Abstract
TaiMed Biologics is developing ibalizumab (Trogarzo™, ibalizumab-uiyk)—a humanised IgG4 monoclonal antibody—as a treatment for HIV-1 infection. Ibalizumab blocks HIV entry into CD4 cells while preserving normal immunological function and is the first CD4-directed post-attachment HIV-1 inhibitor and the first humanised monoclonal antibody for the treatment of HIV/AIDS. This article summarizes the milestones in the development of ibalizumab leading to this first approval in HIV-1 treatment.
Introduction
Ibalizumab (Trogarzo™, ibalizumab-uiyk) is a humanised IgG4 monoclonal antibody being developed by TaiMed Biologics for the treatment of HIV-1 infection. Ibalizumab blocks HIV entry into CD4 cells without impairing normal immunological function. The drug is the first CD4-directed post-attachment HIV-1 inhibitor and the first humanised monoclonal antibody for the treatment of HIV/AIDS. Ibalizumab is approved in the USA for use as part of a combination antiretroviral regimen in heavily treatment-experienced patients with multidrug resistant (MDR) HIV-1 infection failing their current antiretroviral regimen [1]. The recommended dose of ibalizumab is a single intravenous 2000 mg loading dose followed by an intravenous maintenance dose of 800 mg once every 2 weeks [2].
Company Agreements
Ibalizumab was originally developed by Biogen. In the late 1990s Biogen licensed the exclusive worldwide rights to ibalizumab to Tanox Inc. In January 2007 Tanox entered into an agreement with Genentech which subsequently led to Genentech acquiring all shares in Tanox for a total cash price of ≈ $US919 million [3] and—shortly thereafter—Genentech licensing ibalizumab to TaiMed Biologics (TaiMed). In August 2012 TaiMed contracted WuXi PharmaTech to manufacture ibalizumab in support of phase II and III clinical trials [4]. In March 2016 TaiMed entered into a 12-year collaboration agreement with Theratechnologies for the latter to market and distribute ibalizumab in the US and Canada. Theratechnologies made a $US1 million cash payment to TaiMed upon signing the agreement and will pay a further $US1 million as shares at the commercial launch. TaiMed may also receive a further conditional $US8.5 million payment at commercial launch, as well as various milestone payments [5]. In March 2017 this agreement was amended to grant Theratechnologies commercialisation rights for ibalizumab in the EU, Israel, Norway, Russia and Switzerland for a 12-year term following regulatory approval on a country-by-country basis, subject to further upfront and milestone payments.
Patent Information
The patents for ibalizumab expired in Europe, Canada, and Australia in 2011, and expired in the US (US-05871732) in 2016, subject to extensions; with orphan drug status in the US, ibalizumab has an extended period of exclusivity through to 2025 [6]. The patent application in Japan is still pending. Ibalizumab is described and claimed in WO-09209305.
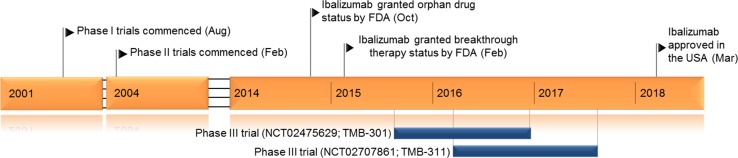
Key milestones in the development of ibalizumab
Scientific Summary
Pharmacodynamics
Ibalizumab binds to domain 2 of the CD4 receptor on the surface opposite both the major histocompatibility complex-class II binding site and the gp120 binding site [7, 8].
