Abstract
Bone resorption by multinucleated osteoclasts is a multistep process involving adhesion to the bone matrix, migration to resorption sites, and formation of sealing zones and ruffled borders. Macrophage colony-stimulating factor (M-CSF) and osteopontin (OPN) have been shown to be involved in the bone resorption process by respective activation of integrin αvβ3 via “inside-out” and “outside-in” signaling. In this study, we investigated the link between signal modulators known to M-CSF- and OPN-induced osteoclast adhesion and spreading. M-CSF- and OPN-induced osteoclast adhesion was achieved via activation of stepwise signals, including integrin αvβ3, PLCγ, PKCδ, and Rac1. Osteoclast spreading induced by M-CSF and OPN was shown to be controlled via sequential activation, consistent with the osteoclast adhesion processes. In contrast to osteoclast adhesion, osteoclast spreading induced by M-CSF and OPN was blocked via activation of PLCγ/PKCα/RhoA signaling. The combined results indicate that osteoclast adhesion and spreading are selectively regulated via PLCγ/PKCα-PKCδ/RhoA-Rac1 signaling.
Keywords: Integrin αvβ3, Osteoclast adhesion, Osteoclast spreading, Phospholipase C, Protein kinase C
INTRODUCTION
Osteoclast-mediated bone resorption is known to be a critical process in the development and physiology of the skeleton (1). Multinucleated mature osteoclasts repeatedly resorb old bone matrix and migrate to future bone resorption sites in a process referred to as the resorption cycle (2, 3). The resorption cycle of osteoclasts plays an important role in bone remodeling (4). In the initial stage of bone resorption, osteoclasts adhere to the bone surface via interactions with integrin, a large family of cell adhesion receptors. Integrin consists of α and β subunits and transmits cell-cell and cell-extracellular matrix (ECM) interactions (5). Binding of integrin to its ligand activates signal transduction pathways, which lead to cell adhesion, spreading, and cytoskeletal reorganization. Among integrin isoforms, integrin αvβ3 is predominantly expressed on the cytoplasmic surface of osteoclasts and interacts with bone matrix proteins such as osteopontin (OPN) and bone sialoprotein II (6) as well as participates in adhesion to bone, cytoskeletal reorganization, and bone resorption. It is also known that macrophage colony-stimulating factor (M-CSF) and hepatocyte growth factor (HGF) mediate osteoclast adhesion and spreading via integrin αvβ3-dependent mode (5). Coupling between αvβ3 and its binding partner triggers multiple signaling factors, such as phosphatidylinositol 3-kinase (PI3K), protein kinase C (PKC), phospholipase C (PLC), proline-rich tyrosine kinase (PYK2), c-Src, and small GTPases (7–12). Specifically, binding of M-CSF to its receptor, c-Fms, activates αvβ3 and subsequent downstream signaling molecules, including PLCγ, PI3K, and small GTPases, via “inside-out” signaling (5, 7, 10). Further, direct interaction of OPN with αvβ3 was found to activate PYK2, c-Src, PI3K, and PLCγ via “outside in” signaling (10, 13) as well as enhance osteoclast survival and function by facilitating the Ca2+-dependent transcription factor NFATc1 pathway (14), which is essential for osteoclast differentiation.
PLCγ is a common downstream effector for integrin αvβ3-and M-CSF-mediated signaling in pre-fusion osteoclasts (10). Suppression of PLCγ2 has been shown to reduce cell adhesion, migration, and bone resorption in osteoclasts (15). PLC generates diacylglycerol and inositol-triphosphate, leading to PKC activation and calcium release from the endoplasmic reticulum (16). PKCα is thought to play a key role in integrin αvβ3-mediated signal transduction, osteoclast migration, and bone resorption (12). On the contrary, the functional roles of other PKC isoforms in osteoclast adhesion and spreading have not been fully understood. PKC is known to activate downstream small GTPase cascades involved in the modulation of integrin-mediated cytoskeletal organization (17, 18). For example, small GTPases (Rho and Rac) have been reported to organize and maintain cellular cytoskeletal structures in osteoclasts (19). Despite osteoclast adhesion and spreading being associated with various signal molecules, sequential coordination between signal modulators in osteoclast adhesion and spreading induced by M-CSF and OPN is not well understood. Here, we observed that consecutive activation of the PLCγ/PKCδ/Rac1 signal axis governs osteoclast adhesion and spreading induced by M-CSF and OPN. Further, the results show that signal activation via PLCγ/PKCα/RhoA plays a negative role in osteoclast spreading but not osteoclast adhesion.
