Summary
The daily light-dark cycles represent a key signal for synchronizing circadian clocks. Both insects and mammals possess dedicated “circadian” photoreceptors but also utilize the visual system for clock resetting. In Drosophila, circadian clock resetting is achieved by the blue-light photoreceptor cryptochrome (CRY), which is expressed within subsets of the brain clock neurons. In addition, rhodopsin-expressing photoreceptor cells contribute to light synchronization. Light resets the molecular clock by CRY-dependent degradation of the clock protein Timeless (TIM), although in specific subsets of key circadian pacemaker neurons, including the small ventral lateral neurons (s-LNvs), TIM and Period (PER) oscillations can be synchronized by light independent of CRY and canonical visual Rhodopsin phototransduction. Here, we show that at least three of the seven Drosophila rhodopsins can utilize an alternative transduction mechanism involving the same α-subunit of the heterotrimeric G protein operating in canonical visual phototransduction (Gq). Surprisingly, in mutants lacking the canonical phospholipase C-β (PLC-β) encoded by the no receptor potential A (norpA) gene, we uncovered a novel transduction pathway using a different PLC-β encoded by the Plc21C gene. This novel pathway is important for behavioral clock resetting to semi-natural light-dark cycles and mediates light-dependent molecular synchronization within the s-LNv clock neurons. The same pathway appears to be responsible for norpA-independent light responses in the compound eye. We show that Rhodopsin 5 (Rh5) and Rh6, present in the R8 subset of retinal photoreceptor cells, drive both the long-term circadian and rapid light responses in the eye.
Keywords: rhodopsin, circadian clock, phototransduction, period, timeless, cryptochrome, phospholipase C
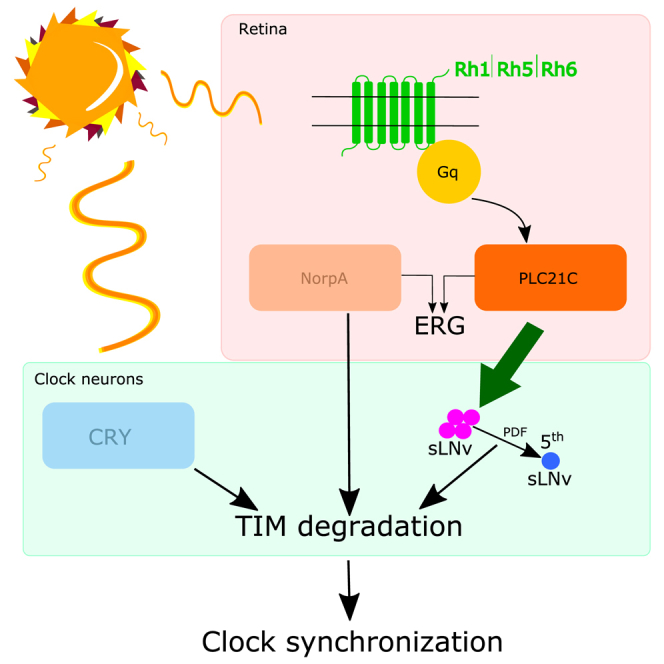
Graphical Abstract
Highlights
-
•
CRYPTOCHROME-independent light input synchronizes key circadian pacemaker neurons
-
•
In the absence of norpA, Plc21C mediates this novel light input
-
•
Rhodopsin 1, Rhodopsin 5, and Rhodopsin 6 can signal via this Gq-dependent pathway
-
•
The same pathway mediates ERG responses from R8 photoreceptors in norpA mutants
Light synchronization of the Drosophila circadian clock relies on cryptochrome and rhodopsin photoreceptors. Ogueta et al. reveal that rhodopsin signaling to the clock involves non-canonical phototransduction, which synchronizes key circadian pacemaker neurons and behavior. In the eye, the same pathway can mediate ERG responses to brief light pulses.
Introduction
Circadian clocks regulate the physiology, behavior, and sleep of organisms in a rhythmic daily fashion. Proper timing of these parameters contributes to overall fitness, and it is, therefore, crucial that circadian clocks are accurately synchronized with the environmental time, dictated by the natural daily cycles of light and temperature [1]. Absence of any environmental time cues reveals the endogenous nature of circadian clock function, realized by rhythmic expression of clock genes. In Drosophila, the key clock genes period and timeless are rhythmically activated by CLOCK (CLK) and CYCLE (CYC), transcription factors belonging to the basic-helix-loop-helix (bHLH)/PAS domain family [2]. Period (PER) and Timeless (TIM) proteins accumulate in the cytoplasm, whereby PER is stabilized by dimerization with TIM. Eventually, PER and TIM translocate to the nucleus and block CLK/CYC activity to shut down their own transcription until eventual degradation of PER and TIM restarts the cycle [2]. These molecular oscillations can be synchronized to the environment by light-induced degradation of TIM, which is mediated by the blue light photoreceptor cryptochrome (CRY) [3, 4]. Upon light exposure, CRY undergoes a conformational change, allowing it to bind to TIM and the F-box protein JETLAG, resulting in the proteasomal degradation of TIM and CRY [5, 6, 7, 8, 9, 10]. Because PER stability depends on the presence and binding to TIM, this pathway efficiently resets the circadian clock to light.
Although the circadian clock mechanism operates in many cells throughout the body, only a few neurons in the fly brain (∼150) express clock genes. These interconnected “clock neurons” form a neuronal network that regulates the fly’s daily behavioral activity rhythm, representing the best studied output rhythm controlled by the clock [11]. Based on the position within the brain and expression of the neuropeptide Pigment Dispersing Factor (PDF), clock neurons are subdivided into PDF-expressing small and large ventral lateral neurons (s-LNvs and l-LNvs, respectively), the fifth PDF-negative s-LNv, the dorsal lateral neurons (LNds), the dorsal neuron groups 1, 2, and 3 (DN1, DN2, and DN3), and the lateral posterior neurons (LPNs) [11]. The l-LNvs are important cells for the regulation of sleep and arousal behavior and are thought to propagate light input to the clock [12, 13, 14, 15]. The s-LNvs are the key neurons driving behavioral rhythms in constant darkness [16] but also regulate anticipatory behavioral activity at dawn, which is why they were also coined Morning (M) cells [17, 18]. The fifth s-LNv and LNd represent the Evening (E) cells, which are important for anticipatory behavioral activity at dusk. The DNs form heterogeneous groups of neurons that receive circadian signals from the other clock neurons but also receive and deliver light and temperature information to the network [19].
CRY is expressed in about 50% of the central brain clock neurons, including all of the LNvs, and subsets of the LNd and DN1. Apart from CRY, circadian clock resetting of behavioral activity and the underlying molecular rhythms in clock neurons is mediated by the compound eyes, the Hofbauer-Buchner eyelet (H-B eyelet), and the ocelli, e.g [4, 20, 21, 22, 23]. At least six different rhodopsins are expressed in the various visual photoreceptors. The major Rhodopsin 1 (Rh1) is present in the outer photoreceptors, while Rh3–Rh6 are expressed in the inner photoreceptors (Rh3 and Rh4 in R7 and Rh5/6 in R8). Rh6 is also the opsin expressed in the H-B eyelet, while the ocelli only contain Rh2 [24, 25]. The expression pattern of the recently characterized Rh7 is less clear, as different studies report expression in the s-LNv and LNd [26], or within the subset of the DN1, and the R8 and H-B-eyelet photoreceptors [27], respectively.
Flies lacking CRY can still synchronize their circadian clock to light-dark (LD) cycles, and the same applies for visually blind flies lacking norpA-encoded phospholipase C-β (PLC-β), an essential enzyme in the canonical phototransduction cascade operating in the visual photoreceptor cells [4]. When visual phototransduction is blocked in parallel with removing CRY (e.g., in norpAP41 cryb double mutants), flies lose the ability to synchronize to low light intensities (5–16 lux); nevertheless, they remain able to slowly synchronize to higher light intensities (400 to 1,000 lux) [4, 22, 28, 29]. In line with these behavioral observations, s-LNvs, LNds, and DN1s exhibit light-synchronized clock protein oscillations in the absence of CRY [4, 20, 30, 31, 32]. Moreover, s-LNvs of norpAP41 cryb mutants can still be synchronized by LD cycles, suggesting that the LNds receive norpA-dependent, and the s-LNvs receive norpA-independent, visual system light input [20, 31]. Szular et al. (2012) showed that Rh5 and Rh6 contribute to norpA-independent behavioral synchronization, raising the exciting possibility that these rhodopsins can use alternative phototransduction pathways and that they are responsible for the CRY-independent light synchronization of TIM and PER expression in the s-LNvs [20, 28].
