Summary
Background
Femoroacetabular impingement syndrome is an important cause of hip pain in young adults. It can be treated by arthroscopic hip surgery, including reshaping the hip, or with physiotherapist-led conservative care. We aimed to compare the clinical effectiveness of hip arthroscopy with best conservative care.
Methods
UK FASHIoN is a pragmatic, multicentre, assessor-blinded randomised controlled trial, done at 23 National Health Service hospitals in the UK. We enrolled patients with femoroacetabular impingement syndrome who presented at these hospitals. Eligible patients were at least 16 years old, had hip pain with radiographic features of cam or pincer morphology but no osteoarthritis, and were believed to be likely to benefit from hip arthroscopy. Patients with bilateral femoroacetabular impingement syndrome were eligible; only the most symptomatic hip was randomly assigned to treatment and followed-up. Participants were randomly allocated (1:1) to receive hip arthroscopy or personalised hip therapy (an individualised, supervised, and progressive physiotherapist-led programme of conservative care). Randomisation was stratified by impingement type and recruiting centre and was done by research staff at each hospital, using a central telephone randomisation service. Patients and treating clinicians were not masked to treatment allocation, but researchers who collected the outcome assessments and analysed the results were masked. The primary outcome was hip-related quality of life, as measured by the patient-reported International Hip Outcome Tool (iHOT-33) 12 months after randomisation, and analysed in all eligible participants who were allocated to treatment (the intention-to-treat population). This trial is registered as an International Standard Randomised Controlled Trial, number ISRCTN64081839, and is closed to recruitment.
Findings
Between July 20, 2012, and July 15, 2016, we identified 648 eligible patients and recruited 348 participants: 171 participants were allocated to receive hip arthroscopy and 177 to receive personalised hip therapy. Three further patients were excluded from the trial after randomisation because they did not meet the eligibility criteria. Follow-up at the primary outcome assessment was 92% (319 of 348 participants). At 12 months after randomisation, mean iHOT-33 scores had improved from 39·2 (SD 20·9) to 58·8 (27·2) for participants in the hip arthroscopy group, and from 35·6 (18·2) to 49·7 (25·5) in the personalised hip therapy group. In the primary analysis, the mean difference in iHOT-33 scores, adjusted for impingement type, sex, baseline iHOT-33 score, and centre, was 6·8 (95% CI 1·7–12·0) in favour of hip arthroscopy (p=0·0093). This estimate of treatment effect exceeded the minimum clinically important difference (6·1 points). There were 147 patient-reported adverse events (in 100 [72%] of 138 patients) in the hip arthroscopy group) versus 102 events (in 88 [60%] of 146 patients) in the personalised hip therapy group, with muscle soreness being the most common of these (58 [42%] vs 69 [47%]). There were seven serious adverse events reported by participating hospitals. Five (83%) of six serious adverse events in the hip arthroscopy group were related to treatment, and the one in the personalised hip therapy group was not. There were no treatment-related deaths, but one patient in the hip arthroscopy group developed a hip joint infection after surgery.
Interpretation
Hip arthroscopy and personalised hip therapy both improved hip-related quality of life for patients with femoroacetabular impingement syndrome. Hip arthroscopy led to a greater improvement than did personalised hip therapy, and this difference was clinically significant. Further follow-up will reveal whether the clinical benefits of hip arthroscopy are maintained and whether it is cost effective in the long term.
Funding
The Health Technology Assessment Programme of the National Institute of Health Research.
Introduction
Femoroacetabular impingement syndrome is a painful disorder of the hip that is caused by a premature contact (impingement) between the femur and acetabulum during hip movements.1, 2 This premature contact typically occurs as a result of certain hip shapes, such as cam or pincer morphology.1 Cam morphology refers to a flattening or convexity at the femoral head neck junction, whereas pincer morphology refers to a focal or global over-coverage of the femoral head by the acetabulum.1 Femoroacetabular impingement syndrome leads to progressive damage within the joint, including the acetabular labrum and articular cartilage,1 and is associated with the development of osteoarthritis of the hip.1, 3
Research in context.
Evidence before this study
Femoroacetabular impingement syndrome is a relatively common non-arthritic cause of hip pain in young adults. It is caused by symptomatic premature contact between the proximal femur and acetabular rim during hip motion, and this phenomenon is associated with certain hip shapes. Repeated impingement leads to damage to the articular cartilage or to acetabular labral tears, and it is these injuries that are thought to be painful. In the past decade, surgeons have developed arthroscopic (ie, keyhole) surgical techniques to treat femoroacetabular impingement syndrome. The rationale is that reshaping the hip and repairing cartilage and labral damage will prevent impingement and relieve symptoms. In 2013, we did a Cochrane systematic review of the effectiveness of hip arthroscopy for femoroacetabular impingement syndrome. We searched MEDLINE (from 1946 to Nov 19, 2013), Embase (1980 to Nov 19, 2013), and the Cochrane Central Register of Controlled Trials (issue 11, 2013), for randomised controlled trials of surgery compared with placebo treatment, non-operative treatment, or no treatment, in any language, in human adults with femoroacetabular impingement syndrome. We found no high-quality evidence examining the effectiveness of surgery for femoroacetabular impingement syndrome. Therefore, we chose to do a pragmatic, multicentre, randomised controlled trial to determine the effectiveness of hip arthroscopy compared with best conservative care.
Added value of this study
To our knowledge, this is the largest randomised controlled trial to show the clinical effectiveness of hip arthroscopy in treating femoroacetabular impingement syndrome. We have shown that patients with femoroacetabular impingement syndrome have better hip-related quality of life at 12 months since randomisation with either hip arthroscopy or best conservative care (personalised hip therapy), and that this quality of life improves significantly more in patients treated hip arthroscopy, exceeding the minimal clinically important difference. Hip arthroscopy was also more expensive than was personalised hip therapy.
Implications of all the available evidence
Over the past 15 years, increasing numbers of patients with femoroacetabular impingement syndrome have been treated with hip arthroscopy. This is the first study to show that hip arthroscopy is more clinically effective, at least in the short term, than best conservative care. Longer-term outcomes are required to establish whether this improvement is sustained and whether surgery is cost-effective. These results should be shared with patients when selecting an appropriate treatment strategy.
Surgery has become an established treatment for femoroacetabular impingement syndrome. The aim of such surgery is to reshape the hip joint to prevent impingement.2 Intra-articular injury, such as a cartilage and labral damage, can be resected, repaired, or reconstructed.2 Initially, open surgery was used to treat femoroacetabular impingement syndrome, but the minimally invasive, so-called keyhole technique known as hip arthroscopy is now being used more frequently.1 This approach has become possible because of advances in technology and surgical techniques. Hip arthroscopy is safer and has a shorter recovery time than does open surgery.4, 5 In the UK in 2013, 1908 operations for femoroacetabular impingement syndrome were done by arthroscopic surgery, compared with only 491 by open surgery.6 Since then, there has been a rapid increase in the use of hip arthroscopy in most countries around the world.4, 5, 6 Non-operative treatments for femoroacetabular impingement syndrome are also available,2 including exercise-based packages of conservative care, delivered by a physiotherapist.7 Potential targets for physiotherapy include the abnormal movement patterns and weakness of hip muscles seen in patients with femoroacetabular impingement syndrome.8, 9
Numerous case series report improvement in patients with femoroacetabular impingement syndrome after open or arthroscopic surgery, or physiotherapy.5, 10 However, a 2014 Cochrane review11 of surgery for treating femoroacetabular impingement syndrome showed that there was no evidence from randomised controlled trials to support these treatments.
In a feasibility study,6 we established that patients were prepared to be recruited, and that surgeons were in equipoise and willing to recruit patients to a randomised controlled trial of hip arthroscopy compared with best conservative care. Our aim was to measure the clinical effectiveness of hip arthroscopy compared with best conservative care in treating patients with femoroacetabular impingement syndrome.
