Abstract
In a previously reported double-blind, randomized controlled trial (RCT), we demonstrated that daily supplementation with anserine (750 mg) and carnosine (250 mg) improves brain blood flow and memory function in elderly people. Here, we conducted a sub-analysis of MRI data and test scores from the same RCT to determine whether anserine/carnosine supplementation specifically benefits elderly people carrying the APOE e4 allele, which is a risk gene for accelerated brain aging and for the onset of Alzheimer’s Disease. We collected data from 68 participants aged 65 years or older who received anserine/carnosine supplementation (ACS) or placebo for 12 months. Subjects were assessed at the start and end of the trial using several neuropsychological tests, including the Wechsler Memory Scale-Logical Memory (WMS-LM). We also collected two types of MRI data, arterial spin labeling (ASL) and diffusion tensor imaging (DTI) at the start and end of the trial. We found that ACS significantly preserved verbal memory (WMS-LM, F[1,65] = 4.2003, p = 0.0445) and blood flow at frontal areas of the brain (FWEcluster level, p < 0.001). Sub-analysis based on the APOE4 genotype showed a significant preservation of blood flow (p = 0.002, by ASL analysis) and white-matter microstructure (p = 0.003, by DTI analysis) at prefrontal areas in APOE4+ subjects in the active group, while there was no significant difference between APOE4- subjects in the active and placebo groups. The effect of ACS in preserving brain structure and function in elderly people carrying APOE4 should be verified by further studies.
Keywords: Alzheimer’s Disease, ASL, DTI, verbal memory, RCT, APOE e4
Alzheimer’s disease (AD) is a chronic neurodegenerative disease that is responsible for 60-70% of dementia [1]. Diagnosis and intervention in the early stages, when symptoms first begin to appear, can reduce the number of individuals with AD. The onset of AD symptoms typically begins with a subtle decline in memory. Lifestyle improvements, such as better nutrition and increased mental and physical activity, [2,3], may slow this process and delay the onset of AD. In previous studies [4,5], we investigated the effect of daily dietary supplements containing anserine (beta-alanyl-3-methyl-L-histidine) and carnosine (beta-alanyl-L-histidine) on the preservation of memory function in healthy elderly people.
Carnosine is an endogenous dipeptide that consists of beta-alanine and histidine and is present in skeletal muscle in the millimolar range and in the vertebrate brain in the hundred-micromolar range [6,7]. High levels of anserine, a natural carnosine derivative, are found in the skeletal breast muscle of chicken. Anserine is equivalent to carnosine in physiological function except that it is not cleaved by human carnosinase [8,9]. Double-blind randomized control trials (RCT) conducted by our group and others have demonstrated that anserine/carnosine supplementation (ACS) preserves verbal memory performance in elderly people [4,5,10]. We assessed verbal episodic memory with the Wechsler Memory Scale (WMS-R LM) [11].
Normal aging is associated with diminished brain perfusion [12]. We previously suggested that ACS may suppress this age-related decline in brain perfusion [5]. The strongest known heritable risk factor for AD is the ε4 allele of apolipoprotein E (APOE) [13-15]; this allele is also a risk factor for an accelerated age-related decline in brain perfusion [16-18].
Here, we assessed whether ACS preserved brain blood flow in healthy elderly subjects carrying the APOE e4 allele. We also examined white-matter abnormalities by diffusion MRI, on which decreased blood flow at the white matter causes abnormal hyperintensity of the white-matter [19,20] and decreased fraction anisotropy values [21,22]. We compared psychological test scores and MRI data between elderly subjects in the ACS-treated (active) and placebo groups according to the presence or absence of the APOE e4 allele.
MATERIALS AND METHODS
Subjects
Eighty-four healthy volunteers from 60-80 years of age were recruited in the Tokyo metropolitan area from April to August of 2014 [5]. Exclusion criteria were 1) a neuropsychiatric disorder or head injury; 2) a local lesion, such as a brain tumor or cerebral infarction, that could affect cognitive function; or 3) claustrophobia or a metal or electrical implant that would prevent obtaining MRI scans. All participants provided informed written consent. The participants visited the study site at the beginning of the study and 3, 6, and 12 months later. Participants were randomized into the ACS or placebo group. RCT-consistent assignment to the ACS or placebo group, determined by age and gender, was performed by Imepro Inc. (Tokyo, Japan). All participants and clinical and coordinating personnel were blinded to the group assignments for the duration of the study. The study was approved in 2012 by the Ethics Committees of the University of Tokyo and the National Center of Neurology and Psychiatry. The present report is an age-restricted sub-analysis from this healthy volunteer study, using data from 68 elderly volunteers who were at least 65 years old and completed the study (Table 1).
Table 1.
Characteristics of participants in a 12-month RCT of ACS.
| Active group | Placebo group | p value | |
|---|---|---|---|
| Age | 71.3 (4.8) | 71.8 (4.8) | 0.70 |
| Gender (M/F) | 14/17 | 15/22 | 0.61 |
| BMI | 22.1 (2.1) | 21.9 (2.3) | 0.67 |
| Education, years | 14.7 (2.0) | 14.3 (3.1) | 0.52 |
| APOE4+ / APOE4- | 8/23 | 4/33 | 0.09 |
Test formula
The test formula was a powder supplied by NH Foods Ltd. of Japan, derived from chicken meat and containing anserine and carnosine at a ratio of 3:1. The safety of this imidazole dipeptide supplement was verified in previous studies [23,24]. Participants in the active group received twice-daily doses of the imidazole dipeptide formula (500 mg/dose). The placebo formula contained an equivalent amount of essential amino acids, specifically L-lysine (43 mg/day) and L-histidine (150 mg/day), which were chosen because the enzymatic digestion of carnosine (250 mg/day) generates L-histidine (150 mg/day) and beta-alanine [5].
