Summary
The patterning of tissues to form subdivisions with distinct and homogeneous regional identity is potentially disrupted by cell intermingling. Transplantation studies suggest that homogeneous segmental identity in the hindbrain is maintained by identity switching of cells that intermingle into another segment. We show that switching occurs during normal development and is mediated by feedback between segment identity and the retinoic acid degrading enzymes, cyp26b1 and cyp26c1. egr2, which specifies the segmental identity of rhombomeres r3 and r5, underlies the lower expression level of cyp26b1 and cyp26c1 in r3 and r5 compared with r2, r4, and r6. Consequently, r3 or r5 cells that intermingle into adjacent segments encounter cells with higher cyp26b1/c1 expression, which we find is required for downregulation of egr2b expression. Furthermore, egr2b expression is regulated in r2, r4, and r6 by non-autonomous mechanisms that depend upon the number of neighbors that express egr2b. These findings reveal that a community regulation of retinoid signaling maintains homogeneous segmental identity.
Keywords: hindbrain segmentation, regional identity, egr2, cell intermingling, cyp26, retinoic acid, community effect, cell identity switching, cell segregation, boundary formation
Graphical Abstract
Highlights
-
•
Some cells intermingle between hindbrain segments and switch identity
-
•
Identity is influenced by the number of neighbors with same or different identity
-
•
Switching is mediated by feedback between segment identity and retinoid signaling
-
•
Community retinoid signaling enables maintenance of homogeneous segments
Addison et al. uncover how segments within the hindbrain maintain a homogeneous identity despite intermingling of cells at early stages. Cells that intermingle into an adjacent segment switch identity since they encounter a different level of retinoic acid signaling from their new neighbors.
Introduction
The complex organization of the adult body arises during development by formation of distinct tissues at different locations, many of which are then further subdivided into domains with a specific regional identity. Such regionalization occurs along the anterior-posterior (A-P) axis of the vertebrate central nervous system to form subdivisions that are demarcated by sharp borders. A-P patterning of the neural epithelium is achieved through graded cell signaling, mediated by retinoic acid (RA) and members of the Fgf and Wnt families (Tuazon and Mullins, 2015), which regulates the spatial expression of transcription factors that specify regional identity (Parker and Krumlauf, 2017, Tumpel et al., 2009). However, at early stages the borders between different subdivisions are ragged and there can be overlapping expression of transcription factors that confer different identities. This imprecision is likely due in part to variability in the formation and interpretation of gradients of signals. In addition, the proliferation and intercalation of cells during tissue growth and morphogenesis can potentially scramble the pattern by causing intermingling of cells between adjacent regions. This raises the questions of how, despite these challenges, a sharp border forms at the interface of subdivisions, and each subdivision acquires a homogeneous regional identity. Insights into underlying mechanisms have come from studies of the vertebrate hindbrain.
The hindbrain is subdivided into seven segments, termed rhombomeres (r1–r7), which underlie the organization and A-P specification of neurons and branchial neural crest cells (Lumsden and Krumlauf, 1996). Regionalization of the hindbrain is established by graded Fgf and RA signaling, which regulates the spatial expression of a network of transcription factors that underlie the formation and A-P identity of hindbrain segments, including Egr2 (Krox20), MafB, and Hox family members (Tumpel et al., 2009). Initially, there is some overlap at borders between hoxb1 expression in r4, and egr2 expression in r3 and r5, which is resolved bdevcel_4183_gr4_4c.eps - y mutual repression such that cells express one or the other transcription factor (Giudicelli et al., 2001, Labalette et al., 2015, Zhang et al., 2012). The borders of egr2 expression in r3 and r5 are ragged when first detected, and then progressively become sharp and straight (Cooke and Moens, 2002, Irving et al., 1996, Kemp et al., 2009). This sharpening is driven by signaling between segmentally expressed Eph receptors and ephrins that segregates cells and prevents intermingling across borders (Cooke et al., 2001, Cooke et al., 2005, Kemp et al., 2009, Xu et al., 1995, Xu et al., 1999), potentially through regulation of cell adhesion, tension, and/or repulsion (Calzolari et al., 2014, Cayuso et al., 2015, Fagotto et al., 2014, Taylor et al., 2017). Computer simulations suggest that cell segregation and the resolution of cell identity have synergistic roles in border sharpening (Wang et al., 2017).
A further mechanism required to establish segments with homogeneous identity was suggested by the results of clonal analyses in the chick hindbrain. Once rhombomeres are seen at the morphological level, intermingling of cells is restricted across segment borders, but the progeny of individual cells labeled at earlier stages can contribute to adjacent segments (Fraser et al., 1990). The finding that some intermingling occurs between hindbrain segments implies that cells that move into another segment acquire an identity in accordance with their new A-P location. Direct evidence for an ability of hindbrain cells to switch A-P identity has come from transplantation experiments in mouse and zebrafish embryos. It was found that when single cells are transplanted between hindbrain segments, they downregulate markers of their site of origin and switch to the identity of their new location (Kemp et al., 2009, Schilling et al., 2001, Trainor and Krumlauf, 2000). In zebrafish, cells can switch identity at early stages of segmentation (11.5 hr post fertilization [hpf]), but this plasticity progressively decreases at later stages (14–16.5 hpf) (Schilling et al., 2001). In contrast to single cells, groups of cells transplanted between segments maintain their original identity, suggestive of a community regulation of cell identity (Schilling et al., 2001, Trainor and Krumlauf, 2000). Such community effects have been found in other contexts to be mediated by positive feedback between transcription factors and intercellular signals that regulate cell identity (Bolouri and Davidson, 2010, Buckingham, 2003, Cossu et al., 1995, Gurdon, 1988, Standley et al., 2001). Through non-autonomous induction of transcription factor expression, this feedback promotes a homogeneous identity within a field of cells (Bolouri and Davidson, 2010). Interestingly, mosaic overexpression of egr2 in the chick hindbrain induces egr2 expression in neighboring cells (Giudicelli et al., 2001), but the molecular basis of this non-autonomous induction is not known.
The findings from transplantation experiments have led to the idea that cell identity switching could act in parallel with cell segregation to establish sharp and homogeneous segments (Cooke and Moens, 2002, Pasini and Wilkinson, 2002). However, it is unclear to what extent intermingling of cells between segments occurs during normal development. egr2 has a key role in hindbrain segmentation through specification of r3 and r5 identity (Schneider-Maunoury et al., 1993, Voiculescu et al., 2001) and is a direct transcriptional regulator of ephA4 (Theil et al., 1998), which underlies cell segregation (Cooke et al., 2005, Xu et al., 1995, Xu et al., 1999). It is therefore likely that intermingling between segments is confined to the time period before there has been sufficient upregulation of EphA4 to drive cell segregation. Consistent with findings in chick (Fraser et al., 1990), some isolated cells expressing egr2 or egr2-cre reporter are detected in even-numbered segments in the mouse hindbrain (Irving et al., 1996, Voiculescu et al., 2001). However, recent work has suggested that there is no intermingling between hindbrain segments in zebrafish, and therefore cell identity switching does not occur (Calzolari et al., 2014). In this study, tracking of cells in time-lapse movies from 11 hpf did not detect intermingling between segments, and fluorescent reporter expression driven downstream of egr2 was not detected in any cells in adjacent segments (Calzolari et al., 2014). However, interpretation of these findings may be limited by timing of the analyses, as mechanisms that restrict cell intermingling may already be in place by 11 hpf and prior to detectable expression of the transgenic reporters.
We set out to analyze the role and mechanisms of cell identity switching in establishment of homogeneous segmental identity. By using genome modification to create an early reporter of egr2 expression, we show that cell intermingling and identity switching occurs during hindbrain segmentation in zebrafish. egr2 expression is regulated by a combination of A-P location and non-autonomous mechanisms that depend upon the number of neighbors that express egr2. We uncover a crucial role of RA-degrading enzymes, cyp26b1 and cyp26c1, which we show are regulated by egr2 and are required for identity switching of r3 and r5 cells that intermingle into adjacent segments. These findings reveal that coupling between segment identity and retinoid signaling enables homogeneous segmental identity to be maintained despite intermingling of cells.
Results
Cell Intermingling and Identity Switching Occurs in the Zebrafish Hindbrain
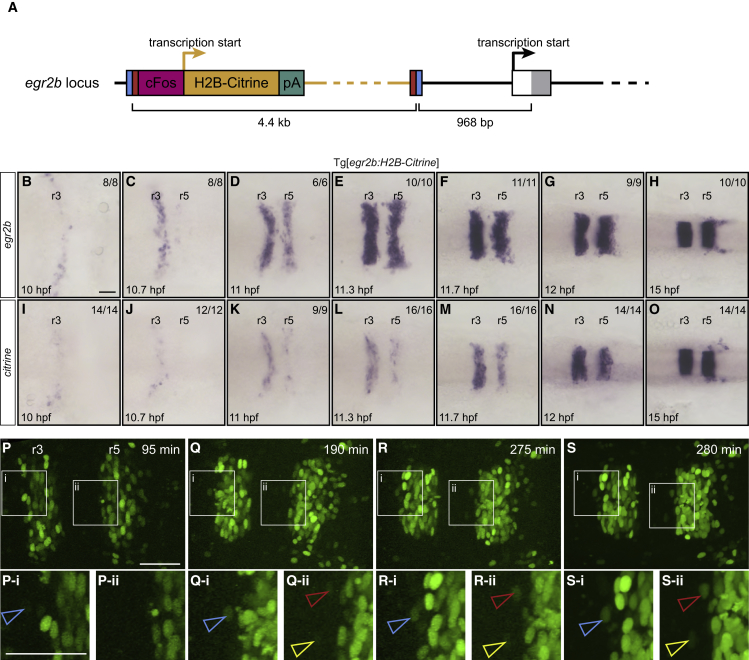
In zebrafish, egr2 is upregulated in prospective r3 and then r5, starting at 10–11 hpf respectively, and the initially ragged borders of egr2 gene expression are sharpened over a 2 hr period (Cooke and Moens, 2002, Kemp et al., 2009, Zhang et al., 2012). Some ectopic egr2-expressing cells are detected in even-numbered segments, which potentially could segregate back to r3 or r5 or switch identity. To create an early-expressed reporter of cells with r3 or r5 identity, we used an enhancer trap strategy in which H2B-Citrine with a minimal promoter was inserted upstream of the egr2b gene by CRISPR/Cas9-mediated recombination (Figure 1A). This was found to drive reporter expression in r3 and r5. To maximize reporter expression, we created a line homozygous for egr2b:H2B-Citrine. In situ hybridization reveals that citrine and egr2b transcripts are concurrently expressed in r3 and r5 from the earliest time that egr2b expression is detected (Figures 1B–1O). Importantly, this analysis reveals that insertion of the reporter gene has not blocked expression of egr2b. Comparison of time-lapse movies with in situ hybridization data reveals that citrine fluorescence is first seen ∼1 hr after detection of egr2 transcripts.
Figure 1.
Generation of egr2b Gene Trap Expressing H2B-Citrine
(A) CRISPR-mediated insertion of a donor construct with cFos minimal promoter and H2B-Citrine upstream of the transcriptional start site of egr2b to generate the Tg[egr2b:H2B-Citrine] line.
(B–O) In situ hybridization to detect egr2b (B–H) and citrine (I–O) transcripts in Tg[egr2b:H2B-Citrine] embryos from 10 to 15 hpf. Embryos are flat-mounted with anterior to the left.
(P–S) Selected frames from a time-lapse movie of a Tg[egr2b:H2B-Citrine] embryo acquired from 12 hpf (t = 0 min) for 280 min. A higher resolution image was captured at the final time point (S). Below each panel is a magnified view of the indicated areas (i, ii), with arrowheads pointing at three examples of H2B-Citrine-expressing cells that are ectopic at the final time point. When first detected, these cells are already surrounded by non-expressing cells. Most of the H2B-Citrine-expressing cells seen posterior to r5 are egr2-expressing neural crest cells. Scale bars: 50 μm.
