Abstract
Ionic liquids are well known and frequently used ‘designer solvents’ for biocatalytic reactions. This review highlights recent achievements in the field of multiphasic ionic liquid-based reaction concepts. It covers classical biphasic systems including supported ionic liquid phases, thermo-regulated multi-component solvent systems (TMS) and polymerized ionic liquids. These powerful concepts combine unique reaction conditions with a high potential for future applications on a laboratory and industrial scale. The presence of a multiphasic system simplifies downstream processing due to the distribution of the catalyst and reactants in different phases.
Keywords: Ionic liquids, Biphasic, Enzyme, Equilibrium, Reaction engineering
Introduction
Within the last decades, biocatalysts became a very powerful alternative to classical chemical and chemical reaction systems (Nestl et al. 2011; Reetz 2013). This includes their use from laboratory up to large-scale applications for the synthesis of bulk chemicals, fine chemicals and agrochemicals (Wenda et al. 2011; Patel 2011; Busacca et al. 2011). Major examples comprise the synthesis of bio-based compounds such as (bio)ethanol, acrylamide, antibiotics and various intermediates for active pharmaceutical ingredients (APIs) (Muñoz Solano et al. 2012). Herein biocatalysts, applied either as isolated enzymes or whole microbial cells, offer significant advantages, e.g. mild reaction conditions, high regio- and enantio-selectivities and a general use of water as solvent. The increasing demand for new bioactive molecules and bio-based products continues to fuel the search for new biocatalysts for the use in synthetic pathways (Renata et al. 2015; Bornscheuer et al. 2012). Already existing biocatalysts can be further improved by protein engineering techniques to adjust certain features such as substrate range (Turner 2003), selectivity (Luetz et al. 2008), solvent compatibility (Reetz 2003), and process stability (Singh et al. 2013). Consequently, biocatalysts are frequently used to enable alternative and more eco-friendly synthetic pathways, which also include an integrated use with classical chemical and catalytic reaction systems (Wohlgemuth 2010).
However, enzymes usually operate best in aqueous media because of their native origin in prokaryotic or eukaryotic cells. Nevertheless, there are various successful examples of biocatalysis in or with organic solvents (Stepankova et al. 2013). These reactions are frequently carried out in two-phase systems consisting of organic solvents and water. Here, the organic phase is often used as a substrate and product reservoir (if the main reactants are only sparingly soluble in water), whereas the aqueous phase contains and stabilizes the enzyme by retaining its hydration shell (Hernández Fernández et al. 2015). In addition, the product can be recovered from the organic layer. The stability of biocatalysts in such classical non-aqueous environments is unfortunately often limited, which pathed the success of ionic liquids (ILs) as substitutes for molecules solvents or volatile organic compounds (VOCs) (Kragl et al. 2002; Sheldon et al. 2002; Oppermann et al. 2011; Zhao and Baker 2013; Sheldon 2014, 2016; Stein and Kragl 2014; Sivapragasam et al. 2016; Itoh 2017).
Since the late 1990s ILs have become a major research topic and the number of scientific publications about these relatively new class of compounds has risen almost exponentially (Itoh 2017; Sivapragasam et al. 2016; Zhao et al. 2013; Sheldon et al. 2002; Kragl et al. 2002; Hallett and Welton 2011). Here ILs are in general characterized as salt-based compounds that include (typically) one cation and one anion with a melting point considerably below 100 °C. Such a low melting point is based on low coulomb-, van-der Waals- and hydrogen bond-interactions (Olivier-Bourbigou et al. 2010). The unique composition of ILs facilitate interesting miscibilities with classical molecular solvents, typically high solubilities of unpolar compounds, low vapor pressure, high ionic conductivity and a general non-flammable nature. Most ILs are based on organic cations in combination with anions with a strongly delocalized negative charge (see below). This highly tunable chemical composition allows high thermal and chemical stability. Various enzymatic reactions in, or strictly speaking in the presence of, ILs were reported in scientific literature (Naushad et al. 2012; Zhao 2010; Moniruzzaman et al. 2010). There is also a large number of reviews covering various aspects over the years (Kragl et al. 2002; Sheldon et al. 2002; Zhao and Baker 2013; Benedetto and Ballone 2016a, b; Sivapragasam et al. 2016; Itoh 2017). Within this short review, we will cover recent developments in the use of ionic liquid in multiphasic solvent systems, which allow the design of specialized biocatalytic reaction concepts.
Biocatalysis with ionic liquids
Biphasic reaction systems
Downstream processing (e.g. separation of products, recovery of the biocatalyst or preferably recycling of reaction streams) is a major challenge in various biotechnological processes. Due to the growing demand for biotechnologically manufactured fine chemicals and biomolecules, this topic was focused on in recent years (Oppermann et al. 2011). Successful examples of the utilization of biphasic reaction systems with ILs are described in the following.
