Antibody Drug Conjugates (ADC).
-
•
Use of TEAA as charge reducing agent improves cysteine-linked ADC characterization.
-
•
TEAA preserves the intact mAb and facilitate easy drug load determination by native MS.
-
•
This method is particularly beneficial for users of low resolution mass spectrometers.
Abstract
Antibody-drug-conjugates (ADC) are a growing class of anticancer biopharmaceuticals. Conjugation of cysteine linked ADCs, requires initial reduction of mAb inter-chain disulfide bonds, as the drugs are attached via thiol chemistry. This results in the active mAb moiety being transformed from a covalently linked tetramer to non-covalently linked complexes, which hinders precise determination of drug load with LC–MS. Here, we show how the addition of the charge reducing agent triethylammonium acetate (TEAA) preserves the intact mAb structure, is well suited to the study of cysteine linked conjugates and facilitates easy drug load determination by direct infusion native MS.
1. Introduction
Antibody-drug-conjugates (ADC) are dynamic and heterogeneous mixtures composed of a monoclonal antibody (mAb) linked via a chemical linker to a biologically active cytotoxic small-molecule drug. [1], [2], [3] The high binding specificity of mAb and targeted receptors make ADCs effective delivery systems for cytotoxic drugs to the tumor cells. In vivo, the ADC is recognized and binds to the receptor on the surface of the targeted cell. Subsequently, the ADC is internalized into the cancer cell and digested in the lysosome, where the cytotoxic drug is released and consequently kills the target cancer cells, thus reducing systemic toxicity to the noncancerous cells [4]. Currently, there are two approved ADC cancer therapies on the market (ADCETRIS for treatment of relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma, and KADCYLA for treatment of HER2 positive metastatic breast cancer) with many more in pre-clinical and clinical development. [5], [6], [7]
At present, the dominant conjugation strategies involve reaction to either lysines or to cysteines. Covalent linking of the cytotoxic drug to the mAb can be achieved via: (1) reaction of lysines [8]—attachment to the epsilon amino group of lysine; (2) reaction of genetically engineered cysteines [9]—attachment to the side chain thiol of unpaired cysteine; or (3) reaction of cysteine residues generated by the reduction of existing interchain disulfide bonds (DSB) [10], [11]. In the latest type of mAb conjugation, the interchain disulfide bridges are partially reduced prior to the conjugation reaction. The resulting mixture consists of ADCs with an even number of conjugated drug molecules ranging from zero to eight. In other words, each disulfide cleavage results in the conjugation of two conjugates. One of the most important attributes of an ADC is the average number of drug molecules bound to the mAb, as it determines the amount of drug delivered to the tumor cells and will influence its potency [12]. Additionally the drug distribution profile of the ADC is key for both safety and efficacy, and needs to be measured accurately and maintained during manufacturing and formulation including from batch to batch [13]. Commonly used methods for ADC drug load assessment and drug-to-antibody ratio (DAR) determination include UV spectroscopy, capillary electrophoresis (CE), HPLC chromatography methods such as hydrophobic interaction chromatography (HIC) and mass spectrometry (MS) [14], [15], [16], [17], [18]. According to a recent survey conducted by Bioanalysis Zone, 82% of those surveyed considered MS to be a rapid technique of choice for evaluation of the quality attributes for ADCs at various stages of the development [19].
The dynamic nature and heterogeneity of ADCs raises significant bioanalytical challenges. In the case of lysine- or engineered cysteine-conjugated ADCs, the interchain DSB between heavy and light chains of a mAb remain intact, and DAR can be determined using liquid chromatography–mass spectrometry (LC–MS) methods employing mobile phase containing organic solvents [17], [20], [21]. ADCs conjugated at the interchain cysteine residues produce a mixture of non-covalent mAb tetramers (2 light chains (LC) and 2 heavy chains (HC) with a variable number of drug molecules attached) after reduction of interchain DSBs, hence the application of more classic LC–MS analytical strategies would result in dissociation of this non-covalent ADC.