Due to the high stability of H2B fusions with fluorescent reporters (Brennand et al., 2007, Ninov et al., 2012), H2B-Citrine will perdure in any cells that have intermingled and downregulated egr2b transcripts. Analysis of time-lapse movies reveals the presence of some isolated cells with Citrine fluorescence within even-numbered segments, which have not segregated back to r3 or r5 by 16 hpf (Figure 1). When these cells are back-tracked, they are found to already be surrounded by non-expressing cells at the earliest time point at which Citrine fluorescence can be detected (Figures 1P–1S). This suggests that intermingling into adjacent segments occurs at early stages, during convergent-extension movements in which there is extensive intercalation of cells (Kimmel et al., 1994). Since egr2 expression is being upregulated during this period of convergent extension (Figures 1B–1E), the 1 hr delay in accumulating sufficient Citrine protein for direct detection may limit the ability to detect cells that have intermingled and then downregulated egr2b transcripts, which have a half-life of ∼30 min (Bouchoucha et al., 2013).
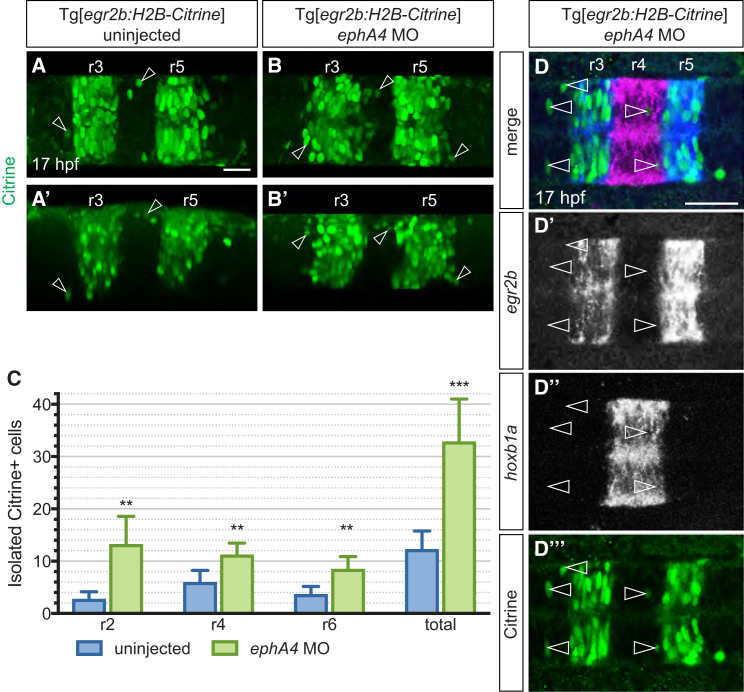
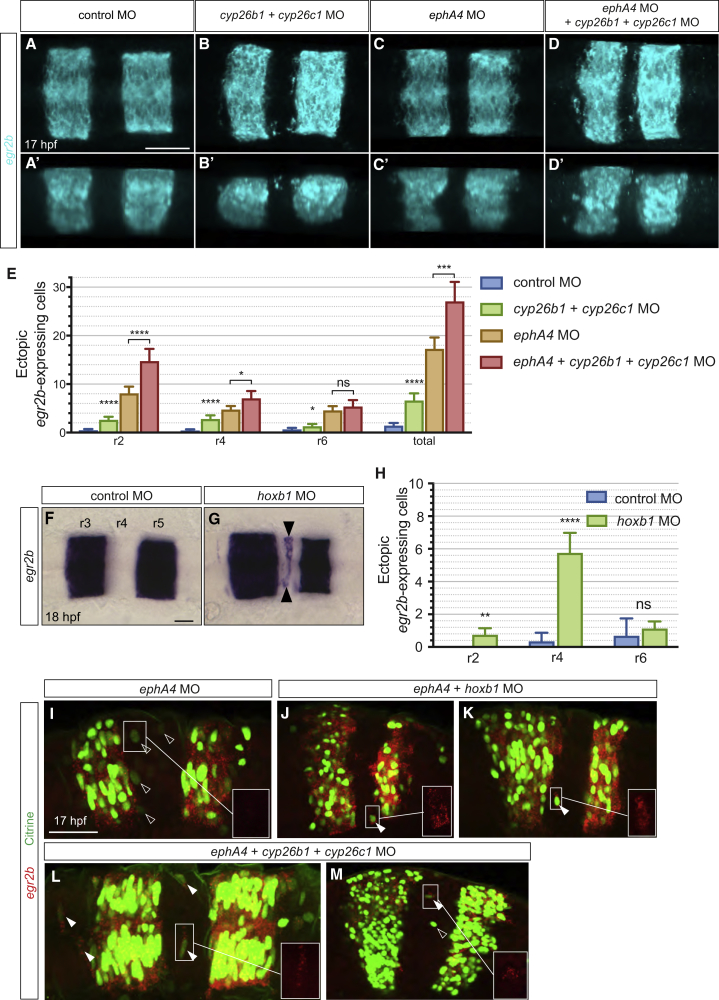
Based on these findings, we increased sensitivity of detection by immunostaining for Citrine protein, and found that at 17 hpf there are 2–6 isolated Citrine-expressing cells per even-numbered segment (Figures 2A and 2C). As predicted from previous studies, knockdown of EphA4 increases cell intermingling between segments, with 8–13 cells expressing Citrine reporter per even-numbered segment (Figures 2B and 2C). To determine whether ectopic Citrine-expressing cells have maintained or changed identity, we carried out in situ hybridization for egr2b and hoxb1a transcripts. We found that Citrine-expressing cells in even-numbered segments do not express egr2b, and when present in r4 express hoxb1a, and have therefore switched to r4 identity (Figure 2D).
Figure 2.
Cell Identity Switching of Ectopic egr2:H2B-Citrine-Expressing Cells
(A and B) Maximum intensity projections of typical uninjected (A) and ephA4 morphant (B) Tg[egr2b:H2B-Citrine] embryos at 17 hpf, with H2B-Citrine protein detected using anti-GFP antibody. Lateral views of the same embryos are shown in (A′) and (B′). Examples of Citrine-positive cells outside r3 and r5 are indicated by arrowheads.
(C) Quantification of isolated ectopic Citrine-positive cells in uninjected and ephA4 morphant embryos. Mean numbers with 95% confidence intervals are shown. Asterisks indicate statistical significance determined by Welsh's t test: r2, p = 0.0014; r4, p = 0.0017; r6, p = 0.0016; total isolated ectopic cells in r2, r4, and r6, p = 0.0002. n = 18 embryos (uninjected), n = 11 embryos (ephA4 morphants).
(D) Double in situ hybridization reveals that isolated Citrine-expressing cells (green in D‴, arrowheads in D, D″) in r2, r4, and r6 of ephA4 morphant Tg[egr2b:H2B-Citrine] embryos have downregulated egr2b transcripts (blue in D, grayscale in D′; 105/121 cells). Citrine-expressing cells located in r4 have upregulated hoxb1a (magenta in D, scale in D″). Single slices from a confocal z stack are shown. Scale bars: 50 μm.
Cell Organization Influences Non-autonomous Induction of egr2b Expression
Our findings reveal that at early stages there is some intermingling of cells expressing egr2b into adjacent segments, and these ectopic cells switch identity. This raises the question of how cell identity switching is regulated. Since the initial pattern of segmental identities is established by graded signaling (Hernandez et al., 2007, White et al., 2007, White and Schilling, 2008), one potential mechanism for switching is that cells respond to the amount of signal present at their new location. However, this model does not explain why groups of transplanted cells do not switch identity (Schilling et al., 2001, Trainor and Krumlauf, 2000). Experiments in chick embryos suggest that egr2 could be a component of a community regulation of cell identity since forced mosaic expression of egr2 in r2, r4, and r6 induces egr2 expression in adjacent cells (Giudicelli et al., 2001). We therefore analyzed whether egr2 expression is influenced by interactions with neighboring cells.
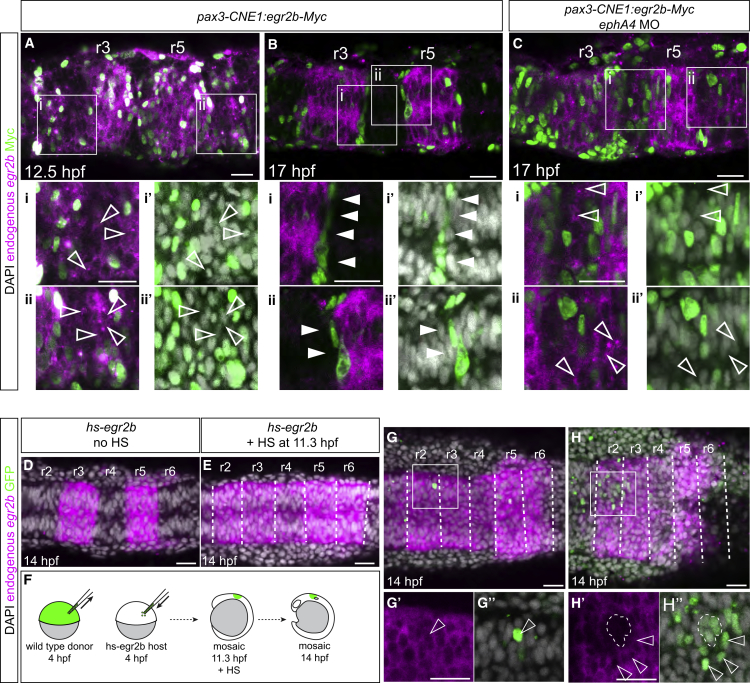
We first tested whether non-autonomous induction of egr2 expression occurs in the zebrafish hindbrain. To achieve sustained expression of myc-tagged Egr2b, we used a pax3 regulatory element (CNE1) that drives gene expression in the dorsal neural epithelium (Moore et al., 2013). Injection of this construct into zebrafish embryos led to mosaic ectopic expression of egr2b. We found that, at 12.5 hpf, cells ectopically expressing egr2b are scattered, and that endogenous egr2b is upregulated in adjacent cells in r2, r4, and r6 (Figure 3A). In contrast, by 17 hpf the egr2b-overexpressing cells have segregated to the borders of even-numbered segments, and non-autonomous induction of egr2b no longer occurs (Figure 3B). One potential explanation for this lack of non-autonomous induction is that it is influenced by the proportion of neighboring cells that express egr2b. Another possibility is that cells no longer respond to signaling from egr2-expressing cells as they have reduced plasticity at the later stage (Schilling et al., 2001). Since segregation of egr2b-expressing cells is mediated by EphA4 (Cooke et al., 2005, Theil et al., 1998, Xu et al., 1995), they can be maintained in a dispersed state by simultaneous knockdown of ephA4. We found that, in this situation, non-autonomous induction of egr2b occurs at 17 hpf (Figure 3C).
Figure 3.
Relationship between Cell Organization and Non-autonomous Induction of egr2 Gene Expression
(A–C) Embryos injected with CNE1:egr2b-Myc and fixed at various time points to detect endogenous egr2b transcripts. At 12 hpf (A), endogenous egr2b gene expression (magenta) is upregulated in cells overexpressing Egr2b-Myc (green nuclei) and non-autonomous upregulation of egr2b is also observed in adjacent cells (hollow arrowheads, Ai and Aii). DAPI staining (grayscale) is shown in (Ai′) and (Aii′). By 17 hpf (B), Egr2b-Myc-expressing cells become segregated to the borders of r3 and r5, and non-autonomous upregulation of egr2b is no longer observed in adjacent cells (Bi and Bii, solid arrowheads). Knockdown of ephA4 (see also Figure S1) in embryos injected with CNE1:egr2b-Myc (C) causes cells overexpressing Egr2b-Myc to remain dispersed throughout the neuroepithelium at 17 hpf and non-autonomous upregulation of egr2b is now observed (Ci and Cii, hollow arrowheads).