Methods
Study design and participants
We did this pragmatic, multicentre, assessor-blind randomised controlled trial in 23 National Health Service (NHS) hospitals in the UK. We treated the initial feasibility study6 as an internal pilot study so that participants who took part in the initial study were included in the main trial recruitment.
Participants were recruited from the specialist hip arthroscopy service at each hospital. Participating surgeons identified eligible patients during routine diagnostic consultations. Assessments were medical history, clinical examination, X-rays, and cross-sectional imaging (MRI, CT, or both). For patients with a diagnosis of femoroacetabular impingement syndrome, the surgeon classified them as having cam (alpha angle >55°), pincer (lateral centre-edge angle >40° or a positive crossover sign), or mixed-type (combination of cam and pincer) impingement.12
Patients were eligible if they had hip pain, radiographic features of cam or pincer morphology, were at least 16 years old, were able to give informed consent, and if the treating surgeon believed that they were likely to benefit from hip arthroscopy. Patients were excluded if they had hip osteoarthritis (Tonnis grade >1 or less than 2 mm of superior joint space on an antero-posterior radiograph); a history of hip pathology such as Perthes' disease, slipped upper femoral epiphysis, or avascular necrosis, or previous hip injury such as acetabular fracture, hip dislocation, or femoral neck fracture; or if they had already had shape-changing surgery (open or arthroscopic) of the hip.13, 14 Patients with bilateral femoroacetabular impingement syndrome were eligible, and only the most symptomatic hip was randomly assigned to treatment and followed up. Trained research associates approached eligible patients to explain the trial and to invite them to participate. All participants gave written informed consent.
Qualitative research, to understand recruitment as it occurred, was integrated into the trial. The findings were used to design a recruiter training and centre support programme that was implemented during the trial to optimise recruitment. The research was based on the QuinteT Recruitment Intervention and continued the work done during the internal pilot trial.15, 16
The study was approved by NHS Research Ethics Service West Midlands (14/WM/0124). An independent trial steering committee and data monitoring committee provided oversight of the progression of the study. The study protocol has previously been published.17
Randomisation and masking
Participants were randomly assigned (1:1) with a computer-generated minimisation (adaptive stratified sampling) algorithm for centre and type of impingement, to receive either hip arthroscopy or best conservative care. All baseline data were collected before randomisation, which was done by the recruiting research associate. Allocation concealment was ensured by use of a secure telephone randomisation service hosted by Warwick Clinical Trials Unit. It was not possible to mask patients or the treating clinicians to their allocation. Researchers who collected outcome assessments and analysed the results were masked to allocation by concealment of treatment.
Procedures
Surgery for femoroacetabular impingement syndrome was done using arthroscopic techniques by a senior surgeon (consultant grade) in the NHS, who was trained and experienced in hip arthroscopy. Further details about surgeons' training and experience, and surgical procedures are shown in the appendix. 27 trial surgeons did the surgery. Hip arthroscopy was done under general anaesthesia in a lateral or supine position. Arthroscopic portals were established in the central and peripheral compartments of the hip under radiographic guidance according to the surgeon's usual practice. Shape abnormalities and consequent labral and cartilage pathology were treated. Adequacy of bony reshaping was assessed by intraoperative image intensifier views or by arthroscopic visualisation of a satisfactory impingement free range of movement of the hip, or both. Patients were allowed home when they could walk safely with crutches (typically within 24 h). Patients were referred to outpatient physiotherapy services for a course of rehabilitation as per usual care for that surgeon. These post-operative physiotherapists were distinct from those providing conservative care, to avoid contamination between groups. Patients had MRI of their hip at least 6 weeks after surgery.
A panel of international experts assessed the fidelity of the surgery (appendix). They reviewed operation notes, intraoperative images, and postoperative MRI scans to subjectively assess whether adequate surgery had been undertaken, according to the protocol. The panel discussed each case and subjectively assessed whether shape abnormalities and intra-articular pathology were treated, and whether there was a sufficient resection to allow impingement-free range of motion. This approach included assessing the proximal femoral and acetabular rim resections and whether the resection edges were smooth.
Personalised hip therapy is a package of physiotherapist-led rehabilitation for femoroacetabular impingement syndrome. It was developed during our feasibility study and tested during the pilot study.6 Although the name for this intervention is new, the care offered was based on a consensus of what physiotherapists, physicians, and surgeons currently regard as best conservative care for femoroacetabular impingement syndrome.7 Personalised hip therapy has four core components: an assessment of pain, function, and range of hip motion; patient education; an exercise programme taught in the clinic and repeated at home, that has the key features of individualisation, progression, and supervision; and help with pain relief, which could include one X-ray or ultrasound-guided intra-articular steroid injection when pain prevents performance of the exercise programme.18
Personalised hip therapy was delivered by at least one physiotherapist at each centre. 47 physiotherapists were trained formally in this protocol through a 1-day workshop and supported to deliver personalised hip therapy through refresher workshops (appendix). At their initial assessment, participants received a personalised hip therapy information pack that described what to expect during the course of their treatment. They then had between six and ten face-to-face contacts with the physiotherapist over 12–24 weeks. Some contacts were allowed by either telephone or email when geographical distance prevented all contacts being carried out face-to-face. Patient-completed exercise diaries were also encouraged to help both patients and physiotherapists monitor progress and adherence with personalised hip therapy.
Physiotherapists recorded full details of their advice and treatments, number and type of treatment contacts, and any non-attendance, on case report forms. These case report forms were reviewed for accuracy in comparison to the usual physiotherapy records at each treatment centre and then assessed for fidelity to the personalised hip therapy protocol by a panel comprising members of the core group who developed the protocol for personalised hip therapy (appendix). The personalised hip therapy panel reviewed case report forms to ensure participants received an adequate number of sessions, all four core components, and that their exercise programme was individualised, supervised, and progressive.
Outcomes
The primary outcome was hip-related quality of life, as measured by the international Hip Outcome Tool (iHOT-33) at 12 months after randomisation.19 iHOT-33 is a patient-derived and patient-reported outcome instrument designed to measure hip-related quality of life in young adults with non-arthritic hip pain. The iHOT-33 consists of four domains: symptoms and functional limitations; sports and recreational physical activities; job-related concerns; and social, emotional, and lifestyle concerns.18 iHOT-33 provides a 100-point score, with 100 representing no pain and perfect function, and lower scores indicating pain and poor function. The instrument has been validated in a relevant population for this trial,19 and has a minimum clinically important difference of 6·1 points.19, 20 Secondary outcomes were health-related quality of life, measured with the EuroQol EQ-5D-5L, the 12-item Short Form Health Survey (SF-12 version 2), adverse events, and health-care resource use, all measured 6 and 12 months after treatment allocation.21, 22, 23 Patients reported adverse events 6 weeks after the start of their intervention (first session of personalised hip therapy, or date of hip arthroscopy). Any serious adverse events were reported by each centre to the Warwick Clinical Trials Unit. Forms for iHOT-33, EQ-5D-5L, SF-12, adverse events, and health-care resource use were collected by postal questionnaires, which were centrally administered. Scores for these measures were collected at the time of consent and again by postal questionnaire at 6 and 12 months after treatment allocation.
Statistical analysis
The planned sample size was 172 participants in each group, based on a standard deviation of iHOT-33 of 16 points and a minimum clinically important difference of 6·1 points, giving a standardised effect size of 0·38. We designed the trial to have 90% power to detect an effect of this size at a two-sided 5% significance level, allowing for up to 15% loss to follow-up at the primary outcome timepoint.
We prepared a full statistical analysis plan before the final analysis. No interim analyses were planned. The plan was approved by the independent data monitoring committee.