The baseline in vitro susceptibility of HIV to ibalizumab was determined in isolates from 38 of 40 heavily pre-treated patients with multidrug resistant HIV-1 entering in the phase III TMB-301 clinical trial. Mean ibalizumab maximum percent inhibition (MPI) of viral replication was 91% overall, and 90–100% against 27 isolates, 80 to 90% against 6 and < 80% against 5. The overall mean fold change in the concentration of drug required to inhibit 50% of the MPI (IChalfmax fold change) [occurring at the midpoint of the dose response curve] was 1.2. Ibalizumab had mean MPI values of 81, 98, 89, and 91% and mean IChalfmax fold changes of 1.3, 0.9, 1.1 and 1.0 against isolates with wild-type susceptibility to nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs) and integrase inhibitors (INIs), respectively, compared to respective mean MPI values of 94, 91, 91, and 92% and mean IChalfmax fold changes of 1.2, 1.2, 1.3, and 1.1 against isolates that were resistant to all NRTIs, NNRTIs, PIs, or INIs. The drug had MPI values of 84–99% and IChalfmax fold change values of 0.7 to 1.4 against 5 of 6 isolates with reduced susceptibility to enfuvirtide at screening; one isolate with reduced susceptibility to enfuvirtide at screening also had reduced susceptibility to ibalizumab (MPI 41%, IChalfmax fold change 6.2). Ibalizumab had MPI values of 94 and 100% against two isolates exhibiting CCR5-dependent replication with reduced susceptibility to maraviroc (MPI 58 and 0%) [9].
The in vitro activity of ibalizumab has also been assessed against a panel of 116 Tier-2 Env-pseudotyped viruses selected to represent envelope diversity by geography, clade, tropism, and stage of infection, including 30 transmitted/founder viruses. Ibalizumab achieved 50 and 80% inhibition of infection in 92 and 65% of these HIV strains, respectively. The median half maximal inhibitory concentration of ibalizumab (0.03 mg/ml) was an order of magnitude lower than those of the HIV-neutralizing monoclonal antibodies PG9 (0.11 mg/ml), and VRC01 (0.22 mg/ml), and two orders of magnitude lower than those of 4E10, 2F5, 2G12 and b12. Analyses of gp160 sequence polymorphism revealed the predominant determinant of resistance to ibalizumab was the absence of a potential asparagine (N)-linked glycosylation site (PNGS) at the variable region (V5) N-terminus. Other independent correlates of resistance were PNGS at position 386 and the side chain length of residue 375. Ibalizumab exhibited complementary resistance to VRC01 and sCD4 which was partly mediated by the V5 PNGS [7]. Loss of V5 PNGS was also associated with resistance to ibalizumab in HIV-1 isolates from patients (n = 14) participating in a phase Ib study [10].
Pharmacokinetics
The pharmacokinetic properties of intravenous ibalizumab have been investigated in an open-label, randomized phase Ib study in patients with HIV-1 infection [11]. Patients were randomised to intravenous ibalizumab 10 mg/kg on day 1 then once weekly for 9 weeks (n = 9) or 10 mg/kg on day one then 6 mg/kg at weeks 1, 3, 5, 7 or 9 (n = 10). A further three (non-randomised) patients received ibalizumab 25 mg/kg on day 1 and weeks 2, 4, 6, and 8. Cmax and AUCall were 402 µg/ml and 3604 µg · day/ml, respectively, in the 10 mg/kg group and 564 µg/ml and 4941 µg · day/ml, respectively, in the 25 mg/kg group. The elimination half-life was 3.3 and 3.1 days in the 10 and 25 mg/kg groups, respectively, volume of distribution at steady state 44 and 50 ml/kg, respectively, and steady state clearance 5.7 and 8.8 ml/day/kg, respectively. Trough ibalizumab serum concentrations were 48, 31 and 96 µg/ml in the 10 mg/kg, 10/6 mg/kg and 25 mg/kg groups respectively prior to week 1 infusion, and 138, 0.2 and 96 µg/ml, respectively, prior to the final dose. Considerable (110%) variability in serum drug concentrations was evident later in the dosing period among the three patients who received ibalizumab 25 mg/kg [11].
