Abstract
Necroptosis is an emerging form of programmed cell death occurring via active and well-regulated necrosis, distinct from apoptosis morphologically, and biochemically. Necroptosis is mainly unmasked when apoptosis is compromised in response to tumor necrosis factor alpha. Unlike apoptotic cells, which are cleared by macrophages or neighboring cells, necrotic cells release danger signals, triggering inflammation, and exacerbating tissue damage. Evidence increasingly suggests that programmed necrosis is not only associated with pathophysiology of disease, but also induces innate immune response to viral infection. Therefore, necroptotic cell death plays both physiological and pathological roles. Physiologically, necroptosis induce an innate immune response as well as premature assembly of viral particles in cells infected with virus that abrogates host apoptotic machinery. On the other hand, necroptosis per se is detrimental, causing various diseases such as sepsis, neurodegenerative diseases and ischemic reperfusion injury. This review discusses the signaling pathways leading to necroptosis, associated necroptotic proteins with target-specific inhibitors and diseases involved. Several studies currently focus on protective approaches to inhibiting necroptotic cell death. In cancer biology, however, anticancer drug resistance severely hampers the efficacy of chemotherapy based on apoptosis. Pharmacological switch of cell death finds therapeutic application in drug- resistant cancers. Therefore, the possible clinical role of necroptosis in cancer control will be discussed in brief.
Keywords: Apoptosis, Cancer, Necroptosis, Necroptosis modulator, Regulator
INTRODUCTION
Cell survival is constantly in dynamic equilibrium with cell death for tissue homeostasis. Aside from cell survival, cell death occurs in various modes such as programmed or random mechanisms upon exposure to various stresses. Programmed cell death or apoptosis is activated via intrinsic or extrinsic pathways in response to death stimuli. Both pathways involve a series of signaling molecules with activated caspases and is termed caspase-dependent cell death. Meanwhile, necroptosis is classified as type III programmed cell death with apoptotic and necrotic features. It is also known as programmed necrosis or caspase-independent cell death according to morphological or molecular features. Initially, necroptosis was considered as undesirable passive cell death. However, it is currently considered as specialized cell death since it is orchestrated under a caspase-compromised condition. Similar to apoptosis, necroptosis is executed via distinctive signaling mechanism comprising a cascade of specified proteins, resulting in regulated necrotic cell death. Despite unknown mechanisms and pathological significance compared with apoptosis, the discovery of pharmacological inhibitors targeting necroptosis has been extensively pursued. Here, we introduce the concept of necroptotic cell death induced under various pathophysiological conditions and delineate the pathological mechanisms unmasking necroptosis in a well-orchestrated fashion. In this review, necroptosis-associated diseases and the underlying pathogenesis will be discussed.
SIGNALING PATHWAYS LEADING TO NECROPTOSIS INDUCTION AND BIOCHEMICAL CHANGES
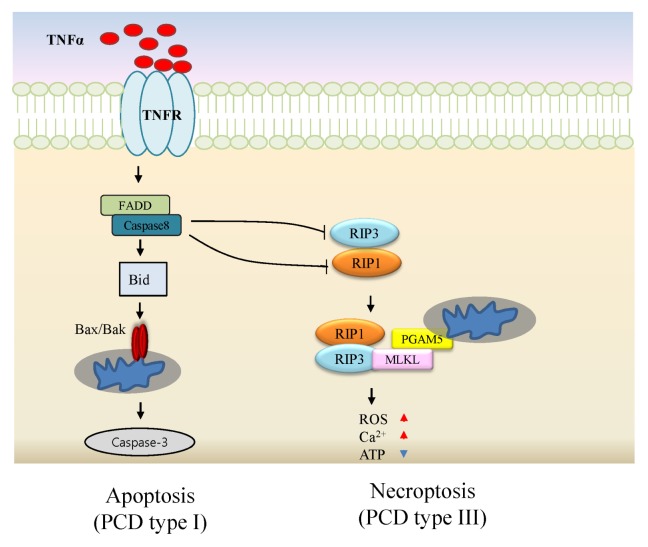
Typically, necroptosis occurs in cells exposed to extrinsic stimuli such as TNFα, FASL, and TRAIL in combination with compromised caspase-8 (C-8). C-8 inhibition is induced by genetic defects, viral proteins, and treatment with a pan-caspase inhibitor zVAD (1–3). Necroptosis-regulating proteins have been identified and biologically validated comprehensively. Genome-wide siRNA screening yielded a few candidate genes associated with necroptosis (4). In addition to RIP1 as a necroptosis regulator, RIP3, mixed lineage kinase domain-like protein (MLKL) and PGAM5 were identified (5–8). The signaling pathway leading to necroptosis is illustrated in Fig. 