Abstract
Breast cancer is the most common malignancy affecting women, but the heterogeneity of the condition is a significant obstacle to effective treatment. Triple negative breast cancers (TNBCs) do not express HER2 or the receptors for estrogen or progesterone, and so often have a poor prognosis. Tumor-infiltrating T cells have been well-characterized in TNBC, and increased numbers are associated with better outcomes; however, the potential roles of B cells and plasma cells have been large. Here, we conducted a retrospective correlative study on the expression of B cell/plasma cell-related genes, and the abundance and localization of B cells and plasma cells within TNBCs, and clinical outcome. We analyzed 269 TNBC samples and used immunohistochemistry to quantify tumor-infiltrating B cells and plasma cells, coupled with NanoString measurement of expression of immunoglobulin metagenes. Multivariate analysis revealed that patients bearing TNBCs with above-median densities of CD38+ plasma cells had significantly better disease-free survival (DFS) (HR = 0.44; 95% CI 0.26–0.77; p = 0.004) but not overall survival (OS), after adjusting for the effects of known prognostic factors. In contrast, TNBCs with higher immunoglobulin gene expression exhibited improved prognosis (OS p = 0.029 and DFS p = 0.005). The presence of B cells and plasma cells was positively correlated (p < 0.0001, R = 0.558), while immunoglobulin gene IGKC, IGHM, and IGHG1 mRNA expression correlated specifically with the density of CD38+ plasma cells (IGKC p < 0.0001, R = 0.647; IGHM p < 0.0001, R = 0.580; IGHG1 p < 0.0001, R = 0.655). Interestingly, after adjusting the multivariate analysis for the effect of intratumoral CD38+ plasma cell density, the expression levels of all three genes lost significant prognostic value, suggesting a biologically important role of plasma cells. Last but not least, the addition of intratumoral CD38+ plasma cell density to clinicopathological features significantly increased the prognostic value for both DFS (ΔLRχ2 = 17.28, p = 1.71E−08) and OS (ΔLRχ2 = 10.03, p = 6.32E−08), compared to clinicopathological features alone. The best combination was achieved by integrating intratumoral CD38+ plasma cell density and IGHG1 which conferred the best added prognostic value for DFS (ΔLRχ2 = 27.38, p = 5.22E−10) and OS (ΔLRχ2 = 21.29, p = 1.03E−08). Our results demonstrate that the role of plasma cells in TNBC warrants further study to elucidate the relationship between their infiltration of tumors and disease recurrence.
Keywords: triple negative breast cancer, plasma cells, B cells, immunohistochemistry, tumor immunology
Introduction
Breast cancer is the most common malignancy in women, affecting approximately 12% of females during their lifetimes (1). The disease exists as numerous heterogeneous subtypes, with tumors classified based on histological or molecular characteristics, which result in distinct biological characteristics and clinical prognoses. Triple negative breast cancers (TNBCs), defined by the absence of hormone receptor (estrogen receptor, progesterone receptor) and cerbB2 (HER2) expression, constitute about 15–20% of all breast cancers (2–4). They pose significant management challenges due to the lack of effective treatment options and often exhibit aggressive clinical behavior (5–9). There is an urgent need to better understand the processes driving progression in these tumors, and to define biomarkers that can transcend their heterogeneity and enable optimal and individualized treatment strategies.
Compared to other breast cancer subtypes, TNBCs exhibit more abundant lymphoid cell infiltrates; various components of which have been variably correlated with better or worse prognosis (10–15). More recent studies have revealed the importance of considering the broader picture of immune cell infiltrates: our group showed that the density of Foxp3+ regulatory T cells is closely correlated with the abundance of CD8+ cytotoxic T cells, and that a high frequency of both cell types predicts better disease-free survival (DFS) and overall survival (OS) in TNBC (16); while Bottai et al. demonstrated that the density of total tumor-infiltrating lymphocytes (TILs) had superior prognostic value compared to the density of CD4+, CD8+, or FOXP3+ lymphocytes, or the expression level of PD-1 or LAG-3 on lymphocytes (17). Thus future progress will require us to understand the full suite of immune cells present in the tumor, their relative abundance, and their functional profile, in order to paint a complete picture of tumor-immune interactions and their effects on disease outcome.
