Abstract
Ionic liquids (ILs) are a vast class of organic non-aqueous electrolytes whose interaction with biomolecules is receiving great attention for potential applications in bio-nano-technology. Recently, it has been shown that ILs can affect protein amyloidogenesis. Whereas some ILs favour the aggregation of proteins into amyloids, others inhibit their formation. Moreover, ILs can dissolve mature fibrils and restore the protein biochemical function. In this letter, we present a brief state-of-the-art summary of this emerging field that holds the promise of important developments both in basic science and in applications from bio-medicine to material science, and bio-nano-technology. The huge variety of ILs offers a vast playground for future studies and potential applications.
Introduction
Protein-protein interactions and their role in several biological functions are fascinating areas of research with potential applications in bio-nano-technology and a non-negligible impact in human wellbeing. Even though the interaction between proteins is essential for life, the aberrant increase in protein-protein interactions can lead to the formation of proteins’ aggregates known as amyloids, which are playing a major role in several diseases such as Alzheimer’s, diabetes type 2 and spongiform encephalopathies. Amyloids are insoluble fibres that can be found in tissues and organs. They are the result of multiple aggregation stages of specific proteins or peptides taking place under different physiological conditions such as pH, temperature and concentration (Hamley 2012). The effect of several inorganic ions, salts, and complexes on the formation and inhibition of amyloids is a widely studied area of research aimed to find solutions to resist various neurodegenerative disorders (Liu et al. 2005; Yeh et al. 2010; Ghalebani et al. 2012; Zhu et al. 2014; Branch et al. 2015; Kong et al. 2015; Yugay et al. 2016). In this respect, ionic liquids (ILs), which can be seen as the organic equivalent of inorganic salts, are the next potential good candidates to be studied.
ILs are a vast class of ionic compounds made by an organic cation and either an organic or inorganic anion; they are liquid around room temperature and have a very low vapour pressure (Welton 1999). Their high tunability and promising biocompatibility attracted the interest of the biophysical community. As a result, several studies have been carried out in the last two decades on the interaction between ILs and biomolecules, such as proteins, biomembranes, nucleic acids, and saccharides (Benedetto and Ballone 2016a, b, 2018; Benedetto 2017; Benedetto and Galla 2017). For the best of our knowledge, the first study on the ability of ILs to stabilize proteins was published in 2000 by Summers and Flowers. In their seminal work, they have shown the renaturation capability of the protic IL ethylammonium nitrate (EAN) on lysozyme (Summers and Flowers 2000). Followed by this work, several investigations on the effects of ILs on proteins have been carried out as a function of concentration, pH, cation’s chain length, anion species, etc. Different proteins like lysozyme, serum albumins, myoglobin and haemoglobin, and α-chymotrypsin in presence of different ILs based on imidazolium, ammonium, phosphonium, and pyridinium were employed for structure, stability, dynamics, and biochemical studies. The diverse number of research works published in this domain is beyond the scope of this letter, to the interested readers, we suggest to start with some of the recent reviews on the topic (Benedetto and Ballone 2016a, b; Kumar et al. 2017; Schroeder 2017). In this letter, instead, we would like to focus on the effect of ILs on amyloids formation and inhibition, which is an emerging topic in the area of IL-protein interaction, holding the promise of important applications in bio-nano-technology.
