Abstract
Diet may modify metabolomic profiles towards higher or lower cardiovascular disease (CVD) risk. We aimed to identify metabolite profiles associated with high adherence to dietary recommendations - the Alternative Healthy Eating Index (AHEI) - and the extent to which metabolites associated with AHEI also predict incident CVD. Relations between AHEI score and 80 circulating lipids and metabolites, quantified by nuclear magnetic resonance metabolomics, were examined using linear regression models in the Whitehall II study (n = 4824, 55.9 ± 6.1 years, 28.0% women) and were replicated in the Cardiovascular Risk in Young Finns Study (n = 1716, 37.7 ± 5.0 years, 56.3% women). We used Cox models to study associations between metabolites and incident CVD over the 15.8-year follow-up in the Whitehall II study. After adjustment for confounders, higher AHEI score (indicating healthier diet) was associated with higher degree of unsaturation of fatty acids (FA) and higher ratios of polyunsaturated FA, omega-3 and docosahexaenoic acid relative to total FA in both Whitehall II and Young Finns studies. A concordance of associations of metabolites with higher AHEI score and lower CVD risk was observed in Whitehall II. Adherence to healthy diet seems to be associated with specific FA that reduce risk of CVD.
Introduction
The benefits of healthy diet are supported by nutritional epidemiological studies on coronary heart diseases1, respiratory diseases2 and healthy old-age phenotypes3. Recent advancements of high-throughput metabolite profiling in large epidemiological studies allow the determination of metabolites predicting the risk for cardiometabolic diseases, providing insights into the molecular mechanisms underlying age-related diseases, such as cardiovascular diseases (CVD)4. It has been hypothesized that metabolites are very responsive to dietary exposure as diet is an important source of metabolite variation and also induces metabolic response.
Few studies have examined the association between overall diet and metabolites and a majority of investigations assessed metabolites via mass spectrometry methods. In the EPIC-Potsdam cohort study of 2380 adults, for example, dietary patterns were derived through reduced rank regression methods to explain the maximum variations of metabolites5 and a weak association between habitual diet and serum metabolites was observed. In a subsample of 1977 participants of the ARIC study, amongst the 336 metabolites assessed, dietary pattern “sugar-rich food and beverages” was associated with 7 unsaturated long-chain fatty acids, five 2-hydroxybutyrate–related metabolites, two sex steroids, five γ-glutamyl dipeptides, and four metabolites in other pathways6 and in the Women’s Health Initiative study, Prudent dietary pattern was associated with 85 metabolites (mostly lipids)7. Another study, carried out on 502 participants from the Prostate, Lung Colorectal and Ovarian Cancer Screening Trial, examined the correlations between 412 metabolites, food groups and the Healthy Eating Index score8. The authors reported that 39 metabolites were associated with 13 dietary groups and concluded that the metabolomic approach might be useful in identifying biomarkers reflecting the effect of nutrition intakes on human metabolism. In agreement with this, results from a study assessing lipoprotein particle subclasses profile via Nuclear Magnetic Resonance (NMR) in 663 adults showed associations between specific dietary patterns (“fish” and “junked food” pattern) and lipoprotein subclasses9. Identifying robust associations between dietary habits and metabolites may offer the possibility to better understand pathways by which overall diet mediates protection against chronic diseases, such as CVD, but none of these studies examined this issue.
In this study, we sought to identify metabolites associated with adherence to a healthy diet and to determine the extent to which these metabolites are also related to reduced risk of CVD. To do so, we assessed adherence to dietary guidelines in a large cohort of British middle-aged men and women from the Whitehall II study10 using the Alternative Healthy Eating Index (AHEI) – a dietary index whose high scores have been shown to be associated with reduced risk of CVD morbidity11 and mortality12. We examined associations of healthy diet with metabolites quantified using a serum NMR metabolomics and replicated the results in an independent cohort, the Cardiovascular Risk in Young Finns Study13. We then determined the extent to which metabolites associated with AHEI were also associated with the risk of developing CVD over 15.8 years of follow-up in the Whitehall II study.
Results
Participant characteristics
A total of 4824 participants from the Whitehall II study were included in the discovery analysis. Characteristics are described in Table 1. Mean concentration of the 80 metabolites are detailed in Supplementary Material-Table A. The mean (±SD) score of AHEI was 50.7 ± 9.8 points. Compared to the 3034 participants who attended the 1997/99 examination but were not included in the present analysis, those included were more likely to be men, white, with high socio-economic status, to practice physical activity and to report higher total energy intake. Participants included were also less likely to be smoker, to use antihypertensive or lipids lowering drugs and showed lower concentrations of triglycerides and lower diastolic blood pressure. No significant difference in AHEI score was observed (Supplementary Material- Table B). Regarding participants included in the Young Finns Study (replication cohort), the latter were younger and showed lower means of AHEI compared to participants included in Whitehall II study (Table 1).
Table 1.
Characteristics of Whitehall II participants and Young Finns Study participants.
| Characteristics | Whitehall II | Young Finns Study | |||
|---|---|---|---|---|---|
| N | % or mean (SD) | N | mean ± SD or ρ* | ||
| Sex | Men | 3483 | 72.2 | 966 | 56.3 |
| Women | 1341 | 27.8 | 750 | 43.7 | |
| Age, years | 4824 | 55.9 (6.1) | 1716 | 37.7 (5.0) | |
| Ethnicity | White | 4541 | 93.9 | 1716 | 1716 (100.0) |
| South Asian | 183 | 3.9 | / | / | |
| Black | 100 | 2.2 | / | / | |
| Smoking habits | Non | 2490 | 52.1 | 886 | 51.6 |
| Former | 1864 | 39.1 | 421 | 24.5 | |
| Current | 470 | 8.9 | 409 | 23.8 | |
| Physical activity MET unit /hours/week | 4824 | 15.6 (14.8) | 1716 | 19.6 (21.5) | |
| Total score in AHEI, points | 4824 | 50.7 (9.8) | 1716 | 46.3 (8.0) | |
| Total energy intake, kcal/day | 4824 | 2233 (683) | 1716 | 2392 (800) | |
| Prevalent type 2 diabetes | No | 4528 | 93.9 | 1665 | 97.0 |
| Yes | 296 | 6.1 | 51 | 3.0 | |
| Systolic blood pressure, mmHg | 4824 | 123.1 (16.5) | 1716 | 120.3 (14.3) | |
| Diastolic blood pressure, mmHg | 4824 | 77.4 (10.5) | 1714 | 75.4 (11.4) | |
| Use of antihypertensive treatment | No | 4 229 | 87.4 | 1604 | 93.5 |
| Yes | 607 | 12.6 | 112 | 6.5 | |
| Triglycerides, mmol/L | 4823 | 1.35 (0.86) | 1714 | 1.38 (0.90) | |
| HDL-cholesterol, mmol/L | 4298 | 1.46 (0.39) | 1708 | 1.34 (0.32) | |
| Use of lipids lowering drugs | No | 4669 | 96.9 | 1686 | 98.2 |
| Yes | 155 | 3.1 | 30 | 1.7 | |
| Body mass index, kg/m² | 4175 | 26.0 (3.9) | 1695 | 25.8 (4.7) | |
Association between AHEI score and metabolites in the Whitehall II study
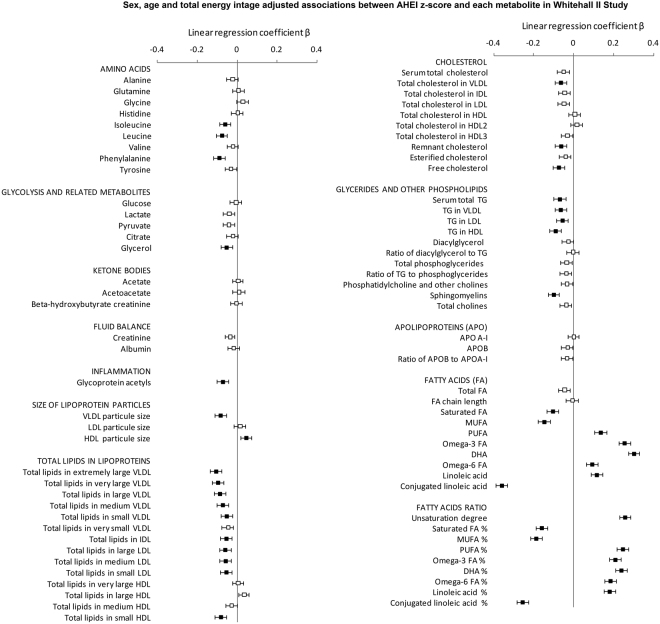
Results of the associations between AHEI z-score and 80 metabolites in Whitehall II study are shown in Fig. 1, (estimates and p values are available in Supplementary Material-Table C). Good adherence to healthy dietary recommendations, as assessed by higher AHEI score, was associated with lower circulating concentrations of specific amino acids (isoleucine, leucine and phenylalanine) and of metabolites related to gluconeogenesis (mainly glycerol) as well as lower chronic inflammation (assessed by glycoprotein acetyls) after accounting for Bonferroni correction for 80 tests. Adherence to healthy diet was also associated with a smaller average size of VLDL particles and larger average size of HDL particles. Regarding lipids in different lipoproteins subclasses, participants with higher AHEI score showed lower concentration of lipids in VLDL, IDL and LDL particles (from large to small) and with lipids in small HDL particles. AHEI score was also inversely associated with concentrations of cholesterol in VLDL, cholesterol not contained in HDL nor LDL (remnant cholesterol) and with free cholesterol. Higher AHEI score was associated with lower triglycerides concentrations in all lipid subfractions and lower circulating sphingomyelins.
