Abstract
Ionic liquids (ILs) are versatile solvents for a broad range of biotechnological applications. Recent experimental and simulation results highlight the potential benefits of dilute ILs in aqueous solution (aqueous ILs) in order to modify protein and DNA structures systematically. In contrast to a limited number of standard co-solutes like urea, ectoine, trimethylamine-N-oxide (TMAO), or guanidinium chloride, the large amount of possible cation and anion combinations in aqueous ILs can be used to develop tailor-made stabilizers or destabilizers for specific purposes. In this review article, we highlight common principles and differences between aqueous ILs and standard co-solutes with a specific focus on their underlying macromolecular stabilization or destabilization behavior. In combination with statistical thermodynamics theories, we present an efficient framework, which is used to classify structure modification effects consistently. The crucial importance of enthalpic and entropic contributions to the free energy change upon IL-assisted macromolecular unfolding in combination with a complex destabilization mechanism is described in detail. A special focus is also set on aqueous IL-DNA interactions, for which experimental and simulation outcomes are summarized and discussed in the context of previous findings.
Keywords: Ionic liquids, Proteins, DNA, Co-solutes, Kirkwood-Buff theory
Introduction
Over the last years, room temperature ionic liquids (ILs) and dilute ILs in aqueous solution (aqueous ILs) were often used as versatile solvents and co-solutes in biotechnological applications (Wilkes 2004; van Rantwijk and Sheldon 2007; Benedetto and Ballone 2015; Egorova et al. 2017; Smiatek 2017). The rising interest in aqueous ILs can be mainly rationalized in terms of a broad impact on several fields of research, ranging from food science and pharmaceutical applications to protein structure crystallography (Patel et al. 2014a). Besides long-time storage (Byrne and Angell 2008) and stimulated biocatalytic activities in neat ILs (van Rantwijk and Sheldon 2007; Zhao 2015b; Benedetto and Ballone 2015; Egorova et al. 2017), aqueous ILs have shown their benefits as promising solutions to systematically stabilize or destabilize protein and enzyme structures (Kumar and Venkatesu 2013; Patel et al. 2014a; Benedetto and Ballone 2015; Egorova et al. 2017; Constantinescu et al. 2007; Weingärtner et al. 2012; Zhao 2015b; Schröder 2017; Smiatek 2017; Saha and Mukherjee 2018). Often studied species are aqueous dialkylimidazolium based aprotic ILs (Constantinescu et al. 2007; Weingärtner et al. 2012; Zhao 2015b; Senske et al. 2016; Schröder 2017; Smiatek 2017), in addition to protic or aminoacid based ILs (Baker et al. 2004; Fujita et al. 2005; Fukumoto et al. 2005; Constantinescu et al. 2007; Mann et al. 2009; Fujita and Ohno 2010; Weingärtner et al. 2012; Patel et al. 2014b; Senske et al. 2016). Although the underlying mechanisms are not yet fully understood, one can assume that the molecular properties of the ILs and thus specific ion effects play a vital role (Fujita et al. 2006; Weingärtner et al. 2012; Senske et al. 2016; Zhao 2015b; Schröder 2017; Smiatek 2017). For instance, it was demonstrated that the denaturation strength of several ions can be ordered within a Hofmeister series (Constantinescu et al. 2007, 2010; Yang 2009; Zhao 2015b), such that the combination of distinct anions and cations reveals different destabilization effects (Zhao 2015b). In view of these findings, a more complex interaction mechanism and protein-binding behavior (Lesch et al. 2015b; Diddens et al. 2017) can be observed when compared with standard co-solutes like urea, guanidinium chloride, trimethylamine-N-oxide (TMAO) or others (Canchi and García 2013a; Rösgen et al. 2005; Harries and Rösgen 2008; Canchi and García 2013b; Collins 2004; Sukenik et al. 2013a; Narayanan Krishnamoorthy et al. 2014; Hahn et al. 2015; Micciulla et al. 2016; Schroer et al. 2016; Oprzeska-Zingrebe and Smiatek 2018).
The particular benefit of using aqueous ILs for structure modification in contrast to standard co-solutes becomes evident with regard to low concentrations that are needed. Significant effects were already reported for concentrations below 1 mol/L (Constantinescu et al. 2007; Smiatek 2017), although also a non-linear concentration-dependent behavior was observed (Constatinescu et al. 2010; Weingärtner et al. 2012; Senske et al. 2016). Due to these reasons, it is of utmost importance to achieve deeper insights into the underlying interaction mechanisms in terms of optimized ion combinations for specific purposes. Besides detailed experimental studies, useful tools are atomistic molecular dynamics (MD) simulations, which were already performed for several aqueous ILs (Micaêlo and Soares 2008; Klähn et al. 2011; Haberler et al. 2011; 2012a; Haberler and Steinhauser 2012; Lesch et al. 2015b; Baker et al. 2015; Chevrot et al. 2016; Tung and Pfaendtner 2016; Diddens et al. 2017). The pronounced interaction between cations and anions and the resulting effect on the unfolding behavior were discussed in more detail in recent publications (Dabirmanesh et al. 2011; Yan et al. 2012; Ajloo et al. 2013; Schröder 2017; Smiatek 2017; Figueiredo et al. 2013; Lesch et al. 2015b; Diddens et al. 2017). Noteworthy, most of these studies focused on hydration and binding properties of the proteins, whereas the stability of protein conformations in presence of aqueous ILs was only sparsely investigated (Lesch et al. 2015b; Chevrot et al. 2016; Tung and Pfaendtner 2016; Diddens et al. 2017).
In addition to stronger effects at lower concentrations when compared with standard co-solutes and the large number of possible ion combinations, one can ask if even further differences or similarities between standard co-solutes and aqueous ILs exist. In a previous review article, we mainly reported on the effects of aqueous ILs on protein structures (Smiatek 2017). Here, we want to focus on a general comparison between aqueous ILs and standard co-solutes, in addition to findings for aqueous IL-DNA interactions. Recent experimental research on the applicability of neat ILs for DNA enabled to establish efficient methods of DNA consecutive reuse and redissolution (Mukesh et al. 2013), electrophoresis (Qin and Li 2003), and isolation and purification (Wang et al. 2007; Fujita and Ohno 2012; Li et al. 2013a; Ghaemi and Absalan 2014; Clark et al. 2016). In the latter case, novel magnetic ILs turn out to be particularly interesting for the purpose of effective extraction and preservation of DNA (Clark et al. 2015a, b, 2016). For industrial purposes, ILs are being currently applied in DNA-based biosensors (Chen et al. 2013; Sun et al. 2013; Machado et al. 2014), or to assist a vast variety of methods in genetic manipulations, including genomic sequencing (Kulkarni and Mukherjee 2016), cloning (Li et al. 2013b), or gene delivery and transformation (Zhang et al. 2009; Soni et al. 2015). Moreover, replacement of standard aqueous solutions by ILs as solvents and catalysts for laboratory purposes enabled to overcome certain technological limitations associated with DNA nanotechnology (Nishimura et al. 2005; Dandia et al. 2013) due to enhanced DNA stability and solubility in ILs (Zhang et al. 2009; Sharma et al. 2015; Jumbri et al. 2016; Mishra et al. 2016). Among others, it has been shown that plasmid DNA demonstrates an increased resistance against enzymatic degradation in aqueous ILs based on choline dihydrogen phosphate when compared to saline buffer (Mazid et al. 2015).
Having in focus the latest achievements in IL-related DNA biotechnology, there appears a particular need to approach the molecular aspects of the interaction between these components and nucleic acids in detail. In this review article, we summarize recent findings on DNA and IL interactions, with main focus on computational results. In contrast to several studies on the features and behavior of proteins and enzymes in aqueous ILs (see Benedetto and Ballone 2015; Egorova et al. 2017; Smiatek 2017; Schröder 2017; Zhao 2015b for a detailed overview), DNA-related studies were only sparsely conducted, and most of the research carried out to date relies on experimental procedures (Cheng et al. 2007; Xie et al. 2008; Singh et al. 2012; Araújo et al. 2013; Sharma et al. 2015; Roy et al. 2016; Pandey et al. 2018) instead of simulation approaches. Despite limitations in the used force fields and models (Dommert et al. 2012; Zeman et al. 2017; Uhlig et al. 2018), the benefits of computer simulations rely in their ability to provide a detailed level of information, which is often not reachable even by advanced experimental procedures.
In order to shed more light on co-solute-assisted structure modification effects, we highlight the use of Kirkwood-Buff (KB) theory in this review, which was recently often applied in the context of experimental and computational studies concerning the properties of aqueous IL solutions (Reid et al. 2015, 2017; Lesch et al. 2015b; Kobayashi et al. 2017; Zeindlhofer et al. 2017, 2018; Smiatek 2017). In general words, the KB approach provides a solid statistical mechanical fundament in order to rationalize structure changes in terms of thermodynamic arguments without further approximations. Moreover, the basic relations of the theory are simple to use, make no assumptions on molecular geometries, and can be applied to standard co-solutes and aqueous ILs simultaneously without further modification. After a brief introduction into the main principles of the theory, we review previous experimental and simulation findings on the interactions between aqueous ILs, standard co-solutes, and macromolecules in ternary solutions. In agreement with KB theory, all literature results reveal the crucial importance of local ion accumulation effects in order to stabilize or destabilize macromolecular structures. Moreover, we also discuss the energetic contributions to the binding mechanism and further report on the properties of aqueous IL-DNA interactions.
The article is organized as follows. In the next section, we highlight main concepts of KB theory in order to rationalize co-solute-assisted structure stabilization and destabilization effects. Hereafter, we review recent findings on enthalpic and entropic structure modification mechanisms, and we discuss typical interaction mechanisms observed for aqueous aprotic ILs in contrast to urea as a standard co-solute. In Section “Aqueous ILs and their influence on DNA structures,” we report on recent results concerning aqueous IL-DNA effects, for which we mostly focus on the outcomes of computational studies. We summarize the main results in the last section.
Thermodynamic aspects of co-solute-assisted structure modification
In this section, we will discuss basic thermodynamic concepts in order to rationalize co-solute-induced changes in macromolecular structures. For this reason, we will briefly introduce the Kirkwood-Buff (KB) theory (Kirkwood and Buff 1951; Ben-Naim 2013; Newman 1994; Pierce et al. 2008), which can be regarded as a meaningful concept in order to study thermodynamic properties of solutions and higher component liquid mixtures. Although already developed more than 60 years ago (Kirkwood and Buff 1951), the KB theory has recently attracted a lot of interest in terms of the analysis of experimental and computer simulation data, distinct co-solute-induced effects, and the study of multicomponent solutions (Smith 1999, 2004, 2006; Shimizu and Smith 2004; Shimizu 2004; Schurr et al. 2005; Rösgen et al. 2004, 2005, 2007; Smith et al. 2013; Smiatek 2014; Baynes and Trout 2003; Pierce et al. 2008; Krishnamoorthy et al. 2016, 2018; Schroer et al. 2016; Micciulla et al. 2016; Reid et al. 2015, 2017; Kobayashi et al. 2017; Martinez and Shimizu 2017; Shimizu and Matubayasi 2018). (We refer the reader to Kirkwood and Buff (1951), Ben-Naim (2013), Pierce et al. (2008), Smiatek (2017), and Newman (1994) for a more detailed introduction into the broad applicability of the theory.) In more detail, the use of the KB theory allows us to describe co-solute-induced structure changes by basic thermodynamic relations, while at the same time avoiding the pitfalls of phenomenological explanations. Even more beneficial, the KB theory makes no assumption on the molecular shape or size, which highlights the full applicability of the approach for all molecules, regardless if aqueous ILs or standard co-solutes.