(D–H) Transient expression of egr2b by heat shock of Tg[hsp70:egr2b-Myc] embryos (hs-egr2b) at 11.3 hpf induces upregulation of endogenous egr2b in r2, r4, and r6 (E); compare with control embryo shown in (D). H2B-GFP labeled wild-type donor cells were transplanted into hs-egr2b embryos at 4 hpf, which were heat shocked at 11.3 hpf to induce widespread expression of egr2b (F). Wild-type donor cells in r2 upregulate endogenous egr2b non-autonomously when isolated or dispersed (G, H, hollow arrowheads) but not when clustered together, indicated by the region encircled by white dashed line in (H). White dashed lines in (E), (G), and (H) indicate presumptive rhombomere borders. Single slices from a confocal z stack are shown. Scale bars: 50 μm.
To further test the relationship between cell organization and non-autonomous egr2 induction, we created embryos that have mosaic ectopic expression of the endogenous egr2 gene. We took advantage of the finding that egr2 gene regulation involves an autoregulatory element that maintains expression following an initial pulse of egr2 expression (Bouchoucha et al., 2013, Chomette et al., 2006). We established a transgenic line with heat-shock-regulated egr2b (hs-egr2b) and used this to induce an early pulse of egr2b expression, which leads to ectopic expression of endogenous egr2b in even-numbered segments (Figures 3D and 3E) (Bouchoucha et al., 2013, Chomette et al., 2006). We transplanted labeled wild-type cells into the hs-egr2 line, induced egr2b expression, and analyzed whether there is non-autonomous induction of egr2 expression in wild-type cells present in even-numbered segments (Figure 3F). We found that wild-type cells express egr2b if intermingled with hs-egr2b cells (Figure 3G), but groups of wild-type cells do not upregulate egr2b (Figure 3H). Taken together, these findings suggest that non-autonomous induction of egr2b expression in even-numbered segments depends upon having a sufficient number of neighbors that are expressing egr2b.
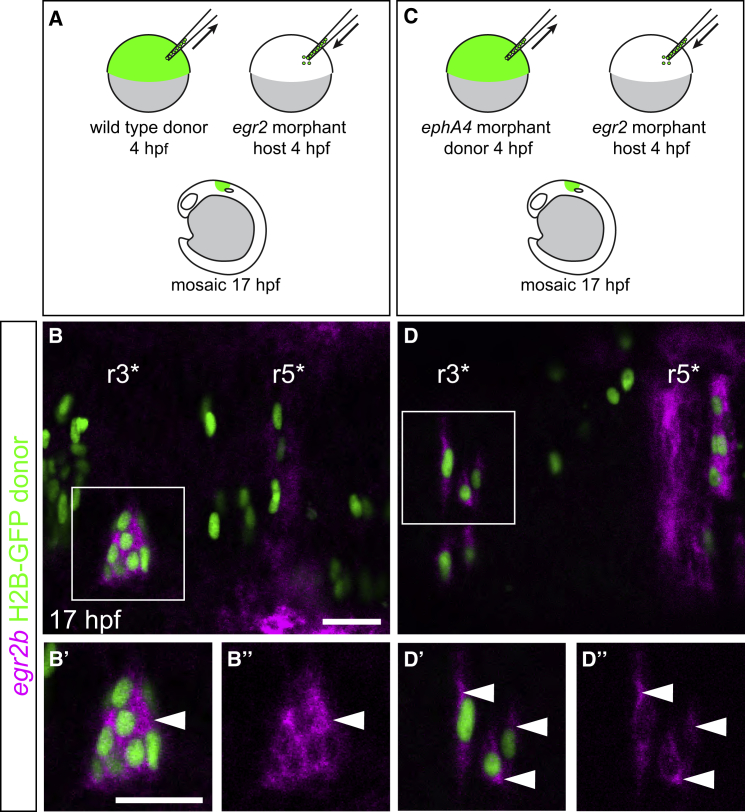
In view of these findings, we wondered whether cells downregulate egr2b expression when surrounded by non-expressing cells, as occurs when they intermingle into adjacent segments. Since autoregulation is required to sustain expression, knockdown of egr2b leads to loss of egr2b expression in r3 and to a lesser extent in r5 by 17 hpf, leading to depletion of r3 and r5 territory, whereas markers of r2 and r4 identity are still expressed (Figure S2) (Bouchoucha et al., 2013, Chomette et al., 2006). By transplanting wild-type cells into egr2b morphant embryos (Figure 4A), we created embryos in which only some cells are competent to maintain egr2b expression. We found that even small groups of wild-type cells present at the border of r2 and r4 are able to express egr2b (r3∗ in Figure 4B). As a further test, we prevented clustering of egr2-expressing cells by carrying out analogous experiments with ephA4 morphant cells transplanted into egr2 morphant embryos (Figure 4C). We found that egr2b-expressing cells were still present, even when organized as single cells at the border of r2 and r4 (Figure 4D). Thus, egr2b expression can be maintained in cells at the normal A-P location of r3 when surrounded by cells which are not expressing egr2b. This suggests that A-P patterning of cell identity is dominant over community signaling.
Figure 4.
Effect of Decreasing the Number of egr2b-Expressing Cells
(A and B) H2B-GFP-labeled wild-type donor cells were transplanted into egr2a plus egr2b morphant hosts (A). Since autoregulation is required to maintain egr2 expression (Figure S2), only the transplanted cells can express egr2 in r3 at this stage. Wild-type donor cells at the position of r3 (denoted r3∗) in egr2 morphants form a small cluster and maintain egr2b expression at 17 hpf (B, white arrowhead).
(C and D) H2B-GFP-labeled ephA4 morphant donor cells were transplanted into egr2 morphant hosts (C). ephA4 morphant donor cells no longer cluster in r3∗ but still maintain egr2b expression (D, white arrowheads). Single slices from a confocal z stack are shown. Scale bars: 50 μm.
Egr2b Regulates the Segmental Expression Level of cyp26b1 and cyp26c1
Our findings raise the question of how egr2 expression can occur in single cells at the correct A-P location but is downregulated in isolated cells present in even-numbered segments, whereas groups of cells can maintain segmental identity in an ectopic location. Previous studies have shown that a high posterior to low anterior gradient of RA has a key role in establishing segmental identity in the hindbrain (Tumpel et al., 2009, White and Schilling, 2008). A gradient of RA is created by a counter-gradient of the RA-degrading enzyme, cyp26a1, which is regulated by RA and Fgf signaling (White et al., 2007). Two other family members, cyp26b1 and cyp26c1, are expressed in dynamic segmental patterns in the hindbrain, and unlike cyp26a1 are not direct targets of RA signaling (Gu et al., 2005, Hernandez et al., 2007, Sirbu et al., 2005, Zhao et al., 2005). This dynamic regulation of cyp26b1 and cyp26c1 expression may also establish a gradient of RA that underlies A-P patterning (Hernandez et al., 2007, Sirbu et al., 2005). Since knockdown of cyp26b1 or cyp26c1 only leads to changes in segment identity when combined with loss of cyp26a1 function (Hernandez et al., 2007), these cyp26 family members have overlapping roles in regulating RA levels. We speculated that cyp26b1 and cyp26c1 may have a further role in which they are regulated by segment identity genes, and act in a homeostatic feedback mechanism that maintains an RA level appropriate for the A-P position. Furthermore, by acting as a sink for RA, Cyp26 enzymes can have non-autonomous effects by influencing RA levels in adjacent cells (Rydeen et al., 2015, Rydeen and Waxman, 2014, White et al., 2007). We therefore wondered whether there is feedback between egr2 and cyp26 genes that contributes to regulation of the segmental identity of cells.
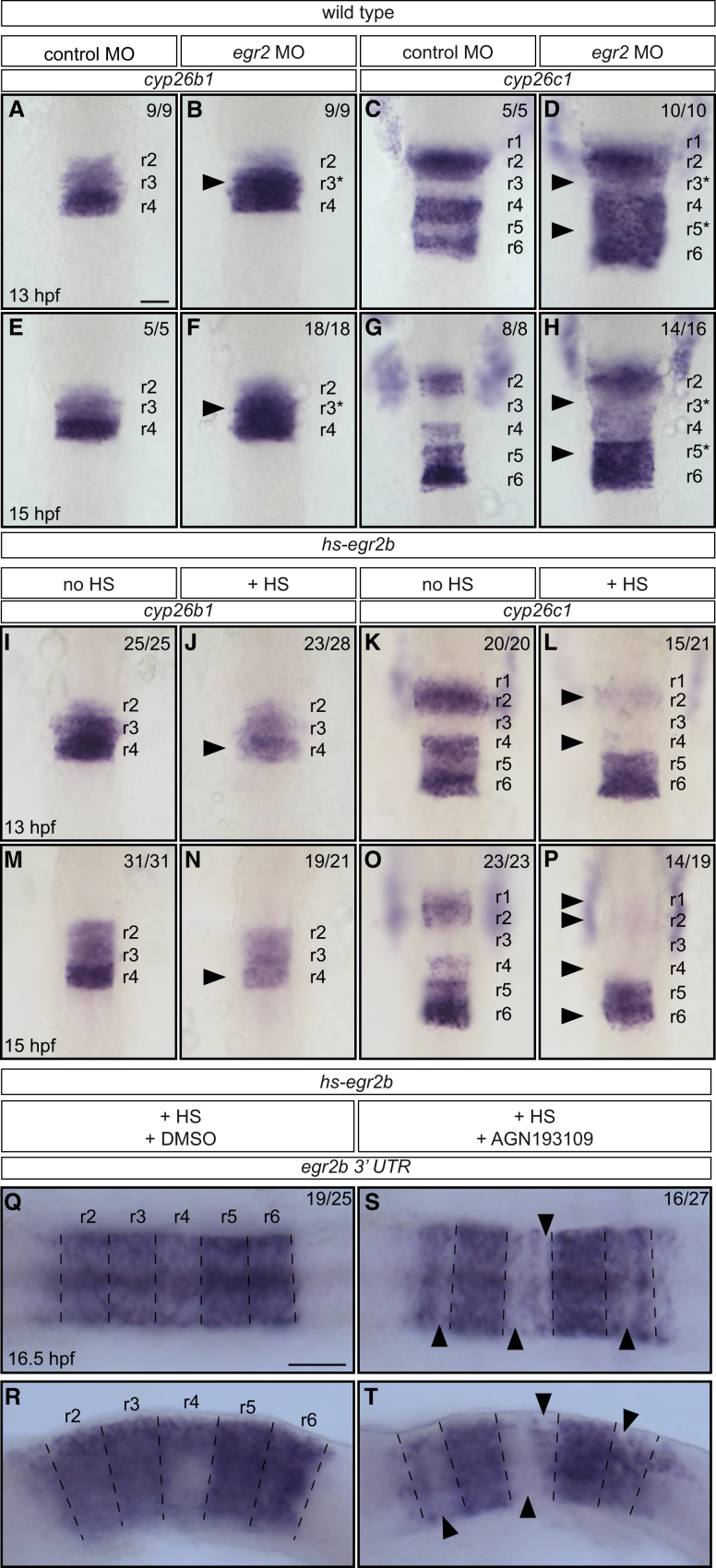
To test this idea, we carried out loss and gain of function experiments to determine whether cyp26b1 and cyp26c1 expression is regulated by egr2. cyp26b1 is expressed in r2-r4, at lower levels in r2 and r3 than in r4 (Figures 5A and 5E), and cyp26c1 is expressed in r2, r4, and r6, with lower expression in r3 and r5 (Figures 5C and 5G). Thus collectively, cyp26b1 and cyp26c1 are expressed at lower levels in r3 and r5 than in r2, r4, and r6. We found that, following egr2 knockdown, cyp26b1 expression in r3 increases to the same level as in r4 (Figures 5B and 5F), and cyp26c1 expression increases in r3 and r5 (Figures 5D and 5H). In gain of function experiments, we induced an early pulse of egr2b expression using the hs-egr2b transgenic line, such that endogenous egr2b is upregulated throughout the hindbrain (Figure 3E). We found that, in this situation, cyp26b1 and cyp26c1 expression is lower in r4, and now at the same level as in r3, and that cyp26c1 expression also decreases in r2 and r6 (Figures 5I–5P). The results of these loss and gain of function experiments reveal that egr2b underlies the lower level of cyp26b1 and cyp26c1 expression in r3 and r5 compared with r2, r4, and r6.