Figure 1.
Age-, sex- and energy intake-adjusted associations between AHEI z-score and metabolites in Whitehall II study. Results are expressed as regression coefficients accompanied with their 95% confidence interval for one standard deviation increment in AHEI diet score. To facilitate comparison, metabolites were square root transformed and standardized to z-scores (mean = 0, SD = 1).  P ≥ 0.0006;
P ≥ 0.0006;  P < 0.0006.
P < 0.0006.
The strongest associations between metabolites and AHEI score were observed for fatty acid measures, especially for monounsaturated and conjugated linoleic acids for which linear regression coefficients were three times higher than for other metabolites on average (Supplementary Material-Table B). Regarding fatty acids, high AHEI score was associated with lower concentrations of saturated and monounsaturated fatty acids. Conversely, participants with higher AHEI score displayed higher concentrations of polyunsaturated fatty acids, including omega-3 (docosahexaenoic acid especially) and omega-6 (linoleic acids) but lower concentrations of conjugated linoleic acids. Analyses for ratios of each fatty acids category relative to total fatty acid concentrations confirmed the association between AHEI score and fatty acids and tended to display even stronger associations. All analyses were repeated by replacing AHEI by AHEI 2010 and similar trends were observed. Results are detailed in Supplementary Material-Table D.
The metabolites associations were only modestly attenuated (30.7% on average) after further adjustment for ethnicity, physical activity, smoking habits and cardiovascular risk factors (including type 2 diabetes, diastolic and systolic blood pressure, use of antihypertensive drugs and use of lipid-lowering drugs). All but one remained statistically significant (Table 2). An additional model in which body mass index (BMI) was added as covariate was performed. Analyses, carried out on the 4175 participants with available data on BMI, showed similar results (Supplementary Material-Table E).
Table 2.
Results of multivariable adjusted linear regression models of the association between AHEI z-score and the 42 selected metabolites in the Whitehall II study and in Young Finns Study.
| Multivariable-adjusted Model* | ||||||
|---|---|---|---|---|---|---|
| Whitehall II (N = 4699) | Young Finns Study (N = 1625) | |||||
| Beta | 95% CI | p | Beta | 95% CI | p | |
| Amino Acids | ||||||
| Isoleucine | −0.044 | −0.072 to −0.017 | 0.002 | 0.011 | −0.034 to 0.056 | 0.64 |
| Leucine | −0.061 | −0.088 to −0.034 | 1*10−5 | 0.010 | −0.035 to 0.055 | 0.65 |
| Phenylalanine | −0.074 | −0.104 to −0.044 | 1*10−6 | −0.038 | −0.090 to 0.014 | 0.15 |
| Glycolysis related metabolites | ||||||
| Glycerol | −0.044 | −0.074 to −0.014 | 0.004 | −0.009 | −0.060 to 0.042 | 0.73 |
| Inflammation | ||||||
| Glycoprotein acetyls | −0.035 | −0.064 to −0.006 | 0.02 | −0.012 | −0.063 to 0.039 | 0.64 |
| Size of lipoprotein particles (Mean diameters) | ||||||
| VLDL particle size | −0.048 | −0.076 to −0.019 | 0.0009 | −0.003 | −0.051 to 0.046 | 0.91 |
| HDL particle size | 0.033 | 0.006 to 0.061 | 0.02 | −0.005 | −0.051 to 0.040 | 0.82 |
| Total lipid concentrations in lipoprotein subclasses | ||||||
| Total lipids in chylomicrons and extremely large VLDL | −0.073 | −0.102 to −0.044 | 8*10−7 | −0.033 | −0.082 to 0.016 | 0.19 |
| Total lipids in very large VLDL | −0.061 | −0.089 to −0.032 | 4*10−5 | −0.022 | −0.070 to 0.027 | 0.38 |
| Total lipids in large VLDL | −0.049 | −0.078 to −0.021 | 0.0007 | −0.006 | −0.054 to 0.042 | 0.81 |
| Total lipids in medium VLDL | −0.036 | −0.067 to −0.008 | 0.01 | 0.001 | −0.048 to 0.048 | 0.99 |
| Total lipids in small VLDL | −0.019 | −0.048 to 0.010 | 0.21 | 0.017 | −0.031 to 0.065 | 0.49 |
| Total lipids in IDL | −0.039 | −0.069 to −0.010 | 0.01 | −0.032 | −0.084 to 0.021 | 0.24 |
| Total lipids in large LDL | −0.045 | −0.075 to −0.015 | 0.003 | −0.032 | −0.084 to 0.021 | 0.24 |
| Total lipids in medium LDL | −0.044 | −0.074 to −0.014 | 0.004 | −0.028 | −0.080 to 0.024 | 0.29 |
| Total lipids in small LDL | −0.039 | −0.069 to −0.009 | 0.01 | −0.028 | −0.080 to 0.023 | 0.28 |
| Total lipids in small HDL | −0.054 | −0.083 to −0.024 | 0.0003 | 0.001 | −0.052 to 0.054 | 0.96 |
| Cholesterol | ||||||
| Cholesterol in VLDL | −0.036 | −0.065 to −0.006 | 0.02 | −0.006 | −0.055 to 0.043 | 0.81 |
| Remnant cholesterol (non−HDL, non−LDL −cholesterol) | −0.041 | −0.071 to −0.011 | 0.007 | −0.021 | −0.072 to 0.029 | 0.41 |
| Free cholesterol | −0.055 | −0.084 to −0.026 | 0.0002 | −0.035 | −0.089 to 0.018 | 0.19 |
| Glycerides and other Phospholipids | ||||||
| Serum total TG | −0.034 | −0.063 to −0.005 | 0.02 | −0.002 | −0.050 to 0.047 | 0.95 |
| TG in VLDL | −0.031 | −0.061 to −0.003 | 0.04 | 0.001 | −0.046 to 0.049 | 0.95 |
| TG in LDL | −0.031 | −0.060 to −0.002 | 0.04 | −0.012 | −0.064 to 0.041 | 0.66 |
| TG in HDL | −0.060 | −0.090 to −0.031 | 5*10−5 | −0.017 | −0.070 to 0.036 | 0.53 |
| Sphingomyelins | −0.081 | −0.110 to −0.053 | 2*10−8 | −0.022 | −0.074 to 0.031 | 0.42 |
| Fatty Acids (FA) | ||||||
| Saturated FA | −0.056 | −0.086 to −0.027 | 0.0002 | −0.029 | −0.080 to 0.022 | 0.27 |
| Monounsaturated FA | −0.069 | −0.098 to −0.040 | 2*10−6 | −0.022 | −0.072 to 0.028 | 0.39 |
| Polyunsaturated FA | 0.076 | 0.047 to 0.106 | 4*10−7 | 0.028 | −0.025 to 0.080 | 0.30 |
| Omega-3 FA | 0.140 | 0.111 to 0.169 | 1*10−20 | 0.097 | 0.045 to 0.149 | 0.0003 |
| docosahexaenoic acid | 0.176 | 0.147 to 0.205 | 1*10−32 | 0.096 | 0.044 to 0.149 | 0.0003 |
| Omega-6 FA | 0.054 | 0.024 to 0.083 | 0.0004 | 0.009 | −0.043 to 0.062 | 0.72 |
| linoleic acid | 0.076 | 0.046 to 0.105 | 6*10−7 | 0.004 | −0.049 to 0.056 | 0.89 |
| Conjugated linoleic acid | −0.198 | −0.227 to −0.169 | 2*10−40 | NA | NA | |
| Fatty acids ratios, relative to total fatty acids | ||||||
| Estimated degree of unsaturation | 0.210 | 0.183 to 0.238 | 1*10−48 | 0.116 | 0.066 to 0.166 | 5*10−6 |
