Abstract
Comparison of chemical catalysis by metal complexes, enzymatic catalysis and whole-cell biocatalysis shows well-addressed advantages of the latter approach. However, a critical limitation in the practical applications originates from the high sensitivity of microorganisms to the toxic effects of organic solvents. In the present review, we consider toxic solvent properties of ionic liquid/water systems towards the development of efficient applications in practical organic transformations.
Keywords: Ionic liquids, Toxicity, Biological activity, Catalysis, Biocatalysis
Introduction
Transition metal catalysis dominates modern industrial and chemical synthesis, both in terms of production volumes and diversity of reactions (hydrocarbon processing, fuel production, fine chemical synthesis, pharmaceutical applications, etc.) (Egorova and Ananikov 2016). Such chemical catalysis has indisputable advantages: it shows good selectivity and can be carried out in a variety of solvents (Table 1). However, known disadvantages include sustainability issues (North and Clark 2016), high cost of metals (Pt, Pd, Rh, Ru, Au, etc.) and the impossibility of easy self-reproduction of catalysts.
Table 1.
Selected key characteristics of different types of catalysis
| Characteristics | Catalysis | ||
|---|---|---|---|
| Chemical | Enzymatic | Whole-cell | |
| Sustainability | Low | High | High |
| Cost | High | High | Low |
| Selectivity | Good | High | High |
| Self-replication | No | No | Yes |
| Solvent tolerance | High | Good | Low |
From this viewpoint, enzymatic catalysis can be seen as a successful alternative to metal catalysis in terms of good sustainability, chemical selectivity and solvent tolerance (Table 1). However, the cost of enzymatic catalysts remains high, and enzymes cannot directly self-replicate.
In this regard, whole-cell biocatalysis presents an excellent opportunity, since it is characterized by high sustainability level, excellent chemical selectivity, relatively low cost and an ability to easily self-replicate. However, the well-addressed critical limitation is a poor solvent tolerance: usually whole-cell systems perform best in aqueous buffered media, while such biocatalytic systems are not usually compatible with regular organic solvents (Table 1). This is a critical limitation from a practical point of view and overcoming this problem may solve a number of challenges in modern research and development.
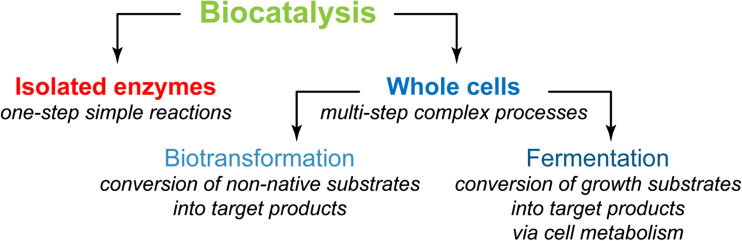
As shown in Table 1, biocatalytic approaches are inherently very efficient and can be divided into isolated enzymes and whole cells (Fig. 1). The former are beneficial in one-step reactions, whereas the latter allow the carrying out of complex multi-step processes. Whole-cell biocatalysis, in turn, can be roughly divided into biotransformation and fermentation processes, though intermediate cases are known (de Carvalho 2011; Schrewe et al. 2013; Ladkau et al. 2014; Wachtmeister and Rother 2016; de Carvalho 2017; Lin and Tao 2017).
Fig. 1.
Biocatalysis is carried out by isolated enzymes or by whole cells. In the latter case, non-native (biotransformation) or native (fermentation) substrates can be used
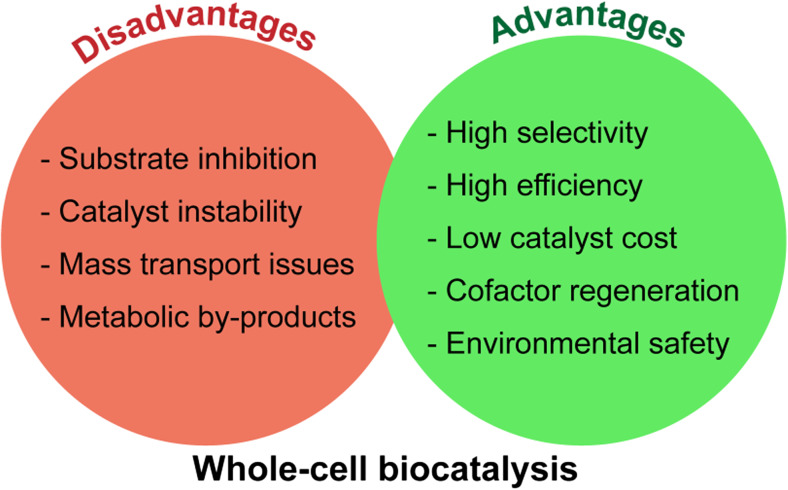
One of the main advantages of enzymes is their ability to function in vitro, under conditions unsuitable for cell growth. Such conditions can be optimized for particular chemical transformation for facilitating product purification. Among the other advantages of enzymes are the reaction specificity and simplicity of the methodology. However, enzymatic protocols often demand preliminary isolation and immobilization procedures, as well as usage of cofactors. Whole-cell biocatalysis provides the means of running highly selective multi-step reactions with cofactor regeneration. Its disadvantages relate to catalyst instability or product inhibition, formation of toxic by-products and mass transport issues (Fig. 2) (de Carvalho 2011; Schrewe et al. 2013; Lin and Tao 2017).
Fig. 2.
Main advantages and disadvantages of whole-cell biocatalysis
The nature of target compounds also imposes its restrictions on the application of whole-cell biocatalysts. Due to hydrophobicity of many products, studies on whole-cell biocatalysis in unconventional, non-aqueous media have recently been attracting much attention. Often, biphasic systems consisting of an aqueous (buffer) phase and an organic phase are used (Wachtmeister and Rother 2016; de Carvalho 2017).
In both chemical catalysis and biocatalysis, the issue of environmental toxicity of catalytic media is of great importance. Since common organic solvents can be dangerous to the environment, recyclable media have been attracting significant attention in recent decades (Zhang 2006). In contrast to chemical catalysts, biocatalytic systems, especially whole-cell ones, can be very sensitive to organic solvents. Therefore, ionic liquids and deep eutectic solvents have been suggested as safer alternatives to common organic reagents (Weuster-Botz 2007; Gorke et al. 2010; Quijano et al. 2010; Xu et al. 2016a).
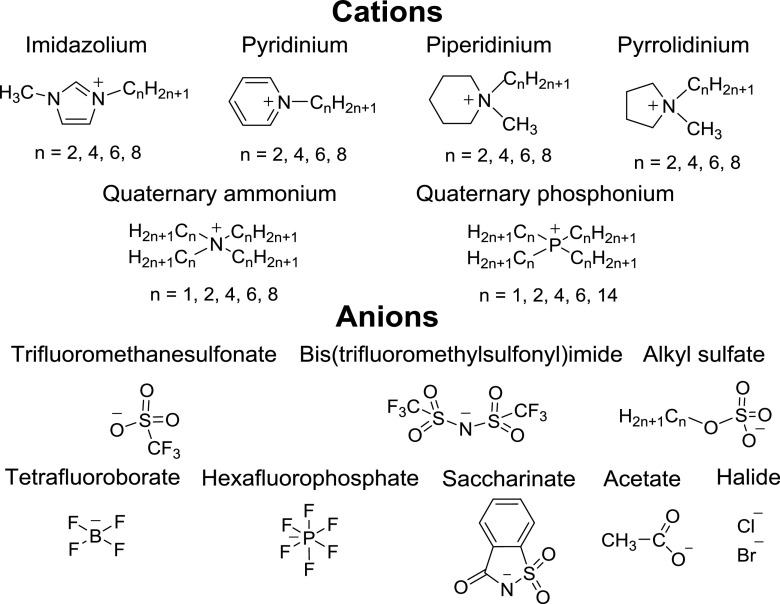
Ionic liquids (ILs), or molten salts, are thermally stable, nonflammable and nonvolatile media prone to micro- and nanostructuring. ILs usually consist of bulky organic cations and weakly coordinating anions (Fig. 3). Remarkable tunability and solvent properties of ILs are being widely employed in dissolution of biomolecules and extraction (Seitkalieva et al. 2015; Egorova et al. 2017; Seitkalieva et al. 2017). The striking solvent properties of ILs can be explained by spatial micro- and nano-heterogeneity of the media which has been established by both theoretical and experimental studies. IL ions in the liquid phase self-assemble into relatively stable amphiphilic nanostructures which are realized via ionic interactions and hydrogen bonds (Wang and Voth 2005; Triolo et al. 2007; Hayes et al. 2015; Egorova et al. 2017).
Fig. 3.
Examples of common cations and anions used in ionic liquids
From the economical viewpoint, ILs also seem rather plausible candidates. In recent years, the cost of IL production has been decreasing, and production prices of US$1–5 per kg of ammonium or imidazolium IL have been reported (Chen et al. 2014; George et al. 2015; Sun et al. 2017). Moreover, IL recycling in biocatalytic applications has been demonstrated (Dennewald et al. 2011).
In spite of their presumable “safety”, ILs can possess significant biological activity (Egorova and Ananikov 2014; Egorova et al. 2017). Therefore, their application in biocatalysis should be a compromise between two factors: (1) solvent properties that benefit the desired chemical reaction/separation, and (2) toxic effects of solvents that inhibit the whole-cell approach. In this review, we briefly discuss both aspects of IL usage in modern whole-cell biocatalysis, while our main attention is paid to the second aspect dealing with the toxicological properties of ILs studied in various whole-cell biocatalytic systems. Our research interests are focused on the toxicity of ILs, and here we analyze the perspectives of application of whole-cell biocatalysts in demanding chemical reactions from the viewpoint of reaction media (solvents).
Ionic liquids in whole-cell biocatalysis
To date, ILs have found applications in various types of whole-cell biocatalytic reactions, among which are biofuel production (Arai et al. 2010; Ouellet et al. 2011; Frederix et al. 2016; Hashmi et al. 2017; Pérez de los Ríos et al. 2017), enantioselective reduction of ketones (Howarth et al. 2001; Pfruender et al. 2004, 2006; Lou et al. 2006; Bräutigam et al. 2007; Hussain et al. 2007; Schroer et al. 2007; Zhang et al. 2008; Bräutigam et al. 2009; Lou et al. 2009a, b and c; Wang et al. 2009, 2013; Dennewald et al. 2011, 2012; Xiao et al. 2012; Zhang et al. 2012; Silva et al. 2013; Zampieri et al. 2013; Li et al. 2015; Xu et al. 2016b, 2017), enantioselective sulfoxidation (Gao et al. 2014), enantioselective hydrolysis (Matsumoto et al. 2014), enantioselective hydroxylation (Cornmell et al. 2008; Wu et al. 2011; Mao et al. 2012, 2013), dehydrogenation (He et al. 2011), double-bond hydrogenation (Lenourry et al. 2005; Castiglione et al. 2017), glycosylation (Schmideder et al. 2016), and nucleoside acylation (Yang et al. 2014) (see Table 2).
Table 2.
