Abstract
Objectives
Mapping of the lymphatic chain for identification of the sentinel lymph node (SLN) is an important aspect of predicting outcomes for breast cancer patients, and it is usually performed as an intraoperative procedure using blue dye and/or radiopharmaceuticals agents. Recently the use of contrast-enhanced ultrasound (CEUS) has been proposed as an alternative imaging technique for this mapping. The objective of this study was to evaluate the use of subdermal administration of the ultrasound contrast agent Sonazoid (GE Healthcare) in terms of patient safety and to select the dose to be used for lymphatic applications in humans.
Methods
This study was performed in 12 female volunteers that received bilateral subdermal injections of Sonazoid (1 or 2 ml doses) in the mid-upper outer quadrant of their breasts at two different time-points. CEUS was performed 0, 0.25, 0.5, 1, 2, 4, 6 and 24 hours post-injection to identify SLNs.
Results
SLNs were identified within the first hour post-injection as enhanced structures and there was no significant difference by dose in the number of SLNs identified (p=0.74). Volunteers only experienced minor adverse experiences (AEs) that resolved completely without intervention by study completion.
Conclusion
The subdermal use of Sonazoid in this study showed only minor local and non-significant AEs that were completely resolved without any intervention. Two different doses were compared with no significant differences observed between them. Hence, the lower dose studied (1 ml) was selected for use in future clinical studies.
Keywords: Ultrasound, contrast agent, breast, sentinel lymph node, safety
Introduction
Lymph node (LN) status is considered an important predictor of long term outcomes for patients with breast cancer [1], which makes their mapping clinically important. This mapping is usually performed as an intraoperative procedure to identify lymph nodes in the draining pathway of a primary tumor [2]. The SLN concept is based on the theory that metastatic cells spread through the lymphatic system with the SLN being the first one in the lymphatic chain. Hence, if the SLN is free of cancer cells, the rest of the lymphatic chain will be free of metastatic disease [3].
Several techniques and imaging agents have been developed to map lymphatic drainage from tumors; the ones currently utilized clinically include the use of blue dye with surgical dissection and injection of radiopharmaceuticals followed by evaluation with a gamma camera (i.e., lymphoscintigraphy) or intraoperatively with a gamma probe (isotope mapping) [4–10]. However, studies of these approaches indicate wide variability in the accuracy for detection of SLNs ranging from 76 to 97% [11–13]. Also, there are limitations and potential adverse effects e.g., the use of blue dye requires surgical dissection that can be extensive - especially if the blue dye passes the SLNs and drain into secondary LNs resulting in a more extensive resection. Blue dye can also cause anaphylactic reactions. Lymphoscintigraphy uses radiation, and while it is low dose it does nonetheless involve exposure not only for the patient but also for the surgical team. Another issue with lymphoscintigraphy is that the detection of SLNs can be incomplete if they are located outside the imaging field or behind another SLN (due to the lack of anatomical information). The material used for lymphoscintography can also pass the SLNs draining into secondary LNs resulting in a larger resection than would otherwise be needed [4, 5, 14, 15]. Hence, there is a need for better mapping of the SLNs to avoid these pitfalls, while at the same time having equal or better efficacy.
Diagnostic ultrasound (US) imaging has been used to evaluate LNs for both benign as well as metastatic disease [16–18]. It has also been found to be a valuable method to guide LN biopsies [19, 20]. Although grayscale US, color flow US and pulsed Doppler have been used alone or in combination to assess LNs for the presence of metastases, US cannot be used for lymphatic mapping (i.e., to identify a tumor’s SLNs), because mapping requires administration of a tracer (e.g., dye or radiopharmaceutical). This paradigm changed when reports on the use of contrast enhanced US (CEUS) to detect lymphatic channels (LCs) and LNs after subdermal injections of microbubble-based US contrast agents (UCAs) in several animal species (termed “lymphosonography”) were produced [14, 15, 21–23]. Our investigations, using a Sinclair swine model with naturally occurring melanoma tumors, established that the accuracy of SLN detection was 82 % for lymphosonography (293/351 SLNs), which was significantly higher than the 63 % achieved with lymphoscintigraphy (231/351 SLNs; p < 0.0001) [15]. One important benefit of lymphosonography is that the UCA remain within the SLNs and doesn’t progress further into the lymphatic system, which is an important aspect, since an unnecessary larger resection is prejudicial for the patients. More recently, some studies have focused on the use of this technique to evaluate the presence of SLNs in humans with breast cancer, where the UCA was injected subdermally around the areola and the LCs were followed to the axilla for the identification of SLNs. These studies demonstrated an accuracy for SLN detection varying from 70 to 100% [24–28].
A number of different UCAs have been tested for lymphosonography, however our animal studies indicated that the best agent available at present for this application is Sonazoid (perfluorobutane microbubbles, GE Healthcare, Oslo, Norway) [14]. The use of subdermal administration of the UCA Sonazoid (GE Healthcare) in terms of patient safety was assessed in this study and the best dose selected (from between two doses used in animals) for translation into lymphatic applications in human patients.
Material and Methods
Subjects
Twelve healthy female volunteers were enrolled in the study that was conducted at Thomas Jefferson University from January to April of 2016. First the volunteers went through a full demographic profile, known drug allergies or intolerances, and a review of their medical/surgical history were recorded. Pregnant women were excluded from this study.