Here, we tested this hypothesis by combining different rhodopsin and phototransduction mutants with the norpAP41 cryb double mutant. We show that norpA- and cry-independent light input specifically targets the s-LNvs by mediating light-dependent degradation of clock proteins within these neurons. norpA- and cry- independent molecular and behavioral synchronization depends on Rh1, Rh5, and Rh6 and also requires Gq as well as the second Drosophila PLC-β, encoded by Plc21C. Electroretinogram (ERG) recordings from the same mutants reveal residual norpA-independent light responses that depend on Rh5 and Rh6 expressed in R8 photoreceptors as well as on Gq and Plc21C.
Results
norpA-Independent Circadian Clock Resetting Requires Gq
As a measure of circadian clock synchronization to light, wild-type and mutant flies were raised in 12-hr:12-hr LD cycles (lights on at 9 a.m.) at a constant temperature of 25°C and 60% relative humidity. During the light part of these LD cycles, 3- to 5-day-old males were individually loaded into glass tubes, and locomotor activity was monitored for 4 days using the same LD cycle (i.e., lights on at 9 a.m.), except that now, light intensity was gradually ramped from 0 to 180 lux and back, using white LEDs (see Figure S1 for light profile and LED spectrum). We avoided using higher light intensities to prevent potential light-independent activation of rhodopsins [33]). To ensure that all mutant (potentially “circadian blind”) genotypes become synchronized during the first 4 days of the experiment, we applied a 25°C:16°C temperature cycle combined with the LD cycle (temperature increase at 9 a.m.). During these conditions, wild-type flies exhibit typical bimodal or crepuscular behavior, with activity peaks in the morning and evening (Figure 1A) [34]. After 4 days of exposure to these combined LD and temperature cycles, a clear synchronized activity peak in all genotypes could be observed (Figures 1 and 2). In contrast to the wild-type controls, most of the visually impaired mutants exhibited a slowly advancing or broadened evening activity peak, which is reminiscent of the wild-type activity peak after exposure to 25°C:16°C temperature cycles in constant darkness (DD) (e.g., Figures 2A–2D) [34]. Although this preferential synchronization to temperature already indicates deficits in light resetting, the synchronized activity peak allowed us in the following to analyze how many days the various genotypes require to resynchronize their behavioral activity pattern to a 6-hr delay of this LD cycle (where the temperature was kept constant at 25°C). Flies were exposed for 7 days to the shifted LD cycle (mimicking a rapid westward time zone shift of 6 hr) before being released into DD conditions at constant 25°C. As previously reported, wild-type flies and norpAP41 mutants require only 1–2 days for resynchronization, whereas cryb and norpAP41 cryb double mutants require at least 5 days [28, 29] (Figures 1A–1D, 2A, and 2E). Additional removal of Rh5 and Rh6 function (norpAP41 Rh52 Rh61 cryb) abolishes the ability to resynchronize completely, confirming that Rh5 and Rh6 operate in a norpA- and cry-independent pathway (Figures 2B and 2E) [28]. We then tested flies lacking Rh1 (encoded by ninaE) in the same norpAP41 cryb background and found that they, too, are not able to resynchronize to LD cycles, suggesting that Rh1 can also signal independently of norpA (Figure 2C). In order to identify where the norpA-dependent and norpA-independent transduction pathways diverge, we analyzed Gq1 mutant flies in the background of the norpAP41 cryb double mutant. These mutants also failed to resynchronize to the shifted LD cycle, indicating that the signaling pathways diverge after activation of Gq and before activation of the norpA-encoded PLC-β (Figure 2D). We note that removal of Rh7 did not further impair re-synchronization in flies lacking norpA and cry function, indicating that the phenotypes described earlier are specific for Rh1, Rh5, Rh6, and Gq [27]. To better visualize re-synchronization of the activity peaks, we also plotted the average daily activity before the shift and during the final 3 days before release into DD next to the actograms. While, in norpAP41 cryb flies, the main evening activity peak before and after the shift occurs almost at the same time with relation to the LD cycle, the activity peak in the various rhodopsin and Gq mutants does not shift or only shifts minimally (Figures 2A–2D). We also quantified this behavior by determining the daily shift of the activity peak, again revealing no, or minimal, behavioral adjustment to the delayed LD cycle in the rhodopsin and Gq mutants (Figure 2E; STAR Methods).
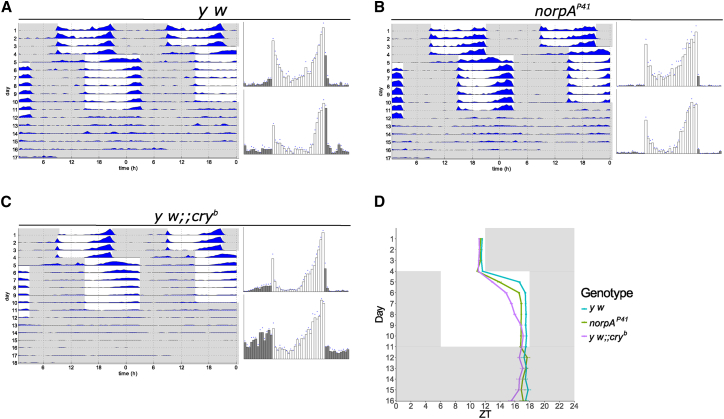
Figure 1.
norpA and cry Mutants Re-entrain More Slowly than Wild-Type
(A–C) Representative actograms (left) of 16 flies for each of the genotypes and histograms (right) of the last 3 days before the shift (top) and after the shift (bottom). (A) y w. (B) norpAP41. (C) y w;; cryb.
(D) Quantification of the position of the evening peaks for the three genotypes for each day of the experiment. While wild-type flies move their evening peak by 5 hr on the first day and 0.8 hr on the second day of the phase shift, norpAP41 mutants need almost 2 days to move their evening peak by 5.6 hr (3 hr on the first day and 2.6 hr on the second). cry mutants shift 5.6 hr in 5 days (1.9 hr on the first day, 1.8 hr on the second, 0.7 hr on the third, 0.4 hr on the fourth, and 0.8 hr on the fifth). y w, n = 58; norpAP41, n = 31; y w;; cryb, n = 56.
See also Figure S1.
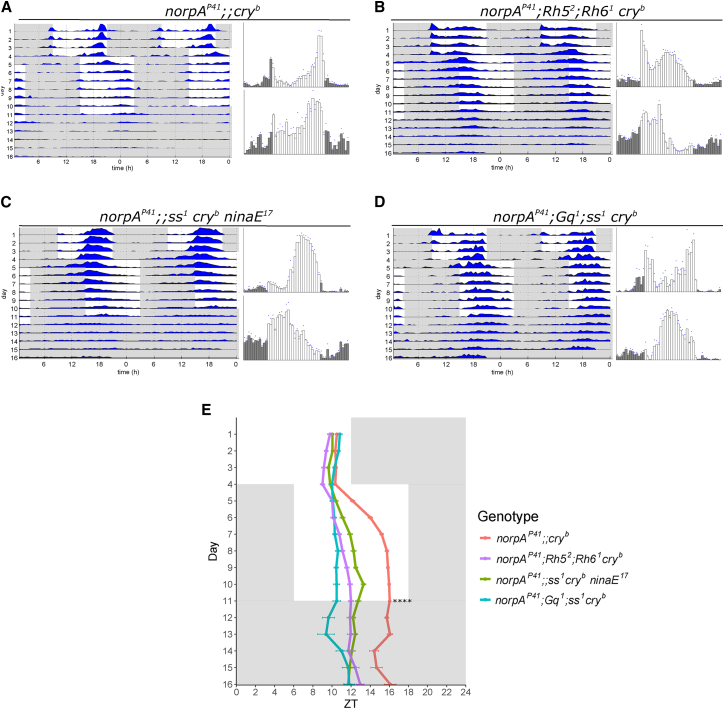
Figure 2.
Rh1, Rh5, Rh6, and Gq Contribute to norpA- and cry-Independent Behavioral Light Resetting
(A–D) Representative actograms (left) of 16 flies for each of the genotypes, and histograms (right) of the last 3 days before the shift (top) and after the shift (bottom). Flies were entrained to a 2-hr ramping 12-hr:12-hr LD cycle in combination with temperature cycles of 25:16°C. After 4 days, the temperature was kept constant at 25°C, and the LD regime was shifted 6 hr. The flies were kept in these new conditions for 7 days, and afterward they were in constant darkness for 5 days. (A) norpAP41;; cryb. (B) norpAP41; Rh52; Rh61cryb. (C) norpAP41;; ss1crybninaE17. (D) norpAP41; Gq1; ss1cryb.