Ionic liquids in protein amyloidogenesis: a brief screenshot of the state-of-the-art
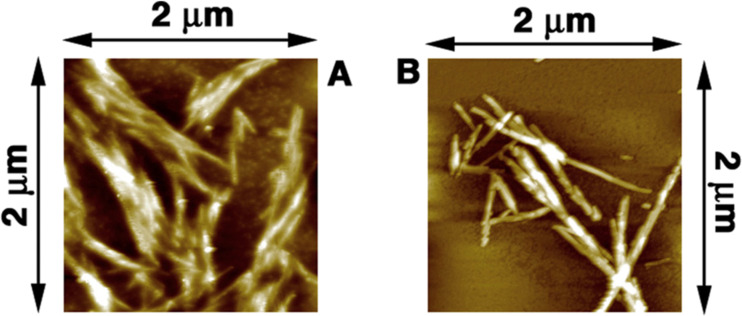
One of the major steps in protein amyloidogenesis consists in the unfolding of the protein itself. If the unfolding is forbidden or partially inhibited, proteins may maintain their biological function and they do not aggregate into amyloids. In a similar way, if amyloid aggregates can be dissolved, and proteins are properly re-folded, the biological function can then be restored. In this respect, Byrne and Angell have shown the ability of common protic ILs, like EAN, to favour the unfolding and aggregation of lysozyme into fibrils, and then promoting their dissolution to restore the protein biochemical function (Byrne et al. 2007; Byrne and Angell 2008, 2009). Following this work, Kalhor et al. (2009) have demonstrated the inhibitory and reversible effect of another protic IL, i.e., tetramethylguanidinium acetate, towards the amyloid formation of lysozyme. Transmission electron microscopy images showed that the fibrils formed in this protic IL were thinner, and optical spectroscopy data revealed that the inhibitory effect was mainly due to the functional carboxy group present in the anionic part of the IL. Takekiyo and co-workers have linked this behaviour to the nanoheterogeneity of the aqueous solutions of ILs (Takekiyo et al. 2012). In their work, they have shown also how an aprotic IL, i.e., 1-butyl-3-methylimidazolium nitrate, is able to inhibit the fibrillation of lysozyme. They had also revealed that at higher IL concentrations non-native (α-helical) structural transformations could occur in proteins (Takekiyo et al. 2013). More recently, Basu et al. (2018) have investigated the ability of another aprotic IL, i.e., 1-butyl-3-methylimidazolium bromide, in suppressing the amyloidogenesis of lysozyme (Fig. 1).
Fig. 1.
Atomic force microscopy image of lysozyme fibrils (a) in the absence and (b) in the presence of the 1-butyl-3-methylimidazolium bromide IL after 8 h of incubation taken from (Basu et al. 2018). The images show the ability of this IL to attenuate the fibrillogenesis of lysozyme, something that may be useful in developing new therapeutical strategies against amyloids. Figure reproduced with permission from the publisher
The refolding ability of EAN on lysozyme pointed out by Byrne and Angell has then been investigated by Mangialardo and co-workers by Raman spectroscopy (Mangialardo et al. 2012). The refolding pathway was studied by monitoring in the Raman spectra (i) the amide bands which show the changes in the protein secondary structure and (ii) the Tyr, Trp doublet which is a signature of the protein tertiary structure. In this study, a clear refolding ability of EAN in comparison to other ILs with higher alkyl chain length has been registered.
The effect of ILs on protein amyloidogenesis has been studied by taking also into account other proteins rather than the only model protein lysozyme. Interestingly, Takekiyo and co-workers (Takekiyo et al. 2016) have shown the high suppression effect of the protic IL EAN on the fibrillation of insulin. Their experimental results have revealed the overriding role of the interaction of ILs with the amino acid residues as a potential reason for the suppressive behaviour. This result, when compared with the one of Byrne and Angell mentioned above, highlights how the effect of ILs on proteins’ amyloidogenesis strongly depends on both the protein and the IL. Pannuru and co-workers have focused on insulin as well, where they have shown the effective role of novel ammonium-based protic ILs to prevent its self-aggregation (Kumar and Venkatesu 2013). By varying anions, they have also observed that bromide and chloride provided the long-term stabilization against aggregation in comparison with various other anions (Kumar and Venkatesu 2014). The fibril inhibition ability of surface-active imidazolium-based ILs on serum albumin proteins has been investigated by Nilmoni and co-workers (Kundu et al. 2017). Their experimental results have revealed the disruption of fibrils by these surface-active ILs due to the hydrophobicity related with the long alkyl chain length of the cation. The effect of ILs on model amyloid fibres has been investigated as well. It has been shown that the amyloid fibrillations in Aβ16–22 and Aβ1–40 peptides is inhibited in triethylammonium mesylate and triethylammonium methane sulfonate, respectively (Debeljuh et al. 2011a, b).