The mean age of the healthy volunteers was 47 years (range: 23–64 years) and everyone provided written informed consent. The study was approved by the University’s Institutional Review Board as well as the United States Food and Drug administration (IND no. 124,465), and was compliant with the Health Insurance Portability and Accountability Act.
The UCA used in this study (Sonazoid) was provided by GE, while Siemens provided the ultrasound scanner utilized. However, the authors had sole control of the data and information provided for publication.
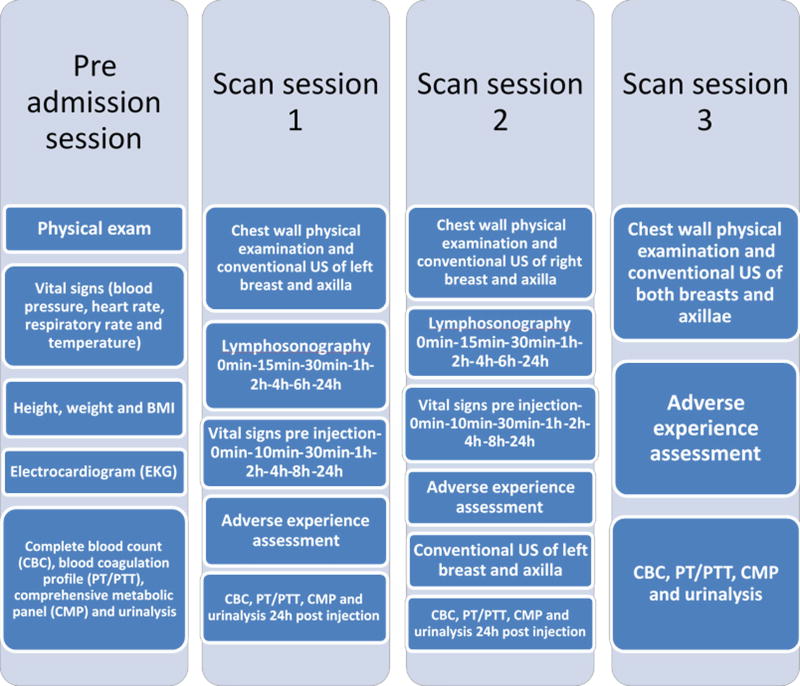
Data Acquisition (Figure 1)
Figure 1.
A schematic chronogram of the study.
The scanning part of the study was performed on three different days. The first day the left breast was injected and scanned. The second day (one week later) involved the right breast being injected and scanned. The left breast was scanned as well, but only to determine if any contrast remained from the first injection/day. The third and final day of scanning was one week from the second study and it did not include UCA injections. Instead, both breasts were scanned to determine if there was any remaining contrast.
The first day of scanning consisted of each volunteer receiving a low (1.0 ml) or high (2.0 ml) dose of Sonazoid injected according to a blinded, randomized allocation schedule. The choice of these two doses as the possible optimal doses to use was based on prior animal studies [14, 15, 22, 23]. As part of the baseline assessments (i.e., prior to UCA injection), the patients had their left breast, chest wall and axilla clinically examined. Also they underwent a grayscale ultrasound baseline examination of their left breast and axilla. First each volunteer received their subdermal, low or high dose of Sonazoid divided into four individual aliquots at four locations (12, 3, 6, and 9 o’clock) around a 2 cm in diameter region in the mid-upper outer quadrant of the left breast. The region around the injection sites was massaged for 5 minutes by an experienced physician (JBL) to accelerate the uptake of Sonazoid into the lymphatic system. CEUS was performed immediately afterwards (i.e., lymphosonography) to identify the number, location and course of the LCs and SLNs using Cadence Pulse Sequencing (CPS; Siemens Healthineers, Mountain View, CA) on an S3000 Helx scanner (Siemens Healthineers, Mountain View, CA) with a high frequency, broad bandwidth (4 – 9 MHz) linear array (the 9L4). CEUS evaluations were repeated at 15 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, 6 hours and 24 hours post Sonazoid administration. For each time-point, the focal zone, scanning depth and time-gain compensation (TGC) were adjusted to optimize visualization of the target region (SLN or LC). No compounding or other image processing techniques were applied. Sagittal and transverse still images and digital clips were acquired during the US examination. The safety and tolerability of Sonazoid was closely monitored throughout this dose-finding study and any adverse events (AEs) were noted.
The second day of scanning (one week later) the upper outer quadrant of the right breast was injected with Sonazoid and lymphosonography was again used to identify the number, location and course of the LCs and SLNs following the same protocol of the first day described in details above. The main difference was that the volunteers who received the low dose during their first day received the high dose this second day and vice versa. During this second study the left breast was also scanned one more time to assess if there was any contrast-enhanced tissues remaining at the one week mark.
The third and last scanning day of the study was performed around one week after the second day and consisted of both breasts being sonographically assessed one last time for the presence of contrast-enhanced tissues.