Inventory of anserine and carnosine in the normal diet
We calculated the amount of anserine and carnosine from the results of a dietary questionnaire, using a semi-quantitative method reported previously [5,25]. At follow-up, the participants filled out a self-administered questionnaire about the frequency of animal meat (chicken, pork, and beef) and fish meat (salmon, red-meat fish represented by tuna, white fish, blue-back fish represented by mackerel, and eel) in their diet over the previous 12 weeks. The representative fish in each category was chosen based on a national consumption survey. The average dietary intake was estimated based on the participants’ responses to the questionnaire and the concentrations of anserine and carnosine in the various animal and fish meats, obtained from our investigations and from Boldyrev et al. [6].
Cognitive testing
We assessed the effect of ACS on cognitive function, mental status, and general health using the following cognitive-evaluation tools and self-reported questionnaires: 1) the Japanese version of the Wechsler Memory Scale-Revised Logical Memory immediate recall (WMS-LM1) and delayed recall (WMS-LM2) tests [5,11], 2) the Japanese version of a cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAScog) [5,26], and 3) the Mini Mental State Examination (MMSE) [5,27]. Mood and subjective states were assessed by the Japanese version of the Beck Depression Inventory (BDI) [5,28,29]. Mental and physical functional well-being was assessed by the Medical Outcomes Study, 36-item Short Form (SF-36) [5,30,31]. We calculated the Mental Health Component Summary (MCS) score and Physical Health Component Summary (PCS) score; higher scores indicated better function. Cognitive and psychological tests were performed under double-blind conditions.
APOE genotyping
Genomic DNA was isolated from blood clots, and the APOE alleles were analyzed by PCR as previously described [32]. The subjects were subgrouped according to whether they carried the ε4 allele (APOE4+ subgroup) or not (APOE4- subgroup), and within each subgroup, the psychological test scores and the changes in scores from baseline to the end of the trial were compared.
MRI analysis
For MRI scans, a 3 T scanner (Siemens, MAGNETOM Verio 3.0T) with a 32-channel phased-array head coil was used. Headgear and earplugs were used to limit head motion and reduce scanner noise. Participants underwent MRI scans prior to beginning the trial and again after 12 months of supplementation. The 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) images were collected using the following parameters: TR = 1900 ms, TE = 2.52 ms, TI = 90 ms, flip angle = 9°, field of view (FoV) = 256 × 256 mm, acquisition matrix = 256 × 256, slice thickness = 1.0 mm, slice gap = 0 mm, slice number = 192 [33]. The 3D pulsed arterial spin labeling (pASL) perfusion images were collected by turbo gradient spin echo using the following parameters: TR = 5000 ms, TE = 38.8 ms, TI = 2350 ms, flip angle = 180°, FoV = 192 × 192 mm, acquisition matrix = 64 × 64, slice thickness = 3.0 mm, slice gap = 1.5 mm, slice number = 40, bolus duration = 700 ms. The 3D pASL data were calculated from the MRI data obtained at the start and end of the trial, and were analyzed using the statistical parametric mapping 12 (SPM12) system. The data were spatially normalized to MNI coordinates using the DARTEL method [34], and smoothed with a full-width parameter at a half-maximal resolution of 12 mm × 12 mm × 12 mm for 3D pASL as previously reported [12]. To compare changes in brain perfusion at the start and end of the 12-month trial, the data were spatially normalized to data from the 3D T1-weighted images, and the smoothed pASL data were subjected to two-way intra-subject analysis assessing the time and group interaction. We also obtained diffusion MRI and T2-weighted fluid-attenuated inversion-recovery (FLAIR) data for each participant. Diffusion tensor images were collected by diffusion-MRI with the following parameters: TR = 14100 ms, TE = 81 ms, flip angle = 90°, FoV = 224 × 224 mm, acquisition matrix = 114 × 114, slice thickness = 2.0 mm, slice number = 75, axes = 30, b-factor = 0, and 1000 s/mm2. T2-weighted FLAIR scans, used to confirm that there were no neurological or inflammatory disorders, were acquired with the following parameters: repetition time TR = 11000 ms, echo time TE = 94 ms, inversion time TI = 2800 ms, 20 axial slices, FoV = 198 × 220 mm. The diffusion tensors were calculated with the Diffusion Toolkit for the voxelwise statistical analysis [35]. Movements and eddy currents corrections were conducted using FSL eddy correct program for the diffusion-weighted images [36]. After correction, diffusion weighted images are skull-stripped with FSL BET program to exclude non-brain voxels from all analyses [37]. Fiber tract analysis and visualization were performed in the TrackVis program.
Statistical analyses
Data were analyzed by a two-way repeated ANOVA or a two-tailed Student’s t-test. A p value less than 0.05 was considered significant. Unless stated otherwise, data were expressed as the mean ± SEM.
RESULTS
Subject data
At the end of the trial, 68 subjects had completed the study, with 12 months of ACS (31 subjects) or placebo (37 subjects) and a battery of tests at the start and end of the trial period. No obvious adverse events were observed. As shown in Table 1, the active and placebo groups did not differ significantly with respect to age, body mass index, sex ratio, or education. Of the subjects, 17.6% were APOE4+, and one subject in the active group and two in the placebo group were APOE4-homozygous (Table 2).
Table 2.
Subjects grouped by APOE genotype.
| APOE4+ group | APOE4- group | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| E4/E4 | E4/E3 | E4/E2 | E3/E3 | E3/E2 | E2/E2 | |
| Active | 1 | 7 | 0 | 23 | 0 | 0 |
| Placebo | 2 | 2 | 0 | 33 | 0 | 0 |
Anserine/carnosine intake
The estimated dietary intake of anserine from animal and fish meat was 341 mg/day in the active group and 362 mg/day in the placebo group (Table 3). The estimated amount of carnosine from the diet was 190 mg/day in the active group, and 191 mg/day in the placebo group. These differences in intake in the normal diet were not significant. ACS provided 750 mg anserine and 250 mg carnosine per day. Thus, the active group ingested approximately three times more anserine/carnosine than the placebo group in this trial.