Although both humoral and cellular arms of the immune system are involved in the development and progression of tumors (18–22), relatively few studies have focused on the role of tumor-infiltrating B cells in tumorigenesis. Furthermore, the success of monoclonal antibody based immunotherapy indicates the potential for harnessing the humoral immune response in breast cancer treatment (21, 23–28). Some studies found that B cells were present and activated in approximately one quarter of breast tumors, and represented up to 40% of the TIL population in some (29–32); in one study, B cells were detected early during tumor development (33, 34), and expression of the B cell marker CD20 was significantly elevated in TNBC compared to other breast cancer subtypes (35).
Following antigen exposure and T cell licensing, B cells differentiate into potent antibody-secreting plasma cells, which no longer express CD20 (36), but can be distinguished by expression of CD38 (20, 21, 37–39). Tumor-infiltrating plasma cells were first reported in the 1980s (40), but have not been well-studied in TNBC. Some studies suggest that higher frequencies of CD138+ B cells, which might be plasma cells, are linked with poorer recurrence-free survival in breast cancers (41); and breast cancers in which 50% or more of stromal TILs are plasma cells were found to have significantly worse disease-free and OS (42). However, molecular studies draw a contradictory conclusion: high expression of groups of B cell/plasma cell genes (collectively termed “metagenes”) has been associated with significantly better prognosis and response to chemotherapy in breast cancer (43–46). In order to make sense of these disparate conclusions, we need to understand the relationship between B cell and plasma cell infiltration in breast cancer, and to examine expression of B cell/plasma cell-related genes in the context of immunohistochemical data.
In this study, we assessed the frequency and localization of B cells and plasma cells within samples from 269 TNBC tumors; we then measured expression of B cell/plasma cell-related genes within matched samples, and asked how these factors were associated with each other and with clinical outcome.
Materials and Methods
Patients and Tumors
A total of 269 archival formalin-fixed paraffin-embedded (FFPE) TNBC specimens from patients diagnosed between 2003 and 2010 at the Department of Anatomical Pathology, Division of Pathology, Singapore General Hospital were analyzed. All samples were obtained before patients underwent chemo- or radio-therapy. Clinicopathological parameters, including patient age, tumor size, histologic growth pattern, grade, and subtype, associated with ductal carcinoma in situ, lymphovascular invasion, and axillary lymph node status were reviewed (Table S1 in Supplementary Material). The age of patients ranged between 28 and 89 years (median 55 years); length of follow-up ranged from 1 to 213 months (mean 101, median 97), with recurrence and death occurring in 29 and 24% of these women, respectively. Tumors were typed, staged, and graded according to World Health Organization, American Society of Clinical Oncology—College of American Pathologists (ASCO-CAP) guidelines (47). The Centralized Institutional Review Board of SingHealth provided ethical approval for the use of patient materials in this study (CIRB Ref: 2013/664/F and 2015/2199).
Tissue Microarray (TMA) Construction
Tumor regions for TMA construction were selected based on pathological assessment of >50% of the sample being tumor area. For each sample, two or three representative tumor cores of 1 mm diameter were transferred from donor FFPE tissue blocks to recipient TMA blocks using a MTA-1 Manual Tissue Arrayer (Beecher Instruments, Sun Prairie, WI, USA). TMAs were constructed as previously described (7).