RESULTS AND DISCUSSION
Osteoclast adhesion and spreading mediated by M-CSF and OPN share integrin αvβ3/PLCγ signaling
Growth factors and extracellular matrix proteins have been identified as activators for inducing cell adhesion and spreading in an integrin-dependent manner (5, 6, 10, 20). We first examined the effects of M-CSF and OPN on osteoclast adhesion and spreading. M-CSF and OPN promoted adhesion and spreading of mature osteoclasts (Fig. 1). M-CSF and OPN in osteoclasts are known to converge at integrin αvβ3 activation via “inside-out” signaling and “outside-in” signaling, respectively (5, 6). Thus, we investigated whether or not enhanced adhesion and spreading induced by M-CSF or OPN in mature osteoclasts are dependent upon integrin αvβ3. Treatment with functional blocking antibodies against integrins αv and β3 suppressed osteoclast adhesion and spreading in response to M-CSF and OPN (Fig. 2A and B). These results indicate that M-CSF and OPN facilitate osteoclast adhesion and spreading via an integrin αvβ3-dependent pathway. Since integrin αvβ3 was reported to activate PLCγ1 and PLCγ2 as well as foster their recruitment to αvβ3 in pre-fusion osteoclasts (10), we also examined whether or not PLCγ activation is required for M-CSF- and OPN-induced adhesion and spreading in osteoclasts. Treatment with PLCγ inhibitor (U73122) blocked M-CSF- and OPN-induced osteoclast adhesion and spreading in a dose-dependent manner (Fig. 2C and D). These results indicate that M-CSF- and OPN-induced osteoclast adhesion and spreading progress via “inside-out” signaling and “outside-in” signaling depending on integrin αvβ3, respectively, and share αvβ3 and PLCγ signaling.
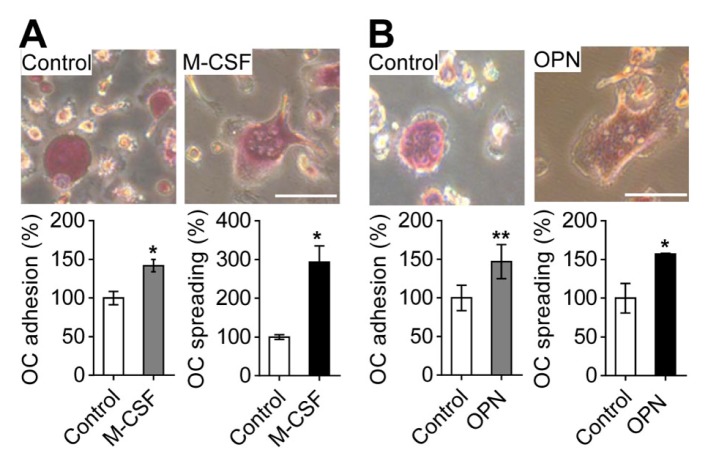
Fig. 1.
M-CSF- and OPN-induced osteoclast adhesion and spreading. (A) Effects of M-CSF on osteoclast adhesion and spreading. For osteoclast adhesion and spreading induced by M-CSF, suspended osteoclasts treated with M-CSF (30 ng/ml) were seeded on culture plates and incubated for 1 h (for cell adhesion) or 4 h (for cell spreading). (B) Effects of OPN on osteoclast adhesion and spreading. For osteoclast adhesion and spreading by OPN, osteoclasts were incubated in OPN-coated culture plates for 1 h (for cell adhesion) or 4 h (for cell spreading). The attached osteoclasts were fixed and stained with TRAP. Osteoclast adhesion and spreading were assessed by counting the number of TRAP-positive multinucleated osteoclasts and by measuring the surface area of osteoclasts, respectively. The upper panel shows representative phase-contrast images. Data are expressed as the mean ± standard deviation (S.D.) (n = 3) and presented as mean percentage relative to control. *P < 0.01; **P < 0.05. Scale bars, 50 μm.