Vital signs, blood analysis and urinalysis
During the study the volunteers had their vital signs (blood pressure, heart rate, respiratory rate and temperature) acquired first as the baseline at the pre admission session, and then at pre-determined intervals (10 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, 8 hours and 24 hours) after the injection of Sonazoid during scanning sessions 1 and 2 as well as finally once again at scan session 3. The volunteers had a complete blood analysis work consisting of complete blood count (CBC), blood coagulation profile (PT/PTT) and comprehensive metabolic panel (CMP) collected during 4 different time-points of the study: pre admission session, scan session1, scan session 2 and scan session 3. Also urinalysis was performed at the same time-points as the blood analysis. All off these values were compared by 2 physicians to the normal range of values within the general population.
Data Analysis
Still images and digital clips from the ultrasound examinations were de-identified and randomized so the readers would not be able to determine the case number. Three independent and blinded readers (two physicians and one physicist; PM, JBL and FF with combined experience in contrast enhanced breast imaging of more than 50 years) analyzed the clips 4–6 months later and identified the number of SLNs observed during all the scanning days and time-points. The number of SLNs and the time where they were first identified were compared between the two doses and between the readers using paired t-tests, ANOVA and kappa. The AEs were analyzed in two different ways, firstly as the individual volunteer experiences during the entire study and secondly by the contrast dose received at that point of the study. Vital signs, blood analysis and urinalysis were compared with the normal range in the general population. All tests were performed using Stata 12.1 (Stata Corp, College Station, TX) with p-values less than 0.05 indicating statistical significance.
Results
SLNs
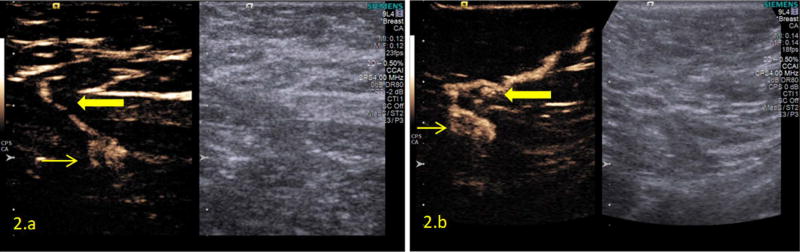
Figure 2 shows an example of SLNs and LCs detected with the low and the high dose (in two different volunteers). A total of 48 SNLs were detected during the entire study, 22 were identified with the 1 ml dose and 26 with the 2 ml dose for averages of 1.8 and 2.2 SLNs per dose, respectively (range from 1 to 4 SLNs detected per injection). There were no significant differences in the number of SLNs detected when doses and readers were compared (p = 0.74 and 0.50, respectively, with kappa values varying from 0.50 to 0.74). However, as different breasts of the same volunteer received different doses, this study is not comparing how the same SLN could be identified by different doses, but rather the ability to visualize SLNs with different doses were compared.
Figure 2.
Dual imaging US consisting of contrast imaging on the left and a grayscale image on the right. (a): 1 ml dose, LC (thick arrow) and SLN (thin arrow). (b): 2 ml dose, LC (thick yellow arrow) and SLN (thin yellow arrow).
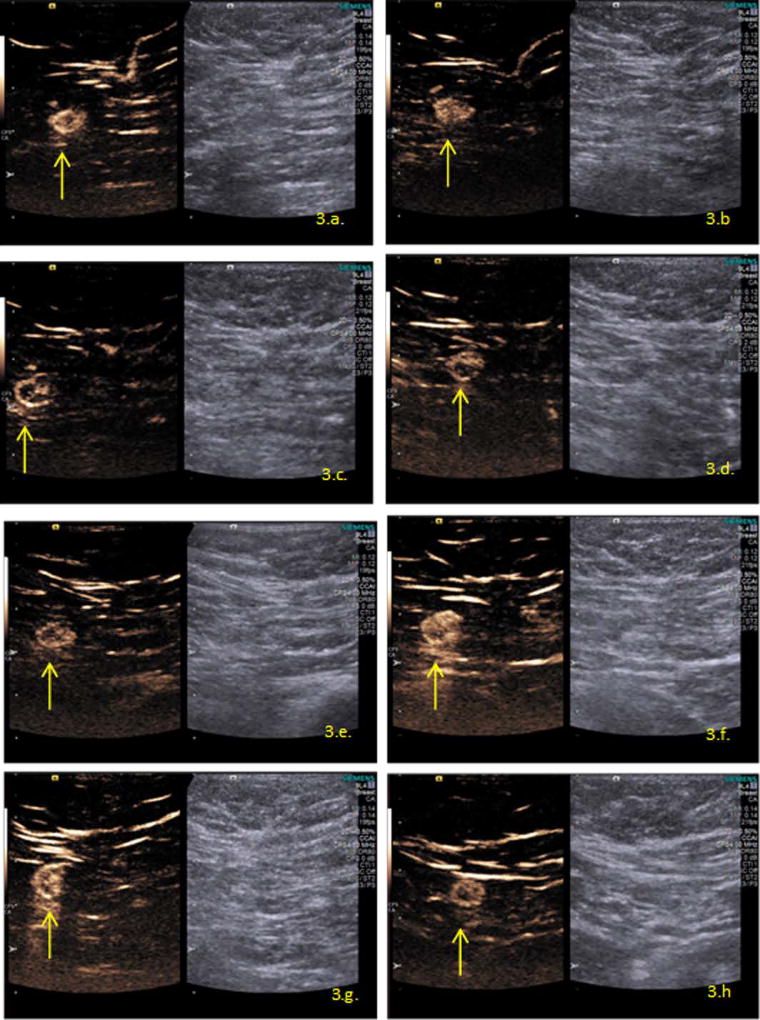
Figure 3 demonstrates the same SLN imaged at the different time-points associated with the 2 ml injection, from immediately after the Sonazoid contrast injection ending at 24 hours post injection. Given the attempt to acquire the best image at any time-point (considering the differences between the intensity of the contrast enhancement observed over time), there are some differences between the images related to focal zones and the gain used.
