Abstract
Room-temperature ionic liquids (RTILs) have attracted considerable attention in recent years due to their versatile properties such as negligible volatility, inflammability, high extractive selectivity and thermal stability. In general, RTILs are organic salts with a melting point below ~100 °C determined by the asymmetry of at least one of their ions. Due to their amphiphilic character, strong interactions with biological materials can be expected. However, rising attention has appeared towards their similarity and interaction with biomolecules. By employing structural modifications, the biochemical properties of RTILs can be designed to mimic lipid structures and to tune their hydrophobicity towards a lipophilic behavior. This is evident for the interaction with lipid-membranes where some of these compounds present membrane-disturbing effects or cellular toxicity. Moreover, they can form micelles or lipid-like bilayer structures by themselves. Both aspects, cellular effects and membrane-forming capacities, of a novel class of lipophilic imidazolium salts will be discussed.
Keywords: Ionic liquid, Imidazolium salt, Micelle, Bilayer, Vesicle, Biomembrane
Introduction
Room-temperature ionic liquids (RTILs) are salts in a liquid state below ~100 °C, which have a hybrid, organic-ionic nature and exhibit remarkable properties, such as negligible volatility and thermal stability, as well as an impressive solvation ability. Some of these compounds show astonishing similarities to biomolecules, similar to lipids that promote attention to investigate their structure-to-function principles and biomolecule interactions. In general, many RTILs serve as designer solvents with specialized properties and applications like the liquid–liquid extraction of antibiotics (Cull et al. 2000) or function as solutes for transition metal catalysis (Wasserscheid and Keim 2000). Based on a large number of cation–anion combinations, RTILs can be tailored for a variety of applications including organic synthesis (Welton 2004), separation (Visser et al. 2001) and electrochemical processes (Kamavaram et al. 2005). In the past few years, ionic liquids and N-heterocyclic carbenes (NHCs) have furthermore gained significant attention in scientific fields such as complex chemistry, material science, drugs and catalysis (Dröge and Glorius 2010; Hallett and Welton 2011; Hopkinson et al. 2014; Richter et al. 2014; Welton 1999). One of the most recent findings in this novel field is that some RTILs can adopt mono- or bilayer arrangements in carbide-derived carbon nanopores that show structural anomalies, such as partially broken columbic orderings, analyzed by X-ray scattering and Monte Carlo simulations (Futamura et al. 2017). The latter work, being at the frontier of recent science, proved the existence of a superionic state and might have further implications for electrochemical applications and the field of charge distributions in close vicinity of surfaces (Futamura et al. 2017; Kondrat and Kornyshev 2011).
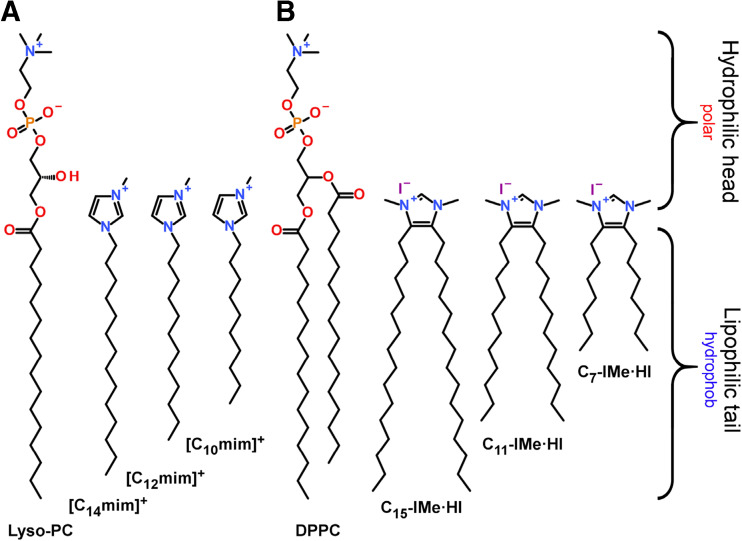
Beside these current studies, we here scrutinize characteristics that are important for the interaction of RTILs with biological membranes and naturally occurring lipids. Among a wide chemical variety, an important class of RTILs are imidazolium cations, carrying aliphatic or aromatic residues at the nitrogen atoms. For instance, transmembrane anion transport properties of adamantyl-functionalized imidazolium salts have been reported in combination with the effect of the flexibility of their linkers to the central imidazolium cation (Gravel and Schmitzer 2015). Interestingly, RTILs could serve as a substitute for aqueous solutions, which embed lipid bilayer membranes. Gayet et al. showed the formation of DPPC lipid vesicles that could be stabilized in the presence of several RTILs such as 1-butyl-3-methylimidazolium tetrafluoroborate (BmimBF4) or RTIL–water mixtures (Gayet et al. 2011). They furthermore demonstrated that the bilayer structure and its stability depends on the RTIL content by applying small-angle neutron scattering and differential scanning calorimetry. Again, this RTIL was modified with alkyl residues at the nitrogen atoms. Instead of employing ionic liquids as a substitute for solvents, RTILs themselves have also been shown to form membranes in aqueous media. An early approach was the ability of RTILs to self-aggregate in relation to the electrostatic or hydrophobic contributions of the isolated cation in water (Blesic et al. 2007). They discovered mixed-micelles formed by [Cnmim]Cl + SDS for n ≈ 6–8 and reported on a rather “pure salt effect” for shorter chain lengths. Liu et al. showed by molecular dynamics simulations the capability of imidazolium-based RTILs [Cnmim]Br (n = 10, 12, 14), that contain a single hydrophobic tail at one nitrogen atom, to form unilamellar vesicles in aqueous solutions without any supplement (Liu et al. 2015). In our approach to focus on similarities to natural lipids, the structure of [C14mim]+ already contained a hydrophilic head and a hydrophobic tail, mimicking to some extent the structure of lyso-phospholipids (Fig. 1a).
Fig. 1.
Lipid mimetic ionic liquids. a [Cnmim]+ (n = 10, 12, 14) salts imitating the structure of lyso-phosphatidylcholine. b (Cn-IMe·HI, n = 7, 11, 15) imidazolium salts resembling the structure of DPPC
The structural flexibility of imidazolium salts was further improved as Richter et al. introduced 4,5-alkyl-N-heterocylcic imidazolium salts. In their work, the hydrophobic residues were connected to the hydrocarbon backbone of the imidazolium cation, whereas the 1,3-nitrogen positions remain accessible for further modifications (Richter et al. 2014). Although the initial design was tailor-made as a precursor for the stabilization of metal-nanoparticles in aqueous solutions, this structure allowed the independent design of the lipid head group-mimicking part and the hydrophobic tail-imitating portion of the molecule. Employing ligand exchange reactions, the imidazolium salt, 1,3-dimethyl-4,5-dipentadecylimidazolium iodide (C15-IMe·HI), could be functionalized to a carbene moiety that associates to palladium-nanoparticles. The long alkyl chains of the molecules then generated a hydrophobic shell around the Pd-particles that prevents their aggregation (Richter et al. 2014). However, this molecule and derivatives thereof (Fig. 1b) exhibit a highly structural similarity to phospholipids, such as DPPC. Following this work, lipid mimetic properties and membrane interactions were further investigated, and the backbone-alkylated imidazolium salts revealed antibacterial, cytotoxic and activities against tumor cells (Wang et al. 2015a, b), to be discussed in detail here.
Additionally, further modifications of the 1,3-nitrogen residues on the imidazolium ring leads to a broad variety of structure designs with manifold properties (Rühling et al. 2017). Interestingly, the introduction of bulky benzyl modifications leading to the 1,3-dibenzyl-4,5- dipentadecylimidazolium salt (C15-IBn·HBr) conserved some structural similarity to lipids, but showed unexpected properties in aqueous solutions. Depending on the bulkiness of the head group and the length of the hydrocarbon chains, lipid-droplet-like emulsions, probably stabilized by π-stacking effects of the aromatic benzyl residues, or even giant vesicles were formed (Drücker et al. 2017). Their membrane interactions were concomitantly studied via a series of artificial lipid bilayer models in vitro.