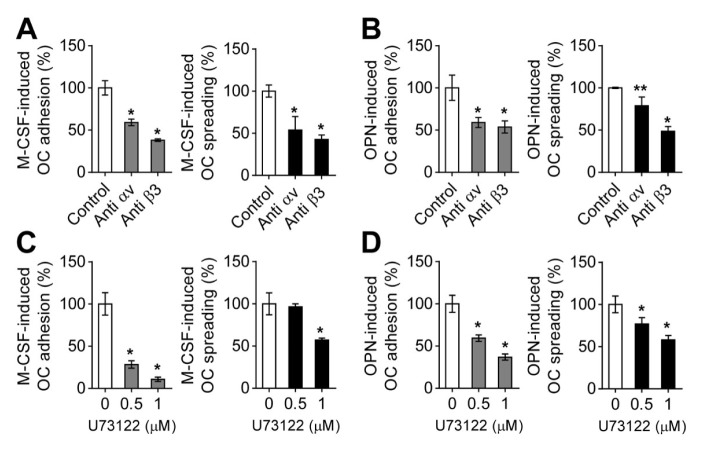
Fig. 2.
Osteoclast adhesion and spreading induced by M-CSF or OPN are dependent on integrin αvβ3 and PLCγ. (A) Effects of functional blocking antibodies against integrins αv and β3 on M-CSF-mediated osteoclast adhesion and spreading. For cell adhesion, suspended osteoclasts treated with anti-integrin αv antibody (10 μg/ml) or anti-integrin β3 antibody (10 μg/ml) for 15 min were exposed to M-CSF (30 ng/ml), seeded on culture plates, and incubated for 1 h. For cell spreading, attached osteoclasts treated with M-CSF for 1 h were incubated with anti-integrin αv antibody (10 μg/ml) or anti-integrin β3 antibody (10 μg/ml) for 3 h. (B) Effects of functional blocking antibodies against integrins αv and β3 on OPN-mediated osteoclast adhesion and spreading. For cell adhesion, suspended osteoclasts treated with the indicated antibodies for 15 min were seeded on OPN-coated culture plates and incubated for 1 h. For cell spreading, the suspended osteoclasts were incubated in OPN-coated culture plates for 1 h and further treated with the indicated antibodies for 3 h. (C and D) Effects of PLCγ inhibitor on osteoclast adhesion and spreading mediated by M-CSF or OPN. Assessment of M-CSF- and OPN-mediated osteoclast adhesion and spreading using PLCγ inhibitor (U73122) was performed according to the same procedures as in (A) and (B). Then, osteoclast adhesion and spreading were evaluated as described in the part of Materials and Methods. Data are expressed as the mean ± standard deviation (S.D.) (n = 3) and presented as mean percentage relative to control. *P < 0.01; **P < 0.05.
PLCγ activates PKCα and PKCδ during osteoclast adhesion and spreading
PKCs are known to be downstream effectors of integrin-mediated PLCγ signaling (21) and M-CSF has been reported to specifically stimulate PKCα and PKCδ among PKC isoforms in osteoclast precursors (22). Here, we investigated the relationship between PLCγ and PKC upon treatment with M-CSF or OPN in mature osteoclasts. PKCα and PKCδ were efficiently translocated into the osteoclastic cytoplasmic membrane in response to M-CSF or OPN (Fig. 3A and B). Moreover, phosphorylation levels of PKCα and PKCδ in membrane fractions were elevated after treatment with M-CSF or OPN. However, active forms of PKCα and PKCδ induced by M-CSF or OPN disappeared after treatment with PLCγ inhibitor (U73122). These findings indicate that M-CSF or OPN induces serial activation of PLCγ and PKCα/PKCδ signal during osteoclast adhesion and spreading. Further, we observed that osteoclast adhesion and spreading induced by M-CSF or OPN were suppressed by treatment with PKCδ inhibitor (rottlerin) but not PKCα inhibitor (Gö6976) (Fig. 3C and D). Interestingly, PKCα inactivation led to an approximately 1.5-fold increase in M-CSF- and OPN-induced osteoclast spreading compared to the control, indicating that PKCα negatively regulates osteoclast spreading. These results indicate that PKCδ is necessary for both osteoclast adhesion and spreading, whereas PKCα plays a negative role in osteoclast spreading.