Native MS provides an alternative approach for intact protein analysis. With appropriate sample preparation, use of aqueous volatile buffers, and suitable tuning of the instrument, it is possible to transfer weakly associated complexes from solution into the gas-phase of a mass spectrometer and obtain insights into complex stoichiometry and protein structure. Up to date, there have been only a handful of reports in the literature employing native MS for intact mass and DAR analysis of these cysteine-linked ADCs [18], [22], [23], [24], [25]. In 2012, Valliere-Douglass et al. [22] presented the first method for the rapid determination of the mass of an intact ADC intact. In this work, ADCs were deglycosylated and then subjected to native-SEC desalting followed by online electrospray MS (ESI-MS) analysis. They reported some dissociation of the non-covalent ADCs into conjugated LCs and HCs and reported this having no effect on the subsequent relative drug load distribution evaluation. A year later, Chen et al. [18] reported a MS method employing enzymatic digestion, followed by nano-ESI and native MS to achieve direct determination of the intact mass and furthermore to calculate the average DAR of the cysteine-linked ADCs. The cytotoxic conjugates investigated, often possess high hydrophobicity which will result in lower proton affinity which may in turn affect the ionisation leading to an under-representation of high-drug load species. To minimize this ion suppression and equalise ionisation efficiency among species with different drug loads by reducing the ADC hydrophobicity, limited enzymatic digestion was performed to cleave the hydrophobic moiety from ADC, while the linker remained attached and was used as an indicative of the drug load. The DAR values obtained post-enzymatic digestion were more comparable with those determined by HIC methods, while DAR values obtained for samples without enzymatic digestion were slightly lower. In 2014, Debaene et. al. [24] developed a semi-quantitative method for determination of average DAR and DAR distribution in cysteine-linked ADCs based on high resolution MS and ion-mobility MS (IM-MS) data. The above mentioned [24] along with other [26] studies of ADCs employed high performance mass spectrometers to generate excellent results and mass spectra with superior resolution. More recently, Marcoux et al. reported on the use of imidazole as a reducing agent to resolve overlapping peak distribution of a lysine-conjugated ADC as an alternative for lower resolution instruments [27]. Native MS has also been utilized in analysis of changing drug load distribution in vivo from plasma samples [23].
Here as an alternative, we have explored the use of a charge reducing agent—triethylammonium acetate (TEAA). By shifting the m/z distribution to lower values of z (and higher m/z) we minimise overlapping ADC peaks and also preserve non-covalent interactions. This approach is potentially more feasible for smaller ADC developers on lower resolution ToF or Q-ToF instruments.
2. Results and discussion
We have investigated several cysteine-linked ADCs using a nano-ESI-Q-ToF mass spectrometer (Ultima API US, Waters Corporation). As the mass of the cytotoxic drug and the linker is relatively small (∼1 kDa) in comparison to the intact mAb (∼150 kDa), to aid the desolvation process and enhance the resolution for accurate peak assignment and mass determination, high acceleration voltages (applied to the source sampling cone) can be applied at the front stages of a mass spectrometer. Often, use of elevated acceleration voltages leads to dissociation of non-covalent complexes. Fig. 1, shows mass spectra of ∼7 μM ADC in 50 mM ammonium acetate acquired at sampling cone voltage of 50 V (a), 100 V (b) and 200 V (c). Use of a lower cone voltage (50–100), allows preservation of the intact ADC as observed in the m/z 5900–8000 spectral region; however, under such conditions base line resolution is not achieved. Moreover, under these more gentle conditions, high levels of residual solvent and salt adducts make accurate mass determination challenging. Elevating the cone voltage to 200 V (Fig. 1c), produces better resolved peaks corresponding to the intact ADC, but also leads to dissociation of the conjugated LCs, detected in the m/z 1500–2700 spectral region.
Fig. 1.
Mass spectra of ∼7 μM ADC acquired on the Q-ToF Ultima API US mass spectrometer at three different acceleration voltages: 50 V (a), 100 V (b) and 200 V (c). Increase of this parameter results in effective in-source salt clean-up during the desolvation process, at the same time leading to dissociation of the non-covalent ADC.