Table 3.
Anserine/carnosine intake from the dieta
| Food | Active Group Ave.±SEM | Placebo Group Ave.±SEM | p value | |
|---|---|---|---|---|
| Anserine | Poultry (656mg/80g) | 120.9±17.6 | 132.6±17.2 | 0.37 |
| (mg/day) | Pork (18.4mg/80g) | 5.1±0.7 | 4.9±0.6 | 0.66 |
| Beef (43mg/80g) | 6.2±1.2 | 6.4±1.3 | 0.79 | |
| Red meat Fish (304mg/80g) | 77.1±10.9 | 71.6±8.9 | 0.5 | |
| Blue back Fish (5.6mg/80g) | 1.6±0.2 | 1.6±0.2 | 0.9 | |
| White Fish (2.3mg/80g) | 130.0±23.4 | 145.1±25.2 | 0.39 | |
| Eel (0mg/80g) | 0±0 | 0±0 | N.D. | |
| Anserine(Total) | 340.9±38.7 | 362.2±342.1 | 0.46 | |
| Carnosine | Poultry (184mg/80g) | 33.9±4.9 | 37.2±4.8 | 0.37 |
| (mg/day) | Pork (246mg/80g) | 68.8±9.5 | 65.7±7.9 | 0.66 |
| Beef (209mg/80g) | 30.0±6.0 | 31.1±6.1 | 0.79 | |
| Red meat Fish (24mg/80g) | 6.1±0.9 | 5.6±0.7 | 0.5 | |
| Blue back Fish (152mg/80g) | 43.5±5.9 | 44.0±5.2 | 0.9 | |
| White Fish (0mg/80g) | 0±0 | 0±0 | N.D. | |
| Eel (336mg/80g) | 7.3±0.8 | 7.6±0.7 | 0.65 | |
| Carnosine(Total) | 189.6±17.3 | 191.3±17.3 | 0.89 |
Estimated intake anserine and carnosine was calculated from the results of a 7-item food frequency questionnaries filled out by each volunteer and the average amount of anserine and carnosine in each type of meat described by Boldyrev et al. (2013)
Figure 4.
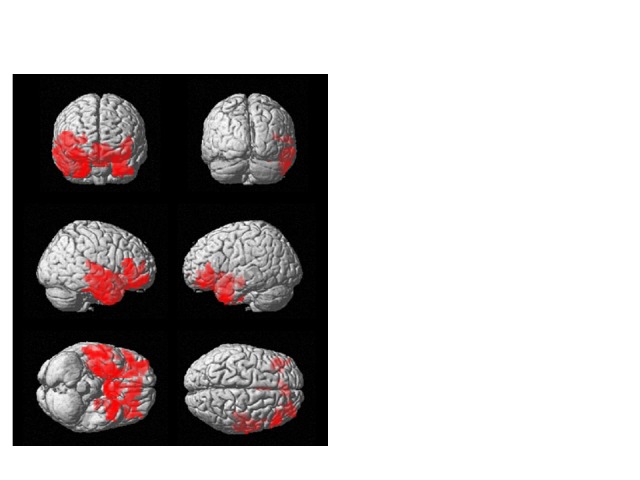
ACS preserves blood flow in the prefrontal brain of elderly people. Location of differential longitudinal changes in brain perfusion (red) in the active and placebo groups, on a brain montage from the SPM platform based on equivalent calculations to those in Fig. 3. In the active group, blood flow in these areas was elevated in the follow-up MRI scan.
Cognitive tests
Cognitive function was assessed using three neuropsychological tests (Table 4). Story B of the WMS-LM2 test was used to assess the delayed recall of verbal memory. Data were analyzed by two-way repeated ANOVA (Time [1st-test or 2nd-test] × Variant [ACS or placebo]). The interaction Time × Variant was significantly different between the ACS and placebo groups (F[1,65] = 4.2003, p = 0.0445; Fig. 1). In addition, at the mid-term test (6 months after the supplementation), we have observed a tendency (p = 0.067) of the cognitive improvement by ACS (Fig. 2). However, there was no difference between the active and placebo groups in the MMSE, the WMS-LM1 to assess the immediate recall of verbal memory, the ADAcog, the SF-36, or the BDI (F[1,65] < 1.0; p > 0.05).
Table 4.
Changes in psychological test scores in the active and placebo groups.
| 1st test | Follow-up | Change | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Active | Placebo | Active | Placebo | Active | Placebo | p value | |
| WMS-LM-1a | 13.3 (3.9) | 15.0 (3.7) | 14.3 (3.9) | 14.3 (4.4) | 0.93 (2.8) | -0.65 (3.6) | 0.054 |
| WMS-LM-2a | 12.6 (4.0) | 14.3 (3.9) | 13.3 (3.9) | 13.5 (4.4) | 0.73 (2.9) | -0.84 (3.3) | 0.044* |
| MMSEb | 27.5 (1.7) | 27.6 (1.9) | 28.2 (2.4) | 28.5 (1.7) | 0.74 (2.0) | 0.92 (1.9) | 0.53 |
| ADASb | 9.3 (5.5) | 8.2 (4.2) | 7.9 (4.6) | 6.8 (4.4) | -1.4 (3.5) | -1.4 (3.3) | 0.92 |
| SF-36 PCSb | 48.0 (7.7) | 49.1 (6.6) | 45.8 (10.2) | 48.1 (5.8) | -2.2 (7.5) | -1.0 (6.1) | 0.45 |
| SF-36 MCSb | 54.3 (4.7) | 52.8 (5.8) | 56.8 (6.9)a | 52.4 (7.0) | 2.3 (7.0) | -0.4 (7.4) | 0.13 |
| BDIb | 8.2 (6.7) | 6.8 (4.3) | 8.0 (6.2) | 7.2 (5.8) | -0.1 (3.7) | 0.4 (4.5) | 0.60 |
Statistically significant.