Features and properties of ibalizumab
| Alternative names | Trogarzo™, ibalizumab-uiyk, TMB-355, TNX-355, TMB-355, Hu5A8, monoclonal antibody 5A8 |
| Class | CD4-directed post-attachment HIV-1 inhibitor, Antiretrovirals, Humanized monoclonal antibodies |
| Mechanism of Action | Binds to domain 2 of the CD4 receptor |
| Route of Administration | Intravenous |
| Pharmacodynamics | Blocks HIV-1 infection in CD4 T-cells |
| Pharmacokinetics | Cmax 402 µg/ml, AUCall 3604 µg · day/ml, t½ 3.3 days, volume of distribution 44 ml/kg, steady state clearance 5.7 ml/day/kg |
| Adverse events | |
| Most frequent | Diarrhoea, dizziness, nausea, rash |
| ATC codes | |
| WHO ATC code | J05A-X (Other antivirals) |
| EphMRA ATC code | J5C4 (HIV antivirals, entry inhibitors) |
| Chemical name | Immunoglobulin G4, anti-(human CD4 (antigen)) (human-mouse monoclonal 5A8 γ4-chain), disulphide with human-mouse monoclonal 5A8 κ-chain, dimer |
When administered as recommended (an initial 2,000 mg loading dose then 800 mg once every 2 weeks), ibalizumab concentrations reached steady-state levels after the first 800 mg maintenance dose with mean concentrations > 30 µg/ml throughout the dosing interval [2].
Therapeutic Trials
Multidrug Resistant HIV-1 Infection
Phase III
Ibalizumab plus an optimised background antiretroviral regimen maintained virologic efficacy in treatment-experienced patients (n = 40) with multidrug resistant HIV-1 infection in an open-label phase III study (TMB-301, NCT02475629). Median viral load and CD4+ T cell count were 4.6 log10 (18% baseline viral load ≥ 100,000 copies/ml) and 73 cells/μl, respectively, at baseline. Resistance to ≥ 3 and 4 antiretroviral drug classes was present in 53 and 35% of patients, respectively, and 13% of patients had HIV-1 resistant to all approved antiretroviral agents. Patients received intravenous ibalizumab at an initial dose of 2000 mg then 800 mg once every two weeks, plus an optimised background regimen, for 24 weeks. Seven days following the initial 2000 mg ibalizumab-loading dose, 33 patients (83%) experienced a ≥ 0.5 log10 decrease in HIV RNA, whereas 1 patient (3%) experienced a ≥ 0.5 log10 decrease in HIV RNA during Control period (p < 0.0001) [12]. Mean viral load was reduced by 1.6 log10 from baseline to week 24 (intention to treat—missing equals failure analysis) in patients receiving ibalizumab. Reductions in viral load of ≥ 1 log10 and ≥ 2 log10 occurred in 55 and 48% of patients, respectively, and 43 and 50% of patients had a viral load < 50 and < 200 HIV RNA copies/ml, respectively [13].
Of patients who completed study TMB-301 (n = 31), only patients from US and Puerto Rico were eligible to enter study TMB-311 (NCT02707861), where they continued to receive ibalizumab 800 mg once every two weeks for up to 48 weeks. This study enrolled 27 patients of whom 59 and 33% had HIV-1 infection resistant to ≥ 3 and ≥ 4 antiretroviral classes, respectively. 7% of patents had HIV-1 resistant to all approved antiretroviral classes. 24 patients received treatment until week 48. The median reduction in viral load from baseline in these 27 patients was 2.5 log10 at both week 24 and 48. 16 (59%) and 17 (63%) patients had a viral load of < 50 and < 200 HIV RNA copies/ml, respectively. All patients with a viral load of < 50 copies/ml at week 24 (n = 15) maintained viral suppression to week 48 [14].