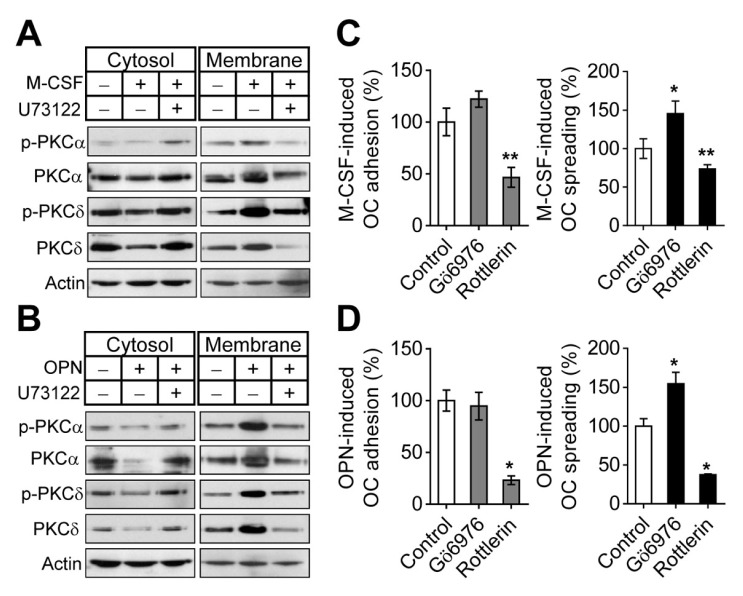
Fig. 3.
PLCγ regulates PKCα and PKCδ in osteoclast adhesion and spreading. (A and B) M-CSF or OPN activates PKCα and PKCδ via PLCγ signaling. (A) Mature osteoclasts were exposed to M-CSF-starved conditions for 4 h, treated with PLCγ inhibitor (U73122; 1 μM) for 30 min, and stimulated with M-CSF (30 ng/ml) for 5 min. (B) M-CSF-starved suspended osteoclasts pretreated with PLCγ inhibitor (U73122; 1 μM) for 15 min were seeded on OPN-coated culture plates and further incubated for 30 min. Fractionated cytosolic and membranous proteins were subjected to immunoblot analysis with the indicated antibodies. Actin was used for a loading control. (C and D) PKCα and PKCδ signals involved in osteoclast adhesion and spreading induced by M-CSF or OPN. Effects of PKCα inhibitor (Gö6976; 1 μM) and PKCδ inhibitor (rottlerin; 5 μM) on M-CSF- and OPN-mediated osteoclast adhesion and spreading were assessed by the same procedures as described in the legend of Fig. 2. Data are expressed as the mean ± S.D. (n = 3) and presented as mean percentage relative to control. *P < 0.01; **P < 0.05.
PKCα and PKCδ selectively regulate activities of RhoA and Rac1 during osteoclast adhesion and spreading
Small GTPases have been reported to regulate integrin-dependent cell morphological changes, including formation of stress fibers, lamellipodia, and filopodia (5, 23, 24). It is also known that small GTPases (Rho and Rac) regulate cell spreading and cytoskeleton organization (2, 19). Since small GTPases are reported to be downstream effectors of the PKC signaling pathway (22, 25), we investigated whether or not M-CSF or OPN is involved in regulating the activities of small GTPases (RhoA and Rac1) in osteoclasts. As shown in Fig. 4A and B, elevation of RhoA activity by M-CSF or OPN was reduced by treatment with either PKCα inhibitor (Gö6976) or PKCδ inhibitor (rottlerin). Specifically, the stimulatory effect of M-CSF and OPN on Rac1 activity was suppressed by treatment with PKCδ inhibitor but not PKCα inhibitor. These results indicate that PKCδ controls both RhoA and Rac1 activities, and PKCα regulates only RhoA activity during M-CSF- and OPN-induced osteoclast stimulation. Further, we observed that osteoclast adhesion and spreading induced by M-CSF or OPN were suppressed by treatment with Rac1 inhibitor (NSC23766) but not RhoA inhibitor (Y27632) (Fig. 4C and D). Particularly, RhoA inactivation led to an approximately 1.5-fold increase in M-CSF- and OPN-induced osteoclast spreading compared to the control, indicating that RhoA signaling is involved in inhibition of osteoclast spreading. The combined results indicate that PKCδ-mediated Rac1 activation is involved in both osteoclast adhesion and spreading, whereas PKCα-mediated RhoA activation negatively regulates osteoclast spreading but not osteoclast adhesion. Seesaw-like crosstalk between extracellular signal-regulated kinase (ERK) and p38 activation has been reported to occur during RANKL-induced osteoclastogenesis (26, 27). Treatment with p38 inhibitors resulted in increased ERK activation, and ERK inhibitors caused an increase in p38 activation. Consistent with these results, it is also possible that the treatment of PKCα or RhoA inhibitors may lead to the activation of PKCδ or Rac1.