For the WMS-LM test combined story B, the 1st test was performed at the second visit (3 months after beginning supplementation). Tests include 68 subjects except for the WMS-LM test, which analyzed only 67 subjects (n = 30 active and 37 placebo) because one subject in the active group did not complete the follow-up WMS-LM.
The 1st test was performed at the first visit; the follow-up test was conducted after 12 months of supplementation (n = 31 active, 37 placebo).
Figure 1.
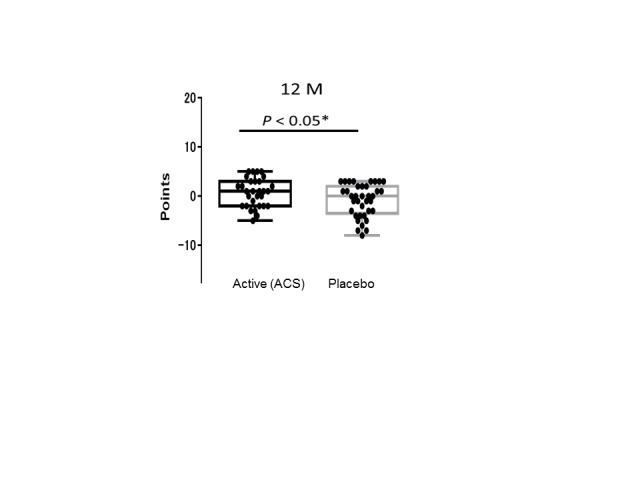
Longitudinal changes in WMS-LM2 scores. A box plot of the WMS-LM2 data in Table 2 for subjects in the active (n = 30) and placebo (n = 37) groups. Each black dot indicates the difference between the first and final test scores for one volunteer. Box shows the 25-75 percentile, and solid bar shows the median. (One subject in the active group could not complete the final WMS-LM2 test.)
Figure 2.
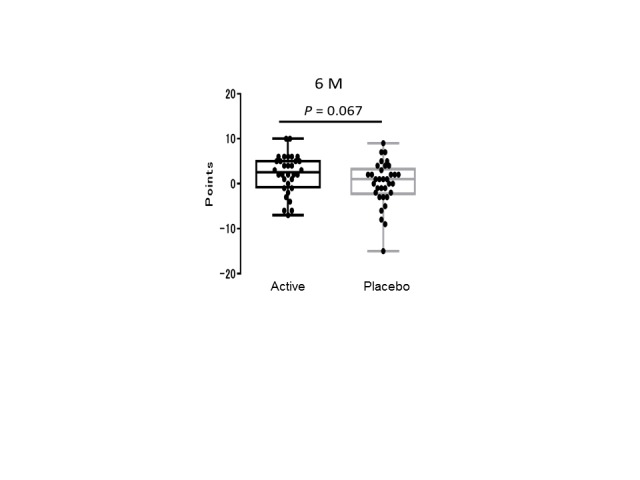
Longitudinal changes in WMS-LM2 scores at the mid-term test. A box plot of the change of WMS-LM2 story A data (the score of 6-month test - the score of the initial test) in the active and placebo groups. Each black dot indicates the difference between the first and final test scores for one volunteer. Box shows the 25-75 percentile, and solid bar shows the median.
MRI analysis
Longitudinal ACS-induced changes in brain perfusion were assessed by comparing the 3D pASL data between the ACS and placebo groups at the beginning and end of the trial. The analysis of whole-brain pASL data revealed a significant preservation of brain perfusion at the medial prefrontal cortex in the ACS group (p < 0.001; Fig. 3 and 4), whereas the inverse calculation did not reveal any significant findings. These changes were also analyzed within the APOE4+ and APOE4- subgroups. We detected significant preservation of blood flow at the prefrontal areas in APOE4+ group (p = 0.002; Fig. 5), but not in APOE4- group. We also compared the DTI data from diffusion MRIs between the ACS and placebo groups at the beginning and follow-up time points, and detected the significant difference between the two groups (p = 0.003; Fig. 6). Fig. 7 shows a tract-graph analysis of APOE4/E4 subject (#75) in the active group with a seed ROI at x=-30; y=30; z=-4, where we detected the difference between the two groups in the previous DTI analysis (Fig. 6). We observed that the longer tract (green) toward the frontal pole seemed to be stronger at the follow-up scan, in comparison with the tract-graph at the start-up scan.
Figure 3.
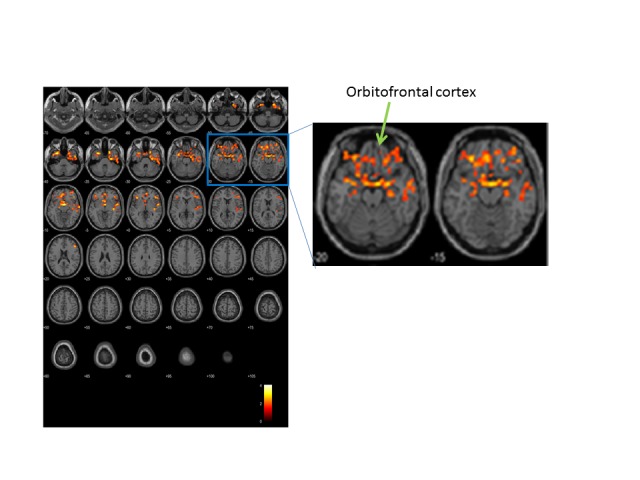
Longitudinal changes in brain perfusion. Brain blood flow was analyzed by ASL. Color indicates regions where changes in brain perfusion differed between the two groups (n = 31 active, 37 placebo). After repeated two-way ANOVA in SPM, the biggest difference was at (x, y, z) = (3, -1, -10) in Montreal Neurological Institute (MNI) coordinate, T=4.09; other two locations were (x, y, z) = (-30, 11, -37) and (24, 11, -43). SPM statistics showed significance between Active and Placebo (Active > Placebo). P(FWE-corr) < 0.001, KE=2857 voxels. Note that the brain locations of the preservation of blood flow by ACS included both sides of temporal lobes, orbitofrontal cortices, dorsolateral prefrontal cortices and anterior cingulate cortices.
