Graphical abstract
Keywords: Secretome, Fetal bovine serum, Tandem mass spectrometry, Mesenchymal stem cells
Highlights
-
Optimized protocol to collect the secretome of human BM-MSC.
-
Truly secreted proteins may be difficult to detect for fetal bovine serum presence.
-
Cells should be transferred into a serum-free medium prior to secretome collection.
Abstract
The analysis of mesenchymal stromal cells secretome is fundamental to identify key players of processes involving these cells. Truly secreted proteins may be difficult to detect in MS based analysis of conditioned media (CM) due to proteins supplemented with fetal bovine serum (FBS). We compared different growth conditions to determine the effect of varying FBS concentration on the number and quantity of truly secreted human proteins vs contaminating bovine proteins. The results suggest that to minimize interference cells should be grown in presence of FBS until confluence and transferred into a serum-free medium prior to secretome collection.
Postnatal stem cells can be obtained from different somatic tissues and have the capacity to self-renew and differentiate into multiple cell types [1]. Mesenchymal stem cells (MSCs) can also modify the immune response and secrete paracrine mediators, reducing inflammation and accelerating tissue regeneration by activation of resident stem cells and mobilization of circulating systemic stem cells through chemotactic signalling [2]. Since the secretome plays a direct role in the biological activities of MSCs, the qualitative and quantitative analysis of the protein components of MSCs secretome is a fundamental step in order to identify key players in the control and regulation of the many biological processes influenced by these cells.
Only a few studies have characterized the cellular secretome in vivo [3], [4] because of the inherent difficulties. A commonly used approach is the analysis of media conditioned by cells in culture (CM) [5], [6], [7], [8]. This model assumes that cells grown in vitro and stimulated using specific factors known to be which cells are exposed under certain conditions in vivo present a secretion phenotype similar to the one in vivo. The obvious advantage of cells in culture is the possibility to study variations of the secretome induced by specific events in a simplified environment and translate these findings for the preparation of material designed for therapeutic applications. Despite the complexity reduction and the experimental advantages offered by in vitro model systems, secretome profiling based on CM analysis presents serious difficulties, such as the low concentration at which the proteins are secreted and the presence of high abundant supplemented proteins fromserum (often fetal bovine serum, FBS) in the CM [8], [9] which often mask the lower abundant proteins secreted by cells, concealing their identification by mass spectrometry [10]. Moreover, in a typical shotgun LC-MS/MS experiment, FBS contaminants are sometimes difficult to discriminate from the human proteins truly secreted by cells, because of shared peptide sequences between species [11].
However, since serum deprivation might slow down and even stop cell proliferation [12], [13] increasing cell death [14], growth media should be supplemented with FBS at least for a defined time frame, in particular until MSCs reached the confluence, prior to secretome collection.
In this study, we compared three different growth conditions to determine the effect of varying FBS concentration in the CM of human bone marrow MSCs (BM-MSC) on the number and quantity of truly secreted human proteins vs contaminating bovine proteins. Ethical Approval for marrow aspiration and stromal cell isolation was obtained from Galway University Hospital Clinical Research Ethics Committees (GUH-CREC).
For this purpose isolated BM-MSC were seeded in tissue culture treated flasks in DMEM 20% FBS and incubated at 37 °C until confluence. After reaching 70⿿80% confluence, the adherent cells were trypsinized, harvested and expanded in larger flasks. Growth medium was substituted with DMEM low glucose supplemented with 25 ng/mL hIL1b, 20 ng/mL hIL6 and 25 ng/mL hTNFa and 0%, 5% or 10% FBS for 24 h. Cells were washed 3 times with DMEM without serum, incubated for 18 h and the conditioned medium was collected and concentrated using Centricon 10 (Millipore) centrifuge filter unit.
Fifty micrograms of total proteins from each experiment were reduced with DTT, alkylated with iodoacetamide, digested with trypsin sequence grade for 16 h at 37 °C using a protein:trypsin ratio of 50:1 (w/w) and desalted using ZipTip (Millipore) as detailed in [15]. NanoLC-ESI-MS/MS analysis was performed on a LTQ Orbitrap Velos (Thermo Fisher Scientific) equipped with a Dionex UltiMate 3000HPLC system. Raw data were analyzed using MaxQuant software (version 1.3.0.5) as detailed in [15], [16]. The acquired MS/MS spectra were analyzed by the Andromeda search engine, against the human Uniprot sequence database (release 2013_05) and the bovine UniProt sequence database (release 2013_05). Comparative analyses were performed using the Perseus software (version 1.4.0.6) (http://coxdocs.org/doku.php?id=perseus:start).