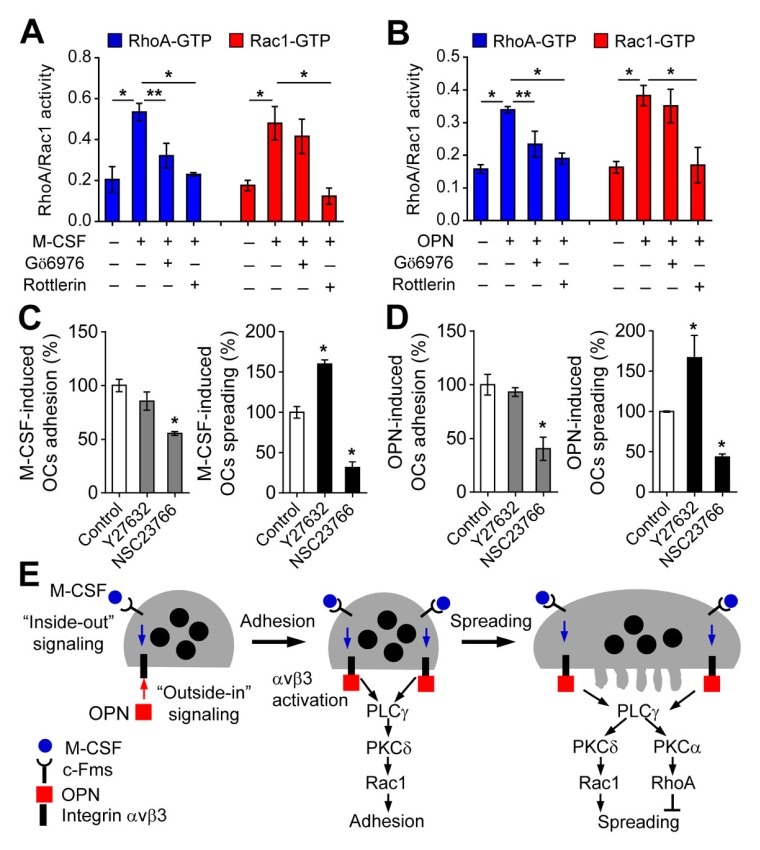
Fig. 4.
Small GTPases activated by M-CSF or OPN modulate osteoclast adhesion and spreading. (A and B) PKCα and PKCδ activated by M-CSF or OPN modulate the activities of RhoA and Rac1. Effects of PKCα inhibitor (Gö6976; 1 μM) and PKCδ inhibitor (rottlerin; 5 μM) on RhoA and Rac1 activities were assessed and data are expressed as the mean ± S.D. (n = 3). *P < 0.01; **P < 0.05. (C and D) The effects of RhoA inhibitor (Y27632; 10 μM) and Rac1 inhibitor (NSC23766; 100 μM) on M-CSF- and OPN-mediated osteoclast adhesion and spreading were assessed by the same procedures as described in the legend of Fig. 2. Data are expressed as the mean ± S.D. (n = 3) and presented as mean percentage relative to control. *P < 0.01; **P < 0.05. (E) Proposed model for M-CSF- and OPN-induced osteoclast adhesion and spreading. Integrin αvβ3 activation in response to M-CSF or OPN induces osteoclast adhesion via PLCγ/PKCδ/Rac1 signal transduction. After cell adhesion, osteoclast spreading was activated via integrin αvβ3-mediated PLCγ/PKCδ/Rac1 signaling but suppressed via PLCγ/PKCα/RhoA signaling.