1. TNFα ligation to its cognate receptor triggers caspase-dependent cell death as a default cell death mode. Caspase-compromised conditions drive cell death via necroptosis with accompanying RIP1 activation (9). Upon stimulation, RIP1 interacts with RIP3 leading to the formation of a necrosome complex via RIP homotypic interaction motif (RHIM) within cells (10). MLKL pseudokinase was identified as a substrate of RIP3 under necroptotic condition. Phosphorylated RIP3 induces MLKL phosphorylation at serine or threonine sites. The RIP3-MLKL complex translocates to mitochondria-associated membrane mediated via phosphoglycerate mutase 5 (PGAM5), which is a necrosome-associated protein regulating dynamic-related protein (Drp1) in turn (8). Finally, Drp1 induces mitochondrial fragmentation, a crucial event for necroptosis (11).
Fig. 1.
Necroptosis regulators and signaling pathways in necroptosis activation. Upon TNF receptor ligation, a signal transduction mediated via RIP1, caspase activation, and tBID cleavage occurs, resulting in apoptotic cell death. Active caspase-8 inhibits necroptosis via cleavage of RIP1 and RIP3. Inhibition of caspase leads to the formation of a physical complex of RIP1 with RIP3 to trigger downstream signaling events including MLKL and PGAM5 recruitment, and transduction of cytosolic death signals to mitochondria, resulting in necroptosis.
Biochemical changes during the early stages of necroptosis include ATP depletion, ROS generation, calcium overload, and loss of mitochondrial permeability transition (12). At the cellular level, necroptosis is characterized by loss of plasma membrane integrity as well as organelle swelling, finally leading to cellular collapse (10). Notably, necroptotic cells release various damage-associated molecular patterns (DAMPs) such as HMGB1, cytokines and histones into extracellular media. Particularly, a disulfide form of HMGB1 by oxidation contributes to the inflammatory response (13). HMGB1 binds to a cognate receptor on endothelial cells and macrophages to transduce cellular responses such as release of proinflammatory cytokines and chemotactic cell migration (14).
NECROPTOSIS: TARGET PROTEINS AND INHIBITORS
As described above, a series of necroptosis-associated proteins were identified and further validated as targets of necroptosis. Subsequently, a few small molecules were successfully discovered, specifically targeting necroptosis proteins. Target-specific small molecules that can modulate necroptosis are listed in Table 1.
Table 1.
Target proteins associated with necroptosis and their specific inhibitors
| Necroptosis Target proteins | Inhibitors | Mode of action/function |
|---|---|---|
| RIP-1 | Nec-1 | Allosteric inhibitor |
| Furo[2,3-d]pyrimidine derivatives | SAR reported | |
| GSK’963 | > 10000-fold selective for RIP1 | |
| RIP-3 | GSK’840, GSK’843, GSK’872 | Caspase activity activated |
| MLKL | Necrosulfonamide | Covalent binding |
| TC13172 | Covalent binding (nanomolar potency) | |
| GW806742X | Binds to pseudokinase domain (ATP-mimetic) | |
| Others | Ponatinib & pazopanib | Interfere with signaling proteins upstream of MLKL |
| Sorafenib | Target unknown | |
| TPCK | UCH-L1 activator | |
| Hydroxyanisole | ROS scavenger | |
| Diphenyleneiodonium (DPI) | ROS suppression |
RIP-1 inhibitors
Necrostatin-1 (Nec-1), 5-(1H-indol-3-ylmethyl)-2-thiohydantoin 1, was first discovered by screening of necroptosis inhibitors and later identified as an allosteric inhibitor of RIP1 via stabilization of an inactive conformation of the kinase domain (KD) (15). It has been widely validated in various necroptosis-associated animal models (16–19). Accordingly, Nec-1 plays a crucial role in prevention or alleviation of necroptotic damage caused by various stimuli via targeting RIP1. Another potent Nec-1 derivative Nec-1s (7-Cl-O-Nec-1) showed greater specificity to RIP1 compared with other kinases. Other inhibitors have been developed as RIP1 inhibitors, including furo[2,3-d]pyrimidines and GSK’963 (20, 21).