As we already mentioned in the introduction, co-solute-assisted changes in macromolecular structures have a broad impact on several disciplines. Moreover, several evolutionary survival strategies strongly rely on the presence of co-solutes (Yancey 2005), such that the influence of harsh environmental conditions on cell metabolism processes is compensated with the help of several low weight molecular compounds (Hahn et al. 2016). Prominent attempts to explain structure modification effects are represented by preferential binding/exclusion approaches (Tanford 1964; Courtenay et al. 2000; Timasheff 2002; Shimizu and Smith 2004; Shimizu 2004), co-solute-solute linkage relations (Wyman 1964; Tanford 1969), transfer free energy models (Auton and Bolen 2004), or solvent-mediated mechanisms (Collins 2004; Marcus 2009). Whereas most of these attempts rely on reasonable assumptions, a rigorous treatment in terms of molecular statistical mechanics theories is often missing (Shimizu 2004). As already mentioned, the KB theory provides a solid theoretical framework in order to underpin recent experimental and computational findings on co-solute-induced effects.
As a starting point for our theoretical considerations, we summarize basic principles in order to rationalize the framework. Most importantly, the KB theory without extensions is not applicable for chemical reactions. Thus, any chemical reaction between co-solutes and the main solute (either polymers, polyelectrolytes, proteins, enzymes, RNA, or DNA) in terms of covalent bond cleavage has to be ignored, which means, at least in our definition, that the co-solutes do not modify the chemical structure of the solute in agreement with experimental findings (Canchi and García 2013a; Zhao 2015b). The corresponding implications lead to the definition of “compatible solutes” (Yancey 2005; Zhao 2005; Smiatek et al. 2012; Hahn et al. 2015, 2016), which means that these compounds do not affect cell metabolism processes.
Furthermore, we consider low or moderate co-solute concentrations, so that the vast amount of the solution is represented by solvent molecules. Here, we do not distinguish between standard co-solutes and aqueous ILs, such that the definition as co-solute is generally applicable. In addition, the amount of macromolecules as main solute is also low, which means that any interaction between the macromolecules can be ignored, and that a sufficient fraction of the solution can be regarded as bulk solution. Despite this requirement, we still consider “macromolecular ensembles” in our approach, where a fraction of macromolecules is in folded state (native state), whereas others remain in different unfolded or denatured states (Smiatek 2017). With regard to this point, we explicitly avoid considerations in terms of kinetically trapped configurations (Bryngelson et al. 1995), and focus our attention on long-time stable thermodynamic states. Thus, we assume that the vast number of possible microstates associated with a given macromolecule can be attributed either to a native or a denatured state, which are separated by a large free energy barrier. Usually, the native state is energetically more favorable than the denatured state, such that the associated free energy landscape can be understood as having only two broad energetic minima. In consequence, the macromolecular states are classified as native (N) or denatured (D), which can be correlated with a simple two-step folding behavior. Thus, the simplified “unfolding reaction” reads

with the chemical equilibrium constant (Atkins and de Paula 2010)
| 1 |
where denotes the chemical activity of the considered state (or species) with the stoichiometric coefficient , which is set to for the denatured state, and νN = − 1 for the native state. In more detail, the chemical activity reads aj = γjxj, with the chemical activity coefficient , and the mole fraction xj = ρj/ρ, where denotes the bulk number density of species or state j in combination with the total bulk number density of the solution, where denotes the number of species or molecules within system volume V. With regard to standard nomenclature, we denote the co-solute (either IL ions or standard co-solute) with index “3,” solvent molecules with index “1,” and the main solute (macromolecule) with index “2.” In consequence, for vanishing macromolecular densities , we can assume ideal conditions with , which allows us to define . Further, the chemical potential is defined by
| 2 |
in which denotes the standard chemical potential, R the universal gas constant, and T the absolute temperature. In accordance with chemical equilibrium () (Atkins and de Paula 2010), it thus follows
| 3 |
which is equivalent to
| 4 |
for infinitely dilute macromolecular solutions, as denoted by the superscript “0.” The relation above highlights the difference in the chemical potentials as the main reason for the stable fraction of native and denatured macromolecules. In order to include co-solute-induced effects, we assume that a finite number density of co-solute species in the solution imposes a modified macromolecular chemical potential
| 5 |
with the associated value of the co-solute, (Rösgen et al. 2004, 2007; Canchi and García 2013a) and
| 6 |
where denotes the co-solute-affected number of structure-modified macromolecules (Smiatek 2017). Furthermore, one can define
| 7 |
in order to yield an explicit expression for the value in Eq. 5. As it was originally discussed in Hall (1971) and recently shown in Smiatek (2017), one can write
| 8 |
which is equivalent to
| 9 |
where denotes the difference in the preferential binding coefficients between the denatured and the native macromolecular state with regard to the number of co-solute molecules and the number of water molecules around the macromolecule. As shown in previous articles (Smiatek 2017; Hall 1971), the corresponding changes mostly occur within a local region around the macromolecule, which can be regarded as the main region of interest in terms of local/bulk partitioning model and preferential binding model (Oprzeska-Zingrebe and Smiatek 2018). In fact, the associated change in the preferential binding coefficients can be directly related to a shift of the chemical equilibrium either to the native or the denatured state, as we will review in more detail in the remainder of this article.
With regard to this point, the Kirkwood-Buff (KB) theory of solutions provides a natural framework in order to describe the influence of co-solutes on the chemical equilibrium in terms of statistical thermodynamics concepts. A central expression of the theory is the Kirkwood-Buff integral (KBI)
| 10 |
which can be interpreted as excess volume of species around component under consideration of the radial distribution functions . It can be shown (Kirkwood and Buff 1951; Ben-Naim 2013) that the KBIs can be also written in terms of molecular fluctuations
| 11 |
with mean particle numbers of species α,β, and with the Kronecker delta function , which is unity for α = β and zero otherwise. In order to establish a connection with the grand canonical partition function , one can use the relation (Ben-Naim 2013)
| 12 |
where denotes constant chemical potentials for all species except , which further yields with , and in combination with Eq. 11 the important relation
| 13 |
which can be applied to evaluate the remaining term in Eq. 7. As it was in more detail outlined in Refs. Ben-Naim (2013), Smith (2006), and Smiatek (2017), the corresponding derivative of the chemical potential can be written as
| 14 |
for typical open ternary solutions with constant T and pressure p, whereas explicit relations for distinct other ensembles were derived in Smith (2006). In consequence, the last relation can be inserted in Eq. 7, which then reads
| 15 |
with
| 16 |
which is always larger than zero for ordinary solutions without spinodal decomposition processes due to stability requirements (Ben-Naim 2013). Furthermore, the corresponding value of a33 often depends on the density of the co-solute species (Rösgen et al. 2004), such that non-linear concentration-dependent effects at higher concentrations are often evident.
A decrease or increase of the macromolecular chemical potential associated with different states (7) is thus solely related to the differences in the preferential binding coefficient, where highlights a preferential binding to the unfolded structure, whereas denotes a preferential exclusion of co-solutes around the denatured state. Hence, stabilizers reveal negative values for the differences of the preferential binding coefficients, while destabilizers have a positive Δν23. This can be equivalently expressed in terms of Eqs. 5 and 6, which also defines the m-value as m = a33Δν23/ρ3. Hence, the m-value crucially depends on the derivative of the chemical potential and the difference in the preferential binding coefficients. In consequence, the corresponding solvation behavior of the co-solute in the solution significantly influences the stabilization or destabilization strength (Rösgen et al. 2004; Krishnamoorthy et al. 2018).
In order to be fully consistent with the KB theory, even the preferential binding coefficient (9) can be written with regard to KB integrals
| 17 |
which highlights the important connection with excess volumes (Smiatek 2017). In summary, all above-discussed relations are fully applicable for standard co-solutes and aqueous ILs. However, it has to be noted that an indistinguishable ion approach is used in our formalism, such that cations and anions are considered as one species of type “3” in accordance with the requirement of electroneutrality (Kusalik and Patey 1987, 1988; Smith 1999). It was recently shown that this approximation is also valid for ILs whose individual species differ significantly in size (Reid et al. 2015, 2017; Kobayashi et al. 2017). Hence, assuming that IL cations and anions contribute equally, one has to define for charged species according to Weerasinghe and Smith (2003), Gee et al. (2011), and Fyta and Netz (2012)
| 18 |
with , where and denote the number of corresponding ion species in the dissociated state, and for cations and anions due to reasons of symmetry (Ben-Naim 2013). The corresponding KB integral for ion-solvent interactions is defined by
| 19 |
with . With regard to charged species, the underlying formalism for solute-ion interactions also changes slightly. For instance, a crucial modification has to be introduced if the macromolecule is charged (Eisenberg 1976). Thus, a Donnan equilibrium condition contributes to the individual preferential binding coefficients (17), which then read
| 20 |
with the absolute charge Z of the macromolecule (Smith 2004; Pierce et al. 2008), and where the superscripts denote the corresponding macromolecular state. However, with regard to the difference in the preferential binding coefficients (17), it follows
| 21 |
which highlights the fact that the absolute charge of the macromolecule for chemical equilibrium shifts can be ignored.
Enthalpic and entropic origins of structure modification
As we have outlined before, the binding behavior of co-solutes significantly influences the macromolecular stabilization or destabilization behavior. If we ignore any energetic contribution from the solvent, one may then ask what is the nature of the binding mechanism between the co-solute and the macromolecule. In fact, the stabilization or destabilization of structures is associated with a free energy change or a change in the chemical potential, respectively, which rely on changes of enthalpic or entropic contributions. Both contributions can vary in their strength, and it is often not obvious which one dominates the associated chemical potential difference of the macromolecular states.
In order to classify different co-solutes in terms of their interaction mechanism, a previous publication (Sukenik et al. 2013a) already reported individual enthalpic and entropic contributions for different co-solutes concerning distinct macromolecular processes. In general, the free energy change of a macromolecular process in presence of co-solutes reads
| 22 |
where is the free energy difference between distinct states in absence of co-solutes, and in presence of co-solutes, respectively. Hence, the corresponding enthalpic and entropic contributions are defined by
| 23 |
with enthalpy and entropy difference , respectively. As meaningful examples, the authors studied the impact of neutral and charged co-solutes on the complexation free energy difference of -cyclodextrin (CD) and adamantane carboxylate (AD) in addition to the unfolding free energy difference of a model peptide (Sukenik et al. 2013a). The corresponding complexation free energy differences for CD/AD complexes in presence of distinct co-solutes are shown at the left side of Fig. 1. If the free energy change is positive with , the corresponding co-solutes can be considered as destabilizers, which are located in the pink shaded regions (regions III and IV), and vice versa for stabilizers (ΔΔG < 0) in regions I and II (blue shaded regions).
Fig. 1.
Entropy versus enthalpy plots for changes in macromolecular processes upon co-solute addition in accordance with Eq. 23. a CD/AD complexation with enthalpy and entropy change. The inset shows the different corresponding regimes on the plot, and the blue and red colors signify stabilizing and destabilizing regions, respectively. More details can be found in the main text. b Folding of a model peptide. The inset depicts the model enthalpic and entropic contributions to charged and non-charged terms of electrolytes on peptide folding. Reproduced from Sukenik et al. (2013a) with permission from the PCCP owner societies
In more detail, region I includes all enthalpic stabilizers (ΔΔG < 0 and ), whereas region II is associated with an entropic stabilization mechanism with and . Although weakly populated in Fig. 1a, region III corresponds to an enthalpic destabilization mechanism (ΔΔG > 0 and ), and for reasons of completeness, all entropic destabilizers are located in region IV with and TΔΔS < 0.