For bone resorption, multinucleated osteoclasts derived from hematopoietic stem cells adhere to the bone surface via integrin αvβ3 present in the cytoplasmic membrane, resulting in the formation of a large and dense F-actin ring called the sealing zone and ruffled border (1, 2, 28). Osteoclast adhesion and subsequent spreading are critical in the initial stage of bone resorption by osteoclasts. Afterward, osteoclasts can resorb old or damaged bone by sequential repeated processes, including cell contraction, cell spreading by disassembling the sealing zone, and cell migration.
Here, we analyzed the regulatory mechanisms underlying osteoclast adhesion and spreading induced by M-CSF or OPN. As summarized in Fig. 4E, integrin αvβ3 in the surface of osteoclasts is activated by binding of M-CSF to c-Fms receptor in a process termed “inside-out” signaling. Further, OPN directly binds to and stimulates integrin αvβ3 in a process termed “outside-in” signaling. Integrin αvβ3 activated by M-CSF or OPN allows the initial adhesion of osteoclasts for bone resorption and subsequent spreading process via PLCγ/PKCδ/Rac1 signaling. Additionally, we observed that PLCγ/PKCα/RhoA signaling stimulated by M-CSF or OPN plays a negative role in osteoclast spreading with no effect on osteoclast adhesion. Overall, our findings suggest that osteoclast adhesion and spreading are differentially regulated via PLCγ/PKCα-PKCδ/RhoA-Rac1 signaling.
MATERIALS AND METHODS
Antibodies and inhibitors
Antibodies specific for phosphorylated-PKCα and phosphorylated-PKCδ were from Cell Signaling (Beverly, MA, USA); antibodies for PKCα, PKCδ, integrins αv and β3, and actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). U73122 was obtained from Sigma-Aldrich (St. Louis, MO, USA). Gö6976, rottlerin, Y27632, and NSC23766 were from Calbiochem (San Diego, CA, USA).
Osteoclast differentiation
Bone marrow-derived monocytes were isolated from the long bones of 6-week-old C57BL/6 male mice by flushing the bone marrow cavity with minimum essential medium-alpha (α-MEM; Hyclone, Logan, Utah, USA). Cells were centrifuged and the red blood cells were removed using red blood cell lysis buffer (Sigma-Aldrich). Next, cells were incubated with α-MEM containing 10% fetal bovine serum (FBS; Hyclone) and M-CSF (5 ng/ml) for 12 h. The non-adherent cells were collected, plated on 100-mm culture dishes, and further cultured for 3 days with α-MEM containing M-CSF (30 ng/ml) to generate bone marrow-derived osteoclast precursors. Osteoclast precursors were differentiated into osteoclasts in α-MEM supplemented with M-CSF (30 ng/ml) and receptor activator of nuclear factor κB ligand (RANKL; 100 ng/ml) for 4 days with a change of medium on day 2.
Cell adhesion and spreading assays
Cell adhesion and spreading assays were performed as previously described (29). For M-CSF-induced osteoclast adhesion and spreading assays, osteoclasts were detached with cell dissociation solution (Sigma-Aldrich) and resuspended in α-MEM. The suspended osteoclasts treated with M-CSF (30 ng/ml) were seeded at a density of 2 × 104 cells per well in 96-well culture plates and then incubated at 37°C for 1 h (for cell adhesion) or 4 h (for cell spreading). For OPN-coated osteoclast adhesion and spreading experiments, 96-well culture plates were coated with OPN (10 μg/ml; Sigma-Aldrich) for 12 h at 4°C. The OPN-coated plates were blocked with 1% bovine serum albumin (BSA) in PBS for 1 h at 37°C and then washed with PBS. The osteoclasts detached from culture dish were seeded at a density of 2 × 104 cells per well in OPN-coated 96-well culture plates and incubated at 37°C 1 h (for cell adhesion) or 4 h (for cell spreading). The attached osteoclasts were fixed with 3.7% (v/v) formaldehyde and stained for tartrate-resistant acid phosphatase (TRAP) with a leukocyte acid phosphatase staining kit provided from Sigma-Aldrich. To assess cell adhesion, TRAP-positive multinucleated osteoclasts were counted under a light microscope. The extent of cell spreading was evaluated by measuring the surface area of osteoclasts using Image-Pro plus software (Version 6.0; Media Cybernetics, Rockville, MD, USA). Data are presented as the mean percentage relative to the control.