Also, the ability of hydrated ILs in re-folding proteins has been proved. For instance, Dhathathreyan and co-workers have observed that reversible and irreversible structural transitions take place in myoglobin while solvated in water solution of amino acid ILs at different concentrations (Sankaranarayanan et al. 2012). At low hydration level of phenylalanine IL, the protein transforms to complete β-sheet from its helical conformation; rehydration reverses the β-sheet to an α-helix. Moreover, Fujita et al. (2016) have shown that hydrated cholinium dihydrogen phosphate is able to dissolve and re-fold the aggregated recombinant cellulase protein from Escherichia coli bacteria.
ILs have also the ability to favour protein amyloidogenesis, rather than only to inhibit it. Several studies, for instance, have shown the role of imidazolium-based ILs in favouring amyloidogenesis (Hwang et al. 2009; Bae et al. 2010, 2011; Debeljuh et al. 2011a). Byrne and co-workers (Debeljuh et al. 2012), moreover, have compared the efficiency of different amine-based ILs on the conversion of Aβ16–22 peptides from monomers to amyloid fibrils; they concluded that the primary amine IL is having higher conversion efficiency than others due to the higher degree of proton transfer. They have also observed that the protic ammonium IL triethylammonium mesylate maintains the native β-barrel structure of the β-lactoglobulin protein at low concentration (20 wt%) even after heating, but at a higher concentration (40 wt%), it induces the formation of amyloids (Byrne et al. 2013). In a recent work, Khan et al. (2017) have demonstrated the role of ammonium-based ILs in favouring the amyloidogenesis of bovine liver catalase.
Table 1 summarizes the above-mentioned data and other studies on the effect of ILs on protein amyloidogenesis.
Table 1.
List of several studies on the effect of ILs on protein amyloidogenesis
| Proteins | Ionic liquids | Techniques | References |
|---|---|---|---|
| Lysozyme | Ethylammonium nitrate | DSC, UV-vis, and fluorescence | Summers and Flowers 2000 |
| Lysozyme | Ethylammonium nitrate | DSC | Byrne et al. 2007 |
| Lysozyme | Ammonium-based protic ionic liquids | DSC, NMR | Byrne and Angell 2008 |
| Lysozyme | Ethylammonium nitrate, triethylammonium mesylate, and triethylammonium triflate | SEM, CD, and fluorescence | Byrne and Angell 2009 |
| Lysozyme | Tetramethylguanidinium cation | TEM, CD, UV-vis, and fluorescence | Kalhor et al. 2009 |
| Lysozyme | 1-butyl-3-methylimidazolium bromide | AFM, CD, UV-vis, and fluorescence | Basu et al. 2018 |
| Lysozyme | 2-methoxy ethyl ammonium nitrate, ethyl ammonium nitrate, propyl ammonium nitrate, and butyl ammonium nitrate | Raman spectroscopy | Mangialardo et al. 2012 |
| Lysozyme | 1-butyl-3-methylimidazolium nitrate | CD, SAXS, FTIR, and Raman spectroscopy | Takekiyo et al. 2012 |
| β-Lactoglobulin | 1-butyl-3-methylimidazolium nitrate, ethyl ammonium nitrate | FTIR, CD | Takekiyo et al. 2013 |
| Several proteins | 1-butyl-3-methylimidazolium thiocyanate | FTIR, CD, and Raman spectroscopy | Takekiyo et al. 2015 |
| Insulin | Ammonium-based protic ionic liquids | UV-vis, fluorescene, CD, and DLS | Kumar and Venkatesu 2013 |
| Insulin | 1-butyl-3-methylimidazolium cation | UV-vis, fluorescene, CD, and DLS | Kumar and Venkatesu 2014 |
| Bovine serum albumin, human serum albumin | 1-methyl-3-octylimidazolium chloride, 1-dodecyl-3-methyllimidazolium chloride, and 1-hexadecyl-3-methyllimidazolium chloride | SEM, fluorescence, FCS, AFM, and surface tension | Kundu et al. 2017 |