Cellular effect and structural features of 4,5-dialkylated imidazolium-based salts
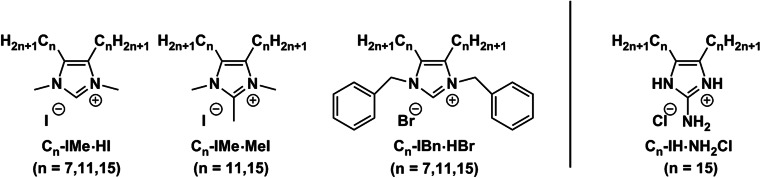
To date, the best-known RTILs are imidazolium-based ionic liquids. In the past few years, a number of studies have focused on 1-alkyl-3-methylimidazolium species (Ranke et al. 2004; Cui et al. 2003; Kumar and Malhotra 2010; Samori et al. 2010; Stolte et al. 2007) which behave as amphipathic compounds and reveal surface activities analogous to quaternary ammonium compounds (QACs). The polar and hydrophilic imidazolium head group is beneficial for an electrostatic interaction with membrane lipids, showing a remarkable membrane activity (Samori et al. 2010; Anderson and Long 2010; Geng et al. 2010; Carson et al. 2009; Rawat and Bohidar 2012). In addition, the long alkyl chain attached to the N1 atom has been shown to effectively enhance surface activity and self-assembly characteristics of 1-alkylimidazolium cations in aqueous solution (Tischer et al. 2012). This mono-alkylated imidazolium species has been reported to exhibit potent cellular toxicity against a panel of tumor cell lines, including human origin (MCF-7, HeLa, HT-29 and Caco-2 cells) (Kumar et al. 2009; Stepnowski et al. 2004; Frade et al. 2007, 2009; García-Lorenzo et al. 2008) as well as animal tumor cell lines (IPC-81 and C6 Glioma) (Ranke et al. 2004, 2007; Stolte et al. 2006). Generally, their cytotoxicity could be directly correlated with their lipophilic properties (Łuczak et al. 2010; Ranke et al. 2004). Hitherto uncharacterized 4,5-dialkylated imidazolium cations, which represent a novel class of imidazolium-based RTILs, have been designed and synthesized (Wang et al. 2015a, b), as mentioned above. However, further structural diversity was introduced by head group modifications to monitor lipid mimetic properties in relation to head group size and hydrophobicity (Wang et al. 2016; Rühling et al. 2017; Drücker et al. 2017). These backbone-alkylated imidazolium RTILs consist, for instance, of a hydrophilic N,N′-disubstituted imidazolium head group and two hydrophobic alkyl chains located at the 4- and 5-positions of the imidazole ring (Fig. 2). Either an N-methyl- or N-benzyl-substituent was employed as a tunable wingtip group of the imidazole core, which allowed modification of steric properties and the hydrophobicity of imidazolium RTILs. In particular, the N-methylated imidazolium head group was additionally equipped with C2-methylation. Varying alkyl chain lengths of n = 7, 11 and 15 were taken into account to cover the different cation lipophilicities. This work combines the structural features of phospholipids and imidazolium cations to mimic the structure and biophysical behavior of membrane lipids (Fig. 2) (Rühling et al. 2017).
Fig. 2.
Chemical structures of investigated 4,5-dialkylated imidazolium-based RTILs. The imidazolium RTILs are abbreviated as Cn-IR·R’A, where n represents the number of carbon atoms in each alkyl chain, I the imidazolium, R the substituent at the N-position, R’ the substituent at the 2-position and A the anion. Modified from Rühling et al. (2017)
The cellular toxicity of these compounds was evaluated in vitro using a panel of cell lines, including the rat C6 Ggioma tumor cell line and cells such as Madin–Darby canine kidney (MDCK II), as well as the mouse embryonic fibroblast cell line (NIH3T3). The half-maximal effective concentrations (EC50) are summarized in Table 1. Remarkably, compared to general mono-alkylated imidazolium species with an EC50 > 1000 μM (Ranke et al. 2004), all studied 4,5-dialkylated imidazolium RTILs reveal an approximate three orders of magnitude higher anti-tumor activity against the C6 glioma cell line. The most potent one is C7-IBn·HBr, displaying the lowest EC50 of ~1.0 μM (Wang et al. 2015a, b). This indicates that the introduction of two hydrophobic alkyl chains into the backbone contributes greatly to improve the activity against tumor-derived cell lines of imidazolium-based RTILs.
Table 1.
CMC [μM] and EC50 [μM] and confidence intervals of all tested 4,5-dialkylimidazolium RTILs
| Abbreviations | CMC/μM | EC50 ± SD/μM | ||
|---|---|---|---|---|
| C6 glioma | MDCK II | NIH3T3 | ||
| C7-IMe·HI | 65.9 (50~80) | 2.3 ± 0.5 | 6.7 ± 0.8 | 4.7 ± 0.8 |
| C7-IBn·HBr | 17.9 (10~20) | 1.0 ± 0.1 | 12.9 ± 2.1 | 2.5 ± 0.4 |
| C11-IMe·HI | 8.5 (5~10) | 10.5 ± 0.3 | 8.9 ± 0.8 | 5.0 ± 0.5 |
| C11-IMe·MeI | 7.9 (5~10) | 5.3 ± 0.5 | 8.4 ± 0.5 | 1.0 ± 0.2 |
| C11-IBn·HBr | 2.3 (2.0~5.0) | 2.0 ± 0.1 | 16.0 ± 5.7 | 4.2 ± 0.5 |
| C15-IH·NH2Cl | 4.1 (2.5~5) | 67.2 ± 3.9 | 32.2 ± 3.3 | 12.2 ± 1.7 |
| C15-IMe·HI | 1.4 (1.0~5.0) | 28.1 ± 0.7 | 15.9 ± 0.5 | 7.7 ± 0.2 |
| C15-IMe·MeI | 0.9 (0.7~1.0) | 16.3 ± 0.6 | 23.6 ± 1.1 | 3.5 ± 0.9 |
| C15-IBn·HBr | 0.5 (0.4~1.3) | 11.3 ± 2.0 | 20.6 ± 4.2 | 8.7 ± 0.7 |
Further, for a given type of head group, the cellular toxicity is decreased with increasing alkyl chain length, which is true for all tested cell lines. In other words, the cytotoxic effect of the 4,5-dialkylimidazolium RTILs carrying the same head group is negatively correlated with their lipophilicity, which is apparently inverse to the earlier reported findings for 1-alkylimidazolium species (Łuczak et al. 2010; Ranke et al. 2004).
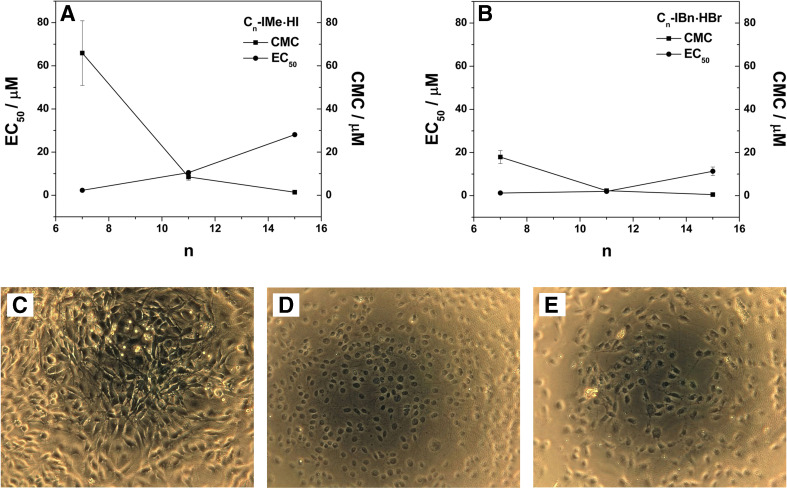
In order to analyze a possible structure-activity relationship for Cn-IMe·HI and Cn-IBn·HBr species to natural occurring lipids, their critical micelle concentration (CMC) values, which also characterize surface activity, were co-plotted with the EC50 values of their toxicity on cells (Fig. 2a, b) (Wang et al. 2015a, b). It appeared that the CMC values are negatively correlated with the EC50 values for both the Cn-IMe·HI and the Cn-IBn·HBr compound series, showing that the elongation of alkyl chains in the backbone result in a declined tendency of their activity against tumor-derived cell lines (Fig. 3a; Wang et al. 2015a, b).