IL-assisted whole-cell biocatalytic reactions
| Reaction | Organism | Ref. |
|---|---|---|
| Biofuel production | Rhizopus oryzae, Aspergillus oryzae, Escherichia coli, Saccharomyces cerevisiae, Scheffersomyces stipitis | (Arai et al. 2010; Ouellet et al. 2011; Frederix et al. 2016; Hashmi et al. 2017; Pérez de los Ríos et al. 2017) |
| Enantioselective reduction of ketones | Escherichia coli, Saccharomyces cerevisiae, Candida parapsilosis, Rhodotorula sp., Trigonopsis variabilis, Lactobacillus kefir, Acetobacter sp., Acinetobacter sp., Cryptococcus sp., Candida albicans, Rhodotorula glutinis, Geotrichum candidum, Micrococcus luteus, Aureobasidium pullulans, Trichosporon capitatum, Rhodococcus erythropolis | (Howarth et al. 2001; Pfruender et al. 2004, 2006; Lou et al. 2006; Bräutigam et al. 2007; Hussain et al. 2007; Schroer et al. 2007; Zhang et al. 2008; Bräutigam et al. 2009; Lou et al. 2009a, b, c; Wang et al. 2009, 2013; Dennewald et al. 2011, 2012; Xiao et al. 2012; Zhang et al. 2012; Silva et al. 2013; Zampieri et al. 2013; Li et al. 2015; Xu et al. 2016b, 2017) |
| Hydrolysis of glycyrrhizin | Penicillium purpurogenum, Escherichia coli, Pichia pastoris | (Chen et al. 2012) |
| Enantioselective hydrolysis | Rhodotorula glutinis | (Matsumoto et al. 2014) |
| Enantioselective hydroxylation | Escherichia coli, Penicillium raistrickii, Aspergillus ochraceus, Rhizopus nigricans | (Cornmell et al. 2008; Wu et al. 2011; Mao et al. 2012, 2013) |
| Enantioselective sulfoxidation | Escherichia coli | (Gao et al. 2014) |
| Dehydrogenation | Arthrobacter simplex | (He et al. 2011) |
| C=C hydrogenation | Escherichia coli, Sporomusa termitida | (Lenourry et al. 2005; Castiglione et al. 2017) |
| Glycosylation | Escherichia coli | (Schmideder et al. 2016) |
| Nucleoside acylation | Pseudomonas fluorescens | (Yang et al. 2014) |
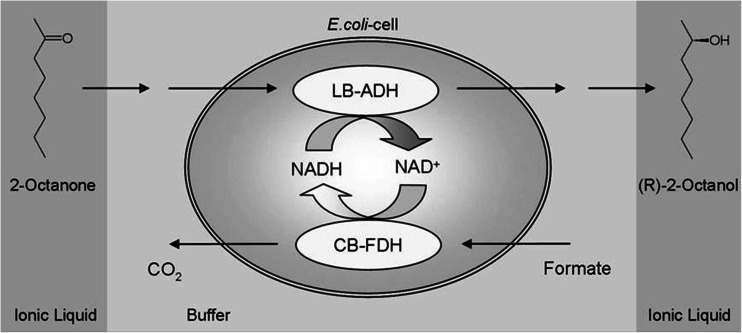
As evidenced by the accumulated reports, enantioselective reduction of ketones is one of the most popular reactions in IL-assisted whole-cell biocatalysis. In this case, ILs are usually employed as a non-aqueous phase in the two-phase systems in which the reaction is carried out (Fig. 4). Biocatalysis is carried out in nearly native conditions in the water phase, in which the toxic effect of the added solvent can be minimized. Extraction of organic product to IL phase simplifies the overall process and facilitates separation. Diffusion of reagents between two phases is an important parameter to connect different stages into a continuous process.
Fig. 4.
Enantioselective whole-cell bioreduction of 2-octanone to (R)-2-octanol in a biphasic system consisting of an aqueous buffer and an IL. LB-ADH alcohol dehydrogenase from Lactobacillus brevis; CB-FDH formate dehydrogenase from Candida boidinii. Reproduced with permission from Bräutigam et al. (2009)
Such IL-containing systems have been successfully used for the asymmetric bioreduction of 2-octanone to (R)-2-octanol (organism: recombinant E.coli; IL: [C6MPyr][NTf2]) (Fig. 4) (Bräutigam et al. 2009; Dennewald et al. 2011, 2012); of 2,5-hexanedione to (2R,5R)-hexanediol (organism: recombinant E.coli; IL: [C4MIM][NTf2]) (Schroer et al. 2007); of 6-Br-β-tetralone to (S)-6-Br-β-tetralol (organisms: Rhodococcus erythropolis, Trichosporon capitatum; IL: [C2MIM][Tos]) (Hussain et al. 2007); of 4′-methoxyacetophenone to (R)- or (S)-1-(4-methoxyphenyl)ethanol (organisms: Trigonopsis variabilis, Rhodotorula sp.; ILs: [HOC2MIM][NO3] (co-solvent), [C4MIM][PF6]) (Lou et al. 2009b, c; Wang et al. 2009); of 4-chloroacetophenone to (R)-1-(4-chlorophenyl)ethanol (organism: Lactobacillus kefir; IL: [C4MIM][NTf2]) (Pfruender et al. 2004); and of ethyl acetoacetate to ethyl (R)-3-hydroxybutyrate (organism: Acetobacter sp.; IL: [C4MIM][PF6]) (Wang et al. 2013).
Another promissing direction of IL application in whole-cell biocatalysis is the production of biofuels, such as biodiesel and bioethanol, from biomass. Due to their unique dissolution properties, ILs have been used for the pretreatment of biomass (Zakrzewska et al. 2011; Wang et al. 2012). Possible positive and negative effects of ILs on biofuel-producing organisms have been studied in several microbial systems. A study on [C4MIM][OAc]-pretreated sugarcane bagasse produced no definite evidences of advantages (disadvantages) of the IL application (Hashmi et al. 2017). In Saccharomyces cerevisiae, residual [C2MIM][OAc] in biomass hydrolyzate inhibited the yeast growth (Ouellet et al. 2011). A [C2MIM][OAc] pretreatment of switchgrass was efficient for lignocellulose disruption, but toxic for Escherichia coli. The authors reported the development of [C2MIM]-resistant E.coli strain possibly providing new advantages in biofuel production (Frederix et al. 2016). The design and selection of IL-resistant microbial strains seem to be the main route to successful application of ILs in modern whole-cell biocatalysis. This subject will be further discussed in the next section.
Currently, Escherichia coli and Saccharomyces cerevisiae are the most studied microorganisms to which IL-containing systems have been applied. Representative examples of such applications are provided in Table 3.
Table 3.
Application of ILs in whole-cell biocatalysis by E.coli and S.cerevisiae
| Reaction | IL system | Ref. |
|---|---|---|
| Escherichia coli (recombinant) | ||
| Benzaldehyde, HCN → (S)-mandelic acid, (S)-mandeloamide | Two-phase system (aqueous phase - 20% (v/v) [C4MPyr][NTf2] or [C4MIM][PF6]) | (Baum et al. 2012) |
| (R)-carvone → (2R,5R)-dihydrocarvone | Two-phase system (aqueous phase - 20% (v/v) [C6MPyr][NTf2] or [C6MIM][PF6] or [C6Py][NTf2]) | (Castiglione et al. 2017) |
| 3-chloro-1-phenyl-1-propanone → (S)-3-chloro-1-phenyl-1-propanol | Two-phase system (aqueous phase - 20% (v/v) [C4MIM][NTf2]) | (Choi et al. 2011) |
| 4-chloroacetophenone → (R)-1-(4-chlorophenyl)ethanol; ethyl 4-chloroacetoacetate → ethyl (S)-4-chloro-3-hydroxybutyrate; phenacyl chloride → (S)-α-chloro-1-phenylethanol | Two-phase system (aqueous phase - 20% (v/v) [C4MIM][NTf2] or [C4MIM][PF6]) | (Bräutigam et al. 2007) |
| Toluene → toluene cis-glycol | Two-phase system (aqueous phase - 23% (v/v) [(C8)3C1N][NTf2] or [C14(C6)3P][NTf2]) | (Cornmell et al. 2008) |
| 2-Octanone → (R)-2-octanol | Two-phase system (aqueous phase - 20-70% (v/v) [C6MPyr][NTf2]) | (Bräutigam et al. 2009; Dennewald et al. 2011, 2012) |
| Biomass → limonene | Biomass pretreatment ([C2MIM][OAc]) | (Frederix et al. 2016) |
| Asymmetric sulfoxidation of sulfides | Two-phase system (aqueous phase: [C14(C6)3P][NTf2] = 10:1) | (Gao et al. 2014) |
| Geraniol → geranyl glucoside | Two-phase system (aqueous phase - 5% (v/v) [C6Pyr][NTf2]) | (Schmideder et al. 2016) |
| 2,5-hexanedione → (2R,5R)-hexanediol | Two-phase system (aqueous phase - 5% (v/v) [C4MIM][NTf2]) | (Schroer et al. 2007) |
| Baeyer–Villiger bioconversion | Three-phase tank bioreactor (air – water – 5-20% (v/v) [C4MPyr][NTf2]) | (Melgarejo-Torres et al. 2011) |
| Saccharomyces cerevisiae | ||
| Biomass → ethanol | Biomass pretreatment ([C2MIM][OAc], [C4MIM][OAc]); as extraction phase ([(C8)3C1N][NTf2], [C14(C6)3P][Cl]) | (Ouellet et al. 2011; Hashmi et al. 2017; Pérez de los Ríos et al. 2017) |
| Ketone bioreduction | [C4MIM][PF6] : water system (10:1) | (Howarth et al. 2001) |
| Benzaldehyde, glucose → (R)-phenylacetylcarbinol | Two-phase system (aqueous phase +5% (v/v) [C4MIM][PF6]) | (Kandar et al. 2015) |
| Acetyltrimethylsilane → (S)-1-trimethylsilylethanol | Two-phase system (aqueous phase: [C4MIM][PF6] = 6:1 (v/v); aqueous phase +10% (v/v) [C4MIM][BF4] as co-solvent) | (Lou et al. 2006) |
| 4-chloroacetoacetate → (S)-4-chloro-3-hydroxybutanoate | Two-phase system (aqueous phase +20% (v/v) [C4MIM][PF6]) | (Pfruender et al. 2006) |
| L-phenylalanine → 2-phenylethanol | Two-phase system (aqueous phase +20% (v/v) [C4MIM][NTf2] or [C3MPy][NTf2] or [(C8)3C1N][NTf2]) | (Sendovski et al. 2010) |
| Reduction of (Z)-C6H5CH=CXC(=O)CH3 (X = Cl, Br) | Two-phase system (aqueous phase +0.5% (v/v) [C4MIM][PF6]) | (Zampieri et al. 2013) |
Indeed, in a carefully optimized process, ILs exhibit a noticeable positive impact on whole-cell biocatalysis and greatly enhance practical usage. The nanoheterogeneous nature of ILs can enhance their application in biocatalysis. In particular, IL-water systems can contain nanobubbles, nanochannels and other structures, depending on the water content (Kashin et al. 2016; Seitkalieva et al. 2017). Protein molecules and even whole bacterial cells can possibly exist in such aqueous compartments or surrounded by water shells without redundant contacts with the IL media (Benedetto and Ballone 2016; Egorova et al. 2017). However, further optimization and application to a diverse range of chemical reactions is limited by the toxic effect of added ILs, which is discussed in more detail in the next section.
Toxicity of ILs towards microbial cells
There are three broad categories of studies on biological effects imposed by ILs on microbial cells: environmental (concerning toxicity towards ecological indicator species), medical (concerning inhibition of antibiotic-resistant pathogenic microorganisms) and biotechnological (concerning IL advantages in biocatalysis). Such separation often seems rather artificial, because eventually all ILs regardless of their application can enter the environment. For example, [C4MIM][PF6] was found to enhance the horizontal transfer of antibiotic resistance genes thus posing an indirect danger to human health (Luo et al. 2014). Therefore, the toxic aspect of these reagents should not be underestimated.
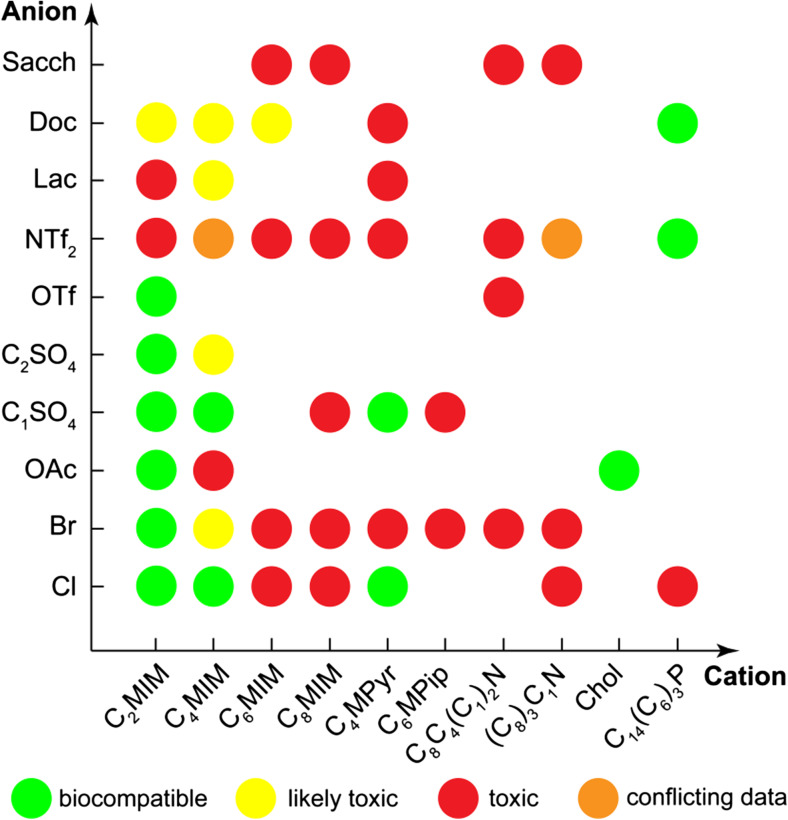
Most publications on the application of ILs in whole-cell biocatalysis address their toxic effects to some degree. These findings are summarized in Table 4. Usually, they do not allow unambiguous recommendations on the choice of ILs for a particular application; however, some general conclusions made for other biological objects are also valid for biocatalytic microorganisms. Thus, the IL toxic effect is thought to depend on the length of the alkyl chain in the cation, as well as on the nature of the cation and anion; the organism nature also plays an important role in the development of toxic effects (Egorova and Ananikov 2014). Figure 5 shows the map of biocompatibility of various ILs for Escherichia coli compiled on the basis of the available data.