In our primary analysis, we investigated differences in the primary outcome measure, (iHOT-33 score at 12 months after randomisation) between the two treatment groups on a intention-to-treat basis, which included all eligible patients randomly assigned to an intervention. We assessed the primary outcome 12 months from treatment allocation rather than from intervention because this was a pragmatic trial design of two different treatment strategies. We used a mixed-effects regression analysis to assess the effects of the interventions on 12-month iHOT-33 scores, after adjusting for the fixed-effects of impingement type, sex, and baseline iHOT-33 score, with recruiting centre included as a random effect to model any potential associations within the recruiting centres. This mixed-effects model was used for all outcome measures.
We drew our primary inferences from the intention-to-treat analysis, irrespective of compliance, without imputation for missing data. We also did several additional analyses. We did a per-protocol analysis, comparing participants who actually received surgery and those who actually received personalised hip therapy. Additionally, we compared participants randomly allocated to hip arthroscopy or personalised hip therapy, who received an allocated treatment that was deemed to be of high fidelity. We did prespecified subgroup analyses for different impingement types (cam, pincer, and mixed) and for patients younger and older than 40 years. We added pairwise interaction terms between treatment group and both impingement type and age group to the mixed-effects model to test for important subgroup effects. In addition to the primary adjusted analysis, we also reported unadjusted differences between groups, and we assessed significance using t tests for normally distributed outcomes. We present treatment effect estimates from the adjusted mixed-effects model (primary analysis) with 95% CIs. All hypothesis testing was at the 5% level, with no adjustments for multiple testing. Unless otherwise indicated, the analysis of secondary outcomes followed the same modelling approach as for the primary outcome. All analyses were on a complete-case basis and where follow-up data were missing, the reasons for missing data were obtained and patterns were investigated to judge the plausibility of missingness assumptions. We did a sensitivity analysis using multiple imputation techniques (imputation using chained equations) to assess the effect of missing data on the primary outcome. Finally, we did a post-hoc sensitivity analysis of the effect of variation in recovery time in the hip arthroscopy group. We compared post-intervention adverse events between groups using Fisher's exact test. We did all analyses using Stata (version 14).24
We did an economic evaluation from the perspective of the NHS and personal social services.25 We estimated economic costs associated with the delivery of the two interventions. Resource use questions completed by participants at each assessment point provided a profile of all hospital inpatient and outpatient service use, community health and social care encounters, prescribed medications, and NHS supplies, such as crutches or home adaptations. We obtained unit costs (2016 £) from primary and secondary sources in accordance with national guidelines and attached them to every item of resource use.24 We used health utilities generated from EQ-5D-5L responses at every timepoint of assessment to estimate quality-adjusted life-year (QALY) profiles for every participant; these QALYs were calculated as the area under the baseline-adjusted utility curve, assuming linear interpolation between utility measurements. We did a bivariate regression of costs and QALYs, with multiple imputation of missing data, with the view to estimating the incremental cost per QALY gained for hip arthroscopy compared with personalised hip therapy. Further details, including sensitivity analyses done to assess the effect of uncertainty surrounding aspects of the economic evaluation, and prespecified subgroup analyses exploring heterogeneity in the cost-effectiveness results, are provided in the appendix. The trial is registered as an International Standard Randomised Controlled Trial, number ISRCTN64081839.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
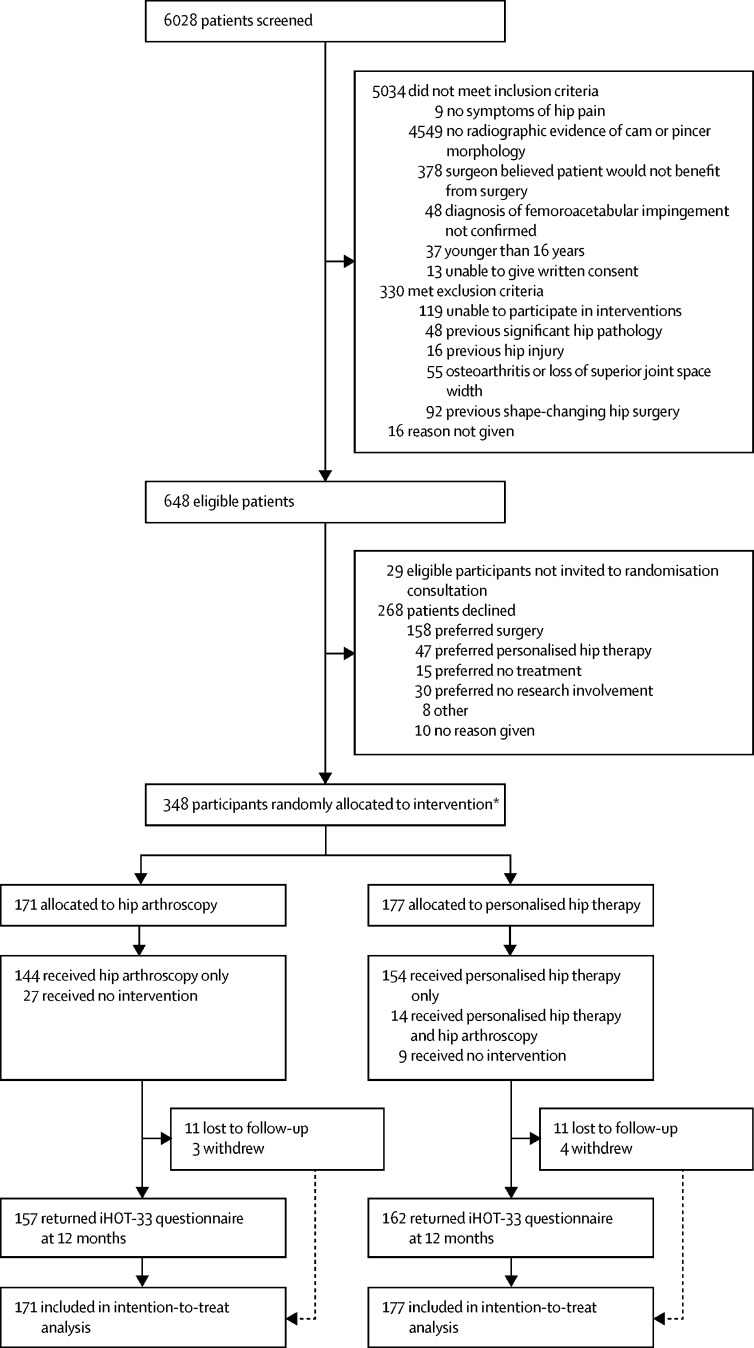
Between July 20, 2012, and July 15, 2016, 648 patients who attended the participating surgeons' hip clinics were deemed eligible (figure 1). Of these patients, 348 (54%) agreed to participate. The mean age of participants was 35·3 years (SD 9·6); the mean age of those who declined to participate was 35·4 years (10·3). The 348 participants were randomly allocated to receive hip arthroscopy (n=171) or personalised hip therapy (n=177). Three additional patients were randomly allocated in error, in each instance contrary to protocol procedures. One participant, who was not eligible, was recruited after miscommunication between the treating surgeon and recruiter; one patient was allocated without signing the consent form; and one patient was deemed eligible by a trainee surgeon, but within a few days the treating surgeon deemed they were not eligible. None of these patients participated in the trial (figure 1).
Figure 1.
Trial profile
iHOT-33=International Hip Outcome Tool. *Three patients were randomly assigned in error but did not receive treatment and were not followed-up.