The biphasic reaction systems considered in this review are mostly composed of an IL and a buffer solution. To form such a two-phase system, both applied liquids need to be in equilibrium with each other and only partially miscible in the given proportions. The solution phase behaviour of such partially miscible mixtures can be described by a binary phase diagram (see Fig. 1). The binodal (coexistence curve) divides the phase diagram into a homogeneous and a heterogeneous region (miscibility gap), thus representing exactly the line of coexistence of both phases. Changing the temperature can shift the balance of the liquid-liquid equilibrium (LLE) to one side or the other. The most common types of miscibility gaps include systems with upper critical solution temperature (UCST) and systems with lower critical solution temperature (LCST). At temperatures above UCST, the system is completely miscible in all proportions, whereas below UCST partial miscibility of the liquids occurs. At temperatures below LCST, the system is completely miscible in all proportions, whereas above LCST partial miscibility of the liquids occurs. At a given temperature and pressure, phase separation occurs if a mixture (e.g. red point in Fig. 1) is located in the biphasic region of a phase diagram. The resulting two phases have different compositions x1 and x2 (red dotted lines). Although the description of IL-based two-phase system as a binary mixture is sufficient for most applications, the exact description of phase systems is a more complicated matter. Correctly, each component in the mixtures needs to be considered (e.g. buffer salts or other components), resulting in ternary or even higher mixtures, etc., as discussed in detail elsewhere (Gutowski et al. 2003; Freire et al. 2012; Bridges et al. 2007; Behr et al. 2011).
Fig. 1.
Illustration of a typical binary solution phase behaviour of partially miscible solutions with an upper critical solution temperature (UCST, left hand side) and a lower critical solution temperature (LCST, right hand side)
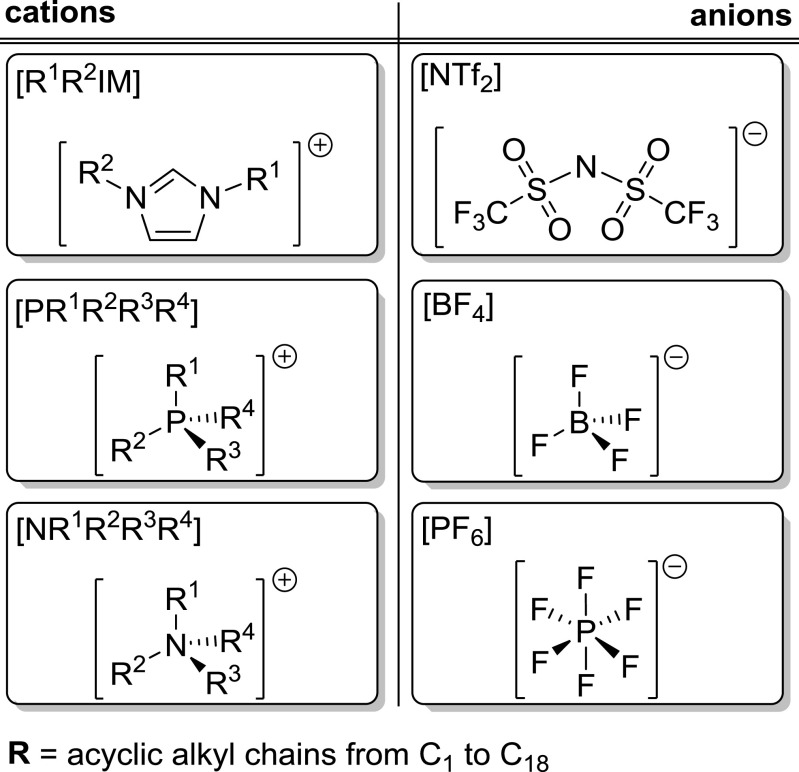
Over the past years, primarily ILs composed of imidazolium [R1R2IM], phosphonium [PR1R2R3R4] and ammonium cations [NR1R2R3R4] together with bis(trifluoromethane)sulfonimide [NTf2], tetrafluoroborate [BF4] and hexafluorophosphate [PF6] anions were used for biphasic IL-based reaction systems. The basic structures are shown in Fig. 2. The secondary, immiscible solvent forming the intended two-phase system is usually water, but also IL-organic solvent-based systems were reported (Hou et al. 2017) . However, the number of water non-miscible ionic liquids is still limited and the majority of the systems is widely miscible with water.
Fig. 2.
Commonly used IL in this review
In Table 1, a number of different systems published recently are listed covering different types of biphasic reaction systems. Besides the two-phase system, the reaction and a brief comment are given. The IL phase is generally designed to solubilize the desired non-polar substrates and products. The second solvent then contains the biocatalytic reaction system (entries 1–15). This includes the use as whole cell biocatalysts and (partly) purified enzymes in free or immobilized form. However, recent investigations include the use of reaction-induced phase changes (entry 16) and supported IL phases (entries 17–18). In addition, recent studies also investigated the influence of such a biphasic reaction systems on whole cell systems (entries 19–20).
Table 1.