Table 4.
Summary of toxicity effects of ILs towards whole-cell biocatalysts (MNTC - maximum non-toxic concentration)
| IL | Effect | Ref. |
|---|---|---|
| Escherichia coli | ||
| [C2MIM][Cl] | Inhibited cell growth at concentrations >200 mM (some mutations increased tolerance); imposed no effect at 2% (v/v) in 24 h | (Wood et al. 2011; Frederix et al. 2014, 2016) |
|
[C2MIM]X X = [Br], [C2SO4], [C1(OC2)3SO4], [Tos] |
Imposed no significant effect at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C2MIM][BF4] | Inhibited cell growth at >500 mg*L−1 in solid culture or at >4000 mg*L−1 in suspension culture; 8-h EC50 30,000-40,000 mg*L−1 | (Lee et al. 2005) |
| [C2MIM][OAc] | Inhibited cell growth at concentrations >150 mM (some mutations increased tolerance) | (Frederix et al. 2016) |
| [C2MIM][C1SO4] | Inhibited cell growth at >100 mg*L−1 in solid culture or at >1000 mg*L−1 in suspension culture; 8-h EC50 2000-3000 mg*L−1 | (Lee et al. 2005) |
|
[C2MIM]X X = [C8SO4], [Lac] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C2MIM][OTf] | 8-h EC50 10,000-30,000 mg*L−1 | (Lee et al. 2005) |
| [C2MIM][NTf2] | Inhibited cell growth at 0.25-40% (v/v) | (Cornmell et al. 2008; Wood et al. 2011) |
| [C2MIM][Doc] | Imposed some effect at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[C4MIM]X X = [Cl], [C1SO4] |
Imposed no effect at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[C4MIM]X X = [I], [OAc] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[C4MIM]X X = [Br], [C2SO4], [C3SO4], [i-C3SO4], [i-C4SO4], [C1OC2SO4], [C2OC2SO4], [Lac], [Doc] |
Imposed some effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C4MIM][BF4] | 8-h EC50 8500-9500 mg*L−1 | (Lee et al. 2005) |
| [C4MIM][PF6] | According to some reports, 20% (v/v) did not impose significant effects in 4-5 h; according to one report, 9% (v/v) inhibited cell growth in 4 h; membrane integrity decreased moderately after 5 h incubation in 20% (v/v); 8-h EC50 3500-4500 mg*L−1 | (Lee et al. 2005; Pfruender et al. 2006; Bräutigam et al. 2007; Baum et al. 2012; Gao et al. 2014) |
| [C4MIM][NTf2] | According to one report, 20% (v/v) did not impose significant effects in 5 h; according to other reports, inhibited cell growth at 0.25-40% (v/v); membrane integrity decreased significantly after 5 h incubation in 20% (v/v); 8-h EC50 100-200 mg*L−1 | (Lee et al. 2005; Pfruender et al. 2006; Bräutigam et al. 2007; Cornmell et al. 2008; Wood et al. 2011; Baum et al. 2012; Gao et al. 2014) |
| [C4MIM][(C2F5)3PF3] | Membrane integrity decreased dramatically after 5 h incubation in 20% (v/v) | (Bräutigam et al. 2007) |
|
[C6MIM]X X = [Cl], [Br], [I], [Sacch] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C6MIM][PF6] | Membrane integrity decreased moderately after 5 h incubation in 20% (v/v); inhibited cell growth at >5000 mg*L−1 in solid culture or at >2000 mg*L−1 in suspension culture; 8-h EC50 50-1050 mg*L−1 | (Lee et al. 2005; Bräutigam et al. 2007) |
| [C6MIM][SbF6] | 8-h EC50 50-1050 mg*L−1 | (Lee et al. 2005) |
| [C6MIM][NTf2] | Membrane integrity decreased significantly after 5 h incubation in 20% (v/v); 8-h EC50 100-200 mg*L−1; inhibited cell growth at 2% (v/v) in 24 h | (Lee et al. 2005; Bräutigam et al. 2007; Wood et al. 2011) |
|
X[Doc] X = [C6MIM], [CnMPy] (n = 4, 6) |
Imposed some effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
X[Cl] X = [C8MIM], [(C8)3C1N] |
Inhibited cell growth at 0.25-40% (v/v) | (Cornmell et al. 2008; Wood et al. 2011) |
|
[C8MIM]X X = [Br], [I], [NTf2], [Sacch] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C8MIM][PF6] | 8-h EC50 100-200 mg*L−1 | (Lee et al. 2005) |
| [C8MIM][C1SO4] | Inhibited cell growth at >200 mg*L−1 in solid culture or at >50 mg*L−1 in suspension culture; 8-h EC50 1000-2000 mg*L−1 | (Lee et al. 2005) |
| [C10MIM][Cl] | Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [PPMIM][SbF6] | 8-h EC50 1000-5000 mg*L−1 | (Lee et al. 2005) |
| [PPMIM][OTf] | 8-h EC50 1000 mg*L−1 | (Lee et al. 2005) |
| [C4MPy][i-(C8)2PO4] | Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C10Py][NTf2] | Inhibited cell growth at 0.25-40% (v/v) | (Cornmell et al. 2008) |
|
[C4MPyr]X X = [Cl], [C1SO4], [(C1)2PO4] |
Imposed no significant effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
X[Br] X = [C4MPyr], [C4MPip] |
Imposed some effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[C4MPyr]X X = [NTf2], [(C2F5)3PF3] |
Membrane integrity decreased significantly after 5 h incubation in 20% (v/v) | (Bräutigam et al. 2007) |
|
[C4MPyr]X X = [Lac], [Doc] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C6MPyr][Br] | Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C6MPyr][NTf2] | Membrane integrity decreased significantly after 5 h incubation in 20% (v/v) | (Bräutigam et al. 2007) |
|
[C6MPip]X X = [Br], [C1SO4] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[C8MPip]X X = [Br], [OAc] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
X[NTf2] X = [(C1OC3)C1Pip], [(HOC2)2C12C1N] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C4(C1)3N][(C1)2PO4] | Imposed no significant effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
X[Br] X = [C4C2(C1)2N], [(HOC3)C2(C1)2N] |
Imposed no significant effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[C4C2(C1)2N]X X = [CnSO4] (n = 2, 4) |
Imposed some effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C8C2(C1)2N][C2SO4] | Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[C8C4(C1)2N]X X = [Br], [I], [NTf2], [Sacch], [i-(C8)2PO4], [Lin] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C10C4(C1)2N][NTf2] | Imposed some effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[(C8)2(C1)2N]X X = [C1SO4], [C2SO4], [C6SO4], [Tos] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[(C8)3C1N]X X = [Br], [Sacch], [i-(C8)2PO4] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [(C8)3C1N][NTf2] | Biocompatible, according to some reports (20% (v/v) did not impose significant effects in 5 h); according to one report, 9% (v/v) inhibited cell growth in 4 h | (Pfruender et al. 2006; Cornmell et al. 2008; Wood et al. 2011; Gao et al. 2014) |
| [Chol][OAc] | Imposed no significant effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[Chol]X X = [C2COO], [C5COO] |
Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [(HOC2)C2(C1)2N][C2SO4] | Imposed no effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [(HOC3)C4(C1)2N][Cl] | Imposed no significant effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
|
[(HOC2)2C4C2N]X X = [Br], [C2SO4] |
Imposed no significant effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [(HOC2)3C1N][C1SO4] | Imposed no effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [(C8)3C1P][C1SO4] | Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C14(C6)3P][Cl] | Inhibited cell growth at 0.25-40% (v/v) | (Cornmell et al. 2008) |
| [C14(C6)3P][NTf2] | Biocompatible | (Cornmell et al. 2008; Gao et al. 2014) |
| [C14(C6)3P][Doc] | Imposed no effect on cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [C14(C8)3P][Doc] | Inhibited cell growth at 2% (v/v) in 24 h | (Wood et al. 2011) |
| [(C1)4C2Gua][(C2F5)3PF3] | Membrane integrity decreased dramatically after 5 h incubation in 20% (v/v) | (Bräutigam et al. 2007) |
| Saccharomyces cerevisiae | ||
| [C2MIM][Cl] | MNTC <6.7 mM | (Santos et al. 2014) |
| [C2MIM][NTf2] | MNTC <2.5 mM | (Santos et al. 2014) |
| [C2MIM][OAc] | Inhibited cell growth at >0.5% (v/v) and reduced glucose consumption at 2% (v/v); inhibited cell growth at 30-35 mM under both aerobic and anaerobic conditions | (Ouellet et al. 2011; Mehmood et al. 2015) |
| [C2MIM][C2SO3] | MNTC <4.5 mM | (Santos et al. 2014) |
| [C2MIM][C2SO4] | MNTC <4.2 mM | (Santos et al. 2014) |
| [C2MIM][C1PO3] | Inhibited cell growth at >0.5% (v/v) | (Mehmood et al. 2015) |
| [C4MIM][PF6] | Biocompatible (20% (v/v) did not impose significant effects in 20 h, but imposed pronounced effect in 72 h); according to another study, inhibited growth significantly at low concentrations | (Lou et al. 2006; Pfruender et al. 2006; Sendovski et al. 2010; Song et al. 2011; Zampieri et al. 2013; Kandar et al. 2015; Pérez de los Ríos et al. 2017) |
| [C4MIM][NTf2] | Biocompatible (20% (v/v) did not impose significant effects in 20 h, but imposed some effect in 72 h); MNTC <2.5 mM | (Pfruender et al. 2006; Sendovski et al. 2010; Santos et al. 2014; Pérez de los Ríos et al. 2017) |
| [C4MIM][BF4] | Moderately inhibited cell growth at 3% (v/v) | (Lou et al. 2006) |
| [CnMIM][PF6] (n = 6, 8) | Inhibited cell growth at 20% (v/v) in 72 h | (Sendovski et al. 2010) |
| [C8MIM][BF4] | Inhibited cell growth at 3% (v/v) | (Pérez de los Ríos et al. 2017) |
| [C8MIM][NTf2] | Biocompatible | (Pérez de los Ríos et al. 2017) |
| [C10MIM][PF6] | Completely inhibited cell growth at 20% (v/v) in 72 h | (Sendovski et al. 2010) |
| [BzMIM][BF4] | Completely inhibited cell growth at 20% (v/v) in 72 h | (Sendovski et al. 2010) |
| [C2Py][NTf2] | Inhibited cell growth at 3% (v/v) | (Pérez de los Ríos et al. 2017) |
| [C3MPip][NTf2] | Imposed no effect on cell growth at 20% (v/v) in 72 h | (Sendovski et al. 2010) |
| [(C8)3C1N][NTf2] | Biocompatible (20% (v/v) did not impose significant effects in 20-72 h) | (Pfruender et al. 2006; Sendovski et al. 2010; Pérez de los Ríos et al. 2017) |
| [Chol][Cl] | MNTC 224 mM | (Santos et al. 2014) |
| [Chol][NTf2] | MNTC 10 mM | (Santos et al. 2014) |
| [(C4)3C1P][C1SO4] | MNTC 1.95 mM | (Santos et al. 2014) |
| [C14(C6)3P][Cl] | Biocompatible | (Pérez de los Ríos et al. 2017) |
|
[C14(C6)3P]X X = [Br], [N(CN)2] |
Inhibited cell growth at 3% (v/v) | (Pérez de los Ríos et al. 2017) |
| [C14(C6)3P][i-(C8)2PO4] | Completely inhibited cell growth at 20% (v/v) in 72 h | (Sendovski et al. 2010) |
| Clostridium sporogenes | ||