Phase II
Treatment with ibalizumab plus an optimised background antiretroviral regimen resulted in significant reductions in viral load in a 24-week randomised, double-blind, phase IIb study (TMB-202, NCT00784147). 113 patients with HIV-1 infection and documented resistance to at least 1 NRTI, 1 NNRTI and 1 PI were randomised to treatment with an optimized background antiretroviral regimen plus intravenous ibalizumab 800 mg once every 2 weeks (n = 59) or 2000 mg once every 4 weeks (n = 54). The primary endpoint was the proportion of patients with viral load < 50 copies/ml at week 24. 44 and 28% of patients, respectively, had a viral load of < 50 copies/ml and 53 and 43%, respectively, had a viral load of < 200 copies/ml at week 24. The median reduction in viral load from baseline to week 24 was 1.6 and 1.5 log10 in ibalizumab 800 and 2000 mg recipients, respectively [15].
Adverse Events
The most common adverse reactions (all grades) reported in ≥ 5% of the 40 patients who participated in trial TMB-301 were diarrhoea (8%), dizziness (8%), nausea (5%), and rash (5%). 90% of adverse reactions reported were mild or moderate in severity. Drug-related severe adverse reactions were observed in two patients; one developed a severe rash and the other developed immune reconstitution inflammatory syndrome manifesting as an exacerbation of progressive multifocal leukoencephalopathy [2].
Key clinical trials of intravenous ibalizumab (TaiMed Biologics)
| Drug(s) | Indication | Phase | Patients | Status | Location (s) | Identifier |
|---|---|---|---|---|---|---|
| Ibalizumab | HIV-1 infection | Ia | 30 | Completed | USA | N/A |
| Ibalizumab plus optimised background regimen | Treatment naïve or previously treated HIV-1 infection | Ib | 22 | Completed | USA | N/A |
| Ibalizumab plus optimised background regimen, placebo | Previously treated HIV-1 infection | IIa | 82 | Completed | USA, Puerto Rico, Canada | NCT00089700, TNX-355.03 |
| Ibalizumab plus optimised background regimen | Previously treated HIV-1 infection | IIb | 113 | Completed | USA, Puerto Rico | NCT00784147, TMB-202 |
| Ibalizumab plus optimised background regimen | Previously treated HIV-1 infection, PI-IND extension of IIb | II | 56 | Completed | USA | NCT01056393 |
| Ibalizumab plus optimised background regimen | Multidrug resistant HIV-1 infection | III | 40 | Completed | USA, Puerto Rico, Taiwan | NCT02475629, TMB-301 |
| Ibalizumab plus optimised background regimen | Multidrug resistant HIV-1 infection | III | 27 | Ongoing | USA, Puerto Rico | NCT02707861, TMB-311 |
Laboratory abnormalities ≥ grade 3 reported in trial TMB-301 included bilirubin ≥ 2.6 times the upper limit of normal (ULN) [5%], direct bilirubin > ULN (3%), creatinine > 1.8 times ULN or 1.5 times baseline (10%), blood glucose > 250 mg/dl (3%), lipase >3.0 times ULN (5%), uric acid > 12 mg/dl (3%), haemoglobin < 8.5 g/dl (3%), platelets < 50000/mm3 (3%), leukocytes < 1.5 × 109 cells/l (5%) and neutrophils < 0.6 × 109 cells/l (5%) [2].
As with all therapeutic proteins, there is potential for ibalizumab to cause immunogenicity. Among patients enrolled in trials TMB-301 and TMB-202, one developed low titre anti-ibalizumab antibodies. No adverse reactions or reduced efficacy was attributed to the positive sample observed in this patient [2].
Ongoing Clinical Trials
The Expanded Access Program for ibalizumab in resistant HIV-1 infection is ongoing and enrolling patients (TMB-311; NCT02707861).
Current Status
Ibalizumab received its first global approval on 6 March 2018 in the US for the treatment of heavily treatment-experienced patients with multidrug resistant HIV-1 infection in combination with other antiretroviral medicines.
Compliance with Ethical Standards
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. A. Markham, a contracted employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Footnotes
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original version of this article was revised due to a retrospective Open Access request.