| Ratio of saturated FA to total FA (%) | −0.164 | −0.194 to −0.134 | 4*10−27 | −0.089 | −0.141 to −0.037 | 0.0008 |
| Ratio of monounsaturated FA to total FA (%) | −0.124 | −0.152 to −0.095 | 2*10−17 | −0.034 | −0.082 to 0.015 | 0.17 |
| Ratio of polyunsaturated FA to total FA (%) | 0.194 | 0.162 to 0.219 | 2*10−41 | 0.082 | 0.033 to 0.131 | 0.0011 |
| Ratio of omega-3 FA to total FA (%) | 0.190 | 0.162 to 0.219 | 1*10−37 | 0.142 | 0.091 to 0.193 | 5*10−8 |
| Ratio of docosahexaenoic acid to total FA (%) | 0.220 | 0.192 to 0.249 | 9*10−52 | 0.139 | 0.090 to 0.189 | 4*10−8 |
| Ratio of omega-6 FA to total FA (%) | 0.137 | 0.109 to 0.166 | 6*10−21 | 0.044 | −0.006 to 0.094 | 0.08 |
| Ratio of linoleic acid to total FA (%) | 0.145 | 0.116 to 0.174 | 1*10−22 | 0.029 | −0.022 to 0.080 | 0.26 |
| Ratio of conjugated linoleic acid to total FA (%) | −0.226 | −0.255 to −0.197 | 7*10−52 | NA | NA | / |
*Multivariable adjusted model: adjusted for age, sex, total energy intake, ethnicity, smoking habits, physical activity, type 2 diabetes, diastolic and systolic blood pressure, use of antihypertensive drugs and use of lipid-lowering drugs. Results were expressed as linear regression coefficients accompanied with their 95% confidence interval. Analyses were carried out on participants for which all metabolites measurement were available.
Because both dietary changes and a modification of circulating metabolites are expected in participants with prevalent CVD, cancer or longstanding illness, we performed sensitivity analyses to assess the extent by which the AHEI-metabolites associations reported here might be explained by these diseases. Analyses repeated after excluding participants (1) reporting history of cardiovascular diseases (2) with a diagnosis of cancer and (3) reporting a longstanding illness indicate that the associations reported were not explained by these chronic diseases (Supplemental Material-Table F).
Replication analysis in the Young Finns study
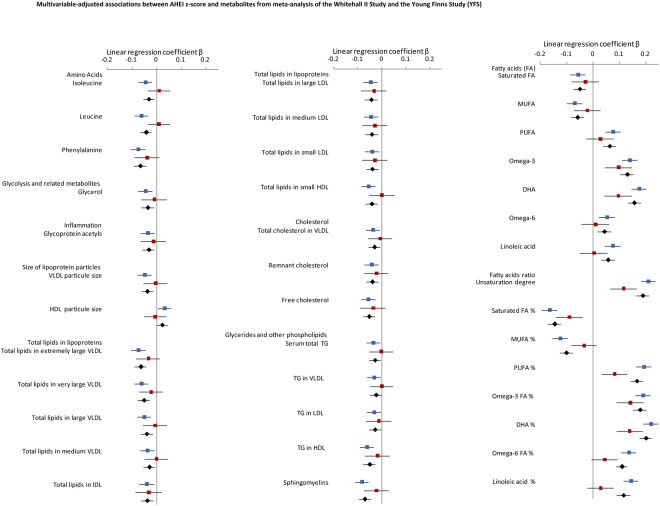
Analyses of the associations between the 41 metabolites (those significantly associated with AHEI score in multivariate model performed in Whitehall II study) and AHEI z-score were repeated in the Young Finns Study whose sample effective was about the third of the Whitehall II effective. Mean concentration of these 41 metabolites in Young Finns are listed in Supplemental Material-Table G. The replication analyses and meta-analyses, displayed in Table 2 and Supplementary Material-Table H respectively and illustrated in Fig. 2 showed that 38 of the 41 diet-metabolites associations were directionally concordant. The only deviating measures are branched amino acids, glycerol and size of HDL particle.
Figure 2.
Multivariable-adjusted associations between AHEI z-score and metabolites from meta-analysis of the Whitehall II Study and the Young Finns Study (YFS).  Whitehall II study;
Whitehall II study;  YFS;
YFS;  Meta-analysis. Linear regression models were adjusted for age, sex, total energy intake, ethnicity, smoking habits, physical activity, type 2 diabetes, diastolic and systolic blood pressure, use of antihypertensive drugs and use of lipid-lowering drugs. Results are expressed as linear regression coefficients accompanied with their 95% confidence interval. To facilitate comparison, metabolites were first square root transformed and then standardized to z-scores (mean = 0, SD = 1).
Meta-analysis. Linear regression models were adjusted for age, sex, total energy intake, ethnicity, smoking habits, physical activity, type 2 diabetes, diastolic and systolic blood pressure, use of antihypertensive drugs and use of lipid-lowering drugs. Results are expressed as linear regression coefficients accompanied with their 95% confidence interval. To facilitate comparison, metabolites were first square root transformed and then standardized to z-scores (mean = 0, SD = 1).
Of the 41 metabolites assessed, AHEI score was significantly associated with two fatty acids and 5 fatty acids ratio (Fig. 2 and Supplementary Table H) confirming the strong associations between good adherence to healthy diet and higher concentrations of omega-3 and docosahexaenoic acid, higher ratios of all polyunsaturated fatty acids ratio (including omega-3, omega-6) and lower ratios of saturated and monounsaturated fatty acids relative to total fatty acids. Regarding the other metabolites, even if the direction of most of associations was similar as observed in Whitehall II, the associations were weaker and did not reach statistical significance in the Young Finns study with much smaller sample size than in Whitehall II.
Metabolites associated with AHEI and predicting cardiovascular disease
We assessed the extent to which each of the 41 metabolites associated with diet score also predicted CVD events. Of the 5481 Whitehall II participants, 697 developed CVD over the 15.8 years of follow-up. Results are presented in Table 3. Metabolites found to significantly predict CVD risk consisted of amino acids, glycoprotein acetyls, size of lipoprotein particule size, total lipids in lipoproteins (except those in IDL and in small HDL), total cholesterol in VLDL particles and triglycerides. Amongst fatty acids, significant association were found for saturated fatty acids and monounsaturated fatty acids. Degree of unsaturation was inversely associated with CVD risk. When ratio of fatty acids categories relative to total fatty acids concentration was considered, significant associations were observed for mono- and poly-unsaturated fatty acids, omega-3 and docosahexaenoic acid, with higher ratio of monounsaturated fatty acids increasing the risk of CVD risk, and higher ratio of polyunsaturated fatty acids, omega-3 and docosahexaenoic acid decreasing CVD risk. Other fatty acids ratios were not found significantly associated with CVD risk.
Table 3.