Figure 5.
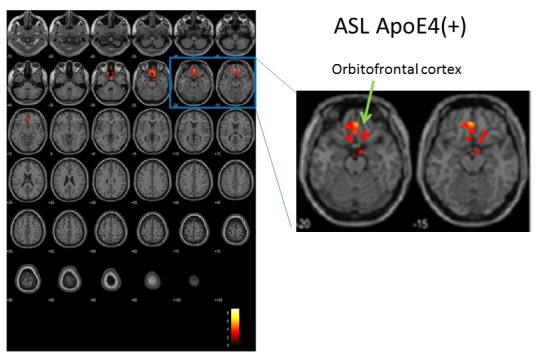
Longitudinal changes in brain perfusion in APOE4+ subjects, assessed by ASL. Color indicates brain regions with differences in longitudinal changes in brain perfusion between the active (n = 8) and placebo (n = 4) groups. After repeated two-way ANOVA in SPM, the biggest difference was at (x, y, z) = (-3, 44, -16), T=9.03. SPM statistics showed significance between Active and Placebo (Active > Placebo). P(FWE-corr) = 0.002, KE=378 voxels.
Figure 6.
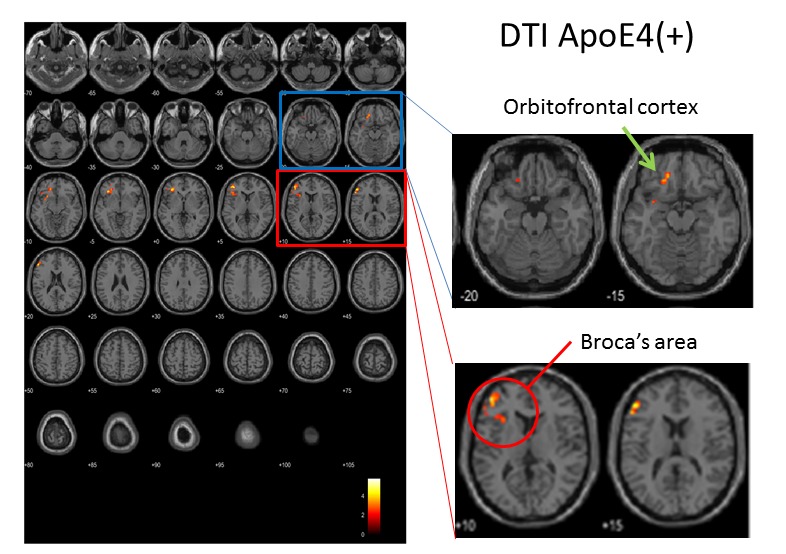
Longitudinal changes in FA (fraction anisotropy) values in APOE4+ subjects, assessed by DTI. Color indicates areas of the brain where FA values differed between the active (n = 8) and placebo (n = 4) groups. After repeated two-way ANOVA in SPM, the biggest difference was at (x, y, z) = (-32, 32, -4), T=5.75; other two locations were (x, y, z) = (-38, 44, 8) and (-50, 36, 18). SPM statistics showed significance between Active and Placebo (Active > Placebo). P(FWE-corr) = 0.003, KE=754 voxels.
Figure 7.
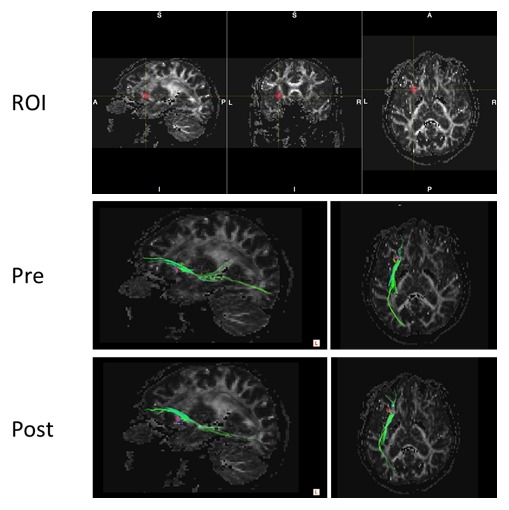
Tract-graph analysis of APOE4/E4 subject (#75) in the active group. Top panel shows a seed ROI (x, y, z) = (-30, 30, -4), Sphere Radius = 3.00 mm. Middle panel: a tract-graph using this spherical seed ROI at the start-up scan (Pre). Bottom panel: a tract-graph using this spherical seed ROI at the follow-up scan (Post). Note the longer tract (green) toward the frontal pole seemed to be stronger at the follow-up scan.
DISCUSSION
Here, we showed that ACS suppresses age-related memory decline and alteration in brain blood flow, in line with previous observations [4,5,10]. However, a new finding in the present study is that ACS has stronger benefits for elderly people bearing the risk allele APOE4, as revealed by both ASL and DTI data. Brain blood flow was preserved in prefrontal areas of the brain in APOE4 carriers after 12 months of ACS. Although considerable research has gone into determining whether DHA can benefit APOE4 carriers, the issue is still in debate [38,39]. ACS is another promising candidate for protecting against the detrimental effect of APOE4 molecules on brain function and structure.