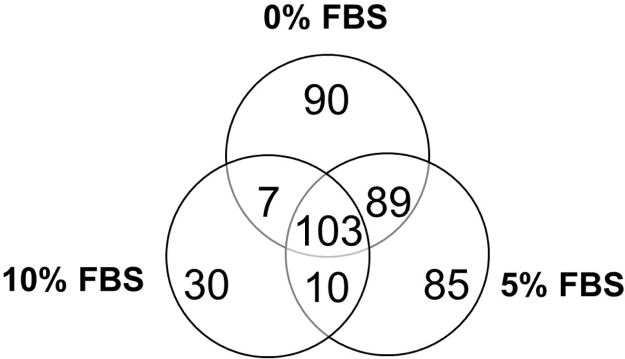
Table 1 summarizes the main results obtained from each hMSC growth condition: 0%, 5% and 10% of FBS. Whereas the total number of identified bovine secreted proteins is approximately the same in each sample, the total number of identified human secreted proteins is about twice when hMSCs were grown in 0% and 5% FBS culture medium compared to cells grown in 10% FBS culture medium. This positive result is due to a very significant decrease in MS signals due to bovine, i. e. contaminating, proteins, as shown in Table 1. Taking the sum of ion intensities of human or bovine proteins as an approximate estimation of protein quantities deriving from the two organisms, it can be observed that 0% FBS maximizes the signals due to human proteins, allowing detection of the less abundant ones. The lists of proteins exclusively expressed in the three different growth conditions are provided as supplementary material in Table S1 (0% vs 5% FBS), S2 (5% vs 10% FBS), and S3 (0% vs 10% FBS). As shown in Fig. 1, significant overlap of proteins identified in the different conditions can be observed; in particular most proteins detected in 10% FBS are also found under the other two conditions.
Table 1.
Protein identification and quantification.
| Culture media [FBS%] | Protein groups human | Protein groups bovine | Sum intensity human | Sum intensity bovine | Ratio intensity human/bovine |
|---|---|---|---|---|---|
| 0% | 289 | 67 | 1.60E + 11 | 1.06E + 10 | 15.05 |
| 5% | 287 | 55 | 7.25E + 10 | 9.67E + 09 | 7.50 |
| 10% | 150 | 63 | 1.04E + 10 | 6.91E + 09 | 1.51 |
Fig. 1.
Venn diagram of human proteins identified in hMSCs secretome obtained from different culture media (0%, 5% and 10% FBS).
To get a more detailed overview of the effect of varying FBS concentration, we compared the results of a gene ontology biological process (GOBP) analysis on the proteins identified in the three conditions (Figure S1). Since a very important parameter to define whether a secretome analysis is reliable is the percentage of ⿿truly⿿ secreted out of the total proteins identified, two very popular prediction programs, SignalP [17] and SecretomeP [18], were used to implement the secretion category of the GOBP analysis. As expected, more than 70% of human identified proteins are predicted as ⿿secreted⿿ under all conditions: 74% (215 proteins) in 0% FBS, 74% (213 proteins) in 5% FBS and 72% (108 proteins) in 10% FBS. In accordance, Figure S1 show that overall that the absence of FBS in the CM does not lead to qualitative changes in the biological processes related to secretome components. Interestingly, cellular component disassembly, death and cell death are little represented biological processes under all conditions, suggesting that the 0% FBS condition does not promote precesses leading to a higher number of lysing cells. Finally, although the function of individual proteins identified is out of the scope of the present communication, it should be noted that several proteins belong to GOBP categories related to response to stimulus.