| [C4MIM][C2SO4] | Inhibited cell growth at 2.0% (w/v) | (Dipeolu et al. 2009) |
| [C4MIM][BF4] | Inhibited cell growth at 0.25-2.0% (w/v) | (Dipeolu et al. 2009) |
| [(HOC2)(C1)2N][OAc] | Inhibited cell growth at 2.0% (w/v) | (Dipeolu et al. 2009) |
| [Chol][C2PO4] | Stimulated cell growth at 0.25-1.0% (w/v) | (Dipeolu et al. 2009) |
| AMMOENG™ 100 | Inhibited cell growth at 0.1-2.0% (w/v) | (Dipeolu et al. 2009) |
| Propionibacterium freudenreichii subsp. freudenreichii | ||
|
[C4MIM]X X = [BF4], [C1SO4], [SCN], [Tos], [NO3] |
48-h IC50 0.5-1.0% (w/v) | (Hajfarajollah et al. 2014) |
| [C6MIM][BF4] | 48-h IC50 0.05% (w/v) | (Hajfarajollah et al. 2014) |
|
[C6MIM]X X = [Tos], [NO3] |
48-h IC50 0.5% (w/v) | (Hajfarajollah et al. 2014) |
| [C8MIM][C1SO4] | 48-h IC50 0.5-1.0% (w/v) | (Hajfarajollah et al. 2014) |
| [C8MIM][SCN] | 48-h IC50 0.1-0.5% (w/v) | (Hajfarajollah et al. 2014) |
| [C8MIM][NO3] | 48-h IC50 0.005% (w/v) | (Hajfarajollah et al. 2014) |
| Rhodococcus erythropolis | ||
| [C2MIM][Tos] | Imposed no significant effect at 50% (v/v) | (Hussain et al. 2007) |
|
[C4MIM]X X = [BF4], [PF6] |
Imposed no significant effect at 50% (v/v) | (Hussain et al. 2007) |
|
[C4MIM]X X = [C8SO4], [MDEGSO4] |
Inhibited cell growth at 50% (v/v) | (Hussain et al. 2007) |
| [(C8)3C1N][NTf2] | Imposed no significant effect at 50% (v/v) | (Hussain et al. 2007) |
| [CABHEM][C1SO4] | Inhibited cell growth at 50% (v/v) | (Hussain et al. 2007) |
| Candida parapsilosis | ||
| [CnMIM][NTf2] (n = 2, 4) | Biocompatible (IL system reduced toxicity of the substrate 4-(trimethylsilyl)-3-butyn-2-one and the product (S)-4-(trimethylsilyl)-3-butyn-2-ol and of the substrate acetyltrimethylsilane and the product (R)-1-trimethylsilylethanol) | (Lou et al. 2009a; Zhang et al. 2012) |
| [CnMIM][PF6] (n = 4, 4(i), 5, 6) | Biocompatible (IL system reduced toxicity of the substrate 4-(trimethylsilyl)-3-butyn-2-one and the product (S)-4-(trimethylsilyl)-3-butyn-2-ol and of the substrate acetyltrimethylsilane and the product (R)-1-trimethylsilylethanol) | (Lou et al. 2009a; Zhang et al. 2012) |
|
X[NTf2] X = [C6MIM], [C4MPyr], [C4MPip], [C6(C4)3P] |
Biocompatible (IL system reduced toxicity of the substrate acetyltrimethylsilane and the product (R)-1-trimethylsilylethanol) | (Zhang et al. 2012) |
| [C7MIM][PF6] | Biocompatible (IL system reduced toxicity of the substrate 4-(trimethylsilyl)-3-butyn-2-one and the product (S)-4-(trimethylsilyl)-3-butyn-2-ol and of the substrate acetyltrimethylsilane) | (Lou et al. 2009a; Zhang et al. 2012) |
| Candida albicans | ||
| [C4MIM][PF6] | No formation of inhibition halo was observed with pure IL in 24 h | (Zampieri et al. 2013) |
| Rhodotorula sp. | ||
| [CnMIM][NTf2] (n = 2, 4) | Imposed some effect on cell viability at 20% (v/v) in 24 h; reduced substrate toxicity (4′-methoxyacetophenone) | (Wang et al. 2009) |
| [C2MIM][NO3] | Imposed no significant effect on cell viability at 10% (v/v) in 24 h | (Lou et al. 2009b) |
|
X[Cl] X = [HOC2MIM], [C4MIM] |
Imposed some effect on cell viability at 10% (v/v) in 24 h | (Lou et al. 2009b) |
| [HOC2MIM][PF6] | Imposed no significant effect on cell viability at 10% (v/v) in 24 h | (Lou et al. 2009b) |
| [HOC2MIM][NO3] | Imposed no significant effect on cell viability at 10% (v/v) in 24 h; reduced substrate toxicity (4′-methoxyacetophenone) | (Lou et al. 2009b) |
| [HOC2MIM][OTf] | Inhibited cell growth at 10% (v/v) in 24 h | (Lou et al. 2009b) |
|
X[NO3] X = [C4MIM], [C4C1MIM] |
Imposed some effect on cell viability at 10% (v/v) in 24 h | (Lou et al. 2009b) |
| [CnMIM][PF6] (n = 4, 5, 6, 7) | Imposed no significant effect on cell viability at 20% (v/v) in 24 h; reduced substrate toxicity (4′-methoxyacetophenone) | (Wang et al. 2009) |
| [CnMIM][Br] (n = 4, 5, 6, 7) | Imposed some effect on cell viability at 10% (v/v) in 24 h | (Lou et al. 2009b) |
| Rhodotorula glutinis | ||
| [C4MIM][PF6] | No formation of inhibition halo was observed with pure IL n 24 h | (Zampieri et al. 2013) |
| Trigonopsis variabilis | ||
| [C2MIM][NO3] | Imposed some effect on cell viability at 5% (v/v) in 24 h; reduced substrate toxicity (4′-methoxyacetophenone) | (Lou et al. 2009c) |
| [HOC2MIM][Cl] | Imposed some effect on cell viability at 5% (v/v) in 24 h; reduced substrate toxicity (4′-methoxyacetophenone) | (Lou et al. 2009c) |
| [HOC2MIM][PF6] | Imposed no significant effect on cell viability at 5% (v/v) in 24 h | (Lou et al. 2009c) |
| [HOC2MIM][NO3] | Imposed no significant effect on cell viability at 5% (v/v) in 24 h; reduced substrate toxicity (4′-methoxyacetophenone) | (Lou et al. 2009c) |
| [HOC2MIM][OTf] | Inhibited cell growth at 5% (v/v) in 24 h | (Lou et al. 2009c) |
| [C4MIM][Cl] | Imposed some effect on cell viability at 5% (v/v) in 24 h | (Lou et al. 2009c) |
| [CnMIM][Br] (n = 4, 5, 6, 7) | Imposed some effect on cell viability at 5% (v/v) in 24 h | (Lou et al. 2009c) |
|
X[NO3] X = [C4MIM], [C4C1MIM] |
Imposed some effect on cell viability at 5% (v/v) in 24 h | (Lou et al. 2009c) |
| Synechocystis sp. | ||
|
X[NTf2] X = [C4MPyr], [C14(C6)3P] |
Imposed no significant effect on cell viability at 25% (w/v) in 24 h | (Lovejoy et al. 2012) |
|
X[Cl] X = [Chol], [(C4)4P] |
Imposed no significant effect on cell viability at 25% (w/v) in 24 h | (Lovejoy et al. 2012) |
| [Chol][NTf2] | Imposed some effect on cell viability at 25% (w/v) in 24 h | (Lovejoy et al. 2012) |
| [(HOC3)3C10P][Br] | Imposed some effect on cell viability at 25% (w/v) in 24 h | (Lovejoy et al. 2012) |
| Penicillium raistrickii | ||
| [C4MIM][PF6] | Inhibited glucose uptake at 20% in 10 h | (Mao et al. 2012) |
| [C4MIM][NTf2] | Imposed little inhibitory effect on glucose uptake at 20% in 10 h | (Mao et al. 2012) |
|
[C6MIM]X X = [PF6], [NTf2] |
Significantly inhibited glucose uptake at 20% in 10 h | (Mao et al. 2012) |
| Penicillium diversum | ||
|
[C2MIM]X X = [Cl], [C2SO4], [SCN], [OAc], [Lac] |
Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Cl] X = [C4MIM], [C4Py], [C4MPyr] |
Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[OAc] X = [C2Py], [C4MPip], [Chol] |
Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Lac] X = [C2Py], [Chol] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [C4MPyr][Lac] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
| [Chol][Cl] | Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [Chol][NTf2] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
| Penicillium variabile | ||
|
[C2MIM]X X = [Cl], [C2SO4], [SCN], [OAc], [Lac] |
Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Cl] X = [C4MIM], [C4Py], [C4MPyr] |
Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[OAc] X = [C2Py], [C4MPip] |
Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
| [C2Py][Lac] | Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [C4MPyr][Lac] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
[Chol]X X = [Cl], [NTf2], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| Penicillium restrictum | ||
|
[C2MIM]X X = [Cl], [C2SO4], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [C2MIM][SCN] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
| [C4MIM][Cl] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
[C2Py]X X = [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [C4Py][Cl] | Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
[C4MPyr] X = [Cl], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [C4MPip][OAc] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
[Chol]X X = [Cl], [NTf2], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| Penicillium corylophilum | ||
|
[C2MIM]X X = [Cl], [C2SO4], [SCN], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [C4MIM][Cl] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[OAc] X = [C2Py], [C4MPip][ |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Lac] X = [C2Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Cl] X = [C4Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
[Chol]X X = [Cl], [NTf2], [OAc] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [Chol][Lac] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
| Penicillium janczewskii | ||
|
[C2MIM]X X = [Cl], [C2SO4], [SCN], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [C2MIM][OAc] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Cl] X = [C4MIM], [C4Py], [C4MPyr], [Chol] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[OAc] X = [C2Py], [C4MPip], [Chol] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Lac] X = [C2Py], [C4MPyr], [Chol] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [Chol][NTf2] | Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| Penicillium brevicompactum | ||
|
[C2MIM]X X = [Cl], [C2SO4], [SCN], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [C2MIM][OAc] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Cl] X = [C4MIM], [C4Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[OAc] X = [C2Py], [C4MPip] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Lac] X = [C2Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
[Chol]X X = [Cl], [NTf2], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| Penicillium adametzii | ||
|
[C2MIM]X X = [Cl], [C2SO4], [SCN], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Cl] X = [C4MIM], [C4Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[OAc] X = [C2Py], [C4MPip] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Lac] X = [C2Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
[Chol]X X = [Cl], [NTf2], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| Penicillium glandicola | ||
|
[C2MIM]X X = [Cl], [C2SO4], [SCN], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Cl] X = [C4MIM], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[OAc] X = [C2Py], [C4MPip] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Lac] X = [C2Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [C4Py][Cl] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