Participants in the two groups were well matched in terms of demographics and pre-randomisation hip-related quality of life, having had symptoms for approximately 3 years (table 1). 14 (8%) participants who were allocated to personalised hip therapy had all or part of this intervention, but then, at their request, went on to have hip arthroscopy within 12 months after randomisation. No patients allocated to hip arthroscopy had personalised hip therapy. For hip arthroscopy, the median time from random assignment to treatment was 122 days (IQR 80–185), and for personalised hip therapy, it was 37 days (22–60). Surgeons did a mean of 112 (SD 55) hip arthroscopies per year during the study. At 12 months after randomisation, 144 (84%) of the 171 participants allocated to receive hip arthroscopy had received it; 27 (16%) had not. 121 (84%) participants who received a hip arthroscopy procedure had postoperative MRI and their case was assessed by the surgical review panel; 105 (87%) of these 121 procedures were deemed to be of high fidelity, and 16 (13%) were deemed unsatisfactory. The reasons for unsatisfactory surgery were an inadequate bony resection of the proximal femur (n=7) and acetabular rim (n=2), a sharp transition from the femoral head to neck as a result of reshaping surgery (n=5), and no reshaping surgery being done because the hip was degenerate (n=2). Nine (5%) of the 177 participants allocated to personalised hip therapy did not receive any treatment by 12 months. Of the patients who received personalised hip therapy (n=154), 107 (70%) were judged to have received the intervention to a high fidelity (appendix). The most common reason for low fidelity of personalised hip therapy was participants not receiving the minimum of six therapy sessions (34 [72%] of 47). Other reasons for low-fidelity personalised hip therapy were no progression of exercises by the physiotherapist (11 [23%] of 47) and the patient not complying with the exercise programme (two [4%] of 47).
Table 1.
Baseline characteristics of the study population
| Hip arthroscopy (n=171) | Personalised hip therapy (n=177) | ||
|---|---|---|---|
| Age (years) | 35·4 (9·7) | 35·2 (9·4) | |
| Sex | |||
| Women | 71 (42%) | 64 (36%) | |
| Men | 100 (58%) | 113 (64%) | |
| Current smoker | |||
| Yes | 31 (18%) | 25 (14%) | |
| No | 136 (80%) | 151 (85%) | |
| Missing data | 4 (2%) | 1 (1%) | |
| Hip side considered for treatment | |||
| Right | 95 (56%) | 103 (58%) | |
| Left | 75 (44%) | 74 (42%) | |
| Participants with bilateral symptoms | 11 (6%) | 18 (10%) | |
| Duration of hip symptoms (months) | 37 (36·6) | 40 (40·8) | |
| Impingement type | |||
| Cam | 129 (75%) | 133 (75%) | |
| Mixed | 29 (17%) | 30 (17%) | |
| Pincer | 13 (8%) | 14 (8%) | |
| Units of alcohol in an average week | 6·2 (8·6) | 6·0 (7·7) | |
| Diabetes | |||
| Yes | 2 (1%) | 4 (2%) | |
| No | 165 (96%) | 171 (97%) | |
| Missing data | 4 (2%) | 2 (1%) | |
| Chronic renal failure | |||
| Yes | 1 (1%) | 0 | |
| No | 166 (97%) | 176 (99%) | |
| Missing data | 4 (2%) | 1 (1%) | |
| Physical activity (UCLA Activity Scale) | 4·3 (2·5) | 4·4 (2·5) | |
| Hip-related quality of life (iHOT-33) | 39·2 (20·9) | 35·6 (18·2) | |
| SF-12 PCS | 44 (7·6) | 44 (5·9) | |
| SF-12 MCS | 42 (7·1) | 42 (7·3) | |
| EQ-5D 3L/5L Index Score | 0·576 (0·26) | 0·557 (0·25) | |
| EQ-5D 5L VAS | 67 (20·2) | 67 (18·7) | |
| Mean lateral centre edge angle (°) | 31 (5) | 31 (5) | |
| Number of participants with LCEA <25° | 7 (4%) | 6 (3%) | |
| Number of participants with LCEA <20° | 0 | 0 | |
| Mean alpha angle (°) measured on antero-posterior radiograph | 61 (17) | 64 (18) | |
Data are mean (SD) or n (%). UCLA=University of California Los Angeles. iHOT-33=International Hip Outcome Tool. SF-12=12-item Short Form Health Survey. PCS=physical component score. MCS=mental component score. VAS=visual analogue score. LCEA=lateral centre edge angle.
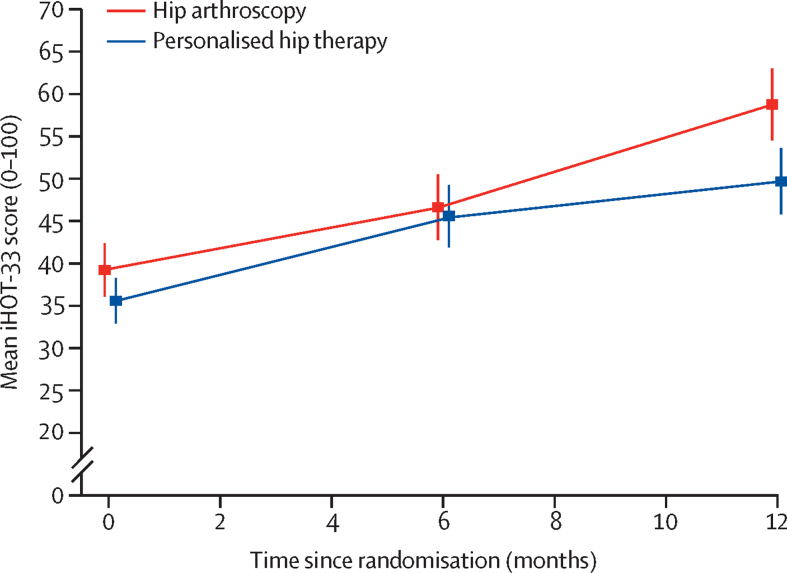
319 (92%) of 348 participants completed the iHOT-33 questionnaires at 12 months after randomisation. Seven (2%) withdrew from follow-up, and 22 (6%) were lost to follow-up. The iHOT-33 score increased in both groups, indicating an improvement in hip-related quality of life (table 2, figure 2). 12 months after randomisation, mean iHOT-33 scores had improved from 39·2 (SD 21) to 58·8 (27) for participants in the hip arthroscopy group, and from 35·6 (18) to 49·7 (25) in the personalised hip therapy group. In the primary intention-to-treat analysis at 12 months, the adjusted estimate of treatment effect measured with iHOT-33 was 6·8 (95% CI 1·7 to 12·0, p=0·0093) in favour of hip arthroscopy, compared with personalised hip therapy. In the per-protocol analysis at 12 months, including participants who received personalised hip therapy (n=154) or hip arthroscopy (n=144), the adjusted estimate of the between-group difference on iHOT-33 was 8·2 (95% CI 2·8 to 13·6) in favour of hip arthroscopy. In the exploratory, pre-specified secondary analysis based on those participants whose treatment was deemed of a high fidelity (hip arthroscopy n=105, personalised hip therapy n=107), the adjusted estimate of between-group difference on the iHOT-33 was 5·8 (95% CI −0·7 to 12·2) in favour of hip arthroscopy. There were no significant between-group differences in SF-12 or EQ-5D-5L scores at 6 or 12 months after randomisation (table 2).
Table 2.