Our research suggests that ACS may be able to suppress progressive memory decline, which often follows the onset of AD, in APOE4+ individuals, who are at a greater risk for AD [14,15]. There were not enough APOE4+ subjects in this study to demonstrate a significant difference in changes of verbal episodic memory score between APOE4+ subjects in the active and placebo groups, but we observed a marked trend toward improvement in the active group (n = 8; avg ± SD = 1.75 ± 2.76) compared to the placebo group (n = 4; -0.25 ± 5.25) in WMS-LM scores. We also observed a trend toward improvement in the MMSE test: the change in score was 0.75 ± 2.12 (n = 8) in the active group and -0.25 ± 1.89 (n = 4) in the placebo group. Among the APOE4-homozygous subjects, one subject was in the active group (Fig. 7); this 70-year-old man (#75) with a family history of dementia improved his verbal episodic memory score from 6 to 13. Two subjects in the placebo group were APOE4 homozygotes. One was a 76-year-old man (#42) whose verbal episodic memory score dropped severely, from 22 to 1, and he was diagnosed with mild cognitive impairment (MCI) after the 12-month trial. The other APOE4-homozygous subject in the placebo group (#38) was a 67-year-old man who maintained his verbal episodic memory score (15 at the first visit, and 17 at the 12-month follow-up visit).
After our 12-month ACS RCT, we conducted a 12-week RCT of ACS in elderly patients with MCI. In the 12-week RCT, we observed that the MMSE scores were preserved and WMS-LM scores improved in MCI subjects carrying the APOE4 allele (n = 8 in the active group and n = 12 in the placebo group; unpublished observation, T.H.). Taken together, these findings suggest that ACS may protect against progressive cognitive decline in elderly individuals with the AD risk allele APOE4.
We also previously investigated the mechanism of the protective effect of ACS on cognitive function in a transgenic AD mouse model. We found that carnosine supplementation prevents memory deficits in the AD mouse, probably by preventing abnormalities in brain blood vessels [40]. Very recently, we obtained preliminary observations suggesting that anserine supplementation may prevent damage to brain microvascular pericytes (unpublished observation, J.K. & T.H. submitted separately). A series of studies by Zlokovic and colleagues indicate that pericyte degeneration contributes to the development of AD, especially in AD patients with the APOE4 allele [41,42]. We can speculate that once ingested, anserine and carnosine inhibit cellular damage to brain microvascular pericytes [15]. However, carnosine and anserine have several biochemical properties that could be involved in this effect, such as their anti-oxidant and anti-glycolytic activity and their ability to chelate metal ions [6,7,43], so further study is needed to determine the precise mechanisms. We are currently in the process of assessing whether ACS can protect against cognitive decline in a model mouse produced by crossing human APOE4/E4 knock-in [44] mice with AD (App/Psen double-transgenic) model mice [45] to generate an App/Psen double-transgenic mouse with a human ApoE4/E4 knock-in.
Normal aging is associated with diminished brain perfusion, which can be measured as cerebral blood flow by ASL [12]. Recent studies show that APOE e4 molecules accelerate the decline in brain blood flow at particular areas, including prefrontal regions [16,18]. In this study, we observed that the brain blood flow was preserved in elderly APOE e4 carriers who took ACS for a 12-month period, suggesting that ACS specifically protects against brain vascular abnormalities related to the APOE e4 molecule, such as those observed in brain microvascular pericytes [41,42]. In addition to ASL, other MRI modalities can also demonstrate the effect of the APOE e4 allele on brain aging, including structural MRI [46], functional MRI [47], and diffusion MRI [20]. In this report, an analysis of diffusion MRI data revealed that ACS protected against or improved white-matter abnormalities in APOE4+ subjects. Several well-designed clinical studies have shown that changes in white-matter microstructure in APOE4+ elderly individuals can be detected by analyzing the FA values after diffusion MRI [48-51]. In this study, the analysis of two MRI modalities, ASL and DTI, revealed that ACS protects against the putative detrimental effect of APOE e4 molecules on brain aging in healthy volunteers without dementia. In a separate 12 weeks-RCT, we have also observed preservation of cognitive functions by ACS in APOE4+ mild cognitive impairment patients (Active N=8, Placebo N=12; T.H. submitted separately).
Limitations
Our study has various limitations. This study was based on a sub-analysis of ApoE4+ subjects within a larger study, and it had a relatively small sample size. Larger studies are still needed to verify the effect of ACS in elderly people carrying the APOE4 allele. In addition, it is possible that alterations in blood flow in the brain occur more rapidly in APOE4+ than in APOE4- individuals, so longer-term investigations should be performed.
Concluding Remarks
This is the first RCT to demonstrate the effect of ACS in healthy APOE4+ volunteers. The results indicate that ACS may protect against age-related structural changes in the brain in elderly people with the APOE4 allele, and thus prevent Alzheimer’s Disease if started before the MCI stage. Furthermore, changes seen on MRI scans, particularly ASL and DTI, are potential biomarkers for assessing these benefits, especially in people carrying the APOE4 allele.
Acknowledgments
The authors thank Ms. Risa Fujinaga for preparing the manuscript.
Footnotes
Disclosure
This study was supported by the Scientific Technique Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry (Grant# 25012A) from the Ministry of Agriculture, Forestry, and Fishery (MAFF), the government of Japan; and by a Grant-in-Aid for Scientific Research on Innovative Areas (Memory Dynamism) from the Japan Society for the Promotion of Science (to T. H.).