Figure 5.
Relationships between egr2, cyp26b1, cyp26c1, and RA Signaling
(A–H) Effect of egr2a plus egr2b knockdown (egr2 morpholino oligonucleotides [MO]) on cyp26b1 and cyp26c1 expression. egr2 knockdown increases the level of cyp26b1 expression in r3 (black arrowhead) at 13 hpf (B) and 15 hpf (F), and of cyp26c1 in r3 and r5 (black arrowheads) at 13 hpf (D) and 15 hpf (H) compared with control embryos (A, C, E and G). r3∗ and r5∗ in (B), (D), (F), and (H) refer to the region specified as r3 or r5 in the presence of egr2.
(I–P) Expression of cyp26b1 and cyp26c1 in control embryos and after induction of widespread egr2 expression by heat shock of hs-egr2b embryos at 11.3 hpf. Widespread expression of egr2b leads to reduction of cyp26b1 expression in r4 (black arrowhead) at 13 hpf (J), and 15 hpf (N) compared with control embryos (I and M). Similarly, cyp26c1 expression is reduced in r2, r4, and, to a lesser extent, in r6 (black arrowheads) at 13 hpf (L) and 15 hpf (P) in comparison with control embryos (K and O).
(Q–T) hs-egr2b embryos subjected to heat shock at 11.3 hpf in the presence (S and T) or absence (Q and R) of the pan-RAR antagonist AGN193109. Endogenous egr2b expression was analyzed at 16.5 hpf. In DMSO-treated control embryos, endogenous egr2b is expressed in r2, r4, and r6, but to a lesser extent in ventral r4 (Q and R). In AGN193109-treated embryos, expression of endogenous egr2b in r2, r4, and r6 is more heterogeneous and there are patches of cells that do not express endogenous egr2b (black arrowheads in S and T).
Embryos in (A)–(P) are flat-mounted with anterior to the top; embryos in (Q) and (S) are flat-mounted with anterior to the left; embryos in (R) and (T) are side-mounted with anterior to the left. Dashed lines in (Q)–(T) indicate presumptive rhombomere borders. Scale bars: 50 μm.
Feedback Occurs between egr2b Expression and RA Signaling
The above findings suggest a model in which the repression of cyp26b1 and cyp26c1 expression by egr2 leads to an increased level of RA, which in turn enables maintenance of egr2 expression. To test this, we analyzed whether RA signaling contributes to the regulation of endogenous egr2b expression in r2, r4, and r6 that is induced by a pulse of hs-egr2b expression. In control embryos, this leads to sustained egr2 expression throughout the hindbrain due to transcriptional autoregulation of the egr2 gene (Bouchoucha et al., 2013, Chomette et al., 2006), which at 16.5 hpf is uniform, except for low levels in ventral r4 (Figures 5Q and 5R). We used a chemical blocker to inhibit retinoic acid receptor (RAR) following the pulse of egr2 expression and found that this does not alter expression in r3 or r5, but has a striking effect in even-numbered segments at 16.5 hpf. We found that there is lower expression of egr2b in even-numbered segments compared with controls, with the strongest decrease in r4 (Figures 5S and 5T). Interestingly, following RAR inhibition, the level of egr2b expression is no longer homogeneous in r2, r4, and r6, and is organized in stripes of high and low expression. These findings suggest that, in addition to direct transcriptional autoregulation, ectopic egr2b expression in r2, r4, and r6 is promoted by positive feedback through downregulation of cyp26b1 and cyp26c1 and increased RA signaling.
cyp26b1 and cyp26c1 Contribute to Cell Identity Switching
The finding that low RA signaling antagonizes the maintenance of egr2 expression in r2, r4, and r6 suggests a potential basis for cell identity switching. r3 or r5 cells that intermingle into even-numbered segments encounter territory with higher cyp26b1 plus cyp26c1 expression and thus lower RA levels, and this may contribute to downregulation of egr2 expression. To test this, we analyzed the effect of knocking down cyp26b1 and cyp26c1. We found that cyp26b1 plus cyp26c1 knockdown leads to an increased number of egr2b-expressing cells in r2, r4, and r6 (Figures 6A, 6B, and 6E), though with only a small increase in r6. This phenotype is also seen in embryos that are null mutants for cyp26c1 (Figure S3). As a further test, we increased the amount of cell intermingling between segments by carrying out analogous experiments with simultaneous knockdown of ephA4. We found that, when cyp26b1 and cyp26c1 knockdown is combined with the loss of ephA4 function, there is a 4-fold increase in the number of ectopic egr2b-expressing cells in r2 and r4, but not in r6, compared with cyp26b1 plus cyp26c1 knockdown only (Figures 6C–6E). These findings support that identity switching of egr2b-expressing cells present in r2 or r4 requires RA signaling regulated by cyp26b1 and cyp26c1.
Figure 6.
cyp26b1, cyp26c1, and hoxb1 Knockdown Increases Ectopic egr2-Expressing Cells
(A–D) egr2b expression in 17 hpf embryos in which cyp26b1 and cyp26c1 were knocked down, compared with control morphants. Dorsal (A–D) and lateral (A′–D′) maximum intensity projections are shown. Knockdown of cyp26b1 and cyp26c1 causes an increased number of egr2b-expressing cells in r2, r4 and r6 (B and B′) compared with control embryos (A and A′); see also Figure S3. Combined knockdown of cyp26b1, cyp26c1, and ephA4 increases the number of egr2b expressing cells in r2 and r4 (D and D′) compared with ephA4 morphant embryos (C and C′) and cyp26b1 plus cyp26c1 knockdown alone (B and B′).
(E) Mean number of egr2b-expressing cells in even-numbered segments with 95% confidence intervals. Since blocking identity switching will favor segregation of cells that have initially intermingled, we counted egr2-expressing cells adjacent to r3 and r5 as well as isolated cells. Asterisks indicate statistical significance determined by Welsh's t test. For cyp26b1 and cyp26c1 morphants (n = 40) compared with control morphants (n = 22): r2, p < 0.0001; r4, p < 0.0001; r6, p = 0.038; all misplaced egr2b-expressing cells, p < 0.0001. For ephA4, cyp26b1, and cyp26c1 triple morphants (n = 21) compared with ephA4 morphants (n = 33): r2, p < 0.0001; r4, p = 0.01; r6, p = 0.31; total, p = 0.0001.
(F–H) Knockdown of hoxb1a and hoxb1b (hoxb1 MO) increases the number of egr2b-expressing cells in r4 (black arrowheads) at 18 hpf (G) compared with control embryos (F). hoxb1 knockdown does not affect cyp26b1 or cyp26c1 expression (Figure S4). (H) Mean number of ectopic egr2b-expressing cells with 95% confidence intervals. Asterisks indicate statistical significance determined by Welsh's t test: r2, p = 0.001; r4, p < 0.0001; r6, p = 0.38. Control embryos, n = 6; hoxb1 morphants, n = 26.
(I–M) Knockdowns in the egr2b:H2B-Citrine line followed by detection of Citrine protein with anti-GFP antibody (green) and egr2b transcripts (red). The box in the bottom right of each panel is a magnified view of the egr2b transcript signal in the indicated area. Following knockdown of ephA4 alone to increase cell intermingling, ectopic Citrine-expressing cells do not express detectable egr2b transcripts (I). egr2b transcripts are detected in ectopic Citrine-expressing cells when ephA4 knockdown is combined with hoxb1 (J and K) or cyp26b1 plus cyp26c1 knockdown (L and M). Empty arrowheads indicate Citrine-expressing cells lacking detectable egr2b transcripts, and filled arrowheads Citrine-expressing cells that express egr2b transcripts. Scale bars: 50 μm.
Our findings raise the question of how retinoid signaling affects egr2 expression in even-numbered segments, since dissection of gene regulatory elements has not found a direct input of RA (Bouchoucha et al., 2013, Chomette et al., 2006). One potential explanation is that RA signaling in even-numbered segments promotes expression of a repressor of egr2 gene expression. hoxb1 is a good candidate to mediate such a mechanism in r4 since it is directly regulated by retinoid signaling (Marshall et al., 1994, Studer et al., 1994, Tumpel et al., 2009) and represses egr2 expression (Giudicelli et al., 2001, Labalette et al., 2015, Zhang et al., 2012). We therefore carried out knockdown of hoxb1a and hoxb1b. We found that hoxb1 knockdown does not alter cyp26b1 or cyp26c1 expression (Figure S4) but leads to an increase in the number of isolated egr2-expressing cells in r4 (Figures 6F–6H).
Taken together, these findings suggest that cyp26b1, cyp26c1, and hoxb1 mediate identity switching of r3 and r5 cells that have intermingled into adjacent segments. To directly test this, we carried out knockdowns in the egr2:H2B-Citrine line followed by detection of citrine protein and egr2 transcripts. As shown previously (Figure 2), egr2 transcripts are not detected in citrine-expressing cells that have intermingled into r4 following ephA4 knockdown (Figure 6I). In contrast, egr2 transcripts are detected in ectopic Citrine-expressing cells following knockdown of hoxb1 (Figures 6J and 6K) or cyp26b1 plus cyp26c1 (Figures 6L and 6M).