Recently reported biphasic hydrophobic IL-based reaction systems in biocatalysis
| Entry | Investigated biphasic reaction systems | Biocatalytic reaction system | Reference |
|---|---|---|---|
| Classical biphasic reaction system | |||
| 1 | [BMIM][PF6]/buffer | β-Glucuronidase (PGUS-E) from Penicillium purpurogenum Li-3; immobilized on zinc oxide nanoparticles | (Kaleem et al. 2017) |
| 2 | [BMIM][NTf2]/buffer | Commercial alcohol dehydrogenase, expressed in E. coli and alanine aminotransferase isolated from porcine heart | (Voges et al. 2017) |
| 3 | [HPYR][NTF]/buffer | Whole cells of Escherichia coli overexpressing a glucosyltransferase from Vitis vinifera | (Schmideder et al. 2016) |
| 4 | Various ILs/buffer | Acetobacter pasteurianus Gim1.158 whole cells | (Xu et al. 2016) |
| 5 | [BMIM][PF6] and [NTf2]/buffer | Baker’s yeast (BY) and old yellow enzyme (OYE1-3) | (Brenna et al. 2014) |
| 6 | Various ILs/buffer | Rhodotorula glutinis whole cells | (Matsumoto et al. 2014) |
| 7 | Various ILs/buffer | Mung bean epoxide hydrolases, cross-linked enzyme aggregates (CLEAs) | (Yu et al. 2014) |
| 8 | Various ILs/various organic solvents | Various lipases | (Fischer et al. 2013) |
| 9 | [BMPY][NTf2], | Candida antarctica lipase B (CalB) | (Pohar et al. 2012) |
| 10 | Various ILs/buffer | Naringinase from Penicillium decumbens | (Temme et al. 2012) |
| 11 | [BMIM][PF6]/buffer | Immobilized Candida parapsilosis CCTCC M203011 whole cells | (Zhang et al. 2012) |
| 12 | [BMIM][NTf2]/buffer | Escherichia coli whole cells co-expressing yeast reductase YOL151W and Bacillus subtilis glucose dehydrogenase | (Choi et al. 2011) |
| 13 | [HMPL][NTF2]/buffer | Escherichia coli whole cells overexpressing the Lactobacillus brevis alcohol dehydrogenase (ADH) and the Candida boidinii formate dehydrogenase (FDH) | (Dennewald et al. 2011) |
| 14 | [BMIM][PF6]/isoamyl alcohol | Candida antarctica lipase B (CalB) | (Eisenmenger and Reyes-De-Corcuera 2010) |
| 15 | [BMIM][PF6]/buffer | Recombinant β-d-glucuronidase from Escherichia coli BL21 (PGUS-E) | (He et al. 2010) |
| Reaction-induced phase change | |||
| 16 | Various Ammoeng ILs/reactant phase | Various lipases | (Devi et al. 2011) |
| Supported ILs | |||
| 17 | Membrane separator containing [BMIM][NTf2] and [HMIM][PF6] | Biocatalytic electrochemical cell (supported ionic liquid membranes (SILMs)) | (Koók et al. 2017) |
| 18 | [OMIM][BF4]/MTBE as transport phase | Candida antarctica lipase B (CalB) | (Sandig et al. 2015) |
| Influence of IL-based biphasic reaction system | |||
| 19 | [BMIM][PF6]/buffer | Effects of [BMIM][PF6] on Saccharomyces cerevisiae | (Song et al. 2011) |
| 20 | Various ILs/buffer | Escherichia coli K-12 agar diffusion tests and growth inhibition tests | (Wood et al. 2011) |
As mentioned above, biphasic reaction systems are herein mainly applied for poorly water soluble, unpolar substrates and products, wherein the IL phase represents the substrate reservoir and in situ-extractant for the product, which also simplifies downstream processing. In addition, the high hydrophobicity of the ILs may also prevent negative effects to the catalytic system, e.g. invasive effects on cellular membranes. For example, Zhang et al. reported the use of various hydrophobic ILs in a biphasic reaction system for the conversion of ATMS (acetyltrimethylsilane) to (R)-1-TMSE ((R)-1-trimethylsilylethanol) (Fig. 3; Table 1, entry 11).
Fig. 3.
Enantioselective reduction of ATMS to (R)-1-TMSE by Candida parapsilosis CCTCC M203011 whole cells in a biphasic IL/buffer system
Both reactants are highly toxic to the used whole cell biocatalyst and the authors investigated various water-immiscible ILs to overcome this limitation, resulting in 1-butyl-3-methylimidazolium hexafluorophosphate [BMIM][PF6]/buffer biphasic system providing optimal conditions. Significantly higher reactant concentration with a high operational stability was found. The immobilized cells retain their high activity in the biphasic system and the presented reaction system was eventually applied at preparative scale with > 90 yield and > 99% e.e.(R).
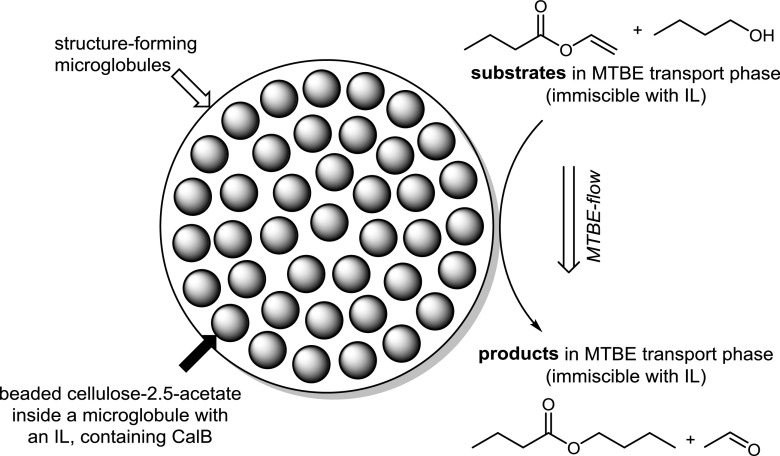
A different approach with a non-aqueous biphasic reaction system was recently presented by Sandig et al. in 2015 (Table 1, entry 18). Here a porous monolithic hybrid support containing immobilized Candida antarctica lipase B (CalB) and octylmethylimidazolium tetrafluoroborate [OMIM][BF4] in swollen cellulose-2.5-acetate beads was applied (Fig. 4). This concept basically represents a supported IL phase (SILP) for CalB, while an external solvent (in this case MTBE) is used as a continuous phase that contains all required reactants. The experimental setup was continuously operated in a 0.46 × 15 cm column at 50 °C achieving a productivity of 58 mmol mg−1 (CalB) min−1, which corresponds in this experiment to a total turnover number (TON) of 3.9 × 107.
Fig. 4.