Conflict of Interest
NH Food Ltd., provided the supplements free of charge. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- [1].Ritchie K, Lovestone S (2002). The dementias. The Lancet, 360:1759-66. [DOI] [PubMed] [Google Scholar]
- [2].Lindenberger U (2014). Human cognitive aging: corriger la fortune? Science, 346:572-8. [DOI] [PubMed] [Google Scholar]
- [3].Cooper JK (2014). Nutrition and the brain: what advice should we give? Neurobiol Aging, (Suppl 2) 35: S79-83. [DOI] [PubMed] [Google Scholar]
- [4].Rokicki J, Li L, Imabayashi E, Kaneko J, Hisatsune T, Matsuda H (2015). Daily Carnosine and Anserine Supplementation Alters Verbal Episodic Memory and Resting State Network Connectivity in Healthy Elderly Adults. Front Aging Neurosci, 7:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hisatsune T, Kaneko J, Kurashige H, Cao Y, Satsu H, Totsuka M, Katakura Y, Imabayashi E, Matsuda H (2016). Effect of Anserine/Carnosine Supplementation on Verbal Episodic Memory in Elderly People. J Alzheimers Dis, 50:149-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boldyrev AA, Aldini G, Derave W (2013). Physiology and pathology of carnosine. Physiol Rev, 93:1803-1845. [DOI] [PubMed] [Google Scholar]
- [7].Hipkiss AR (2014). Aging risk factors and Parkinson’s disease: contrasting roles of common dietary constituents. Neurobiol Aging, 35:1469-72. [DOI] [PubMed] [Google Scholar]
- [8].Yeum K-J, Orioli M, Regazzoni L, Carini M, Rasmussen H, Russell RM, Aldini G (2010). Profiling histidine dipeptides in plasma and urine after ingesting beef, chicken or chicken broth in humans. Amino Acids, 38:847-858. [DOI] [PubMed] [Google Scholar]
- [9].Kubomura D, Matahira Y, Masui A, Matsuda H (2009). Intestinal Absorption and Blood Clearance of L-Histidine-Related Compounds after Ingestion of Anserine in Humans and Comparison to Anserine-Containing Diets. J Agric Food Chem, 57:1781-1785. [DOI] [PubMed] [Google Scholar]
- [10].Szcześniak D, Budzen S, Kopec W, Rymaszewska J (2014). Anserine and carnosine supplementation in the elderly: Effects on cognitive functioning and physical capacity. Arch. Gerontol Geriatr, 59:485-490. [DOI] [PubMed] [Google Scholar]
- [11].Kawano N, Awata S, Ijuin M, Iwamoto K, Ozaki N (2013). Necessity of normative data on the Japanese version of the Wechsler Memory Scale-Revised Logical Memory subtest for old-old people. Geriatr Gerontol Int, 13:726-30. [DOI] [PubMed] [Google Scholar]
- [12].Liu Y, Zhu X, Feinberg D, Guenther M, Gregori J, Weiner MW, Schuff N (2012). Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn Reson Med, 68:912-22. [DOI] [PubMed] [Google Scholar]
- [13].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA, 278:1349-56. [PubMed] [Google Scholar]
- [14].Giri M, Zhang M, Lü Y (2016). Genes associated with Alzheimer’s disease: an overview and current status. Clin Interv Aging, 11:665-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tai LM, Thomas R, Marottoli FM, Koster KP, Kanekiyo T, Morris AWJ, Bu G (2016). The role of APOE in cerebrovascular dysfunction. Acta Neuropathol, 131:709-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM (2010). APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol, 67:93-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gietl AF, Warnock G, Riese F, Kälin AM, Saake A, Gruber E, Leh SE, Unschuld PG, Kuhn FP, Burger C, Mu L, Seifert B, Nitsch RM, Schibli R, Ametamey SM, Buck A, Hock C (2015). Regional cerebral blood flow estimated by early PiB uptake is reduced in mild cognitive impairment and associated with age in an amyloid-dependent manner. Neurobiol Aging, 36:1619-28. [DOI] [PubMed] [Google Scholar]
- [18].Michels L, Warnock G, Buck A, Macauda G, Leh SE, Kaelin AM, Riese F, Meyer R, O’Gorman R, Hock C, Kollias S, Gietl AF (2016). Arterial spin labeling imaging reveals widespread and Aβ-independent reductions in cerebral blood flow in elderly apolipoprotein epsilon-4 carriers. J Cereb Blood Flow Metab, 36:581-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sato Y, Chin Y, Kato T, Tanaka Y, Tozuka Y, Mase M, Ageyama N, Ono F, Terao K, Yoshikawa Y, Hisatsune T (2009). White matter activated glial cells produce BDNF in a stroke model of monkeys. Neurosci Res, 65:71-78. [DOI] [PubMed] [Google Scholar]
- [20].Cox SR, Ritchie SJ, Dickie DA, Pattie A, Royle NA, Corley J, Aribisala BS, Harris SE, Valdés Hernández M, Gow AJ, Muñoz Maniega S, Starr JM, Bastin ME, Wardlaw JM, Deary IJ (2017). Interaction of APOE e4 and poor glycemic control predicts white matter hyperintensity growth from 73 to 76. Neurobiol Aging, 54:54-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chin Y, Sato Y, Mase M, Kato T, Herculano B, Sekino M, Ohsaki H, Ageyama N, Ono F, Terao K, Yoshikawa Y, Hisatsune T (2010). Transient decrease in cerebral motor pathway fractional anisotropy after focal ischemic stroke in monkey. Neurosci Res, 66:406-411. [DOI] [PubMed] [Google Scholar]
- [22].Chin Y, Kishi M, Sekino M, Nakajo F, Abe Y, Terazono Y, Ohsaki H, Kato F, Koizumi S, Gachet C, Hisatsune T (2013). Involvement of glial P2Y1 receptors in the cognitive deficits after focal cerebral stroke in a rodent model. J Neuroinflammation, 10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aoyagi S, Sugino T, Kajimoto Y, Nishitani M (2008a). Safety of long-term administration of CBEX-Dr-containing drink of healthy people. Jpn Pharmacol Ther, 36:213-24. [Google Scholar]
- [24].Aoyagi S, Sugino T, Kajimoto Y, Nishitani M (2008b). Safety of excess administration of CBEX-Dr-containing drink of healthy people. Jpn Pharmacol Ther, 36:225-35. [Google Scholar]
- [25].Kiyohara Y, Shinohara A, Kato I, Shirota T, Kubo M, Tanizaki Y, Fujishima M, Iida M (2003). Dietary factors and development of impaired glucose tolerance and diabetes in a general Japanese population: The Hisayama Study. J Epidemiology, 13:251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Homma A, Fukuzawa K, Tsukada Y, Ishii T, Hasegawa K, Mohs RC (1992). Development of a Japanese version of Alzheimer’s disease Assessment Scale (ADAS). Jpn J Geriatr Psychiatry, 3:647-55. [Google Scholar]