Discussion
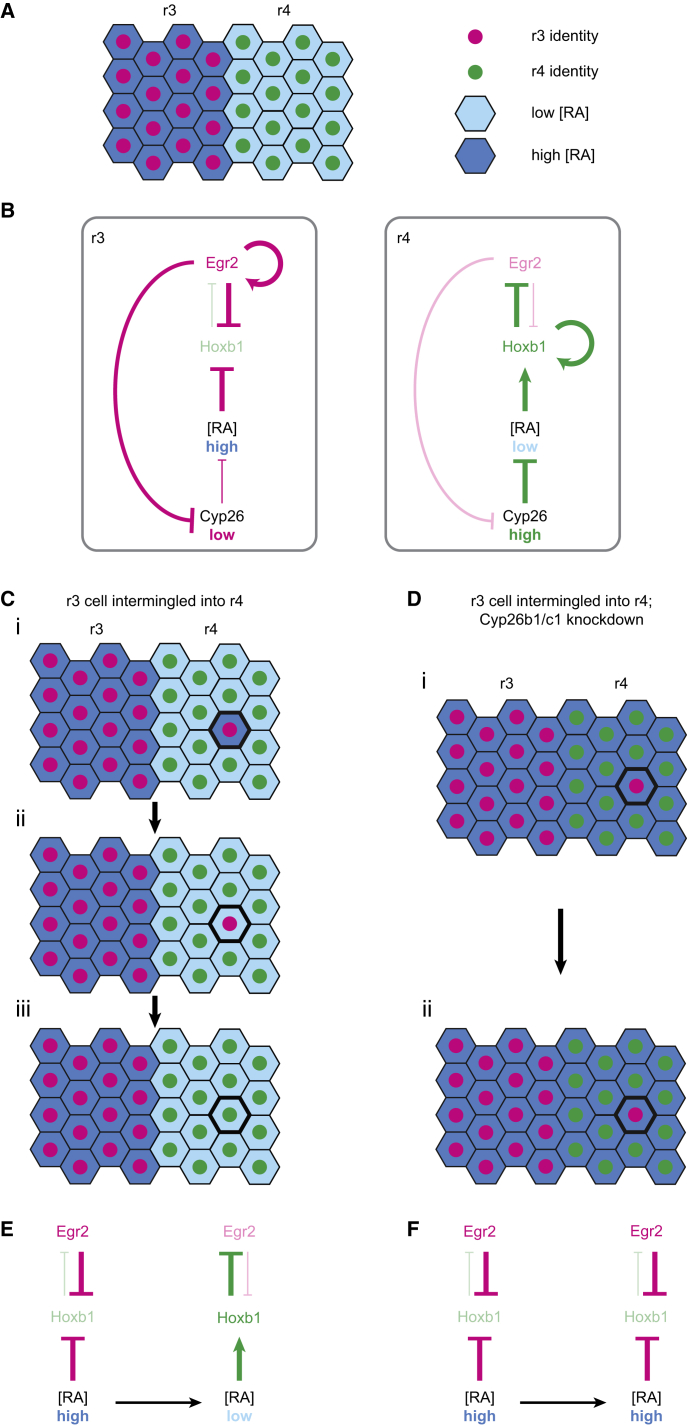
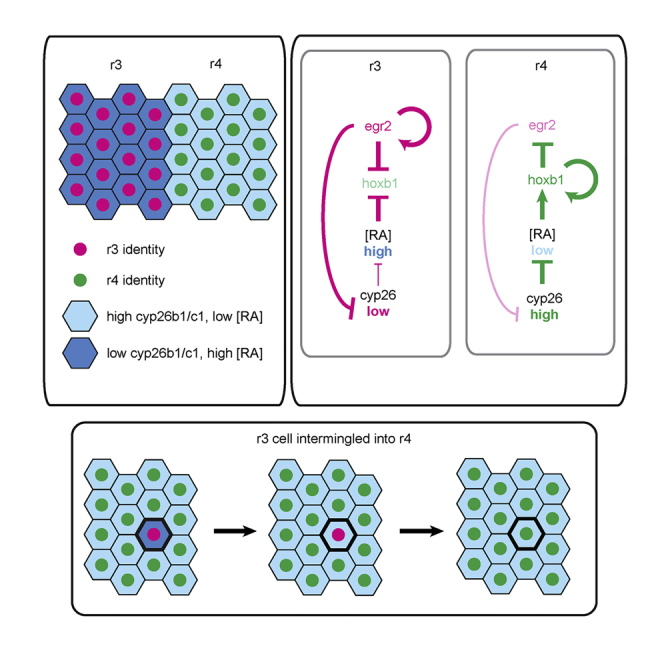
Transplantation experiments in the hindbrain revealed plasticity in cell fate specification, which could enable homogeneous segmental identity to be maintained despite intermingling of cells across segment borders. To study whether cell intermingling and identity switching occurs during normal development in zebrafish, we created an early reporter of egr2b expression by insertion of H2B-Citrine into the egr2b gene locus. We find that cell identity switching occurs during hindbrain segmentation and show that this is regulated by coupling between segment identity and retinoid signaling. Our findings suggest a model in which r3 and r5 cells that intermingle into adjacent segments switch identity since they encounter cells with a higher expression level of cyp26b1 and cyp26c1 (Figure 7).
Figure 7.
Model of cyp26b1, cyp26c1, and hoxb1 in Cell Identity Switching
(A) Segmental regulation of low cyp26b1 and cyp26c1 expression in r3 (magenta) and high expression in r4 (green) maintains high [RA] in r3 and low [RA] in r4.
(B) In r3, egr2 represses cyp26b1 and cyp26c1 expression, which in turn keeps RA levels high, repressing hoxb1 expression. Egr2 also directly autoregulates its own expression and represses hoxb1 expression. In r4, high cyp26b1 and cyp26c1 expression maintains a low level of RA, permitting expression of hoxb1. Hoxb1 autoregulates its own expression and represses expression of egr2.
(C and E) When an r3 cell intermingles into r4, the surrounding cells have high cyp26b1 and cyp26c1 expression, which reduces the level of RA within the ectopic r3 cell. This promotes upregulation of hoxb1 in the ectopic r3 cell, which represses egr2 expression, causing the r3 cell to change identity to r4.
(D and F) When an r3 cell intermingles into r4, but cyp26b1 and cyp26c1 are knocked down, the surrounding r4 cells no longer have high cyp26b1/c1 expression, so RA levels remain high in the ectopic r3 cell. Consequently, egr2 is not downregulated in the ectopic r3 cell (F).
Cell Intermingling and Identity Switching
Cell lineage analysis in the chick hindbrain found that the clonal progeny of cells labeled before morphological boundaries are seen can contribute to adjacent segments, but when cells are labeled after boundary formation clones are confined to single segments (Fraser et al., 1990). These findings can be explained by the hierarchical relationships between genes that regulate segmental identity and cell segregation. The egr2 gene specifies r3 and r5 identity (Schneider-Maunoury et al., 1993, Voiculescu et al., 2001), which is coupled to A-P specification through reciprocal interactions with hox genes (Sham et al., 1993, Tumpel et al., 2009), and is also a direct transcriptional regulator of ephA4 (Theil et al., 1998). Signaling between ephA4 in r3/r5 and ephrins expressed in r2/r4/r6 underlies morphological boundary formation and the restriction of cell intermingling across borders (Cooke et al., 2005, Sela-Donenfeld et al., 2009, Xu et al., 1995). It is therefore likely that intermingling between segments is largely confined to the period before EphA4 has been sufficiently upregulated to mediate cell segregation. In zebrafish, this period is also when convergent extension of the neural epithelium occurs, which through cell intercalation drives extensive intermingling along the A-P axis (Kimmel et al., 1994). Based on these considerations, detection of intermingling between segments requires analysis with early reporters, and can explain why it was not observed in a study that used reporters expressed one step downstream of egr2 (Calzolari et al., 2014). This latter study used either an egr2 autoregulatory element driving GFP or a Gal4 gene trap in egr2b that drives expression of UAS-mCherry, and reporter expression was first seen in r5 at 12.8 hpf, ∼2 hr after egr2b transcripts are detected. Since sharp borders have formed by this stage, it is likely that intermingling between segments has been restricted by the time that reporter expression is detected. The reporter line that we created enables direct detection of H2B-Citrine ∼1 hr after egr2b expression, and sensitivity was further increased by immunodetection of citrine protein.
We find isolated cells expressing H2B-Citrine reporter in even-numbered segments, which do not have a detectable level of egr2b transcripts, and express hoxb1a when present in r4. Thus, r3 and r5 cells that have intermingled into adjacent segments have switched identity to that of their new neighbors. One potential mechanism for switching is that, at the new location, cells respond to graded signals that specify A-P position. However, this does not account for the finding that groups of cells retain their original identity when transplanted to another segment (Schilling et al., 2001, Trainor and Krumlauf, 2000). The finding that forced mosaic expression of egr2 in even-numbered segments can non-autonomously induce egr2 expression (Giudicelli et al., 2001) suggests that this transcription factor regulates community signaling. We find that non-autonomous induction occurs when cells with forced egr2 expression are intermingled with r2, r4, or r6 cells, but not when egr2-expressing and non-expressing cells are segregated. The induction of egr2 expression in r2, r4, and r6 cells thus depends upon the number of egr2-expressing cells that they contact, suggesting that segmental identity is influenced by short-range interactions in which the majority wins.
Roles of cyp26b1 and cyp26c1
Previous studies have shown that the A-P specification of hindbrain segments is mediated by a posterior to anterior gradient of RA generated by Cyp26 enzymes. There is a distinct expression and regulation of different cyp26 family members in the hindbrain. cyp26a1 is expressed in an anterior to posterior gradient that forms at early stages and is directly regulated by RA and FGF signaling (White et al., 2007). In contrast, cyp26b1 and cyp26c1 are not directly regulated by RA, and are expressed in dynamic segmental patterns (Hernandez et al., 2007, Sirbu et al., 2005). Since cyp26b1 and cyp26c1 knockdown only has a strong effect on A-P patterning when combined with cyp26a1 loss of function, these family members are thought to have parallel roles. Our findings provide evidence for a distinct role of cyp26b1 and cyp26c1, in which they underlie identity switching of egr2-expressing cells that have intermingled into even-numbered segments.
We show that egr2 underlies the lower level of cyp26b1 and cyp26c1 expression in r3 and r5 compared with r2, r4, and r6. Since Egr2 is a transcriptional activator, the repression of cyp26b1 and cyp26c1 expression may be indirect, but there is evidence that in some contexts Egr2 can act as a repressor (Desmazieres et al., 2009, Mager et al., 2008, Sevetson et al., 2000). It is currently not known how cyp26b1 or cyp26c1 expression is regulated in r2, r4, and r6. Although we find that hoxb1 knockdown has no effect on cyp26b1 or cyp26c1 expression, it is possible that other hox genes are involved. The expression pattern of cyp26b1 and cyp26c1 suggests that, following initial patterning of the hindbrain by graded RA, a more complex pattern is formed with higher RA levels in odd- compared with even-numbered segments. The currently available transcriptional reporter lines are only sensitive enough to detect the high RA levels present in the spinal cord (reviewed by Schilling et al., 2016). Techniques for direct detection of RA have revealed a gradient in the hindbrain (Shimozono et al., 2013, Sosnik et al., 2016), but, as the RA levels detected are noisy at the spatial resolution of hindbrain segments, it is unclear whether there is a continuously decreasing gradient or a more complex pattern.
A consequence of coupling cyp26b1 and cyp26c1 expression levels to segment identity (Figures 7A and 7B) is that, when r3 or r5 cells intermingle into adjacent segments, they will initially retain low expression of cyp26b1 and cyp26c1, and are moving into territory with higher cyp26b1/c1 expression. Previous work has shown that, in addition to a strong cell autonomous effect on RA levels, Cyp26 enzymes have a weak non-autonomous effect on adjacent cells, presumably by acting as a local sink for RA (Rydeen et al., 2015, Rydeen and Waxman, 2014, White et al., 2007). This predicts that, when single cells intermingle into an adjacent segment, the amount of RA they experience can be influenced by the level of Cyp26 enzymes in their neighbors. We find that knockdown of cyp26b1 plus cyp26c1 leads to an increase in the number of ectopic egr2-expressing cells in r2 and r4, although not in r6. Furthermore, there is a greater number of ectopic egr2-expressing cells when intermingling is increased by simultaneous loss of ephA4 function. Thus, cyp26b1 and cyp26c1 enable the identity switching of r3 and r5 cells that have intermingled into adjacent segments. The reason why they are required in r2 and r4, but not r6, is unclear, but may relate to the anterior to posterior progression of cyp26b1 and cyp26c1 expression, which is upregulated in r2–r4 at 10 hpf, and in r6 at 11 hpf (Gu et al., 2005, Hernandez et al., 2007). This suggests that another mechanism acts to mediate identity switching in r6 during early stages when intermingling between segments occurs.
Further evidence for how egr2b is regulated when expressed in r2, r4, or r6 came from use of a heat shock inducible line to induce transient egr2b expression, which due to autoregulation is then maintained throughout the hindbrain. Since ectopic egr2b expression downregulates cyp26b1 and cyp26c1 expression in r2, r4, and r6, this is predicted to increase RA signaling to a similar level as occurs in r3 and r5. We find that inhibition of RAR function disrupts the maintenance of egr2b expression in even-numbered segments, leading to a heterogeneous mixture of expressing and non-expressing cells. Taken together, these findings reveal that low RA signaling (high cyp26b1/c1 or inhibition of RAR) promotes downregulation of egr2b expression in even-numbered segments, and that high RA (knockdown of cyp26b1/c1, or overexpression of egr2, which represses cyp26b1 and cyp26c1) enables maintenance of egr2 expression. We therefore propose that an egr2-expressing cell that intermingles into an adjacent segments is surrounded by cells with a higher level of cyp26b1/c1, which non-autonomously decreases RA levels in the egr2-expressing cell and leads to identity switching (Figure 7C). In contrast, such identity switching does not occur when an egr2-expressing cell intermingles into territory in which cyp26b1/c1 levels have been lowered by gene knockdown (Figure 7D).