We have found a significant improvement in cysteine linked ADC intact mass analysis with the addition of the charge reducing agent TEAA which enables preservation of non-covalently bound mAbs allowing direct DAR evaluation [28]. Addition of certain salts to the sample buffer has been reported to both reduce the charge of the ions but also to increase the stability of protein complexes in the gas phase. [29], [30], [31], [32] The charge reducing nature of alkylated ammonium ions is mainly based on its higher gas-phase basicity relative to ammonium acetate (the latter is commonly used as a salt in native MS experiments). The gas-phase basicity of the ionic species present in the solution controls how much charge is emitted during the electrospray process. Small ionic electrolytes with higher gas-phase basicity compete for charges with ionised sites of the protein and effectively remove the charge from protein species [33], [34], [35], [36]. Moreover, reducing the charge increases the energy barrier of unfolding and subsequent complex dissociation [32], [37].
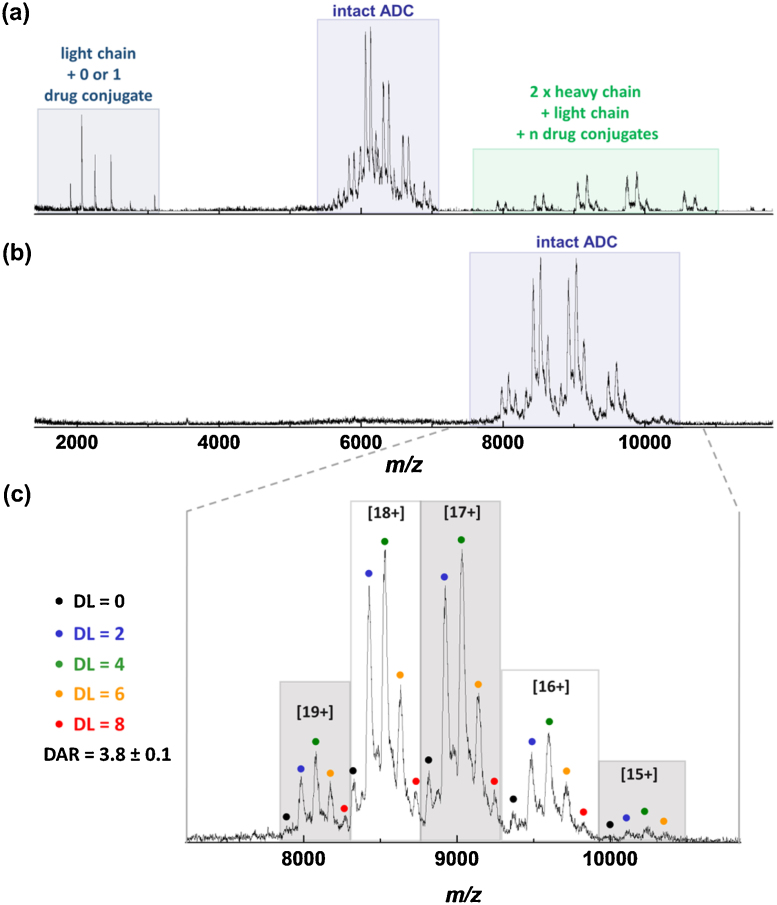
We have applied this to several ADCs provided by Piramal Healthcare [28]. Fig. 2a, shows a mass spectrum of ∼7 μM ADC in 100 mM ammonium acetate buffer acquired at sampling cone voltage held at 200 V. Peaks corresponding to different forms of the intact ADC complex are observed in the region m/z 5500–7000. Additionally a significant amount of in source dissociation products are produced, one light chain + 1 drug molecule fragments (m/z 1800–3500) and two heavy chains + one light chain + n drug molecules fragments (m/z 7800–11000), making DAR determination challenging. When TEAA buffer is added to the sample solution (Fig. 2b), the intact ADC species are now observed in a higher m/z range (7800–10500), and no dissociation products are present suggesting the complex is now significantly stabilised against dissociation. Moreover, upon addition of TEAA, the charge state envelope previously centered at [24+] (Fig. 2a) is now centered at the [17+] charge state (Fig. 2b). This shift to a lower average charge state, also helps to resolve overlapping peaks, since the lower the value of z, the better the separation in a charge state envelope and as a consequence spectra interpretation and DAR derivation is rendered more straightforward. The lack of subunit dissociation upon addition of 10% TEAA to the buffer solution and application of high acceleration voltage was also observed for other cysteine-linked ADCs, sample mass spectra are presented in Fig. S1.