| Myoglobin | 1-ethyl-3-methylimidazolium phenylalanine | QCM, UV-vis, fluorescence, SEM, and CD | Sankaranarayanan et al. 2012 |
| Insulin | 1-butyl-3-methylimidazolium thiocyanate; ethyl ammonium nitrate; and propyl ammonium nitrate | FTIR | Takekiyo et al. 2016 |
| α-Synuclein and α-tandem | 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | Chromatography, TEM, CD, fluorescence | Bae et al. 2010 |
| α-Synuclein | Imidazolium-based ionic liquids | Fluorescence | Hwang et al. 2009 |
| α-Lactalbumin, Ca2 binding protein | 1-butyl-3-methylimidazolium cation | Flow cytometry, TEM, and fluorescence | Bae et al. 2011 |
| Aβ(16–22) | Ethylammonium mesylate; diethylammonium mesylate; and triethylammonium mesylate | TEM, CD, and fluorescence | Debeljuh et al. 2012 |
| Aβ (16–22) | Triethylammonium lactate; triethylammonium trifluoroacetate; triethylammonium mesylate; triethylammonium dihydrogen sulfate; triethylammonium hydrogen sulfate; and triethylammonium triflate | TEM, CD, and fluorescence | Debeljuh et al. 2011a |
| Aβ(1–40) | Triethylammonium methanesulfonate | CD, TEM | Debeljuh et al. 2011b |
| β-Lactoglobulin | Triethylammonium acetate; triethylammonium trifluoroacetate; triethylammonium mesylate; and trimethylammonium sulfate | TEM, CD, and fluorescence | Byrne et al. 2013 |
| Bovine liver catalase | Dodecyltrimethylammonium bromide, Tetradecyltrimethylammonium bromide | TEM, CD, fluorescence, UV-vis, isothermal titration calorimetry, DLS, and RLS | Khan et al. 2017 |
| Enzymes | 1-butyl-3-methylimidazolium tetrafluoroborate | TEM, SEM | Kim et al. 2012 |
| Recombinant protein (E. coli) | Cholinium dihydrogen phosphate | CD, and fluorescence | Fujita et al. 2016 |
| Several proteins | 1-alkyl-3-methylimidazolium nitrate | FTIR, UV | Takekiyo et al. 2014 |
| β-Casein | 1-octyl-3-methylimidazolium bromide, 1-butyl-3-methylimidazolium bromide | UV-vis, fluorescence, microcalorimetry, DLS, TEM, and conductivity measurement | Liu et al. 2014 |
| Bovine serum albumin | 1-dodecyl-3-methylimidazolium chloride; amide functionalized 1-dodecyl-3-methylimidazolium chloride; ester functionalized 1-dodecyl-3-methylimidazolium chloride | Isothermal titration calorimetry, DLS, CD, fluorescence, SEM, confocal LSM, and FTIR | Singh and Kang 2015 |
| Recombinant plasminogen activator | N-ethyl-N-methylimidazolium cation | UV-vis, DSC | Buchfink et al. 2010 |
| Heparin | 1-alkyl-3-methylimidazolium chloride | DLS, CD, FTIR, and Raman spectroscopy | Rawat and Bohidar 2015 |
| Ribonuclease A | Choline dihydrogenphosphate, 1-ethyl-3-methylimidazolium dicyanamide | CD, DSC | Constatinescu et al. 2010 |
| Collagen | 1-butyl-3-methylimidazolium chloride; 1-ethyl-3-methylimidazolium chloride; and 1,3-dimethylimidazolium chloride | SEM, CD, UV-vis, thermoporometry, DSC, FTIR, and optical microscopy | Mehta et al. 2014 |
| Collagen | Tributyl methyl phosphoniummethyl sulfate, Tributyl ethyl phosphonium diethylphosphate | Viscometry, UV-vis, fluorescence, CD, FTIR, optical microscopy, MD simulation, and dielectric spectroscopy | Tarannum et al. 2016 |
| Collagen | 1-butyl-3-methylimidazolium chloride | Optical microscopy, FTIR, XRD, and SEM | Meng et al. 2012 |
| Collagen | Bischoline sulfate, 1-butyl-3-methylimidazolium dimethyl phosphate | Optical microscopy, CD, DSC, NMR, and impedance | Tarannum et al. 2018 |
Conclusions and future outlook