Figure 3.
SLN (yellow arrow) shown on CEUS after contrast injection of 2 ml at different time-points. (a): immediately post injection, 0 minutes. (b): 15 minutes post injection. (c): 30 minutes post injection. (d): 1 hour post injection. (e): 2 hours post injection. (f): 4 hours post injection. (g): 6 hours post injection. (h): 24 hours post injection.
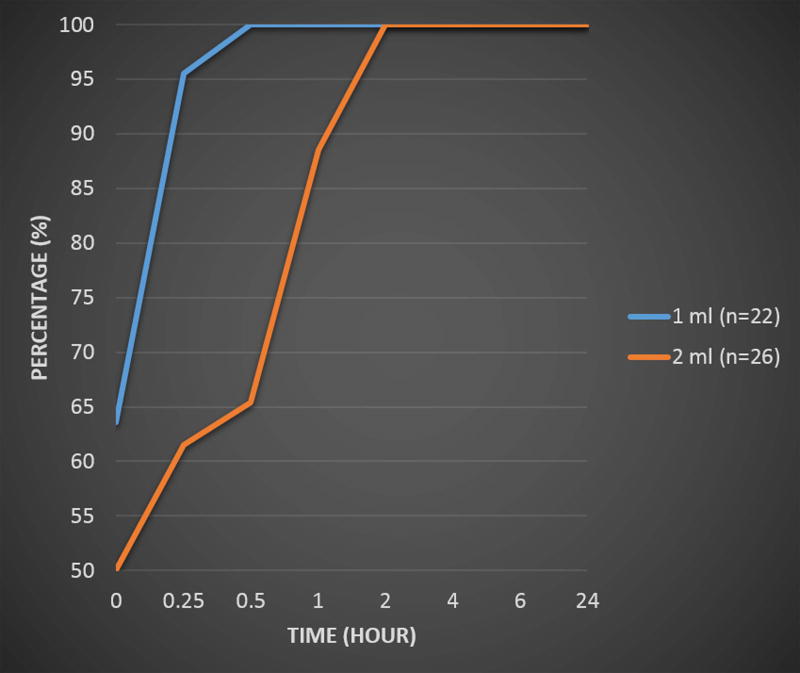
The time for the initial detection of SLNs based on dose is shown in Figure 4. For the 1 ml dose 100% of the SLNs were detected within the first hour, while for the 2 ml dose 89% of the SLNs were detected in that time period. There were no significant differences between doses or readers for the time of detection (p > 0.57), with kappa values showing the agreement between readers for the time for the initial detection varying from 0.51 to 0.62.
Figure 4.
Average time to the first detection of the SLNs by dose.
Adverse experiences
Only minor local and non-significant AEs confined to the injection site and surrounding area were encountered by the volunteers. All AEs were completely resolved without any intervention by the time the study was completed. Table 1 details the AEs that the volunteers encountered that were considered to be related to the injection of contrast. The data is shown as total volunteers and also per dose to demonstrate that there were no significant differences between doses when AEs were compared (p > 0.50).
Table 1.
Adverse experiences per volunteers and per dose
| Adverse experience | Volunteer (n=12) |
1 ml (n=12) |
2 ml (n=12) |
|---|---|---|---|
| Redness | 10 (83%) | 10 (83%) | 7 (58%) |
| Pain | 4 (33%) | 2 (17%) | 3 (25%) |
| Bruise | 3 (25%) | 2 (17%) | 1 (8%) |
| Burning sensation | 2 (17%) | 1 (8%) | 2 (17%) |
| Numbness | 1 (8%) | 1 (8%) | 1 (8%) |
| Tingling sensation | 1 (8%) | 0 (0%) | 1 (8%) |
Three volunteers reported other AEs that were not considered related to the contrast injections (and therefore were not included in Table 1). These were: cough (n=3), toothache (n=2), sore throat (n=1) and bronchitis (n=1).
Vital signs, blood analysis and urinalysis
The analysis of the vital signs showed no significance alterations in the volunteer’s blood pressure, heart rate, respiratory rate and temperature when pre and post injections values were compared (p > 0.50). The mean values of the vital signs are summarized in Table 2. Likewise, the blood analysis and urinalysis showed no significance differences when pre and post injections values (p > 0.50) and the values remained in the normal range when compared with the general population during the entire study (Tables 3 and 4).
Table 2.
Average vital signs across all volunteers and both dosages.
| Blood pressure (mmHg) |
Heart rate (bpm) |
Respiratory rate (bpm) |
Temperature (C) |
|
|---|---|---|---|---|
| Pre injection | 115/71 | 72 | 18 | 36.3 |
| 10 min | 114/67 | 70 | 18 | 36.3 |
| 30 min | 115/69 | 71 | 18 | 36.3 |
| 1 h | 118/71 | 73 | 18 | 36.1 |
| 2 h | 117/67 | 71 | 18 | 36.4 |
| 4 h | 118/66 | 72 | 17 | 36.4 |
| 8 h | 121/69 | 71 | 18 | 36.5 |
| 24 h | 117/71 | 70 | 17 | 36.5 |
Table 3.