Fig. 3.
Cellular interactions of Cn-IMe·HI and Cn-IBn·HBr (n = 7, 11, 15). Both CMC and EC50 values against C6 glioma cell line plotted as a function of the number of carbon atoms (n) in each alkyl chain for a Cn-IMe·HI and b Cn-IBn·HBr (n = 7, 11, 15). Microscopy-phase-contrast images of C6 glioma cells: c untreated, d treated by 1% Triton X-100 for 10 min and e treated by 100 μM C11-IMe·HI for 24 h. Modified from Wang et al. (2015a, b)
Interestingly, the EC50 value (~10.5 μM) of C11-IMe·HI (Fig. 3a) is very close to its CMC value (~8.5 μM) and almost overlapping in the case of the C11-IBn·HBr compound with an EC50 (~2.0 μM) and CMC (~2.3 μM) (Fig. 2b). This suggests that the micellization plays a critical role in their cellular toxicity (Wang et al. 2015a, b). The micelle formation and the CMC values might indicate an effective adsorption for C11 derivatives at the cell–media interface, thus facilitating the fusion into the cell membrane. A strong membrane destabilization or disruption is potentially given by the hydrophobic mismatch between the medium length C11 alkyl chains and long alkyl chains of membrane lipids. This effect, presumably rising at a specific chain length range of 10~12 carbon atoms, is well supported by earlier results with respect to the biological activities of long-chain imidazolium cationic surfactants (Pernak et al. 2003). In order to visually present the cellular toxicity of C11 imidazolium salt species by wide-field microscopy using phase-contrast imaging, C6 glioma cells were treated with 100 μM C11-IMe·HI for 24 h (Fig. 3c–e). The intact cell membranes of untreated C6 glioma cells are presented in Fig. 3c. However, after lysis of cell membranes by Triton X-100, only the nuclei of the tumor cells can be identified (Fig. 3d). After treatment with 100 μM C11-IMe·HI, the plasma membranes of C6 glioma cells have nearly disappeared (Fig. 3e), which display a similar cell morphology compared to the control shown in Fig. 3d (Wang et al. 2015a, b). This demonstrates that C11-IMe·HI act as a detergent and exhibits strong lytic activity on the cell membranes.
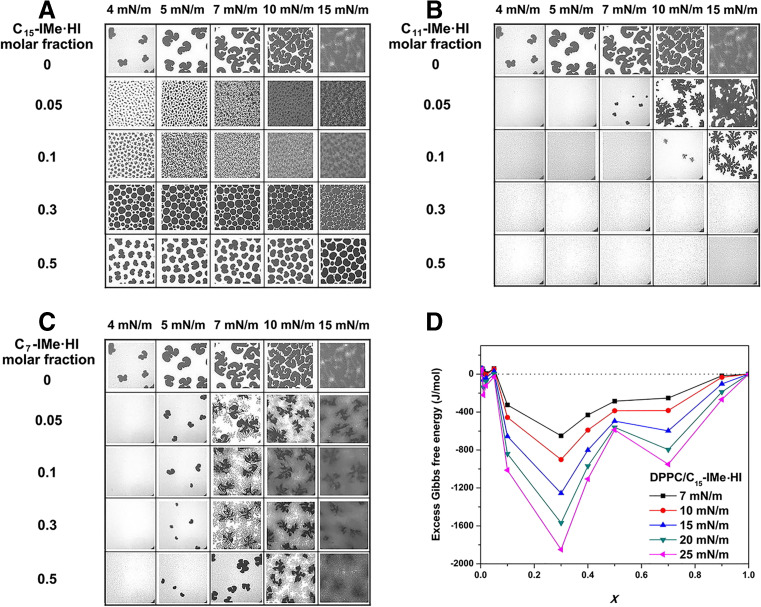
As reported by Ranke et al. (2004), the single, long alkyl chain at the imidazolyl-1-position may intercalate into the cell membranes, which could then promote a strong membrane disruption. However, backbone-alkylated imidazolium RTILs are structurally even closer to the natural phospholipid framework. Thus, it could be speculated that they might exert their potent cellular effects (mentioned above) along distinct pathways differing from those of 1-alkylimidazolium salts. To verify this hypothesis, a series of investigations of the interaction between Cn-IMe·HI (n = 7, 11, 15) and monolayers using the Langmuir technique in vitro were carried out (Fig. 4) (Wang et al. 2016). DPPC, a zwitterionic phospholipid, was used as a representative model membrane lipid to establish binary monolayers at the air-water interface. It appears that C15-IMe·HI components generate significant concentration-dependent modifications of the monolayer structure below 15 mN/m (Fig. 4a.). With increasing addition of C15-IMe·HI (molar ratio from 0.05 to 0.3), a drastic rigidifying effect on the DPPC monolayer is shown by the increasing appearance of lipid condensed domains (dark area). At the 0.3 M fraction of the C15 compound, the most remarkable rigidification is reached, accompanied by the formation of a Langmuir film with the best thermodynamic stability (minimal ΔGex = −1849 J/mol, Fig. 4d) (Wang et al. 2016). This implies that C15-IMe·HI has good affinity to mix with DPPC, leading to a readily miscible lipid monolayer at the air-water interface. Furthermore, its capability to mix well with membrane lipids (such as DPPC) might correlate with a lower cellular toxicity (i.e. by higher EC50 values) (Table 1; Wang et al. 2015a, b, 2016). Instead, the integrity of the lipid film is severely disrupted by the incorporation of C11-IMe·HI, showing lowered line tensions between domains (irregular appearance of domains) (Fig. 4b). These findings thus suggest, that the capability of these imidazolium salts to interact, i.e. intercalate into the membrane, may play along with the cellular toxicity and membrane disturbance. In fact, the medium length C11 compound shows a stronger cellular effect than the long chain C15, which is in line with the domain disturbance mentioned above (Fig. 4). This is also in agreement with the findings from the cytotoxicity assays by Wang et al. (2015a, b) (Fig. 3c–e) showing membrane lysis. However, the short chain C7-IMe·HI exhibits negligible membrane activity (Fig. 4c), although it is the most toxic compound in the Cn-IMe·HI series. Therefore, it can be concluded that, for a given type of head group (particularly -Me), a negative correlation between the cellular toxicity of 4,5-dialkylated imidazolium RTILs and their lipophilicity, i.e. the capability to intercalate in dependence of alkyl chain length, is evident.
Fig. 4.
Interactions of Cn-IMe·HI (n = 7, 11, 15) on a DPPC containing monolayer. Epi-fluorescence images of the monolayers of DPPC mixed with different molar fractions of a C15-IMe·HI, b C11-IMe·HI, and c C7-IMe·HI at the air-water interface at 293 K. All samples were mixed with 0.5 mol% of the fluorescent dye BODIPY-PC. The frame size of all images is 120 × 120 μm. d The excess Gibbs free energy (ΔGex) of mixing versus composition plots of the binary mixed monolayers of DPPC/C15-IMe·HI at several, constant surface pressures at 293 K. Modified from Wang et al. (2016)
Further, their cellular toxic effect is moderated by another important factor, the hydrophobicity and size of the imidazolium head group. Here, a guanidinium RTIL (C15-IH·NH2Cl) bearing a highly hydrophilic imidazolium head group (Fig. 2, fourth structure), where the nitrogen atoms are reduced and the carbon C2 is modified by an amine group, showed the lowest toxicity (EC50 ~67.2 μM), accompanied by a relatively high water solubility (CMC ~4.1 μM) (Rühling et al. 2017). However, Cn-IMe·MeI (n = 11, 15) ,with a trimethylated and hence more hydrophobic imidazolium head group, have a relatively high activity against C6 glioma cells in comparison to the structurally related Cn-IMe·HI compounds. Here, the cytotoxic effect is enhanced 1.72-fold and 1.98-fold in the cases of n = 11 and n = 15, respectively (Rühling et al. 2017, oral communication). By contrast, the N-benzylation of the imidazole structure did more effectively improve the anti-tumor activity towards C6 glioma cells (Table 2) (Rühling et al. 2017).