Hybrid support containing immobilized Candida antarctica lipase B (CalB) and octylmethylimidazolium tetrafluoroborate ([OMIM][BF4]) in cellulose-2.5-acetate beads
A product-based-induced phase change during the reaction was evaluated in detail by Devi et al. for the enzymatic methanolysis of rapeseed oil (transesterification) (Fig. 5; Table 1, entry 16) to produce fatty acid methyl ester (FAME, biodiesel). This reaction system facilitates 98% biodiesel yield in combination with a simplified downstream processing via phase separation.
Fig. 5.
Methanolysis of triglycerides forming biodiesel; phase separation was obtained by addition of Ammoeng 102 or Ammoeng 120
In this study, the authors investigated 24 commercially available ILs with different cations and anions and evaluated the reaction characteristics and phase behaviour. Finally, they selected the most promising candidates Ammoneng 102 and Ammoeng 120. This reaction system shows the unique property to spontaneously separate into immiscible phases during the reaction. The formation of mono- and diglycerides initially yields an intermittent ternary phase system with an oil phase, mesophase and IL phase. After completion of the reaction, the reaction system separates into a binary phase system of an oil phase (FAME) and IL phase (MeOH, glycerol and IL), which simplifies downstream processing to a large extent.
Thermo-regulated multi-component solvent systems
In contrast to classical biphasic systems, thermo-regulated multi-component systems facilitate a temperature-induced phase change, which is based on the corresponding phase diagram (see Fig. 1). By a change of temperature, the reaction systems can be switched between a one- and two-phase system. Such systems are also called thermomorphic systems. For example, Mai et al. have developed such a temperature-regulated two-phase system composed of amino acid-based IL and water (LCST-type, see above) (Mai and Koo 2014). The system was used for an enzymatic in situ penicillin G hydrolysis (see Scheme 1) and product separation. In a phosphate buffer with 30 wt% IL (tetra-n-butylphosphonium trifluoromethanesulfonylleucine, [TBP][Tf-Leu], the reaction was carried out at 37 °C with a hydrolysis yield of 87.1%. After reaction, the system was heated to 42 °C and a phase separation occurred. The ILs could be reused for at least five cycles without significant loss in hydrolysis efficiency when fresh enzyme was added.
Scheme 1.
Enzymatic hydrolysis of penicillin G (Mai and Koo 2014)
Lozano et al. used a similar reaction system with the IL 1-methyl-3-octadecylimidazolium bis(trifluoromethylsulfonyl)imide [C18MIM] [NTf2] and the biocatalyst Novozym 435 for the methanolysis of triolein to produce biodiesel (Fig. 6) (Lozano et al. 2010).
Fig. 6.
Methanolysis of triolein (A) and structure of IL [C18C1IM] [NTf2] (B) by Lozano et al. (2010)
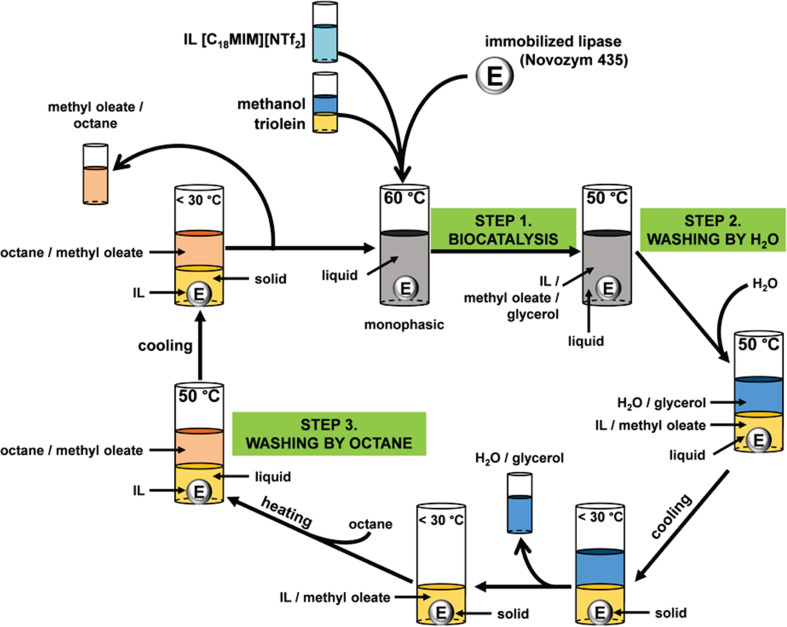
The reaction including the downstream processing pathway is shown in Fig. 7. The initial reaction was initiated by addition of triolein and methanol to [C18MIM][NTf2]. The system was subsequently heated to 60 °C and became monophasic due to its UCST-type behaviour. Afterwards, Novozym 435 was added to initiate the reaction (step 1, Fig. 7) and after the reaction, water was added at 50 °C to extract methanol and glycerol, resulting in a two-phase system (step 2, Fig. 7). After cooling, the aqueous phase was removed and, as a second extraction step, octane was added to the remaining solid IL phase. After heating to 50 °C, the biodiesel (methyl oleate) was extracted (step 3, Fig. 7) at 30 °C. Finally, the residual IL layer was dried under a vacuum to remove traces of octane. A new process cycle was started by adding methanol and triolein and at least seven runs were performed, and no loss of activity of biocatalytic reaction was observed. Also, the IL [C16BIM][NTf2] (de Diego et al. 2011a, b) was used with a similar experimental procedure for the synthesis of biodiesel from triolein/methanol. The concept was even expanded to utilize vegetable and waste cooking oils as substrates with ILs [C16BIM][NTf2] and [NC1C1C1C18][NTf2] (Lozano et al. 2016).