Blood analysis averaged for all volunteers across the 3 scanning sessions. The range of normal values are provided in parentheses.
| Blood analysis | Pre admission session |
Scan session1 |
Scan session2 |
Scan session 3 |
|---|---|---|---|---|
| Sodium (135–146 mmol/L) | 140 ± 2.6 | 139 ± 1.4 | 140 ± 2.1 | 139 ± 1.3 |
| Potassium (3.5–5.0 mmol/L) | 4.5 ± 0.3 | 4.4 ± 0.2 | 4.5 ± 0.2 | 4.3 ± 0.3 |
| Chloride (89–109 mmol/L) | 102 ±2.7 | 101 ± 1.2 | 102 ± 1.2 | 102 ± 1.9 |
| CO2 (24–32 mmol/L) | 25 ± 1.1 | 25 ± 2.5 | 25 ± 1.9 | 25 ± 2.0 |
| Anion gap (4–16 mmol/L) | 13 ± 2.2 | 12 ± 1.9 | 13 ± 1.3 | 13 ± 1.8 |
| Urea-N (7–26 mg/dL) | 13 ±4.3 | 12 ± 3.1 | 12 ± 3.4 | 13 ± 3.2 |
| Protein (6.0–8.5 g/dL) | 7.1 ± 0.5 | 6.9 ± 0.4 | 6.9 ± 0.5 | 6.7 ± 0.4 |
| Albumin (3.2–4.9 g/dL) | 4.4 ± 0.3 | 4.2 ± 0.2 | 4.2 ± 0.3 | 4.1 ± 0.2 |
| Calcium (8.5–10.3 mg/dL) | 9.5 ± 0.3 | 9.3 ± 0.4 | 9.3 ± 0.4 | 9.2 ± 0.5 |
| Creatinine (0.7–1.4 mg/dL) | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| Total bilirubin (0.1–0.9 mg/dl) | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Alkaline phos [ALP] (29–92 IU/L) | 64 ± 16.2 | 63 ± 14.4 | 62 ± 14.1 | 62 ± 16.1 |
| AST [SGOT] (7–35 IU/L) | 19 ± 5.2 | 16 ± 2.2 | 16 ± 3.1 | 17 ± 2.7 |
| ALT [SGPT] (<30 IU/L) | 19 ± 11.1 | 17 ± 7.1 | 16 ± 6.2 | 17 ± 5.3 |
| Phosphate (2.4–4.5 mg/dL) | 3.7 ± 0.5 | 3.6 ± 0.3 | 3.6 ± 0.6 | 3.6 ± 0.5 |
| Glucose (70–100 mg/dL) | 88 ± 10.5 | 92 ± 7.9 | 91 ± 11.0 | 93 ± 11.7 |
| WBC count (4–11 B/L) | 7.4 ± 1.6 | 6.7 ± 1.7 | 6.8 ± 1.6 | 7.1 ± 1.4 |
| RBC count (3.7–5.2 t/L) | 4.6 ± 0.3 | 4.6 ± 0.4 | 4.6 ± 0.2 | 4.3 ± 0.4 |
| Hemoglobin (12.5–15 g/dL) | 12.9 ± 1.1 | 12.8 ± 0.9 | 12.9 ± 0.9 | 12.2 ± 1.0 |
| Hematocrit (36–46 %) | 39.7 ±2.4 | 38.9 ± 2.2 | 39.3 ± 2.4 | 37.1 ± 3.0 |
| MCV (80–99 fl) | 87 ± 5.8 | 86 ± 5.0 | 86 ± 5.6 | 86 ± 5.6 |
| MCH (26–34 pg) | 28.2 ± 2.3 | 28.2 ± 2.3 | 28.2 ± 2.4 | 28.3 ± 2.4 |
| MCHC (32.0–37.5 g/dL) | 32.5 ± 1.3 | 32.9 ± 1.0 | 32.8 ± 1.2 | 32.9 ± 1.3 |
| RBC distribution wid (11.0–15.8 %) | 13.8 ± 1.2 | 13.6 ± 1.2 | 13.6 ± 1.4 | 13.7 ± 1.4 |
| Platelet (140–400 B/L) | 294 ± 51.3 | 270 ± 50.0 | 273 ± 66.2 | 253 ± 42.5 |
| MPV (9–13 fl) | 10.4 ± 0.8 | 10.2 ± 0.7 | 10.3 ± 0.9 | 10.3 ± 0.8 |
| Neutrophils (40–73 %) | 55.4 ± 7.6 | 51.8 ± 8.8 | 51.2 ± 6.8 | 54.6 ± 8.5 |
| Lymphocytes (20–44%) | 33.3 ± 6.7 | 35.5 ±8.5 | 36.7 ± 6.1 | 33.3 ± 9.3 |
| Monocytes (3–13%) | 8.3 ± 1.7 | 8.6 ± 2.2 | 8.6 ± 2.3 | 9.1 ± 1.5 |
| Eosinophils (0–6 %) | 2.3 ± 1.4 | 3 ± 1.6 | 2.6 ± 1.0 | 2.3 ± 1.2 |
| Basophils (0–3 %) | 0.4 ± 0.3 | 0.4 ± 0.3 | 0.6 ± 0.4 | 0.4 ± 0.3 |
Table 4.