Relationship between RA Signaling and egr2 Expression
Since dissection of gene regulatory elements has not found any direct input of RA into regulation of egr2 (Bouchoucha et al., 2013, Chomette et al., 2006), our findings raise the question of how changes to RA levels affect egr2 gene expression in even-numbered segments. We tested a role of hoxb1, since it represses egr2 expression (Giudicelli et al., 2001, Labalette et al., 2015, Zhang et al., 2012) and is directly upregulated in r4 and repressed in r3 and r5 by retinoid signaling (Lee and Skromne, 2014, Marshall et al., 1994, Studer et al., 1994, Tumpel et al., 2009). Consistent with previous studies (Labalette et al., 2015, Zigman et al., 2014), we find that knockdown of hoxb1 genes leads to an increase in the number of egr2-expressing cells in r4. We therefore propose that lower RA levels maintained by cyp26b1 and cyp26c1 in r4 enable upregulation of hoxb1 expression in r3 and r5 cells that have intermingled, which in turn represses egr2b expression (Figures 7C and 7E). Following knockdown of cyp26b1 and cyp26c1, there is a higher level of RA in r4, which does not promote upregulation of hoxb1, and consequently egr2b expression is maintained (Figures 7D and 7F).
Distinct Regulation of egr2 at Normal and Ectopic Locations
Whereas single ectopic cells downregulate egr2, we find that isolated cells can express egr2 when present at the normal A-P position of r3. This suggests that A-P regulation of egr2 expression is dominant over the proposed community signaling. Similarly, inhibition of RAR at late stages leads to downregulation of ectopic egr2 expression in r2, r4, and r6 but does not disrupt egr2 expression in r3 and r5. These findings are consistent with the mechanisms that regulate egr2 gene expression. Due to the combinatorial input of Fgf signaling and Hox and Nlz transcription factors, an initiator element that regulates egr2 expression is strongly activated in r3 but has weak activity in r2 and r4 (Labalette et al., 2015). This generates sufficient Egr2 protein in r3 to maintain and amplify subsequent expression through an autoregulatory element (Bouchoucha et al., 2013, Chomette et al., 2006). Our findings suggest that strong activation of the initiator element plus autoregulation is able to maintain egr2 gene expression even in single r3 cells flanked by r2 and r4 cells, which can have non-autonomous effects on RA levels. In contrast, r3 or r5 cells that intermingle into adjacent segments are in territory with low activation of the initiator element, and thus the non-autonomous effects of cyp26b1 and cyp26c1 are able to antagonize the maintenance of egr2 expression.
Community Regulation of Cell Identity
In classical models of community effects, a transcription factor that specifies cell identity upregulates expression of a signaling molecule that can induce expression of the transcription factor in neighboring cells (Bolouri and Davidson, 2010, Buckingham, 2003, Standley et al., 2001). Our findings provide evidence for a related mechanism, in which coupling between segment identity and the expression level of retinoid degrading enzymes sets the amount of RA signaling in a group of cells. The coupling of cyp26b1 and cyp26c1 expression to segment identity can account for the finding that groups of cells maintain their original identity when transplanted to another segment (Schilling et al., 2001, Trainor and Krumlauf, 2000). Consistent with this, mosaic overexpression of cyp26c1 induced ectopic hoxb1 gene expression when present in groups of cells but not in single cells (Lee and Skromne, 2014). Interestingly, r3 and r5 gene expression occurred in groups of cells in adjacent segments following overexpression of truncated EphA4 (Xu et al., 1995), which is predicted to greatly increase cell intermingling by both blocking Eph receptor activation and activating ephrin reverse signaling. The extent of intermingling that occurs during normal development, or even single Eph receptor knockdown, leads to isolated ectopic cells that come under the influence of the level of cyp26b1/c1 expression and RA degradation in neighboring cells. In this mechanism, cell identity regulation depends upon how many neighbors are of the same or different type, and thus switching does not occur at borders of segregated cell populations. This may provide a more reliable mechanism for switching than responding to an RA gradient, which is not able to specify identity along the A-P axis with single-cell precision (Zhang et al., 2012).
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Digoxigenin-AP (in situ) | Roche | Cat# 11093274910; RRID: AB_514497 |
| Anti-Fluorescein-AP (in situ) | Roche | Cat# 11426338910; RRID: AB_514504 |
| Anti-GFP (IF) | Torrey Pines Biolabs | Cat# TP401 071519; RRID: AB_10013661 |
| Anti-Myc (IF) | Santa Cruz Biotechnology | Cat# sc-40; RRID: AB_627268 |
| Anti-Rabbit Alexa Fluor 488 (IF) | Thermo Fisher Scientific | Cat# R37116; RRID: AB_2556544 |
| Anti-Mouse Alexa Fluor 488 (IF) | Thermo Fisher Scientific | Cat# R37120; RRID: AB_2556548 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| BCIP | Roche | Cat# 11383221001 |
| NBT | Roche | Cat# 11383213001 |
| AGN193109 | Santa Cruz Biotechnology | Cat# sc-210768 |
| Fast Blue BB | Sigma | Cat# F3378 |
| NAMP (Naphthol AS-MX phosphate) | Sigma | Cat# N5000 |
| Fast Red TR/Naphthol AS-MX Tablets | Sigma | Cat# F4648 |
| Cas9 NLS | New England Biolabs | Cat# M0646M |
| Experimental Models: Organisms/Strains | ||
| Zebrafish Danio rerio: WT | N/A | N/A |
| Zebrafish Danio rerio: Tg [egr2b:H2B-Citrine] | This study | N/A |
| Zebrafish Danio rerio: Tg [CNE1:egr2b-Myc] | This study | N/A |
| Zebrafish Danio rerio: Tg [HS:egr2b-Myc] | This study | N/A |
| Oligonucleotides | ||
| See supplementary table | This study | N/A |
| Recombinant DNA | ||
| pCS2b1a: probe synthesis hoxb1a: linearize KpnI: polymerase T3 | McClintock et al., 2001 | N/A |
| pBS-hoxa2: probe synthesis hoxa2: linearize KpnI: polymerase T3 | Prince et al., 1998 | N/A |
| pCS2-cyp26b1: probe synthesis cyp26b1: linearize BglII: polymerase Sp6 | Hernandez et al., 2007 | N/A |
| pCS2-cyp26c1: probe synthesis cyp26c1: linearize SmaI: polymerase T7 | Hernandez et al., 2007 | N/A |
| pBS-krox-20: full-length probe synthesis egr2b: linearize PstI: polymerase: T3 | Oxtoby & Jowett, 1993 | N/A |
| pBS-krox-20: 3′ UTR probe synthesis egr2b: linearize SphI: polymerase: T3 | Oxtoby & Jowett, 1993 | N/A |
| pT3Ts-Tol2: mRNA synthesis Tol2: linearize SpeI: polymerase T3 | Balciunas et al., 2006 | N/A |
| pDONR221 | Thermo Fisher Scientific | Cat# 12536017 |
| p5E-hsp70l | Kwan et al., 2007 | N/A |
| p3E-MTpA | Kwan et al., 2007 | N/A |
| pDestTol2pACryGFP | Berger and Currie, 2013 | N/A |
| pCS2-H2B-citrine: probe synthesis citrine: linearize XmaI: polymerase T3 | Megason, 2009 | N/A |
| pCS2-H2B-EGFP: mRNA synthesis H2B-eGFP: linearize NotI: polymerase Sp6 | Megason, 2009 | N/A |
| pMiniTol2-Pax3CNE1-TKprom-Gal4-UAS:H2Bcitrine | Moore et al., 2013 | N/A |
| pDR274: linearize BsaI-HF | Hwang et al., 2013 | N/A |
| MLM3613: mRNA synthesis cas9: linearize PmeI: polymerase T7 | Hwang et al., 2013 | N/A |
| Software and Algorithms | ||
| FIJI (ImageJ) | Schneider et al., 2012 | N/A |
| Fluorender | N/A | N/A |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, David G. Wilkinson (david.wilkinson@crick.ac.uk).
Experimental Model and Subject Details
Maintenance of Zebrafish Strains
Wild type and transgenic zebrafish embryos were obtained by natural spawning and raised at 28.5°C as described (Westerfield, 2007). Embryos were staged by hours post-fertilization (hpf) at 28.5°C and/or morphological features (Kimmel et al., 1995). The zebrafish work was carried out under a UK Home Office Licence under the Animals (Scientific Procedures) Act 1986 and underwent full ethical review.
Method Details
Constructs and Transgenesis
pMiniTol2-Pax3CNE1-TKprom-Gal4-UAS:Egr2b-Myc (referred to as CNE1:egr2b-Myc) was created by replacing the H2B-citrine coding sequence from pMiniTol2-Pax3CNE1-TKprom-Gal4-UAS:H2B-citrine (Moore et al., 2013) with the egr2b coding sequence and C-terminal Myc tag, amplified from hsp70:Egr2b-Myc-ACG. For transient transgenesis, 15-18 pg DNA and 36 pg Tol2 transposase RNA were injected into one-cell stage embryos.
The HS:egr2b-Myc;αCrystallin-GFP expression vector was generated using plasmids from the Tol2Kit (Kwan et al., 2007) and pDestTol2pACryGFP destination vector (Addgene plasmid #64022) (Berger and Currie, 2013). The egr2b middle entry vector (pME-egr2b) was created by BP recombination between the pDONR-221 vector and the egr2b coding sequence (Oxtoby and Jowett, 1993) flanked by attB sites. The mBait-H2B-Citrine donor plasmid was generated by insertion of a cFos minimal promoter, mBait gRNA target site, H2B-Citrine coding sequence and SV40 polyadenylation signal into a pBluescript II KS backbone.
The Tg[egr2b:H2B-Citrine] line (fci3) was generated by CRISPR/Cas9-mediated insertion using the strategy described in (Kimura et al., 2014). Embryos were injected at the one cell stage with Cas9 mRNA (350 pg), mBait gRNA (50 pg), gRNA (100 pg) targeting a sequence 893 bp upstream of the transcriptional start site of Egr2b (ATTCTGAGCTATCCAGTACGG), and mBait-H2B-Citrine donor plasmid (10-20 pg). CRISPR/Cas9-mediated mutation of the ephA4 and cyp26c1 genes was carried out using the gRNAs described in Table S1. The hs-egr2b line (fci4) was generated by injection of an Hsp70:Egr2b-Myc;αCrystallin-GFP expression vector.