Association between baseline metabolites and incident cardiovascular disease over 15.8 years of follow-up in the Whitehall II study.
| Association with CVD risk (total N = 5481; N incident cases = 697) | |||
|---|---|---|---|
| Hazard Ratio* | 95% CI | p | |
| Amino Acids | |||
| Isoleucine | 1.14 | 1.06 to 1.24 | 0.001 |
| Leucine | 1.13 | 1.04 to 1.22 | 0.003 |
| Phenylalanine | 1.14 | 1.07 to 1.23 | 0.000 |
| Glycolysis related metabolites | |||
| Glycerol | 1.06 | 0.98 to 1.14 | 0.14 |
| Inflammation | |||
| Glycoprotein acetyls | 1.20 | 1.11 to 1.30 | 3*10−6 |
| Size of lipoprotein particles (Mean diameters) | |||
| VLDL particle size | 1.12 | 1.03 to 1.21 | 0.007 |
| HDL particle size | 0.86 | 0.79 to 0.94 | 0.0006 |
| Total lipid concentrations in lipoprotein subclasses | |||
| Total lipids in chylomicrons and extremely large VLDL | 1.09 | 1.01 to 1.18 | 0.02 |
| Total lipids in very large VLDL | 1.09 | 1.01 to 1.17 | 0.03 |
| Total lipids in large VLDL | 1.12 | 1.03 to 1.21 | 0.005 |
| Total lipids in medium VLDL | 1.12 | 1.04 to 1.21 | 0.004 |
| Total lipids in IDL | 1.07 | 0.99 to 1.16 | 0.003 |
| Total lipids in large LDL | 1.09 | 1.01 to 1.18 | 0.01 |
| Total lipids in medium LDL | 1.09 | 1.01 to 1.18 | 0.08 |
| Total lipids in small LDL | 1.08 | 1.00 to 1.17 | 0.03 |
| Total lipids in small HDL | 0.97 | 0.90 to 1.05 | 0.02 |
| Cholesterol | |||
| Cholesterol in VLDL | 1.09 | 1.01 to 1.18 | 0.03 |
| Remnant cholesterol (non-HDL, non-LDL -cholesterol) | 1.07 | 0.99 to 1.16 | 0.07 |
| Free cholesterol | 1.04 | 0.96 to 1.12 | 0.36 |
| Glycerides and other Phospholipids | |||
| Serum total TG | 1.15 | 1.07 to 1.24 | 0.0002 |
| TG in VLDL | 1.14 | 1.05 to 1.23 | 0.0013 |
| TG in LDL | 1.19 | 1.11 to 1.28 | 3*10−6 |
| TG in HDL | 1.13 | 1.04 to 1.22 | 0.0021 |
| Sphingomyelins | 0.98 | 0.90 to 1.06 | 0.56 |
| Fatty Acids (FA) | |||
| Saturated FA | 1.09 | 1.01 to 1.18 | 0.02 |
| Monounsaturated FA | 1.12 | 1.04 to 1.21 | 0.004 |
| Polyunsaturated FA | 1.05 | 0.97 to 1.13 | 0.26 |
| Omega-3 FA | 0.97 | 0.90 to 1.05 | 0.43 |
| docosahexaenoic acid | 0.96 | 0.89 to 1.04 | 0.33 |
| Omega-6 FA | 1.06 | 0.98 to 1.14 | 0.14 |
| linoleic acid | 1.06 | 0.98 to 1.14 | 0.14 |
| Conjugated linoleic acid | 1.05 | 0.97 to 1.13 | 0.23 |
| Fatty acids ratios, relative to total fatty acids | |||
| Estimated degree of unsaturation | 0.90 | 0.83 to 0.97 | 0.009 |
| Ratio of saturated FA to total FA (%) | 1.01 | 0.93 to 1.08 | 0.90 |
| Ratio of monounsaturated FA to total FA (%) | 1.11 | 1.03 to 1.21 | 0.007 |
| Ratio of polyunsaturated FA to total FA (%) | 0.90 | 0.84 to 0.97 | 0.009 |
| Ratio of omega-3 FA to total FA (%) | 0.90 | 0.83 to 0.97 | 0.008 |
| Ratio of docosahexaenoic acid to total FA (%) | 0.90 | 0.83 to 0.97 | 0.009 |
| Ratio of omega-6 FA to total FA (%) | 0.93 | 0.87 to 1.01 | 0.083 |
| Ratio of linoleic acid to total FA (%) | 0.96 | 0.89 to 1.04 | 0.28 |
| Ratio of conjugated linoleic acid to total FA (%) | 1.03 | 0.95 to 1.11 | 0.53 |
*Cox regression models were performed to estimate association between each metabolites and risk of CVD onset over the 16-y of follow-up. Models were adjusted for age, sex, total energy intake, ethnicity, smoking habits, physical activity, type 2 diabetes, diastolic and systolic blood pressure, use of antihypertensive drugs and use of lipid-lowering drugs.
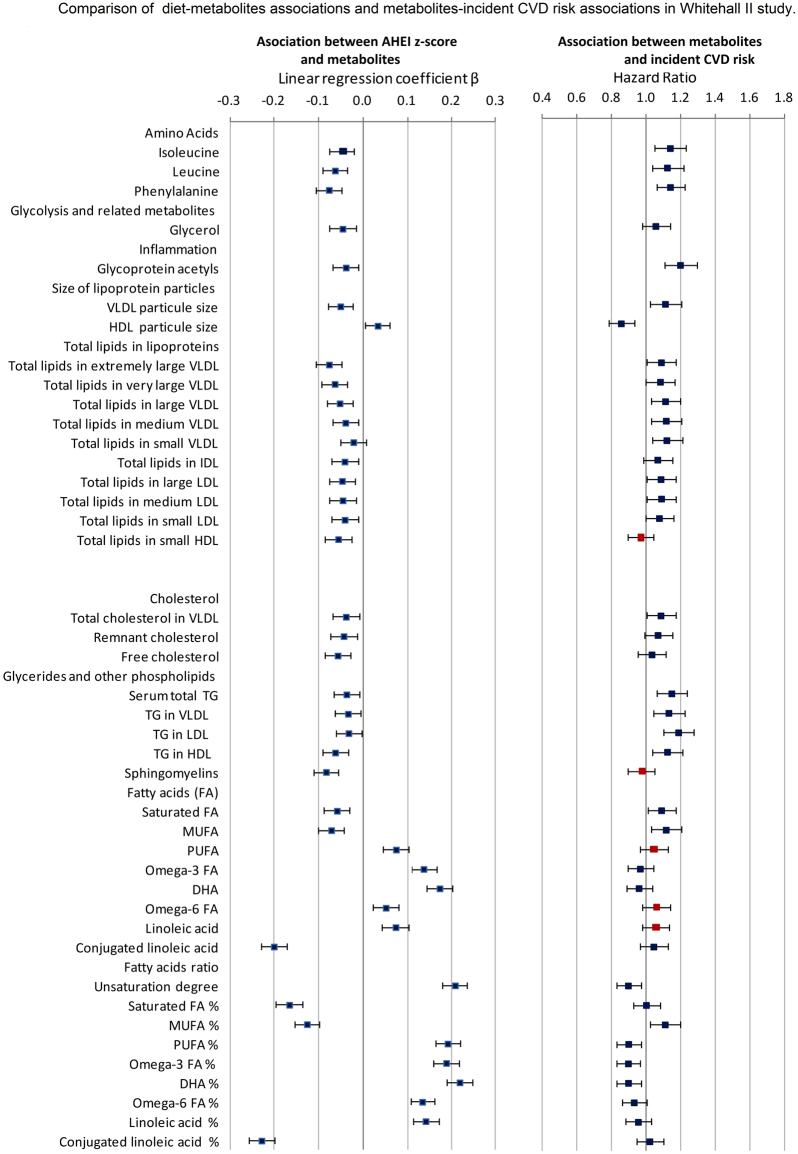
Figure 3 illustrates whether metabolites associated with poor adherence to healthy dietary guidelines were also related to higher CVD risk. Of the 41 diet-metabolites and metabolites-CVD risk associations assessed, only 5 were directionally discordant and concerned polyunsaturated fatty acids, omega 6 and linoleic acids whose higher blood concentrations were associated with higher CVD risk but without reaching statistical significance. Discordance in terms of direction of association was also observed for total lipids in small HDL and sphingomyelin (Fig. 3).
Figure 3.
Comparison of diet-metabolites associations and metabolites-incident CVD risk associations in Whitehall II study.  Associations directionally concordant.