Urinalysis averaged for all volunteers across the 3 scanning sessions. Normal ranges or values are provided in parentheses.
| Urinalysis | Pre admission session |
Scan session 1 |
Scan session2 |
Scan session 3 |
|---|---|---|---|---|
| Spec gravity (1.01–1.03) | 1.01 ± 0.01 | 1.02 ± 0.01 | 1.01 ± 0.01 | 1.02 ± 0.01 |
| pH (5.0–8.0) | 5.7 ± 0.8 | 5.9 ±0.9 | 6.0 ± 0.9 | 5.8 ± 0.6 |
| Protein (neg) | neg | neg | neg | neg |
| Glucose (neg) | neg | neg | neg | neg |
| Ketones (neg) | neg | neg | neg | neg |
| Bilirubin (neg) | neg | neg | neg | neg |
| Blood (neg) | neg | neg | neg | neg |
| Nitrite (neg) | neg | neg | neg | neg |
| Urobilinogen (normal) | normal | normal | normal | normal |
| Leuk est (neg) | neg | neg | neg | neg |
| Color (yellow) | yellow | yellow | yellow | yellow |
| Appearance (clear) | clear | clear | clear | clear |
Discussion
The presence and extent of axillary LN involvement remains the most powerful predictor of recurrence and survival in breast cancer [29, 30]. Studies have shown that the presence of regional metastases within the axillary basin decreases a patient’s 5-year survival by approximately 28–40% [30]. There are a few factors that can be use as predictors of node metastases such as tumor size, lymphovascular invasion, tumor grade, and patient age. However, currently there is no combination of predictors of axillary node status that can replace the histopathologic examination of the LNs [29]. A accurate assessment of the nodes is important not only for staging and prognosis, but also for guiding treatment selection [29]. The accurate assessment of possible affected LNs that need to be removed is essential so that only the affected LNs are removed in order to minimize the anatomic disruption caused by axillary LN dissection, which can result in lymphedema, nerve injury, shoulder dysfunction, and other complications that may compromise functionality and quality of life [29]. This is particularly troublesome when considering the fact that LN metastases are found in only 40% of patients who undergo axillary LN dissection. The remaining patients derive no therapeutic benefit from the procedure, whereas all patients are exposed to the complications described above [30].
Ultrasound examination is an economically viable, safe, and well-accepted technique for both patients and health care providers. Our studies has shown that CEUS represents a practical method for mapping the lymphatic drainage of tumors in real time and that could enable targeted preoperative ultrasound-guided biopsy of SLNs in patients with breast cancer. However, no dedicated safety studies have, to the best of our knowledge, been reported. Hence, this study aimed to evaluate the subdermal use of Sonazoid in CEUS to identify SLNs and therefore, to be used in the future as an alternative to blue dye and/or radiopharmaceuticals. Safety of UCA by intra-vascular or intra-vesical administration has been studied extensively [31–36] and the safety profile is excellent with low incidence of AEs. The side-effects reported with UCAs are, in general, non-serious, transient and resolve spontaneously without residual effects [31–36].
Recently a few studies focused on the use of UCAs to evaluate the presence of SLNs in humans with breast cancer, where the UCA was injected subdermally around the areola (unlike this study) and the LCs were followed to the axilla for the identification of SLNs [24–28, 37]. These studies demonstrated an accuracy of SLN detection varying from 70 to 100%. This variation may be due to the different locations on the breast of the injection, as well as the time of the injection [24–28, 37]. Safety assessments were not the main point of these studies, but nonetheless the investigators reached the same conclusion as our study in that only a few minor AEs were observed and these completely resolved on their own without any intervention [24–28].
Our study showed similar findings when safety was evaluated, where the few AEs observed were minor and completely resolved on their own without any intervention. That finding was independent of the Sonazoid dosage used. Also when we compared the number of SLNs that were identified by the two dosages there was no significant difference. Therefore, using the concept of ALARA (As Low As Reasonably Achievable), we selected the lower dose of 1 ml as the best dose in the present study. We acknowledge that not all possible doses were evaluated, but between the two doses analyzed there was no difference in their accuracy and therefore, the lower dose was chosen for future studies in breast cancer patients.
In conclusion the aim of this study was to evaluate the subdermal use of Sonazoid for SLN identification with the focus on the safety of this method for lymphosonography and results showed that the volunteers only had minor local and non-significant AEs confined to the injection site and surrounding area that were completely resolved without any intervention by the time the study was completed. Two different doses were compared and there was no significant differences in the number of SLNs detected (p = 0.74). Therefore the lower dose of 1 ml was selected as the best dose (of the two studied here) and that it will be the dose used in future clinical studies.
Acknowledgments
The authors would like to thank all the volunteers and the staff at the Clinical Research Unit at Thomas Jefferson University for their part in this study. This work was supported by NIH grants R01 CA100370 and R01 CA172336. Sonazoid was supplied by GE Healthcare, Oslo, Norway, while the S3000 Helx scanner was provided by Siemens Healthineers, Mountain View, CA.