Table 2.
Comparison of cellular toxic efficiency between Cn-IMe·HI and Cn-IBn·HBr species
| C6 glioma | MDCK II | NIH3T3 | |
|---|---|---|---|
| n= 7 | 2.3 | 0.52 | 1.9 |
| n = 11 | 5.3 | 0.56 | 1.2 |
| n = 15 | 2.5 | 1.2 | 0.89 |
The most significant effect of N-benzylation on cellular toxicity appears for a alkyl chain length of n = 11, where it is 5.3-fold higher in comparison to N-methylation of the imidazolium ring (Table 2). This might be interpreted by the different stability of micelles in solution (Table 1). These results suggest that the head group hydrophobicity and size of imidazolium-based RTILs could influence the probability to interact and intercalate into cellular bilayers. A head group with enhanced hydrophobicity but lower size favors a higher partitioning probability into the hydrophobic interior of the cell membrane, followed by membrane disturbance or disruption.
Taken together, the cellular toxicity effect of the 4,5-dialkylated imidazolium RTILs discussed here strongly depend on both the alkyl chain length as well as the head group type. Specifically, their activity against C6 glioma tumor-derived cells in vitro can be summarized in the following order:
Membrane interactions of 4,5-dialkylated imidazolium-based salts
By employing different techniques, it could be demonstrated that the 1,3-dimethyl-4,5-dialkylimidazolium salts (Cn-IMe·HI, n = 7, 11, 15) strongly interact with artificial and cellular bilayer membranes (Drücker et al. 2017; Wang et al. 2015a, b). The combination of the quartz crystal microbalance technique and fluorescent membrane probes allowed further determination of fundamental membrane interactions and bilayer properties of the pure compound. Lipid monolayer insertion properties and monolayer-domain rigidifications were probed by surfactant mimicking Langmuir film measurements as discussed before (Wang et al. 2016; Rühling et al. 2017). Additionally, the application of laser scanning confocal microscopy demonstrated vesicle formation and bilayer membrane domain fluidization of the long alkyl chain C15-IMe·HI.
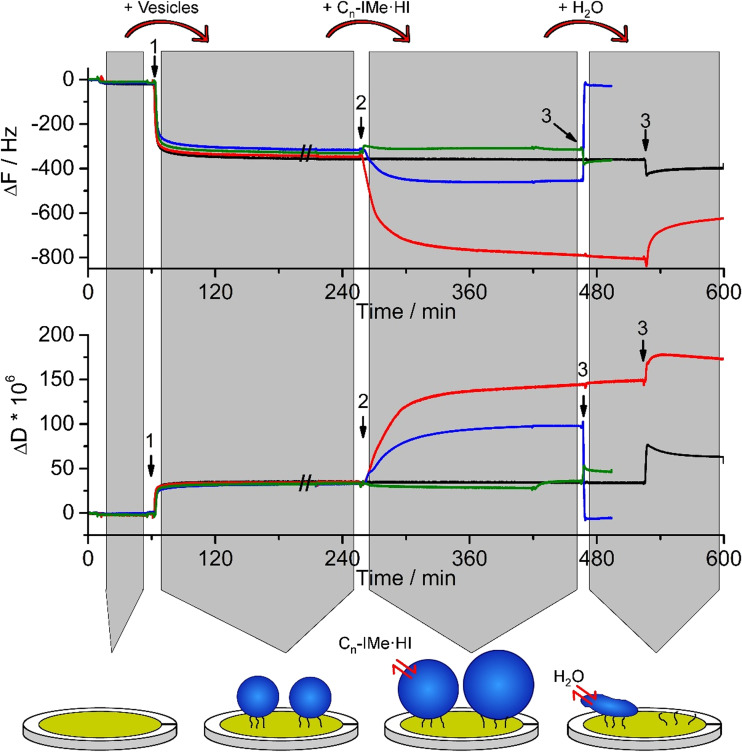
The quartz crystal microbalance technique with dissipation monitoring (QCM-D) is a versatile method to analyze surface interactions at the fluid/solid interface. It has been frequently used to analyze the contact of biomolecules, such as peptide nucleic acids or hemoglobin with membrane surfaces (Höök et al. 1998; Janshoff et al. 2000). The simultaneous measurement of the sensor’s frequency shift (ΔF = F(t) – F0), representing changes in associated mass, and dissipation (ΔD = D(t) – D0), which provides information about the viscoelastic properties delivers two important parameters to characterize the interaction of RTILs with membrane models (Sauerbrey 1959; Janshoff et al. 2000; Fatisson et al. 2011).
Since C15-IMe·HI has a strong structural resemblance to naturally occurring phospholipids (Fig. 1), a charged, polar head group and two hydrophobic alkyl chains, it is interesting to gain further insights into functional similarities. Therefore, negatively charged POPC/POPS (4:1) vesicle membranes were incubated with suspensions of Cn-IMe·HI (n = 7, 11, 15) imidazolium salts in a tris-buffered milieu, pH 7.4 (TBS) that roughly mimics cellular conditions (Fig. 5).
Fig. 5.
Bilayer interactions of Cn-IMe·HI on POPC/POPS (4:1) vesicles probed by QCM-D. Large unilamellar vesicles of POPC/POPS (4:1, 200 nm) were surface-tethered by biotin-streptavidin linkers on a self-assembled monolayer coated sensor surface (arrows 1). Following equilibration with tris-buffered-saline, pH 7.4, suspensions of 0.1 mM (Cn-IMe·HI, n = 7, 11, 15) imidazolium salts were incubated, which induced diverse membrane interactions (arrows 2). Remarkably, the C15 (red) and C11 (blue) compounds lead to an increase in surface associated mass, indicated by ΔΔF and an increased viscoelasticity of the associated layers (ΔΔD), whereas the short chain C7 (green) imidazolium salt did not much alter vesicle membranes. After another equilibration step with buffer for 30–60 min, rinsing with pure water applied hypo-osmotic conditions (arrows 3). The osmotic gradient across the vesicle membrane resulted in a severe loss of vesicle mass after interaction with the C11 compound. Vesicles incubated with the C15 imidazolium salt partially lost some mass and vesicles treated by the C7 compound did not considerably change their mass. As a control, ~0.5 mg/ml POPC:POPS (4:1), 200-nm vesicles were also applied (black). The cartoon represents the effects of imidazolium salt on the vesicles (e.g. C11-IMe·HI). Modified from Drücker et al. (2017) and Wang et al. (2015a, b)
Following the equilibration of the sensor’s surface in TBS buffer, 200-nm POPC/POPS (4:1) vesicles were tethered on the surface via streptavidin/biotin linkers. The adsorption showed a frequency shift of ΔΔF = 311 ± 16 Hz (Drücker et al. 2017; Wang et al. 2015a, b), accompanied by a moderate change in dissipation (Fig. 5, arrows 1). The latter results from the fact that surface-associated vesicles show a much larger loss of resonance energy due to their viscoelastic properties than, for instance, solid supported bilayers (Drücker et al. 2014). With respect to that comparison, the subsequent incubation of Cn-IMe·HI (n = 7, 11, 15) imidazolium salts revealed a much more enhanced effect. First, the long chain C15 and medium length C11 imidazolium compounds showed a huge additional increase in the frequency shift (ΔΔFC15 = 445 ± 8 Hz), which demonstrates the accumulation of a large mass in relation to the initial mass of liposomes (Fig. 5, arrows 2). Second, the interaction of these imidazolium salts with the vesicle membrane showed an even larger gain in the dissipation shift than the adsorption of vesicles in the first place. This effect was mostly pronounced for the C15 compound and appeared to be proportional to chain length (Drücker et al. 2017). It is likely that the simple intercalation of the imodazolium salts into the vesicle bilayer, which leads to the formation of a mixed membrane, is not sufficient to explain such large shifts. Rather than detecting only the addition of membrane mass, it seems to be reasonable that the intercalation of these imidazolium salts moreover altered the viscoelastic properties by generating packing defects in the bilayer. This is in good agreement with the fact that the C11 and C7 imidazolium compounds cannot fully penetrate the membrane and hence led to packing defects as shown for a DPPC bilayer simulated by molecular dynamics (Wang et al. 2016). Whereas in contrast to that, the long tail C15-IMe·HI compound induced to the formation of a gel-phase in the DPPC mixed bilayer. Following the interactions of Cn-IMe·HI (n = 7, 11, 15) imidazolium salts with the POPC/POPS (4:1) vesicle membranes, the application of hypo-osmotic conditions (Fig. 5, arrows 3), showed the almost complete loss of vesicle membranes in case of prior C11 compound interactions. Vesicle membranes, which have been incubated with the C15 compound, were partially disrupted, whereas the C7 imidazolium salt did not much affect the membranes at all. This is in good agreement with the difference in head/tail size proportions of these compounds in relation to phosphatidylcholine lipids. The long tail C15-IMe·HI provided the least hydrophobic mismatch between alkyl-chains and the palmitoyl- or oleoyl-groups of the membrane lipids, whereas C11-IMe·HI exhibits a much larger hydrophobic mismatch, resulting in a stronger disintegration of vesicle bilayers.