[Chol]X X = [Cl], [NTf2], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| Penicillium olsonii | ||
|
[C2MIM]X X = [Cl], [C2SO4], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| [C2MIM][SCN] | Inhibited cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Cl] X = [C4MIM], [C4Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[OAc] X = [C2Py], [C4MPip] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Lac] X = [C2Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
[Chol]X X = [Cl], [NTf2], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| Penicillium glabrum | ||
|
[C2MIM]X X = [Cl], [C2SO4], [SCN], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Cl] X = [C4MIM], [C4Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[OAc] X = [C2Py], [C4MPip] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
X[Lac] X = [C2Py], [C4MPyr] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
|
[Chol]X X = [Cl], [NTf2], [OAc], [Lac] |
Imposed no inhibitory effect on cell growth at 50 mM | (Petkovic et al. 2009) |
| Aspergillus ochraceus | ||
| [C3MIM][PF6] | Imposed little inhibitory effect on glucose consumption at 20% in 12 h; alleviated toxicity of the substrate 16α,17-epoxyprogesterone | (Mao et al. 2013) |
|
X[NTf2] X = [C3MIM], [C4MIM], [C6MIM], [AMIM] |
Significantly inhibited glucose consumption at 20% in 10 h | (Mao et al. 2013) |
| [CnMIM][PF6] (n = 4, 6) | Significantly inhibited glucose consumption at 20% in 10 h | (Mao et al. 2013) |
| Aspergillus brasiliensis | ||
| [C2MIM][Cl] | MNTC 426 mM | (Santos et al. 2014) |
| [C2MIM][NTf2] | MNTC 160 mM | (Santos et al. 2014) |
| [C2MIM][C2SO3] | MNTC >581 mM | (Santos et al. 2014) |
| [C2MIM][C2SO4] | MNTC >540 mM | (Santos et al. 2014) |
| [C4MIM][NTf2] | MNTC 150 mM | (Santos et al. 2014) |
| [Chol][Cl] | MNTC 448 mM | (Santos et al. 2014) |
| [Chol][NTf2] | MNTC 74 mM | (Santos et al. 2014) |
| [Chol][OAc] | MNTC 196 mM | (Santos et al. 2014) |
| [(C4)3C1P][C1SO4] | MNTC 8 mM | (Santos et al. 2014) |
| Lactobacillus kefir | ||
| [C4MIM][PF6] | 20% (v/v) imposed no damage to the cell membrane in 5 h | (Pfruender et al. 2004) |
| [C4MIM][NTf2] | 20% (v/v) imposed no damage to the cell membrane in 5 h and alleviated the harmful effect of the substrate 4-chloroacetophenone | (Pfruender et al. 2004) |
| [(C8)3C1N][NTf2] | 20% (v/v) imposed no damage to the cell membrane in 5 h | (Pfruender et al. 2004) |
| Lactobacillus delbrueckii subsp. delbrueckii | ||
| [C2MIM][Cl] | MNTC 53 mM | (Santos et al. 2014) |
|
X[NTf2] X = [C2MIM], [Chol] |
MNTC <5 mM | (Santos et al. 2014) |
| [C2MIM][C2SO3] | MNTC 72 mM | (Santos et al. 2014) |
| [C2MIM][C2SO4] | MNTC 67.5 mM | (Santos et al. 2014) |
| [C4MIM][NTf2] | MNTC <4.7 mM | (Santos et al. 2014) |
| [Chol][Cl] | MNTC 112 mM | (Santos et al. 2014) |
| [Chol][OAc] | MNTC 24.5 mM | (Santos et al. 2014) |
| [(C4)3C1P][C1SO4] | MNTC 8 mM | (Santos et al. 2014) |
| Bacillus subtilis | ||
| [C2MIM][Cl] | MNTC 426 mM | (Santos et al. 2014) |
| [C2MIM][NTf2] | MNTC <5 mM | (Santos et al. 2014) |
| [C2MIM][C2SO3] | MNTC 581 mM | (Santos et al. 2014) |
| [C2MIM][C2SO4] | MNTC 270 mM | (Santos et al. 2014) |
| [C4MIM][NTf2] | MNTC <4.7 mM | (Santos et al. 2014) |
| [Chol][Cl] | MNTC 28 mM | (Santos et al. 2014) |
| [Chol][NTf2] | MNTC 10 mM | (Santos et al. 2014) |
| [Chol][OAc] | MNTC 24.5 mM | (Santos et al. 2014) |
| [(C4)3C1P][C1SO4] | MNTC <3.75 mM | (Santos et al. 2014) |
| Bacillus coagulans | ||
|
[C2MIM]X X = [Cl], [OAc] |
Significantly inhibited cell growth at 236 mM | (Simmons et al. 2014) |
| Streptomyces drozdowikzii | ||
| [C2MIM][Cl] | MNTC 27 mM | (Santos et al. 2014) |
|
X[NTf2] X = [C2MIM], [Chol] |
MNTC <5 mM | (Santos et al. 2014) |
| [C2MIM][C2SO3] | MNTC 36 mM | (Santos et al. 2014) |
| [C2MIM][C2SO4] | MNTC 34 mM | (Santos et al. 2014) |
| [C4MIM][NTf2] | MNTC <4.7 mM | (Santos et al. 2014) |
| [Chol][Cl] | MNTC 112 mM | (Santos et al. 2014) |
| [Chol][OAc] | MNTC 24 mM | (Santos et al. 2014) |
| [(C4)3C1P][C1SO4] | MNTC <3.75 mM | (Santos et al. 2014) |
| Pseudomonas aeruginosa | ||
| [C2MIM][Cl] | MNTC 852.5 mM | (Santos et al. 2014) |
| [C2MIM][NTf2] | MNTC 10 mM | (Santos et al. 2014) |
| [C2MIM][C2SO3] | MNTC 145 mM | (Santos et al. 2014) |
| [C2MIM][C2SO4] | MNTC 135 mM | (Santos et al. 2014) |
| [C4MIM][NTf2] | MNTC 300 mM | (Santos et al. 2014) |
| [Chol][Cl] | MNTC 448 mM | (Santos et al. 2014) |
| [Chol][NTf2] | MNTC 74 mM | (Santos et al. 2014) |
| [Chol][OAc] | MNTC 98 mM | (Santos et al. 2014) |
| [(C4)3C1P][C1SO4] | MNTC 15 mM | (Santos et al. 2014) |
| Pseudomonas fluorescens | ||
| [CnMIM][BF4] (n = 2, 4, 6, 8) | Inhibited cell growth at 5% (v/v) in 48 h | (Yang et al. 2014) |
| [C4MIM][PF6] | Inhibited cell growth at 5% (v/v) in 48 h | (Yang et al. 2014) |
| Yarrowia lipolytica | ||
| [C2MIM][Cl] | MNTC 235 mM | (Santos et al. 2014) |
| [C2MIM][NTf2] | MNTC 10 mM | (Santos et al. 2014) |
| [C2MIM][C2SO3] | MNTC 145 mM | (Santos et al. 2014) |
| [C2MIM][C2SO4] | MNTC 270 mM | (Santos et al. 2014) |
| [C4MIM][NTf2] | MNTC 5 mM | (Santos et al. 2014) |
| [Chol][Cl] | MNTC 448 mM | (Santos et al. 2014) |
| [Chol][NTf2] | MNTC 37 mM | (Santos et al. 2014) |
| [Chol][OAc] | MNTC 196 mM | (Santos et al. 2014) |
| [(C4)3C1P][C1SO4] | MNTC 7.8 mM | (Santos et al. 2014) |
| Kluyveromyces marxianus | ||
| [C2MIM][Cl] | MNTC <6.7 mM | (Santos et al. 2014) |
|
X[NTf2] X = [CnMIM] (n = 2, 4), [Chol] |
MNTC <2.5 mM | (Santos et al. 2014) |
| [C2MIM][C2SO3] | MNTC <4.5 mM | (Santos et al. 2014) |
| [C2MIM][C2SO4] | MNTC <4.2 mM | (Santos et al. 2014) |
| [Chol][Cl] | MNTC 224 mM | (Santos et al. 2014) |
| [Chol][OAc] | MNTC 98 mM | (Santos et al. 2014) |
| [(C4)3C1P][C1SO4] | MNTC <3.75 mM | (Santos et al. 2014) |
| Rhizopus oryzae | ||
| [C2MIM][Cl] | MNTC >852.5 mM | (Santos et al. 2014) |
| [C2MIM][NTf2] | MNTC 20 mM | (Santos et al. 2014) |
| [C2MIM][C2SO3] | MNTC 290 mM | (Santos et al. 2014) |
| [C2MIM][C2SO4] | MNTC >540 mM | (Santos et al. 2014) |
| [C4MIM][NTf2] | MNTC >300 mM | (Santos et al. 2014) |
| [Chol][Cl] | MNTC >896 mM | (Santos et al. 2014) |
| [Chol][NTf2] | MNTC 74 mM | (Santos et al. 2014) |
| [Chol][OAc] | MNTC 392 mM | (Santos et al. 2014) |
| [(C4)3C1P][C1SO4] | MNTC 8 mM | (Santos et al. 2014) |
| Acetobacter sp. | ||
|
X[Cl] X = [HOC2MIM], [CnMIM] (n = 2, 4) |
Imposed some effect on metabolic activity at 5% (v/v) in 24 h; alleviated toxicity of the substrate 4-(trimethylsilyl)-3-butyn-2-one; increased membrane permeability | (Xiao et al. 2012) |
| [HOC2MIM][NO3] | Imposed no significant effect on metabolic activity at 5% (v/v) in 24 h; alleviated toxicity of the substrate 4-(trimethylsilyl)-3-butyn-2-one; increased membrane permeability | (Xiao et al. 2012) |
| [HOC2MIM][OTf] | Inhibited cell growth at 5% (v/v) in 24 h; increased membrane permeability | (Xiao et al. 2012) |
|
[CnMIM]X (n = 2, 4) X = [Br], [NO3] |
Imposed some effect on metabolic activity at 5% (v/v) in 24 h; alleviated toxicity of the substrate 4-(trimethylsilyl)-3-butyn-2-one; increased membrane permeability | (Xiao et al. 2012) |
| [C4MIM][PF6] | Imposed no significant effect on metabolic activity in 12 h; alleviated toxicity of the substrate ethyl acetoacetate | (Wang et al. 2013) |
| [CnMIM][NTf2] (n = 4, 6) | Imposed some effect on metabolic activity in 12 h; alleviated toxicity of the substrate ethyl acetoacetate | (Wang et al. 2013) |
| [CnMIM][PF6] (n = 4(i), 5, 6, 7) | Imposed some effect on metabolic activity in 12 h; alleviated toxicity of the substrate ethyl acetoacetate | (Wang et al. 2013) |
| [C4C1MIM][NO3] | Imposed some effect on metabolic activity at 5% (v/v) in 24 h; increased membrane permeability | (Xiao et al. 2012) |
| [CnMIM][Br] (n = 5, 6, 7) | Imposed some effect on metabolic activity at 5% (v/v) in 24 h; increased membrane permeability | (Xiao et al. 2012) |
| Acinetobacter sp. | ||
|
[C2MIM]X X = [Cl], [BF4], [NO3] |
Imposed some effect on metabolic activity in 22 h; increased membrane permeability | (Xu et al. 2016b) |
|
[HOC2MIM]X X = [Cl], [OTf] |
Imposed some effect on metabolic activity in 22 h; increased membrane permeability | (Xu et al. 2016b) |
|
X[NO3] X = [HOC2MIM], [HOOC2MIM] |
Imposed no significant effect on metabolic activity in 22 h; increased membrane permeability | (Xu et al. 2016b) |
| [C2C1MIM][NO3] | Imposed some effect on metabolic activity in 22 h; increased membrane permeability | (Xu et al. 2016b) |
| [CnMIM][Cl] (n = 4, 7) | Inhibited metabolic activity in 22 h; increased membrane permeability | (Xu et al. 2016b) |
| Geotrichum candidum | ||
| [C4MIM][PF6] | No formation of inhibition halo was observed with pure IL in 24 h | (Zampieri et al. 2013) |
| Micrococcus luteus | ||
| [C4MIM][PF6] | No formation of inhibition halo was observed with pure IL in 24 h | (Zampieri et al. 2013) |
| Aureobasidium pullulans | ||
|
X[BF4] X = [C4MIM], [C8Py] |
Inhibited metabolic activity in 4 h | (Zhang et al. 2008) |
|
X[PF6] X = [C4MIM], [C4C1MIM] |
Imposed no effect on metabolic activity in 4 h | (Zhang et al. 2008) |
| [C4MIM][OTf] | Inhibited metabolic activity in 4 h | (Zhang et al. 2008) |
Fig. 5.
Biocompatibility of selected ILs for E.coli (based on data from Table 4)
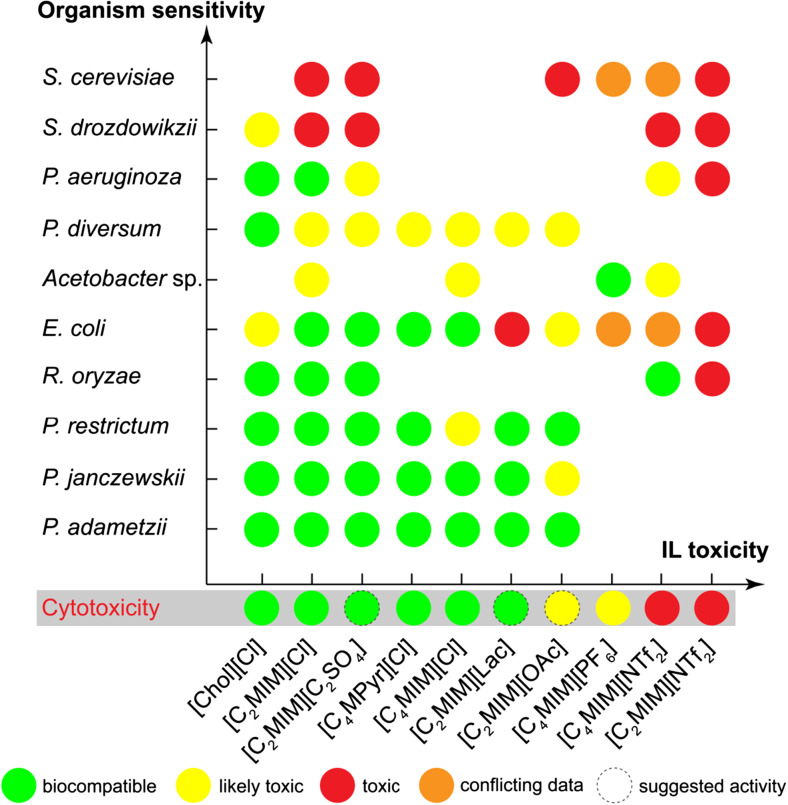
Further analysis involves different microorganisms and shows the relative tolerance of frequently used biocatalytic systems towards the most studied ILs (Fig. 6). According to the existing data, cholinium ILs and imidazolium ILs with short alkyl chains represent the systems of choice for application in whole-cell biocatalysis. As for the IL anions, chloride seems the best choice for the selected microorganisms. Regarding the organisms, the Penicillium genus shows the highest IL tolerance, whereas S.cerevisiae demonstrates high sensitivity to most ILs.