Patient-reported outcome measures
|
Hip arthroscopy (n=171) |
Personalised hip therapy (n=177) |
Unadjusted difference | Adjusted difference (95% CI) | p value | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | ||||
| iHOT-33 | |||||||
| 6 months | 46·6 (25) | 161 | 45·6 (23) | 154 | 1·0 | −0·7 (−5·2 to 3·7) | 0·743 |
| 12 months* | 58·8 (27) | 158 | 49·7 (25) | 163 | 9·1 | 6·8 (1·7 to 12·0) | 0·0093 |
| EQ-5D-5L (utility) | |||||||
| 6 months | 0·544 (0·26) | 144 | 0·573 (0·23) | 147 | −0·029 | −0·042 (−0·088 to 0·005) | 0·081 |
| 12 months | 0·615 (0·25) | 152 | 0·578 (0·24) | 147 | 0·037 | 0·020 (−0·027 to 0·067) | 0·397 |
| EQ-5D VAS | |||||||
| 6 months | 67·8 (19·3) | 145 | 70·3 (19·3) | 145 | −2·5 | −2·1 (−5·7 to 1·4) | 0·241 |
| 12 months | 71·9 (20·7) | 150 | 69·2 (19·4) | 145 | 2·7 | 2·6 (−1·2 to 6·4) | 0·180 |
| SF-12 PCS | |||||||
| 6 months | 43·4 (7·0) | 146 | 44·2 (6·6) | 142 | −0·8 | −0·7 (−2·1 to 0·7) | 0·304 |
| 12 months | 45·1 (6·3) | 145 | 44·2 (6·4) | 132 | 1·0 | 1·1 (−0·2 to 2·5) | 0·099 |
| SF-12 MCS | |||||||
| 6 months | 42·1 (7·3) | 146 | 42·1 (7·2) | 142 | −0·1 | −0·1 (−1·5 to 1·3) | 0·929 |
| 12 months | 43·2 (7·1) | 145 | 42·6 (6·9) | 132 | 0·6 | 0·4 (−1·2 to 2·0) | 0·589 |
iHOT-33=International Hip Outcome Tool. VAS=visual analogue score. PCS=physical component score. MCS=mental component score.
Primary outcome.
Figure 2.
Changes in mean iHOT-33 score from baseline to 6 and 12 months after randomisation
Error bars are 95% CIs. iHOT-33=International Hip Outcome Tool.
In the prespecified subgroup analyses, the between-group difference for iHOT-33 was 5·0 (95% CI −1·2 to 11·3) in participants younger than 40 years and 10·9 (1·7 to 20·1) in those older than 40 years (pinteraction=0·3023) in favour of hip arthroscopy; the difference was 8·3 (95% CI 2·5 to 14·2) in patients with cam morphology, 1·1 (–11·5 to 13·7) in those with mixed cam and pincer morphology, and 4·0 (–14·6 to 22·7) in those with pincer morphology (pinteraction=0·5672), in favour of hip arthroscopy.
Among patients who received their allocated intervention, there were 147 patient-reported adverse events (in 100 [73%] of 138 patients) in the hip arthroscopy group versus 102 (in 88 [60%]) of 146 patients in the personalised hip therapy group. The most frequently reported adverse event was muscle soreness, reported by 58 (42%) patients in the hip arthroscopy group and 69 (47%) patients in the personalised hip therapy group (table 3). At 12 months, seven serious adverse events had been reported by participating hospitals. Six of these were among the participants in the hip arthroscopy group: one patient was not discharged from the day surgery unit and required an overnight admission, one patient had scrotal haematoma requiring readmission, two patients had superficial wound infections that required oral antibiotics, one patient had a hip joint infection that required further surgery and ultimately a total hip replacement, and one patient had a fall that was unrelated to hip arthroscopy. One participant in the personalised hip therapy group developed biliary sepsis that was unrelated to treatment. There were no treatment-related deaths.
Table 3.
Patient-reported adverse events
| Hip arthroscopy (n=138)* | Personalised hip therapy (n=146)† | p value | |
|---|---|---|---|
| Muscle soreness at 6 weeks after intervention | 58 (42%) | 69 (47%) | 0·40 |
| Numbness in groin, leg, or foot | 35 (25%) | NA | NA |
| Hip pain or stiffness at 6 weeks after intervention | 13 (9%) | 8 (6%) | 0·26 |
| Unscheduled hospital appointments | 13 (9%) | 6 (4%) | 0·096 |
| Superficial wound problems | 9‡ (7%) | NA | NA |
| Hip joint infection | 1 (1%) | NA | NA |
| Fracture | 0 | NA | NA |
| Deep-vein thrombosis | 0 | NA | NA |
| Other adverse events potentially related to intervention | 8 (6%; 2 numbness proximal thigh, 1 scrotal infection, 1 scrotal bruising, 1 labial swelling, 1 ankle pain, 1 erratic International Normalised Ratio, 1 nausea secondary to analgesia, 1 numbness to tip of tongue for 2 weeks after operation) | 1 (1%; muscle spasms) | 0·017 |
| Other adverse events not related to intervention | 10 (7%; 3 knee pain, 2 lower back pain, 1 shingles, 1 urinary tract infection, 1 essential thrombocythaemia, 1 hernia surgery, 1 contralateral foot pain) | 18 (13%; 7 lower back pain, 2 knee pain, 2 road traffic collisions, 2 abdominal pain under investigation, 1 viral illness, 1 endometriosis, 1 chronic pain referred to rheumatologist, 1 skin discoloration, 1 multiple sclerosis) | 0·17 |
NA=not applicable.
Six of 144 patients who received hip arthroscopy within 12 months did not return an adverse events form.
Eight of 154 patients who received personalised hip therapy only did not return an adverse events form.
Four patients required antibiotics.
There was a low level of missing item-level data (eg, iHOT-33 0·6%) in all patient-reported outcome measures at all timepoints; after imputation for missing data, the adjusted estimate of treatment effect was similar, at 6·6 (95% CI 1·7 to 11·4) points in favour of hip arthroscopy. In a post-hoc analysis there was no significant difference in iHOT-33 at 12 months for patients in the hip arthroscopy group who were treated within 6 months of randomisation versus those treated 6 months or more after randomisation (0·9 [95% CI −10·7 to 8·8]). We assessed model assumptions, including assessment of quantile-quantile plots, which were deemed adequate. The mean cost of hip arthroscopy was £3042 (35% staff time, 28% surgical devices and anaesthetic drugs, 19% theatre-running costs, and 18% bed-day costs). Participants in the personalised hip therapy group attended a mean of six physiotherapy sessions (average duration of 30 min), generating mean total treatment costs of £155 per participant. The adjusted incremental cost of hip arthroscopy compared with personalised hip therapy during the 12-month follow-up was £2372, with incremental QALYs of −0·015 (representing a net QALY loss). Personalised hip therapy was more cost-effective than hip arthroscopy at 12 months (appendix).
Discussion
The UK FASHIoN study is the first randomised controlled trial to provide evidence that hip arthroscopy is effective in patients with femoroacetabular impingement syndrome. In this pragmatic trial, we found that iHOT-33 scores improved for patients in both groups; 12 months after randomisation, there was a mean adjusted difference of 6·8 points in the iHOT-33 score between patients allocated to receive hip arthroscopy and those allocated to receive personalised hip therapy, in favour of hip arthroscopy. This is a statistically significant difference that also exceeded the minimum clinically important difference for iHOT-33. These results are consistent with the hypothesis that hip arthroscopy is more clinically effective than best conservative care.
There have been many observational studies showing benefit from hip arthroscopy; however, these studies generally did not have control groups for comparison, and are at high risk of bias.6 Results from a Cochrane systematic review done by members of our group11 showed that there had been no previous relevant randomised controlled trials. Since then, one recent randomised controlled trial26 has reported no difference between hip arthroscopy and conservative care. This study was small, was done in a military setting, with a single surgeon in a single centre, and with a very high rate of crossover (70%) from conservative care to hip arthroscopy.26 When the authors did a per-protocol comparison of those who had hip arthroscopy (n=66) with those who had conservative care (n=14), they reported that “power was lost making type II errors possible”.26 They concluded that “large cohorts across multiple sites are needed to make definitive conclusions”.26 Our trial is larger and therefore has greater power to detect between-group differences, and was done in 23 centres with a more generalisable patient population.
There were no differences between groups in the secondary outcome measure of general health-related quality of life (EQ-5D-5L and SF-12). This finding could either be because treatment for femoroacetabular impingement syndrome does not have an effect on health-related quality of life or because the measures we used are not sufficiently sensitive to detect the changes that occur. A further possibility is that the trial was not sufficiently powered to detect changes in health-related quality of life.