ILs are a vast class of organic salts, which shows high affinity with proteins and, more in general, biomolecules and biosystems. In this letter, we had the opportunity to enjoy and discover their quite broad effect on protein amyloidogenesis (Fig. 2). Whereas some ILs are able to inhibit the amyloidogenesis and to dissolve the mature fibrils back to the native protein structures restoring also their biochemical function, other ILs are acting in the opposite direction by favouring the aggregation of proteins into amyloids. Moreover, other ILs are able to make the amyloidogenesis reversible by first favouring the unfolding of proteins and their aggregation into fibrils, and then promoting the fibrils’ dissolution and the refolding of the proteins. Another key ingredient is the presence of water, since it has been shown that in some cases the effect of ILs on protein amyloidogenesis changes by changing the amount of water surrounding the protein. Interestingly, in some cases, ILs that behave in one way with one specific protein show a totally opposite effect on a different protein. This points out the extremely important role of the specificity of the chemical-physical interactions, something that on the one hand makes hard to a priori predict the behaviour of a given IL on a specific protein, but on the other hand represents a vast playground for applications in bio-medicine, pharmacology, diagnostic, therapeutics, material science, food science, and, more in general, bio-nano-technology. In this respect, the huge variety of ILs including, in particular, ILs based on amino acids (Benedetto et al. 2014) and surface-active ILs, together with their tuneable character offer an almost unlimited scenario of combinations and opportunities.
Fig. 2.
A screenshot of ionic liquids in protein amyloidogenesis
Acknowledgments
The authors thank Prof. Pietro Ballone for fruitful discussions. A.B. acknowledges the additional support provided by the School of Physics and the School of Chemistry, University College Dublin, Ireland, and the Laboratory for Neutron Scattering, Paul Scherrer Institute, Switzerland.
Funding
A.B. receives support from the Science Foundation Ireland (SFI) under the Starting Investigator Research Grant 15-SIRG-3538.
Compliance with ethical standards
Conflicts of interest
Visakh VS Pillai declares that he has no conflict of interest. Antonio Benedetto declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Ionic Liquids and Biomolecules’ edited by Antonio Benedetto and Hans-Joachim Galla
References
- Bae SY, Kim S, Hwang H, Kim HK, Yoon HC, Kim JH, Lee SY, Kim TD. Amyloid formation and disaggregation of alpha-synuclein and its tandem repeat (alpha-TR) Biochem Biophys Res Commun. 2010;400:531–536. doi: 10.1016/j.bbrc.2010.08.088. [DOI] [PubMed] [Google Scholar]
- Bae SY, Kim S, Lee BY, Kim KK, Kim TD. Amyloid formation using 1-butyl-3-methyl-imidazolium based ionic liquids. Anal Biochem. 2011;419:354–356. doi: 10.1016/j.ab.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Basu A, Bhattacharya SC, Kumar GS. Influence of the ionic liquid 1-butyl-3-methylimidazolium bromide on amyloid fibrillogenesis in lysozyme: evidence from photophysical and imaging studies. Int J Biol Macromol. 2018;107:2643–2649. doi: 10.1016/j.ijbiomac.2017.10.152. [DOI] [PubMed] [Google Scholar]
- Benedetto A. Room-temperature ionic liquids meet bio-membranes: the state-of-the-art. Biophys Rev. 2017;9:309–320. doi: 10.1007/s12551-017-0279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto A, Ballone P. Room temperature ionic liquids meet bio-molecules: a microscopic view of structure and dynamics. ACS Sustain Chem Eng. 2016;4:392–412. [Google Scholar]