Associations directionally concordant.  Associations directionally discordant. On the left hand size: Linear regression models estimating the associations between AHEI z-score and the 41 selected metabolites performed in 4824 participants and adjusted for age, sex, total energy intake, ethnicity, smoking habits, physical activity, type 2 diabetes, diastolic and systolic blood pressure, use of antihypertensive drugs and use of lipid-lowering drugs. Results are expressed as linear regression coefficients accompanied with their 95% confidence interval. To facilitate comparison, metabolites were first square root transformed and then standardized to z-scores (mean = 0, SD = 1). On the right hand size: Cox proportional hazards regression models estimating the association between the selected 41 metabolites and the risk of incident CVD over the 15.8 years of follow-up, performed in 5840 Whitehall II participant, adjusted for age, sex, total energy intake, ethnicity, smoking habits, physical activity, type 2 diabetes, diastolic and systolic blood pressure, use of antihypertensive medication. Results are expressed as Hazard Ratio accompanied with their 95% confidence interval. To facilitate comparison, metabolites were first square root transformed and then standardized to z-scores (mean = 0, SD = 1).
Associations directionally discordant. On the left hand size: Linear regression models estimating the associations between AHEI z-score and the 41 selected metabolites performed in 4824 participants and adjusted for age, sex, total energy intake, ethnicity, smoking habits, physical activity, type 2 diabetes, diastolic and systolic blood pressure, use of antihypertensive drugs and use of lipid-lowering drugs. Results are expressed as linear regression coefficients accompanied with their 95% confidence interval. To facilitate comparison, metabolites were first square root transformed and then standardized to z-scores (mean = 0, SD = 1). On the right hand size: Cox proportional hazards regression models estimating the association between the selected 41 metabolites and the risk of incident CVD over the 15.8 years of follow-up, performed in 5840 Whitehall II participant, adjusted for age, sex, total energy intake, ethnicity, smoking habits, physical activity, type 2 diabetes, diastolic and systolic blood pressure, use of antihypertensive medication. Results are expressed as Hazard Ratio accompanied with their 95% confidence interval. To facilitate comparison, metabolites were first square root transformed and then standardized to z-scores (mean = 0, SD = 1).
Discussion
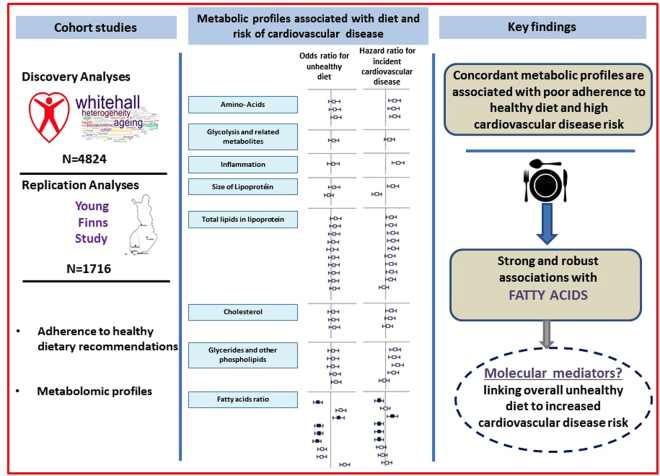
The present study based on metabolic profiling analyses identified and replicated metabolites associated with the adherence to dietary recommendations provided by the Alternative Healthy Eating Index after taking into account potential confounders and multiple testing in two population-based studies - the Whitehall II and the Cardiovascular Risk in Young Finns. A key finding of these analyses concerns the metabolic profiles of fatty acids associated with diet score. Furthermore, our study highlights the concordance between metabolites profile associated with low adherence to healthy diet and the metabolites profile associated with 15.8-year risk of CVD in Whitehall II participants by showing that an increased risk of CVD onset was associated with high levels of saturated and monounsaturated fatty acids and a decreased risk of CVD was associated with a higher ratio of polyunsaturated fatty acids, omega-3 and docosahexaenoic acid relative to total fatty acids concentrations (Fig. 4).
Figure 4.
Metabolomic profiles associated with low adherence to healthy dietary guidelines and with the risk of incident cardiovascular diseases -. The metabolic profiling analyses identified 41 metabolites associated with the adherence to healthy diet in Whitehall II study. Replication analyses in the Young Finns Study showed that most of these diet-metabolites associations were directionally concordant. We then assessed the extent to which each of the 41 metabolites associated with diet score also predicted CVD events over the 15.8 years of Whitehall II Study follow-up. Results showed that most of metabolites associated with poor adherence to healthy dietary guidelines are also related to higher CVD risk and consisted of amino acids, glycoprotein acetyls, size of lipoprotein particule size, lipids in lipoproteins, cholesterol and triglycerides and fatty acids. These findings highlight a specific fatty acid patterns robustly associated with both adherence to healthy diet and reduced risk of CVD. These specific fatty acids pattern consisted of lower levels of saturated and monounsaturated fatty acids and higher ratio of polyunsaturated fatty acids, omega-3 and docosahexaenoic acid relative to total fatty acids concentrations, possibly representing a molecular link between healthy diet and lower cardiovascular disease risk.
Our metabolic profiling analyses identified 41 metabolites associated with the adherence to healthy diet. The strongest associations between metabolites and AHEI score were observed for fatty acid measures. We reported a robust and positive association between AHEI scores and degree of unsaturation of fatty acids, ratio and concentrations of polyunsaturated fatty acids including omega-3 (docosahexaenoic acid in particular, brought by fatty fish intake but also oil supplements), omega-6 (linoleic acids found in nuts, fatty seeds and their derived vegetable oil). Conversely, a negative association was found between AHEI scores and ratio (and concentrations) of saturated (found in dairy products, fatty products, processed food and fatty meat intakes) and monounsaturated fatty acids (affected by vegetable oils, lean meat but also produced endogenously by the desaturation of dietary saturated fatty acids4,14) and conjugated linoleic acids (found in ruminant meat and dairy products15). This fatty acids pattern associated with AHEI score was directionally concordant with fatty acids pattern (except for omega-6) associated with incident CVD in Whitehall II study. Our results are also concordant with previous findings from observational studies suggesting associations of higher levels of omega-316 and linoleic acid17 with lower coronary heart disease events and an increased disease risk in relation to high levels of monounsaturated fatty acids4,18. The strong association found between fatty acids and diet in the Whitehall II and Young Finns studies and the fact that similar metabolic profile of fatty acids was associated with incident CVD suggest that these specific fatty acids are potential molecular mediators between unhealthy diet and increased CVD risk. Even if recent randomized trials19,20 did not indicate a beneficial impact of replacing dietary saturated fatty acids with polyunsaturated ones on CVD risk, our work suggests that the better understanding of the mechanisms underlying the variability of these fatty acids may be helpful in explaining how overall diet might be linked to CVD development.
We identified detailed lipid profiles associated with a good adherence to AHEI recommendations. Using NMR spectroscopy, we were able to determine the lipoprotein subclasses distribution as well as their lipid composition. We found that participants with high score in AHEI had a lipid profile characterized by lower concentrations of lipids in chylomicrons and extremely large, very large and large VLDL, as well as small HDL compared to participants with low score in AHEI. Higher amounts of lipids packaged into chylomicrons may reflect higher ingestion of lipids through the diet and postprandial lipidemia, an established risk factor for CVD21,22. Since chylomicrons and VLDL are competitive substrates for triglyceride hydrolysis by lipoprotein lipase in adipose and muscle tissues, higher amounts of circulating chylomicrons are usually associated with predominance of oversized VLDL particles. This specific metabolic profile of lipids in participants with low AHEI scores has also been linked to an increased risk of artherosclerosis and premature CVD23–25. The predominance of large VLDL has also been linked to metabolically unhealthy individuals, regardless of BMI and metabolic health definition21.
Even if the NMR metabolomics platform featured here is not designed for novel biomarker discovery and includes less metabolites than mass spectrometry-based platforms, the panel of biomarkers covers a wide range of potential relevant biomarkers for diet-CVD associations, including amino acids, glycolysis related metabolites, inflammation, lipids and cholesterol, glycerides and other phospholipids, and fatty acids. The possibility to quantify these measures robustly in a single experiment26 is important to determine their relative importance for diet and CVD risk.