Adverse events in the hip arthroscopy group were more frequent than in the personalised hip therapy group. However, there was only one serious surgical complication in which a patient developed a hip joint infection. In one systematic review of 36 761 cases,27 hip arthroscopy had a reported complication rate of 3·3%, with a rate of major complications of 0·2%; our study findings are consistent with this.
Our within-trial health economic evaluation suggests that hip arthroscopy is not cost-effective by comparison with personalised hip therapy. However, our economic models were only able to assess cost-effectiveness at 12 months from randomisation. This finding must also be set in the context of the high initial treatment costs of hip arthroscopy, the treatment timing (long delay in patients receiving hip arthroscopy, reducing the period of potential benefit during follow-up), and the period of economic inactivity during postoperative recovery (appendix). There could be long-term benefits from treatment that were not assessed in this economic analysis. We plan further follow-up points at 2, 3, 5, and 10 years, which will inform the lifetime cost-effectiveness of both surgery and personalised hip therapy, and whether treatment effects are maintained or if further treatments are required. Comparison of the rates of hip replacement in both groups will also help establish whether surgery affects the risk of osteoarthritis.1, 3
Strengths of this trial include the consent to participate rate among eligible patients (54%) and the follow-up rate (92%). Both of these values are high compared with similar trials in orthopaedics, and especially with trials of surgery versus no surgery, contributing to external and internal validity.28 The integrated qualitative research optimised recruitment, as it has done in other trials.15 This trial was thoroughly pragmatic, exploring the effectiveness of a strategy of offering hip arthroscopy compared with conservative care in the everyday reality of a national health service, where patients do not always receive or comply with the treatment they are offered, where surgeons and physiotherapists have varying levels of training, skill, and expertise, where postoperative care is variable, and where there are waiting lists for treatment. The large number of centres (n=23), surgeons (n=27), and physiotherapists (n=43) involved is a strength, which contributes to the generalisability of our findings. The comparator for this trial was personalised hip therapy, which we consider to be the best conservative care that can realistically be provided in the NHS for these patients. Personalised hip therapy was designed through international consensus and developed, supported, and tested in similar ways to other physiotherapist-led conservative care protocols.29 It meets the standards expected of a complex intervention in a randomised controlled trial, and was delivered by musculoskeletal physiotherapists who attended additional training and support events.6, 7, 30
Limitations of this trial include that participants and treating clinicians were not masked to treatment allocation. A blinded allocation trial, with a placebo control, would have been better suited to measuring the underlying effect of surgery. In our trial, the research question was whether hip arthroscopy or best conservative care was the most effective treatment strategy, leading to an inevitable absence of blinding. Data collection and analysis were done without revealing treatment allocation. An unexpected difficulty was the frequent delay in delivery of surgery for those patients allocated to hip arthroscopy. We had anticipated that this delay would be less than 3 months in most patients because when we designed the trial, there was a strongly enforced NHS target to treat patients within 18 weeks from referral to surgery. In fact, during the study, this target was a challenge in many hospitals. Patients allocated to hip arthroscopy therefore often had longer times to treatment, and because the primary outcome was measured 12 months after randomisation, these patients were often still within a few months (and in some cases a few weeks) of their operation when we measured the primary outcome. Because of these delays, patients in the hip arthroscopy group had, on average, less time to recover before the primary outcome measurement than did patients in the personalised hip therapy group. We compared the outcome of hip arthroscopy participants who had surgery in the first 6 months after randomisation with those who had surgery in the second 6 months. There was no significant difference between these groups, suggesting that the systematic difference in time to treatment between groups does not account for the treatment effect. Our inferences about the effectiveness of hip arthroscopy compared with personalised hip therapy are limited to data collected 12 months after randomisation; long-term follow-up is required to establish if this effect is maintained and if further treatments are required.
The fact that not all surgery or personalised hip therapy was deemed to be of a high fidelity is also a reflection of the real-world setting in which this trial was done. Some surgery was not satisfactory, and some participants allocated to personalised hip therapy did not engage with it or complete it: our fidelity assessment showed high-fidelity treatment in 87% of patients receiving hip arthroscopy and 70% of patients receiving personalised hip therapy. However, these proportions are comparable with other studies and reflect the pragmatism of our trial.31 We took great effort to minimise crossover in our trial, using techniques developed in our feasibility study. No participants allocated to receive hip arthroscopy received personalised hip therapy; 14 participants allocated to personalised hip therapy subsequently changed their mind and decided to have surgery within 12 months. We do not believe that these crossovers can account for the results of this trial; indeed, we would expect such crossovers to dilute and so reduce our estimate of the real underlying effect of hip arthroscopy.
Personalised hip therapy is believed to work by improving muscle control, strength around the hip, and movement patterns, leading to the avoidance of hip impingement. Surgery is thought to work by reshaping the bone to prevent impingement and by treating painful injuries to articular cartilage and labrum. In our trial, the observed effect of hip arthroscopy over conservative care might be attributable to the surgical procedure, the placebo effect of surgery (given the unblinded nature of this trial), post-surgical rehabilitation, or a combination of these factors. The results of our subgroup analysis of patients with only cam morphology are suggestive of an increased treatment effect of hip arthroscopy compared with other shapes. This finding would support the idea that the removal of a cam shape has a specific therapeutic effect. The low number of patients with pincer or mixed cam and pincer morphology in this study means we are less certain about the influence of reshaping the acetabular rim. Ultimately, we do not fully understand the mechanism of benefit from hip arthroscopy or personalised hip therapy. Future research should focus on investigating these mechanisms further, and which patients would benefit most from hip arthroscopy or personalised hip therapy.
We have shown that offering hip arthroscopy to patients with femoroacetabular impingement syndrome led to better patient-assessed function 12 months after randomisation, compared with best conservative care. This improvement comes at a cost; our study does not show cost-effectiveness of hip arthroscopy compared with conservative care within the first 12 months.
Acknowledgments
Acknowledgments
We are indebted to all patients who participated in the trial. We are grateful to the members of the trial steering committee, data monitoring and safety committee, and members of the UK FASHIoN Study Group for their contributions to this study. UK FASHIoN was jointly sponsored by the University of Warwick and the University Hospitals of Coventry and Warwickshire NHS Trust. This work is supported by the Health Technology Assessment Programme of the NIHR (feasibility and pilot trial grant number 10/41/02, full trial grant number 13/103/02). The UK Comprehensive Research Network also supported this trial. JLD is supported by the MRC ConDuCT Hub for Trials Methodology Research (MR/K025643/1), and the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) West at University Hospitals Bristol NHS Foundation Trust, and is an NIHR Senior Investigator (NF-SI-0507-10213). SP is an NIHR Senior Investigator (NF-SI-0616-10103). NEF is an NIHR Senior Investigator, and was supported through an NIHR Research Professorship (NIHR-RP-011-015). This project benefited from facilities funded through Birmingham Science City Translational Medicine Clinical Research and Infrastructure Trials Platform, with support from Advantage West Midlands and the Wolfson Foundation. The views expressed are those of the authors, and not necessarily those of the NHS, the NIHR, or the Department of Health.
Contributors
DRG, PDHW, RH, NEF, CEH, JLD, AR, and SP did the feasibility and pilot trial and wrote the grant application for this trial. DRG was the chief investigator. NEF and PDHW led development of personalised hip therapy intervention, and NEF, JS, and EJD supported participating physiotherapists in its delivery. EJD and RH were responsible for administrative set-up and performance of the trial, and centre staff training. CEH and EJD were responsible for collating and analysing the radiological data. NRP and JG provided statistical input to design, performance, and analysis of the trial. SP and FA designed and did the health economic analysis. AR, MJ, and JLD did the qualitative research recruitment intervention. DRG, JG, and NRP had access to all study data. DRG drafted the final report; all of the FASHIoN investigators have been involved in revising the report, and all of the authors have seen and approved the final version. DRG is the guarantor for this study and decided to submit the manuscript.