With regard to the data shown in Fig. 1, it can be thus concluded that most simple salts at low concentration reveal an entropically driven stabilization mechanism concerning the CD/AC complexation behavior. An entropically unfavorable release of counterions upon complexation in presence of excess salt can be regarded as main reason for this observation. Strong ion pair formers like LiCl and KF instead (Collins 2004) or salts at higher concentrations (Sukenik et al. 2013a) show an enthalpic destabilization mechanism. Hence, the number of free and unpaired ions, as well as the concentration of the co-solutes imposes a pivotal influence on the complexation behavior and the underlying stabilization mechanism. These conclusions are also supported by betaine as a zwitterionic net neutral molecular enthalpic destabilizer, which reveals an enthalpic destabilization mechanism. In agreement with comparable results for urea (Micciulla et al. 2016) and TMAO (Schroer et al. 2016), it can be assumed that strong dispersion interactions can be observed for betaine with the individual CD and AD molecules, such that a larger accessible surface area with regard to unbound CD and AC molecules is favored.
In contrast to macromolecular association processes, typical results for a model protein unfolding free energy change in presence of distinct co-solutes are shown on the right side of Fig. 1. As can be seen, most co-solutes are stabilizers, which either show enthalpic (region I) or entropic stabilization mechanisms (region II). In fact, large co-solutes like PEG at low concentration, which are often considered as macromolecular crowders are located in region II, whereas smaller co-solutes like polyols or PEG at higher concentrations are located in region I, revealing an enthalpic stabilization of macromolecular folded states (Sukenik et al. 2013b). In more detail, enthalpic stabilization has its origin in favorable interaction energies between the macromolecule and the co-solute, while entropic stabilization purely relies on excluded-volume effects. Noteworthy, co-solutes located in region II are usually large molecules, which significantly reduce the available free volume and thus modify the entropy for macromolecular unfolding. In order to make this point more clear, these co-solutes are usually also called “macromolecular crowders.” A recent review article discusses the principles of entropic stabilization in more detail (Sapir and Harries 2017). In contrast, most salts at all concentrations are located in region IV, highlighting an entropic destabilization mechanism, but it has to be noted that the corresponding differences to enthalpic stabilization (region IV) are rather marginal due to . The entropic destabilization mechanism thus can be attributed to an electrostatic binding of the ions to the charged macromolecular surface and an ion-specific accumulation around the uncharged regions. Both contributions compensate each other to a large extent, such that the remaining entropic contribution associated with the co-solute accumulation behavior dominates. Furthermore, it was speculated that also a modified water order, as it is known in presence of some salts (Collins 2004), contributes to this effect (Sukenik et al. 2013a). The associated implications in terms of Hofmeister and ion-specific effects were discussed in a series of articles (for an overview, we refer the reader to Marcus 2009; Kunz 2010; Lo Nostro and Ninham 2012; Okur et al. 2017), and the related small enthalpy/entropy differences can be regarded as the underlying reason for our still limited understanding.
Interestingly, one of the strongest protein destabilizers, guanidinum hydrochloride (GnHCl), shows a pronounced enthalpic destabilization effect (region III). Due to recent experimental and simulation results (Heyda et al. 2017; Xia et al. 2012; Zheng et al. 2016; Jha and Marqusee 2014), it can be expected that the large size of the guanidinium cation promotes both favorable electrostatic and dispersion interactions with the protein, such that the observed strong destabilization effect becomes reasonable. Previous findings by others and us (Smiatek 2014; Micciulla et al. 2016; Schroer et al. 2016; Horinek and Netz 2011; Rodríguez-Ropero and van der Vegt 2015; Oprzeska-Zingrebe and Smiatek 2018) highlighted comparable results for urea, which is also known as a strong protein denaturant. Furthermore, the inset on the right side of Fig. 1 clearly shows that the presence of electrostatic interactions between charged residues and charged co-solutes is crucial in order to establish enthalpic destabilization effects. Related measurements reveal that a neglect of electrostatic interactions changes the underlying behavior of the co-solutes, such that these compounds show an enthalpic stabilization effect instead. The compensation of both contributions results in the aforementioned entropic destabilization mechanism.
Based on these findings, we can conclude that the most potent and polar protein denaturants are located in region III as enthalpic destabilizers. In consequence, the pronounced contributions from both electrostatic and dispersion interactions favor a preferential binding of these molecules to macromolecular surfaces (Smiatek 2014; Micciulla et al. 2016; Horinek and Netz 2011; Rodríguez-Ropero and van der Vegt 2015; Oprzeska-Zingrebe and Smiatek 2018). As we have already discussed in the previous section, most preferential binders favor an unfolding of the macromolecule in order to increase the local co-solute concentration around the macromolecule with regard to a larger solvent-accessible surface area. In conjunction with the relatively large and charged guanidinium ion, and the corresponding similarities with aqueous IL cations, we conclude that most common aprotic aqueous IL cations like dialkylimidazolium are also located in region III in agreement with previous simulation outcomes (Lesch et al. 2015b; Diddens et al. 2017). Despite this simple argument, it was already found that the properties of the solute as well as the co-solute concentration significantly influence the observed binding behavior (Rösgen et al. 2004, 2007; Sukenik et al. 2013a; Smiatek 2014; Micciulla et al. 2016, 2018; Kanduč et al. 2017).
Hence, it can be assumed that aqueous ILs as complex and molecular salts also reveal a plethora of strong specific binding mechanisms. A previous publication by us has focused on this topic for imidazolium-based ILs at low concentration in water and their influence on the unfolding behavior of a simple hairpin peptide (Lesch et al. 2015b; Diddens et al. 2017). In more detail, we studied 1-ethyl-3-methylimidazolium (EMIM) cations in combination with tetrafluoroborate (BF4), acetate (ACE), and chloride ions (CL) as anions. Our findings (Lesch et al. 2015b; Diddens et al. 2017) reveal a decrease in the unfolding strength with the order ACE BF4 CL. Closely related with the previous discussion, we further studied the enthalpic contributions to the binding energy between the ions and the hairpin peptide. The results are shown in Fig. 2.
Fig. 2.
Top: Potential energies between a hairpin peptide in its denatured state and the individual ion species for EMIM/ACE, EMIM/BF4 and EMIM/CL with different anions acetate (ACE), chloride (CL), and tetrafluoroborate (BF4). The interaction energies are separated into Lennard-Jones (LJ), electrostatic (Coulomb), and total (total) energies as given by the sum of Lennard-Jones and Coulomb energies between the peptide and EMIM (EMIM) or the corresponding anion (anion) species, respectively. The red bar reveals the total potential energy (total (IL)) between the peptide and both ion species. Reproduced from Diddens et al. (2017) with permission from the PCCP owner societies
As can be seen, most imidazolium-based aqueous ILs show a strong Coulomb and van der Waals interaction energy with the moderately charged hairpin peptide (Z = − 4e). The related total potential interaction energies strongly differ between the individual ILs, although all aqueous ILs include identical EMIM cations. As can be seen, the contributions in terms of short-range Lennard-Jones (LJ), meaning dispersion, and electrostatic interactions concerning EMIM cations and the peptide are roughly comparable. In consequence, the variation in total potential binding energies among the aqueous ILs is mostly imposed by distinct anions. In more detail, larger anions reveal more negative values in terms of Coulomb interactions, whereas the LJ contributions are negligible. Based on these findings and further analysis (Diddens et al. 2017), which will be discussed in the following section, we can assume that dialkylimidazolium-based aqueous ILs can be considered as enthalpic destabilizers in good agreement with previous experimental results (Constantinescu et al. 2007; Weingärtner et al. 2012; Senske et al. 2016).
Complex destabilization mechanism for aqueous ILs
As it was discussed in the previous section, the destabilization mechanisms of the considered aqueous ILs are dominated by anion-specific effects, which influence the overall interaction energy in combination with LJ and electrostatic interactions associated with the cations. As it was pointed out in Fig. 2 for dialkylimidazolium-based ILs, smaller anions show minor or nearly negligible energetic contributions to the free energy change, such that the enthalpic contribution to the free energy change is solely dominated by cation-protein interactions. Based on these outcomes and further analysis, we are able to propose a complex destabilization mechanism, as it was in more detail discussed in Lesch et al. (2015b) and Diddens et al. (2017).
The basic outcomes of the KB theory underpin that a preferential binding of co-solutes induces a destabilization of peptide structures. Under this assumption, we can expect that both ion species involved as destabilizers accumulate in the local volume around the peptide and specifically around the denatured state. As an initial step, the effective accumulation of bulky EMIM ions due to strong LJ and Coulomb interactions induces the formation of positively charged cation layers around the native state of the peptide. This assumption is evidenced by the pronounced value for LJ and Coulomb interactions shown in Fig. 2. In order to compensate the effective net charge, anions from the solution are attracted due to long-range electrostatic interactions in combination with hydrophobic effects (Haberler et al. 2012b; Tung and Pfaendtner 2016), which form the second sheath around the macromolecules as it was also observed for bulk ILs (Lesch et al. 2015a, 2016). However, the accumulated ions need to form mixed shells in order to minimize unfavorable electrostatic interactions with like-wise charged species, and further need to maximize electrostatic interactions with the partially charged peptide.
This formation of mixed cation-anion shells around the native state, as shown on the left side of Fig. 3, can be regarded as the main driving force for the unfolding mechanism. In more detail, larger anions occupy more space and thus significantly perturb the local organization of the EMIM cations, which results in a volume increase of the ion cloud around the peptide. As a consequence, the protein unfolds in order to provide a higher amount of accessible surface area promoting short-distance contacts for both ion species. As one would expect, this effect is significantly more pronounced for larger anions like BF4 or acetate anions, whereas smaller anions like chloride can even fill gaps in the EMIM layer, such that only a weak unfolding tendency can be observed (Diddens et al. 2017). In agreement with the KB theory and as can be seen on the right side in Fig. 3, the unfolded state thus shows a higher number of mixed ion species in the local volume around the denatured peptide when compared with the native state.
Fig. 3.