Fig. 6.
Biocompatibility of most studied ILs with most demanded microorganisms (based on data from Table 4 and Egorova and Ananikov (2014) and Radosevic et al. (2015)
Toxicity of ILs presumably manifests due to their interaction with the cell membrane. ILs with long alkyl chains supposedly penetrate into the lipid bilayer thus disturbing its structure (Jeong et al. 2012; Petkovic et al. 2012; Benedetto and Ballone 2016; Benedetto 2017; Egorova et al. 2017). Upon the insertion of IL cations, the membrane starts bending which supposedly leads to morphological damage (Yoo et al. 2016). In the case of imidazolium ILs, the imidazolium ring interacts with the lipid head group, whereas the alkyl side chain forms contacts with the lipid tail. Longer alkyl chains facilitates the deeper penetration of IL into the lipid bilayer (Yoo et al. 2014).
Presumably, in the case of microorganisms, the damage to the cell membrane is one of the main reasons of IL toxicity. Thus, it was shown that [CnMIM][Cl] with long alkyl chains (n = 6, 8, 10) caused membrane damage, whereas cholinium ILs were less toxic, though their toxicity increased upon increasing the length of the alkanoate anion. [CnMIM][Cl] also up-regulated the expression of genes involved in the biosynthesis of saturated fatty acids (Jing et al. 2014a, b; Hartmann et al. 2015). A study on the accumulation of [P6,6,6,14][NTf2] in Escherichia coli found the IL in the membrane fraction, but not in the cytoplasm (Cornmell et al. 2008).
Another important issue is the ability of ILs to penetrate into the cellular nucleus (Chattoraj et al. 2016). ILs can bind to nucleic acids via electrostatic interactions with phosphate groups and via hydrogen bonds with nucleobases. Various ILs have been shown to stabilize DNA in its native B structure (Chandran et al. 2012; Jumbri et al. 2014; Egorova et al. 2017). Some ILs (e.g., guanidinium ILs) also cause DNA compaction (Satpathi et al. 2015; Benedetto and Ballone 2016). Though no direct evidence has yet been obtained, such a strong impact of ILs on the DNA structure suggests the possibility of harmful effects on cell viability and metabolism.
From the viewpoint of the development of resistant microbial strains, studies on transcriptome and proteome of IL-treated microorganisms are of primary importance. Such works are scarce, but some interesting results have been obtained thus far.
In Aspergillus nidulans, exposure to [Chol][Cl] or [C2MIM][Cl] led to significant changes in the expression of metabolism-related genes. The ILs activated the mechanisms of detoxification, but these responses depended on the IL nature. Thus, primary metabolism was activated by [Chol][Cl], but was inhibited by [C2MIM][Cl], presumably because choline could be utilized by cells. Both ILs enhanced the production of acetyl-CoA, which was the precursor of various secondary metabolites. In Neurospora crassa, [Chol][Cl] and [C2MIM][Cl] also induced the expression of various stress-responsive proteins and proteins participating in the biosynthesis of unusual metabolites (Martins et al. 2013; Alves et al. 2016). The authors suggested that ILs could be applied for diversifying or regulating the repertoire of metabolites produced by microbes.
The lignocellulolytic bacterium Enterobacter lignolyticus (strain SCF1) was tolerant up to 0.5 M [C2MIM][Cl], possibly due to an increase of the content of cyclopropane fatty acids in the membrane, down-regulation of membrane porins and up-regulation of multidrug efflux pumps and osmoprotectant transporters. According to the authors, the response differed from general stress and was unique for the IL studied. They suggested an IL tolerance model, which included: (1) reducing cell permeability via fast cell membrane remodeling and porin down-regulation; (2) decreasing the intracellular IL concentration via multidrug efflux pump up-regulation; and (3) alleviating harmful osmotic pressure effects of IL via enhanced scavenging of biocompatible solutes (Khudyakov et al. 2012). When two IL resistance genes encoding an inner membrane transporter and its IL-inducible repressor from Enterobacter lignolyticus were transferred to biofuel-producing Escherichia coli, it acquired the IL resistance (Ruegg et al. 2014). Overall, these findings suggest a promising opportunity for designing IL-tolerant biocatalytic microorganisms.
Conclusions
In modern chemistry, whole-cell biocatalysis can become a ‘magic’ alternative to traditional chemical synthesis (Table 1). However, low aqueous solubility of target compounds and the necessity to use organic solvents hinder its successful widespread application. ILs with their unique solvent properties have been proposed as a more efficient and safe option for biocatalysis. However, in spite of the first encouraging results, we have not gained enough information for the rational design of IL-containing whole-cell biocatalytic systems. There is evidence of the possibility of selecting IL-resistant microorganisms, which can enjoy the benefits of ILs without suffering from their toxic effects. Nevertheless, such endeavors should be undertaken with extreme caution, because acquired resistance of a microbial species to a chemical can pose potential dangers to human health. Thus, prior to cultivating IL-tolerant strains of bacteria and fungi, attention should be paid to the mechanisms of IL toxicity which have not yet been fully elucidated.
Whereas the advantages of ILs can undoubtedly be used in whole-cell biocatalytic systems, the biological activity of these compounds should also be assessed with the utmost care. Indeed, more detailed studies on the biological activity and toxic effects of ILs towards microorganisms should be carried out. The progress in this area will to a large extent determine the practical impact of water/IL systems for whole-cell biocatalysis.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (grant 16-29-10804).
Abbreviations
- AMMOENG™ 100
Cocosalkylpenthaethoxymethylammonium methylsulfate
IL cations
- [CnMIM]
1-alkyl-3-methylimidazolium
- [HOC2MIM]
(2-hydroxy)ethyl-3-methylimidazolium
- [CnC1MIM]
1-alkyl-2,3-dimethylimidazolium
- [PPMIM]
1-phenylpropyl-3-methylimidazolium
- [AMIM]
1-allyl-3-methylimidazolium
- [BzMIM]
1-benzyl-3-methylimidazolium
- [HOOC2MIM]
1-carboxymethyl-3-methylimidazolium
- [CnPy]
N-alkylpyridinium
- [CnMPy]
N-alkyl-3-methyl-pyridinium
- [CnMPyr]
N-alkyl-N-methylpyrrolidinium
- [CnMPip]
N-alkyl-N-methylpiperidinium
- [(C1OC3)C1Pip]
N-methyl-N-(1-methoxypropyl)-piperidinium
- [CnCnCnCnN]
N,N,N,N-tetraalkylammonium
- [Chol]
cholinium, N-(2-hydroxy)ethyl- N,N,N-trimethylammonium
- [(HOC2)(C1)2N]
N,N-dimethyl-N-ethanolammonium
- [(HOC2)2C4C2N]
N,N-di(2-hydroxy)ethyl- N-butyl-N-ethylammonium
- [(HOC3)C2(C1)2N]
N-(3-hydroxy)propyl- N-ethyl-N,N-dimethylammonium
- [(HOC3)C4(C1)2N]
N-(3-hydroxy)propyl- N-butyl-N,N-dimethylammonium
- [(HOC2)3C1N]
Tris(2-hydroxyethyl)methylammonium
- [CnCnCnCnN]
Tetraalkylphosphonium
- [(HOC3)3C10P]
Decyltris(3-hydroxypropyl)phosphonium
- [(C1)4C2Gua]
N,N,N’,N’-tetramethyl-N”-ethylguanidinium
- [CABHEM]
PEG-5 cocomonium
IL anions
- [BF4]
Tetrafluoroborate
- [PF6]
Hexafluorophosphate
- [OTf]
Trifluoromethanesulfonate
- [NTf2]
Bis(trifluoromethylsulfonyl)imide
- [(C2F5)3PF3]
Tris(pentafluoroethyl)trifluorophosphate
- [N(CN)2]
Dicyanamide
- [SCN]
Thiocyanate
- [NO3]
Nitrate
- [OAc]
Acetate
- [C2COO]
Propanoate
- [C5COO]
Hexanoate
- [C1PO3]
Methylphosphonate
- [C2PO4]
Dimethylphosphate
- [i-(C8)2PO4]
Bis(2,4,4-trimethylpentyl)phosphinate
- [C2SO3]
Ethylsulfonate
- [CnSO4]
Alkylsulfate
- [CnOCnSO4]
2-alkoxyalkylsulfate
- [C1(OC2)3SO4]
2-[2-(2-methoxy)ethoxy]ethoxyethylsulfate
- [Tos]
Tosylate, p-toluenesulfonate
- [MDEGSO4]
Ethylenglycolmonomethylethersulfate
- [SbF6]
Hexafluoroantimonate
- [Lac]
Lactate
- [Sacch]
Saccharinate
- [Doc]
Docusate
- [Lin]
Linoleate
Compliance with ethical standards
Conflict of interest
Ksenia S. Egorova declares that she has no conflict of interest. Valentine P. Ananikov declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Ionic Liquids and Biomolecules’ edited by Antonio Benedetto and Hans-Joachim Galla.