Trial steering committee
Ashley Blom (chair), Richard Villar, Alan Girling, Jeremy Fry, David Ralph, Ceri Jones, and Graham Hewitt.
Data monitoring committee
Lee Shepstone (chair), Simon Donell, and Nicholas Mohtadi.
UK FASHIoN Study Group
Administrative staff: Siobhan Stevens, Elke Gemperle-Mannion, and Jaclyn Brown (Warwick Clinical Trials Unit). Fidelity review panel members: Marc Philippon (USA), Martin Beck (Switzerland), John O'Donnell (Australia), David Robinson (UK), Ivor Hughes (UK). Australian FASHIoN: David Hunter and Kim Bennell. FASHIoN surgeons: Christopher Edward Bache, Callum McBryde, and Angelos Politis (The Royal Orthopaedic Hospital NHS Foundation Trust); Marcus Bankes and Marc George (Guys and St Thomas Hospital NHS Foundation Trust); Gavin Bartlett and Mark Norton (Royal Cornwall Hospitals NHS Trust); Tim Board, Aslam Mohammed, and Asim Rajpura (Wrightington, Wigan and Leigh NHS Foundation Trust); Michael Cronin (University Hospitals Coventry and Warwickshire NHS Trust); Wael Dandachli and Johan Witt (University College London Hospitals NHS Trust); Stephen Eastaugh-Waring (North Bristol NHS Trust); Max Fehily (Spire Manchester Hospital); Darren Fern (Ramsay Duchy Hospital); Richard Field and Giles Stafford (Southwest London Elective Orthopaedic Centre); Aresh Hashimi-Nejad and Tahir Khan (Royal National Orthopaedic Hospital NHS Trust); Venu Kavathapu (King's College Hospital NHS Foundation Trust); Nigel Kiely and John Paul Whitaker (Robert Jones and Agnes Hunt Orthopaedic and District Hospital); Paul Latimer (Yeovil District Hospital NHS Trust); Sanjeev Madan (Doncaster and Bassetlaw Hospitals NHS Foundation Trust); Ajay Malviya (Northumbria NHS Trust); Sanjeev Patil (NHS Greater Glasgow and Clyde); Manoj Ramachandran (Royal London Hospital, Bart's Health NHS Trust); Seb Sturridge (Frimley Health NHS Foundation Trust); Philip Thomas (Cardiff and the Vale University Health Board); Craig White (South Tees Hospitals NHS Foundation Trust); Matthew Wilson (Royal Devon and Exeter NHS Trust); John Paul Whitaker (Wrexham Maelor Hospital); Mark Williams (Plymouth Hospitals NHS Trust). FASHIoN physiotherapists: Emma Jones, Simon Baker, Joanna Stanton, and Charlotte Nicholls (Yeovil District Hospital); Alison Smeatham (Royal Devon & Exeter NHS Foundation Trust); Lucie Gosling, Katte MacFarlane, and Fraser Pressdee (The Royal Orthopaedic Hospital NHS Foundation Trust); Gareth Dickinson and Karen Boulton (Frimley Health NHS Trust); Jill Goss (Epsom and St Hellier NHS Trust); Rina Venter (Guys and St Thomas Hospital NHS Foundation Trust); Jamila Kassam (Royal London Hospital, Bart's Health NHS Trust); Rachel Simmons, Kathryn Poll, and Thomas Bergmann (University College London Hospitals NHS Trust); Margaret Pilkington, Jo Armstrong, and Daniel Wright (Wrightington, Wigan and Leigh NHS Foundation Trust); Philippa Dolphin and Kelly Bainbridge (James Cook University Hospital); Miles Callum, Anthony Lewis, and Evonne Smith (Wansbeck General Hospital); Veronica Cornes and Ivor Hughes (University Hospitals Coventry and Warwickshire NHS Trust); Joanna Benfield, Katie Monnington, and Emma Stewart (Royal National Orthopaedic Hospital); Steven Borrill (Doncaster and Bassetlaw Hospitals NHS Foundation Trust); Megan Pinches, Sam Dawson, and Noel Harding (Robert Jones and Agnes Hunt Orthopaedic Hospital); Matthew Willis (Wrexham Maelor Hospital); Dani Moore (Kings College Hospital); Andrew MacCauley (St Austell Community Hospital); David Cooke (Royal Cornwall Hospitals NHS Trust); Rebecca Fleck (University Hospitals Cardiff); Julliet Ball (North Bristol NHS Trust); Peter Morrison (NHS Greater Glasgow and Clyde); and Michael Kennedy (Spire Manchester Hospital). FASHIoN recruiting research nurses and research associates: Sylvia Turner, Charlotte Bryant, Kirsten Harris, Rebecca McKeown, and Louise Clarkson (University Hospitals Coventry and Warwickshire); Alison Lewis and Rebecca Rowland-Axe (Yeovil District Hospital); Anna Grice and Gayle Githens-Mazer (Royal Devon and Exeter NHS Trust); Helen Aughwan, Faye Moore, and Eleanor Keeling (The Royal Orthopaedic Hospital NHS Trust); Justine Amero and Stephanie Atkinson (Frimley Park NHS Foundation Trust); Lynne Graves, Anna Fouracres, and Fiona Hammonds (Royal Cornwall Hospitals NHS Trust); Jas Curtis (South West London Elective Orthopaedic Centre); Lisa Brackenridge (University College London Hospital); Tracey Taylor (Wrightington, Wigan and Leigh NHS Foundation Trust); Christine Dobb (Northumbria NHS Trust); Joanna Whitworth and Thelma Commey (Doncaster and Bassetlaw Hospitals NHS Foundation Trust); Vasanti Limbani (Royal National Orthopaedic Hospital NHS Trust); Alanna Milne (South Tees Hospitals NHS Foundation Trust); Heather Maclintock (Wrexham Maelor Hospital); Claire Cleary (Cardiff and the Vale University Health Board); Helen Murray (NHS Greater Glasgow and Clyde); Maria Dubia and Abdulkerim Gokturk (King's College Hospital NHS Foundation Trust); and Rachel Bray (North Bristol NHS Trust).
Declaration of interests
DRG reports grants from the National Institute of Health Research (NIHR) during the conduct of the study, and personal fees from Stryker UK, outside the submitted work; he is also a board member of the International Society of Hip Arthroscopy, and is a consultant surgeon who routinely performs hip arthroscopy. PDHW, MJ, JLD, CEH, NRP, and NEF report grants from the NIHR Health Technology Assessment Programme during the conduct of this study. All other authors declare no competing interests.
Contributor Information
Damian R Griffin, Email: damian.griffin@warwick.ac.uk.