RIP3 inhibitors
Recent studies proposed that necroptosis is mediated in a RIP1- or RIP3-dependent manner (22, 23). Unlike RIP1, RIP3 is essential for necroptosis, but not apoptosis, and targeting RIP3 appears to be more specific in controlling necroptosis. Interestingly, silencing of RIP3 substantially protected cells and tissues against necroptosis (24). Small molecules such as GSK’840, GSK’843 and GSK’872 have been reported to suppress RIP3-dependent necroptosis (25). Mechanistically, these molecules form a complex with RIP3 to restore caspase activity. Consequently, RIP3 inhibitors potentially protect cells from diverse stimuli than RIP1 inhibitors.
MLKL inhibitors
Necrosulfonamide ((E)-N-(4-(N-(3-methoxypyrazin-2-yl) sulfamoyl) phenyl)-3-(5-nitrothiophene-2-yl) acrylamide, NAS) protects cells against TNF-induced necroptosis via covalent modification of a cysteine in MLKL (7). A compound TC13172 is an MLKL inhibitor with single nanomolar potency. It induced covalent binding at Cys-86 of MLKL (26). In addition, GW806742X targets the pseudo-kinase domain of MLKL with nanomolar inhibitory activity, protecting against necroptosis, although it shows off-target effects against other kinases (26).
Additional agents have been developed as inhibitors of necroptosis acting directly via targets that have yet to be designated. Ponatinib and pazopanib (27) are two anti-cancer agents identified from a representative panel of United States Food and Drug Administration (FDA)-approved drugs through phenotypic screening. Both drugs abrogate phosphorylation of MLKL during TNFα-induced necroptosis, suggesting interference with the signaling proteins upstream of MLKL. However, they do not rescue cells from apoptosis. Interestingly, ponatinib inhibits both RIP1 and RIP3 while pazopanib acts against preferential inhibitor of RIP1. Further, sorafenib is a multi-targeting kinase inhibitor with potent inhibitory activity against B-RAK, and is widely used for the treatment of acute leukemia. It blocks signaling target proteins upstream of MLKL such as RIP1 and RIP3, although its real target remains elusive (28). Therefore, sorafenib can be harnessed for fine-tuning of necroptosis-inducing agents.
TPCK
N-tosyl-l-phenylalanine-chloromethyl ketone (TPCK), a serine protease inhibitor, protects cells from TNF-mediated necroptosis. Using a TPCK probe, HtrA2/Omi has been identified as a target and it acts as an activator of ubiquitin C-terminal hydrolase (UCH-L1) resulting in necroptosis (29).
ROS scavengers
Reactive oxygen species (ROS) play a role in diverse signaling pathways, due to high reactivity with biomolecules such as proteins, DNA and lipids. Upon exposure of L929 cells to TNFα, necroptotic signaling generates ROS via mitochondrial transport chain but not cytosolic enzyme (30), which was strongly supported by reports suggesting that butylated hydroxyanisole blocks ROS accumulation and cell death (31). An NADPH oxidase inhibitor diphenyleneiodonium (DPI) protects renal tubular epithelial cells against necroptosis by blocking ROS generation (32).