Fig. 2.
Mass spectra of ∼7 μM ADC in 100 mM ammonium acetate (a) and in 100 mM ammonium acetate + 10% TEAA (b) acquired on the Ultima API US mass spectrometer where the sampling cone voltage is held at 200 V. Addition of TEAA shifts the intact protein peaks to a higher m/z region and preserves non-covalent interactions. (c) Zoom of (b) the intact ADC mass region and assignment of ADC species with different drug load (DL); the average DAR = 3.8 ± 0.1.
The average DAR values for the ADC shown in Fig. 2. have been calculated in the absence (Fig. 2a) and in the presence of TEAA (Fig. 2b) based on data acquired under identical instrumental conditions (acceleration voltage of 200 V). The DAR value based on data acquired in the presence of TEAA was found to be 3.8 ± 0.1; this value is slightly lower than the solution based value of 4.0 ± 0.1 as determined with HIC-HPLC. This discrepancy is likely due to the ionisation efficiency of the highly conjugated species. Similar to that reported by Chen et. al. [18], we have found that physiochemical properties for example the hydrophobicity of the drug moiety may influence the ionisation efficiency and alter proton affinity which in turn affects the ionisation leading to an under-representation of high-drug load species; which provides an explanation for the discrepancy between MS-based and HIC-HPLC based DAR value. This deviation could be possibly minimized by performing enzymatic digestion to remove the drug molecule only and reduce ionisation suppression of highly-conjugated species. From the mass spectrum shown in Fig. 2c, we can clearly see that ADC with 4 drug molecules (DL = 4) is the most abundant, followed by ADCs with 2 drug molecules (DL = 2), then 6 drug molecules (DL = 6); and a smaller amount of an ADC with 8 drug molecules (DL = 8) and unconjugated ADC (DL = 0) (each about 20%). The average DAR value in the absence of TEAA (Fig. 2a) was calculated to be 3.5 ± 0.2. Application of high acceleration voltages may cause the dissociation of non-covalent complexes. In the case of cysteine-linked ADCs, we have observed dissociation of the light chains (Fig. 2a, S1a and c). The DAR value here was calculated based on the peaks corresponding to the intact species only and is significantly lower than the HIC-HPLC-based DAR or even MS-TEAA-based DAR. The observed dissociation will progressively under-represented conjugated species as the number of conjugates increases. Addition of TEAA to the analysis buffer, here ammonium acetate, preserves intact the entire ADC population even upon application of a high acceleration voltage resulting in DAR values that are closer to the solution based DAR (Fig. 2).
3. Conclusions
Superior resolution of large intact molecules with similar masses can be achieved at lower m/z values using high resolution instruments, such as modified Ortibraps [38]. Instrument and operational costs are significantly higher in comparison to standard Q-ToF instruments. Moreover, such high resolution instrumentation still often requires custom modification in order to effectively transmit large proteins such as ADCs. Our native mass spectrometry approach offers a fast, cost effective method for intact ADC analysis without the use of HPLC solvents. The application of high acceleration voltages aids the desolvation process and in-source salt clean up, while addition of TEAA buffer helps to preserve non-covalent complexes and shifts the charge state envelope towards higher m/z region. The sample preparation requires addition of 10% TEAA (by volume) to ADCs contained in ammonium acetate buffer. Additionally, deglycosylation or proteolytic removal of the drug, as reported by others [18], [39], [40] could be performed to enhance the resolution further. We envision this approach being particularly beneficial to users of lower resolution MS platforms, as well as with conjugates of low mass.