Immunohistochemistry Analysis of TMAs
Tissue microarray sections of 4 µm thickness were incubated with antibodies specific for CD3, CD8, CD20, CD38, and Foxp3, as well as ER, PR, and HER2 (Table S2 in Supplementary Material). We also labeled some sections with antibodies specific for epidermal growth factor receptor (EGFR), cytokeratins (CK) 14, and high molecular weight (clone 34βE12) to identify TNBCs that possess the basal-like phenotype, according to previously published protocols (7, 48). Appropriate positive and negative controls were included. Scoring of antibody-labeled sections was carried out for nuclear ER and PR, membrane HER2 and EGFR, and cytoplasmic CK14 and 34βE12 positivity. To generate the score, images of labeled slides were captured using a ScanScope XT device (Aperio Technologies, Vista, CA, USA) or an IntelliSite Ultra-Fast Scanner (Philips, Eindhoven, Netherlands) before viewing by two pathologists blinded to the clinicopathological and survival information. ASCO-CAP guidelines were used to define positivity cutoffs for the tumors: for ER, PR, CK14, EGFR, and 34βE12, a positive result was defined by the presence of at least 1% of tumor cells displaying any intensity of unequivocal staining, and for HER2, tumor positivity was defined by more than 10% of tumor cells exhibiting 3+ membrane staining (49). Equivocal HER2 cases were tested and confirmed by fluorescence in situ hybridization based on the ASCO/CAP guidelines (50, 51).
Tumor-infiltrating lymphocytes expressing CD20 (B cells) or CD38 (plasma cells) were identified within the stromal and intratumoral regions separately. Plasma cells were presented as the percentage of the intratumoral or stromal areas occupied by the respective cell population, based on published methods (52, 53). Tumors were then divided into “high” and “low” with respect to a particular cell population, when the percentage of the intratumoral or stromal areas occupied by cells labeled for either CD38 (plasma cells) or CD20 (B cells) was above or on/below the median, respectively. Furthermore, cutoff median percentages used were also compatible to the accepted clinical pathological practices: 5% for intratumoral CD38+ plasma cells and CD20+ B cells, and 1% for stromal CD38+ plasma cells and CD20+ B cells.
RNA Extraction, NanoString Measurement of Gene Expression, and Analysis
RNA was extracted from unlabeled FFPE sections of 10 µm thickness using the RNeasy FFPE kit (Qiagen, Hilden, Germany) on a QIAcube automated sample preparation system (Qiagen, Hilden, Germany) and was quantified by an Agilent 2100 Bioanalyzer system (Agilent, Santa Clara, CA, USA). A total of 100 ng of functional RNA (>300 nucleotides) was assayed on the nCounter MAX Analysis System (NanoString Technologies, Seattle, WA, USA). The NanoString counts were normalized using the positive control probes as well as the housekeeping genes, as previously reported (16). The count data were then logarithmically transformed prior to further analysis. p Values <0.05 were deemed to be statistically significant.
Gene Heat Map, Validation, Follow-Up, and Statistical Analysis
Follow-up data were obtained from medical records. DFS and OS were defined as the time from diagnosis to recurrence or death/date of last follow-up, respectively. Statistical analysis was performed using SPSS for Windows, Version 23. The relationship between clinicopathological parameters and the frequency of CD38+ plasma cells and CD20+ B cells was tested using χ2 and Fisher’s exact tests. Survival outcomes were estimated with the Kaplan–Meier analysis and compared between groups with log-rank statistics. Multivariate Cox regression was carried out to evaluate the effect of various tissue compartmentalization of CD38 and CD20 status, as well as NanoString counts of IGKC, IGHM, and IGHG1, with survival after adjusting for clinicopathological parameters, including patient age, tumor size, tumor grade, and lymph node status. Models were compared using the reduction in the log-likelihood of the models (ΔLRχ2) using a likelihood ratio test. A p value <0.05 is defined as statistical significant.