References
- Alves PC, Hartmann DO, Nunez O, Martins I, Gomes TL, Garcia H, et al. Transcriptomic and metabolomic profiling of ionic liquid stimuli unveils enhanced secondary metabolism in Aspergillus nidulans. BMC Genomics. 2016;17:284. doi: 10.1186/s12864-016-2577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Nakashima K, Tanino T, Ogino C, Kondo A, Fukuda H. Production of biodiesel fuel from soybean oil catalyzed by fungus whole-cell biocatalysts in ionic liquids. Enzym Microb Technol. 2010;46:51–55. [Google Scholar]
- Baum S, van Rantwijk F, Stolz A. Application of a recombinant Escherichia coli whole-cell catalyst expressing hydroxynitrile lyase and nitrilase activities in ionic liquids for the production of (S)-mandelic acid and (S)-mandeloamide. Adv Synth Catal. 2012;354:113–122. [Google Scholar]
- Benedetto A. Room-temperature ionic liquids meet bio-membranes: the state-of-the-art. Biophys Rev. 2017;9:309–320. doi: 10.1007/s12551-017-0279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto A, Ballone P. Room temperature ionic liquids meet biomolecules: a microscopic view of structure and dynamics. ACS Sustain Chem Eng. 2016;4:392–412. [Google Scholar]
- Bräutigam S, Bringer-Meyer S, Weuster-Botz D. Asymmetric whole cell biotransformations in biphasic ionic liquid/water-systems by use of recombinant Escherichia coli with intracellular cofactor regeneration. Tetrahedron Asymmetry. 2007;18:1883–1887. [Google Scholar]
- Bräutigam S, Dennewald D, Schürmann M, Lutje-Spelberg J, Pitner W-R, Weuster-Botz D. Whole-cell biocatalysis: evaluation of new hydrophobic ionic liquids for efficient asymmetric reduction of prochiral ketones. Enzym Microb Technol. 2009;45:310–316. [Google Scholar]
- Castiglione K, Fu Y, Polte I, Leupold S, Meo A, Weuster-Botz D. Asymmetric whole-cell bioreduction of (R)-carvone by recombinant Escherichia coli with in situ substrate supply and product removal. Biochem Eng J. 2017;117:102–111. [Google Scholar]
- Chandran A, Ghoshdastidar D, Senapati S. Groove binding mechanism of ionic liquids: a key factor in long-term stability of DNA in hydrated ionic liquids? J Am Chem Soc. 2012;134:20330–20339. doi: 10.1021/ja304519d. [DOI] [PubMed] [Google Scholar]
- Chattoraj S, Amin MA, Mohapatra S, Ghosh S, Bhattacharyya K. Cancer cell imaging using in situ generated gold nanoclusters. ChemPhysChem. 2016;17:61–68. doi: 10.1002/cphc.201500731. [DOI] [PubMed] [Google Scholar]
- Chen J-Y, Kaleem I, He D-M, Liu G-Y, Li C. Efficient production of glycyrrhetic acid 3-O-mono-β-d-glucuronide by whole-cell biocatalysis in an ionic liquid/buffer biphasic system. Process Biochem. 2012;47:908–913. [Google Scholar]
- Chen L, Sharifzadeh M, Mac Dowell N, Welton T, Shah N, Hallett JP (2014) Inexpensive ionic liquids: [HSO4]−−based solvent production at bulk scale. Green Chem 16:3098–3106
- Choi HJ, Uhm K-N, Kim H-K. Production of chiral compound using recombinant Escherichia coli cells co-expressing reductase and glucose dehydrogenase in an ionic liquid/water two phase system. J Mol Catal B Enzym. 2011;70:114–118. [Google Scholar]
- Cornmell RJ, Winder CL, Schuler S, Goodacre R, Stephens G. Using a biphasic ionic liquid/water reaction system to improve oxygenase-catalysed biotransformation with whole cells. Green Chem. 2008;10:685–691. [Google Scholar]
- de Carvalho CCCR. Enzymatic and whole cell catalysis: finding new strategies for old processes. Biotechnol Adv. 2011;29:75–83. doi: 10.1016/j.biotechadv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- de Carvalho CCCR. Whole cell biocatalysts: essential workers from nature to the industry. Microb Biotechnol. 2017;10:250–263. doi: 10.1111/1751-7915.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennewald D, Pitner W-R, Weuster-Botz D. Recycling of the ionic liquid phase in process integrated biphasic whole-cell biocatalysis. Process Biochem. 2011;46:1132–1137. [Google Scholar]
- Dennewald D, Hortsch R, Weuster-Botz D. Evaluation of parallel milliliter-scale stirred-tank bioreactors for the study of biphasic whole-cell biocatalysis with ionic liquids. J Biotechnol. 2012;157:253–257. doi: 10.1016/j.jbiotec.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Dipeolu O, Green E, Stephens G. Effects of water-miscible ionic liquids on cell growth and nitro reduction using Clostridium sporogenes. Green Chem. 2009;11:397–401. [Google Scholar]
- Egorova KS, Ananikov VP. Toxicity of ionic liquids: eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization. ChemSusChem. 2014;7:336–360. doi: 10.1002/cssc.201300459. [DOI] [PubMed] [Google Scholar]
- Egorova KS, Ananikov VP (2016) Which metals are green for catalysis? Comparison of the toxicities of Ni, Cu, Fe, Pd, Pt, Rh, and Au salts. Angew Chem Int Ed 55:12150–12162 [DOI] [PubMed]
- Egorova KS, Gordeev EG, Ananikov VP. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev. 2017;117:7132–7189. doi: 10.1021/acs.chemrev.6b00562. [DOI] [PubMed] [Google Scholar]
- Frederix M, Hütter K, Leu J, Batth TS, Turner WJ, Rüegg TL, et al. Development of a native Escherichia coli induction system for ionic liquid tolerance. PLoS ONE. 2014;9:e101115. doi: 10.1371/journal.pone.0101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederix M, Mingardon F, Hu M, Sun N, Pray T, Singh S, et al. Development of an E. coli strain for one-pot biofuel production from ionic liquid pretreated cellulose and switchgrass. Green Chem. 2016;18:4189–4197. [Google Scholar]
- Gao P, Li A, Lee HH, Wang DIC, Li Z. Enhancing enantioselectivity and productivity of P450-catalyzed asymmetric sulfoxidation with an aqueous/ionic liquid biphasic system. ACS Catal. 2014;4:3763–3771. [Google Scholar]
- George A, Brandt A, Tran K, Zahari SMSNS, Klein-Marcuschamer D, Sun N, et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015;17:1728–1734. [Google Scholar]
- Gorke J, Srienc F, Kazlauskas R. Toward advanced ionic liquids. Polar, enzyme-friendly solvents for biocatalysis. Biotechnol Bioprocess Eng. 2010;15:40–53. doi: 10.1007/s12257-009-3079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajfarajollah H, Mokhtarani B, Sharifi A, Mirzaei M, Afaghi A. Toxicity of various kinds of ionic liquids towards the cell growth and end product formation of the probiotic strain, Propionibacterium freudenreichii. RSC Adv. 2014;4:13153–13160. [Google Scholar]
- Hartmann DO, Shimizu K, Siopa F, Leitão MC, Afonso CAM, Canongia Lopes JN, et al. Plasma membrane permeabilisation by ionic liquids: a matter of charge. Green Chem. 2015;17:4587–4598. [Google Scholar]
- Hashmi M, Shah A, Hameed A, Ragauskas A. Enhanced production of bioethanol by fermentation of autohydrolyzed and C4mimOAc-treated sugarcane bagasse employing various yeast strains. Energies. 2017;10:1207. [Google Scholar]
- Hayes R, Warr GG, Atkin R. Structure and nanostructure in ionic liquids. Chem Rev. 2015;115:6357–6426. doi: 10.1021/cr500411q. [DOI] [PubMed] [Google Scholar]
- He J-Y, Wang P, Yang Y-F, Xie S-L. Enhanced whole-cell biodehydrogenation of 11β-hydroxyl medroxyprogesterone in a biphasic system containing ionic liquid. Biotechnol Bioprocess Eng. 2011;16:852–857. [Google Scholar]
- Howarth J, James P, Dai JF. Immobilized baker’s yeast reduction of ketones in an ionic liquid, [bmim]PF6 and water mix. Tetrahedron Lett. 2001;42:7517–7519. [Google Scholar]
- Hussain W, Pollard DJ, Lye GJ. The bioreduction of a β-tetralone to its corresponding alcohol by the yeast Trichosporon capitatum MY1890 and bacterium Rhodococcus erythropolis MA7213 in a range of ionic liquids. Biocatal Biotransform. 2007;25:443–452. [Google Scholar]
- Jeong S, Ha SH, Han S-H, Lim M-C, Kim SM, Kim Y-R, et al. (2012) Elucidation of molecular interactions between lipid membranes and ionic liquids using model cell membranes. Soft Matter 8:5501–5506
- Jing C, Hu H, Guo M, Chen X, Li T. Cytotoxicity of 1-octyl-3-methylimidazolium chloride on Escherichia coli DH5α. Toxin Rev. 2014;33:91–94. [Google Scholar]
- Jing C, Mu L, Ren T, Li B, Chen S, Nan W. Effect of 1-octyl-3-methylimidazolium chloride on cell replication and membrane permeability of Escherichia coli DH5alpha. Bull Environ Contam Toxicol. 2014;93:60–63. doi: 10.1007/s00128-014-1269-7. [DOI] [PubMed] [Google Scholar]
- Jumbri K, Rahman MBA, Abdulmalek E, Ahmad H, Micaelo NM. An insight into structure and stability of DNA in ionic liquids from molecular dynamics simulation and experimental studies. Phys Chem Chem Phys. 2014;16:14036–14046. doi: 10.1039/c4cp01159g. [DOI] [PubMed] [Google Scholar]
- Kandar S, Suresh AK, Noronha SB. (R)-PAC biosynthesis in [BMIM][PF6]/aqueous biphasic system using Saccharomyces cerevisiae BY4741 cells. Appl Biochem Biotechnol. 2015;175:1771–1788. doi: 10.1007/s12010-014-1394-0. [DOI] [PubMed] [Google Scholar]
- Kashin AS, Galkin KI, Khokhlova EA, Ananikov VP. Direct observation of self-organized water-containing structures in the liquid phase and their influence on 5-(hydroxymethyl)furfural formation in ionic liquids. Angew Chem Int Ed. 2016;55:2161–2166. doi: 10.1002/anie.201510090. [DOI] [PubMed] [Google Scholar]
- Khudyakov JI, D’Haeseleer P, Borglin SE, Deangelis KM, Woo H, Lindquist EA et al (2012) Global transcriptome response to ionic liquid by a tropical rain forest soil bacterium, Enterobacter lignolyticus. Proc Natl Acad Sci U S A 109:E2173–E2182 [DOI] [PMC free article] [PubMed]
- Ladkau N, Schmid A, Buhler B. The microbial cell-functional unit for energy dependent multistep biocatalysis. Curr Opin Biotechnol. 2014;30:178–189. doi: 10.1016/j.copbio.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Lee S-M, Chang W-J, Choi A-R, Koo Y-M. Influence of ionic liquids on the growth of Escherichia coli. Korean J Chem Eng. 2005;22:687–690. [Google Scholar]
- Lenourry A, Gardiner J, Stephens G. Hydrogenation of C-C double bonds in an ionic liquid reaction system using the obligate anaerobe, Sporomusa termitida. Biotechnol Lett. 2005;27:161–165. doi: 10.1007/s10529-004-7662-2. [DOI] [PubMed] [Google Scholar]
- Li J, Wang P, Huang J, Sun J. Design and application of a novel ionic liquid with the property of strengthening coenzyme regeneration for whole-cell bioreduction in an ionic liquid-distilled water medium. Bioresour Technol. 2015;175:42–50. doi: 10.1016/j.biortech.2014.10.059. [DOI] [PubMed] [Google Scholar]
- Lin B, Tao Y. Whole-cell biocatalysts by design. Microb Cell Factories. 2017;16:106. doi: 10.1186/s12934-017-0724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou W-Y, Zong M-H, Smith TJ (2006) Use of ionic liquids to improve whole-cell biocatalytic asymmetric reduction of acetyltrimethylsilane for efficient synthesis of enantiopure (S)-1-trimethylsilylethanol. Green Chem 8:147–155
- Lou W-Y, Chen L, Zhang BB, Smith TJ, Zong MH. Using a water-immiscible ionic liquid to improve asymmetric reduction of 4-(trimethylsilyl)-3-butyn-2-one catalyzed by immobilized Candida parapsilosis CCTCC M203011 cells. BMC Biotechnol. 2009;9:90. doi: 10.1186/1472-6750-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou W-Y, Wang W, Li RF, Zong MH. Efficient enantioselective reduction of 4′-methoxyacetophenone with immobilized Rhodotorula sp. AS2.2241 cells in a hydrophilic ionic liquid-containing co-solvent system. J Biotechnol. 2009;143:190–197. doi: 10.1016/j.jbiotec.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Lou W-Y, Wang W, Smith TJ, Zong M-H. Biocatalytic anti-Prelog stereoselective reduction of 4′-methoxyacetophenone to (R)-1-(4-methoxyphenyl)ethanol with immobilized Trigonopsis variabilis AS2.1611 cells using an ionic liquid-containing medium. Green Chem. 2009;11:1377–1384. [Google Scholar]