- [27].Uchida K, Shan L, Suzuki H, Tabuse Y, Nishimura Y, Hirokawa Y, Mizukami K, Akatsu H, Meno K, Asada T (2015). Amyloid-β sequester proteins as blood-based biomarkers of cognitive decline. Alzheimers Dement: Diagnosis Assessment Disease Monitoring, 1:270-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Beck AT, Steer RA, Brown GK (1996). Manual for the Beck Depression Inventory, 2nd Ed., Pearson, Texas. [Google Scholar]
- [29].Kojima M, Furukawa TA (2003). Japanese manual of the Beck Depression Inventory, 2nd ed., Nihon Bunka Kagakusha, Tokyo. [Google Scholar]
- [30].Ware JE, Sherbourne CD (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care, 30:474-83. [PubMed] [Google Scholar]
- [31].Lu Y, Nyunt MS, Gwee X, Feng L, Feng L, Kua EH, Kumar R, Ng TP (2012). Life event stress and chronic obstructive pulmonary disease (COPD): associations with mental well-being and quality of life in a population-based study. BMJ Open, 2: e001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang YG, Kim JY, Park SJ, Kim SW, Jeon OH, Kim DS (2007). Apolipoprotein E genotyping by multiplex tetra-primer amplification refractory mutation system PCR in single reaction tube. J Biotechnol, 131:106-10. [DOI] [PubMed] [Google Scholar]
- [33].Matsuda H (2013). Voxel-based morphometry of brain MRI in normal aging and Alzheimer’s Disease. Aging Dis, 4:29-37. [PMC free article] [PubMed] [Google Scholar]
- [34].Ashburner J (2007). A fast-diffeomorphic image registration algorithm. Neuroimage, 38: 95-113. [DOI] [PubMed] [Google Scholar]
- [35].Wang R, Benner T, Sorensen AG, Wedeen VJ (2007). Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med, 15: 3720 [Google Scholar]
- [36].Andersson JL, Sotiropoulos SN (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 125:1063-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Smith SM (2002). Fast robust automated brain extraction. Hum Brain Mapp, 17:143-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Daiello LA, Gongvatana A, Dunsiger S, Cohen RA, Ott BR; Alzheimer’s Disease Neuroimaging Initiative. (2015). Association of fish oil supplement use with preservation of brain volume and cognitive function. Alzheimers Dement, 11:226-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yassine HN, Braskie MN, Mack WJ, Castor KJ, Fonteh AN, Schneider LS, Harrington MG, Chui HC (2017). Association of Docosahexaenoic Acid Supplementation with Alzheimer Disease Stage in Apolipoprotein E ε4 Carriers: A Review. JAMA Neurol, 74:339-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Herculano B, Tamura M, Ohba A., Shimatani M, Kutsuna N, Hisatsune T (2013). β-alanyl-L-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis, 33:983-997. [DOI] [PubMed] [Google Scholar]
- [41].Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV (2012). Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature, 485:512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV (2016). Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab, 36:216-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hipkiss AR (2017). On the Relationship between Energy Metabolism, Proteostasis, Aging and Parkinson’s Disease: Possible Causative Role of Methylglyoxal and Alleviative Potential of Carnosine. Aging Dis, 8:334-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hamanaka H, Katoh-Fukui Y, Suzuki K, Kobayashi M, Suzuki R, Motegi Y, Nakahara Y, Takeshita A, Kawai M, Ishiguro K, Yokoyama M, Fujita SC (2000). Altered cholesterol metabolism in human apolipoprotein E4 knock-in mice. Hum Mol Genet, 9:353-61. [DOI] [PubMed] [Google Scholar]
- [45].Jankowsky JL, Slunt HH, Ratoviski T, Jenkins NA, Copeland NG, Borchelt DR (2001). Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomolecular Engineering, 17:157-165. [DOI] [PubMed] [Google Scholar]
- [46].Fujishima M, Kawaguchi A, Maikusa N, Kuwano R, Iwatsubo T, Matsuda H (2017). Sample Size Estimation for Alzheimer’s Disease Trials from Japanese ADNI Serial Magnetic Resonance Imaging. J Alzheimers Dis, 56:75-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rajah MN, Wallace LMK, Ankudowich E, Yu EH, Swierkot A, Patel R, Chakravarty MM, Naumova D, Pruessner J, Joober R, Gauthier S, Pasvanis S (2017). Family history and APOE4 risk for Alzheimer’s disease impact the neural correlates of episodic memory by early midlife. Neuroimage Clin, 14:760-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kljajevic V, Meyer P, Holzmann C, Dyrba M, Kasper E, Bokde AL, Fellgiebel A, Meindl T, Hampel H, Teipel S; EDSD study group (2014). The ε4 genotype of apolipoprotein E and white matter integrity in Alzheimer’s disease. Alzheimers Dement, 10:401-4. [DOI] [PubMed] [Google Scholar]
- [49].Adluru N, Destiche DJ, Lu SY, Doran ST, Birdsill AC, Melah KE, Okonkwo OC, Alexander AL, Dowling NM, Johnson SC, Sager MA, Bendlin BB (2014). White matter microstructure in late middle-age: Effects of apolipoprotein E4 and parental family history of Alzheimer’s disease. Neuroimage Clin, 4:730-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Racine AM, Adluru N, Alexander AL, Christian BT, Okonkwo OC, Oh J, Cleary CA, Birdsill A, Hillmer AT, Murali D, Barnhart TE, Gallagher CL, Carlsson CM, Rowley HA, Dowling NM, Asthana S, Sager MA, Bendlin BB, Johnson SC (2014). Associations between white matter microstructure and amyloid burden in preclinical Alzheimer’s disease: A multimodal imaging investigation. Neuroimage Clin, 4:604-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Reinvang I, Espeseth T, Westlye LT (2013). APOE-related biomarker profiles in non-pathological aging and early phases of Alzheimer’s disease. Neurosci Biobehav Rev, 37:1322-35. [DOI] [PubMed] [Google Scholar]