(E) Comparison of the position of the evening peak for each of the genotypes for each of the days of the experiment. While norpAP41;; cryb flies shift their evening peak by 5.7 ± 0.2 hr during the 7 days of the new regime, none of the other mutants are able to adapt to the new conditions (norpAP41; Rh52; Rh61cryb, 3.0 ± 0.3 hr; norpAP41; Gq1; cryb, 0.5 ± 0.4 hr; and norpAP41;; ninaE17cryb, 2.9 ± 0.3 hr). norpAP41;; cryb, n = 40; norpAP41; Rh52; Rh61cryb, n = 55; norpAP41; Gq1; ss1cryb, n = 41; and norpAP41;;ss1crybninaE17, n = 57.
Error bars represent SEM. ∗∗∗∗p < 0.0000001.
See also Figures S1A, S1B, and S3.
norpA- and cry-Independent Light Signaling Targets the s-LNv Pacemaker Neurons
The ability of norpAP41 cryb double mutants to resynchronize activity rhythms to LD cycles is presumably mediated by molecular oscillations in at least some of the clock neurons that are known to control these rhythms. Indeed, a previous study showed that PER oscillations in the s-LNvs of norpAP41 cryb flies are synchronized during LD cycles [20]. Therefore, we determined whether PER oscillations could be synchronized by LD cycles (at constant temperature) in clock neurons of norpAP41 cryb flies and in flies that additionally lacked rhodopsins or Gq. In agreement with the previous study [20], norpAP41 cryb double mutants maintain robust PER oscillations during the LD cycle in the s-LNvs. Indeed, we show here that, in contrast to the l-LNvs, the four PDF-expressing s-LNvs and the fifth PDF-negative s-LNv maintain light-synchronized oscillations in norpAP41 cryb (Figures 3A–3D). In contrast, flies lacking additionally Rh5 and Rh6, as well as those lacking Gq, do not show rhythmic accumulation of PER, presumably due to the lack of light-mediated degradation during the day (see high PER levels at zeitgeber time [ZT] 10 in Figure 3). We also analyzed PER levels in s-LNvs at normal peak (ZT22) and trough (ZT10) time points in flies lacking Rh1, in addition to PLC-β and CRY, and again saw maintained high PER levels at ZT10, suggesting a block of light-dependent degradation (Figure 3). We also determined PER levels in the other clock neuronal groups but did not observe any consistent differences between norpAP41 cryb flies and those lacking rhodopsins or Gq in addition (Figure S2). These results strongly suggest that the main target for the norpA- and cry-independent light input are the PDF+ and PDF− s-LNvs and that light-synchronized oscillations in these neurons are responsible for the behavioral synchronization of norpAP41 cryb flies.
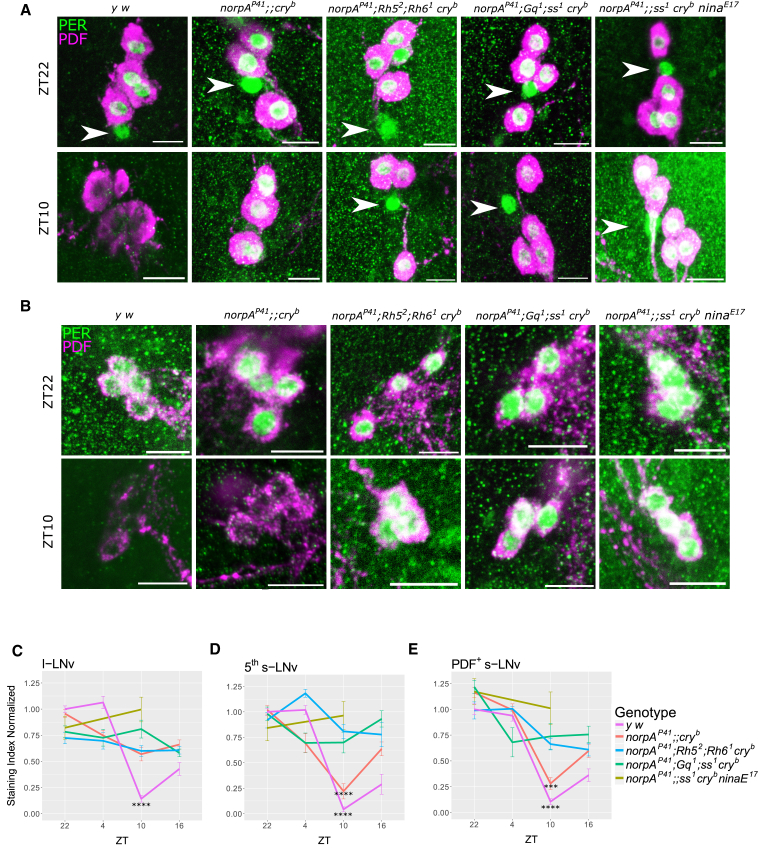
Figure 3.
Rh1, Rh5, Rh6, and Gq Are Required for Synchronized PER Oscillations in the s-LN Clock Neurons
(A and B) Representative images of the staining at ZT22 and ZT10 of the l-LNvs and the fifth s-LNv (A; fifth s-LNv marked with arrowheads) and the s-LNvs (B), with PER antibody indicated in green and PDF antibody indicated in magenta to identify the fifth PDF-negative s-LNv. Scale bars, 10 μm.
(C–E) Quantification of PER expression in the l-LNv (C), fifth s-LNv (D), and PDF+ s-LNv (E). Note that PER oscillations in the PDF+ and PDF− s-LNvs are synchronized to the LD cycle in y w and norpAP41;; cryb flies, but not in any of the other genotypes, presumably because of PER is not degraded during the day (compare PER values at ZT10 between the genotypes: PER levels in y w and norpAP41;; cryb s-LNvs are significantly lower compared to any of the mutants; ∗∗∗∗p < 0.0000001 and ∗∗∗p < 0.0001, respectively). For ZT22 and ZT10 time points, between 12 (norpAP41;; ninaE17ss1cryb) and 51 (y w) brain hemispheres were imaged in 2–5 independent experiments. For ZT4 and ZT16 time points, between 6 (norpAP41; Gq1; ss1cryb) and 19 (y w) hemispheres were analyzed in 3 independent experiments (see Table S1 for exact numbers).
Error bars indicate SEM.
Rh5 and Rh6 Dominate the Residual ERG Responses in norpA Mutant Flies
Our results indicate a norpA- and cry-independent role for Rh1, Rh5, Rh6, and Gq in behavioral and molecular light resetting of the Drosophila clock. All of these genes and proteins are expressed in the retinal photoreceptors; Rh1 is expressed in the outer photoreceptor cells R1–R6; Rh5 and Rh6 are expressed in the inner R8 cells; and Gq is expressed in all photoreceptors. In addition Rh6 is expressed in the H-B eyelet, e.g [35], but although the Rh5 promoter is also active in this structure, Rh5 protein could not be detected in the eyelet [25, 28]. Therefore, we speculated that the most relevant tissue for the circadian clock function of Rh1, Rh5, Rh6 and Gq was the retina. In an attempt to demonstrate the involvement of retinal rhodopsins in norpA-independent phototransduction, we performed ERG recordings with flies lacking norpA as well as several rhodopsins and Gq. All genotypes carrying norpAP41 were also mutant for cry (i.e., they were the same lines we used for the behavioral analysis), though it has recently been reported that the lack of CRY does not influence the ERG [36].
norpAP41 is a true loss-of-function allele [28] and was reported to lack all ERG responses to light [37] (Figure 4A). Upon closer inspection, however, small (<1 mV) ERG responses to bright illumination are detectable in norpAP41 mutant flies (Figures 4B and 4C). As an extracellular potential, photoreceptor depolarization in the ERG is manifested as a maintained hyperpolarizing (negative) response, while positive-going “on” and negative-going “off” transients reflect transient activation of postsynaptic interneurons. In norpAP41, a small maintained hyperpolarization of up to ∼1 mV was detected in response to a 2-s bright step of light, which then decayed very slowly (Figures 4B, 4C, and S1C). Surprisingly, this response was largely unaffected (if anything, slightly enhanced) in norpAP41 ninaE17 double mutants lacking visual pigment in the major R1–R6 class (Figures 4B and 4C). By contrast, in norpAP41 Rh52 Rh61 treble mutants lacking visual pigment in all R8 cells, the negative-going ERG was largely abolished, leaving a small, predominantly positive-going response (Figures 4B and 4C). This suggests that the R8 cells contribute most of the receptor component of the residual ERG in norpAP41 flies. ERG responses in norpAP41 Gq1 were similar in amplitude compared to norpAP41 alone, but the duration of the response was significantly shortened (Figures 4B and 4D). This shows that Gq influences the duration of the norpA-independent residual ERG response, in line with observations from whole-cell patch-clamp recordings from R1–R6 photoreceptors [38]. While the underlying physiological mechanisms for these effects are not known, the results, nevertheless, indicate an involvement of Rh5, Rh6, and Gq in the norpA-independent ERG response.