Fig. 7.
Reaction and recovery pathway of the carried out transesterification of triolein by Lozano et al. (2010)
Furthermore, Lozano et al. published the biocatalytic direct esterification of flavor alcohols (isoamyl alcohol, nerol, citronellol or geraniol) and aliphatic carboxylic acids (acetic acid, propionic acid, butyric acid and valeric acid) with immobilized Candida antarctica lipase B (Novozym 435) with the IL [NC1C1C1C16]][NTf2] (see Fig. 8) (Lozano et al. 2012). The reactions were carried out in temperature-switchable IL/solid phases system leading to easy product recovery. Sixteen different flavor esters were synthesized via this approach.
Fig. 8.
Flavor esters synthesized in a temperature-switchable IL/solid phase system used by Lozano et al. (2012)
For the direct esterification reaction, the desired alcohol and carboxylic acid were added as a second phase to solid IL [NC1C1C1C16]][NTf2] (Fig. 9). The reaction was started by adding Novozym 435 and subsequent heating to 50 °C resulting in a monophasic and fully clear reaction solution. After 14 h, the reaction mixtures were cooled and consecutively centrifuged four times at room temperature, 21, 10 and 4 °C, resulting in a bottom solid phase containing IL and immobilized enzyme and a clear top IL-free liquid phase containing products and non-reacted substrates. The flavor ester and the non-reacted substrates were decanted and the remaining IL/enzyme solid phase was conditioned under a vacuum to remove any remaining product/substrate traces. By adding a new liquid phase of alcohol and carboxylic acid, a new process cycle can be initiated at least for seven times. Product yields up to > 99% were achieved. Unfortunately, a scale up of the whole reaction protocol to produce several grams or even kilograms was not shown in the study.
Fig. 9.
Scheme of the cyclic protocol used by Lozano et al. (2012)
Further investigations include the biocatalytic synthesis of anisyl acetate fragrance with ILs [NC1C1C1C16][NTf2] and [NC1C1C1C18][NTf2] as reaction/separation media under conventional and microwave heating (Lozano et al. 2014), and the biocatalytic synthesis of monoacylglycerides by the direct esterification of fatty acids with glycerol in different ILs (Lozano et al. 2017).
Polymerized ionic liquids
In contrast to classical biphasic systems, recently, a novel catalytic concept involving cross-linked ILs in gel-like compositions was reported in literature (Claus et al. 2018). Herein typically free radical polymerization of vinyl groups at the cation of the IL is polymerized, e.g. 1-vinyl-3-ethylimidazolium together with a cross-linker, which creates a three-dimensional network and consequently encapsulates in some form the corresponding biocatalyst. Usually, additional surfactants are preferred to generate a microencapsulation before curing and thus solidifying the IL to an IL-polymer-gel, which is typically referred to as polymerized ILs (PILs) (Fig. 10). This concept was used for the encapsulation of Candida rugose lipase by Moniruzzaman et al. (2012), horseradish peroxidase by Nakashima et al. (2009) and glucose oxidase by Sánchez-Paniagua López et al. (2006).
Fig. 10.
Schematic representation of the incorporation of enzymes into polymerized ILs
Generally, slightly lower activities were found with these enzyme preparations, which seems to originate mainly from the entrapment procedure. The presence of free radicals during the radical polymerization might affect the encapsulated enzyme, and the formed polymer layer around the biocatalyst will eventually cause some kind of diffusion limitation.
We have recently immobilized raw extract of CaLB in hydrogels based on [VEIm][Br] and compared in the kinetic resolution of 1-phenylethanol with commercially available immobilisates such as Novozym 435 (Grollmisch et al. submitted).
Conclusions
The use of ILs is a powerful alternative to classical organic solvents in biocatalysis and thus avoids the use of hazardous compounds. In this review, we summarize the use of ILs in multiphasic IL-based systems such as classical biphasic solvent systems (liquid-liquid), thermo-regulated multi-component solvent systems (temperature-induced phase changes) and polymerized ILs (solid-liquid). An overview of recent developments in the use of ILs-solvent mixtures with respect to a defined biocatalytic reaction has been presented in detail. However, it should be kept in mind that the interaction of ILs especially with proteins is better described using the Hofmeister series rather than treating the IL similar to a molecular solvent and using octanol-water distribution coefficients respectively the logP-concept (Yang 2009).
Due to the ionic structure within the huge group of ILs, these reaction concepts facilitate unique conditions with a high potential for future applications on a laboratory and industrial scale. This primarily includes their use for the design of very stable biocatalyst preparations in combination with a simplified downstream processing due to the presence of phase boundary. Examples are applications in biodiesel and flavor ester synthesis. Typical transfer limitations can be also overcome by the use of supported IL phases, which combine the classical advantages of biocatalyst immobilization with the advantageous properties of ILs.
Future research will most likely concentrate on further developments of polymerized ILs and specifically tailor-made ILs for existing processes to enhance productivity and atom efficiency of biocatalytic reactions. In addition, concept to recycle and re-use ILs should be developed to improve the commercial applicability of the shown ILs. Herein multiphasic approaches, e.g. by using water-immiscible ILs, will be preferred.
Compliance with ethical standards
Funding information
The authors received funding from the German Federal Ministry of Education and Research (BMBF—Bundesministerium für Bildung und Forschung; project number: 031A123) and German Research Foundation (Deutsche Forschungsgemeinschaft, grant numbers: 252186816 and 386850916).