In conclusion, this analysis allows to define an optimized protocol to collect the secretome of human BM-MSC in order to detect the differential expression of secreted proteins induced by exposing cells to specific stimulation conditions in future studies.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Acknowledgments
This project was funded in part by ⿿Piano di Sviluppo Unimi 2014⿿ from Università degli Studi di Milano, Italy. We thank Nicoletta Leveratto and Francesca Grassi Scalvini for their skillful technical assistance.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.euprot.2016.01.005.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Roobrouck V.D.1, Clavel C., Jacobs S.A., Ulloa-Montoya F., Crippa S., Sohni A., Roberts S.J., Luyten F.P., Van Gool S.W., Sampaolesi M., Delforge M., Luttun A., Verfaillie C.M. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cells. 2011;29(5):82–87. doi: 10.1002/stem.633. [DOI] [PubMed] [Google Scholar]
- 2.Amable P.R., Teixeira M.V., Carias R.B., Granjeiro J.M., Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jelly. Stem Cell Res. Ther. 2014;5:53–66. doi: 10.1186/scrt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celis J.E., Gromov P., Cabezon T., Moreira J.M., Ambartsumian N., Sandelin K., Rank F., Gromova I. Proteomic characterization of the interstitial fluid perfusing the breast tumor microenvironment: a novel resource for biomarker and therapeutic target discovery. Mol. Cell. Proteomics. 2004;3:327–344. doi: 10.1074/mcp.M400009-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y., Elmets C.A., Smith J.W., Liu Y.T., Chen Y.R., Huang C.P., Zhu W., Ananthaswamy H.N., Gallo R.L., Huang C.M. Quantitative proteomes and in vivo secretomes of progressive and regressive UV-induced fibrosarcoma tumor cells: mimicking tumor microenvironment using a dermis-based cell-trapped system linked to tissue chamber. Proteomics. 2007;7:4589–4600. doi: 10.1002/pmic.200700425. [DOI] [PubMed] [Google Scholar]
- 5.Xue H., Lu B., Lai M. The cancer secretome: a reservoir of biomarkers. J. Transl. Med. 2008;6:52. doi: 10.1186/1479-5876-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson J.C., Mateos A., Pepperkok R. Maturation of the mammalian secretome. Genome Biol. 2007;8(4):211. doi: 10.1186/gb-2007-8-4-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;13:2196–2211. doi: 10.1016/j.biochi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Finoulst I., Vink P., Rovers E., Pieterse M., Pinkse M., Bos E., Verhaert P. Identification of low abundant secreted proteins and peptides from primary culture supernatants of human T-cells. J. Proteomics. 2011;75:23–33. doi: 10.1016/j.jprot.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 9.Colzani M., Waridel P., Laurent J., Faes E., Ruegg C., Quadroni M. Metabolic labeling and protein linearization technology allow the study of proteins secreted by cultured cells in serum-containing media. J. Proteome Res. 2009;8:4779–4788. doi: 10.1021/pr900476b. [DOI] [PubMed] [Google Scholar]
- 10.Pellitteri-Hahn M.C., Warren M.C., Didier D.N., Winkler E.L., Mirza S.P., Greene A.S. Improved mass spectrometric proteomic profiling of the secretome of rat vascular endothelial cells. J. Proteome Res. 2006;5:2861–2864. doi: 10.1021/pr060287k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin J., Kim G., Kabir M.H., Park S.J., Lee S.T., Lee C. Use of composite protein database including search result sequences for mass spectrometric analysis of cell secretome. PLoS ONE. 2015;10(3):e0121692. doi: 10.1371/journal.pone.0121692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper S. Reappraisal of serum starvation, the restriction point, G0, and G1 phase arrest points. FASEB J. 2003;17:333–340. doi: 10.1096/fj.02-0352rev. [DOI] [PubMed] [Google Scholar]
- 13.Shin J.S., Hong S.W., Lee S.L., Kim T.H., Park I.C., An S.K., Lee W.K., Lim J.S., Kim K.I., Yang Y., Lee S.S., Jin D.H., Lee M.S. Serum starvation induces G1 arrest through suppression of Skp2-CDK2 and CDK4 in SK-OV-3 cells. Int. J. Oncol. 2008;32:435–439. [PubMed] [Google Scholar]
- 14.Hasan N.M., Adams G.E., Joiner M.C. Effect of serum starvation on expression and phosphorylation of PKC-alpha and p53 in V79 cells: implications for cell death. Int. J. Cancer. 1999;80:400–405. doi: 10.1002/(sici)1097-0215(19990129)80:3<400::aid-ijc11>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Tamplenizza M., Lenardi C., Maffioli E., Nonnis S., Negri A., Forti S., Sogne E., De Astis S., Matteoli M., Schulte C., Milani P., Tedeschi G. Nitric oxide synthase mediates PC12 differentiation induced by the surface topography of nanostructured TiO2. J. Nanobiotechnol. 2013;11:11–35. doi: 10.1186/1477-3155-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 17.Petersen T.N., Brunak S., Von Heijne G., Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 18.Dyrløv Bendtsen J., Juhl Jensen L., Blom N., Von Heijne G., Brunak S. Feature based prediction of non-classical and leaderless protein secretion. Protein Eng. Des. Sel. 2004;17(4):349–356. doi: 10.1093/protein/gzh037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.