Acknowledgments
We thank Dr. Xavier Despinoy from Piramal Healthcare for reading of the manuscript and providing us with ADC samples, what lead to development of this method. We are also grateful to MRC for award of Industrial Case Studentship to KJP in collaboration with UCB Pharma. We also thank the British Mass Spectrometry Society for a grant that allowed us to purchase our nanospray tip puller still going strong after 13 years of pulling.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.euprot.2016.02.004.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Chari R.V.J., Miller M.L., Widdison W.C. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew. Chem. 2014;53(15):3796–3827. doi: 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]
- 2.Beck A. Review of antibody–drug conjugates, methods in molecular biology series. mAbs. 2014;6(1):30–33. [Google Scholar]
- 3.Sievers E.L., Senter P.D. Antibody–drug conjugates in cancer therapy. Ann. Rev. Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- 4.Wu A.M., Senter P.D. Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005;23(9):1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 5.Senter P.D., Sievers E.L. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 2012;30(7):631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 6.Leal M., Sapra P., Hurvitz S.A., Senter P., Wahl A., Schutten M., Shah D.K., Haddish-Berhane N., Kabbarah O. Antibody–drug conjugates: an emerging modality for the treatment of cancer. Ann. N. Y. Acad. 2014;1321(August):41–54. doi: 10.1111/nyas.12499. [DOI] [PubMed] [Google Scholar]
- 7.Deonarain M.P., Yahioglu G., Stamati I., Marklew J. Emerging formats for next-generation antibody drug conjugates. Exp. Opin. Drug Discov. 2015;10(5):463–481. doi: 10.1517/17460441.2015.1025049. [DOI] [PubMed] [Google Scholar]
- 8.Hamann P.R., Hinman L.M., Hollander I., Beyer C.F., Lindh D., Holcomb R., Hallett W., Tsou H.R., Upeslacis J., Shochat D., Mountain A., Flowers D.A., Bernstein I. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody–calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjugate Chem. 2002;13(1):47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
- 9.Junutula J.R., Raab H., Clark S., Bhakta S., Leipold D.D., Weir S., Chen Y., Simpson M., Tsai S.P., Dennis M.S., Lu Y., Meng Y.G., Ng C., Yang J., Lee C.C., Duenas E., Gorrell J., Katta V., Kim A., McDorman K., Flagella K., Venook R., Ross S., Spencer S.D., Wong W.L., Lowman H.B., Vandlen R., Sliwkowski M.X., Scheller R.H., Polakis P., Mallet W. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 2008;26(8):925–932. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 10.Trail P.A., Willner D., Lasch S.J., Henderson A.J., Hofstead S., Casazza A.M., Firestone R.A., Hellstrom I., Hellstrom K.E. Cure of xenografted human carcinomas by BR96-doxorubicin immunoconjugates. Science. 1993;261(5118):212–215. doi: 10.1126/science.8327892. [DOI] [PubMed] [Google Scholar]
- 11.Francisco J.A., Cerveny C.G., Meyer D.L., Mixan B.J., Klussman K., Chace D.F., Rejniak S.X., Gordon K.A., DeBlanc R., Toki B.E., Law C.L., Doronina S.O., Siegall C.B., Senter P.D., Wahl A.F. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102(4):1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 12.Hamblett K.J., Senter P.D., Chace D.F., Sun M.M.C., Lenox J., Cerveny C.G., Kissler K.M., Bernhardt S.X., Kopcha A.K., Zabinski R.F., Meyer D.L., Francisco J.A. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 2004;10(20):7063–7070. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- 13.Teicher B.A., Chari R.V.J. Antibody conjugate therapeutics: challenges and potential. Clin. Cancer Res. 2011;17(20):6389–6397. doi: 10.1158/1078-0432.CCR-11-1417. [DOI] [PubMed] [Google Scholar]