Conflict of interest
Lars-Erik Meyer declares that he has no conflict of interest. Jan von Langermann declares that he has no conflict of interest. Udo Kragl declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Ionic Liquids and Biomolecules’ edited by Antonio Benedetto and Hans-Joachim Galla.
References
- Behr A, Johnen L, Daniel B. A liquid immobilisation concept for enzymes by thermomorphic solvent systems. Green Chem. 2011;13:3168. [Google Scholar]
- Benedetto A, Ballone P (2016a) Room temperature ionic liquids interacting with bio-molecules: an overview of experimental and computational studies. Philos Mag 96:870
- Benedetto A, Ballone P (2016b) Room temperature ionic liquids meet biomolecules: a microscopic view of structure and dynamics. ACS Sustainable Chem Eng 4:392
- Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K. Engineering the third wave of biocatalysis. Nature. 2012;485:185. doi: 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- Brenna E, Crotti M, Gatti FG, Manfredi A, Monti D, Parmeggiani F, Santangelo S, Zampieri D. Enantioselective synthesis of (R)-2-arylpropanenitriles catalysed by ene-reductases in aqueous media and in biphasic ionic liquid-water systems. ChemCatChem. 2014;6:2425. [Google Scholar]
- Bridges NJ, Gutowski KE, Rogers RD. Investigation of aqueous biphasic systems formed from solutions of chaotropic salts with kosmotropic salts (salt–salt ABS) Green Chem. 2007;9:177. [Google Scholar]
- Busacca CA, Fandrick DR, Song JJ, Senanayake CH. The growing impact of catalysis in the pharmaceutical industry. Adv Synth Catal. 2011;353:1825. [Google Scholar]
- Choi HJ, Uhm K-N, Kim H-K. Production of chiral compound using recombinant Escherichia coli cells co-expressing reductase and glucose dehydrogenase in an ionic liquid/water two phase system. J Mol Catal B: Enzym. 2011;70:114. [Google Scholar]
- Claus J, Sommer FO, Kragl U. Ionic liquids in biotechnology and beyond. Solid State Ionics. 2018;314:119. [Google Scholar]
- Dennewald D, Pitner W-R, Weuster-Botz D. Recycling of the ionic liquid phase in process integrated biphasic whole-cell biocatalysis. Process Biochem. 2011;46:1132. [Google Scholar]
- Devi BLAP, Guo Z, Xu X (2011) Characterization of ionic liquid-based biocatalytic two-phase reaction system for production of biodiesel. AIChE J 57:1628
- de Diego T, Manjón A, Lozano P, Iborra JL. A recyclable enzymatic biodiesel production process in ionic liquids. Bioresour Technol. 2011;102:6336. doi: 10.1016/j.biortech.2011.02.071. [DOI] [PubMed] [Google Scholar]
- de Diego T, Manjón A, Lozano P, Vaultier M, Iborra JL. An efficient activity ionic liquid-enzyme system for biodiesel production. Green Chem. 2011;13:444. [Google Scholar]
- Eisenmenger MJ, Reyes-De-Corcuera JI. Enhanced synthesis of isoamyl acetate using an ionic liquid–alcohol biphasic system at high hydrostatic pressure. J Mol Catal B: Enzym. 2010;67:36. [Google Scholar]
- Fischer F, Happe M, Emery J, Fornage A, Schütz R. Enzymatic synthesis of 6- and 6′-O-linoleyl-α-d-maltose: From solvent-free to binary ionic liquid reaction media. J Mol Catal B Enzym. 2013;90:98. [Google Scholar]
- Freire MG, Cláudio AFM, Araújo JMM, Coutinho JAP, Marrucho IM, Canongia Lopes JN, Rebelo LPN. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem Soc Rev. 2012;41:4966. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- Grollmisch, A.; Kragl, U.; Großeheilmann, J. SynOpen, submitted
- Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J Am Chem Soc. 2003;125:6632. doi: 10.1021/ja0351802. [DOI] [PubMed] [Google Scholar]
- Hallett JP, Welton T. Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem Rev. 2011;111:3508. doi: 10.1021/cr1003248. [DOI] [PubMed] [Google Scholar]
- He D-M, Kaleem I, Qin S-Y, Dai D-Z, Liu G-Y, Li C. Biosynthesis of glycyrrhetic acid 3-O-mono-β-d-glucuronide catalyzed by β-d-glucuronidase with enhanced bond selectivity in an ionic liquid/buffer biphasic system. Process Biochem. 2010;45:1916. [Google Scholar]
- Hernández Fernández FJ, Pérez de los Ríos A, Quesada-Medina J, Sánchez-Segado S (2015) Ionic Liquids as Extractor Agents and Reaction Media in Ester Synthesis. ChemBioEng Rev 2:44
- Hou Q, Li W, Zhen M, Le Liu, Chen Y, Yang Q, Huang F, Zhang S, Ju M (2017) An ionic liquid–organic solvent biphasic system for efficient production of 5-hydroxymethylfurfural from carbohydrates at high concentrations. RSC Adv 7:47288
- Itoh T. Ionic liquids as tool to improve enzymatic organic synthesis. Chem Rev. 2017;117:10567. doi: 10.1021/acs.chemrev.7b00158. [DOI] [PubMed] [Google Scholar]
- Kaleem I, Rasool A, Lv B, Riaz N, Hassan JU, Manzoor R, Li C. Immobilization of purified β-glucuronidase on ZnO nanoparticles for efficient biotransformation of glycyrrhizin in ionic liquid/buffer biphasic system. Chem Eng Sci. 2017;162:332. [Google Scholar]