Spatial distribution for EMIM (red clouds) and BF anions with a concentration of c = 0.57 mol/L around the native (left side) and the denatured state of a β −hairpin peptide (right side). Reproduced from Diddens et al. (2017) with permission from the PCCP owner societies
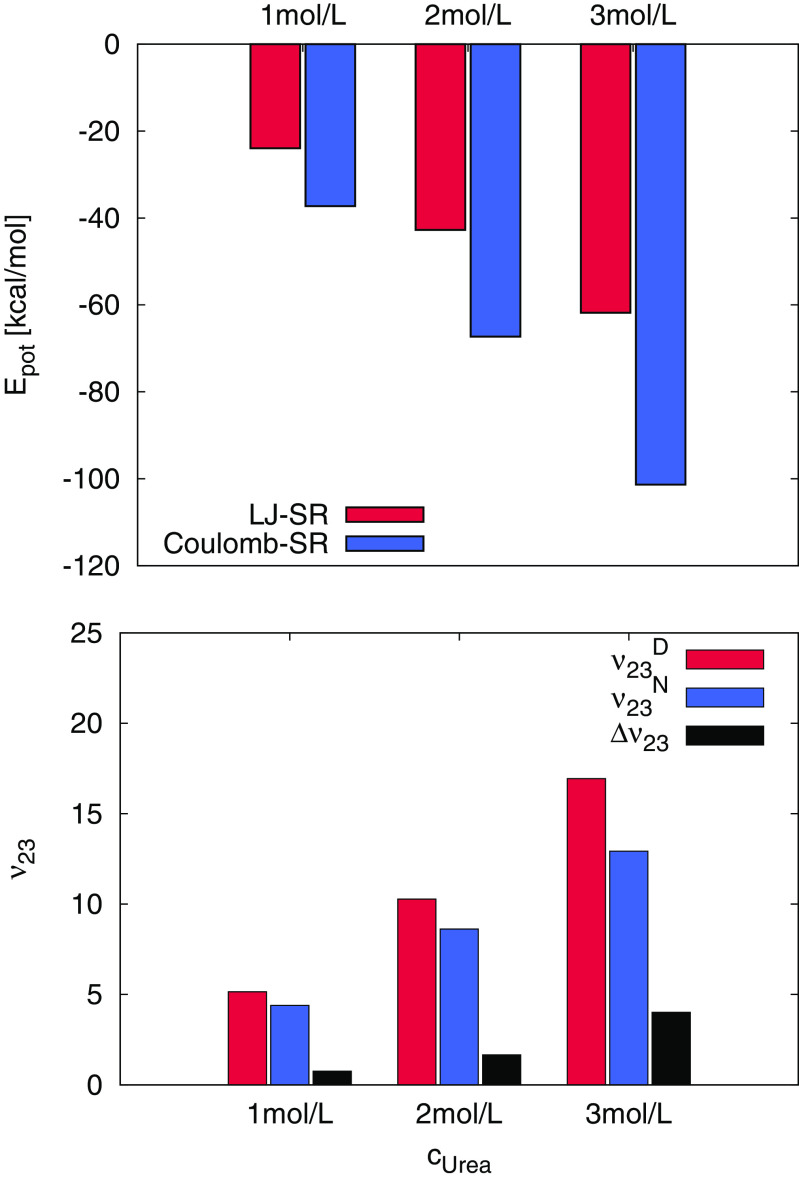
Hence, the unfolding mechanism induced by aqueous ILs follows a more complex binding behavior in contrast to standard co-solutes (Rodríguez-Ropero and van der Vegt 2015; Micciulla et al. 2016; Oprzeska-Zingrebe and Smiatek 2018). As an example, urea molecules interact directly with protein or oligonucleotide residues in terms of LJ or electrostatic interactions, while at the same time replacing water molecules in the first solvation shell (Rodríguez-Ropero and van der Vegt 2015; Micciulla et al. 2016; Oprzeska-Zingrebe and Smiatek 2018). The corresponding nearly linear increase of LJ and electrostatic interactions between urea molecules and a short DNA hairpin structure for increasing urea concentration is shown in Fig. 4. As can be seen, the unfolded state is energetically more favorable, such that urea can be regarded as an enthalpic destabilizer. Moreover, we observe a linearly increasing value of Δν23, which implies that the denaturation strength increases for higher urea concentrations (Oprzeska-Zingrebe and Smiatek 2018). In contrast to uncharged proteins and polymers (Horinek and Netz 2011; Rodríguez-Ropero and van der Vegt 2015; Micciulla et al. 2016), the findings for urea-DNA hairpin binding energies also indicate a significantly higher contribution of dipolar electrostatic interactions in comparison with LJ binding energies (Oprzeska-Zingrebe and Smiatek 2018). Furthermore, and in agreement with the KB theory, it can be seen in the bottom of Fig. 4 that the values of the preferential binding coefficients imply a higher affinity for urea towards the denatured state. In addition, even the differences of the preferential binding coefficients show a linear increase with urea concentration, which demonstrates a less complex destabilization mechanism when compared with aqueous ILs.
Fig. 4.
Top: Lennard-Jones (LJ-SR) and electrostatic short-range (Coulomb-SR) energies between urea and DNA for native and unfolded 1KR8 DNA oligonucleotide with regard to different urea concentrations curea. Bottom: Preferential binding coefficients to the denatured state (red bars) and the native state (blue bars) of a DNA hairpin peptide for different urea concentrations. The difference in the preferential binding coefficient is shown as black bar. Figure adapted from results published in Oprzeska-Zingrebe and Smiatek (2018)
Aqueous ILs and their influence on DNA structures
In comparison with simple proteins and peptides, much less is known on the properties of DNA structures in aqueous IL and standard co-solute solution. This can be mostly attributed to the limited number of computational studies in the field. Jumbri et al. employed MD simulations combined with fluorescence spectroscopy and circular dichroism to study the influence of neat and aqueous ILs on the structure and stability of DNA (Jumbri et al. 2014). The authors concluded, that the native DNA B-conformation is retained in aqueous dialkyimidazolium bromide ILs mainly due to the formation of hydration shells around DNA phosphate groups. Since stronger hydration shells seem to minimize the binding ability of IL cations to negatively charged DNA phosphate groups, the studied aqueous ILs at low water concentrations turn out to favor native DNA conformation. Moreover, stable duplex conformations were observed even at 373.15 K, which is noticeably higher than average B-DNA melting temperatures in physiological or laboratory conditions (Khandelwal and Bhyravabhotla 2010). Additionally, the computed interaction energies revealed that the dominant DNA-stabilizing interaction can be attributed to electrostatic attraction between DNA phosphate groups and IL cations. These results are consistent with previous research by He et al., who investigated the interaction between 1-dodecyl-3-methylimidazolium bromide IL and DNA in dilute NaBr solution (He et al. 2013) with the application of various experimental techniques and MD simulations. The authors approximated the DNA/IL system with a coarse-grained model, where DNA is described as an anionic polyelectrolyte and the IL species as cationic surfactants. Further isothermal titration calorimetry measurements complemented by MD simulations revealed that the accumulation of IL ions on DNA chains is driven both by enthaplic and entropic contributions. Besides electrostatic attraction between charged DNA groups and organic ions in the mixture, also hydrophobic interactions among alkyl chains contribute to the aggregation of IL species at the DNA surface. Another interesting observation was that distinct dialkylimidazolium-based aqueous ILs (Ding et al. 2010) induce conformational changes in DNA structures (He et al. 2013), in agreement with previous results for protein conformations (Lesch et al. 2015b; Diddens et al. 2017). A further contribution to the understanding of the mechanism of neat IL binding to DNA comes from MD simulations by Cardoso and Micaelo (2011). The authors studied several imidazolium-, pyridinium-, pyrrolidinium-, oxazolium-, and ammonium-based ILs and their influence on Dickerson-Drew B-DNA. The corresponding results revealed the importance of hydrogen bonding between IL anions and adenine (A) - thymine (T) and cytosine (C) -guanine (G) nucleobase pairs, respectively. Interestingly, all IL cations showed electrostatic attraction to the negatively charged DNA backbone in addition to hydrogen bonding and nonbonded edge-to-face interactions with nucleobases (Cardoso and Micaelo 2011).
A less toxic alternative to imidazolium-based ILs, morpholinium IL N-ethyl-N-methyl-morpholinium bromide ([Mor1,2][Br]), was also investigated via spectroscopic and molecular docking methods by Pabbathi and Samanta (2015), which showed a retention of a stable B-DNA form in the aforementioned IL. In more detail, the morpholinium cation binds to the DNA minor grooves in agreement with the imidazolium cation, but with a slightly lower binding affinity. This effect has been attributed to the non-planar form of the [Mor1,2]+ cation, yielding a reduced number of interactions between [Mor1,2]+ and DNA (Pabbathi and Samanta 2015).
An interesting view on DNA stabilization mechanisms in various aqueous ILs is also presented in the work of Chandran et al. (2012). In these and further publications mentioned below, the authors argue that the presence of ILs in water inhibits catalytic reactions, such that most DNA structures are stabilized in aqueous ILs. Using MD simulations and spectroscopic experiments, the authors showed that IL ions, entering DNA minor grooves, contributed significantly to macromolecular stability via polar and hydrophobic interactions. A water cage around DNA, especially in the minor groove, was thus being disrupted by IL ions, which evokes a partial dehydration. In consequence, hydrolytic reactions leading to DNA denaturation were obstructed, which contributed to the long-term DNA stability in aqueous ILs (Chandran et al. 2012).
Another evidence on the favorable environment provided by aqueous ILs comes from the work by Tateishi-Karimata and Sugimoto (2012, 2014) and Nakano et al. (2014). Due to a low water activity in aqueous protic ILs, securing a mild hydrogen bonding environment, the rate of hydrolytic reactions in the system was effectively slowed down (Tateishi-Karimata and Sugimoto 2012; Nakano et al. 2014). This mechanism contributed significantly to the enhanced stability of double-helical DNA in aqueous ILs based on choline in agreement with previous experimental findings (Vijayaraghavan et al. 2010). A further insight into the DNA sequence binding specificity of choline-based ILs is presented in another work by Tateishi-Karimata et al. (2012, 2014) and Nakano et al. (2014). Here, the thermodynamic stability of DNA base pairs in a representative IL, choline dihydrogen phosphate, was investigated. In a combined experimental and MD simulation approach it was shown, that choline ions bind strongly via hydrogen bonds to duplex DNA minor grooves including A-T regions, but only weakly to G-C rich regions. However, in single-stranded DNA, the binding preference was reversed and the interaction of choline ions with G-C rich strands was favored (Tateishi-Karimata and Sugimoto 2012). Although under physiological conditions G-C rich DNA is more stable than A-T rich DNA, choline cations effectively destabilize G-C duplexes due to a preferential binding to guanine (Tateishi-Karimata and Sugimoto 2012). Concerning A-T rich regions, choline ions exhibit significantly stronger affinity for the minor groove than for other groove areas (Nakano et al. 2014; Saha et al. 2016; Haque et al. 2017). By binding preferentially to atoms involved in G-C bonding, choline ions also inhibit the formation of hydrogen bonds between G-C base pairs (Nakano et al. 2014).
In fact, the minor groove has been proved to be the main target of numerous ILs regardless of their cationic moiety, with the prevalence of electrostatic interactions and only minor contribution from hydrophobic interactions (Haque et al. 2017). The problem of possible cross-interactions between DNA, ILs, and water has been approached in the computational study by Saha et al. (2016). Using all-atom MD simulations followed by mean residence time calculations, the authors explored not only thermodynamic behavior, but also the dynamics of interactions in aqueous ILs. It was found, that the slower dynamics of water near G-C base pairs also slowed down the dynamics of IL cations, leading to an entropically unfavorable state compared to A-T-rich regions (Saha et al. 2016). The authors of the study argue that slower relaxation times can be brought into agreement with a more rigid hydration shell, such that reorganization processes or thermal escape of water molecules are hindered. The associated influence of restricted water motion on the free solvation entropy was also discussed in Kumar et al. (2006, 2009) and Smiatek (2014). Thus, the interaction of IL ions near G-C base pairs is not only enthalpically disfavored due to their lower affinity to IL cations, but is also missing an entropic stabilization in consequence of slower dynamics. On the contrary, binding of IL cations to A-T base pairs is driven by both enthalpic and entropic contributions, which results in stronger binding free energies. The authors postulate that in aqueous ILs, both enthalpy and entropy favor binding to A-T and disfavor binding to C-G base pairs (Saha et al. 2016).
In 2012, Fujita et al. observed experimentally that a G-quadruplex structure is stabilized by the combination of protic cholinium cations and dihydrogen phosphate anions (Fujita and Ohno 2012). Further spectroscopic and computational studies by Satpathi et al. revealed that particular ILs can even stabilize and form G-quadruplex structures in the absence of typical monovalent quadruplex-forming alkali metal ions (Satpathi et al. 2016). Thermodynamic analyses combined with MD simulations by Tateishi-Karimata et al. showed that in certain aqueous protic ILs, choline dihydrogen phosphate, DNA i-motifs are more stable than G-quadruplexes (Tateishi-Karimata et al. 2015). It was shown that choline tends to bind to the loop regions of the i-motifs, thus contributing to the enhanced stability of their structure. Although i-motifs tend to dissociate upon deprotonation of cytosine components (Smiatek and Heuer 2014), in choline dihydrogen phosphate, i-motifs retained their stability even at physiological pH values (Tateishi-Karimata et al. 2015). It was suggested that favorable enthalpic interactions contribute to the increased i-motif stability (Tateishi-Karimata et al. 2015).