References
- 1.Tokin CA, Cope FO, Metz WL, Blue MS, Potter BM, Abbruzzese BC, et al. The efficacy of Tilmanocept in sentinel lymph mode mapping and identification in breast cancer patients: a comparative review and meta-analysis of the 99mTc-labeled nanocolloid human serum albumin standard of care. Clinical & experimental metastasis. 2012;29:681–6. doi: 10.1007/s10585-012-9497-x. [DOI] [PubMed] [Google Scholar]
- 2.Leong SP, Kim J, Ross M, Faries M, Scoggins CR, Metz WLR, et al. A phase 2 study of 99mTc-tilmanocept in the detection of sentinel lymph nodes in melanoma and breast cancer. Annals of surgical oncology. 2011;18:961–9. doi: 10.1245/s10434-010-1524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emerson DK, Limmer KK, Hall DJ, Han S-H, Eckelman WC, Kane CJ, et al. A receptor-targeted fluorescent radiopharmaceutical for multireporter sentinel lymph node imaging. Radiology. 2012;265:186–93. doi: 10.1148/radiol.12120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldfarb LR, Alazraki NP, Eshima D, Eshima LA, Herda SC, Halkar RK. Lymphoscintigraphic identification of sentinel lymph nodes: clinical evaluation of 0.22-micron filtration of Tc-99m sulfur colloid. Radiology. 1998;208:505–9. doi: 10.1148/radiology.208.2.9680583. [DOI] [PubMed] [Google Scholar]
- 5.Hill AD, Mann GB, Borgen PI, Cody HS. Sentinel lymphatic mapping in breast cancer 1. Journal of the American College of Surgeons. 1999;188:545–9. doi: 10.1016/s1072-7515(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 6.Yudd AP, Kempf JS, Goydos JS, Stahl TJ, Feinstein RS. Use of sentinel node lymphoscintigraphy in malignant melanoma. Radiographics: a review publication of the Radiological Society of North America, Inc. 1998;19:343–53. doi: 10.1148/radiographics.19.2.g99mr12343. discussion 54-6. [DOI] [PubMed] [Google Scholar]
- 7.Bostick PJ, Giuliano AE. Vital dyes in sentinel node localization. Seminars in nuclear medicine: Elsevier. 2000:18–24. doi: 10.1016/s0001-2998(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 8.Eshima S, Fauconnier T, Eshima L, Thornback JR. Radiopharmaceuticals for Lymphoscintigraphy: Including dusimetry and radiation considerations. Seminars in nuclear medicine: Elsevier. 2000:25–32. doi: 10.1016/s0001-2998(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 9.Giménez RB-E, Dolores Hernández, Margarita Carbonell, Maria A. Martínez, Carlos Guillén, Carlos Vázquez, Julia. Anaphylaxis after peritumoral injection of sulphan blue 1% for identification of the sentinel node in lymphatic mapping of the breast. The European journal of surgery. 2001;167:921–3. doi: 10.1080/110241501753361622. [DOI] [PubMed] [Google Scholar]
- 10.De Kanter A, Menke-Pluijmers M, Henzen-Logmans S, Van Geel A, van Eijck C, Wiggers T, et al. Reasons for failure to identify positive sentinel nodes in breast cancer patients with significant nodal involvement. European Journal of Surgical Oncology (EJSO) 2006;32:498–501. doi: 10.1016/j.ejso.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. New England Journal of Medicine. 2003;349:546–53. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Uematsu M, Saito T, Ishikawa H, Tamaki K, Tamai S, et al. Sentinel Lymph Node Identification for Patients with Breast Cancer Using Large-Size Radiotracer Particles: Technetium-99m–Labeled Tin Colloids Produced Excellent Results. The breast journal. 2001;7:388–91. doi: 10.1046/j.1524-4741.2001.07602.x. [DOI] [PubMed] [Google Scholar]
- 13.Camp E, Cendan J, Feezor R, Lind D. The hottest sentinel lymph node is not always the positive node. The American surgeon. 2004;70:475. [PubMed] [Google Scholar]
- 14.Goldberg BB, Merton DA, Liu J-B, Murphy G, Forsberg F. Contrast-Enhanced Sonographic Imaging of Lymphatic Channels and Sentinel Lymph Nodes. Journal of ultrasound in medicine. 2005;24:953–65. doi: 10.7863/jum.2005.24.7.953. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg BB, Merton DA, Liu J-B, Forsberg F, Zhang K, Thakur M, et al. Contrast-Enhanced Ultrasound Imaging of Sentinel Lymph Nodes After Peritumoral Administration of Sonazoid in a Melanoma Tumor Animal Model. Journal of Ultrasound in Medicine. 2011;30:441–53. doi: 10.7863/jum.2011.30.4.441. [DOI] [PubMed] [Google Scholar]
- 16.Evans KD, Boyd A. Sonographic and Vascular Assessment of Axillary Lymph Nodes: A Review. Journal of Diagnostic Medical Sonography. 2007;23:63–72. [Google Scholar]
- 17.Cho N, Moon WK, Han W, Park IA, Cho J, Noh D-Y. Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results. American Journal of Roentgenology. 2009;193:1731–7. doi: 10.2214/AJR.09.3122. [DOI] [PubMed] [Google Scholar]
- 18.Voit C, Van Akkooi AC, Schäfer-Hesterberg G, Schoengen A, Kowalczyk K, Roewert JC, et al. Ultrasound morphology criteria predict metastatic disease of the sentinel nodes in patients with melanoma. Journal of Clinical Oncology. 2010;28:847–52. doi: 10.1200/JCO.2009.25.7428. [DOI] [PubMed] [Google Scholar]
