Glucagon-like peptide 1 (GLP1) functions as an incretin, which promotes insulin secretion in response to oral glucose. Multiple pharmaceutical companies have developed GLP1 receptor agonists, including six drugs approved to treat diabetes and/or obesity. These drugs provide multiple therapeutic benefits, including improved glycemic control and weight loss. In this issue of Lancet Diabetes & Endocrinology, Bethel et al.1 report a meta-analysis of cardiovascular outcome studies with four GLP1 receptor agonists2–5, concluding that GLP1 receptor agonists provide consistent reduction of cardiovascular risk notwithstanding the fact that only the studies with liraglutide2 and semaglutide3 met prespecified criteria for statistical significance. The ELIXA study with lixisenatide4 did not reveal any trend toward cardioprotection. The EXSCEL study of exenatide represented a “near miss” with respect to statistical significance (hazard ratio of 0.93, 95% confidence interval, 0.83–1.00)5. Had the upper bound of the 95% confidence interval been 0.99 rather than 1.00, the study might have been judged to have met its primary end-point. Although the American Statistical Association has emphasized the limitations of the p<0.05 criterion6, regulatory agencies tend to take a legalistic approach and enforce p-values strictly.
While the meta-analysis emphasized commonalities among the four cardiovascular outcome studies, this Commentary takes a complementary approach by discussing differentiation among GLP1 receptor agonist drugs. Multiple head-to-head trials of GLP1 receptor agonists demonstrated varying degrees of HbA1c-lowering. To account for differences in study design, we carried out a ‘normalization’ procedure based on pairwise comparisons within individual head-to-head studies (Fig. 1), which suggests the following rank order for mean glycemic efficacy for approved doses of each drug:
Figure. Magnitude of cardioprotection is correlated with HbA1c-lowering.
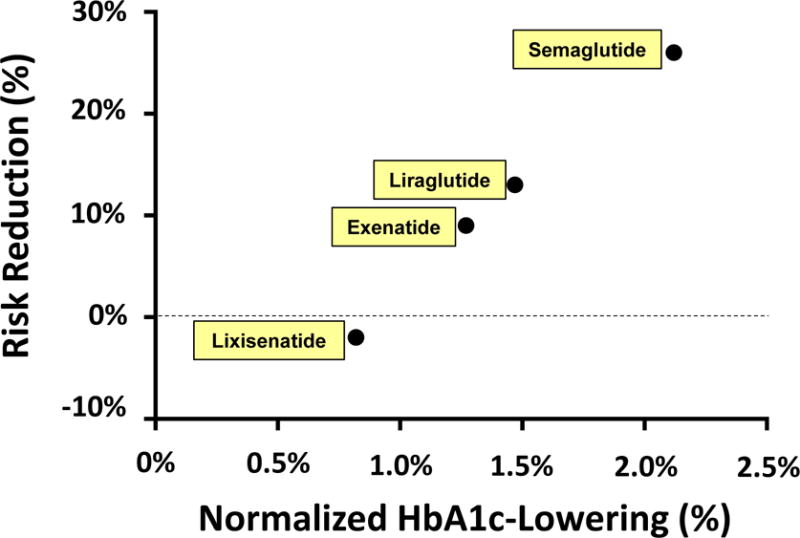
- Normalized HbA1c-lowering for short-acting exenatide/BYETTA was defined as 1.00%.
- Normalized HbA1c-lowering for lixisenatide was calculated based on head-to-head comparative efficacy data obtained in the GetGoal-X study7. Observed HbA1c-lowering was 0.96% for short-acting exenatide/BYETTA and 0.79% for lixisenatide, corresponding to an Efficacy Ratio of 0.82 (=0.79% / 0.96%). A normalized HbA1c-lowering for lixisenatide of 0.82% was calculated by multiplying the Efficacy Ratio (0.82) times the normalized HbA1c-lowering for short-acting exenatide (1.00%).
- Normalized HbA1c-lowering for long-acting exenatide/BYDUREON was calculated in a similar fashion based on comparative efficacy data in the DURATION-1 study8. This yielded a value of 1.27% as the normalized HbA1c-lowering for long-acting exenatide [BYETTA].
- The DURATION-69 and SUSTAIN-310 studies reported Efficacy Ratios of 1.16 and 1.67 for liraglutide and semaglutide, respectively, relative to extended-release exenatide/BYDUREON. These calculations yield values for normalized HbA1c-lowering of 1.47% and 2.12% for liraglutide and semaglutide, respectively.
What accounts for the differences in mean HbA1c-lowering? Available data suggest that the different clinical profiles are determined primarily by dose selection. Pharmaceutical companies conduct Phase 2 dose-ranging studies to select dose(s) to be investigated in Phase 3 studies. Health Authorities decide which dose(s) to approve for marketing. Typically, doses were selected to optimize the balance between glycemic efficacy vs. side effects. If the selected dose is near the top of the dose-response curve, this will favor greater glycemic efficacy. If a lower dose is selected (e.g., to minimize nausea and vomiting), this will result in less glycemic efficacy. Some companies invest considerable time and money to select the optimal dose by conducting large studies of multiple closely spaced doses. Other companies save money by conducting smaller studies of widely spaced doses. This cost-saving approach runs the risk of not selecting the optimal dose and not achieving a best-in-class clinical profile.