PHYSIOLOGICAL AND PATHOLOGICAL SIGNIFICANCE OF NECROPTOSIS
Necroptosis was originally considered as undesirable cell death upon exposure to stimuli, inducing tissue damage. Furthermore, it is the alternative cell death induced under conditions of defective apoptosis. However, growing evidence suggests that necroptosis is mediated via an orchestrated and specialized pathway (33–35). Well-regulated cell death occurs in a range of biological phenomena such as development, immunology and differentiation. Further, extrinsic apoptosis and necroptosis contribute to host defense mechanism against microbial infection. Viruses such as adenoviruses, poxviruses, and herpes viruses evade host apoptosis machinery, and perpetuate if host cells only execute caspase-dependent default cell death (36–38). For instance, vaccinia viruses encode a caspase 8 inhibitor to block apoptotic cell death upon infection. Under this caspase-compromised condition, cells are committed to alternative necroptosis (6). The resulting necroptosis is vital to provoke innate immune response by killing virus-infected cells and releasing danger signals from host cells into external milieu. Furthermore, necroptosis in T cells regulates antigen-activated T-cell proliferation and survival. Caspase-8 negatively regulates necroptosis, promoting survival of activated T cells under physiological conditions. In mice lacking caspase-8, T cells fail to show immune response when infected with murine hepatitis virus (39).
Pathologically, evidence suggests that necroptotic cell death leads to various diseases. Sepsis is mainly caused by Gram-negative bacteria, which release endotoxin that elicits systemic inflammation via release of TNFα and IL-1 inflammatory cytokines. Necroptosis occurs in ischemia-reperfusion (I/R) injury and neurodegenerative diseases. During restoration of blood flow into tissues, tissue damage occurs with severe neutrophil infiltration and cytokine production. Furthermore, necroptosis is involved in traumatic brain and spinal cord injuries (19, 40). Excitotoxicity, Huntington’s disease and retinal degeneration are closely associated with necroptosis. Exposure to a specific RIP inhibitor Nec-1 effectively protects cells from necroptosis. Nec-1 protects hippocampal HT-22 cells against glutamate-induced oxytosis (41). Inhibition of RIP1 kinase or RIP3 silencing significantly rescues necroptotic cell death (42). Furthermore, Nec-1 reduces or delays necroptotic damage in transgenic mice expressing mutant Huntingtin protein, astrocytes from amytrophic lateral sclerosis, and retinal pigment epithelium (42–44). Interestingly, microbial infection induces necroptosis in host cells. Microbial proliferation occurs by circumventing default programmed cell death in the host. Necroptosis is induced by viral infections such as vaccinia virus (VV). VV expresses an inhibitor of caspase-1 and -8, diverting the host response toward necroptosis in a RIP3-dependent pathway (45). Further, Sendai virus induces necroptosis in neuroblastoma cells (17). Macrophage infected with S. typhimurium induces necroptotic cell death in a RIP1-and RIP3-dependent manner.
PERSPECTIVES OF NECROPTOSIS AND CONCLUSIONS
Necroptosis has been recognized as an alternative to apoptosis when cells are exposed to various stimuli under specific conditions.
As mentioned above, it induces a variety of pathological conditions including septic shock, acute pancreatitis and neuronal degeneration. Apoptotic death of damaged cells is removed by phagocytosis in macrophages or neighboring cells. Conversely, because loss of membrane integrity occurs in cells undergoing necroptosis, intracellular substances including heat-shock proteins and HMGB1 are released into extracellular media to provoke inflammation and immune responses.
In response to infectious viruses or intracellular bacterial pathogens escaping apoptotic cell death, host cells actively switch to necroptosis, causing premature assembly of virus particles or bacteria progeny and release of critical components triggering an immune response (46–48).
Similarly, necroptosis was demonstrated under conditions of chronic sterile inflammation such as alcoholic-induced liver injury and atherosclerosis (18, 49). Under these pathological conditions, necroptotic cells release DAMPS to trigger sterile inflammation, via unknown mechanism.
A potent and specific regulation of necroptosis is therefore, needed. Indeed, pharmacological blockade of necroptosis is of primary concern in the treatment of various diseases. Further studies will be extensively undertaken to identify the target molecules mediating the signal transduction leading to necroptosis and facilitate the discovery of mechanism-based inhibitors.