In contrast to POPC/POPS (4:1) membranes, a neutrally charged model of pure POPC liposomes showed less prominent interactions with these imidazolium salts, therefore suggesting that the positive charge of Cn-IMe·HI salts facilitates surface contact to the bilayer and promotes membrane intercalation (Drücker et al. 2017).
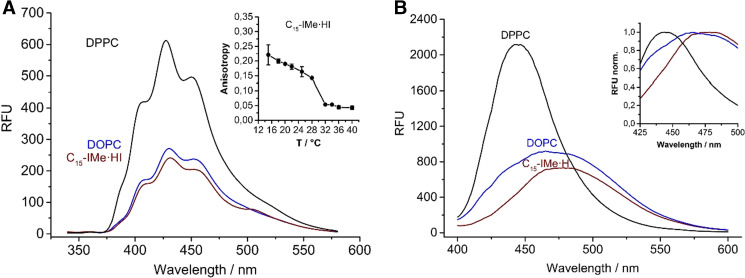
The C15-IMe·HI imidazolium salt interacted starkly with vesicle membranes, thus proposing that the structure-to-function relationship of this compound matches best to natural phospholipids. Surprisingly, it could be further demonstrated that it incorporates fluorescent membrane probes, such as 1,6-diphenyl-1,3,5-hexatriene (DPH) or Laurdan. These probes change their fluorescence intensity and anisotropy in dependence of water contact, and report on membrane dynamics and fluidity when they are embedded within the bilayer (Peng et al. 2012; Parasassi et al. 1998).
The fluorescence spectra of these two probes incorporated in pure C15-IMe·HI, exhibit strong similarity to spectra of the same probes embedded in a DOPC reference bilayer (Fig. 6a, b). This demonstrates two interesting findings. First, the fluorescence intensity suggests that it can stabilize bilayer membranes and, furthermore, that these membranes show more similarity to fluid DOPC rather than ordered DPPC bilayers. Moreover, a change in the florescence anisotropy of DPH showed a phase transition at 28–32 °C (Drücker et al. 2017).
Fig. 6.
Characterization of membranes formed by pure C15-IMe·HI using fluorophore probes. a Fluorescence spectra of DPH incorporated into C15-IMe·HI membranes. Vesicles of DOPC and DPPC, which incorporated the probe under similar conditions, were used as a reference. Membranes formed by C15-IMe·HI are likely to have similar properties as DOPC, because their spectra show similar intensity and appearance. The fluorescence anisotropy of DPH showed a transition at 28-32 °C. b Fluorescence spectra of Laurdan in C15-IMe·HI and DOPC and DPPC reference membranes. The inset shows normalized intensities to present the difference in the Laurdan maximum intensity shift. Modified from Drücker et al. (2017)
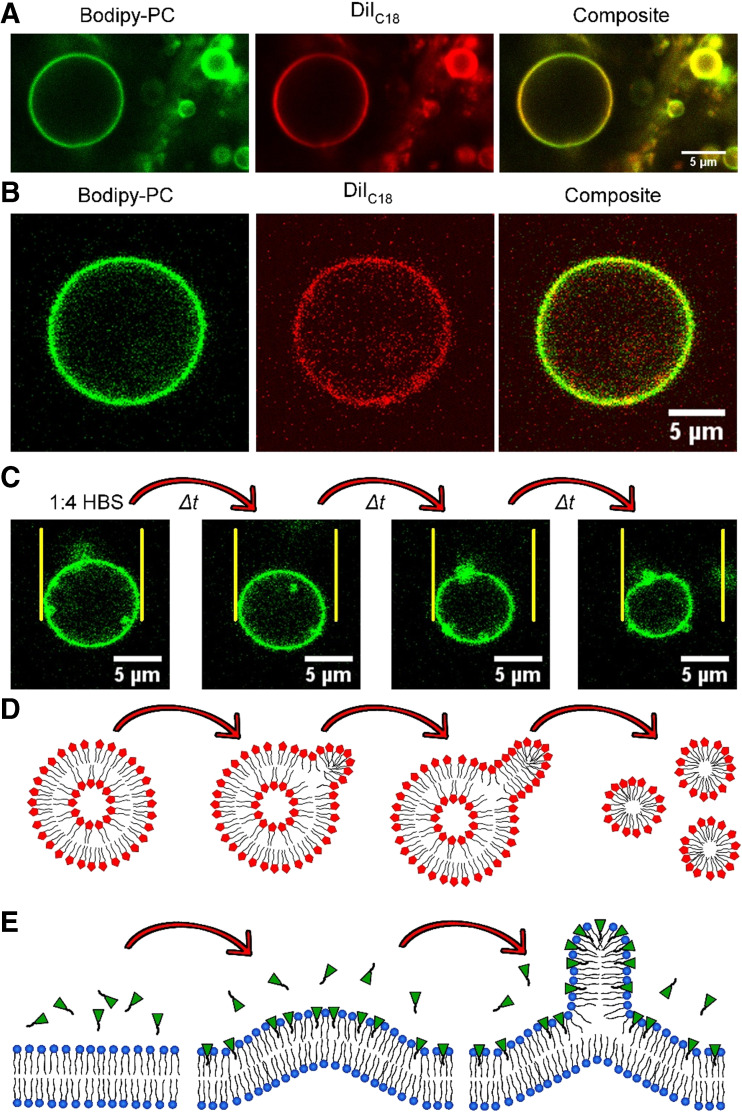
Visual proof that the Cn-IMe·HI imidazolium salts (n = 11, 15) can indeed form bilayer membranes was provided by the attempt to generate giant vesicles in sucrose or HBS, pH 7.4, employing confocal laser scanning microscopy (Fig. 7a, b). The vesicle membranes appeared to be uniform and consisted of one homogeneous phase at room temperature, indicated by phase selective dyes like Bodipy-PC or DiIC18 (Drücker et al. 2017). If domains in a membrane exist, it is expected that those dyes exhibit a liquid ordered (lo) or liquid disordered (ld) domain preference (Baumgart et al. 2007; Klymchenko et al. 2014). Although vesicles formed by C15-IMe·HI appeared to be stable, the C11-IMe·HI compound forms kinetically driven, unstable giant vesicles. The latter could be observed, when vesicles of C11-IMe·HI were formed in sucrose, diluted 1:4 by HBS, and instantly captured by fluorescence microscopy (Fig. 7c). Note the equidistant yellow lines, highlighting the shrinkage in diameter during 110 s (Fig. 7c). The total amount of the C11-IMe·HI membrane surface that could be observed by microscopy was decreasing, hence suggesting that micelle nucleation leads to the formation of non-observable smaller structures. This phenomenon could be explained by the relatively high CMC of ~8.5 μM for this compound (Wang et al. 2015a, b). Consequently, the C11 compound vesicles become more unstable after dilution than micelles. The hydrophobic tail is shorter than that of the C15 compound, which could explain the preferential transformation. The shrinking process possibly starts with C11 imidazolium salt protrusions at the surface of giant vesicles, which eventually leads to micelle nucleation, elongation and the subsequent detachment of micelles (Fig. 7d).