- Benedetto A, Ballone P. Room temperature ionic liquids meet bio-molecules: a microscopic view of structure and dynamics. Phi Mag. 2016;96:870–894. [Google Scholar]
- Benedetto A, Ballone P (2018) Room-temperature ionic liquids and bio-membranes: setting the stage for applications in pharmacology, bio-medicine, and bio-nano-technology. Langmuir. 10.1021/acs.langmuir.7b04361 [DOI] [PubMed]
- Benedetto A, Bodo E, Gontrani L, Ballone P, Caminiti R. Amino acid anions in organic ionic compounds. An ab initio study of selected ion pairs. J Phys Chem. 2014;118:2471–2486. doi: 10.1021/jp412281n. [DOI] [PubMed] [Google Scholar]
- Benedetto A, Galla HJ. Overview of the “Ionic Liquids meet Biomolecules” session at the 19th international IUPAB and 11th EBSA congress. Biophys Rev. 2017;9:279–281. doi: 10.1007/s12551-017-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch T, Girvan P, Barahona M, Ying L. Introduction of a fluorescent probe to amyloid-beta to reveal kinetic insights into its interactions with copper (II) Angew Chem Int Ed. 2015;54:1227–1230. doi: 10.1002/anie.201408810. [DOI] [PubMed] [Google Scholar]
- Buchfink R, Tischer A, Patil G, Rudolph R, Lange C. Ionic liquids as refolding additives: variation of the anion. J Biotechnol. 2010;150:64–72. doi: 10.1016/j.jbiotec.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Byrne N, Angell CA. Protein unfolding and the “tuning in” of reversible intermediate states, in protic ionic liquid media. J Mol Biol. 2008;378:707–714. doi: 10.1016/j.jmb.2008.02.050. [DOI] [PubMed] [Google Scholar]
- Byrne N, Angell CA. Formation and dissolution of hen egg white lysozyme amyloid fibrils in protic ionic liquids. Chem Comm. 2009;0:1046–1048. doi: 10.1039/b817590j. [DOI] [PubMed] [Google Scholar]
- Byrne N, Barrow C, Mccluskey A (2013) Solvent induced changes in the conformational state of β–Lactoglobulin and the influence of protic ionic liquids. J Mol Eng Mater 1:1250004
- Byrne N, Wang LM, Belieres JP, Angell CA. Reversible folding–unfolding, aggregation protection, and multi-year stabilization, in high concentration protein solutions, using ionic liquids. Chem Comm. 2007;0:2714–2716. doi: 10.1039/b618943a. [DOI] [PubMed] [Google Scholar]
- Constatinescu D, Herrmann C, Weingartner H. Patterns of protein unfolding and protein aggregation in ionic liquids. Phys Chem Chem Phys. 2010;12:1756–1763. doi: 10.1039/b921037g. [DOI] [PubMed] [Google Scholar]
- Debeljuh N, Barrow CJ, Byrne N. The impact of ionic liquids on amyloid fibrilization of Abeta16-22: tuning the rate of fibrilization using a reverse Hofmeister strategy. Phys Chem Chem Phys. 2011;13:16534–16536. doi: 10.1039/c1cp22256b. [DOI] [PubMed] [Google Scholar]
- Debeljuh N, Barrow CJ, Henderson L, Byrne N. Structure inducing ionic liquids-enhancement of alpha helicity in the abeta (1-40) peptide from Alzheimer’s disease. Chem Comm. 2011;47:6371–6373. doi: 10.1039/c1cc10377f. [DOI] [PubMed] [Google Scholar]
- Debeljuh N, Varghese S, Barrow CJ, Byrne N. Role of cation in enhancing the conversion of the Alzheimer’s peptide into amyloid fibrils using protic ionic liquids. Aust J Chem. 2012;65:1502–1506. [Google Scholar]
- Fujita K, Kajiyama M, Liu Y, Nakamura N, Ohno H. Hydrated ionic liquids as a liquid chaperon for refolding of aggregated recombinant protein expressed in Escherichia coli. Chem Comm. 2016;52:13491–13494. doi: 10.1039/c6cc06999a. [DOI] [PubMed] [Google Scholar]