A correction to this article is available online at https://doi.org/10.1007/s40265-018-0926-2.
Change history
5/30/2018
The article Ibalizumab: First Global Approval, written by Anthony Markham, was originally published Online First without open access.
References
- 1.US FDA. FDA approves new HIV treatment for patients who have limited treatment options [media release]. 6 Mar 2018. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm599657.htm.
- 2.US FDA. TROGARZO™ (ibalizumab-uiyk): US prescribing Information. 2018. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761065lbl.pdf. Accessed 21 Mar 2018.
- 3.Genentech Inc. Genentech announces expiration of Hart-Scott-Rodino waiting period and completes acquisition of Tanox [media release]. 3 Aug 2007. http://www.gene.com.
- 4.TaiMed Biologics. WuXi PharmaTech to manufacture ibalizumab for TaiMed Biologics [media release]. 28 Aug 2012. http://www.taimedbiologics.com.
- 5.Theratechnologies, TaiMed Biologics. Theratechnologies and TaiMed Biologics sign exclusive marketing and distribution agreement for ibalizumab [media release]. 18 Mar 2016. http://www.theratech.com.
- 6.US FDA. Orphan Drug Designations and Approvals. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=321910. Accessed 10 Apr 2018.
- 7.Pace CS, Fordyce MW, Franco D, et al. Anti-CD4 monoclonal antibody ibalizumab exhibits breadth and potency against HIV-1, with natural resistance mediated by the loss of a V5 glycan in envelope. J Acquir Immune Defic Syndr. 2013;62(1):1–9. doi: 10.1097/QAI.0b013e3182732746. [DOI] [PubMed] [Google Scholar]
- 8.Freeman MM, Seaman MS, Rits-Volloch S, et al. Crystal structure of HIV-1 primary receptor CD4 in complex with a potent antiviral antibody. Structure. 2010;18(12):1632–1641. doi: 10.1016/j.str.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinheimer S, Cohen Z, Marsolais C, et al. Ibalizumab susceptibility in patient HIV isolates resistant to antiretrovirals [abstract no. 561]. In: 25th Conference on Retroviruses and Opportunistic Infections. 2018.
- 10.Toma J, Weinheimer SP, Stawiski E, et al. Loss of asparagine-linked glycosylation sites in variable region 5 of human immunodeficiency virus type 1 envelope is associated with resistance to CD4 antibody ibalizumab. J Virol. 2011;85(8):3872–3880. doi: 10.1128/JVI.02237-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson JM, Kuritzkes DR, Godofsky E, et al. Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 2009;53(2):450–457. doi: 10.1128/AAC.00942-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalezari J, Fessel WJ, Schrader S, et al. Primary efficacy endpoint and safety results of ibalizumab in a phase 3 study of heavily treatment-experienced patients with multidrug-resistant human immunodeficiency virus-1 infection [abstract no. LB-6 plus oral presentation]. Open Forum. Infectious Diseases. 2016;3(Suppl. 1):S1. [Google Scholar]
- 13.Lewis S, Fessel J, Emu B, et al. Long-acting ibalizumab in patients with multi-drug resistant HIV-1: a 24-week study [abstract no. 438 plus poster 449LB]. In: 24th conference on retroviruses and opportunistic infections. 2017.
- 14.Emu B, Fessel WJ, Schrader S, et al. Forty-eight-week safety and efficacy on-treatment analysis of ibalizumab in patients with multi-drug resistant HIV-1 [abstract no. 1686 plus oral presentation]. Open Forum. Infect Dis. 2017;4(Suppl. 1):S38–S39. [Google Scholar]
- 15.Khanlou H, Gathe JJ, Schrader S, et al. Safety, efficacy, and pharmacokinetics of ibalizumab in treatment-experienced HIV-1 infected patients: a phase 2b study [abstract no. H2-794b plus oral presentation]. In: 51st Interscience Conference on Antimicrobial Agents and Chemotherapy. 2011.