On the other hand, necroptosis induction can be actively harnessed via molecular switch or unmasking. Generally, cancer cells grow in an uncontrolled manner and further acquire mechanisms to evade cell death intrinsically or extrinsically. Chemotherapy or radiotherapy is mainly based on apoptosis through caspase activation. However, many cancers have developed strategies to disarm apoptotic machinery, including dysregulated apoptosis, activation of pro-survival signaling pathways and upregulation of drug transporters. Altered apoptosis contributes to drug resistance of cancer during chemotherapy. To overcome drug-resistant cancers, alternative cell death mechanisms such as necroptosis or autophagy can be considered. In previous reports, cancer cells that are refractory to apoptotic agents were shown to succumb to necroptosis-inducing agents (50). It is conceivable that resistance to apoptosis can be overcome by necroptosis, because necroptotic pathway is distinct from apoptotic mechanisms. Furthermore, several reports suggest that necroptosis contributes to suppression of tumorigenesis. Particularly, mutations in the CYLD gene aggressively facilitate carcinoma via upregulation of angiogenic factors (51). Further, RIP3 gene polymorphisms occur in non-Hodgkin’s lymphoma and levels of a spliced variant RIP3-γ are relatively high in colon and lung cancers (52, 53).
Practically, a few inducers of necroptosis have been reported to trigger necroptotic cell death in malignant cancers although their targets remain to be identified. Caspase activation is a prerequisite for apoptosis induction, and apoptotic cell death fails if caspases are blocked or compromised by unknown mechanisms. Under those conditions, cells activate necroptosis in response to cell death stimuli. In fact, a natural product shikonin induces necroptotic cell death in MCF-7 breast cancer cells that express Bcl-2 or Bcl-xL, which acquire multidrug resistance. Obatoclax, an antagonist of Bcl-2 family members, promotes necroptosis based on autophagy in acute lymphoblastic leukemia resistant to glucocorticoids. In addition, combined treatment of pancaspase inhibitors with 5-fluorouracil drives necroptosis in colorectal cancer (54). Furthermore, necroptotic cells trigger adaptive immunity in dendritic cells (DCs), which in turn activate CD8+ T cells for antitumor immunity (55).
However, necroptosis-based cancer therapy still remains elusive. It has been demonstrated that necroptosis in tumor microenvironment contributes to inflammation and cancer metastasis (16). Furthermore, the low expression of key modulators of necroptosis in specific cancers fails to induce necroptosis (56–58), resulting in cancer evasion.
In conclusion, suppression or enhancement of necroptosis is therapeutically effective in specific diseases. A comprehensive insight into the underlying mechanisms is needed to facilitate the diagnosis, biomarkers, and drug development in necroptosis-associated diseases. Most studies have focused on the identification of specific inhibitors targeting necroptosis, and their underlying mechanisms of regulation. As a result, a few small molecules have been discovered from the chemical library, and optimized for further clinical use.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19:2003–2014. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FK. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 2015;6:e1636. doi: 10.1038/cddis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miao B, Degterev A. Methods to analyze cellular necroptosis. Methods Mol Biol. 2009;559:79–93. doi: 10.1007/978-1-60327-017-5_6. [DOI] [PubMed] [Google Scholar]
- 4.Hitomi J, Christofferson DE, Ng A, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YS, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Wang H, Wang Z, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Feoktistova M, Leverkus M. Programmed necrosis and necroptosis signalling. FEBS J. 2015;282:19–31. doi: 10.1111/febs.13120. [DOI] [PubMed] [Google Scholar]