Results
High Intratumoral Plasma Cell Density Is Associated With Longer Time to Relapse in TNBC
Previous studies have relied upon CD138 as a plasma cell marker, however, as this molecule is also expressed on some tumor cells, we used CD38 to discriminate plasma cells within tumors (54–57). Our previous study showed that the prognostic value of T cells in breast cancer varied depending on their localization within the tumor (16). In this study, we labeled TNBC sections for CD20 or CD38 and quantified the area of positive labeling within the intratumoral and stromal areas separately. Samples were then grouped according to whether their intratumoral or stromal B cell or plasma cell densities were high (above median), or low (on/below median). Representative images of high and low CD38+ plasma cell and CD20+ B cell TNBC sections are shown in Figure 1. Univariate analyses did not reveal any association between the high/low density of B cells or plasma cells in either the intratumoral or stromal regions with clinicopathological features of the TNBC sample cohort (Table S1 in Supplementary Material), and in agreement with our previous study (16). However, there was clear evidence of a significant positive correlation between the densities of intratumoral CD20+ B cells and intratumoral CD38+ plasma cells (p < 0.0001, R = 0.558) (Table S3 in Supplementary Material).
Figure 1.
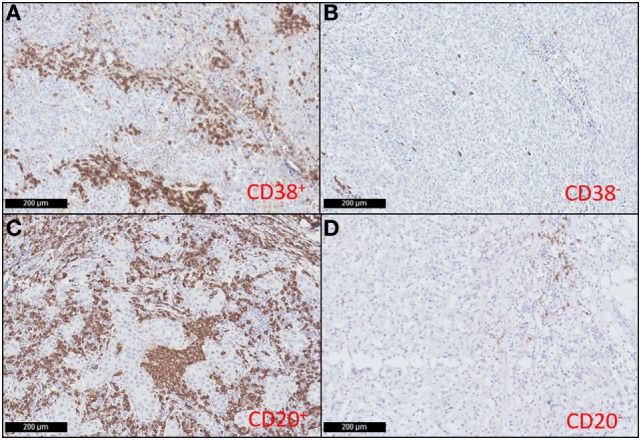
CD38+ plasma cells and CD20+ B cells infiltrate triple negative breast cancers (TNBC). Representative immunohistochemical staining showing high (A) and low (B) CD38+ plasma cell density; and high (C) and low (D) CD20+ B cell density in TNBC sections (magnification: 100×).
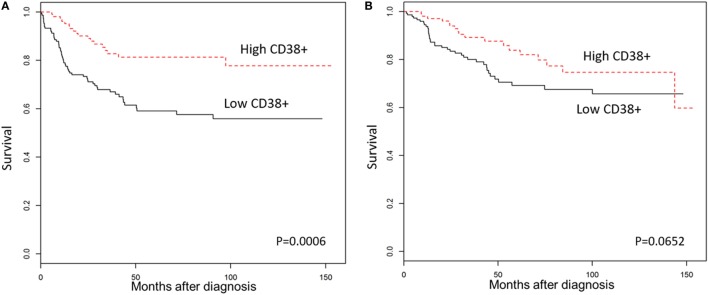
We then explored the association between plasma cell density of the tumors and the clinical outcomes in TNBC patients. As shown in Figure 2, Kaplan–Meier survival analysis revealed significantly better DFS in TNBC patients within the “high intratumoral CD38+ plasma cell” group compared to the “low CD38+ intratumoral plasma cell” group (p = 0.0006); while OS was not significantly different between groups (p = 0.0652), and the density of stromal plasma cells did not affect survival outcomes (Table S4 in Supplementary Material). Multivariate analysis further supported the fact that high density of intratumoral CD38+ plasma cells in TNBC was associated with a significantly better DFS (HR = 0.44; 95% CI 0.26–0.77; p = 0.004), which was also evident at every 1 percent increment level of CD38+ plasma cell denstity (Table 1). In other words, every incremental 1 percent was associated with better DFS (HR = 0.95; 95% CI 0.93–0.98; p = 0.002).
Figure 2.
High CD38+ plasma cell density is associated with better survival in triple negative breast cancers (TNBC). Kaplan–Meier analysis of survival outcomes in women with high vs. low densities of intratumoral CD38+ plasma cells. (A) Disease-free survival; (B) overall survival.
Table 1.