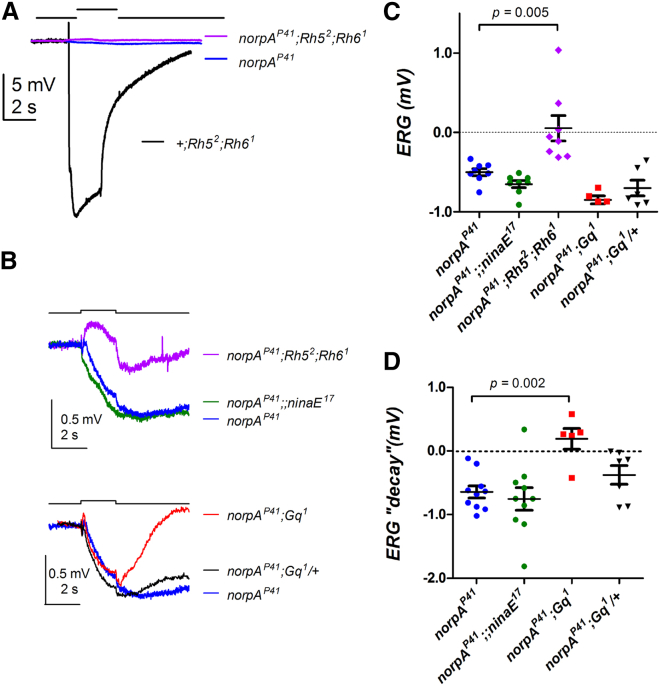
Figure 4.
Rh5 and Rh6 Dominate the norpA-Independent ERG Responses
(A) ERG in response to a 2-s bright white light flash (bar, equivalent to ∼107 effective photons per photoreceptor) in norpAP41 (blue), Rh52; Rh61 (black), and norpAP41; Rh52; Rh61 (purple) mutants.
(B) ERG responses in response to a 2-s bright white flash. Top traces recorded from norpAP41 flies (blue), norpAP41; Rh52; Rh61 flies lacking both R8 opsins (purple), and norpAP41;; ninaE17 mutant flies lacking the R1–R6 opsin (green). Lower traces recorded from norpAP41 flies (blue; same as upper panel), norpAP41; Gq1 flies (red), and norpAP41; Gq1/CyO flies (black; sibling controls from same vials). Each trace is a “grand” average from responses from 5 to 10 flies, each of which was itself averaged from responses to at least 4 flashes repeated at 5-min intervals.
(C) Summary of response amplitudes averaged over the last 500 ms of the 2-s flash. Responses in norpAP41; Rh52; Rh61 were significantly (p = 0.005) more positive than norpAP41 controls. Other genotypes were not significantly different (one-way ANOVA with Dunnett’s multiple comparison).
(D) Summary of response amplitudes averaged between 2 and 4 s after termination of the stimulus. Responses in norpAP41; Gq1 recovered significantly more quickly (p = 0.002). All genotypes were also homozygous for the cryb allele, but CRY does not influence the ERG response [36].
See also Figure S1C.
PLC21C-Encoded PLC-β Participates in Circadian Light Resetting and ERG Responses
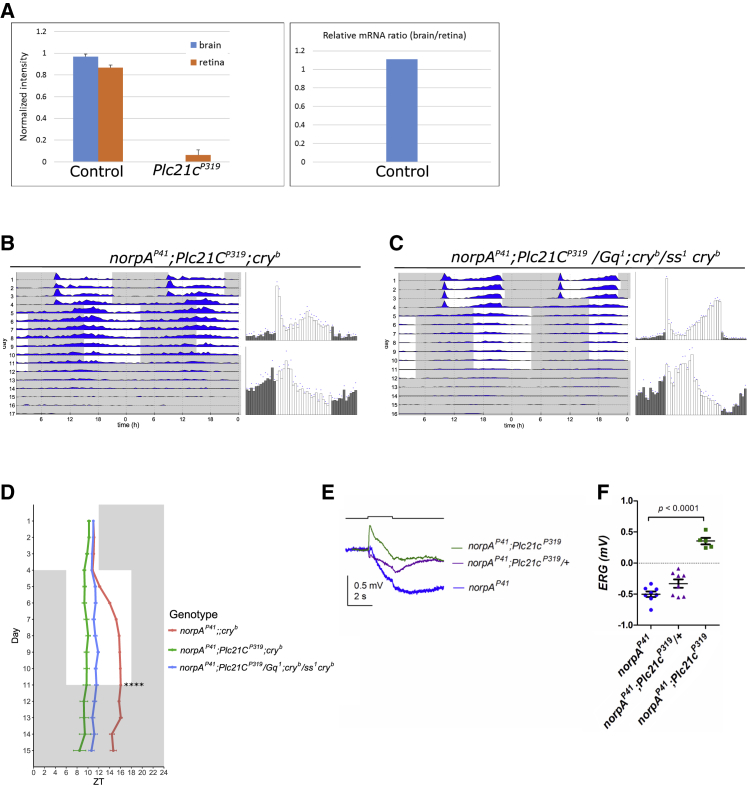
A candidate for the alternative Gq target is PLC21C, the second PLC-β encoded in the Drosophila genome. PLC21C is enriched in heads and the CNS [39], and we show here that Plc21C mRNA is also expressed in the retina (Figure 5A). To test whether Plc21C participates in norpA-independent circadian photoreception, we combined the Plc21CP319 allele with norpAP41 and cryb. Plc21CP319 is a strong hypomorphic allele, which drastically reduces the amplitude of electroantennogram recordings in response to odor stimulation [39, 40]. Surprisingly, the treble-mutant flies did not synchronize to shifted LD cycles, similar to introducing Gq1, ninaE17, or Rh52 Rh61 into the same background (Figures 5B and 5D; compare to Figure 2). To confirm that Plc21C and Gq operate in the same pathway, we also tested the light-resetting ability of transheterozygous norpAP41 Gq1/Plc21CP314 cryb flies. Although these flies carry one wild-type copy of the Gq and Plc21C genes, they are not able to resynchronize to the shifted LD cycle, demonstrating that both genes genetically interact and strongly indicating that Gq activates PLC21C (Figures 5C and 5D). The same strategy (with identical alleles) has been used previously to demonstrate that Gq activates Plc21C in the olfactory system [41]. Next, we performed ERG recordings from norpAP41 Plc21CP319 cryb flies and found that, similar to norpAP41 Rh52 Rh61 cryb flies, they only showed a small positive-going response to brief light pulses (Figures 5E and 5F). These data suggest that Plc21C contributes to circadian light resetting and norpA-independent ERG response, presumably in response to Rh5/Rh6-mediated activation of Gq.
Figure 5.
PLC21C Participates in Circadian Light Resetting and ERG Responses
(A) Determination of Plc21C expression levels in brains and retinas of control and mutant flies by semi-qRT-PCR. Data were normalized against the ribosomal gene rp49.
(B and C) Representative actograms (left) of 16 flies for each of the genotypes, and histograms (right) of the last 3 days before the shift (top) and after the shift (bottom). (B) norpAP41; Plc21CP319; cryb. (C) norpAP41; Plc21CP319/Gq1; cryb/ ss1cryb.
(D) Phase determination of evening activity peaks for norpAP41;; cryb (n = 40, same as in Figure 2), norpAP41; Plc21CP319; cryb (n = 36), and norpAP41; Plc21CP319/Gq1; cryb/ ss1cryb (n = 28) flies. In the 7 days of the new light regime, neither norpAP41; Plc21CP319; cryb flies nor norpAP41; Plc21CP319/Gq1; cryb/ ss1cryb flies are able to shift their main activity peak.
(E) ERG in response to a 2-s bright white light flash in norpAP41 (blue), norpAP41; Plc21CP319/+; cryb (purple), and norpAP41; Plc21CP319; cryb (green) flies. Each trace is a “grand” average from responses from 5–10 flies, each of which was itself averaged from responses to at least 4 flashes repeated at 5-min intervals.
(F) Summary of response amplitudes averaged over the last 500 ms of the 2-s flash. norpAP41; Plc21CP319; cryb no longer showed any hyperpolarizing component at this state (p < 0.0001, one-way ANOVA with Dunnett’s multiple comparison). norpAP41; Plc21CP319/+ heterozygotes also showed a reduced hyperpolarizing component, though this was only marginally significant on this sample (p = 0.06).