- Ghalebani L, Wahlström A, Danielsson J, Wärmländer SKTS, Gräslund A. pH-dependence of the specific binding of Cu(II) and Zn(II) ions to the amyloid-beta peptide. Biochem Biophys Res Commun. 2012;421:554–560. doi: 10.1016/j.bbrc.2012.04.043. [DOI] [PubMed] [Google Scholar]
- Hamley W. The amyloid beta peptide: a chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem Rev. 2012;112:5147–5192. doi: 10.1021/cr3000994. [DOI] [PubMed] [Google Scholar]
- Hwang H, Choi H, Kim HK, Jo DH, Kim TD. Ionic liquids promote amyloid formation from a-synuclein. Anal Biochem. 2009;386:293–295. doi: 10.1016/j.ab.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Kalhor HR, Kamizi M, Akbari J, Heydari A. Inhibition of amyloid formation by ionic liquids: ionic liquids affecting intermediate oligomers. Biomacromolecules. 2009;10:2468–2475. doi: 10.1021/bm900428q. [DOI] [PubMed] [Google Scholar]
- Khan MV, Zaman M, Chandel T, Siddiqui MK, Ajmal MR, Abdelhameed AS, Khan RH (2017) Cationic surfactant mediated fibrillogenesis in bovine liver catalase: a biophysical approach. J Biomol Struct Dyn doi. 10.1080/07391102.2017.1363085 [DOI] [PubMed]
- Kim S, Bae SY, Lee BY, Kim TD. Coaggregation of amyloid fibrils for the preparation of stable and immobilized enzymes. Anal Biochem. 2012;421:776–778. doi: 10.1016/j.ab.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Kong X, Zhao Z, Lei X, Zhang B, Dai D, Jiang L. Interaction of metal ions with the his13-his14 sequence relevant to Alzheimer’s disease. J Phys Chem A. 2015;119:3528–3534. doi: 10.1021/acs.jpca.5b01443. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bisht M, Venkatesu P. Biocompatibility of ionic liquids towards protein stability: a comprehensive overview on the current understanding and their implications. Int J Biol Macromol. 2017;96:611–651. doi: 10.1016/j.ijbiomac.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Kumar A, Venkatesu P. Prevention of insulin self-aggregation by a protic ionic liquid. RSC Adv. 2013;3:362–367. [Google Scholar]
- Kumar A, Venkatesu P. The stability of insulin in the presence of short alkyl chain imidazolium-based ionic liquids. RSC Adv. 2014;4:4487–4499. [Google Scholar]
- Kundu S, Banerjee C, Sarkar N. Inhibiting the fibrillation of serum albumin proteins in the presence of surface active ionic liquids (SAILs) at low pH: spectroscopic and microscopic study. J Phys Chem B. 2017;121:7550–7560. doi: 10.1021/acs.jpcb.7b03457. [DOI] [PubMed] [Google Scholar]
- Liu W, Cellmer T, Keerl D, Prausnitz JM, Blanch HW. Interactions of lysozyme in guanidinium chloride solutions from static and dynamic light-scattering measurements. Biotechnol Bioeng. 2005;90:482–490. doi: 10.1002/bit.20442. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang L, Mao H, Guo R (2014) Comparative studies on the interaction of [C4mim]Br, and [C8mim]Br with β-casein micelles. Colloids Surf A 441:581–588
- Mangialardo S, Gontrani L, Leonelli F, Caminiti R, Postorino P. Role of ionic liquids in protein refolding: native/fibrillary versus treated lysozyme. RSC Adv. 2012;2:12329–12336. [Google Scholar]
- Mehta A, Rao JR, Fathima NN. Effect of ionic liquids on the different hierarchical order of type I collagen. Colloids Surf B Biointerfaces. 2014;117:376–382. doi: 10.1016/j.colsurfb.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Menga Z, Zheng X, Tang K, Liu J, Maa Z, Zhao Q. Dissolution and regeneration of collagen fibers using ionic liquid. Int J Biol Macromol. 2012;51:440–448. doi: 10.1016/j.ijbiomac.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Rawat K, Bohidar HB. Heparin-like native protein aggregate dissociation by 1-alkyl-3-methyl imidazolium chloride ionic liquids. Int J Biol Macromol. 2015;73:23–30. doi: 10.1016/j.ijbiomac.2014.10.057. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan K, Sathyaraj G, Nair BU, Dhathathreyan A. Reversible and irreversible conformational transitions in myoglobin: role of hydrated amino acid ionic liquid. J Phys Chem B. 2012;116:4175–4180. doi: 10.1021/jp300596z. [DOI] [PubMed] [Google Scholar]