- 10.Belizario J, Vieira-Cordeiro L, Enns S. Necroptotic Cell Death Signaling and Execution Pathway: Lessons from Knockout Mice. Mediators Inflamm. 20152015 doi: 10.1155/2015/128076. 128076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank S, Gaume B, Bergmann-Leitner ES, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/S1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 12.Vanlangenakker N, Vanden Berghe T, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2008;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- 13.Janko C, Filipovic M, Munoz LE, et al. Redox modulation of HMGB1-related signaling. Antioxid Redox Signal. 2014;20:1075–1085. doi: 10.1089/ars.2013.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern D, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev. 2002;54:1615–1625. doi: 10.1016/S0169-409X(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 15.Degterev A, Maki JL, Yuan J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013;20:366. doi: 10.1038/cdd.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu ZY, Wu B, Guo YS, et al. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am J Cancer Res. 2015;5:3174–3185. [PMC free article] [PubMed] [Google Scholar]
- 17.Nomura M, Ueno A, Saga K, Fukuzawa M, Kaneda Y. Accumulation of cytosolic calcium induces necroptotic cell death in human neuroblastoma. Cancer Res. 2014;74:1056–1066. doi: 10.1158/0008-5472.CAN-13-1283. [DOI] [PubMed] [Google Scholar]
- 18.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Wang H, Tao Y, Zhang S, Wang J, Feng X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience. 2014;266:91–101. doi: 10.1016/j.neuroscience.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Bandyopadhyay D, Berger SB, et al. Discovery of small molecule RIP1 kinase inhibitors for the treatment of pathologies associated with necroptosis. ACS Med Chem Lett. 2013;4:1238–1243. doi: 10.1021/ml400382p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger SB, Harris P, Nagilla R, et al. Characterization of GSK’963: a structurally distinct, potent and selective inhibitor of RIP1 kinase. Cell Death Discov. 2015;1:15009. doi: 10.1038/cddiscovery.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Shim JH, Cho YS. Distinctive roles of receptor-interacting protein kinases 1 and 3 in caspase-independent cell death of L929. Cell Biochem Funct. 2014;32:62–69. doi: 10.1002/cbf.2972. [DOI] [PubMed] [Google Scholar]
- 24.Fettweis G, Di Valentin E, L’Homme L, et al. RIP3 antagonizes a TSC2-mediated pro-survival pathway in glioblastoma cell death. Biochim Biophys Acta. 20171864:113–124. doi: 10.1016/j.bbamcr.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Mandal P, Berger SB, Pillay S, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan B, Liu L, Huang S, et al. Discovery of a new class of highly potent necroptosis inhibitors targeting the mixed lineage kinase domain-like protein. Chem Commun (Camb) 2017;53:3637–3640. doi: 10.1039/C7CC00667E. [DOI] [PubMed] [Google Scholar]
- 27.Fauster A, Rebsamen M, Huber KV, et al. A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis. 2015;6:e1767. doi: 10.1038/cddis.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldmann F, Schenk B, Martens S, Vandenabeele P, Fulda S. Sorafenib inhibits therapeutic induction of necroptosis in acute leukemia cells. Oncotarget. 2017;8:68208–68220. doi: 10.18632/oncotarget.19919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosna J, Voigt S, Mathieu S, et al. The proteases HtrA2/Omi and UCH-L1 regulate TNF-induced necroptosis. Cell Commun Signal. 2013;11:76. doi: 10.1186/1478-811X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ardestani S, Deskins DL, Young PP. Membrane TNF-alpha-activated programmed necrosis is mediated by ceramide-induced reactive oxygen species. J Mol Signal. 2013;8:12. doi: 10.1186/1750-2187-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y, Choksi S, Shen HM, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 32.Dong W, Li Z, Chen Y, et al. NADPH oxidase inhibitor, diphenyleneiodonium prevents necroptosis in HK-2 cells. Biomed Rep. 2017;7:226–230. doi: 10.3892/br.2017.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulda S. Regulation of necroptosis signaling and cell death by reactive oxygen species. Biol Chem. 2016;397:657–660. doi: 10.1515/hsz-2016-0102. [DOI] [PubMed] [Google Scholar]
- 34.Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135:1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Zhang K, Shen H, Yao X, Sun Q, Chen G. Necroptosis: A novel manner of cell death, associated with stroke (Review) Int J Mol Med. 2018;41:624–630. doi: 10.3892/ijmm.2017.3279. [DOI] [PubMed] [Google Scholar]