Multivariate analysis of intratumoral CD38+ plasma cell and CD20+ B cell density with survival outcomes in triple negative breast cancer (TNBC) patients.
| Hazard ratio | 95% confidence interval | pValue | |
|---|---|---|---|
| Disease-free survival (DFS) | |||
| Intratumoral CD38+ plasma cell TNBCs High vs. low |
0.44 | (0.26, 0.77) | 0.004* |
| Intratumoral CD38+ plasma cell TNBCs (every 1 percent) | 0.95 | (0.93, 0.98) | 0.002* |
| Intratumoral CD20+ B cell TNBCs High vs. low |
0.50 | (0.25, 0.99) | 0.046* |
| Intratumoral CD20+ B cell TNBCs (every 1 percent) | 0.98 | (0.95, 1.01) | 0.228 |
| Overall survival (OS) | |||
| Intratumoral CD38+ plasma cell TNBCs High vs. low |
0.66 | (0.36, 1.2) | 0.171 |
| Intratumoral CD38+ plasma cell TNBCs (every 1 percent) | 0.98 | (0.96, 1.01) | 0.187 |
| Intratumoral CD20+ B cell TNBCs High vs. low |
0.42 | (0.19, 0.97) | 0.042* |
| Intratumoral CD20+ B cell TNBCs (every 1 percent) | 1.00 | (0.96, 1.03) | 0.792 |
Analysis was adjusted for tumor size, grade, age, and lymph node status.
*Statistically significant.
Multivariate analysis similarly showed that high intratumoral CD20+ B cell density was associated with better OS (HR = 0.42; 95% CI 0.19–0.97; p = 0.042) and DFS (HR = 0.50; 95% CI 0.25–0.99; p = 0.046), but these effects were not detectable at 1 percent incremental levels (Table 1). Patients with TNBC bearing high densities of CD20+ B cells in stromal regions also showed significantly better OS and DFS (Table S4 in Supplementary Material). However, incremental stromal CD20+ B cells achieved significance for DFS but not OS.
We then asked about the interaction of intratumoral B cell/plasma cell densities in TNBC by comparing survival outcomes between four combinatorial phenotype groups (Table 2): “High intratumoral CD20+ B cell and high CD38+ plasma cell TNBCs” (n = 33), “High intratumoral CD20+ B cell and low CD38+ plasma cell TNBCs” (n = 17), “Low intratumoral CD20+ B cell and high CD38+ plasma cell TNBCs” (n = 10), and “Low intratumoral CD20+ B cell and low CD38+ plasma cell TNBCs” (n = 33). Multivariate analysis showed that patients having high intratumoral B cell and plasma cell densities had significantly better OS (HR = 0.26; 95% CI 0.09–0.75; p = 0.013) and DFS (HR = 0.24; 95% CI 0.09–0.64; p = 0.004) compared to patients with low densities of intratumoral B cells and plasma cells (Table 2). When considering the same combinatorial phenotypes for the stromal compartment, only “High stromal CD20+ B cells and low CD38+ plasma cells TNBCs” were significantly associated with both OS and DFS (Table S5 in Supplementary Material).
Table 2.
Multivariate analysis of combinatorial intratumoral B cell/plasma cell density phenotypes with survival outcomes in triple negative breast cancers (TNBC).
| Reference to: low intratumoral CD20+ B cell and low intratumoral CD38+ plasma cell TNBCs | Nnumber 63 | Hazard ratio | 95% confidence interval | pValue |
|---|---|---|---|---|
| Disease-free survival (DFS) | ||||
| High intratumoral CD20+ B cell and low CD38+ plasma cell TNBCs | 17 | 0.71 | (0.29, 1.76) | 0.460 |
| Low intratumoral CD20+ B cell and high CD38+ plasma cell TNBCs | 10 | 0.29 | (0.07, 1.23) | 0.093 |
| High intratumoral CD20+ B cell and high CD38+ plasma cell TNBCs | 33 | 0.24 | (0.09, 0.64) | 0.004* |
| Overall survival (OS) | ||||
| High intratumoral CD20+ B cell and low CD38+ plasma cell TNBCs | 17 | 0.34 | (0.08, 1.48) | 0.150 |
| Low intratumoral CD20+ B cell and high CD38+ plasma cell TNBCs | 10 | 0.18 | (0.02, 1.36) | 0.096 |
| High intratumoral CD20+ B cell and high CD38+ plasma cell TNBCs | 33 | 0.26 | (0.09, 0.75) | 0.013* |
*Statistically significant.