With reference to the presented results, the following conclusion can be drawn. Many ionic liquids contribute to the increased DNA stability without changing its structure (Cardoso and Micaelo 2011; Chandran et al. 2012; Jumbri et al. 2014, 2016; Pabbathi and Samanta 2015; Zhao 2015a; Mishra et al. 2016), whereas some other tend to destabilize DNA (Mukesh et al. 2013). In particular cases, even a small difference in ionic composition of ILs can change the characteristics of its interaction with DNA drastically. An exemplary choline-based IL, choline-indole-3-butyrate, led to DNA structural degradation, whereas choline-indole-3-acetate did not (Mukesh et al. 2013). Although the interaction between DNA and ILs occurs predominantly via electrostatic attraction of organic cations with phosphate backbones (Cheng et al. 2007; Ding et al. 2010; Wang et al. 2011; Cardoso and Micaelo 2011; Chandran et al. 2012; He et al. 2013; Jumbri et al. 2014, 2016; Sharma et al. 2015; Zhao 2015a; Saha et al. 2016) hydrophobic and polar interactions also play a vital role (Ding et al. 2010; Wang et al. 2011; Singh et al. 2012; He et al. 2013; Zhao 2015a; Jumbri et al. 2016; Haque et al. 2017). The contribution from hydrogen bonding, especially of anions, to DNA stabilization is less pronounced (Cheng et al. 2007; Cardoso and Micaelo 2011; Tateishi-Karimata and Sugimoto 2012; Nakano et al. 2014; Sharma et al. 2015; Zhao 2015a; Haque et al. 2017). In this regard, the interaction of DNA with ILs seems to occur via similar mechanisms as the interaction with small polar molecules like urea or zwitterionic ectoine. Latest computational studies by us on the interaction of urea with short DNA oligonucleotide show that the urea-DNA binding is driven mainly by electrostatic energies complemented by short-range Lennard-Jones contributions (see Fig. 4) (Oprzeska-Zingrebe and Smiatek 2018). Comparable to DNA-protic IL attraction, hydrogen bonding also assists binding of urea to DNA (Oprzeska-Zingrebe and Smiatek 2018). However, a significant contribution to DNA stabilization in ILs comes from the favorable entropic change associated with solvent effects in the system. Especially in aqueous ILs, low water activity near DNA surfaces associated with the change in hydration pattern supports its structure remarkably (Vijayaraghavan et al. 2010; Tateishi-Karimata and Sugimoto 2012; Nakano et al. 2014; Marušič et al. 2015; Saha et al. 2016). Thus, one can observe both enthalpic and entropic contributions to DNA-IL interactions (Saha et al. 2016). On the contrary, urea binding to DNA is driven mostly by conservative interactions (Oprzeska-Zingrebe and Smiatek 2018). As already mentioned, urea replaces successively water molecules in the first solvation shell around DNA, but without perturbing the structure of hydrated water. Thus, entropic contributions in case of urea binding can be disregarded (Oprzeska-Zingrebe and Smiatek 2018).
In summary, the broad range of investigated ILs hinders the definition of general principles. As main outcomes, electrostatic interactions dominate the binding characteristics. Furthermore, the water behavior also plays a pivotal role. In agreement with proteins, it was often observed that protic ILs tend to stabilize DNA structures, whereas aprotic aqueous ILs reveal destabilization effects (Smiatek 2017). Moreover, it was shown that specific ILs promote a preferential interaction with certain nucleobase components. Hence, most effects are comparable to the influence of aqueous ILs on proteins, but certain differences can be observed, which can be mainly associated with pronounced electrostatic interactions.
Summary and conclusion
In this review, we discussed typical effects and binding mechanisms between aqueous ILs or standard co-solutes and macromolecular structures. As a standard theoretical framework, we introduced the Kirkwood-Buff (KB) theory for a reliable understanding of co-solute-assisted structure stabilization and destabilization effects. The theory is broadly applicable and makes no assumptions on the molecular geometries, while providing at the same time a rationale for the observed effects without further approximations. However, it becomes clear that the KB theory can only provide a general framework in order to rationalize co-solute-induced effects, which has to be modified if specific interactions like hydrogen bonds become important. Despite this limitation, it provides a reasonable approach in order to highlight the similarities and differences between aqueous ILs and standard co-solutes in terms of their binding behavior.
With regard to previous results on protein structures, one can distinguish co-solutes in terms of their enthalpically or entropically dominated interaction mechanism. Entropic stabilization or destabilization can be mostly attributed to excluded-volume effects or compensating enthalpic interactions, respectively, such that macromolecular unfolding is either promoted or surpressed. In contrast, but with comparable consequences, enthalpic mechanisms rely on the presence of significant conservative interactions between the macromolecule and the surrounding co-solute species. Due to the presence of strong electrostatic and dispersion interactions, one can assume that enthalpic contributions dominate the IL binding behavior (Diddens et al. 2017). In contrast to standard co-solutes like urea, the combination of two species in aqueous ILs also provides a more complex destabilization mechanism. Comparable conclusions can be drawn for aqueous ILs and their effects on oligonucleotides. Unfortunately, the ions differ broadly between the investigated systems, such that general principles are hard to define. Most research was performed for DNA in neat ILs, which reveals a completely different behavior when compared with dilute aqueous ILs. Despite recent intensive research on the field of DNA behavior in the presence of ILs, many aspects still remain unaddressed. Elucidating all the molecular details of DNA-IL interactions will require in-depth computational and experimental studies in the future.
The current lack of understanding highlights that only a few aspects of this fascinating topic are understood, which hinder a tailor-made functionalization for specific purposes. It is thus of utmost importance to advance our knowledge by performing additional experiments and simulations in order to establish main principles.
Acknowledgements
The authors acknowledge helpful discussions with Diddo Diddens, Volker Lesch, Andreas Heuer, Hans-Joachim Galla, Julian Michalowsky, Miriam Kohagen, Frank Uhlig, Johannes Zeman, Maria Fyta, Takeshi Kobayashi, Anand Narayanan Krishnamoorthy, Samantha Micciulla, Martin Grininger, Wilhelm-Maximilian Hützler, Marc-Benjamin Hahn, Tihomir Solomun, Heinz Sturm, Martin Schroer, Christian Schröder, and Christian Holm.
Funding information
This work was funded by the Deutsche Forschungsgemeinschaft through the Sonderforschungsbereich 716 (SFB 716)
Compliance with ethical standards
Conflict of interest
Ewa Anna Oprzeska-Zingrebe declares that she has no conflicts of interest. Jens Smiatek declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on “Ionic Liquids and Biomolecules” edited by Antonio Benedetto and Hans-Joachim Galla
References
- Ajloo D, Sangian M, Ghadamgahi M, Evini M, Saboury AA. Effect of two imidazolium derivatives of ionic liquids on the structure and activity of adenosine deaminase. Int J Biol Macromol. 2013;55:47–61. doi: 10.1016/j.ijbiomac.2012.12.042. [DOI] [PubMed] [Google Scholar]
- Araújo J, Pereiro A, Alves F, Marrucho I, Rebelo L. Nucleic acid bases in 1-alkyl-3-methylimidazolium acetate ionic liquids: a thermophysical and ionic conductivity analysis. J Chem Thermodyn. 2013;57:1–8. [Google Scholar]
- Atkins PW, de Paula J. Physical chemistry. Oxford: Oxford Univ Press; 2010. [Google Scholar]
- Auton M, Bolen DW. Additive transfer free energies of the peptide backbone unit that are independent of the model compound and the choice of concentration scale. Biochemistry. 2004;43(5):1329–1342. doi: 10.1021/bi035908r. [DOI] [PubMed] [Google Scholar]
- Baker SN, McCleskey TM, Pandey S, Baker GA. Fluorescence studies of protein thermostability in ionic liquids. Chem Commun. 2004;8:940–941. doi: 10.1039/b401304m. [DOI] [PubMed] [Google Scholar]
- Baker JL, Furbish J, Lindberg GE. Influence of the ionic liquid [c4 mpy][tf2n] on the structure of the miniprotein trp-cage. J Mol Graph Model. 2015;62:202–212. doi: 10.1016/j.jmgm.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Baynes BM, Trout BL. Proteins in mixed solvents: a molecular-level perspective. J Phys Chem B. 2003;107(50):14058–14067. [Google Scholar]
- Ben-Naim A. Statistical thermodynamics for chemists and biochemists. Berlin: Springer Science & Business Media; 2013. [Google Scholar]
- Benedetto A, Ballone P. Room temperature ionic liquids meet biomolecules: a microscopic view of structure and dynamics. ACS Sustain Chem Eng. 2015;4(2):392–412. [Google Scholar]
- Bryngelson JD, Onuchic JN, Socci ND, Wolynes PG. Funnels, pathways, and the energy landscape of protein folding: a synthesis. Prot Struct Funct Bioinf. 1995;21(3):167–195. doi: 10.1002/prot.340210302. [DOI] [PubMed] [Google Scholar]
- Byrne N, Angell CA. Protein unfolding, and the ‘tuning in’ of reversible intermediate states, in protic ionic liquid media. J Mol Biol. 2008;378(3):707–714. doi: 10.1016/j.jmb.2008.02.050. [DOI] [PubMed] [Google Scholar]
- Canchi DR, García AE. Cosolvent effects on protein stability. Ann Rev Phys Chem. 2013;64:273–293. doi: 10.1146/annurev-physchem-040412-110156. [DOI] [PubMed] [Google Scholar]
- Canchi DR, García AE. Cosolvent effects on protein stability. Annu Rev Phys Chem. 2013;64:273–293. doi: 10.1146/annurev-physchem-040412-110156. [DOI] [PubMed] [Google Scholar]
- Cardoso L, Micaelo NM. DNA molecular solvation in neat ionic liquids. Chemphyschem. 2011;12(2):275–277. doi: 10.1002/cphc.201000645. [DOI] [PubMed] [Google Scholar]
- Chandran A, Ghoshdastidar D, Senapati S. Groove binding mechanism of ionic liquids: a key factor in long term stability of DNA in hydrated ionic liquids? J Am Chem Soc. 2012;134(50):20330–20339. doi: 10.1021/ja304519d. [DOI] [PubMed] [Google Scholar]
- Chen M, Xiong H, Wen W, Zhang X, Gu H, Wang S. Electrochemical biosensors for the assay of DNA damage initiated by ferric ions catalyzed oxidation of dopamine in room temperature ionic liquid. Electrochim Acta. 2013;114:265–270. [Google Scholar]