Morpholino Injection
Morpholino oligonucleotides (MOs) were obtained from GeneTools (Oregon, USA) and were aliquoted and stored at room temperature in glass vials. MOs were injected into the yolk of blastomeres at the 1-4 cell stage in combination with a p53 translation-blocking MO to block off–target effects mediated by activation of apoptotic pathways (Gerety and Wilkinson, 2011, Robu et al., 2007). 4 ng MO was injected, except for ephA4 MO for which 5 ng was used. The MOs used are as follows: egr2b translation-blocking MO (AGTTTTAGCTGTCATCGTGAAGTCC); egr2a translation-blocking MO (CATGTGCTCCATGTTGGGAAGATTT); ephA4 (Cooke et al., 2005); cyp26b1 and cyp26c1 (Hernandez et al., 2007); hoxb1a and hoxb1b (McClintock et al., 2002); p53 (Langheinrich et al., 2002). Control morphant embryos were injected with a corresponding amount of the standard control MO (GeneTools). egr2a and egr2b MO block egr2b autoregulation and cause loss of r3 and r5 as occurs in an egr2b zebrafish mutant (Bouchoucha et al., 2013) and egr2 mouse mutant (Schneider-Maunoury et al., 1993, Voiculescu et al., 2001). All other MOs used have previously been characterized and their efficacy and specificity demonstrated as follows. ephA4 MO blocks generation of EphA4 protein and disrupts the sharpening of the r2/r3, r3/r4 and r5/r6 borders (Cooke et al., 2005, Terriente et al., 2012); we show that the same phenotype occurs in an ephA4 mutant (fci2) which has a 4 bp deletion at residue 158 in the ligand-binding domain (Figure S1). cyp26b1 MO and cyp26c1 MO cause posteriorisation of the hindbrain when combined with a cyp26a1 mutant, and this is phenocopied by chemical blocking of Cyp26 enzymes (Hernandez et al., 2007). We show that the phenotype of ectopic egr2-expressing cells seen in morphants also occurs in cyp26c1 mutant embryos (Figure S3). hoxb1a MO disrupts hoxb1a autoregulation, and hoxb1b MO causes a decreased size of r4 and increase in r3 (McClintock et al., 2002); the same phenotypes but more severe are seen in hoxb1a and hoxb1b mutants (Weicksel et al., 2014, Zigman et al., 2014). p53 MO blocks activation of apoptotic pathways, as also occurs in p53 mutant zebrafish (Gerety and Wilkinson, 2011, Langheinrich et al., 2002, Robu et al., 2007).
Pharmacological and Heat Shock Treatments
To block RA signaling, dechorionated embryos were treated with 10 μM AGN193109 in 2% DMSO in 0.65x Danieau’s solution; control embryos were treated with 2% DMSO in 0.65x Danieau’s solution. For induction of heat shock regulated constructs, embryos were placed at 36°C for 30 min in pre-warmed 0.65 x Danieau's solution at the stages indicated.
Cell Transplantation
Donor embryos were injected at the 1 cell stage with 100 pg H2B-eGFP RNA. Cell transplantation was performed at 4 hpf, with donor cells targeted to the future hindbrain based on fate maps (Kimelman and Martin, 2012).
In Situ Hybridization and Immunocytochemistry
Embryos of the desired stage were fixed in 4% paraformaldehyde/PBS for 3 h at room temperature or overnight at 4°C. Fixed embryos were stored in 100% methanol at −20°C prior to processing by in situ hybridization or immunocytochemistry. Probes used for in situ hybridization have been previously described: egr2b (Oxtoby and Jowett, 1993); citrine (Megason, 2009); hoxb1a (McClintock et al., 2001); cyp26b1 (Zhao et al., 2005); cyp26c1 (formerly known as cyp26d1) (Gu et al., 2005). Antisense riboprobes were labeled with digoxigenin-UTP or fluorescein-UTP. In situ hybridization and color development with BCIP/NBT or Fast Blue was conducted as previously described (Lauter et al., 2011, Xu et al., 1994). Two color fluorescent in situ hybridization was carried out using Fast Blue and Fast Red substrates. After BCIP/NBT color development, embryos were re-fixed, cleared in 70% glycerol/PBS, and mounted for imaging using a Zeiss Axioplan2 with Axiocam HRc camera. In some experiments (Figures 6I–6M), egr2b transcripts were detected by hybridization chain reaction (HCR) using the protocol detailed in (Choi et al., 2016). A kit containing a DNA probe set, a DNA HCR amplifier, and hybridization, wash and amplification buffers was purchased from Molecular Instruments (molecularinstruments.org). The egr2b probes initiate B5 (Alexa594) amplifier.
For immunocytochemistry, embryos were fixed for 2 h at room temperature in 4% paraformaldehyde, washed in PBT, then dechorionated and blocked for 1 h in 5% goat serum in PBT. Primary antibodies were used at the following concentrations in 2.5% goat serum: rabbit anti-GFP (1:500; 1:400 after in situ hybridization); Myc (1:400 after in situ hybridization). Secondary antibodies were used at the following concentrations: goat anti-rabbit Alexa Fluor® 488 (IgG H + L) (1:500 dilution); goat anti-mouse Alexa Fluor® 488 (IgG H + L) (1:500 dilution). Embryos were incubated in DAPI to stain nuclei. Embryos were then cleared in 70% glycerol/PBS, and mounted for imaging on a Leica TCS SP2 confocal microscope or Zeiss LSM700 confocal microscope.
Live Imaging and Analysis
Dechorionated embryos were embedded in 0.6% low melting agarose (Sigma)/0.5x Danieau's solution within a 1% agarose (Bio-Rad Laboratories Inc.)/0.5x Danieau's solution-coated chambered coverslip with individual embryo-shaped wells filled with 0.5x Danieau's solution. Embryos were imaged using an inverted Zeiss LSM710 confocal microscope with 20x lens, NA 0.8. Z-stacks with a slice depth of 2 μm were acquired every 3 minutes. ImageJ (NIH) was used for image processing. The Correct 3D Drift ImageJ plug-in (Parslow et al., 2014) was used to correct for 3D drift in time lapse movies. FluoRender v. 2.20.0 (University of Utah) was used to create 3D projections.
Quantification and Statistical Analysis
Quantification of Ectopic Cells
The number of cells in r2, r4 and r6 that express egr2b transcripts and/or H2B-citrine reporter was manually counted from confocal stacks. Statistical significance was determined using Welsh's T test in GraphPad Prism 7 as indicated in the Figure legends.
Acknowledgments
We thank Jean-Paul Vincent, Andrew Oates, and Greg Elgar for advice; James Briscoe and Caroline Hill for comments on the manuscript; and James Briscoe also for providing the CNE1 construct. We also thank the staff of the Biological Research Facility and the Light Microscopy Technology Platform at the Francis Crick Institute for their invaluable support. This work was supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001217), the UK Medical Research Council (FC001217), and the Wellcome Trust (FC001217).
Author Contributions
Conceptualization, D.G.W., M.A., and Q.X.; Methodology, D.G.W., M.A., Q.X., and J.C.; Investigation, M.A., Q.X., and J.C.; Writing – Original Draft, D.G.W.; Writing – Review & Editing, D.G.W., M.A., and Q.X.; Funding Acquisition, D.G.W.; Supervision, D.G.W.
Declaration of Interests
The authors declare no competing interests.
Published: May 3, 2018
Footnotes
Supplemental Information includes four figures and can be found with this article online at https://doi.org/10.1016/j.devcel.2018.04.003.
Supplemental Information
References
- Balciunas D., Wangensteen K.J., Wilber A., Bell J., Geurts A., Sivasubbu S., Wang X., Hackett P.B., Largaespada D.A., McIvor R.S. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet. 2006;2:e169. doi: 10.1371/journal.pgen.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J., Currie P.D. 503unc, a small and muscle-specific zebrafish promoter. Genesis. 2013;51:443–447. doi: 10.1002/dvg.22385. [DOI] [PubMed] [Google Scholar]
- Bolouri H., Davidson E.H. The gene regulatory network basis of the “community effect,” and analysis of a sea urchin embryo example. Dev. Biol. 2010;340:170–178. doi: 10.1016/j.ydbio.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchoucha Y.X., Reingruber J., Labalette C., Wassef M.A., Thierion E., Desmarquet-Trin Dinh C., Holcman D., Gilardi-Hebenstreit P., Charnay P. Dissection of a Krox20 positive feedback loop driving cell fate choices in hindbrain patterning. Mol. Syst. Biol. 2013;9:690. doi: 10.1038/msb.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand K., Huangfu D., Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 2007;5:e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M. How the community effect orchestrates muscle differentiation. Bioessays. 2003;25:13–16. doi: 10.1002/bies.10221. [DOI] [PubMed] [Google Scholar]
- Calzolari S., Terriente J., Pujades C. Cell segregation in the vertebrate hindbrain relies on actomyosin cables located at the interhombomeric boundaries. EMBO J. 2014;33:686–701. doi: 10.1002/embj.201386003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayuso J., Xu Q., Wilkinson D.G. Mechanisms of boundary formation by Eph receptor and ephrin signaling. Dev. Biol. 2015;401:122–131. doi: 10.1016/j.ydbio.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Choi H.M., Calvert C.R., Husain N., Huss D., Barsi J.C., Deverman B.E., Hunter R.C., Kato M., Lee S.M., Abelin A.C. Mapping a multiplexed zoo of mRNA expression. Development. 2016;143:3632–3633. doi: 10.1242/dev.140137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomette D., Frain M., Cereghini S., Charnay P., Ghislain J. Krox20 hindbrain cis-regulatory landscape: interplay between multiple long-range initiation and autoregulatory elements. Development. 2006;133:1253–1262. doi: 10.1242/dev.02289. [DOI] [PubMed] [Google Scholar]
- Cooke J., Moens C., Roth L., Durbin L., Shiomi K., Brennan C., Kimmel C., Wilson S., Holder N. Eph signalling functions downstream of Val to regulate cell sorting and boundary formation in the caudal hindbrain. Development. 2001;128:571–580. doi: 10.1242/dev.128.4.571. [DOI] [PubMed] [Google Scholar]
- Cooke J.E., Kemp H.A., Moens C.B. EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr. Biol. 2005;15:536–542. doi: 10.1016/j.cub.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Cooke J.E., Moens C.B. Boundary formation in the hindbrain: Eph only it were simple. Trends Neurosci. 2002;25:260–267. doi: 10.1016/s0166-2236(02)02134-3. [DOI] [PubMed] [Google Scholar]
- Cossu G., Kelly R., Didonna S., Vivarelli E., Buckingham M. Myoblast differentiation during mammalian somitogenesis is dependent upon a community effect. Proc. Nat. Acad. Sci. USA. 1995;92:2254–2258. doi: 10.1073/pnas.92.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmazieres A., Charnay P., Gilardi-Hebenstreit P. Krox20 controls the transcription of its various targets in the developing hindbrain according to multiple modes. J. Biol. Chem. 2009;284:10831–10840. doi: 10.1074/jbc.M808683200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F., Winklbauer R., Rohani N. Ephrin-Eph signaling in embryonic tissue separation. Cell Adh. Migr. 2014;8:308–326. doi: 10.4161/19336918.2014.970028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser S., Keynes R., Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990;344:431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- Gerety S.S., Wilkinson D.G. Morpholino artifacts provide pitfalls and reveal a novel role for pro-apoptotic genes in hindbrain boundary development. Dev. Biol. 2011;350:279–289. doi: 10.1016/j.ydbio.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicelli F., Taillebourg E., Charnay P., Gilardi-Hebenstreit P. Krox-20 patterns the hindbrain through both cell-autonomous and non cell-autonomous mechanisms. Genes Dev. 2001;15:567–580. doi: 10.1101/gad.189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X.X., Xu F., Wang X.L., Gao X., Zhao Q.S. Molecular cloning and expression of a novel CYP26 gene (cyp26d1) during zebrafish early development. Gene Expr. Patterns. 2005;5:733–739. doi: 10.1016/j.modgep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B. A community effect in animal development. Nature. 1988;336:772–774. doi: 10.1038/336772a0. [DOI] [PubMed] [Google Scholar]
- Hernandez R.E., Putzke A.P., Myers J.P., Margaretha L., Moens C.B. Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development. 2007;134:177–187. doi: 10.1242/dev.02706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W.Y., Fu Y., Reyon D., Maeder M.L., Tsai S.Q., Sander J.D., Peterson R.T., Yeh J.R., Joung J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving C., Nieto M.A., DasGupta R., Charnay P., Wilkinson D.G. Progressive spatial restriction of Sek-1 and Krox-20 gene expression during hindbrain segmentation. Dev. Biol. 1996;173:26–38. doi: 10.1006/dbio.1996.0004. [DOI] [PubMed] [Google Scholar]