- Schroder C. Proteins in ionic liquids: current status of experiments and simulations. Top Curr Chem (Z) 2017;375:25. doi: 10.1007/s41061-017-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Kang TS. Ionic liquid surfactant mediated structural transitions and selfassembly of bovine serum albumin in aqueous media: effect of functionalization of ionic liquid surfactants. J Phys Chem B. 2015;119:10573–10585. doi: 10.1021/acs.jpcb.5b04854. [DOI] [PubMed] [Google Scholar]
- Summers CA, Flowers RA. Protein renaturation by the liquid organic salt ethylammonium nitrate. Protein Sci. 2000;9:2001–2008. doi: 10.1110/ps.9.10.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekiyo T, Fukudome K, Yamazaki K, Abe H, Yoshimura Y. Protein aggregation and partial globular state in aqueous 1-alkyl-3-methylimidazolium nitrate solutions. Chem Phys Lett. 2014;602:22–27. [Google Scholar]
- Takekiyo T, Koyama Y, Yamazaki K, Abe H, Yoshimura Y. Ionic liquid-induced formation of the α-helical structure of β-lactoglobulin. J Phys Chem B. 2013;117:10142–10148. doi: 10.1021/jp405834n. [DOI] [PubMed] [Google Scholar]
- Takekiyo T, Yamaguchi E, Abe H, Yoshimura Y. Suppression effect on the formation of insulin amyloid by the use of ionic liquids. ACS Sustain Chem Eng. 2016;4:422–428. [Google Scholar]
- Takekiyo T, Yamaguchi E, Yoshida K, Kato M, Yamaguchi T, Yoshimura Y. Interaction site between the protein aggregates and thiocyanate ion in aqueous solution: a case study of 1-Butyl-3-methylimidazolium thiocyanate. J Phys Chem B. 2015;119:6536−6544. doi: 10.1021/acs.jpcb.5b01650. [DOI] [PubMed] [Google Scholar]
- Takekiyo T, Yamazaki K, Yamaguchi E, Abe H, Yoshimura Y. High ionic liquid concentration-induced structural change of protein in aqueous solution: a case study of lysozyme. J Phys Chem B. 2012;116:11092–11097. doi: 10.1021/jp3057064. [DOI] [PubMed] [Google Scholar]
- Tarannum A, Adams A, Blümich B, Fathima NN. Impact of ionic liquids on the structure and dynamics of collagen. J Phys Chem B. 2018;122:1060–1065. doi: 10.1021/acs.jpcb.7b09626. [DOI] [PubMed] [Google Scholar]
- Tarannum A, Muvva C, Mehta A, Rao JR, Fathima NN. Phosphonium based ionic liquids-stabilizing or destabilizing agents for collagen? RSC Adv. 2016;6:4022–4033. [Google Scholar]
- Welton FT. Room-temperature ionic liquids solvents for synthesis and catalysis. Chem Rev. 1999;99:2071–2084. doi: 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- Yeh V, Broering JM, Romanyuk A, Chen B, Chernoff YO, Bommarius AS. The hofmeister effect on amyloid formation using yeast prion protein. Protein Sci. 2010;19:47–56. doi: 10.1002/pro.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugay D, Goronzy DP, Kawakami LM, Claridge SA, Song TB, Yan Z, Xie YH, Gilles JR, Yang Y, Weiss PS. Copper ion binding site in β-amyloid peptide. Nano Lett. 2016;16:6282–6289. doi: 10.1021/acs.nanolett.6b02590. [DOI] [PubMed] [Google Scholar]
- Zhu L, Han Y, He C, Huang X, Wang Y. Disaggregation ability of different chelating molecules on copper ion-triggered amyloid fibres. J Phys Chem B. 2014;118:9298–9305. doi: 10.1021/jp503282m. [DOI] [PubMed] [Google Scholar]