- Cheng DH, Chen XW, Wang JH, Fang ZL. An abnormal resonance light scattering arising from ionic-liquid/DNA/ethidium interactions. Chem Eur J. 2007;13(17):4833–4839. doi: 10.1002/chem.200601544. [DOI] [PubMed] [Google Scholar]
- Chevrot G, Fileti EE, Chaban VV. Protein remains stable at unusually high temperatures when solvated in aqueous mixtures of amino acid based ionic liquids. J Mol Model. 2016;22(11):258. doi: 10.1007/s00894-016-3123-9. [DOI] [PubMed] [Google Scholar]
- Clark KD, Nacham O, Yu H, Li T, Yamsek MM, Ronning DR, Anderson JL. Extraction of DNA by magnetic ionic liquids: tunable solvents for rapid and selective DNA analysis. Anal Chem. 2015;87(3):1552–1559. doi: 10.1021/ac504260t. [DOI] [PubMed] [Google Scholar]
- Clark KD, Yamsek MM, Nacham O, Anderson JL. Magnetic ionic liquids as PCR-compatible solvents for dna extraction from biological samples. Chem Commun. 2015;51(94):16771–16773. doi: 10.1039/c5cc07253k. [DOI] [PubMed] [Google Scholar]
- Clark KD, Sorensen M, Nacham O, Anderson JL. Preservation of DNA in nuclease-rich samples using magnetic ionic liquids. RSC Adv. 2016;6(46):39846–39851. [Google Scholar]
- Collins KD. Ions from the hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process. Methods. 2004;34(3):300–311. doi: 10.1016/j.ymeth.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Constantinescu D, Weingärtner H, Herrmann C. Protein denaturation by ionic liquids and the hofmeister series: a case study of aqueous solutions of ribonuclease a. Angew Chem Int Ed. 2007;46(46):8887–8889. doi: 10.1002/anie.200702295. [DOI] [PubMed] [Google Scholar]
- Constatinescu D, Herrmann C, Weingärtner H. Patterns of protein unfolding and protein aggregation in ionic liquids. Phys Chem Chem Phys. 2010;12(8):1756–1763. doi: 10.1039/b921037g. [DOI] [PubMed] [Google Scholar]
- Courtenay E, Capp M, Anderson C, Record M. Vapor pressure osmometry studies of osmolyte-protein interactions: implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry. 2000;39(15):4455–4471. doi: 10.1021/bi992887l. [DOI] [PubMed] [Google Scholar]
- Dabirmanesh B, Daneshjou S, Sepahi AA, Ranjbar B, Khavari-Nejad RA, Gill P, Heydari A, Khajeh K. Effect of ionic liquids on the structure, stability and activity of two related $\alpha $α-amylases. Int J Biol Macromol. 2011;48(1):93–97. doi: 10.1016/j.ijbiomac.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Dandia A, Singh R, Saini D. Ionic liquid-mediated three-component synthesis of fluorinated spiro-thiazine derivatives and their antimycobacterial and DNA cleavage activities. J Chem Sci. 2013;125(5):1045–1053. [Google Scholar]
- Diddens D, Lesch V, Heuer A, Smiatek J. Aqueous ionic liquids and their influence on peptide conformations: denaturation and dehydration mechanisms. Phys Chem Chem Phys. 2017;19(31):20430–20440. doi: 10.1039/c7cp02897k. [DOI] [PubMed] [Google Scholar]
- Ding Y, Zhang L, Xie J, Guo R. Binding characteristics and molecular mechanism of interaction between ionic liquid and DNA. J Phys Chem B. 2010;114(5):2033–2043. doi: 10.1021/jp9104757. [DOI] [PubMed] [Google Scholar]
- Dommert F, Wendler K, Berger R, Delle Site L, Holm C. Force fields for studying the structure and dynamics of ionic liquids: a critical review of recent developments. ChemPhysChem. 2012;13(7):1625–1637. doi: 10.1002/cphc.201100997. [DOI] [PubMed] [Google Scholar]
- Egorova KS, Gordeev EG, Ananikov VP. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev. 2017;117(10):7132–7189. doi: 10.1021/acs.chemrev.6b00562. [DOI] [PubMed] [Google Scholar]
- Eisenberg H. Biological macromolecules and polyelectrolytes in solution. Oxford: Clarendon Press; 1976. [Google Scholar]
- Figueiredo AM, Sardinha J, Moore GR, Cabrita EJ. Protein destabilisation in ionic liquids: the role of preferential interactions in denaturation. Phys Chem Chem Phys. 2013;15(45):19632–19643. doi: 10.1039/c3cp53395f. [DOI] [PubMed] [Google Scholar]
- Fujita K, Ohno H. Enzymatic activity and thermal stability of metallo proteins in hydrated ionic liquids. Biopolymers. 2010;93(12):1093–1099. doi: 10.1002/bip.21526. [DOI] [PubMed] [Google Scholar]
- Fujita K, Ohno H. Stable G-quadruplex structure in a hydrated ion pair: cholinium cation and dihydrogen phosphate anion. Chem Commun. 2012;48:5751–5753. doi: 10.1039/c2cc30554b. [DOI] [PubMed] [Google Scholar]
- Fujita K, MacFarlane DR, Forsyth M. Protein solubilising and stabilising ionic liquids. Chem Commun. 2005;38:4804–4806. doi: 10.1039/b508238b. [DOI] [PubMed] [Google Scholar]
- Fujita K, Forsyth M, MD R, Reid RW, Elliott GD. Unexpected improvement in stability and utility of cytochrome c by solution in biocompatible ionic liquids. Biotechnol Bioeng. 2006;94:1209–1213. doi: 10.1002/bit.20928. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Yoshizawa M, Ohno H. Room temperature ionic liquids from 20 natural amino acids. J Am Chem Soc. 2005;127(8):2398–2399. doi: 10.1021/ja043451i. [DOI] [PubMed] [Google Scholar]
- Fyta M, Netz RR. Ionic force field optimization based on single-ion and ion-pair solvation properties: going beyond standard mixing rules. J Chem Phys. 2012;136(12):124103. doi: 10.1063/1.3693330. [DOI] [PubMed] [Google Scholar]
- Gee MB, Cox NR, Jiao Y, Bentenitis N, Weerasinghe S, Smith PE. A kirkwood-buff derived force field for aqueous alkali halides. J Chem Theory Comput. 2011;7(5):1369–1380. doi: 10.1021/ct100517z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemi M, Absalan G. Study on the adsorption of DNA on Fe3O4 nanoparticles and on ionic liquid-modified Fe3O4 nanoparticles. Microchim Acta. 2014;181(1-2):45–53. [Google Scholar]
- Haberler M, Steinhauser O. On the influence of of hydrated ionic liquids on the dynamical structure of model proteins: a computational study. Phys Chem Chem Phys. 2012;13(40):17994–18004. doi: 10.1039/c1cp22266j. [DOI] [PubMed] [Google Scholar]
- Haberler M, Schröder C, Steinhauser O. Solvation studies of a zinc finger protein in hydrated ionic liquids. Phys Chem Chem Phys. 2011;13(15):6955–6969. doi: 10.1039/c0cp02487b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberler M, Schröder C, Steinhauser O. Hydrated ionic liquids with and without solute: the influence of water content and protein solutes. J Chem Theory Comput. 2012;8(10):3911–3928. doi: 10.1021/ct300191s. [DOI] [PubMed] [Google Scholar]
- Haberler M, Schröder C, Steinhauser O. Hydrated ionic liquids with and without solute: the influence of water content and protein solutes. J Chem Theo Comput. 2012;8(10):3911–3928. doi: 10.1021/ct300191s. [DOI] [PubMed] [Google Scholar]
- Hahn MB, Solomun T, Wellhausen R, Hermann S, Seitz H, Meyer S, Kunte HJ, Zeman J, Uhlig F, Smiatek J, Sturm H. Influence of the compatible solute ectoine on the local water structure: implications for the binding of the protein g5p to dna. J Phys Chem B. 2015;119(49):15212–15220. doi: 10.1021/acs.jpcb.5b09506. [DOI] [PubMed] [Google Scholar]
- Hahn MB, Uhlig F, Solomun T, Smiatek J, Sturm H. Combined influence of ectoine and salt: spectroscopic and numerical evidence for compensating effects on aqueous solutions. Phys Chem Chem Phys. 2016;18(41):28398–28402. doi: 10.1039/c6cp05417j. [DOI] [PubMed] [Google Scholar]
- Hall D. Kirkwood-buff theory of solutions. An alternative derivation of part of it and some applications. Transact Farad Soc. 1971;67:2516–2524. [Google Scholar]
- Haque A, Khan I, Hassan SI, Khan MS. Interaction studies of cholinium-based ionic liquids with calf thymus DNA: spectrophotometric and computational methods. J Mol Liq. 2017;237:201–207. [Google Scholar]
- Harries D, Rösgen J. A practical guide on how osmolytes modulate macromolecular properties. Methods Cell Biol. 2008;84:679–735. doi: 10.1016/S0091-679X(07)84022-2. [DOI] [PubMed] [Google Scholar]
- He Y, Shang Y, Liu Z, Shao S, Liu H, Hu Y. Interactions between ionic liquid surfactant C(12)mim Br and DNA in dilute brine. Colloids Surf B. 2013;101:398–404. doi: 10.1016/j.colsurfb.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Heyda J, Okur HI, Hladílkova J, Rembert KB, Hunn W, Yang T, Dzubiella J, Jungwirth P, Cremer PS. Guanidinium can both cause and prevent the hydrophobic collapse of biomacromolecules. J Am Chem Soc. 2017;139(2):863–870. doi: 10.1021/jacs.6b11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinek D, Netz RR. Can simulations quantitatively predict peptide transfer free energies to urea solutions? Thermodynamic concepts and force field limitations. J Phys Chem A. 2011;115(23):6125–6136. doi: 10.1021/jp1110086. [DOI] [PubMed] [Google Scholar]
- Jha SK, Marqusee S. Kinetic evidence for a two-stage mechanism of protein denaturation by guanidinium chloride. Proc Natl Acad Sci USA. 2014;111(13):4856–4861. doi: 10.1073/pnas.1315453111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumbri K, Rahman MBA, Abdulmalek E, Ahmad H, Micaelo NM. An insight into structure and stability of DNA in ionic liquids from molecular dynamics simulation and experimental studies. PCCP. 2014;16(27):14036–14046. doi: 10.1039/c4cp01159g. [DOI] [PubMed] [Google Scholar]
- Jumbri K, Ahmad H, Abdulmalek E, Rahman MBA. Binding energy and biophysical properties of ionic liquid-DNA complex: understanding the role of hydrophobic interactions. J Mol Liq. 2016;223:1197–1203. [Google Scholar]
- Kanduċ M, Chudoba R, Palczynski K, Kim WK, Roa R, Dzubiella J. Selective solute adsorption and partitioning around single pnipam chains. Phys Chem Chem Phys. 2017;19(8):5906–5916. doi: 10.1039/c6cp08366h. [DOI] [PubMed] [Google Scholar]
- Khandelwal G, Bhyravabhotla J. A phenomenological model for predicting melting temperatures of DNA sequences. PLoS One. 2010;5(8):e12433. doi: 10.1371/journal.pone.0012433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood JG, Buff FP. The statistical mechanical theory of solutions. I. J Chem Phys. 1951;19(6):774–777. [Google Scholar]