- Koók L, Nemestóthy N, Bakonyi P, Göllei A, Rózsenberszki T, Takács P, Salekovics A, Kumar G, Bélafi-Bakó K. On the efficiency of dual-chamber biocatalytic electrochemical cells applying membrane separators prepared with imidazolium-type ionic liquids containing [NTf2]- and [PF6]-anions. Chem Eng J. 2017;324:296. [Google Scholar]
- Kragl U, Eckstein M, Kaftzik N. Enzyme catalysis in ionic liquids. Curr Opin Biotechnol. 2002;13:565. doi: 10.1016/s0958-1669(02)00353-1. [DOI] [PubMed] [Google Scholar]
- López MS-P, Mecerreyes D, López-Cabarcos E, López-Ruiz B. Amperometric glucose biosensor based on polymerized ionic liquid microparticles. Biosens Bioelectron. 2006;21:2320. doi: 10.1016/j.bios.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Lozano P, Bernal JM, Piamtongkam R, Fetzer D, Vaultier M. One-phase ionic liquid reaction medium for biocatalytic production of biodiesel. ChemSusChem. 2010;3:1359. doi: 10.1002/cssc.201000244. [DOI] [PubMed] [Google Scholar]
- Lozano P, Bernal JM, Navarro A. A clean enzymatic process for producing flavour esters by direct esterification in switchable ionic liquid/solid phases. Green Chem. 2012;14:3026. [Google Scholar]
- Lozano P, Bernal J, Lajarin A, Romera D, Garcia-Verdugo E, Sanchez-Gomez G, Pucheault M, Vaultier M, Burguete M, Luis S. A green approach for producing solvent-free anisyl acetate by enzymecatalyzed direct esterification in sponge-like ionic liquids under conventional and microwave heating. Curr Green Chem. 2014;1:145. [Google Scholar]
- Lozano P, Gomez C, Nicolas A, Polo R, Nieto S, Bernal JM, García-Verdugo E, Luis SV. Clean enzymatic preparation of oxygenated biofuels from vegetable and waste cooking oils by using spongelike ionic liquids technology. ACS Sustainable Chem Eng. 2016;4:6125. [Google Scholar]
- Lozano P, Gomez C, Nieto S, Sanchez-Gomez G, García-Verdugo E, Luis SV. Highly selective biocatalytic synthesis of monoacylglycerides in sponge-like ionic liquids. Green Chem. 2017;19:390. [Google Scholar]
- Luetz S, Giver L, Lalonde J (2008) Engineered enzymes for chemical production. Biotechnol Bioeng 101:647 [DOI] [PubMed]
- Mai NL, Koo Y-M. Enzymatic hydrolysis of penicillin and in situ product separation in thermally induced reversible phase-separation of ionic liquids/water mixture. Enzyme Microb Technol. 2014;63:34. doi: 10.1016/j.enzmictec.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Sugimoto T, Ishiguro Y, Yamaguchi H, Kondo K. Effect of organic solvents and ionic liquids on resolution of 2-epoxyhexane by whole cells of Rhodotorula glutinis in a two-liquid phase system. J Chem Technol Biotechnol. 2014;89:522. [Google Scholar]
- Moniruzzaman M, Nakashima K, Kamiya N, Goto M. Recent advances of enzymatic reactions in ionic liquids. Biochem Eng J. 2010;48:295. [Google Scholar]
- Moniruzzaman M, Ino K, Kamiya N, Goto M. Lipase incorporated ionic liquid polymers as active, stable and reusable biocatalysts. Org Biomol Chem. 2012;10:7707. doi: 10.1039/c2ob25529d. [DOI] [PubMed] [Google Scholar]
- Muñoz Solano D, Hoyos P, Hernáiz MJ, Alcántara AR, Sánchez-Montero JM. Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. Bioresour Technol. 2012;115:196. doi: 10.1016/j.biortech.2011.11.131. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Kamiya N, Koda D, Maruyama T, Goto M. Enzyme encapsulation in microparticles composed of polymerized ionic liquids for highly active and reusable biocatalysts. Org Biomol Chem. 2009;7:2353. doi: 10.1039/b823064a. [DOI] [PubMed] [Google Scholar]
- Naushad M, Alothman ZA, Khan AB, Ali M. Effect of ionic liquid on activity, stability, and structure of enzymes: a review. Int J Biol Macromol. 2012;51:555. doi: 10.1016/j.ijbiomac.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Nestl BM, Nebel BA, Hauer B. Recent progress in industrial biocatalysis. Curr Opin Chem Biol. 2011;15:187. doi: 10.1016/j.cbpa.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Olivier-Bourbigou H, Magna L, Morvan D (2010) Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl Catal A 373:1
- Oppermann S, Stein F, Kragl U. Ionic liquids for two-phase systems and their application for purification, extraction and biocatalysis. Appl Microbiol Biotechnol. 2011;89:493. doi: 10.1007/s00253-010-2933-4. [DOI] [PubMed] [Google Scholar]
- Patel RN. Biocatalysis: Synthesis of key intermediates for development of pharmaceuticals. ACS Catal. 2011;1:1056. [Google Scholar]
- Pohar A, Žnidaršič-Plazl P, Plazl I. Integrated system of a microbioreactor and a miniaturized continuous separator for enzyme catalyzed reactions. Chem Eng J. 2012;189-190:376. [Google Scholar]
- Reetz MT. Biocatalysis in organic chemistry and biotechnology: past, present, and future. J Am Chem Soc. 2013;135:12480. doi: 10.1021/ja405051f. [DOI] [PubMed] [Google Scholar]