In contrast to other NMR methodologies of advanced lipoprotein profiling27, the platform used in this study provides quantification of many fatty-acid measures, some abundant proteins, and a broad range of low-molecular-weight metabolites together with very detailed lipoprotein subclasses profiling28. This simultaneous quantification of circulating biomarkers across multiple pathways provide a very detailed picture of a person’s metabolic state27; we found that in particular fatty acids and lipids components metabolites play a role in both overall unhealthy diet and incidence of CVD events.
Beyond the lipid and fatty acids components, we showed that amino acid components – phenylalanine, leucine and isoleucine - were also associated with both lower AHEI score and increased incident CVD risk. These amino acids have previously been associated with higher risks of developing type 2 diabetes29–31. Branched-chain and aromatic amino acid are affected by intakes of animal (pork, beef, chicken, eggs and dairy products) and plant (soy beans, rice, corn, wheat) protein32. However, our analyses did not allow to assess the associations of these amino acids in the diet-CVD association according to their plant or animal origins. Further analyses to examine this question would be relevant in a context where beneficial effects of plant protein on cardiometabolic diseases has been reported33.
Our study has both strengths and limitations. First, the assessment of dietary intake using a semi-quantitative food frequency questionnaire covered only specific foods and is recognized to be less precise than dietary assessment by the food diary method. However, in a large sample size cohort study, the use of food frequency questionnaires is particularly adapted and a commonly used method. Second, we assessed healthy diet through using the AHEI score which is a summary measure of the degree to which an individual’s diet conforms to the serving recommendations of the US Department of Agriculture Food Guide Pyramid and the US Dietary Guidelines for Americans11. By being based on a set of specific and limited food groups, AHEI does not cover all aspects of “healthy” diet and may not be adapted to dietary habits in all populations. However, high scores on this index have been shown to be associated with reduced risk of CVD11, and type 2 diabetes34. The use of AHEI in the present analyses is particularly relevant, as previous findings from the Whitehall II study suggest that adherence to the AHEI may reduce the long-term risk of all-cause and cardiovascular mortality12 and to be related to an almost 2-fold higher odds of reversing the metabolic syndrome35, a condition known to predict cardiovascular morbidity and mortality36. Third, AHEI provides an overall measure of the extent to which a person adheres healthy dietary guidelines in terms of the intake of vegetables, fruits, nuts and soy, white vs red meat, trans-fat, polyunsaturated and saturated fatty acids, multivitamin, alcohol and cereal fiber. Fourth, to counteract the problem of multiple comparisons we applied a stringent Bonferroni correction which reduces the probability of false significant findings but might increase the probability of false negative results, since many of the examined metabolites and lipid components are strongly correlated with one another. Additionally, we adjusted our analyses for correlated measures such as blood pressure that may artificially reduce the associations’ estimates. Fifth, with an epidemiological observational framework, our observations may be partly explained by unmeasured confounders such as gut microbiota which can potentially influence metabolite variability as well as dietary behaviors. However, by carrying out our analyses on a larger sample size population study compared to previous studies on the same topic and by replicating our findings in another cohort study while previous reports were based on single cohort studies, bring strength to the validity of our observations. The NMR platform used has also limitations. The metabolic profile measured through this platform provided fasting steady-state levels of metabolites. The fact that metabolites related to carbohydrate and protein intakes might be less detectable in fasting state than lipids and fatty acids might explain why metabolites found to be associated with high quality diet were lipids and fatty acids while significant associations with branched amino acids, metabolites related to glycolysis were scarce and most of them were not confirmed in the replication analyses. Furthermore, as glycolysis related metabolites and some amino-acids, lipids and specific fatty acids are produced endogenously with different rates depending on issues, such as individual’s metabolic state, the NMR metabolite measures reflect both metabolites’ exogeneous intake and their endogenous synthesis; they cannot be viewed as markers of specific dietary intakes. A further limitation is that the NMR platform does not include many metabolites from vegetables, fruits, nuts and soy. Further research examining the association of dietary exposure to a wider range of metabolites is needed.
Conclusions
Our metabolic profiling study enabled us to identify and replicate a number of metabolites robustly associated with adherence to dietary recommendations provided by the Alternative Healthy Eating Index. A key finding of these analyses concerns the metabolic profiles of fatty acids (higher ratio of polyunsaturated fatty acids, omega-3, omega-6 and lower ratio of saturated, monounsaturated and conjugated fatty acids relative to total fatty acids) associated with AHEI score in Whitehall II Study and in Young Finns Study. Our report also highlights the high overlap in metabolites associated with low adherence to healthy dietary guidelines and those predicting long-term risk of CVD in Whitehall II. By showing that an increased risk of CVD onset was associated with high levels of saturated and monounsaturated fatty acids and a decreased risk of CVD was associated with higher ratio of polyunsaturated fatty acids, omega-3 and docosahexaenoic acid relative to total fatty acids concentrations, our findings suggest that these specific fatty acids might be important molecular mediators linking overall unhealthy diet to increased CVD risk.
Methods
Study samples
Participants of the discovery cohort were drawn from the Whitehall II cohort study10, an on-going prospective cohort study of adults recruited from 20 London-based Civil Service departments in 198510. Of these, 10 308 (6,895 men and 3,413 women, aged 35 to 55) enrolled, a response proportion of 73%. The baseline medical examination (phase 1) took place during 1985/88, and subsequent phases including both clinical examination and self-administrated questionnaire have taken place approximately every 5 years. The subjects included in the metabolites-diet association analyses (n = 4824) was a sample of men (n = 3483) and women (n = 1341) who participated in the 1997/99 clinical examination, and whose serum sample was profiled using NMR metabolomics and had complete data on diet and covariates assessed in 1997/99. Participants gave full informed written consent to participate in the study and ethical approval was obtained from the University College London Hospital committee on the Ethics of Human Research. All research was performed in accordance with relevant guidelines/regulations.
Replication analyses were based on the 2001 survey of the Cardiovascular Risk in Young Finns Study originally designed to study associations of childhood risk factors to disease in adulthood (youngfinnsstudy.utu.fi)13. The baseline study conducted in 1980 included n = 3596 children and adolescents aged 3–18. The 2283 individuals participating in 2001 survey (response rate 64%)13,37 were representative of the baseline cohort13. Among these, n = 2247 individuals provided an overnight fasting blood samples, and the resulting serum samples were stored at −80 °C prior to metabolic profiling by serum NMR metabolomics which was complete for 2161 participants. We further excluded from the present analyses participants with missing data on dietary variables, and main covariates including age sex, total energy intake, alcohol consumption, smoking status, physical activity index assessed by metabolic equivalent of task, systolic and diastolic blood pressure (mm Hg), use of antihypertensive drugs and type 2 diabetes. Assessment of these variables have been described elsewhere13,38,39. All participants gave written informed consent, and the study was approved by the ethics committees of each of the five participating medical university sites in Finland.
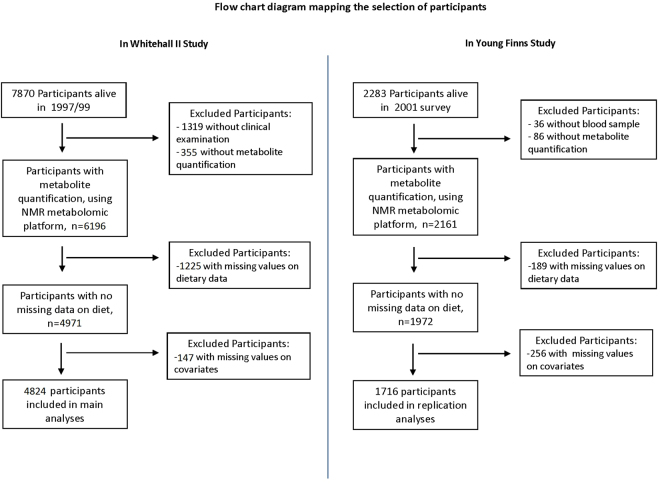
The flow chart diagrams mapping the selection of Whitehall II and Young Finns Study participants are provided in Fig. 5.
Figure 5.
Flow chart diagram mapping the inclusion of Whitehall II and Young Finns Study participants.