- Lovejoy KS, Davis LE, McClellan LM, Lillo AM, Welsh JD, Schmidt EN, et al. Evaluation of ionic liquids on phototrophic microbes and their use in biofuel extraction and isolation. J Appl Phycol. 2012;25:973–981. [Google Scholar]
- Luo Y, Wang Q, Lu Q, Mu Q, Mao D. An ionic liquid facilitates the proliferation of antibiotic resistance genes mediated by class I integrons. Environ Sci Technol Lett. 2014;1:266–270. [Google Scholar]
- Mao S, Hu X, Hua B, Wang N, Liu X, Lu F. 15α-hydroxylation of a steroid (13-ethyl-gon-4-en-3,17-dione) by Penicillium raistrickii in an ionic liquid/aqueous biphasic system. Biotechnol Lett. 2012;34:2113–2117. doi: 10.1007/s10529-012-1016-2. [DOI] [PubMed] [Google Scholar]
- Mao S, Hua B, Wang N, Hu X, Ge Z, Li Y, et al. 11α hydroxylation of 16α, 17-epoxyprogesterone in biphasic ionic liquid/water system by Aspergillus ochraceus. J Chem Technol Biotechnol. 2013;88:287–292. [Google Scholar]
- Martins I, Hartmann DO, Alves PC, Planchon S, Renaut J, Leitao MC et al (2013) Proteomic alterations induced by ionic liquids in Aspergillus nidulans and Neurospora crassa. J Proteomics 94:262–278 [DOI] [PubMed]
- Matsumoto M, Sugimoto T, Ishiguro Y, Yamaguchi H, Kondo K (2014) Effect of organic solvents and ionic liquids on resolution of 2-epoxyhexane by whole cells of Rhodotorula glutinis in a two-liquid phase system. J Chem Technol Biotechnol 89:522–527
- Mehmood N, Husson E, Jacquard C, Wewetzer S, Buchs J, Sarazin C, et al. Impact of two ionic liquids, 1-ethyl-3-methylimidazolium acetate and 1-ethyl-3-methylimidazolium methylphosphonate, on Saccharomyces cerevisiae: metabolic, physiologic, and morphological investigations. Biotechnol Biofuels. 2015;8:17. doi: 10.1186/s13068-015-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgarejo-Torres R, Torres-Martínez D, Gutiérrez-Rojas M, Gómez de Jesús A, Lye GJ, Huerta-Ochoa S. Regime analysis of a Baeyer–Villiger bioconversion in a three-phase (air–water–ionic liquid) stirred tank bioreactor. Biochem Eng J. 2011;58-59:87–95. [Google Scholar]
- North M, Clark JH, eds. (2016). Sustainable Catalysis With Non-endangered Metals. Parts 1 and 2. Royal Society of Chemistry, Cambridge
- Ouellet M, Datta S, Dibble DC, Tamrakar PR, Benke PI, Li C, et al. Impact of ionic liquid pretreated plant biomass on Saccharomyces Cerevisiae growth and biofuel production. Green Chem. 2011;13:2743–2749. [Google Scholar]
- Pérez de los Ríos A, Hernández-Fernández FJ, Zapata Henríquez PA, Missoun F, Hernández-Fernández J, Ortiz-Martínez V et al (2017) Keys for bioethanol production processes by fermentation and ionic liquid extraction. ACS Sustain Chem Eng 5:6986–6993
- Petkovic M, Ferguson J, Bohn A, Trindade J, Martins I, Carvalho MB, et al. Exploring fungal activity in the presence of ionic liquids. Green Chem. 2009;11:889–894. [Google Scholar]
- Petkovic M, Hartmann DO, Adamová G, Seddon KR, Rebelo LPN, Pereira CS. Unravelling the mechanism of toxicity of alkyltributylphosphonium chlorides in Aspergillus nidulans conidia. New J Chem. 2012;36:56–63. [Google Scholar]
- Pfruender H, Amidjojo M, Kragl U, Weuster-Botz D. Efficient whole-cell biotransformation in a biphasic ionic liquid/water system. Angew Chem Int Ed. 2004;43:4529–4531. doi: 10.1002/anie.200460241. [DOI] [PubMed] [Google Scholar]
- Pfruender H, Jones R, Weuster-Botz D. Water immiscible ionic liquids as solvents for whole cell biocatalysis. J Biotechnol. 2006;124:182–190. doi: 10.1016/j.jbiotec.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Quijano G, Couvert A, Amrane A. Ionic liquids: applications and future trends in bioreactor technology. Bioresour Technol. 2010;101:8923–8930. doi: 10.1016/j.biortech.2010.06.161. [DOI] [PubMed] [Google Scholar]
- Radosevic K, Bubalo MC, Srcek VG, Grgas D, Dragicevic TL, Redovnikovic IR. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol Environ Saf. 2015;112:46–53. doi: 10.1016/j.ecoenv.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Ruegg TL, Kim EM, Simmons BA, Keasling JD, Singer SW, Lee TS, et al. An auto-inducible mechanism for ionic liquid resistance in microbial biofuel production. Nat Commun. 2014;5:3490. doi: 10.1038/ncomms4490. [DOI] [PubMed] [Google Scholar]
- Santos AG, Ribeiro BD, Alviano DS, Coelho MAZ. Toxicity of ionic liquids toward microorganisms interesting to the food industry. RSC Adv. 2014;4:37157–37163. [Google Scholar]
- Satpathi S, Sengupta A, Hridya VM, Gavvala K, Koninti RK, Roy B, et al. A green solvent induced DNA package. Sci Rep. 2015;5:9137. [Google Scholar]
- Schmideder A, Priebe X, Rubenbauer M, Hoffmann T, Huang F-C, Schwab W, et al. Non-water miscible ionic liquid improves biocatalytic production of geranyl glucoside with Escherichia coli overexpressing a glucosyltransferase. Bioprocess Biosyst Eng. 2016;39:1409–1414. doi: 10.1007/s00449-016-1617-6. [DOI] [PubMed] [Google Scholar]
- Schrewe M, Julsing MK, Bühler B, Schmid A. Whole-cell biocatalysis for selective and productive C-O functional group introduction and modification. Chem Soc Rev. 2013;42:6346–6377. doi: 10.1039/c3cs60011d. [DOI] [PubMed] [Google Scholar]
- Schroer K, Tacha E, Lütz S. Process intensification for substrate-coupled whole cell ketone reduction by in situ acetone removal. Org Process Res Dev. 2007;11:836–841. [Google Scholar]
- Seitkalieva MM, Kachala VV, Egorova KS, Ananikov VP. Molecular extraction of peptides in ionic liquid systems. ACS Sustain Chem Eng. 2015;3:357–364. [Google Scholar]
- Seitkalieva MM, Kashin AS, Egorova KS, Ananikov VP (2017) Micro-scale processes occurring in ionic liquid–water phases during extraction. Sep Purif Technol:ePub ahead of print. 10.1016/j.seppur.2017.06.056
- Sendovski M, Nir N, Fishman A. Bioproduction of 2-phenylethanol in a biphasic ionic liquid aqueous system. J Agric Food Chem. 2010;58:2260–2265. doi: 10.1021/jf903879x. [DOI] [PubMed] [Google Scholar]
- Silva VD, Carletto JS, Carasek E, Stambuk BU, Nascimento MG. Asymmetric reduction of (4S)-(+)-carvone catalyzed by baker’s yeast: a green method for monitoring the conversion based on liquid–liquid–liquid microextraction with polypropylene hollow fiber membranes. Process Biochem. 2013;48:1159–1165. [Google Scholar]
- Simmons CW, Reddy AP, Vandergheynst JS, Simmons BA, Singer SW. Bacillus coagulans tolerance to 1-ethyl-3-methylimidazolium-based ionic liquids in aqueous and solid-state thermophilic culture. Biotechnol Prog. 2014;30:311–316. doi: 10.1002/btpr.1859. [DOI] [PubMed] [Google Scholar]
- Song X-L, Ye S-Y, Xie R, Yin L, Shi X, Luo S-C. Effects of bmim[PF6] treatments with different concentrations on microbial activity of Saccharomyces cerevisiae. Korean J Chem Eng. 2011;28:1902–1907. [Google Scholar]
- Sun J, Konda NVSNM, Parthasarathi R, Dutta T, Valiev M, Xu F, et al. One-pot integrated biofuel production using low-cost biocompatible protic ionic liquids. Green Chem. 2017;19:3152–3163. [Google Scholar]
- Triolo A, Russina O, Bleif HJ, Di Cola E. Nanoscale segregation in room temperature ionic liquids. J Phys Chem B. 2007;111:4641–4644. doi: 10.1021/jp067705t. [DOI] [PubMed] [Google Scholar]
- Wachtmeister J, Rother D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr Opin Biotechnol. 2016;42:169–177. doi: 10.1016/j.copbio.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Voth GA. Unique spatial heterogeneity in ionic liquids. J Am Chem Soc. 2005;127:12192–12193. doi: 10.1021/ja053796g. [DOI] [PubMed] [Google Scholar]
- Wang W, Zong M-H, Lou W-Y. Use of an ionic liquid to improve asymmetric reduction of 4′-methoxyacetophenone catalyzed by immobilized Rhodotorula sp. AS2.2241 cells. J Mol Catal B Enzym. 2009;56:70–76. [Google Scholar]
- Wang H, Gurau G, Rogers RD. Ionic liquid processing of cellulose. Chem Soc Rev. 2012;41:1519–1537. doi: 10.1039/c2cs15311d. [DOI] [PubMed] [Google Scholar]
- Wang X-T, Yue D-M, Zong M-H, Lou W-Y. Use of ionic liquid to significantly improve asymmetric reduction of ethyl acetoacetate catalyzed by Acetobacter sp. CCTCC M209061 cells. Ind Eng Chem Res. 2013;52:12550–12558. [Google Scholar]
- Weuster-Botz D. Process intensification of whole-cell biocatalysis with ionic liquids. Chem Rec. 2007;7:334–340. doi: 10.1002/tcr.20130. [DOI] [PubMed] [Google Scholar]
- Wood N, Ferguson JL, Gunaratne HQN, Seddon KR, Goodacre R, Stephens GM. Screening ionic liquids for use in biotransformations with whole microbial cells. Green Chem. 2011;13:1843–1851. [Google Scholar]
- Wu D-X, Guan Y-X, Wang H-Q, Yao S-J. 11α-hydroxylation of 16α,17-epoxyprogesterone by Rhizopus nigricans in a biphasic ionic liquid aqueous system. Bioresour Technol. 2011;102:9368–9373. doi: 10.1016/j.biortech.2011.07.060. [DOI] [PubMed] [Google Scholar]
- Xiao Z-J, Du P-X, Lou W-Y, Wu H, Zong M-H. Using water-miscible ionic liquids to improve the biocatalytic anti-Prelog asymmetric reduction of prochiral ketones with whole cells of Acetobacter sp. CCTCC M209061. Chem Eng Sci. 2012;84:695–705. [Google Scholar]
- Xu P, Zheng G-W, Du P-X, Zong M-H, Lou W-Y. Whole-cell biocatalytic processes with ionic liquids. ACS Sustain Chem Eng. 2016;4:371–386. [Google Scholar]
- Xu Z, Wu Q, Yang M, Wang S, Wang Z, Xu X. Efficient asymmetric biosynthesis of (R)-(−)-epinephrine in hydrophilic ionic liquid-containing systems. RSC Adv. 2016;6:102292–102295. [Google Scholar]
- Xu J, Zhou S, Zhao Y, Xia J, Liu X, Xu J, et al. Asymmetric whole-cell bioreduction of sterically bulky 2-benzoylpyridine derivatives in aqueous hydrophilic ionic liquid media. Chem Eng J. 2017;316:919–927. [Google Scholar]
- Yang MY, Wu H, Lian Y, Li XF, Ren Y, Lai FR, et al. Using ionic liquids in whole-cell biocatalysis for the nucleoside acylation. Microb Cell Factories. 2014;13:143. doi: 10.1186/s12934-014-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo B, Shah JK, Zhu Y, Maginn EJ. Amphiphilic interactions of ionic liquids with lipid biomembranes: a molecular simulation study. Soft Matter. 2014;10:8641–8651. doi: 10.1039/c4sm01528b. [DOI] [PubMed] [Google Scholar]
- Yoo B, Jing B, Jones SE, Lamberti GA, Zhu Y, Shah JK, et al. Molecular mechanisms of ionic liquid cytotoxicity probed by an integrated experimental and computational approach. Sci Rep. 2016;6:19889. doi: 10.1038/srep19889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska ME, Bogel-Łukasik E, Bogel-Łukasik R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural-a promising biomass-derived building block. Chem Rev. 2011;111:397–417. doi: 10.1021/cr100171a. [DOI] [PubMed] [Google Scholar]
- Zampieri DS, de Paula BRS, Zampieri LA, Vale JA, Rodrigues JAR, Moran PJS. Enhancements of enantio and diastereoselectivities in reduction of (Z)-3-halo-4-phenyl-3-buten-2-one mediated by microorganisms in ionic liquid/water biphasic system. J Mol Catal B. 2013;85-86:61–64. [Google Scholar]
- Zhang ZC. Catalysis in ionic liquids. Adv Catal. 2006;49:153–237. [Google Scholar]
- Zhang F, Ni Y, Sun ZH, Zheng P, Lin WQ, Zhu P, et al. Asymmetric reduction of ethyl 4-chloro-3-oxobutanoate to ethyl (S)-4-chloro-3-hydroxybutanoate catalyzed by Aureobasidium pullulans in an aqueous/ionic liquid biphase system. Chin J Catal. 2008;29:577–582. [Google Scholar]
- Zhang BB, Cheng J, Lou WY, Wang P, Zong MH. Efficient anti-Prelog enantioselective reduction of acetyltrimethylsilane to (R)-1-trimethylsilylethanol by immobilized Candida parapsilosis CCTCC M203011 cells in ionic liquid-based biphasic systems. Microb Cell Factories. 2012;11:108. doi: 10.1186/1475-2859-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]