- 14.Beck A., Wagner-Rousset E., Ayoub D., Van Dorsselaer A., Sanglier-Cianferani S. Characterization of therapeutic antibodies and related products. Analytical Chemistry. 2013;85(2):715–736. doi: 10.1021/ac3032355. [DOI] [PubMed] [Google Scholar]
- 15.Thompson N.J., Rosati S., Heck A.J.R. Performing native mass spectrometry analysis on therapeutic antibodies. Methods. 2014;65(1):11–17. doi: 10.1016/j.ymeth.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Beck A., Terral G., Debaene F., Wagner-Rousset E., Marcoux J., Janin-Bussat M.-C., Colas O., Van Dorsselaer A., Cianférani S. Cutting-edge mass spectrometry methods for the multi-level structural characterization of antibodydrug conjugates. Expert review of proteomics. 2015;13(2):157–183. doi: 10.1586/14789450.2016.1132167. [DOI] [PubMed] [Google Scholar]
- 17.Wakankar A., Chen Y., Gokarn Y., Jacobson F.S. Analytical methods for physicochemical characterization of antibody drug conjugates. MAbs. 2011;3(2):161–172. doi: 10.4161/mabs.3.2.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J., Yin S., Wu Y., Ouyang J. Development of a native nanoelectrospray mass spectrometry method for determination of the drug-to-antibody ratio of antibody–drug conjugates. Anal. Chem. 2013;85(3):1699–1704. doi: 10.1021/ac302959p. [DOI] [PubMed] [Google Scholar]
- 19.Bioanalysis Zone Antibody– Drug Conjugate Survey Infographic. http://www.bioanalysis-zone.com/2015/05/26/antibody-drug-conjugate-survey-infographic/ (accessed 02.07.15)
- 20.Lazar A.C., Wang L.T., Blattler W.A., Amphlett G., Lambert J.M., Zhang W. Analysis of the composition of immunoconjugates using size-exclusion chromatography coupled to mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19(13):1806–1814. doi: 10.1002/rcm.1987. [DOI] [PubMed] [Google Scholar]
- 21.Xu K., Liu L., Saad O.M., Baudys J., Williams L., Leipold D., Shen B., Raab H., Junutula J.R., Kim A., Kaur S. Characterization of intact antibody–drug conjugates from plasma/serum in vivo by affinity capture capillary liquid chromatography–mass spectrometry. Anal. Biochem. 2011;412(1):56–66. doi: 10.1016/j.ab.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Valliere-Douglass J.F., McFee W.A., Salas-Solano O. Native Intact mass determination of antibodies conjugated with monomethyl auristatin E and F at interchain cysteine residues. Anal. Chem. 2012;84(6):2843–2849. doi: 10.1021/ac203346c. [DOI] [PubMed] [Google Scholar]
- 23.Hengel S.M., Sanderson R., Valliere-Douglass J., Nicholas N., Leiske C., Alley S.C. Measurement of in vivo drug load distribution of cysteine-linked antibody–drug conjugates using microscale liquid chromatography mass spectrometry. Anal. Chem. 2014;86(7):3420–3425. doi: 10.1021/ac403860c. [DOI] [PubMed] [Google Scholar]
- 24.Debaene F., Boeuf A., Wagner-Rousset E., Colas O., Ayoub D., Corvaïa N., Van Dorsselaer A., Beck A., Cianférani S. Innovative native MS methodologies for Antibody Drug Conjugate Characterization: high resolution native MS and IM-MS for average DAR and DAR distribution assessment. Anal. Chem. 2014;86(21):10674–10683. doi: 10.1021/ac502593n. [DOI] [PubMed] [Google Scholar]
- 25.Valliere-Douglass J.F., Hengel S.M., Pan L.Y. Approaches to interchain cysteine-linked ADC characterization by mass spectrometry. Mol. Pharm. 2015;12(6):1774–1783. doi: 10.1021/mp500614p. [DOI] [PubMed] [Google Scholar]
- 26.Dyachenko A., Wang G., Belov M., Makarov A., de Jong R.N., van den Bremer E.T.J., Parren P.W.H.I., Heck A.J.R. Tandem native mass-spectrometry on antibody–drug conjugates and submillion Da antibody–antigen protein assemblies on an Orbitrap EMR equipped with a high-mass quadrupole mass selector. Anal. Chem. 2015;87(12):6095–6102. doi: 10.1021/acs.analchem.5b00788. [DOI] [PubMed] [Google Scholar]