See also Figure S1.
Discussion
The daily environmental changes of light and darkness arguably represent the most reliable circadian clock resetting cue for all organisms exposed to these natural LD cycles. Not surprisingly, various photoreceptors and photopigments contribute to light synchronization in plants and animals, with multiple light-input pathways existing within one species, e.g [20, 41, 42]. In Drosophila, next to the well-established role of the blue light photoreceptor CRY, expressed within subsets of the circadian clock neurons, the visual system also contributes to daily light resetting, e.g [4, 20]. Contribution of the visual system involves canonical rhodopsin signaling, which depends on norpA-encoded PLC-β, and which is important for synchronization to low-light LD cycles [4, 22]. On the other hand, Rh5 and Rh6 have been shown to be part of a norpA-independent pathway, contributing to the synchronization of medium-intensity-light LD cycles (∼400 lux) and suggesting the existence of non-canonical rhodopsin phototransduction in Drosophila [28]. Here, we demonstrate that Rh1, Rh5, Rh6, Gq, and Plc21C are required for light synchronization of molecular and behavioral circadian rhythms in the absence of CRY and canonical visual phototransduction.
Molecular Synchronization by Light in the Absence of CRY and Canonical Phototransduction
Ultimately, all resetting cues that have the potency to stably synchronize clock-controlled behavioral rhythms must affect the molecular oscillations in at least a subset of the clock neurons. The main target of light signals is the clock protein TIM, which is degraded after light-dependent interaction with the photoreceptor CRY and the F-box protein JET [5, 8, 9, 10]. However, CRY is only expressed in about 50% of the ∼150 clock neurons in the fly brain [43, 44], and light-dependent synchronization of clock protein cycling still occurs in some of clock neurons in cry mutants, including the s-LNv, LNd, and DN1 neurons that normally express cry [4, 20, 23, 30, 31, 32, 45]. Furthermore, TIM is CRY-independently degraded in s-LNv and LNd clock neurons after artificial excitation of the PDF+ LNv [46]. This degradation requires PDF signaling from s-LNv to LNd and the E3 ubiquitin ligase CUL-3, which is also required for TIM degradation in constant darkness [46, 47]. Taken together, these findings show that both light-dependent and light-independent TIM degradation mechanisms exist, which do not depend on CRY and, presumably, are responsible for the synchronized TIM and PER oscillations in norpA cry double mutants. Here, we reveal that light-synchronized PER oscillations in the four PDF+ s-LNvs and the PDF− fifth s-LNv are abolished by blocking norpA- and cry-independent photoreception. In other words, we show that Rh1, Rh5, Rh6, and Gq are part of the CRY-independent molecular resetting mechanism targeting the s-LNv pacemaker neurons. Interestingly, CRY-independent light-synchronized oscillations in the fifth s-LNv, but not in the PDF+ s-LNvs, depend on PDF signaling [30, 32, 45]. This means that the rhodopsin- and Gq-dependent (but norpA-independent) mechanism signals light information most likely directly to the PDF+ s-LNv, resulting in CRY-independent molecular clock resetting in all s-LNvs.
Role of PLC21C in Rhodopsin-Mediated Phototransduction
We show here that the norpA-independent pathway depends on Gq, which is also central to the canonical phototransduction pathway. This means that downstream of Gq activation by Rh5, Rh6, and Rh1, alternative effector molecules signal light information to the circadian clock neurons. Our data suggest that the alternative Gq target is PLC21C, the second PLC-β encoded in the fly genome [39]. This is a surprising finding, because PLC21C has not previously been implicated in photoreceptor function, although it has been shown to function in olfactory signal transduction in the antennae downstream of Gq [40]. In addition, Plc21c is expressed in the CNS and, presumably, within subsets of the clock neurons (the PDF expressing LNv), where this enzyme has recently been implicated in light resetting of the circadian clock by brief light pulses [26]. Ni et al. (2017) suggest that PLC21C is activated by Rh7, which, they show, is expressed in the LNv contributing to clock resetting after exposure to brief light pulses. Nevertheless, Rh7 mutants do not show any re-synchronization defects when exposed to shifted white-light LD cycles, as we applied here [27]. Rh7 mutants also did not increase the synchronization deficits of single cry or norpA cry double mutants [27], showing that Rh7 does not contribute to norpA-independent light resetting. Moreover, PLC21C has been shown to act downstream of Go in LNvs to set the (light-independent) free-running period length to 24 hr in response to GABAergic input [48]. Considering the LNv-specific and light-independent functions associated with PLC21C, combined with its known role in olfactory sensory neurons, the new role for this enzyme in the norpA-independent light-input pathway was unexpected and warrants further investigation.
Which Photoreceptor Cells Utilize the Novel Transduction Pathway?
Based on our behavior and molecular light-resetting results, it is likely that the Rh1-expressing photoreceptor cells R1–R6, as well as the Rh5- and Rh6-expressing R8 photoreceptors, contribute to norpA-independent clock resetting. It is also possible that the R7 cells expressing the UV-sensitive opsins Rh3 and Rh4, as well as blue-light-sensitive Rh2-expressing ocelli, participate in norpA-independent light synchronization, but to test their contribution, experiments need to be performed with the specific light spectra in contrast to the white LED light regime used in the present study.
The neuronal connection between the retinal photoreceptors and the circadian clock neurons is currently unknown, but presumably is indirect; e.g [12]. In addition to the R8 cells, 4 photoreceptive cells constituting the H-B eyelet express Rh6 and have been implicated in light signaling to the circadian clock [20, 23, 25, 28]. In fact, a recent study revealed direct synaptic contacts between the H-B eyelet axons and the dendritic fields of the LNv neurons [35]. Moreover, artificial excitation of all Rh6-expressing cells resulted in Ca2+ and cyclic AMP (cAMP) increases in the PDF+ s-LNv, but not the l-LNv, consistent with our results showing that Rh6 contributes to molecular clock resetting in the s-LNv [35] (Figure 3). Therefore, we conclude that, most likely, both retinal and H-B eyelet photoreceptors utilize norpA-independent light signaling, targeting the s-LNv.
norpA-Independent Features of the ERG Responses
Removal of Rh5 and Rh6 function strongly attenuated the residual hyperpolarizing component of the ERG responses of norpA mutants to bright white-light flashes, suggesting that the R8 cells substantially contribute to the remaining light sensitivity in the absence of norpA. It also suggests that this remaining sensitivity contributes to both behavioral and molecular light resetting of the circadian clock (Figures 2 and 3). Removal of Gq also affected the norpA ERG: although the kinetics and degree of the initial negative response, reflecting depolarization of the (R8) photoreceptor cells, were similar in norpA single and norpA Gq double mutants, responses lasted considerably longer in the single mutants (Figures 4B and 4D). Although we would have expected a stronger effect on the initial response, our results show that a Gq-dependent component contributes to the residual light responses of norpA mutants, and it is possible that this Gq function correlates with the role of Gq in behavioral and molecular synchronization (Figure 2, Figure 3). The weaker effects (compared to Rh5 and Rh6 removal) could be explained by the fact that the Gq1 allele is a severe hypomorph [49], whereas Rh52 and Rh61 alleles are true loss-of-function mutants. Also, we cannot rule out the possibility that part of the residual norpA ERG sensitivity is independent of Gq, although the severe phenotype observed in Plc21C mutants suggests that it solely depends on Gq activation by Rh5 and Rh6.
Requirement of Rh1, Rh5, and Rh6 for Molecular and Behavioral Synchronization
Surprisingly, removal of Rh1 did not noticeably alter the residual norpA ERG response (Figure 4B), suggesting that the fly’s major rhodopsin signals almost exclusively via norpA-encoded PLC-β. But why did we observe a norpA-independent function for Rh1 in behavioral and molecular synchronization (Figures 2 and 3)? The ninaE17 cryb and Gq1 cryb flies also carry the spineless1 (ss1) allele, and loss-of-function ss alleles have been shown to drastically reduce Rh4 and Rh6 expression in R7 and R8 cells, respectively [50]. In principle, this raises the possibility that the lack of synchronization observed in both genotypes could be caused by a reduction in Rh6 rather than the loss of Rh1 or reduction in Gq. However, ss1 is a weak allele, and although Rh6 expression has not been investigated, it shows only a mild reduction of Rh4 expression in R7 cells (data not shown); also, it does not show the antennal transformations and tarsal deletions associated with strong ss alleles [51]. In addition, we found no difference in the slow-synchronization phenotype between norpAP41 cryb mutants with and without ss1 (Figure S3), unlike norpAP41 Rh61 cryb mutants, which show a decrease in synchronization speed compared to norpAP41 cryb [20, 29]. Taken together, we are therefore confident that the behavioral and molecular synchronization defects observed in norpAP41 ninaE17 ss1 cryb and norpAP41 Gq1 ss1 cryb flies are, indeed, caused by the lack or reduction of Rh1 and Gq, respectively.