Fig. 7.
Vesicles formed by Cn-IMe·HI imidazolium salts and models of RTIL-membrane interactions. a Giant vesicle formed by pure C15-IMe∙HI in hepes buffered saline, pH 7.4, monitored by confocal laser scanning microscopy. b Giant vesicle formed by C11-IMe∙HI in 320 mM sucrose. c Shrinkage of C11-IMe∙HI vesicles after dilution (1:4) with hepes buffer. Total duration: 110 s. Note the equidistant yellow lines, marking the vesicle size at the beginning of that measurement and the relative shrinkage over time. d: Model of the probable mechanism of C11-IMe∙HI vesicle instability after dilution. e Incubation of the ionic liquid [C4mim]Cl (green) on POPC membranes (cartoon). Modified from Drücker et al. (2017) and Yoo et al. (2016)
A somewhat similar process was determined by Yoo et al. using coarse-grained molecular dynamics simulations of 1-butyl-3-methylimidazolium chloride, [C4mim]Cl, in interaction with POPC bilayers (Yoo et al. 2016). They concluded that the short tail cationic [C4mim]Cl could insert into one leaflet of a planar bilayer altering the leaflet strain and the membrane’s bending modulus (RTIL to lipid ratio 2000:2304). The asymmetric insertion of [C4mim]Cl promotes a monotonic increase in leaflet strain, which then causes bilayer buckling until micelle nucleation occurs at the saturation limit (Fig. 7e). Furthermore, they also simulated POPC vesicles, which could also insert [C4mim]Cl, leading to a discontinuity in the vesicle sphericity. This then promoted nucleation and elongation of mixed POPC-[C4mim]Cl-containing micelles (Yoo et al. 2016).
Another intriguing property of RTILs and their salt-precursors is their impact on membranes containing spatial domains. It has been shown that Cn-IMe·HI (n = 7, 11, 15) imidazolium salts exhibit strong activity against tumor-derived cells, and that 100 μM C11-IMe·HI led to the disruption of C6 glioma cell plasma membranes (Fig. 3) (Wang et al. 2015a, b). Beside membrane transduction of Cn-IMe·HI (n = 7, 11, 15) imidazolium salts and other as yet unknown intracellular factors that promote activities against tumor cell lines, the disintegration of functional cell membrane platforms or domains (lipid rafts) may be another determinant of cell death. It is commonly discussed that cellular plasma membranes contain spatial and timely fluctuating lipid platforms that were enriched in certain lipids, proteins and cholesterol (Sezgin et al. 2017; Simons and Gerl 2010). Moreover, Wang et al. could also demonstrate, that Cn-IMe·HI (n = 7, 11, 15) and other structurally related imidazolium salts change the interfacial line tensions of DPPC domains on a Langmuir monolayer model as mentioned above (Fig. 4) (Wang et al. 2016; Rühling et al. 2017).
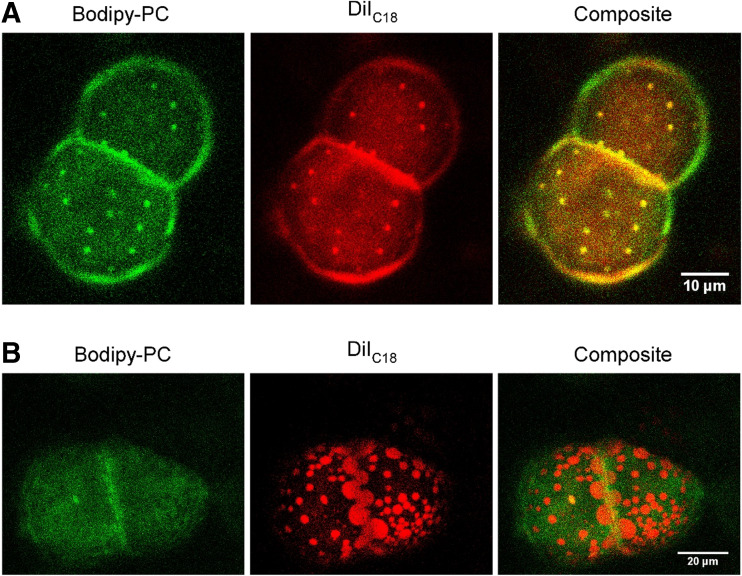
Employing giant vesicles of DOPC/N-stearoyl-D-erythro-sphingosylphosphorylcholine (SSM)/cholesterol (1:1:1) and DOPC/SSM/Chol/C15-IMe·HI (33:23:33:10) at T = 38.0 °C showed an intriguing fluidization of lipid domains (Fig. 8a,b).
Fig. 8.
Membrane fluidization induced by C15-IMe·HI on giant vesicles. a Giant vesicles of DOPC/SSM/Chol (1:1:1) doped with 0.4 mol% Bodipy-PC and 0.4 mol% DiIC18 at T = 38 °C using confocal laser scanning microscopy. Note the small protrusions marked by both dyes. b The incorporation of 10% C15-IMe∙HI results in giant vesicles of DOPC/SSM/Chol/C15-IMe∙HI (33:23:33:10), which present fluidized, larger domains rich in DiIC18 at T = 38 °C. Note that the specificity of the dyes changed. DiIC18 shows high preference for one domain wherein Bodipy-PC is slightly excluded. Modified from Drücker et al. (2017)
Doped with Bodipy-PC and DiIC18, vesicles of DOPC/SSM/cholesterol (1:1:1), showed small domains which protrude out of the surface and exhibit co-localization of both markers (Fig. 8a). After incorporation of 10% C15-IMe·HI, domains appeared to be larger, flat within the bilayer plane, and the dyes showed opposing domain preference. Therefore, a hydrophobic mismatch of the imidazolium salt at domain borders reduced the interfacial energy and provoked bilayer fluidization (Fig. 8b).
Based on that wealth of evidence, another pathway of how the interaction of Cn-IMe·HI (n = 7, 11, 15) imidazolium salts can injure tumor cells (Fig. 3) (C6 glioma; Wang et al. 2015a, b) became apparent. The intercalation of C15-IMe·HI into laterally separated bilayer membranes changed the interfacial energy at domain borders and hence may result in a loss of function on plasma membrane raft domains. Since raft domain integrity is vital for cellular signaling and endo- or exocytosis-related trafficking (Sezgin et al. 2017; Simons and Gerl 2010), the change of cell domain morphology and hence the loss functionality may stimulate cell death.
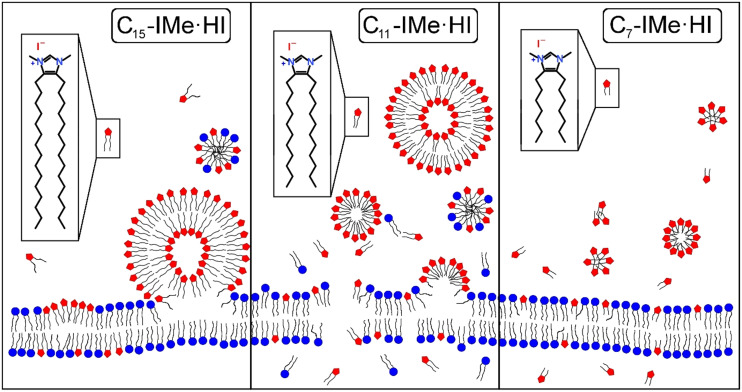
The major interactions of Cn-IMe·HI (n = 7, 11, 15) imidazolium salts with naturally occurring lipid bilayer membranes is briefly summarized by the following cartoon (Fig. 9). The long chain C15-IMe·HI likely had the strongest intermolecular and intramolecular Van der Waals interactions with itself and lipid alkyl chains, promoting the formation of giant vesicles and the fusion to bilayer membranes. It could intercalate, probably form domains, and induce the creation of mixed micelles. The medium chain length C11-IMe·HI compound forms thermodynamically unstable vesicles, which can transiently transform into micelles after dilution. According to the imperfect hydrophobic interactions and its cationic charged head group, it stimulates disintegration of bilayer and plasma membranes. Based on QCM-D results with tethered vesicles and the cytotoxicity in vitro, discussed above, the short tail C7-IMe·HI imidazolium salt evidently passes bilayer membranes without disruption. It cannot form giant vesicles.