Assessment of clinical characteristics
In the Whitehall II study, socio-demographic, health behaviors and health status factors assessed in 1997/99 were considered. Socio-demographic factors included sex, age and ethnicity (white/South Asian/Black). Health behaviors consisted of smoking status (current/former/non smoker), total energy intake (in kcal per day, estimated from the food frequency questionnaire) and physical activity. Based on the physical activity questionnaire that consisted of 20 items on frequency and duration of participation in walking, cycling, sports, gardening, housework, and home maintenance, frequency and duration of each activity were combined to compute Metabolic Equivalent of Task (MET) units/hours/week of moderate to vigorous physical activity40. Health status factors considered were those related to cardiovascular risk factors. They included measures of systolic and diastolic blood pressure, use of antihypertensive drugs; type 2 diabetes (diagnosed according to the WHO definition);and use of lipid-lowering drugs. In the Young Finns Study, corresponding assessment of socio-demographic, health behaviors and clinical characteristics was undertaken13,38.
Metabolite quantification
A high-throughput NMR metabolomics platform28 was used for the quantification of metabolites from serum samples4. We focused on 80 lipid and abundant metabolite measures listed in Supplemental Material-Table A. All metabolites were measured in a single experimental setup that allows for the simultaneous quantification of both routine lipids, total lipid concentrations of 14 lipoprotein subclasses, fatty acid composition such as MUFA and PUFA, various glycolysis precursors, ketone bodies, and amino acids in absolute concentration units. The NMR metabolomics platform has previously been used in various epidemiological studies4,41, details of the experimentation have been described4 and the method has recently been reviewed28,42,43.
Dietary assessment
Dietary intake was assessed using a semi-quantitative food-frequency questionnaire (FFQ) including 127 food items as described previously12,44. The validity and reliability of the FFQ in terms of nutrients and food consumption have been documented in detail elsewhere44,45. The AHEI score11 - a score reflecting dietary guidelines adapted to the UK framework - was implemented in Whitehall II and Young Finns Study cohorts. It was based on the intake of 9 dietary components: (1) vegetables, (2) fruits, (3) nuts and soy, (4) the ratio of white (seafood and poultry) to red meat, (5) trans-fat, (6) the ratio of polyunsaturated to saturated fatty acids, (7) long-term multivitamin use (<5 or ≥5 y), (8) alcohol consumption and (9) cereal fiber. Each component had the potential to contribute 0 to 10 points to the total score, with the exception of multivitamin use, which contributed either 2.5 or 7.5 points. All the component scores were summed to obtain a total AHEI score ranging from 2.5 to 87.5 with higher scores denoting a healthier diet. Means of AHEI score and its components for both cohorts are detailed in Supplementary Material-Table I. AHEI was defined a priori based upon previous knowledge. In 2012, a new measure of the AHEI has been proposed – the AHEI 2010. This index has also been implemented, It includes 11 components, its distribution is detailed in Supplementary Material-Table J.
Ascertainment of incident cardiovascular disease
Whitehall II participants were linked to electronic medical records to ascertain cardiovascular disease, including coronary heart disease and stroke. Records for the first included hospitalisations from coronary heart disease as a primary or secondary diagnosis (defined using ICD-9 codes 410-414 and ICD-10 codes I20-I25 or procedures K40-K49, K50, K75, U19) and coronary deaths (defined using ICD-9 codes 410-414 and ICD-10 codes I20-I25 in death certificates). Data on stroke included records on hospitalizations due to stroke as a primary or secondary diagnosis and stroke deaths (defined using ICD-9 codes 430, 431, 434, 436 and ICD-10-codes I60, I61, I63, I64). The Young Finns Study participants were too young to have CVD events (less than 20 events during the 12 years follow-up).
Statistical analyses
All metabolite concentrations were squared root transformed prior to analyses to obtain approximately normal distribution. The metabolite measures were subsequently standardized using z-score (mean = 0, standard deviation = 1). The overall AHEI scores, normally distributed in the two cohorts, were analyzed as continuous variable using z-scores too. Associations between AHEI z-score and each metabolite were assessed by performing linear regression models first adjusted for age, sex and total energy intake. Metabolites found significantly associated with AHEI score at p < 0.0006 (Bonferroni correction of p < 0.05 accounting for 80 independent tests) were selected for further testing, including replication analysis and associations with CVD event risk. For the selected metabolites, linear regression models further adjusted for ethnicity, smoking habits, physical activity, systolic and diastolic blood pressure, use of antihypertensive drugs, type 2 diabetes and use of lipid-lowering drugs were performed. These analyses were repeated after taking into account BMI. In sensitivity analyses these multivariable adjusted models estimating the association between AHEI z-score and metabolites were repeated (1) in participants free of cardiovascular diseases in 1997/99 (i.e. clinically verified non-fatal myocardial infarction or definite angina), (2) in participants without prevalent cancer and (3) after excluding participants who self-reported longstanding illness.
To examine whether the findings of the associations between AHEI and metabolites in Whitehall II study were replicable, we used data from the Young Finns Study and applied similar multivariable linear regression models. As for analyses in Whitehall II, metabolites were square rooted and z-scores were computed and AHEI was treated as a z-score. The results from individual cohorts were then combined by using inverse variance fixed effect meta-analysis.
To assess the extent to which metabolites associated with diet score were also those predictive of CVD events, we conducted Cox proportional hazards regression models for each of the selected metabolites as predictors of incident CVD events (adjusted for similar risk factors as those considered in the above-mentioned analyses). To do so we selected the 5840 Whitehall II participants for whom quantification of metabolites and clinical characteristics were available in 1997/99 and a follow-up of cardiovascular diseases over the 16-year follow-up. Participants with prevalent CVD were excluded to concentrate on associations with first onset of CVD.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
We thank all of the participating civil service departments and their welfare, personnel, and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team. The Whitehall II Study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants and data entry staff, who make the study possible. The Whitehall II study has been supported by grants from the UK Medical Research Council (K013351 and MR/R024227/1); the British Heart Foundation (PG/11/63/29011 and RG/13/2/30098); the British Health and Safety Executive; the British Department of Health; the National Heart, Lung, and Blood Institute (R01HL036310); the National Institute on Aging, National Institute of Health (R01AG013196, R01 AG034454); the Economic and Social Research Council (ES/J023299/1). The Young Finns Study has been financially supported by the Academy of Finland: grants 286284 (T.L.), 134309, 126925, 121584, 124282, 129378 (Salve), 117787 (Gendi), and 41071 (Skidi); the Social Insurance Institution of Finland; Kuopio, Tampere and Turku University Hospital Medical Funds (grant X51001 for T.L.); Juho Vainio Foundation; Paavo Nurmi Foundation; Finnish Foundation of Cardiovascular Research; Finnish Cultural Foundation; Tampere Tuberculosis Foundation (T.L.); Emil Aaltonen Foundation (T.L.); Yrjö Jahnsson Foundation (T.L.). and Signe ja Ane Gyllenberg’s Foundation (T.L). Tasnime Akbaraly is supported by the Medical Research Council (K013351). Peter Würtz is supported by the Novo Nordisk Foundation and the Academy of Finland (294834). Martin Shipley is supported by the British Heart Foundation. Steven Humphries was supported by the BHF (PG08/008) and the National Institute for Health Research University College London Hospitals Biomedical Research Centre. Rita Haapakoski was supported by the European Commission LIFEPATH project (Horizon 2020 grant 633666). Mika Ala-Korpela has been supported by the Sigrid Juselius Foundation and the Strategic Research Funding from the University of Oulu, Finland. He works in a unit that is supported by the University of Bristol and UK Medical Research Council (MC_UU_12013/1). The Baker Institute is supported in part by the Victorian Government’s Operational Infrastructure Support Program. Mika Kivimaki is supported by the Medical Research Council (K013351 and MR/R024227/1), UK, NordForsk, the Nordic Programme on Health and Welfare, the Academy of Finland (311492) and a Helsinki Institute of Life Science fellowship. The funding organization or sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions
T.A., P.W. and M.Ki. designed the research. T.A. analysed data and performed statistical analyses. M.Ki. supervised the study. T.A. wrote the first draft. A.J.K., P.S., and M.A.-K. designed and performed the metabolomics analyses. T.A., P.W., A.S.M., M.J.S., R.H., M.L., C.D., M.Ka., T.L., V.M., A.H., S.H.E., A.J.K., P.S., O.R., M.A.-K., and M.Ki. made a critical revision of the manuscript for important intellectual content and T.A. had primary responsibility for final content.