Although we see a correlation between the circadian assays and the ERG recordings with regard to Rh5, Rh6, Gq, and PLC21C function, it is questionable to what extent results obtained from the two assays are related. For example, ERGs are responses measured to a 2-s flash of bright light, whereas the circadian assays apply 12-hr:12-hr ramped LD cycles, with light intensities ranging from 0 to 180 lux. There could, however, be a physiologically more interesting explanation for the discrepancy that we observe between the experiments involving removal of Rh1. Namely, the lack of molecular and behavioral synchronization suggests that that integration of signals from both R1–R6 and R8 is required for circadian clock entrainment to occur. This might be implemented either by a common downstream interneuron or, for example, by R1–R6 signals being fed into R8, which would then, in effect, also be acting as an interneuron (although these signals would presumably have to be “silent” at the level of the ERG to explain the lack of effect in the norpAP41 ninaE17 double mutant). Such a situation would have interesting parallels with circadian entrainment in mammals, which is mediated both by melanopsin-expressing, intrinsically light-sensitive retinal ganglion cells (RGCs) and by the rods and cones [42]. Interestingly, the RGCs act both as photoreceptor cells and as interneurons for propagating the rod and cone signals.
Conclusions
We have shown that non-canonical phototransduction involving Rh1, Rh5, Rh6, Gq, and PLC21C can contribute to circadian clock resetting in Drosophila in the absence of NORPA. This novel pathway targets a specific subgroup of clock neurons, the s-LNvs, and synchronizes clock protein oscillations in these cells independent of the cell-autonomous circadian photoreceptor CRY. Remarkably, except for Rh1, the same factors also contribute to residual, norpA-independent, visual system light sensitivity to brief flashes of light, highlighting the versatility and biological significance of this novel pathway.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-PERIOD | Stanewsky lab, University of Münster [52], | N/A |
| monoclonal mouse anti-PDF C7 | DSHB | Cat# PDF C7, RRID:AB_760350 |
| goat anti-rabbit AlexaFluor 488 nm | Molecular Probes | Cat# A-11034, RRID:AB_2576217 |
| goat anti mouse AlexaFluor 647 nm | Molecular Probes | Cat# A32728, RRID:AB_2633277 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| RNAlater | Ambion | Cat# AM7020 |
| Vectashield | VectorLabs | Cat# H-1000 |
| Critical Commercial Assays | ||
| RNeasy kit | QIAGEN | Cat# 74104 |
| RT-PCR Kit | Applied Biosystems | Cat# 4368813 |
| Experimental Models: Organisms/Strains | ||
| D. melanogaster: y w | Stanewsky lab, University of Münster [4], | N/A |
| D. melanogaster: y w;;ss1cryb | Stanewsky lab; University of Münster [4], | N/A |
| D. melanogaster: y w;; cryb | Rouyer lab, CNRS, Gif-sur-Yvette [28], | N/A |
| D. melanogaster: ninaE17 | Helfrich-Förster lab, University of Würzburg [53], | N/A |
| D. melanogaster: Rh52 | Desplan lab, New York University [54], | N/A |
| D. melanogaster: Rh61 | Desplan lab, New York University [55], | N/A |
| D. melanogaster: Gq1 | Hasan lab, National Centre for Biological Sciences, Bangalore [49], | N/A |
| D. melanogaster: Plc21CP319 | Hasan lab, National Centre for Biological Sciences, Bangalore [40], | N/A |
| Oligonucleotides | ||
| rp49 sense 5′-CGATATGCTAAGCTGTCGCACA-3′ | [56] | N/A |
| rp49 antisense 5′-CGCTTGTTCGATCCGTAACC-3′ | [56] | N/A |
| PLC21C sense 5′-CCGCTTTTGGGGTTCTCTCT-3′ | Stanewsky lab, University of Münster, this paper | N/A |
| PLC21C antisense 5′-TCTGGTCGACCCAGTAG AGG-3′ |
Stanewsky lab, University of Münster, this paper | N/A |
| Software and Algorithms | ||
| MATLAB | Mathworks, Inc | Version 2014a |
| Flytoolbox | Levine Lab, University of Toronto [57], | stanewsky@uni-muenster.de |
| FIJI | ImageJ | https://imagej.nih.gov/ij/ |
| GIMP | GNU Image Manipulation Program | https://www.gimp.org/ |
| LED controller | [58] | https://github.com/PolygonalTree/Led-control |
| pClamp | Molecular Devices | Version 10 |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ralf Stanewsky (stanewsky@uni-muenster.de)
Experimental Model and Subject Details
Flies
Flies were raised with a 12-h:12-h LD cycle on standard Drosophila medium (0.7% agar, 1.0% soy flour, 8.0% polenta/maize, 1.8% yeast, 8.0% malt extract, 4.0% molasses, 0.8% propionic acid, 2.3% nipagen) at 25°C and were collected ∼3–5 d post eclosion. y w flies, as well as norpAP41 and cryb flies have been described previously [4]. The Rh52 and Rh61 alleles harbor intragenic deletions of coding sequences [54, 55], while Gq1 is a strong hypomorph [49]. The loss-of-function allele ninaE17 removes large parts (1.6 kb) of the Rh1 DNA coding region [53]. The hypomorphic allele Plc21CP319 harbors a P-element insertion in the first intron of the Plc21C gene resulting in blunted antennal olfactory responses and reduced Plc21C mRNA levels [40].
Method Details
Behavior analysis
3 to 5 day old male flies were individually recorded using the Drosophila Activity Monitor System (DAM2; Trikinetics) in tubes containing food consisting of 2% agar and 4% sucrose. The monitors were located inside a temperature-controlled incubator, where cold white LED strips (5050 surface-mount device cool white LED strips, 3000-4000 K, Figure S1B) were attached. The LEDs were controlled using an Arduino with a custom made shield, enabling the Arduino to switch the lights on and off in conjunction with an open source desktop app [58]. As shown in Figure S1 lights were ramped for 2 hours from 0 to 60 μWatt/cm2 (∼180 lux) at the beginning of the day and in the opposite direction in the evening. For the first 4 days, the flies were kept at the same regime that they were raised in, with the addition of a temperature cycle of 25°C during ‘lights on’ and 16°C during ‘lights off’. On the last day before the change, the temperature was kept constant at 25°C. On that day, the night was prolonged by 6 hours. The flies were in the new regime for 7 days, after which they were released into DD for another 5 days. The actograms and histograms were generated in MATLAB using the flytoolbox [57]. The histograms of the second LD phase were done from the data of the last 3 days, to avoid any transients. The quantification of the phase was done as in [28], where in a custom made Excel macro, individual histograms for each day were assessed to determine the evening activity peak. The averages, SEM and plotting of the results were done in R.
Electroretinogram recordings
Electroretinograms (ERG) were recorded as described previously (e.g [59]) from young (2-5 days old) dark-reared flies of either sex immobilized with low melting point wax in truncated pipette tips. Recordings were made with low resistance (∼10 MΩ) glass microelectrodes filled with fly Ringer (140 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 4 mM MgCl2) ‘punched’ through the cornea into the retina, with a similar electrode inserted into the head capsule near the ocelli as reference. Light stimulation came from a “warm white” power LED (Cairn Research, Figure S1C) delivered to within ∼5 mm of the eye via a liquid-filled light guide (diameter 5 mm). Intensity of the stimulus was approximately 107 effectively absorbed photons per second per photoreceptor. All flies were on a white-eyed (w1118) background.