Fig. 9.
Model representing conceivable interactions of Cn-IMe·HI imidazolium salts with bilayer membranes. The long chain C15-IMe·HI imidazolium salt forms vesicles under buffered conditions and can fuse with membranes. Charge interactions of positive imidazolium salt head groups and zwitterionic or negatively charged lipids led to the intercalation into the membrane. This is likely to be accompanied by the formation of mixed micelles. The medium chain length C11-IMe·HI imidazolium salt can form vesicles and micelles which disintegrate and lyse bilayer membranes by fusion and incorporation. It also may form mixed micelles. The short chain C7-IMe·HI imidazolium salt forms micelles and cannot stabilize giant vesicles in solution. It is likely to pass membranes without disruption. Modified from Drücker et al. (2017)
Conclusion
This overview summarizes some of the new interesting findings of imidazolium salts in the rising field of ionic liquids and their biomembrane interactions as well as biomolecule, lipid- and membrane-mimetic properties. Motivated by the biological activities of imidazolium-based ionic liquids and the structural features of natural membrane lipids, a novel class of 4,5-dialkylated imidazolium salts are discussed. The investigation of their biophysical properties greatly assists in capturing some details that determine their potential biological effects. Recent work has opened a new path to apply a series of bio-mimetic, 4,5-dialkylimidazolium salts as a new class of anti-tumor candidates in medical chemistry. Although some of the imidazolium salts discussed here showed strong activity against a few tumor-derived cell lines, a further investigation employing non-tumor cells as well as additional cancer types may answer whether a more sophisticated clinical approach, and drug discovery studies of RTILs could become interesting in future approaches. Additionally, attention deserves to be paid towards the detailed investigation of potential mechanisms that govern their biological effects and selectivity.
Additionally, new emerging approaches, such as the catalysis in drug production and the novel “active pharmaceutical ingredient-ionic liquid” concept that suggest the employment of traditional drugs in the form of ionic liquids (Egorova et al. 2017), provide powerful potentials for upcoming sophisticated applications.
Compliance with ethical standards
Conflict of interest
Da Wang declares that he has no conflict of interest. Hans-Joachim Galla declares that he has no conflict of interest. Patrick Drücker declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Ionic Liquids and Biomolecules’ edited by Antonio Benedetto and Hans-Joachim Galla.
References
- Anderson EB, Long TE. Imidazole- and imidazolium-containing polymers for biology and material science applications. Polymer. 2010;51:2447–2454. [Google Scholar]
- Baumgart T, Hunt G, Farkas ER, Webb WW, Feigenson GW. Fluorescence probe partitioning between Lo/Ld phases in lipid membranes. Biochim Biophys Acta. 2007;1768(9):2182–2194. doi: 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesic M, Marques MH, Plechkova NV, Seddon KR, Rebelo LPN, Lopes A. Self-aggregation of ionic liquids: micelle formation in aqueous solution. Green Chem. 2007;9(5):481–490. [Google Scholar]
- Carson L, Chau PKW, Earle MJ, Gilea MA, Gilmore BF, Gorman SP, McCann MT, Seddon KR. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem. 2009;11:492–497. [Google Scholar]
- Cui B, Zheng BL, He K, Zheng QY. Imidazole alkaloids from Lepidium meyenii. J Nat Prod. 2003;66:1101–1103. doi: 10.1021/np030031i. [DOI] [PubMed] [Google Scholar]
- Cull SG, Holbrey JD, Vargas-Mora V, Seddon KR, Lye GJ. Room-temperature ionic liquids as replacements for organic solvents in multiphase bioprocess operations. Biotechnol Bioeng. 2000;69(2):227–233. [PubMed] [Google Scholar]
- Dröge T, Glorius F. The measure of all rings - N-heterocyclic Carbenes. Angew Chem Int Ed. 2010;49(39):6940–6952. doi: 10.1002/anie.201001865. [DOI] [PubMed] [Google Scholar]
- Drücker P, Rühling A, Grill D, Wang D, Draeger A, Gerke V, Glorius F, Galla H-J. Imidazolium salts mimicking the structure of natural lipids exploit remarkable properties forming lamellar phases and giant vesicles. Langmuir. 2017;33(6):1333–1342. doi: 10.1021/acs.langmuir.6b03182. [DOI] [PubMed] [Google Scholar]
- Drücker P, Grill D, Gerke V, Galla H-J. Formation and characterization of supported lipid bilayers containing phosphatidylinositol-4,5-bisphosphate and cholesterol as functional surfaces. Langmuir. 2014;30(49):14877–14886. doi: 10.1021/la503203a. [DOI] [PubMed] [Google Scholar]
- Egorova KS, Gordeev EG, Ananikov VP. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev. 2017;117(10):7132–7189. doi: 10.1021/acs.chemrev.6b00562. [DOI] [PubMed] [Google Scholar]
- Fatisson J, Azari F, Tufenkji N. Real-time QCM-D monitoring of cellular responses to different cytomorphic agents. Biosens Bioelectron. 2011;26(7):3207–3212. doi: 10.1016/j.bios.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Frade RFM, Matias A, Branco LC, Afonso CAM, Duarte CMM. Effect of ionic liquids on human colon carcinoma HT29 and CaCo-2 cell lines. Green Chem. 2007;9:873–877. [Google Scholar]
- Frade RFM, Rosatella AA, Marques CS, Branco LC, Kulkarni PS, Mateus NMM, Afonso CAM, Duarte CMM. Toxicological evaluation on human colon carcinoma cell line (CaCo-2) of ionic liquids based on imidazolium, guanidinium, ammonium, phosphonium, pyridinium and pyrrolidinium cations. Green Chem. 2009;11:1660–1665. [Google Scholar]
- Futamura R, Iiyama T, Takasaki Y, Gogotsi Y, Biggs MJ, Salanne M, Segalini J, Simon P, Kaneko K (2017) Partial breaking of the Coulombic ordering of ionic liquids confined in carbon nanopores. Nat Mater. 10.1038/nmat4974. Advance online publication [DOI] [PMC free article] [PubMed]
- García-Lorenzo A, Tojo E, Tojo J, Teijeira M, Rodríguez-Berrocal FJ, González MP, Martínez-Zorzano VS. Cytotoxicity of selected imidazolium-derived ionic liquids in the human Caco-2 cell line. Sub-structural toxicological interpretation through a QSAR study. Green Chem. 2008;10:508–516. [Google Scholar]
- Gayet F, Marty J-D, Brûlet A, Viguerie N L-d. Vesicles in ionic liquids. Langmuir. 2011;27(16):9706–9710. doi: 10.1021/la2015989. [DOI] [PubMed] [Google Scholar]
- Geng F, Zheng L, Yu L, Li G, Tung C. Interaction of bovine serum albumin and long-chain imidazolium ionic liquid measured by fluorescence spectra and surface tension. Process Biochem. 2010;45:306–311. [Google Scholar]
- Gravel J, Schmitzer AR. Transmembrane anion transport mediated by adamantyl-functionalized imidazolium salts. Supramol Chem. 2015;27(5–6):364–371. [Google Scholar]
- Hallett JP, Welton T. Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem Rev. 2011;111:3508–3576. doi: 10.1021/cr1003248. [DOI] [PubMed] [Google Scholar]
- Hopkinson MN, Richter C, Schedler M, Glorius F. An overview of N-heterocyclic carbenes. Nature. 2014;510(7506):485–496. doi: 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]