Intratumoral CD38+ Plasma Cell Density Is an Independent Prognostic Marker in TNBC
We previously reported the prognostic influence of intratumoral CD20+ B cell and CD3+ T cell density in TNBC (16). Therefore, we performed further multivariate analyses on TNBC with high or low intratumoral CD38+ plasma cells, adjusted for the effects of B and T cell density. We found that the density of intratumoral CD38+ plasma cells was an independent prognostic marker for both DFS (HR = 0.28; 95% CI 0.11–0.71; p = 0.007) and OS (HR = 0.28; 95% CI 0.10–0.82; p = 0.020) (Table 3). Furthermore, adjusting for the effects of CD20+ B cell density similarly identified intratumoral CD38+ plasma cell density as an independent prognostic marker (Table S6 in Supplementary Material). One percent incremental increases in CD38+ plasma cell density showed significant prognostic value in DFS, but not OS, even after adjustment for the density of CD3+ T cells and CD20+ B cells (Table 3), or for CD20+ B cells alone (Table S6 in Supplementary Material). These findings were confirmed using CD38 gene expression data from a publicly available database [METABRIC, EGAS00001001753 from the European Genome–phenome Archive (58)], which revealed a significant association between increasing CD38 expression and both DFS (HR = 0.82; 95% CI 0.68–0.97, p = 0.0229) and OS (HR = 0.83; 95% CI 0.72–0.97, p = 0.0191) in 320 cases of TNBC (Table 4). Besides the METABRIC cohort (58) we also analyzed gene expression and survival of TNBCs from The Cancer Genome Atlas (59) which was obtained from cBioPortal (60, 61) for validation purposes, after filtering for TNBC samples. However, CD38 gene expression in this TNBC cohort (n = 89) is not a prognostic marker (p = 0.235) which might be due to the small sample size.
Table 3.
Multivariate analysis showed that high intratumoral CD38+ plasma cell populations are significantly associated with longer disease-free survival (DFS), compared to low intratumoral CD38+ plasma cells populations in triple negative breast cancers (TNBCs), with the median cutoff and with every 1 percent increase.
| Hazard ratio | 95% confidence interval | pValue | |
|---|---|---|---|
| DFS | |||
| Intratumoral CD38+ plasma cells TNBCs High vs. low |
0.28 | (0.11, 0.71) | 0.007* |
| Intratumoral CD38+ plasma cells TNBCs (every 1 percent) | 0.95 | (0.91, 1.00) | 0.031* |
| Overall survival (OS) | |||
| Intratumoral CD38+ plasma cells TNBCs High vs. low |
0.28 | (0.10, 0.82) | 0.020* |
| Intratumoral CD38+ plasma cells TNBCs (every 1 percent) | 0.96 | (0.91, 1.01) | 0.126 |
Intratumoral CD38+ plasma cells are associated with better OS with the median cutoff (multivariate analysis adjusted for tumor size, grade, age, and lymph node status, intratumoral CD20+ B cells, and CD3+ T cells).
*Statistically significant.
Table 4.
Analysis of CD38 expression level and survival outcomes in triple negative breast cancers (TNBC) using data from the European Genome–phenome Archive.
| Hazard ratio | 95% confidence interval | pValue | |
|---|---|---|---|
| Disease-free survival (DFS) | |||
| CD38 (every 1 unit) | 0.82 | (0.68, 0.97) | 0.0229* |
| Overall survival (OS) | |||
| CD38 (every 1 unit) | 0.83 | (0.72, 0.97) | 0.0191* |
*Statistically significant.