Immunohistochemistry
To study PER levels, immunohistochemical analysis were performed as previously described [60]. Briefly, prior to collection, young adult males were entrained for 3 days under the same 12:12 hr LD conditions as were used for the behavior experiments. At the determined time points the flies were fixed in 4% PFA for 4h at 4°C. After fixation, the samples were washed 6 times with 0.1 M phosphate buffer (pH 7.4) with 0.1% Triton X-100 (PBS-T) at room temperature (RT). Brains were dissected in PBS, were then blocked with 10% goat serum in 0.5% PBS-T for 2 hr at RT and stained with pre-absorbed rabbit anti-PER (1:1000) [52] and monoclonal mouse anti-PDF C7 (DSHB, 1:200) in 10% goat serum in 0.5% PBST for at least 48h at 4°C. After washing 3 times by PBS-T, the samples were incubated at 4°C overnight with goat anti-rabbit AlexaFluor 488 nm (1:250) and goat anti mouse AlexaFluor 647 nm (Molecular Probes) in PBS-T. Brains were washed 3 times in PBS-T before being mounted in Vectashield. The images were taken using a Leica SP8 confocal microscope, keeping the same conditions for each experiment. Clock neurons were identified either directly (s-LNv and l-LNv) via expression of the neuropeptide PDF, or by their position within the brain with respect to the PDF+ LNv cell bodies and PDF processes projecting from the s-LNv into the dorsal protocerebrum (for 5th-LNv, LNd, DN1, and DN2). For time course quantification, pixel intensity of mean and background staining in each neuronal group was measured by FIJI [61]. For each cell three measurements were taken, as well as 3 measurements of the background for the corresponding slice. The data were analyzed and represented using R. After background subtraction, the measurements were normalized to the values of each of the neuronal groups obtained for y w at ZT22. The images shown in Figure 3 A and B were processed with GIMP.
RNA isolation and RT-PCR
To extract the RNA, 20-50 flies of the indicated genotype were collected in 2 mL RNAlater (Ambion) supplemented with 100 μl 0.1% PBST to improve RNAlater penetration. Flies were incubated overnight at 4°C, before 30 retinas and 20 brains were dissected in cold RNAlater. Total RNA was extracted using the RNeasy kit (QIAGEN) following the manufacturer’s instructions. The eluted RNA was immediately used for cDNA synthesis using the Reverse Transcription Reagents Kit (Applied Biosystems) using 1 μg of total RNA as a template. The cDNA was diluted and amplified via PCR with the following oligonucleotides: rp49 sense 5′-CGATATGCTAAGCTGTCGCACA-3′, rp49 antisense 5′-CGCTTGTTCGATCCGTAACC-3′ (as in [56]), PLC21C sense 5′-CCGCTTTTGGGGTTCTCTCT-3′ and PLC21C antisense 5′-TCTGGTCGACCCAGTAGAGG-3′. Bands were separated on 2% agarose gels and their intensities measured using ImageJ FIJI plugin and normalized against the intensity value of rp49.
Quantification and Statistical Analysis
Statistical significance was calculated in R by one-way ANOVA followed by Tukey’s test for post hoc analysis (Figures 2E, 3C–E, 5D, S2), or by Dunnet’s multiple comparison (Figures 4C, 4D, 5F).
Data and Software Availability
Raw data and locomotor activity analysis code will be provided upon request by Lead Contact, Ralf Stanewsky (stanewsky@uni-muenster.de)
Acknowledgments
We thank Charlotte Helfrich-Förster, Gaiti Hasan, Claude Desplan, and Francois Rouyer for fly stocks and Luis Garcia for developing the programmable LED system. This work was supported by Biotechnology and Biological Sciences Research Council grant BB/J018589/2 and by the European Union (FP7 Integrated Training Network INsecTIME: PITN-GA-2012-316790 to R.S. and a Marie Curie WHRI-Academy COFUND fellowship: PCOFUND-GA-2013-608765 to M.O.) and a Biotechnology and Biological Sciences Research Council grant BB/M007006/1 to R.C.H.
Author Contributions
M.O. performed, analyzed, and interpreted all behavioral and immunohistological experiments. R.C.H. performed, analyzed, and interpreted all ERG experiments. R.S. designed and interpreted experiments and wrote the paper (with help from M.O. and R.C.H).
Declaration of Interests
The authors declare no competing interests.
Published: May 17, 2018
Footnotes
Supplemental Information includes three figures and one table and can be found with this article online at https://doi.org/10.1016/j.cub.2018.04.016.
Supplemental Information
References
- 1.Chaix A., Zarrinpar A., Panda S. The circadian coordination of cell biology. J. Cell Biol. 2016;215:15–25. doi: 10.1083/jcb.201603076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardin P.E. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv. Genet. 2011;74:141–173. doi: 10.1016/B978-0-12-387690-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emery P., So W.V., Kaneko M., Hall J.C., Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 4.Stanewsky R., Kaneko M., Emery P., Beretta B., Wager-Smith K., Kay S.A., Rosbash M., Hall J.C. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 5.Busza A., Emery-Le M., Rosbash M., Emery P. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science. 2004;304:1503–1506. doi: 10.1126/science.1096973. [DOI] [PubMed] [Google Scholar]
- 6.Czarna A., Berndt A., Singh H.R., Grudziecki A., Ladurner A.G., Timinszky G., Kramer A., Wolf E. Structures of Drosophila cryptochrome and mouse cryptochrome1 provide insight into circadian function. Cell. 2013;153:1394–1405. doi: 10.1016/j.cell.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Dissel S., Codd V., Fedic R., Garner K.J., Costa R., Kyriacou C.P., Rosato E. A constitutively active cryptochrome in Drosophila melanogaster. Nat. Neurosci. 2004;7:834–840. doi: 10.1038/nn1285. [DOI] [PubMed] [Google Scholar]
- 8.Koh K., Zheng X., Sehgal A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science. 2006;312:1809–1812. doi: 10.1126/science.1124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peschel N., Veleri S., Stanewsky R. Veela defines a molecular link between Cryptochrome and Timeless in the light-input pathway to Drosophila’s circadian clock. Proc. Natl. Acad. Sci. USA. 2006;103:17313–17318. doi: 10.1073/pnas.0606675103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peschel N., Chen K.F., Szabo G., Stanewsky R. Light-dependent interactions between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless. Curr. Biol. 2009;19:241–247. doi: 10.1016/j.cub.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Peschel N., Helfrich-Förster C. Setting the clock--by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 2011;585:1435–1442. doi: 10.1016/j.febslet.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Muraro N.I., Ceriani M.F. Acetylcholine from Visual Circuits Modulates the Activity of Arousal Neurons in Drosophila. J. Neurosci. 2015;35:16315–16327. doi: 10.1523/JNEUROSCI.1571-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parisky K.M., Agosto J., Pulver S.R., Shang Y., Kuklin E., Hodge J.J., Kang K., Liu X., Garrity P.A., Rosbash M., Griffith L.C. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang Y., Griffith L.C., Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc. Natl. Acad. Sci. USA. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheeba V., Fogle K.J., Kaneko M., Rashid S., Chou Y.-T., Sharma V.K., Holmes T.C. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr. Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renn S.C., Park J.H., Rosbash M., Hall J.C., Taghert P.H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 17.Grima B., Chélot E., Xia R., Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 18.Stoleru D., Peng Y., Agosto J., Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 19.Beckwith E.J., Ceriani M.F. Communication between circadian clusters: The key to a plastic network. FEBS Lett. 2015;589:3336–3342. doi: 10.1016/j.febslet.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Helfrich-Förster C., Winter C., Hofbauer A., Hall J.C., Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249–261. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 21.Rieger D., Stanewsky R., Helfrich-Förster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J. Biol. Rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- 22.Saint-Charles A., Michard-Vanhée C., Alejevski F., Chélot E., Boivin A., Rouyer F. Four of the six Drosophila rhodopsin-expressing photoreceptors can mediate circadian entrainment in low light. J. Comp. Neurol. 2016;524:2828–2844. doi: 10.1002/cne.23994. [DOI] [PubMed] [Google Scholar]
- 23.Veleri S., Rieger D., Helfrich-Förster C., Stanewsky R. Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J. Biol. Rhythms. 2007;22:29–42. doi: 10.1177/0748730406295754. [DOI] [PubMed] [Google Scholar]