- Kemp H.A., Cooke J.E., Moens C.B. EphA4 and EfnB2a maintain rhombomere coherence by independently regulating intercalation of progenitor cells in the zebrafish neural keel. Dev. Biol. 2009;327:313–326. doi: 10.1016/j.ydbio.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Martin B.L. Anterior-posterior patterning in early development: three strategies. Wiley Interdiscip. Rev. Dev. Biol. 2012;1:253–266. doi: 10.1002/wdev.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel C.B., Warga R.M., Kane D.A. Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development. 1994;120:265–276. doi: 10.1242/dev.120.2.265. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Hisano Y., Kawahara A., Higashijima S. Efficient generation of knock-in transgenic zebrafish carrying reporter/driver genes by CRISPR/Cas9-mediated genome engineering. Sci. Rep. 2014;4:6545. doi: 10.1038/srep06545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan K.M., Fujimoto E., Grabher C., Mangum B.D., Hardy M.E., Campbell D.S., Parant J.M., Yost H.J., Kanki J.P., Chien C.B. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Labalette C., Wassef M.A., Desmarquet-Trin Dinh C., Bouchoucha Y.X., Le Men J., Charnay P., Gilardi-Hebenstreit P. Molecular dissection of segment formation in the developing hindbrain. Development. 2015;142:185–195. doi: 10.1242/dev.109652. [DOI] [PubMed] [Google Scholar]
- Langheinrich U., Hennen E., Stott G., Vacun G. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr. Biol. 2002;12:2023–2028. doi: 10.1016/s0960-9822(02)01319-2. [DOI] [PubMed] [Google Scholar]
- Lauter G., Soll I., Hauptmann G. Two-color fluorescent in situ hybridization in the embryonic zebrafish brain using differential detection systems. BMC Dev. Biol. 2011;11:43. doi: 10.1186/1471-213X-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Skromne I. Retinoic acid regulates size, pattern and alignment of tissues at the head-trunk transition. Development. 2014;141:4375–4384. doi: 10.1242/dev.109603. [DOI] [PubMed] [Google Scholar]
- Lumsden A., Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- Mager G.M., Ward R.M., Srinivasan R., Jang S.W., Wrabetz L., Svaren J. Active gene repression by the Egr2/NAB complex during peripheral nerve myelination. J. Biol. Chem. 2008;283:18187–18197. doi: 10.1074/jbc.M803330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H., Studer M., Pöpperl H., Aparicio S., Kuroiwa A., Brenner S., Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994;370:567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- McClintock J.M., Carlson R., Mann D.M., Prince V.E. Consequences of Hox gene duplication in the vertebrates: an investigation of the zebrafish Hox paralogue group 1 genes. Development. 2001;128:2471–2484. doi: 10.1242/dev.128.13.2471. [DOI] [PubMed] [Google Scholar]
- McClintock J.M., Kheirbek M.A., Prince V.E. Knockdown of duplicated zebrafish hoxb1 genes reveals distinct roles in hindbrain patterning and a novel mechanism of duplicate gene retention. Development. 2002;129:2339–2354. doi: 10.1242/dev.129.10.2339. [DOI] [PubMed] [Google Scholar]
- Megason S.G. In toto imaging of embryogenesis with confocal time-lapse microscopy. Methods Mol. Biol. 2009;546:317–332. doi: 10.1007/978-1-60327-977-2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Ribes V., Terriente J., Wilkinson D., Relaix F., Briscoe J. Distinct regulatory mechanisms act to establish and maintain Pax3 expression in the developing neural tube. PLoS Genet. 2013;9:e1003811. doi: 10.1371/journal.pgen.1003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninov N., Borius M., Stainier D.Y. Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development. 2012;139:1557–1567. doi: 10.1242/dev.076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxtoby E., Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H.J., Krumlauf R. Segmental arithmetic: summing up the Hox gene regulatory network for hindbrain development in chordates. Wiley Interdiscip. Rev. Dev. Biol. 2017;6:e286. doi: 10.1002/wdev.286. [DOI] [PubMed] [Google Scholar]
- Parslow A., Cardona A., Bryson-Richardson R.J. Sample drift correction following 4D confocal time-lapse imaging. J. Vis. Exp. 2014:e51086. doi: 10.3791/51086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini A., Wilkinson D.G. Stabilizing the regionalisation of the developing vertebrate central nervous system. Bioessays. 2002;24:427–438. doi: 10.1002/bies.10085. [DOI] [PubMed] [Google Scholar]
- Prince V.E., Moens C.B., Kimmel C.B., Ho R.K. Zebrafish hox genes: expression in the hindbrain region of wild-type and mutants of the segmentation gene, valentino. Development. 1998;125:393–406. doi: 10.1242/dev.125.3.393. [DOI] [PubMed] [Google Scholar]
- Robu M.E., Larson J.D., Nasevicius A., Beiraghi S., Brenner C., Farber S.A., Ekker S.C. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydeen A., Voisin N., D'Aniello E., Ravisankar P., Devignes C.S., Waxman J.S. Excessive feedback of Cyp26a1 promotes cell non-autonomous loss of retinoic acid signaling. Dev. Biol. 2015;405:47–55. doi: 10.1016/j.ydbio.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydeen A.B., Waxman J.S. Cyp26 enzymes are required to balance the cardiac and vascular lineages within the anterior lateral plate mesoderm. Development. 2014;141:1638–1648. doi: 10.1242/dev.105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T.F., Prince V., Ingham P.W. Plasticity in zebrafish hox expression in the hindbrain and cranial neural crest. Dev. Biol. 2001;231:201–216. doi: 10.1006/dbio.2000.9997. [DOI] [PubMed] [Google Scholar]
- Schilling T.F., Sosnik J., Nie Q. Visualizing retinoic acid morphogen gradients. Methods Cell Biol. 2016;133:139–163. doi: 10.1016/bs.mcb.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Maunoury S., Topilko P., Seitanidou T., Levi G., Cohen-Tannoudji M., Pournin S., Babinet C., Charnay P. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993;75:1199–1214. doi: 10.1016/0092-8674(93)90329-o. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D., Kayam G., Wilkinson D.G. Boundary cells regulate a switch in the expression of FGF3 in hindbrain rhombomeres. BMC Dev. Biol. 2009;9:16. doi: 10.1186/1471-213X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevetson B.R., Svaren J., Milbrandt J. A novel activation function for NAB proteins in EGR-dependent transcription of the luteinizing hormone beta gene. J. Biol. Chem. 2000;275:9749–9757. doi: 10.1074/jbc.275.13.9749. [DOI] [PubMed] [Google Scholar]
- Sham M.H., Vesque C., Nonchev S., Marshall H., Frain M., Das Gupta R., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox-20 regulates Hoxb-2 (Hox2.8) during hindbrain segmentation. Cell. 1993;72:183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Shimozono S., Iimura T., Kitaguchi T., Higashijima S., Miyawaki A. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature. 2013;496:363–366. doi: 10.1038/nature12037. [DOI] [PubMed] [Google Scholar]
- Sirbu I.O., Gresh L., Barra J., Duester G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development. 2005;132:2611–2622. doi: 10.1242/dev.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnik J., Zheng L., Rackauckas C.V., Digman M., Gratton E., Nie Q., Schilling T.F. Noise modulation in retinoic acid signaling sharpens segmental boundaries of gene expression in the embryonic zebrafish hindbrain. Elife. 2016;5:e14034. doi: 10.7554/eLife.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley H.J., Zorn A.M., Gurdon J.B. eFGF and its mode of action in the community effect during Xenopus myogenesis. Development. 2001;128:1347–1357. doi: 10.1242/dev.128.8.1347. [DOI] [PubMed] [Google Scholar]
- Studer M., Pöpperl H., Marshall H., Kuroiwa A., Krumlauf R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science. 1994;265:1728–1732. doi: 10.1126/science.7916164. [DOI] [PubMed] [Google Scholar]
- Taylor H.B., Khuong A., Wu Z., Xu Q., Morley R., Gregory L., Poliakov A., Taylor W.R., Wilkinson D.G. Cell segregation and border sharpening by Eph receptor-ephrin-mediated heterotypic repulsion. J. R. Soc. Interface. 2017;14 doi: 10.1098/rsif.2017.0338. 20170338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terriente J., Gerety S.S., Watanabe-Asaka T., Gonzalez-Quevedo R., Wilkinson D.G. Signalling from hindbrain boundaries regulates neuronal clustering that patterns neurogenesis. Development. 2012;139:2978–2987. doi: 10.1242/dev.080135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil T., Frain M., Gilardi-Hebenstreit P., Flenniken A., Charnay P., Wilkinson D.G. Segmental expression of the EphA4 (Sek-1) receptor tyrosine kinase in the hindbrain is under direct transcriptional control of Krox-20. Development. 1998;125:443–452. doi: 10.1242/dev.125.3.443. [DOI] [PubMed] [Google Scholar]
- Trainor P., Krumlauf R. Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm. Nat. Cell. Biol. 2000;2:96–102. doi: 10.1038/35000051. [DOI] [PubMed] [Google Scholar]
- Tuazon F.B., Mullins M.C. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Semin. Cell Dev. Biol. 2015;42:118–133. doi: 10.1016/j.semcdb.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpel S., Wiedemann L.M., Krumlauf R. Hox genes and segmentation of the vertebrate hindbrain. Curr. Top. Dev. Biol. 2009;88:103–137. doi: 10.1016/S0070-2153(09)88004-6. [DOI] [PubMed] [Google Scholar]
- Voiculescu O., Taillebourg E., Pujades C., Kress C., Buart S., Charnay P., Schneider-Maunoury S. Hindbrain patterning: Krox20 couples segmentation and specification of regional identity. Development. 2001;128:4967–4978. doi: 10.1242/dev.128.24.4967. [DOI] [PubMed] [Google Scholar]
- Wang Q.X., Holmes W.R., Sosnik J., Schilling T., Nie Q. Cell sorting and noise-induced cell plasticity coordinate to sharpen boundaries between gene expression domains. PLoS Comput. Biol. 2017;13:e1005307. doi: 10.1371/journal.pcbi.1005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weicksel S.E., Gupta A., Zannino D.A., Wolfe S.A., Sagerstrom C.G. Targeted germ line disruptions reveal general and species-specific roles for paralog group 1 hox genes in zebrafish. BMC Dev. Biol. 2014;14:25. doi: 10.1186/1471-213X-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. University of Oregon; 2007. The Zebrafish Book. [Google Scholar]
- White R.J., Nie Q., Lander A.D., Schilling T.F. Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biol. 2007;5:e304. doi: 10.1371/journal.pbio.0050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.J., Schilling T.F. How degrading: Cyp26s in hindbrain development. Dev. Dyn. 2008;237:2775–2790. doi: 10.1002/dvdy.21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Alldus G., Holder N., Wilkinson D.G. Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hindbrain. Development. 1995;121:4005–4016. doi: 10.1242/dev.121.12.4005. [DOI] [PubMed] [Google Scholar]
- Xu Q., Holder N., Patient R., Wilson S.W. Spatially regulated expression of three receptor tyrosine kinase genes during gastrulation in the zebrafish. Development. 1994;120:287–299. doi: 10.1242/dev.120.2.287. [DOI] [PubMed] [Google Scholar]
- Xu Q., Mellitzer G., Robinson V., Wilkinson D.G. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- Zhang L., Radtke K., Zheng L., Cai A.Q., Schilling T.F., Nie Q. Noise drives sharpening of gene expression boundaries in the zebrafish hindbrain. Mol. Syst. Biol. 2012;8:613. doi: 10.1038/msb.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q.S., Dobbs-McAuliffe B., Linney E. Expression of cyp26b1 during zebrafish early development. Gene Expr. Patterns. 2005;5:363–369. doi: 10.1016/j.modgep.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Zigman M., Laumann-Lipp N., Titus T., Postlethwait J., Moens C.B. Hoxb1b controls oriented cell division, cell shape and microtubule dynamics in neural tube morphogenesis. Development. 2014;141:639–649. doi: 10.1242/dev.098731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.