Subcellular fractionation and immunoblot analysis
Subcellular fractionation of cytosolic and membrane proteins was carried out using a plasma membrane protein extraction kit (Biovision, Milpitas, CA, USA) according to the manufacturer’s protocols as described previously (29). After cells were lysed with homogenization buffer (Biovision) and centrifuged at 12,000 × g for 30 min, the supernatants were collected as the cytosolic fraction. The pellets were solubilized with RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP40, 1% SDS, 1 mM PMSF, 1 mM Na3VO4, 1 mM glycerol phosphate, and protease inhibitor cocktail) and sonicated for 30 s on ice. After centrifugation at 10,000 × g for 30 min, the supernatants were collected as the membrane fraction. The fractionated proteins were denatured using SDS sample buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 0.1% bromophenol blue, and 50 mM dithiothreitol) and subjected to 10% SDS-PAGE. The separated proteins were electro-transferred to nitrocellulose membranes and probed with primary antibodies and appropriate HRP-conjugated secondary antibodies. All blots were then developed with ECL reagent (Abfrontier, Seoul, Korea).
Small GTPases activation assays
Small GTPase activation assays were performed using a small GTPase G-LISA activation assay kit (Cytoskeleton Inc., Denver, CO, USA) according to the manufacturer’s procedures. To assess relative activities of small GTPases (RhoA and Rac1), the resultant absorbance was read at 450 nm using an ELISA microplate reader model 680 (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All values are presented as means ± S.D. (n = 3). The difference among multiple groups was analyzed using one-way ANOVA analysis with Tukey’s test. P value of < 0.05 was considered to be significant. These analyses were performed using the program GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA).
ACKNOWLEDGEMENTS
This work was supported by grants from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, Family Affairs, Republic of Korea (No. HI15C2164 to D.J.), and the National Research Foundation of Korea (No. 2015R1A5A2 009124 and 2016R1A2B2012108 to D.J. and 2016R1A6A 3A11930818 to K.L.; Research Fellow).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 2.Touaitahuata H, Blangy A, Vives V. Modulation of osteoclast differentiation and bone resorption by Rho GTPases. Small GTPases. 2014;5:e28119. doi: 10.4161/sgtp.28119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgess D, Machuca-Gayet I, Blangy A, Jurdic P. Podosome organization drives osteoclast-mediated bone resorption. Cell Adh Migr. 2014;8:191–204. doi: 10.4161/cam.27840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285:25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faccio R, Novack DV, Zallone A, Ross FP, Teitelbaum SL. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by beta3 integrin. J Cell Biol. 2003;162:499–509. doi: 10.1083/jcb.200212082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross FP, Chappel J, Alvarez JI, et al. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993;268:9901–9907. [PubMed] [Google Scholar]
- 7.Ross FP, Teitelbaum SL. alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 8.Pfaff M, Jurdic P. Podosomes in osteoclast-like cells: structural analysis and cooperative roles of paxillin, proline-rich tyrosine kinase 2 (Pyk2) and integrin alphaVbeta3. J Cell Sci. 2001;114:2775–2786. doi: 10.1242/jcs.114.15.2775. [DOI] [PubMed] [Google Scholar]
- 9.Lakkakorpi PT, Wesolowski G, Zimolo Z, Rodan GA, Rodan SB. Phosphatidylinositol 3-kinase association with the osteoclast cytoskeleton, and its involvement in osteoclast attachment and spreading. Exp Cell Res. 1997;237:296–306. doi: 10.1006/excr.1997.3797. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura I, Lipfert L, Rodan GA, Le TD. Convergence of alpha(v)beta(3) integrin- and macrophage colony stimulating factor-mediated signals on phospholipase Cgamma in prefusion osteoclasts. J Cell Biol. 2001;152:361–373. doi: 10.1083/jcb.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, Udagawa N, Nakamura I, et al. The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. J Cell Sci. 1995;108(Pt 6):2285–2292. doi: 10.1242/jcs.108.6.2285. [DOI] [PubMed] [Google Scholar]