- Renata H, Wang ZJ, Arnold FH. Expanding the enzyme universe: accessing non-natural reactions by mechanism-guided directed evolution. Angew Chem Int Ed Engl. 2015;54:3351. doi: 10.1002/anie.201409470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein F, Kragl U (2014) Biocatalytic reactions in ionic liquids. In: Plechkova NV, Seddon KR (eds) Ionic liquids further unCOILed, vol 5. John Wiley & Sons, Inc, Hoboken, pp 193–216
- Sandig B, Michalek L, Vlahovic S, Antonovici M, Hauer B, Buchmeiser MR. A monolithic hybrid cellulose-2.5-acetate/polymer bioreactor for biocatalysis under continuous liquid-liquid conditions using a supported ionic liquid phase. Chem Eur J. 2015;21:15835. doi: 10.1002/chem.201501618. [DOI] [PubMed] [Google Scholar]
- Schmideder A, Priebe X, Rubenbauer M, Hoffmann T, Huang F-C, Schwab W, Weuster-Botz D. Non-water miscible ionic liquid improves biocatalytic production of geranyl glucoside with Escherichia coli overexpressing a glucosyltransferase. Bioprocess Biosyst Eng. 2016;39:1409. doi: 10.1007/s00449-016-1617-6. [DOI] [PubMed] [Google Scholar]
- Sheldon, R. A. CHAPTER 2. Biocatalysis in ionic liquids. In Catalysis in ionic liquids; Hardacre, C., Parvulescu, V., Eds.; Catalysis series; Royal Society of Chemistry: Cambridge, 2014; pp 20–43
- Sheldon RA. Biocatalysis and biomass conversion in alternative reaction media. Chem Eur J. 2016;22:12984. doi: 10.1002/chem.201601940. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Lau RM, Sorgedrager MJ, van Rantwijk F, Seddon KR. Biocatalysis in ionic liquids. Green Chem. 2002;4:147. [Google Scholar]
- Singh R, Tiwari M, Singh R, Lee J-K (2013) From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes. Int J Mol Sci 14:1232 [DOI] [PMC free article] [PubMed]
- Sivapragasam M, Moniruzzaman M, Goto M. Recent advances in exploiting ionic liquids for biomolecules: Solubility, stability and applications. Biotechnol J. 2016;11:1000. doi: 10.1002/biot.201500603. [DOI] [PubMed] [Google Scholar]
- Song X-L, Ye S-Y, Xie R, Yin L, Shi X, Luo S-C, Korean J. Effects of bmim[PF6] treatments with different concentrations on microbial activity of Saccharomyces cerevisiae. Chem Eng. 2011;28:1902. [Google Scholar]
- Stepankova V, Bidmanova S, Koudelakova T, Prokop Z, Chaloupkova R, Damborsky J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013;3:2823. [Google Scholar]
- Temme H, Dethloff O, Pitner W-R, Fischer S, Scheurich R, Schulte M, Niemeyer B. Identification of suitable ionic liquids for application in the enzymatic hydrolysis of rutin by an automated screening. Appl Microbiol Biotechnol. 2012;93:2301. doi: 10.1007/s00253-011-3749-6. [DOI] [PubMed] [Google Scholar]
- Turner NJ (2003) Directed evolution of enzymes for applied biocatalysis. Trends Biotechnol 21:474 [DOI] [PubMed]
- Voges M, Fischer C, Wolff D, Held C. Influence of natural solutes and ionic liquids on the yield of enzyme-catalyzed reactions: Measurements and predictions. Org Process Res Dev. 2017;21:1059. [Google Scholar]
- Wenda S, Illner S, Mell A, Kragl U. Industrial biotechnology - the future of green chemistry. Green Chem. 2011;13:3007. [Google Scholar]
- Wohlgemuth R (2010) Biocatalysis--key to sustainable industrial chemistry. Curr Opin Biotechnol 21:713 [DOI] [PubMed]
- Wood N, Ferguson JL, Gunaratne HQN, Seddon KR, Goodacre R, Stephens GM. Screening ionic liquids for use in biotransformations with whole microbial cells. Green Chem. 2011;13:1843. [Google Scholar]
- Xu P, Du P-X, Zong M-H, Li N, Lou W-Y. Combination of deep eutectic solvent and ionic liquid to improve biocatalytic reduction of 2-octanone with Acetobacter pasteurianus GIM1.158 cell. Sci Rep. 2016;6:26158. doi: 10.1038/srep26158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysis. J Biotechnol. 2009;144:12. doi: 10.1016/j.jbiotec.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Yu C-Y, Wei P, Li X-F, Zong M-H, Lou W-Y. Using ionic liquid in a biphasic system to improve asymmetric hydrolysis of styrene oxide catalyzed by Cross-Linked Enzyme Aggregates (CLEAs) of Mung Bean epoxide hydrolases. Ind Eng Chem Res. 2014;53:7923. [Google Scholar]
- Zhang B-B, Cheng J, Lou W-Y, Wang P, Zong M-H. Efficient anti-prelog enantioselective reduction of acetyltrimethylsilane to (R)-1-trimethylsilylethanol by immobilized Candida parapsilosis CCTCC M203011 cells in ionic liquid-based biphasic systems. Microb Cell Fact. 2012;11:108. doi: 10.1186/1475-2859-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. Methods for stabilizing and activating enzymes in ionic liquids-a review. J Chem Technol Biotechnol. 2010;85:891. [Google Scholar]
- Zhao H, Baker GA. Ionic liquids and deep eutectic solvents for biodiesel synthesis: a review. J Chem Technol Biotechnol. 2013;88:3–12. [Google Scholar]