Competing Interests
T.A., A.S.-M., M.J.S., R.H., M.L., C.D., M.Ka., T.L., V.M., A.H., S.H.E., O.R., M.A.-K., M.Ki. declare no conflict of interest. P.W., A.J.K. and P.S. are shareholders and employees of Brainshake Ltd, a company offering NMR-based metabolite profiling.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26441-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. Jama. 2002;288:2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 2.Varraso, R. et al. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ (Clinical research ed.)350, h286 (2015). [DOI] [PMC free article] [PubMed]
- 3.Akbaraly T, et al. Does overall diet in midlife predict future aging phenotypes? A cohort study. Am J Med. 2013;126:411–419 e413. doi: 10.1016/j.amjmed.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wurtz P, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131:774–785. doi: 10.1161/CIRCULATIONAHA.114.013116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floegel A, et al. Variation of serum metabolites related to habitual diet: a targeted metabolomic approach in EPIC-Potsdam. European journal of clinical nutrition. 2013;67:1100–1108. doi: 10.1038/ejcn.2013.147. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Yu B, Alexander D, Steffen LM, Boerwinkle E. Human metabolome associates with dietary intake habits among African Americans in the atherosclerosis risk in communities study. American journal of epidemiology. 2014;179:1424–1433. doi: 10.1093/aje/kwu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandler PD, et al. Abstract P278: Metabolomic Profiles Associated with Dietary Patterns in Women. Circulation. 2016;133:AP278–AP278. [Google Scholar]
- 8.Guertin KA, et al. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr. 2014;100:208–217. doi: 10.3945/ajcn.113.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogl LH, et al. Association between habitual dietary intake and lipoprotein subclass profile in healthy young adults. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2013;23:1071–1078. doi: 10.1016/j.numecd.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Marmot M, Brunner E. Cohort Profile: the Whitehall II study. Int J Epidemiol. 2005;34:251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 11.McCullough ML, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 12.Akbaraly TN, et al. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr. 2011;94:247–253. doi: 10.3945/ajcn.111.013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raitakari OT, et al. Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol. 2008;37:1220–1226. doi: 10.1093/ije/dym225. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, et al. Monounsaturated fatty acids generated via stearoyl CoA desaturase-1 are endogenous inhibitors of fatty acid amide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18832–18837. doi: 10.1073/pnas.1309469110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silveira MB, Carraro R, Monereo S, Tebar J. Conjugated linoleic acid (CLA) and obesity. Public health nutrition. 2007;10:1181–1186. doi: 10.1017/S1368980007000687. [DOI] [PubMed] [Google Scholar]
- 16.Superko HR, Superko SM, Nasir K, Agatston A, Garrett BC. Omega-3 fatty acid blood levels: clinical significance and controversy. Circulation. 2013;128:2154–2161. doi: 10.1161/CIRCULATIONAHA.113.002731. [DOI] [PubMed] [Google Scholar]
- 17.Wu JH, et al. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: the Cardiovascular Health Study. Circulation. 2014;130:1245–1253. doi: 10.1161/CIRCULATIONAHA.114.011590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stegemann C, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129:1821–1831. doi: 10.1161/CIRCULATIONAHA.113.002500. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury R, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Annals of internal medicine. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 20.Vafeiadou K, et al. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am J Clin Nutr. 2015;102:40–48. doi: 10.3945/ajcn.114.097089. [DOI] [PubMed] [Google Scholar]
- 21.Phillips CM, Perry IJ. Lipoprotein particle subclass profiles among metabolically healthy and unhealthy obese and non-obese adults: Does size matter? Atherosclerosis. 2015;242:399–406. doi: 10.1016/j.atherosclerosis.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 22.Pechlaner R, et al. Very-Low-Density Lipoprotein-Associated Apolipoproteins Predict Cardiovascular Events and Are Lowered by Inhibition of APOC-III. Journal of the American College of Cardiology. 2017;69:789–800. doi: 10.1016/j.jacc.2016.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arsenault BJ, et al. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Atherosclerosis. 2009;206:276–281. doi: 10.1016/j.atherosclerosis.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 24.Garvey WT, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo M, Pernice V, Frasheri A, Berneis K. Atherogenic lipoprotein phenotype and LDL size and subclasses in patients with peripheral arterial disease. Atherosclerosis. 2008;197:237–241. doi: 10.1016/j.atherosclerosis.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Soininen P, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. The Analyst. 2009;134:1781–1785. doi: 10.1039/b910205a. [DOI] [PubMed] [Google Scholar]
- 27.Wurtz P, et al. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. American journal of epidemiology. 2017;186:1084–1096. doi: 10.1093/aje/kwx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soininen P, Kangas AJ, Wurtz P, Suna T, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circulation. Cardiovascular genetics. 2015;8:192–206. doi: 10.1161/CIRCGENETICS.114.000216. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, et al. Metabolite profiles and the risk of developing diabetes. Nature medicine. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stancakova A, et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes. 2012;61:1895–1902. doi: 10.2337/db11-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng, Y. et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol (2016). [DOI] [PMC free article] [PubMed]
- 32.Kohlmeier, M. Nutrient Metabolism, (Elsevier’s science, 2003).
- 33.Patel H, Chandra S, Alexander S, Soble J, Williams KA., Sr. Plant-Based Nutrition: An Essential Component of Cardiovascular Disease Prevention and Management. Current cardiology reports. 2017;19:104. doi: 10.1007/s11886-017-0909-z. [DOI] [PubMed] [Google Scholar]
- 34.Fung TT, McCullough M, van Dam RM, Hu FB. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care. 2007;30:1753–1757. doi: 10.2337/dc06-2581. [DOI] [PubMed] [Google Scholar]
- 35.Akbaraly TN, et al. Overall diet history and reversibility of the metabolic syndrome over 5 years: the Whitehall II prospective cohort study. Diabetes Care. 2010;33:2339–2341. doi: 10.2337/dc09-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundstrom J, et al. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. Bmj. 2006;332:878–882. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nuotio J, et al. Cardiovascular risk factors in 2011 and secular trends since 2007: the Cardiovascular Risk in Young Finns Study. Scandinavian journal of public health. 2014;42:563–571. doi: 10.1177/1403494814541597. [DOI] [PubMed] [Google Scholar]
- 38.Wurtz, P. et al. Metabolic profiling of alcohol consumption in 9778 young adults. Int J Epidemiol (2016). [DOI] [PMC free article] [PubMed]
- 39.Wurtz P, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS medicine. 2014;11:e1001765. doi: 10.1371/journal.pmed.1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamer M, et al. Physical activity patterns over 10 years in relation to body mass index and waist circumference: the Whitehall II cohort study. Obesity (Silver Spring, Md.) 2013;21:E755–761. doi: 10.1002/oby.20446. [DOI] [PubMed] [Google Scholar]
- 41.Inouye M, et al. Metabonomic, transcriptomic, and genomic variation of a population cohort. Molecular systems biology. 2010;6:441. doi: 10.1038/msb.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rankin NJ, et al. The emergence of proton nuclear magnetic resonance metabolomics in the cardiovascular arena as viewed from a clinical perspective. Atherosclerosis. 2014;237:287–300. doi: 10.1016/j.atherosclerosis.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mallol R, Rodriguez MA, Brezmes J, Masana L, Correig X. Human serum/plasma lipoprotein analysis by NMR: application to the study of diabetic dyslipidemia. Progress in nuclear magnetic resonance spectroscopy. 2013;70:1–24. doi: 10.1016/j.pnmrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Brunner E, Stallone D, Juneja M, Bingham S, Marmot M. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. The British journal of nutrition. 2001;86:405–414. doi: 10.1079/BJN2001414. [DOI] [PubMed] [Google Scholar]
- 45.Bingham SA, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol. 1997;26(Suppl 1):S137–151. doi: 10.1093/ije/26.suppl_1.S137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.