- 27.Marcoux J., Champion T., Colas O., Wagner-Rousset E., Corvaia N., Van Dorsselaer A., Beck A. Native mass spectrometry and ion mobility characterization of trastuzumab emtansine, a lysine-linked antibody drug conjugate. Protein Sci. 2015;24:1210–1223. doi: 10.1002/pro.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacholarz K. University of Edinburgh; Edinburgh, United Kingdom: 2015. PhD Thesis—Investigation of Large Proteins and Multimeric Protein Complex Structures with Mass Spectrometry Techniques. [Google Scholar]
- 29.Pagel K., Hyung S.-J., Ruotolo B.T., Robinson C.V. Alternate dissociation pathways identified in charge-reduced protein complex ions. Anal. Chem. 2010;82(12):5363–5372. doi: 10.1021/ac101121r. [DOI] [PubMed] [Google Scholar]
- 30.Zhou M., Dagan S., Wysocki V.H. Protein subunits released by surface collisions of noncovalent complexes: nativelike compact structures revealed by ion mobility mass spectrometry. Angew. Chem. Int. Ed. 2012;51(18):4336–4339. doi: 10.1002/anie.201108700. [DOI] [PubMed] [Google Scholar]
- 31.Mehmood S., Marcoux J., Hopper J.T.S., Allison T.M., Liko I., Borysik A.J., Robinson C.V. Charge reduction stabilizes intact membrane protein complexes for mass spectrometry. J. Am. Chem. Soc. 2014;136(49):17010–17012. doi: 10.1021/ja510283g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall Z., Politis A., Bush M.F., Smith L.J., Robinson C.V. Charge-state dependent compaction and dissociation of protein complexes: insights from ion mobility and molecular dynamics. J. Am. Chem. Soc. 2012;134(7):3429–3438. doi: 10.1021/ja2096859. [DOI] [PubMed] [Google Scholar]
- 33.Catalina M.I., van den Heuvel R.H.H., van Duijn E., Heck A.J.R. Decharging of globular proteins and protein complexes in electrospray. Chem. Eur. J. 2005;11(3):960–968. doi: 10.1002/chem.200400395. [DOI] [PubMed] [Google Scholar]
- 34.Lemaire D., Marie G., Serani L., Laprevote O. Stabilization of gas-phase noncovalent macromolecular complexes in electrospray mass spectrometry using aqueous triethylammonium bicarbonate buffer. Anal. Chem. 2001;73(8):1699–1706. doi: 10.1021/ac001276s. [DOI] [PubMed] [Google Scholar]
- 35.Hogan C.J., Jr., Carroll J.A., Rohrs H.W., Biswas P., Gross M.L. Combined charged residue-field emission model of macromolecular electrospray ionization. Anal. Chem. 2009;81(1):369–377. doi: 10.1021/ac8016532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogan C.J., Jr., Carroll J.A., Rohrs H.W., Biswas P., Gross M.L. Charge carrier field emission determines the number of charges on native state proteins in electrospray ionization. J. Am. Chem. Soc. 2008;130(22):6926–6927. doi: 10.1021/ja801280c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wanasundara S.N., Thachuk M. Free energy barrier estimation for the dissociation of charged protein complexes in the gas phase. J. Phys. Chem. A. 2009;113(16):3814–3821. doi: 10.1021/jp8094227. [DOI] [PubMed] [Google Scholar]
- 38.Rosati S., van den Bremer E.T.J., Schuurman J., Parren P.W.H.I., Kamerling J.P., Heck A.J.R. In-depth qualitative and quantitative analysis of composite glycosylation profiles and other micro-heterogeneity on intact monoclonal antibodies by high-resolution native mass spectrometry using a modified Orbitrap. MAbs. 2013;5(6):917–924. doi: 10.4161/mabs.26282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandoval W.N., Arellano F., Arnott D., Raab H., Vandlen R., Lill J.R. Rapid removal of N-linked oligosaccharides using microwave assisted enzyme catalyzed deglycosylation. Int. J. Mass Spectrom. 2007;259(1–3):117–123. [Google Scholar]
- 40.Arnott D.P., Lill J., Sandoval W.N., Vandlen R.L. Official Gazette of the United States Patent and Trademark Office Patents; 2012. Microwave Assisted Deglycosylation of Proteins for Molecular Weight Determination by Mass Spectrometry. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.