FASHIoN Study Group:
Siobhan Stevens, Elke Gemperle-Mannion, Jaclyn Brown, Marc Philippon, Martin Beck, John O'Donnell, David Robinson, Ivor Hughes, David Hunter, Kim Bennell, Christopher Edward Bache, Callum McBryde, Angelos Politis, Marcus Bankes, Marc George, Gavin Bartlett, Mark Norton, Tim Board, Aslam Mohammed, Asim Rajpura, Michael Cronin, Wael Dandachli, Johan Witt, Stephen Eastaugh-Waring, Max Fehily, Darren Fern, Richard Field, Giles Stafford, Aresh Hashemi-Nejad, Tahir Khan, Venu Kavathapu, Nigel Kiely, John Paul Whitaker, Paul Latimer, Sanjeev Madan, Ajay Malviya, Sanjeev Patil, Manoj Ramachandran, Seb Sturridge, Phillip Thomas, Craig White, Matthew Wilson, Mark Williams, Emma Jones, Simon Baker, Joanna Stanton, Charlotte Nicholls, Alison Smeatham, Lucie Gosling, Katte MacFarlane, Fraser Pressdee, Gareth Dickinson, Karen Boulton, Jill Goss, Rina Venter, Jamila Kassam, Rachel Simmons, Kathryn Poll, Thomas Bergmann, Margaret Pilkington, Jo Armstrong, Daniel Wright, Philippa Dolphin, Kelly Bainbridge, Miles Callum, Anthony Lewis, Evonne Smith, Veronica Cornes, Joanna Benfield, Katie Monnington, Emma Stewart, Steven Borrill, Megan Pinches, Sam Dawson, Noel Harding, Matthew Willis, Dani Moore, Andrew MacCauley, David Cooke, Rebecca Fleck, Julliet Ball, Peter Morrison, Michael Kennedy, Sylvia Turner, Charlotte Bryant, Kirsten Harris, Rebecca McKeown, Louise Clarkson, Alison Lewis, Rebecca Rowland-Axe, Anna Grice, Gayle Githens-Mazer, Helen Aughwan, Faye Moore, Eleanor Keeling, Justine Amero, Stephanie Atkinson, Lynne Graves, Anna Fouracres, Fiona Hammonds, Jas Curtis, Lisa Brackenridge, Tracey Taylor, Christine Dobb, Joanna Whitworth, Thelma Commey, Vasanti Limbani, Heather Maclintock, Alanna Milne, Claire Cleary, Helen Murray, Maria Dubia, Abdulkerim Gokturk, and Rachel Bray
Supplementary Material
References
- 1.Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Related Res. 2003;417:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 2.Griffin DR, Dickenson EJ, Agricola R. The 2016 Warwick Agreement on femoroacetabular impingement. Br J Sports Med. 2016;50:1169–1176. doi: 10.1136/bjsports-2016-096868. [DOI] [PubMed] [Google Scholar]
- 3.Agricola R, Heijboer MP, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Waarsing JH. Cam impingement causes osteoarthritis of the hip: a nationwide prospective cohort study (CHECK) Ann Rheum Dis. 2012;72:918–923. doi: 10.1136/annrheumdis-2012-201643. [DOI] [PubMed] [Google Scholar]
- 4.Sampson TG. Arthroscopic treatment of femoroacetabular impingement. Tech Orthop. 2005;20:56–62. [PubMed] [Google Scholar]
- 5.Matsuda DK, Carlisle JC, Arthurs SC, Wierks CH, Philippon MJ. Comparative systematic review of the open dislocation, mini-open, and arthroscopic surgeries for femoroacetabular impingement. Arthroscopy. 2011;27:252–269. doi: 10.1016/j.arthro.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Griffin DR, Wall PD, Realpe A. UK FASHIoN: Feasibility study of a randomised controlled trial of arthroscopic surgery for hip impingement compared with best conservative care. Health Technol Assess. 2016;20:1–172. doi: 10.3310/hta20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall PDH, Dickenson EJ, Robinson D. Conservative treatment for femoroacetabular impingement syndrome: personalised hip therapy and the FASHIoN trial. Br J Sports Med. 2016;50:1217–1223. doi: 10.1136/bjsports-2016-096368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond LE, Dobson FL, Bennell KL, Wrigley TV, Hodges PW, Hinman RS. Physical impairments and activity limitations in people with femoroacetabular impingement: a systematic review. Br J Sports Med. 2014;49:230–242. doi: 10.1136/bjsports-2013-093340. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy MJ, Lamontagne M, Beaulé PE. Femoroacetabular impingement alters hip and pelvic biomechanics during gait: walking biomechanics of FAI. Gait Posture. 2009;30:41–44. doi: 10.1016/j.gaitpost.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5:418–426. doi: 10.1016/j.pmrj.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Wall PD, Brown JS, Parsons N, Buchbinder R, Costa ML, Griffin D. Surgery for treating hip impingement (femoroacetabular impingement) Cochrane Database Syst Rev. 2014;9 doi: 10.1002/14651858.CD010796.pub2. CD010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nepple JJ, Prather H, Trousdale RT. Diagnostic imaging of femoroacetabular impingement. J Am Acad Orthop Surg. 2013;21(suppl):S20–S26. doi: 10.5435/JAAOS-21-07-S20. [DOI] [PubMed] [Google Scholar]
- 13.Tönnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am. 1999;81:1747–1770. doi: 10.2106/00004623-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Philippon M, Briggs K, Yen Y-M, Kuppersmith D. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction. Minimum two years follow up. J Bone Joint Surg Br. 2009;91:16–23. doi: 10.1302/0301-620X.91B1.21329. [DOI] [PubMed] [Google Scholar]
- 15.Donovan JL, Rooshenas L, Jepson M. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI) Trials. 2016;17:283. doi: 10.1186/s13063-016-1391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Realpe A, Adams A, Wall P, Griffin D, Donovan JL. A new simple six-step model to promote recruitment to RCTs was developed and successfully implemented. J Clin Epidemiol. 2016;76:166–174. doi: 10.1016/j.jclinepi.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin DR, Dickenson EJ, Wall PD. Protocol for a multi-centre, parallel-arm, 12-month, randomised controlled trial of arthroscopic surgery versus conservative care for femoroacetabular impingement syndrome (FASHIoN) BMJ Open. 2016;6:e012453. doi: 10.1136/bmjopen-2016-012453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warwick Clinical Trials Unit FASHIoN—studying hip impingement. www.warwick.ac.uk/ukfashion
- 19.Mohtadi NG, Griffin DR, Pedersen ME. The development and validation of a self-administered quality-of-life outcome measure for young, active patients with symptomatic hip disease: the International Hip Outcome Tool (iHOT-33) Arthroscopy. 2012;28:595–610. doi: 10.1016/j.arthro.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Phillips L, Mohtadi N, Chan D. The responsiveness and minimal clinically important difference (MCID) of the Mahorn quality of life tool. Arthroscopy. 2011;27:e23. [Google Scholar]
- 21.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 22.Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey) J Health Serv Res Policy. 1997;2:14–18. doi: 10.1177/135581969700200105. [DOI] [PubMed] [Google Scholar]
- 23.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 24.StataCorp L. Stata Press; College Station: 2015. Stata treatment-effects reference manual. [Google Scholar]
- 25.NICE . National Institute of Health and Care Excellence; London: 2013. Guide to the methods of technology appraisal 2013 (PMG9) [PubMed] [Google Scholar]
- 26.Mansell NS, Rhon DI, Meyer J, Slevin JM, Marchant BG. Arthroscopic surgery or physical therapy for patients with femoroacetabular impingement syndrome: a randomized controlled trial with 2-year follow-up. Am J Sports Med. 2018 doi: 10.1177/0363546517751912. DOI:10.1177/0363546517751912 published online Feb 1. [DOI] [PubMed] [Google Scholar]
- 27.Nakano N, Lisenda L, Jones T, Loveday D, Khanduja V. Complications following arthroscopic surgery of the hip: a systematic review of 36761 cases. Bone Joint J. 2017;99:1577–1583. doi: 10.1302/0301-620X.99B12.BJJ-2017-0043.R2. [DOI] [PubMed] [Google Scholar]
- 28.Walters SJ, dos Anjos Henriques-Cadby IB, Bortolami O. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open. 2017;7:e015276. doi: 10.1136/bmjopen-2016-015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster NE, Healey EL, Holden MA. A multicentre, pragmatic, parallel group, randomised controlled trial to compare the clinical and cost-effectiveness of three physiotherapy-led exercise interventions for knee osteoarthritis in older adults: the BEEP trial protocol (ISRCTN: 93634563) BMC Musculoskelet Disord. 2014;15:254. doi: 10.1186/1471-2474-15-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hay EM, Foster NE, Thomas E. Effectiveness of community physiotherapy and enhanced pharmacy review for knee pain in people aged over 55 presenting to primary care: pragmatic randomised trial. BMJ. 2006;333:995. doi: 10.1136/bmj.38977.590752.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.