- 24.Salcedo E., Huber A., Henrich S., Chadwell L.V., Chou W.-H., Paulsen R., Britt S.G. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J. Neurosci. 1999;19:10716–10726. doi: 10.1523/JNEUROSCI.19-24-10716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helfrich-Förster C., Edwards T., Yasuyama K., Wisotzki B., Schneuwly S., Stanewsky R., Meinertzhagen I.A., Hofbauer A. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J. Neurosci. 2002;22:9255–9266. doi: 10.1523/JNEUROSCI.22-21-09255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni J.D., Baik L.S., Holmes T.C., Montell C. A rhodopsin in the brain functions in circadian photoentrainment in Drosophila. Nature. 2017;545:340–344. doi: 10.1038/nature22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kistenpfennig C., Grebler R., Ogueta M., Hermann-Luibl C., Schlichting M., Stanewsky R., Senthilan P.R., Helfrich-Förster C. A New Rhodopsin Influences Light-dependent Daily Activity Patterns of Fruit Flies. J. Biol. Rhythms. 2017;32:406–422. doi: 10.1177/0748730417721826. [DOI] [PubMed] [Google Scholar]
- 28.Szular J., Sehadova H., Gentile C., Szabo G., Chou W.-H.H., Britt S.G., Stanewsky R. Rhodopsin 5- and Rhodopsin 6-mediated clock synchronization in Drosophila melanogaster is independent of retinal phospholipase C-β signaling. J. Biol. Rhythms. 2012;27:25–36. doi: 10.1177/0748730411431673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emery P., Stanewsky R., Helfrich-Förster C., Emery-Le M., Hall J.C., Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 30.Cusumano P., Klarsfeld A., Chélot E., Picot M., Richier B., Rouyer F. PDF-modulated visual inputs and cryptochrome define diurnal behavior in Drosophila. Nat. Neurosci. 2009;12:1431–1437. doi: 10.1038/nn.2429. [DOI] [PubMed] [Google Scholar]
- 31.Yoshii T., Hermann-Luibl C., Kistenpfennig C., Schmid B., Tomioka K., Helfrich-Förster C. Cryptochrome-dependent and -independent circadian entrainment circuits in Drosophila. J. Neurosci. 2015;35:6131–6141. doi: 10.1523/JNEUROSCI.0070-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Lear B.C., Seluzicki A., Allada R. The CRYPTOCHROME photoreceptor gates PDF neuropeptide signaling to set circadian network hierarchy in Drosophila. Curr. Biol. 2009;19:2050–2055. doi: 10.1016/j.cub.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen W.L., Kwon Y., Adegbola A.A., Luo J., Chess A., Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- 34.Yoshii T., Vanin S., Costa R., Helfrich-Förster C. Synergic entrainment of Drosophila’s circadian clock by light and temperature. J. Biol. Rhythms. 2009;24:452–464. doi: 10.1177/0748730409348551. [DOI] [PubMed] [Google Scholar]
- 35.Schlichting M., Menegazzi P., Lelito K.R., Yao Z., Buhl E., Dalla Benetta E., Bahle A., Denike J., Hodge J.J., Helfrich-Förster C., Shafer O.T. A Neural Network Underlying Circadian Entrainment and Photoperiodic Adjustment of Sleep and Activity in Drosophila. J. Neurosci. 2016;36:9084–9096. doi: 10.1523/JNEUROSCI.0992-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzotta G., Rossi A., Leonardi E., Mason M., Bertolucci C., Caccin L., Spolaore B., Martin A.J., Schlichting M., Grebler R. Fly cryptochrome and the visual system. Proc. Natl. Acad. Sci. USA. 2013;110:6163–6168. doi: 10.1073/pnas.1212317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearn M.T., Randall L.L., Shortridge R.D., Burg M.G., Pak W.L. Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutants. J. Biol. Chem. 1996;271:4937–4945. doi: 10.1074/jbc.271.9.4937. [DOI] [PubMed] [Google Scholar]
- 38.Hardie R.C., Martin F., Chyb S., Raghu P. Rescue of light responses in the Drosophila “null” phospholipase C mutant, norpAP24, by the diacylglycerol kinase mutant, rdgA, and by metabolic inhibition. J. Biol. Chem. 2003;278:18851–18858. doi: 10.1074/jbc.M300310200. [DOI] [PubMed] [Google Scholar]
- 39.Shortridge R.D., Yoon J., Lending C.R., Bloomquist B.T., Perdew M.H., Pak W.L. A Drosophila phospholipase C gene that is expressed in the central nervous system. J. Biol. Chem. 1991;266:12474–12480. [PubMed] [Google Scholar]
- 40.Kain P., Chakraborty T.S., Sundaram S., Siddiqi O., Rodrigues V., Hasan G. Reduced odor responses from antennal neurons of G(q)alpha, phospholipase Cbeta, and rdgA mutants in Drosophila support a role for a phospholipid intermediate in insect olfactory transduction. J. Neurosci. 2008;28:4745–4755. doi: 10.1523/JNEUROSCI.5306-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Millar A.J. Input signals to the plant circadian clock. J. Exp. Bot. 2004;55:277–283. doi: 10.1093/jxb/erh034. [DOI] [PubMed] [Google Scholar]
- 42.Lucas R.J., Lall G.S., Allen A.E., Brown T.M. How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Prog. Brain Res. 2012;199:1–18. doi: 10.1016/B978-0-444-59427-3.00001-0. [DOI] [PubMed] [Google Scholar]
- 43.Benito J., Houl J.H., Roman G.W., Hardin P.E. The blue-light photoreceptor CRYPTOCHROME is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J. Biol. Rhythms. 2008;23:296–307. doi: 10.1177/0748730408318588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshii T., Todo T., Wülbeck C., Stanewsky R., Helfrich-Förster C. Cryptochrome is present in the compound eyes and a subset of Drosophila’s clock neurons. J. Comp. Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- 45.Im S.H., Li W., Taghert P.H. PDFR and CRY signaling converge in a subset of clock neurons to modulate the amplitude and phase of circadian behavior in Drosophila. PLoS ONE. 2011;6:e18974. doi: 10.1371/journal.pone.0018974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo F., Cerullo I., Chen X., Rosbash M. PDF neuron firing phase-shifts key circadian activity neurons in Drosophila. eLife. 2014;3:e02780. doi: 10.7554/eLife.02780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grima B., Dognon A., Lamouroux A., Chélot E., Rouyer F. CULLIN-3 controls TIMELESS oscillations in the Drosophila circadian clock. PLoS Biol. 2012;10:e1001367. doi: 10.1371/journal.pbio.1001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahdal D., Reeves D.C., Ruben M., Akabas M.H., Blau J. Drosophila pacemaker neurons require g protein signaling and GABAergic inputs to generate twenty-four hour behavioral rhythms. Neuron. 2010;68:964–977. doi: 10.1016/j.neuron.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott K., Becker A., Sun Y., Hardy R., Zuker C. Gq α protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron. 1995;15:919–927. doi: 10.1016/0896-6273(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 50.Wernet M.F., Mazzoni E.O., Çelik A., Duncan D.M., Duncan I., Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gramates L.S., Marygold S.J., Santos G.D., Urbano J.-M., Antonazzo G., Matthews B.B., Rey A.J., Tabone C.J., Crosby M.A., Emmert D.B., the FlyBase Consortium FlyBase at 25: looking to the future. Nucleic Acids Res. 2017;45(D1):D663–D671. doi: 10.1093/nar/gkw1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanewsky R., Frisch B., Brandes C., Hamblen-Coyle M.J., Rosbash M., Hall J.C. Temporal and spatial expression patterns of transgenes containing increasing amounts of the Drosophila clock gene period and a lacZ reporter: mapping elements of the PER protein involved in circadian cycling. J. Neurosci. 1997;17:676–696. doi: 10.1523/JNEUROSCI.17-02-00676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Tousa J.E., Baehr W., Martin R.L., Hirsh J., Pak W.L., Applebury M.L. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi S., Wolf R., Desplan C., Heisenberg M. Motion vision is independent of color in Drosophila. Proc. Natl. Acad. Sci. USA. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cook T., Pichaud F., Sonneville R., Papatsenko D., Desplan C. Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev. Cell. 2003;4:853–864. doi: 10.1016/s1534-5807(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 56.Sehadova H., Glaser F.T., Gentile C., Simoni A., Giesecke A., Albert J.T., Stanewsky R. Temperature entrainment of Drosophila’s circadian clock involves the gene nocte and signaling from peripheral sensory tissues to the brain. Neuron. 2009;64:251–266. doi: 10.1016/j.neuron.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 57.Levine J.D., Funes P., Dowse H.B., Hall J.C. Advanced analysis of a cryptochrome mutation’s effects on the robustness and phase of molecular cycles in isolated peripheral tissues of Drosophila. BMC Neurosci. 2002;3:5. doi: 10.1186/1471-2202-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogueta M., Garcia Rodriguez L. Open Source LED controller for circadian experiments. bioRxiv. 2017 [Google Scholar]
- 59.Satoh A.K., Xia H., Yan L., Liu C.-H., Hardie R.C., Ready D.F. Arrestin translocation is stoichiometric to rhodopsin isomerization and accelerated by phototransduction in Drosophila photoreceptors. Neuron. 2010;67:997–1008. doi: 10.1016/j.neuron.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harper R.E.F., Dayan P., Albert J.T., Stanewsky R. Sensory Conflict Disrupts Activity of the Drosophila Circadian Network. Cell Rep. 2016;17:1711–1718. doi: 10.1016/j.celrep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.