- 19.Kunte C, Schuh T, Eberle JY, Baumert J, Konz B, Volkenandt M, et al. The Use of High-Resolution Ultrasonography for Preoperative Detection of Metastases in Sentinel Lymph Nodes of Patients with Cutaneous Melanoma. Dermatologic Surgery. 2009;35:1757–65. doi: 10.1111/j.1524-4725.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- 20.Voit CA, van Akkooi AC, Schäfer-Hesterberg G, Schoengen A, Schmitz PI, Sterry W, et al. Rotterdam Criteria for sentinel node (SN) tumor burden and the accuracy of ultrasound (US)-guided fine-needle aspiration cytology (FNAC): can US-guided FNAC replace SN staging in patients with melanoma? Journal of Clinical Oncology. 2009;27:4994–5000. doi: 10.1200/JCO.2008.19.0033. [DOI] [PubMed] [Google Scholar]
- 21.Mattrey RF, Kono Y, Baker K, Peterson T. Sentinel lymph node imaging with microbubble ultrasound contrast material. Academic radiology. 2002;9:S231–S5. doi: 10.1016/s1076-6332(03)80444-0. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg BB, Merton DA, Liu J-B, Thakur M, Murphy GF, Needleman L, et al. Sentinel Lymph Nodes in a Swine Model with Melanoma: Contrast-enhanced Lymphatic US 1. Radiology. 2004;230:727–34. doi: 10.1148/radiol.2303021440. [DOI] [PubMed] [Google Scholar]
- 23.Liu J-B, Merton DA, Berger AC, Forsberg F, Witkiewicz A, Zhao H, et al. Contrast-enhanced sonography for detection of secondary lymph nodes in a melanoma tumor animal model. Journal of Ultrasound in Medicine. 2014;33:939–47. doi: 10.7863/ultra.33.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omoto K, Matsunaga H, Take N, Hozumi Y, Takehara M, Omoto Y, et al. Sentinel node detection method using contrast-enhanced ultrasonography with sonazoid in breast cancer: preliminary clinical study. Ultrasound in medicine & biology. 2009;35:1249–56. doi: 10.1016/j.ultrasmedbio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Sever A, Jones S, Cox K, Weeks J, Mills P, Jones P. Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasonography in patients with breast cancer. British Journal of Surgery. 2009;96:1295–9. doi: 10.1002/bjs.6725. [DOI] [PubMed] [Google Scholar]
- 26.Sever AR, Mills P, Jones SE, Cox K, Weeks J, Fish D, et al. Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. American Journal of Roentgenology. 2011;196:251–6. doi: 10.2214/AJR.10.4865. [DOI] [PubMed] [Google Scholar]
- 27.Sever A, Mills P, Jones S, Mali W, Jones P. Sentinel node identification using microbubbles and contrast-enhanced ultrasonography. Clinical radiology. 2012;67:687–94. doi: 10.1016/j.crad.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Cox CE, Pendas S, Cox JM, Joseph E, Shons AR, Yeatman T, et al. Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Annals of surgery. 1998;227:645. doi: 10.1097/00000658-199805000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyman GH, Giuliano AE, Somerfield MR, Benson AB, III, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. Journal of clinical oncology. 2005;23:7703–20. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 31.Albrecht T, Blomley M, Bolondi L, Claudon M, Correas J-M, Cosgrove D, et al. Guidelines for the use of contrast agents in ultrasound-january 2004. Ultraschall in der Medizin-European Journal of Ultrasound. 2004;25:249–56. doi: 10.1055/s-2004-813245. [DOI] [PubMed] [Google Scholar]
- 32.Blomley M, Claudon M, Cosgrove D. WFUMB Safety Symposium on Ultrasound Contrast Agents: clinical applications and safety concerns. Ultrasound in medicine & biology. 2007;33:180–6. doi: 10.1016/j.ultrasmedbio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)-update 2008. Ultraschall in der Medizin-European Journal of Ultrasound. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 34.Piscaglia F, Nolsøe C, Dietrich Ca, Cosgrove D, Gilja O, Nielsen MB, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall in der Medizin-European Journal of Ultrasound. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 35.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012. Ultraschall in der Medizin-European Journal of Ultrasound. 2013;34:11–29. doi: 10.1055/s-0032-1325499. [DOI] [PubMed] [Google Scholar]
- 36.Rosado E, Riccabona M. Off-Label Use of Ultrasound Contrast Agents for Intravenous Applications in Children. Journal of Ultrasound in Medicine. 2016;35:487–96. doi: 10.7863/ultra.15.02030. [DOI] [PubMed] [Google Scholar]
- 37.Cox K, Sever A, Jones S, Weeks J, Mills P, Devalia H, et al. Validation of a technique using microbubbles and contrast enhanced ultrasound (CEUS) to biopsy sentinel lymph nodes (SLN) in pre-operative breast cancer patients with a normal grey-scale axillary ultrasound. European Journal of Surgical Oncology (EJSO) 2013;39:760–5. doi: 10.1016/j.ejso.2013.03.026. [DOI] [PubMed] [Google Scholar]