- Höök F, Rodahl M, Kasemo B, Brzezinski P. Structural changes in hemoglobin during adsorption to solid surfaces: effects of pH, ionic strength, and ligand binding. Proc Natl Acad Sci U S A. 1998;95(21):12271–12276. doi: 10.1073/pnas.95.21.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janshoff A, Galla H-J, Steinem C. Piezoelectric mass-sensing devices as biosensors-an alternative to optical biosensors? Angew Chem Int Ed. 2000;39(22):4004–4032. doi: 10.1002/1521-3773(20001117)39:22<4004::aid-anie4004>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Kamavaram V, Mantha D, Reddy RG. Recycling of aluminum metal matrix composite using ionic liquids: effect of process variables on current efficiency and deposit characteristics. Electrochim Acta. 2005;50:3286–3295. [Google Scholar]
- Klymchenko, Andrey S, Kreder R. Fluorescent probes for lipid rafts: from model membranes to living cells. Chem Biol. 2014;21(1):97–113. doi: 10.1016/j.chembiol.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Kondrat S, Kornyshev AA. Superionic state in double-layer capacitors with nanoporous electrodes. J Phys Condens Matter. 2011;23:022201. doi: 10.1088/0953-8984/23/2/022201. [DOI] [PubMed] [Google Scholar]
- Kumar RA, PapaÏconomou N, Lee JM, Salminen J, Clark DS, Prausnitz JM. In vitro cytotoxicities of ionic liquids effect of cation rings functional groups and anions. Environ Toxicol. 2009;24:388–395. doi: 10.1002/tox.20443. [DOI] [PubMed] [Google Scholar]
- Kumar V, Malhotra SV. Ionic liquids as pharmaceutical salts: a historical perspective. ACS Symp Ser. 2010;1038:1–12. [Google Scholar]
- Liu X, Zhou G, Huo F, Wang J, Zhang S. Unilamellar vesicle formation and microscopic structure of ionic liquids in aqueous solutions. J Phys Chem C. 2015;120:659–667. [Google Scholar]
- Łuczak J, Jungnickel C, Łącka I, Stolte S, Hupka J. Antimicrobial and surface activity of 1-alkyl-3-methylimidazolium derivatives. Green Chem. 2010;12:593–601. [Google Scholar]
- Parasassi T, Krasnowska EK, Bagatolli L, Gratton E. Laurdan and Prodan as polarity-sensitive fluorescent membrane probes. J Fluoresc. 1998;8(4):365–373. [Google Scholar]
- Peng A, Pisal DS, Doty A, Balu-Iyer SV. Phosphatidylinositol induces fluid phase formation and packing defects in phosphatidylcholine model membranes. Chem Phys Lipids. 2012;165(1):15–22. doi: 10.1016/j.chemphyslip.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernak J, Sobaszkiewicz K, Mirska I. Anti-microbial activities of ionic liquids. Green Chem. 2003;5:52–56. [Google Scholar]
- Ranke J, Moelter K, Stock F, Bottin-Weber U, Poczobutt J, Hoffmann J, Ondruschka B, Filser J, Jastorff B. Biological effects of imidazolium ionic liquids with varying chain lengths in acute Vibrio fischeri and WST-1 cell viability assays. Ecotoxicol Environ Saf. 2004;58:396–404. doi: 10.1016/S0147-6513(03)00105-2. [DOI] [PubMed] [Google Scholar]
- Ranke J, Müller A, Bottin-Weber U, Stock F, Stolte S, Arning J, Stoermann R, Jastorff B. Lipophilicity parameters for ionic liquid cations and their correlation to in vitro cytotoxicity. Ecotoxicol Environ Saf. 2007;67:430–438. doi: 10.1016/j.ecoenv.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Rawat K, Bohidar HB. Universal charge quenching and stability of proteins in 1-methyl-3-alkyl(Hexyl/Octyl) imidazolium chloride ionic liquid solutions. J Phys Chem B. 2012;116:11065–11074. doi: 10.1021/jp3049108. [DOI] [PubMed] [Google Scholar]
- Richter C, Schaepe K, Glorius F, Ravoo BJ. Tailor-made N-heterocyclic carbenes for nanoparticle stabilization. Chem Commun. 2014;50(24):3204–3207. doi: 10.1039/c4cc00654b. [DOI] [PubMed] [Google Scholar]
- Rühling A, Wang D, Ernst JB, Wulff S, Honeker R, Richter C, Ferry A, Galla H-J, Glorius F. Influence of the headgroup of azolium-based lipids on their biophysical properties and cytotoxicity. Chem Eur J. 2017;23(25):5920–5924. doi: 10.1002/chem.201604182. [DOI] [PubMed] [Google Scholar]
- Samori C, Malferrari D, Valbonesi P, Montecavalli A, Moretti F, Galletti P, Sartor G, Tagliavini E, Fabbri E, Pasteris A. Introduction of oxygenated side chain into imidazolium ionic liquids: evaluation of the effects at different biological organization levels. Ecotoxicol Environ Saf. 2010;73:1456–1464. doi: 10.1016/j.ecoenv.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Sauerbrey G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z Phys A. 1959;155(2):206–222. [Google Scholar]
- Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. 2017;18(6):361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11(10):688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- Stepnowski P, Skladanowski AC, Ludwiczak A, Laczyńska E. Evaluating the cytotoxicity of ionic liquids using human cell line HeLa. Hum Exp Toxicol. 2004;23:513–517. doi: 10.1191/0960327104ht480oa. [DOI] [PubMed] [Google Scholar]
- Stolte S, Arning J, Bottin-Weber U, Matzke M, Stock F, Thiele K, Uerdingen M, Welz-Biermann U, Jastorff B, Ranke J. Anion effects on the cytotoxicity of ionic liquids. Green Chem. 2006;8:621–629. [Google Scholar]
- Stolte S, Arning J, Bottin-Weber U, Müller A, Pitner WR, Welz-Biermann U, Jastorff B, Ranke J. Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids. Green Chem. 2007;9:760–767. [Google Scholar]
- Tischer M, Pradel G, Ohlsen K, Holzgrabe U. Quaternary ammonium salts and their antimicrobial potential: targets or nonspecific interactions? ChemMedChem. 2012;7:22–31. doi: 10.1002/cmdc.201100404. [DOI] [PubMed] [Google Scholar]
- Visser A, Swatloski RP, Reichert WM, Mayton R, Sheff S, Wierzbicki A, Davis JH, Rogers RD. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem Commun. 2001;1:135–136. [Google Scholar]
- Wang D, Richter C, Rühling A, Drücker P, Siegmund D, Metzler-Nolte N, Glorius F, Galla H-J. A remarkably simple class of Imidazolium-based lipids and their biological properties. Chem Eur J. 2015;21(43):15123–15126. doi: 10.1002/chem.201502333. [DOI] [PubMed] [Google Scholar]
- Wang D, de Jong DH, Rühling A, Lesch V, Shimizu K, Wulff S, Heuer A, Glorius F, Galla H-J. Imidazolium-based lipid analogues and their interaction with Phosphatidylcholine membranes. Langmuir. 2016;32(48):12579–12592. doi: 10.1021/acs.langmuir.6b02496. [DOI] [PubMed] [Google Scholar]
- Wang D, Richter C, Rühling A, Hüwel S, Glorius F, Galla H-J. Anti-tumor activity and cytotoxicity in vitro of novel 4,5-dialkylimidazolium surfactants. Biochem Biophys Res Commun. 2015;467:1033–1038. doi: 10.1016/j.bbrc.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Wasserscheid P, Keim W. Ionic liquids—new “solutions” for transition metal catalysis. Angew Chem Int Ed. 2000;39(21):3772–3789. doi: 10.1002/1521-3773(20001103)39:21<3772::aid-anie3772>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Welton T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev. 1999;99(8):2071–2084. doi: 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- Welton T. Ionic liquids in catalysis. Coord Chem Rev. 2004;248:2459–2477. [Google Scholar]
- Yoo B, Zhu Y, Maginn EJ. Molecular mechanism of ionic-liquid-induced membrane disruption: morphological changes to bilayers, multilayers, and vesicles. Langmuir. 2016;32:5403–5411. doi: 10.1021/acs.langmuir.6b00768. [DOI] [PubMed] [Google Scholar]