- Klähn M, Lim GS, Sedurama n A, Wu P. On the different roles of anions and cations in th e solvation of enzymes in ionic liquids. Phys Chem Chem Phys. 2011;13(4):1649–1662. doi: 10.1039/c0cp01509a. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Reid JE, Shimizu S, Fyta M, Smiatek J. The properties of residual water molecules in ionic liquids: a comparison between direct and inverse Kirkwood–Buff approaches. Phys Chem Chem Phys. 2017;19:18924–18937. doi: 10.1039/c7cp03717a. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy AN, Zeman J, Holm C, Smiatek J. Preferential solvation and ion association properties in aqueous dimethyl sulfoxide solutions. Phys Chem Chem Phys. 2016;18(45):31312–31322. doi: 10.1039/c6cp05909k. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy AN, Holm C, Smiatek J (2018) The influence of co-solutes on the chemical equilibrium — a Kirkwood-Buff theory for ion pair association-dissociation processes in ternary electrolyte solutions. in revision
- Kulkarni M, Mukherjee A. Ionic liquid prolongs DNA translocation through graphene nanopores. RSC Adv. 2016;6(51):46019–46029. [Google Scholar]
- Kumar A, Venkatesu P. Prevention of insulin self-aggregation by a protic ionic liquid. RSC Adv. 2013;3:362–367. [Google Scholar]
- Kumar P, Franzese G, Buldyrev SV, Stanley HE. Molecular dynamics study of orientational cooperativity in water. Phys Rev E. 2006;73:0415051–0415058. doi: 10.1103/PhysRevE.73.041505. [DOI] [PubMed] [Google Scholar]
- Kumar P, Buldyrev SV, Stanley HE. A tetrahedral entropy for water. Proc Natl Acad Sci USA. 2009;106:22130–22134. doi: 10.1073/pnas.0911094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz W (2010) Specific ion effects. World Scientific
- Kusalik PG, Patey G. The thermodynamic properties of electrolyte solutions: some formal results. J Chem Phys. 1987;86(9):5110–5116. [Google Scholar]
- Kusalik PG, Patey G. On the molecular theory of aqueous electrolyte solutions. i. The solution of the rhnc approximation for models at finite concentration. J Chem Phys. 1988;88(12):7715–7738. [Google Scholar]
- Lesch V, Heuer A, Holm C, Smiatek J. Solvent effects of 1-ethyl-3-methylimidazolium acetate: solvation and dynamic behavior of polar and apolar solutes. Phys Chem Chem Phys. 2015;17(13):8480–8490. doi: 10.1039/c4cp05312e. [DOI] [PubMed] [Google Scholar]
- Lesch V, Heuer A, Tatsis VA, Holm C, Smiatek J. Peptides in the presence of aqueous ionic liquids: tunable co-solutes as denaturants or protectants? Phys Chem Chem Phys. 2015;17(39):26049–26053. doi: 10.1039/c5cp03838c. [DOI] [PubMed] [Google Scholar]
- Lesch V, Heuer A, Holm C, Smiatek J. Properties of apolar solutes in alkyl imidazolium-based ionic liquids: the importance of local interactions. ChemPhysChem. 2016;17(3):387–394. doi: 10.1002/cphc.201501021. [DOI] [PubMed] [Google Scholar]
- Li T, Joshi MD, Ronning DR, Anderson JL. Ionic liquids as solvents for in situ dispersive liquid–liquid microextraction of DNA. J Chromatogr A. 2013;1272:8–14. doi: 10.1016/j.chroma.2012.11.055. [DOI] [PubMed] [Google Scholar]
- Li X, Ma J, Lei W, Li J, Zhang Y, Li Y. Cloning of cytochrome P450 3A137 complementary DNA in silver carp and expression induction by ionic liquid. Chemosphere. 2013;92(9):1238–1244. doi: 10.1016/j.chemosphere.2013.04.055. [DOI] [PubMed] [Google Scholar]
- Lo Nostro P, Ninham BW. Hofmeister phenomena: an update on ion specificity in biology. Chem Rev. 2012;112(4):2286–2322. doi: 10.1021/cr200271j. [DOI] [PubMed] [Google Scholar]
- Machado I, Özalp VC, Rezabal E, Schäfer T. DNA aptamers are functional molecular recognition sensors in protic ionic liquids. Chem Eur J. 2014;20(37):11820–11825. doi: 10.1002/chem.201403354. [DOI] [PubMed] [Google Scholar]
- Mann JP, McCluskey A, Atkin R. Activity and thermal stability of lysozyme in alkylammonium formate ionic liquids—influence of cation modification. Green Chem. 2009;11(6):785–792. [Google Scholar]
- Marcus Y. Effect of ions on the structure of water: structure making and breaking. Chem Rev. 2009;109(3):1346–1370. doi: 10.1021/cr8003828. [DOI] [PubMed] [Google Scholar]
- Martinez L, Shimizu S. Molecular interpretation of preferential interactions in protein solvation: a solvent-shell perspective by means of minimum-distance distribution functions. J Chem Theory Comput. 2017;13(12):6358–6372. doi: 10.1021/acs.jctc.7b00599. [DOI] [PubMed] [Google Scholar]
- Marušič M, Tateishi-Karimata H, Sugimoto N, Plavec J. Structural foundation for DNA behavior in hydrated ionic liquid: an NMR study. Biochimie. 2015;108:169–177. doi: 10.1016/j.biochi.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Mazid RR, Cooper A, Zhang Y, Vijayaraghavan R, MacFarlane DR, Cortez-Jugo C, Cheng W. Enhanced enzymatic degradation resistance of plasmid DNA in ionic liquids. RSC Adv. 2015;5(54):43839–43844. [Google Scholar]
- Micaêlo NM, Soares CM. Protein structure and dynamics in ionic liquids. Insights from molecular dynamics simulation studies. J Phys Chem B. 2008;112(9):2566–2572. doi: 10.1021/jp0766050. [DOI] [PubMed] [Google Scholar]
- Micciulla S, Michalowsky J, Schroer MA, Holm C, von Klitzing R, Smiatek J. Concentration dependent effects of urea binding to poly (n-isopropylacrylamide) brushes: a combined experimental and numerical study. Phys Chem Chem Phys. 2016;18:5324–5335. doi: 10.1039/c5cp07544k. [DOI] [PubMed] [Google Scholar]
- Mishra A, Ekka MK, Maiti S. Influence of ionic liquids on thermodynamics of small molecule DNA interaction: the binding of ethidium bromide to calf thymus DNA. J Phys Chem B. 2016;120(10):2691–2700. doi: 10.1021/acs.jpcb.5b11823. [DOI] [PubMed] [Google Scholar]
- Mukesh C, Mondal D, Sharma M, Prasad K. Rapid dissolution of DNA in a novel bio-based ionic liquid with long-term structural and chemical stability: successful recycling of the ionic liquid for reuse in the process. Chem Commun. 2013;49(61):6849–6851. doi: 10.1039/c3cc42829j. [DOI] [PubMed] [Google Scholar]
- Nakano M, Tateishi-Karimata H, Tanaka S, Sugimoto N. Choline ion interactions with DNA atoms explain unique stabilization of A–T base pairs in DNA duplexes: a microscopic view. J Phys Chem B. 2014;118(2):379–389. doi: 10.1021/jp406647b. [DOI] [PubMed] [Google Scholar]
- Narayanan Krishnamoorthy A, Holm C, Smiatek J. Local water dynamics around antifreeze protein residues in the presence of osmolytes: the importance of hydroxyl and disaccharide groups. J Phys Chem B. 2014;118(40):11613–11621. doi: 10.1021/jp507062r. [DOI] [PubMed] [Google Scholar]
- Newman KE. Kirkwood–buff solution theory: derivation and applications. Chem Soc Rev. 1994;23(1):31–40. [Google Scholar]
- Nishimura N, Nomura Y, Nakamura N, Ohno H. DNA strands robed with ionic liquid moiety. Biomaterials. 2005;26(27):5558–5563. doi: 10.1016/j.biomaterials.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Okur HI, Hladílková J, Rembert KB, Cho Y, Heyda J, Dzubiella J, Cremer PS, Jungwirth P. Beyond the hofmeister series: ion-specific effects on proteins and their biological functions. J Phys Chem B. 2017;121(9):1997–2014. doi: 10.1021/acs.jpcb.6b10797. [DOI] [PubMed] [Google Scholar]
- Oprzeska-Zingrebe EA, Smiatek J (2018) Preferential binding of urea to single-stranded DNA structures:a molecular dynamics study. Biophys J (to be published) [DOI] [PMC free article] [PubMed]
- Pabbathi A, Samanta A. Spectroscopic and molecular docking study of the interaction of DNA with a morpholinium ionic liquid. J Phys Chem B. 2015;119(34):11099–11105. doi: 10.1021/acs.jpcb.5b02939. [DOI] [PubMed] [Google Scholar]
- Pandey PK, Rawat K, Aswal VK, Kohlbrecher J, Bohidar HB. Imidazolium based ionic liquid induced DNA gelation at remarkably low concentration. Coll Surf. 2018;538:184–191. [Google Scholar]
- Patel R, Kumari M, Khan AB. Recent advances in the application of ionic liquids in protein stability and activity: a review. Appl Biochem Biotechnol. 2014;172:3701–3720. doi: 10.1007/s12010-014-0813-6. [DOI] [PubMed] [Google Scholar]
- Patel R, Kumari M, Khan AB. Recent advances in the application of ionic liquids in protein stability and activity: a review. Appl Biochem Biotechnol. 2014;172:3701–3720. doi: 10.1007/s12010-014-0813-6. [DOI] [PubMed] [Google Scholar]
- Pierce V, Kang M, Aburi M, Weerasinghe S, Smith PE. Recent applications of kirkwood-buff theory to biological systems. Cell Biochem Biophys. 2008;50:1–22. doi: 10.1007/s12013-007-9005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Li SFY. Electrophoresis of DNA in ionic liquid coated capillary. Analyst. 2003;128:37–41. doi: 10.1039/b208724c. [DOI] [PubMed] [Google Scholar]
- Reid JE, Walker AJ, Shimizu S. Residual water in ionic liquids: clustered or dissociated? Phys Chem Chem Phys. 2015;17(22):14710–14718. doi: 10.1039/c5cp01854d. [DOI] [PubMed] [Google Scholar]
- Reid JESJ, Gammons RJ, Slattery JM, Walker AJ, Shimizu S (2017) Interactions in water–ionic liquid mixtures: comparing protic and aprotic systems. J Phys Chem B. 10.1021/acs.jpcb.6b10562 [DOI] [PubMed]
- Rodríguez-Ropero F, van der Vegt NF. On the urea induced hydrophobic collapse of a water soluble polymer. Phys Chem Chem Phys. 2015;17(13):8491–8498. doi: 10.1039/c4cp05314a. [DOI] [PubMed] [Google Scholar]
- Rösgen J, Pettitt BM, Bolen DW. Uncovering the basis for nonideal behavior of biological molecules. Biochemistry. 2004;43(45):14472–14484. doi: 10.1021/bi048681o. [DOI] [PubMed] [Google Scholar]
- Rösgen J, Pettitt BM, Bolen DW. Protein folding, stability, and solvation structure in osmolyte solutions. Biophys J. 2005;89(5):2988–2997. doi: 10.1529/biophysj.105.067330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösgen J, Pettitt BM, Bolen DW. An analysis of the molecular origin of osmolyte-dependent protein stability. Protein Sci. 2007;16(4):733–743. doi: 10.1110/ps.062671607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Banerjee P, Dutta R, Kundu S, Sarkar N. Probing the interaction between a DNA nucleotide (adenosine-5 ’-monophosphate disodium) and surface active ionic liquids by rotational relaxation measurement and fluorescence correlation spectroscopy. Langmuir. 2016;32(42):10946–10956. doi: 10.1021/acs.langmuir.6b02794. [DOI] [PubMed] [Google Scholar]
- Saha D, Mukherjee A (2018) Effect of water and ionic liquids on biomolecules. Biophys Rev, 1–14 [DOI] [PMC free article] [PubMed]
- Saha D, Kulkarni M, Mukherjee A. Water modulates the ultraslow dynamics of hydrated ionic liquids near CG rich DNA: consequences for DNA stability. Phys Chem Chem Phys. 2016;18(47):32107–32115. doi: 10.1039/c6cp05959g. [DOI] [PubMed] [Google Scholar]
- Sapir L, Harries D. Wisdom of the crowd. Bunsen-Magazin. 2017;19:152–162. [Google Scholar]
- Satpathi S, Kulkarni M, Mukherjee A, Hazra P. Ionic liquid induced G-quadruplex formation and stabilization: spectroscopic and simulation studies. Phys Chem Chem Phys. 2016;18(43):29740–29746. doi: 10.1039/c6cp05732b. [DOI] [PubMed] [Google Scholar]