- 36.Benedict CA, Norris PS, Prigozy TI, et al. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and -2. J Biol Chem. 2001;276:3270–3278. doi: 10.1074/jbc.M008218200. [DOI] [PubMed] [Google Scholar]
- 37.Nichols DB, De Martini W, Cottrell J. Poxviruses Utilize Multiple Strategies to Inhibit Apoptosis. Viruses. 2017;9 doi: 10.3390/v9080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jerome KR, Fox R, Chen Z, Sears AE, Lee H, Corey L. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J Virol. 1999;73:8950–8957. doi: 10.1128/jvi.73.11.8950-8957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu JVWeist BM, van Raam BJ, et al. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci U S A. 2011;108:15312–15317. doi: 10.1073/pnas.1102779108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YQ, Wang L, Zhang MY, et al. Necrostatin-1 suppresses autophagy and apoptosis in mice traumatic brain injury model. Neurochem Res. 2012;37:1849–1858. doi: 10.1007/s11064-012-0791-4. [DOI] [PubMed] [Google Scholar]
- 41.Xu X, Chua CC, Kong J, et al. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem. 2007;103:2004–2014. doi: 10.1111/j.1471-4159.2007.04884.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhu S, Zhang Y, Bai G, Li H. Necrostatin-1 ameliorates symptoms in R6/2 transgenic mouse model of Huntington’s disease. Cell Death Dis. 2011;2:e115. doi: 10.1038/cddis.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Re DB, Le Verche V, Yu C, et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81:1001–1008. doi: 10.1016/j.neuron.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami Y, Matsumoto H, Roh M, et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2014;21:270–277. doi: 10.1038/cdd.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veyer DL, Carrara G, Maluquer de Motes C, Smith GL. Vaccinia virus evasion of regulated cell death. Immunol Lett. 2017;186:68–80. doi: 10.1016/j.imlet.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sridharan H, Upton JW. Programmed necrosis in microbial pathogenesis. Trends Microbiol. 2014;22:199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Kaminskyy V, Zhivotovsky B. To kill or be killed: how viruses interact with the cell death machinery. J Intern Med. 2010;267:473–482. doi: 10.1111/j.1365-2796.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 49.Lin J, Li H, Yang M, et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Han W, Li L, Qiu S, et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther. 2007;6:1641–1649. doi: 10.1158/1535-7163.MCT-06-0511. [DOI] [PubMed] [Google Scholar]
- 51.Alameda JP, Moreno-Maldonado R, Navarro M, et al. An inactivating CYLD mutation promotes skin tumor progression by conferring enhanced proliferative, survival and angiogenic properties to epidermal cancer cells. Oncogene. 2010;29:6522–6532. doi: 10.1038/onc.2010.378. [DOI] [PubMed] [Google Scholar]
- 52.Yang Y, Hu W, Feng S, Ma J, Wu M. RIP3 beta and RIP3 gamma, two novel splice variants of receptor-interacting protein 3 (RIP3), downregulate RIP3-induced apoptosis. Biochem Biophys Res Commun. 2005;332:181–187. doi: 10.1016/j.bbrc.2005.04.114. [DOI] [PubMed] [Google Scholar]
- 53.Wu W, Liu P, Li J. Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol. 2012;82:249–258. doi: 10.1016/j.critrevonc.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Oliver Metzig M, Fuchs D, Tagscherer KE, Grone HJ, Schirmacher P, Roth W. Inhibition of caspases primes colon cancer cells for 5-fluorouracil-induced TNF-alpha-dependent necroptosis driven by RIP1 kinase and NF-kappaB. Oncogene. 2016;35:3399–3409. doi: 10.1038/onc.2015.398. [DOI] [PubMed] [Google Scholar]
- 55.Najafov A, Chen H, Yuan J. Necroptosis and Cancer. Trends Cancer. 2017;3:294–301. doi: 10.1016/j.trecan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu W, Zhu H, Fu Y, et al. Clinical significance of down-regulated cylindromatosis gene in chronic lymphocytic leukemia. Leuk Lymphoma. 2014;55:588–594. doi: 10.3109/10428194.2013.809077. [DOI] [PubMed] [Google Scholar]
- 57.Feng X, Song Q, Yu A, Tang H, Peng Z, Wang X. Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma. 2015;62:592–601. doi: 10.4149/neo_2015_071. [DOI] [PubMed] [Google Scholar]
- 58.Ruan J, Mei L, Zhu Q, Shi G, Wang H. Mixed lineage kinase domain-like protein is a prognostic biomarker for cervical squamous cell cancer. Int J Clin Exp Pathol. 2015;8:15035–15038. [PMC free article] [PubMed] [Google Scholar]