Based on this interpretation, we hypothesized that cardiovascular protection might follow the same dose-response curve as observed for HbA1c-lowering. To test this hypothesis, we plotted hazard ratios for cardiovascular risk reduction as a function of normalized HbA1c-lowering observed in various head-to-head trials7–12. HbA1c-lowering provides a pharmacodynamic biomarker to place the approved dose on the dose-response curve for GLP1 receptor activation. Despite limitations of this analysis (especially, reliance on an approach to normalize values of HbA1c) we observed a remarkable degree of correlation (r=0.998) between the point estimate for cardiovascular risk reduction and normalized HbA1c-lowering (Fig. 1). This analysis suggests that the lack of apparent cardioprotection in ELIXA may be caused by selection of a lixisenatide dose that is too far to the left on the dose-response curve. Critical differences in the study design might also have contributed to the absence of cardioprotection with lixisenatide. Whereas ELIXA was conducted in the setting of acute coronary syndrome4, LEADER, SUSTAIN-6, and EXSCEL were conducted in a chronic setting2,3,5.
Other factors also have potential to impact the clinical profile. GLP1 receptor agonists vary greatly with respect to pharmacokinetic profile. Short-acting compounds tend to have large peak:trough ratios. If peak drug levels are too high, this predisposes to nausea and vomiting. If trough drug levels are too low, this compromises glycemic efficacy. Furthermore, GLP1 receptor agonists vary widely with respect to molecular weight, ranging from 3751 daltons for liragutide to 72,970 daltons for albigutide. Large compounds might be impaired in their ability to access certain target cells – e.g., CNS cells protected by the blood-brain barrier. Finally, the six approved GLP1 receptor agonists are based on two distinct molecular structures. Whereas liraglutide, semaglutide, dulaglutide, and albiglutide are covalently modified analogs of human GLP1, exenatide and lixisenatide are based on the structure of Gila monster exendin-4. Exendin-4 possesses a nine amino acid C-terminal extension which is absent from GLP1. This C-terminal nonapeptide increases the affinity for binding to GLP1 receptors13. It is theoretically possible that this enhanced binding interaction might alter signaling downstream from the GLP1 receptor.
Finally, one additional point is worthy of emphasis. All four cardiovascular outcome studies focus on analysis of mean data, and implicitly support a “one size fits all” therapeutic approach. However, there is considerable inter-individual variation with respect to the magnitude of clinical benefit, suggesting that a personalized medicine strategy would enable physicians to select the best drug for each patient. For example, both lirgalutide and exenatide provided obese patients (BMI >30) with greater cardiovascular risk reduction; whereas patients with BMI <30 derived little or no cardioprotection2,3. Future research will be necessary to clarify how BMI or other clinical criteria should be taken into account when predicting the magnitude of clinical benefit likely to be provided by specific diabetes drugs.
Acknowledgments
SIT acknowledges grant support from the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK072488) and the American Diabetes Association (1-16-ICTS-112).
Footnotes
Disclosures
Dr. Taylor reports personal fees from Ionis Pharmaceuticals, grants from Regeneron, ownership of stock in Celgene, Abbott Laboratories, and Amgen outside the submitted work; and he was previously employed at Bristol-Myers Squibb (2002–2013). BMS acquired the exenatide program as part of the acquisition of Amylin, but Dr. Taylor did not participate in the exenatide program during his time at BMS. He has divested all BMS stock a couple of years ago. Also, BMS sold the exenatide program to Astra Zeneca. Dr. Taylor has not had any relationship with Astra Zeneca’s exenatide program for four years.
Author’s Contributions
SIT was responsible for all aspects of this manuscript – specifically, drafting the text, literature search, and drawing the Figure.
References
- 1.Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with GLP-1 receptor agonists: A meta-analysis. Lancet Diabetes Endocrinol. 2017 doi: 10.1016/S2213-8587(17)30412-6. in press. [DOI] [PubMed] [Google Scholar]
- 2.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 5.Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228–39. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasserstein RL, Lazar NA. The ASA’s Statement on p-Values: Context, Process, and Purpose. The American Statistician. 2016;70:129–33. [Google Scholar]
- 7.Rosenstock J, Raccah D, Koranyi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X) Diabetes Care. 2013;36:2945–51. doi: 10.2337/dc12-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–50. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 9.Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–24. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 10.Novo-Nordisk. Semaglutide demonstrated superior improvements in glycaemic control vs sitagliptin (SUSTAIN 2) and exenatide ER (SUSTAIN 3) in two clinical trials in adults with type 2 diabetes. 2016 http://pressnovonordisk-uscom/News-Releases?item=122862. downloaded 11/05/2017.
- 11.Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349–57. doi: 10.1016/S0140-6736(14)60976-4. [DOI] [PubMed] [Google Scholar]
- 12.Pratley RE, Nauck MA, Barnett AH, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289–97. doi: 10.1016/S2213-8587(13)70214-6. [DOI] [PubMed] [Google Scholar]
- 13.Doyle ME, Theodorakis MJ, Holloway HW, Bernier M, Greig NH, Egan JM. The importance of the nine-amino acid C-terminal sequence of exendin-4 for binding to the GLP-1 receptor and for biological activity. Regul Pept. 2003;114:153–8. doi: 10.1016/s0167-0115(03)00120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