- Schröder C. Proteins in ionic liquids: current status of experiments and simulations. Top Curr Chem. 2017;375(2):25. doi: 10.1007/s41061-017-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer MA, Michalowsky J, Fischer B, Smiatek J, Grübel G. Stabilizing effect of tmao on globular pnipam states: preferential attraction induces preferential hydration. Phys Chem Chem Phys. 2016;18(46):31459–31470. doi: 10.1039/c6cp05991k. [DOI] [PubMed] [Google Scholar]
- Schurr JM, Rangel DP, Aragon SR. A contribution to the theory of preferential interaction coefficients. Biophys J. 2005;89(4):2258–2276. doi: 10.1529/biophysj.104.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senske M, Constantinescu-Aruxandei D, Havenith M, Herrmann C, Weingärtner H, Ebbinghaus S. The temperature dependence of the hofmeister series: thermodynamic fingerprints of cosolute–protein interactions. Phys Chem Chem Phys. 2016;18(43):29698–29708. doi: 10.1039/c6cp05080h. [DOI] [PubMed] [Google Scholar]
- Sharma M, Mondal D, Singh N, Trivedi N, Bhattab J, Prasad K. High concentration DNA solubility in bio-ionic liquids with long-lasting chemical and structural stability at room temperature. RSC Adv. 2015;5:40546–40551. [Google Scholar]
- Shimizu S. Estimating hydration changes upon biomolecular reactions from osmotic stress, high pressure, and preferential hydration experiments. Proc Natl Acad Sci USA. 2004;101(5):1195–1199. doi: 10.1073/pnas.0305836101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Matubayasi N. A unified perspective on preferential solvation and adsorption based on inhomogeneous solvation theory. Physica A. 2018;492:1988–1996. [Google Scholar]
- Shimizu S, Smith DJ. Preferential hydration and the exclusion of cosolvents from protein surfaces. J Chem Phys. 2004;121(2):1148–1154. doi: 10.1063/1.1759615. [DOI] [PubMed] [Google Scholar]
- Singh PK, Sujana J, Mora AK, Nath S. Probing the dna-ionic liquid interaction using an ultrafast molecular rotor. J Photochem Photobiol A. 2012;246:16–22. [Google Scholar]
- Smiatek J. Osmolyte effects: impact on the aqueous solution around charged and neutral spheres. J Phys Chem B. 2014;118(3):771–782. doi: 10.1021/jp410261k. [DOI] [PubMed] [Google Scholar]
- Smiatek J. Aqueous ionic liquids and their influence on protein structures: an overview on recent theoretical and experimental results. J Phys Condens Matter. 2017;29:233001. doi: 10.1088/1361-648X/aa6c9d. [DOI] [PubMed] [Google Scholar]
- Smiatek J, Heuer A. Deprotonation mechanism of a single-stranded DNA i-motif. RSC Adv. 2014;4:17110–17113. [Google Scholar]
- Smiatek J, Harishchandra RK, Rubner O, Galla HJ, Heuer A. Properties of compatible solutes in aqueous solution. Biophys Chem. 2012;160(1):62–68. doi: 10.1016/j.bpc.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Smith PE. Computer simulation of cosolvent effects on hydrophobic hydration. J Phys Chem B. 1999;103(3):525–534. [Google Scholar]
- Smith PE. Cosolvent interactions with biomolecules: relating computer simulation data to experimental thermodynamic data. J Phys Chem B. 2004;108:18716–18724. [Google Scholar]
- Smith PE. Chemical potential derivatives and preferential interaction parameters in biological systems from Kirkwood-Buff theory. Biophys J. 2006;91(3):849–856. doi: 10.1529/biophysj.105.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PE, Matteoli E, O’Connell JP (2013) Fluctuation theory of solutions: applications in chemistry, chemical engineering, and biophysics. CRC Press
- Soni SK, Sarkar S, Mirzadeh N, Selvakannan PR, Bhargava SK. Self-assembled functional nanostructure of plasmid DNA with ionic liquid Bmim PF6 : enhanced efficiency in bacterial gene transformation. Langmuir. 2015;31(16):4722–4732. doi: 10.1021/acs.langmuir.5b00402. [DOI] [PubMed] [Google Scholar]
- Sukenik S, Sapir L, Gilman-Politi R, Harries D. Diversity in the mechanisms of cosolute action on biomolecular processes. Farad Discuss. 2013;160:225–237. doi: 10.1039/c2fd20101a. [DOI] [PubMed] [Google Scholar]
- Sukenik S, Sapir L, Harries D. Balance of enthalpy and entropy in depletion forces. Curr Opin Coll Interface Sci. 2013;18:495–501. [Google Scholar]
- Sun W, Zhang Y, Hu A, Lu Y, Shi F, Lei B, Sun Z. Electrochemical DNA biosensor based on partially reduced graphene oxide modified carbon ionic liquid electrode for the detection of transgenic soybean A2704-12 gene sequence. Electroanal. 2013;25(6):1417–1424. [Google Scholar]
- Tanford C. Isothermal unfolding of globular proteins in aqueous urea solutions. J Am Chem Soc. 1964;86(10):2050–2059. [Google Scholar]
- Tanford C. Extension of the theory of linked functions to incorporate the effects of protein hydration. J Mol Biol. 1969;39(3):539–544. doi: 10.1016/0022-2836(69)90143-0. [DOI] [PubMed] [Google Scholar]
- Tateishi-Karimata H, Sugimoto N. A-T base pairs are more stable than G-C base pairs in a hydrated ionic liquid. Angew Chem Int Ed. 2012;51(6):1416–1419. doi: 10.1002/anie.201106423. [DOI] [PubMed] [Google Scholar]
- Tateishi-Karimata H, Sugimoto N. Structure, stability and behaviour of nucleic acids in ionic liquids. Nucleic Acids Res. 2014;42(14):8831–8844. doi: 10.1093/nar/gku499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi-Karimata H, Nakano M, Pramanik S, Tanaka S, Sugimoto N. i-motifs are more stable than G-quadruplexes in hydrated ionic liquid. Chem Commun. 2015;51(32):6909–6912. doi: 10.1039/c5cc00666j. [DOI] [PubMed] [Google Scholar]
- Timasheff SN. Protein hydration, thermodynamic binding, and preferential hydration. Biochemistry. 2002;41(46):13473–13482. doi: 10.1021/bi020316e. [DOI] [PubMed] [Google Scholar]
- Tung HJ, Pfaendtner J. Kinetics and mechanism of ionic-liquid induced protein unfolding: application to the model protein hp35. Mol Syst Des Eng. 2016;1(4):382–390. [Google Scholar]
- Uhlig F, Zeman J, Smiatek J, Holm C (2018) First-principles parameterization of polarizable coarse-grained force fields for ionic liquids. J Chem Theory Comput. 10.1021/acs.jctc.7b00903 [DOI] [PubMed]
- van Rantwijk F, Sheldon RA. Biocatalysis in ionic liquids. Chem Rev. 2007;107(6):2757–2785. doi: 10.1021/cr050946x. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan R, Izgorodin A, Ganesh V, Surianarayanan M, MacFarlane DR. Long-term structural and chemical stability of DNA in hydrated ionic liquids. Angew Chem Int Ed. 2010;49(9):1631–1633. doi: 10.1002/anie.200906610. [DOI] [PubMed] [Google Scholar]
- Wang JH, Cheng DH, Chen XW, Du Z, Fang ZL. Direct extraction of double-stranded DNA into ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate and its quantification. Anal Chem. 2007;79(2):620–625. doi: 10.1021/ac061145c. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang J, Zhang S. Binding Gibbs energy of ionic liquids to calf thymus DNA: a fluorescence spectroscopy study. PCCP. 2011;13(9):3906–3910. doi: 10.1039/c0cp01815e. [DOI] [PubMed] [Google Scholar]
- Weerasinghe S, Smith PE. A Kirkwood–uff derived force field for sodium chloride in water. J Chem Phys. 2003;119(21):11342–11349. [Google Scholar]
- Weingärtner H, Cabrele C, Herrmann C. How ionic liquids can help to stabilize native proteins. Phys Chem Chem Phys. 2012;14(2):415–426. doi: 10.1039/c1cp21947b. [DOI] [PubMed] [Google Scholar]
- Wilkes JS. Properties of ionic liquid solvents for catalysis. J Mol Catal A. 2004;214(1):11–17. [Google Scholar]
- Wyman J., Jr Linked functions and reciprocal effects in hemoglobin: a second look. Adv Prot Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- Xia Z, Das P, Shakhnovich EI, Zhou R. Collapse of unfolded proteins in a mixture of denaturants. J Am Chem Soc. 2012;134(44):18266–18274. doi: 10.1021/ja3031505. [DOI] [PubMed] [Google Scholar]
- Xie YN, Wang SF, Zhang ZL, Pang DW. Interaction between room temperature ionic liquid bmim BF4 and DNA investigated by electrochemical micromethod. J Phys Chem B. 2008;112(32):9864–9868. doi: 10.1021/jp803655t. [DOI] [PubMed] [Google Scholar]
- Yan H, Wu J, Dai G, Zhong A, Chen H, Yang J, Han D. Interaction mechanisms of ionic liquids [cnmim] br (n = 4, 6, 8, 10) with bovine serum albumin. J Lumin. 2012;132(3):622–628. [Google Scholar]
- Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208(15):2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- Yang Z. Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysis. J Biotechnol. 2009;144(1):12–22. doi: 10.1016/j.jbiotec.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Zeindlhofer V, Khlan D, Bica K, Schröder C. Computational analysis of the solvation of coffee ingredients in aqueous ionic liquid mixtures. RSC Adv. 2017;7(6):3495–3504. doi: 10.1039/c6ra24736a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeindlhofer V, Berger M, Steinhauser O, Schröder C. A shell-resolved analysis of preferential solvation of coffee ingredients in aqueous mixtures of the ionic liquid 1-ethyl-3-methylimidazolium acetate. J Chem Phys. 2018;148(19):193819. doi: 10.1063/1.5009802. [DOI] [PubMed] [Google Scholar]
- Zeman J, Uhlig F, Smiatek J, Holm C. A coarse-grained polarizable force field for the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J Phys Condens Matter. 2017;29:504004. doi: 10.1088/1361-648X/aa99c4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen X, Lan J, You J, Chen L. Synthesis and biological applications of imidazolium-based polymerized ionic liquid as a gene delivery vector. Chem Biol Drug Des. 2009;74(3):282–288. doi: 10.1111/j.1747-0285.2009.00858.x. [DOI] [PubMed] [Google Scholar]
- Zhao H. Effect of ions and other compatible solutes on enzyme activity, and its implication for biocatalysis using ionic liquids. J Mol Cat B. 2005;37(1-6):16–25. [Google Scholar]
- Zhao H. DNA stability in ionic liquids and deep eutectic solvents. J Chem Technol Biotechnol. 2015;90(1):19–25. doi: 10.1002/jctb.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. Protein stabilization and enzyme activation in ionic liquids: specific ion effects. J Chem Technol Biotechnol. 2015;91:25–50. doi: 10.1002/jctb.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Borgia A, Buholzer K, Grishaev A, Schuler B, Best RB. Probing the action of chemical denaturant on an intrinsically disordered protein by simulation and experiment. J Am Chem Soc. 2016;138(36):11702–11713. doi: 10.1021/jacs.6b05443. [DOI] [PMC free article] [PubMed] [Google Scholar]