- 12.Rucci N, DiGiacinto C, Orru L, Millimaggi D, Baron R, Teti A. A novel protein kinase C alpha-dependent signal to ERK1/2 activated by alphaVbeta3 integrin in osteoclasts and in Chinese hamster ovary (CHO) cells. J Cell Sci. 2005;118:3263–3275. doi: 10.1242/jcs.02436. [DOI] [PubMed] [Google Scholar]
- 13.Horne WC, Sanjay A, Bruzzaniti A, Baron R. The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol Rev. 2005;208:106–125. doi: 10.1111/j.0105-2896.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanabe N, Wheal BD, Kwon J, et al. Osteopontin signals through calcium and nuclear factor of activated T cells (NFAT) in osteoclasts: a novel RGD-dependent pathway promoting cell survival. J Biol Chem. 2011;286:39871–39881. doi: 10.1074/jbc.M111.295048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epple H, Cremasco V, Zhang K, Mao D, Longmore GD, Faccio R. Phospholipase Cgamma2 modulates integrin signaling in the osteoclast by affecting the localization and activation of Src kinase. Mol Cell Biol. 2008;28:3610–3622. doi: 10.1128/MCB.00259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 17.Tatin F, Varon C, Genot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–781. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- 18.Jung SY, Kim OB, Kang HK, Jang DH, Min BM, Yu FH. Protein kinase Calpha/beta inhibitor Go6976 promotes PC12 cell adhesion and spreading through membrane recruitment and activation of protein kinase Cdelta. Exp Cell Res. 2013;319:153–160. doi: 10.1016/j.yexcr.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Ory S, Munari-Silem Y, Fort P, Jurdic P. Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J Cell Sci. 2000;113(Pt 7):1177–1188. doi: 10.1242/jcs.113.7.1177. [DOI] [PubMed] [Google Scholar]
- 20.Teti A, Taranta A, Migliaccio S, et al. Colony stimulating factor-1-induced osteoclast spreading depends on substrate and requires the vitronectin receptor and the c-src proto-oncogene. J Bone Miner Res. 1998;13:50–58. doi: 10.1359/jbmr.1998.13.1.50. [DOI] [PubMed] [Google Scholar]
- 21.Miranti CK, Ohno S, Brugge JS. Protein kinase C regulates integrin-induced activation of the extracellular regulated kinase pathway upstream of Shc. J Biol Chem. 1999;274:10571–10581. doi: 10.1074/jbc.274.15.10571. [DOI] [PubMed] [Google Scholar]
- 22.Kim JM, Kim MY, Lee K, Jeong D. Distinctive and selective route of PI3K/PKCalpha-PKCdelta/RhoA-Rac1 signaling in osteoclastic cell migration. Mol Cell Endocrinol. 2016;437:261–267. doi: 10.1016/j.mce.2016.08.042. [DOI] [PubMed] [Google Scholar]
- 23.Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110(Pt 6):707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- 24.Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J Cell Sci. 2004;117:1259–1268. doi: 10.1242/jcs.00997. [DOI] [PubMed] [Google Scholar]
- 25.Kandabashi T, Shimokawa H, Miyata K, et al. Evidence for protein kinase C-mediated activation of Rho-kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol. 2003;23:2209–2214. doi: 10.1161/01.ATV.0000104010.87348.26. [DOI] [PubMed] [Google Scholar]
- 26.Hotokezaka H, Sakai E, Kanaoka K, et al. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J Biol Chem. 2002;277:47366–47372. doi: 10.1074/jbc.M208284200. [DOI] [PubMed] [Google Scholar]
- 27.Lee K, Chung YH, Ahn H, Kim H, Rho J, Jeong D. Selective Regulation of MAPK Signaling Mediates RANKL-dependent Osteoclast Differentiation. Int J Biol Sci. 2016;12:235–245. doi: 10.7150/ijbs.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura I, Pilkington MF, Lakkakorpi PT, et al. Role of alpha(v)beta(3) integrin in osteoclast migration and formation of the sealing zone. J Cell Sci. 1999;112(Pt 22):3985–3993. doi: 10.1242/jcs.112.22.3985. [DOI] [PubMed] [Google Scholar]
- 29.Jung SY, Kim JM, Kang HK, Jang DH, Min BM. A biologically active sequence of the laminin alpha2 large globular 1 domain promotes cell adhesion through syndecan-1 by inducing phosphorylation and membrane localization of protein kinase Cdelta. J Biol Chem. 2009;284:31764–31775. doi: 10.1074/jbc.M109.038547. [DOI] [PMC free article] [PubMed] [Google Scholar]