Higher Expression of IgG Genes Is Associated With Improved Survival Outcomes in TNBC
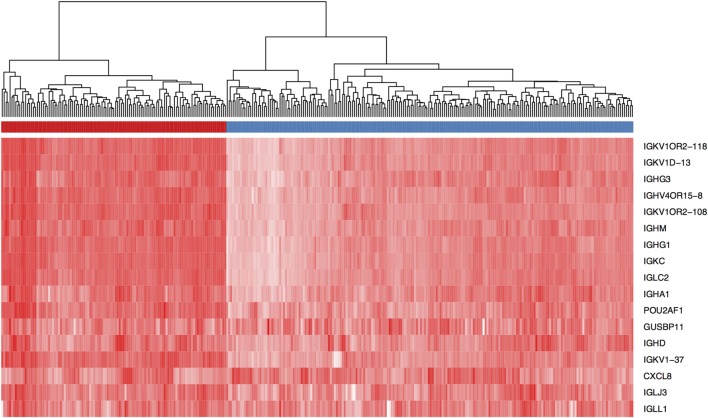
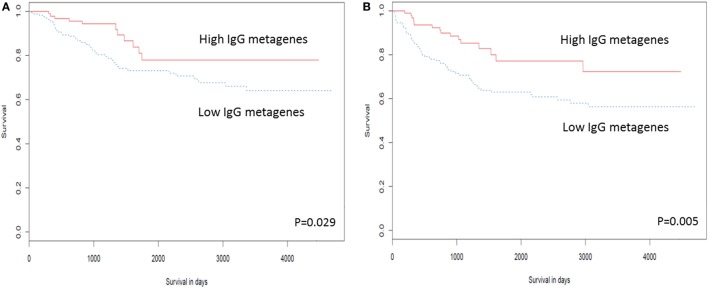
Several studies have examined the link between expression of a panel of B cell-related genes, termed metagenes, and breast cancer prognosis (43–46). We selected 16 IgG genes from a previously published metagene panel (62), for which the expression was likely to reflect functions of B cells and plasma cells. Expression levels of 9 of the 16 IgG genes were positively correlated with better OS, while expression of 11 of the 16 was correlated with DFS (Table S7 in Supplementary Material). The combination of these 16 IgG genes with CXCL8 has been suggested as a prognostic marker in breast cancer in general (44, 63); we, therefore, examined expression of this metagene in our TNBC cohort. Unsupervised hierarchical analysis revealed the existence of two clusters of TNBC (Figure 3): cluster 1 (red) contained TNBC with higher metagene expression and exhibited significantly better OS (p = 0.029) and DFS (p = 0.005) than did the low metagene-expressing cluster 2 (blue) (Figure 4). Of the IgG genes within the metagene panel, three genes: IGKC, IGHM, and IGHG1, have been reported to pre-dominate in both lymph node-negative breast cancer, and TNBC (43, 64–66). We found that expression levels of these three genes significantly and positively correlated with the abundance of CD38+ plasma cells in our TNBC samples (IGKC p < 0.0001, R = 0.647; IGHM p < 0.0001, R = 0.580; IGHG1 p < 0.0001, R = 0.655, Table S3 in Supplementary Material); and were associated with incrementally increasing DFS in multivariate analysis adjusted for tumor size, grade, age, and lymph node status (Table 5). Increasing IGHG1 expression was also associated with better OS (Table 5). Interestingly, after adjusting the multivariate analysis for the effect of intratumoral CD38+ plasma cell density, the expression levels of all three genes lost significant prognostic value (Table S8 in Supplementary Material), suggesting a direct role of plasma cells. However, if the analysis was adjusted for intratumoral CD20+ B cell density, some of the genes retained significant prognostic impact (Table S9 in Supplementary Material), such as IGHM and IGHG1.
Figure 3.
Expression level of a panel of 16 IgG genes and CXCL8 defines two groups of triple negative breast cancer (TNBC) patients. Unsupervised hierarchical clustering using Euclidean distance revealed the existence of two TNBC patient clusters (red and blue) based on expression intensity of the 17 genes listed. The heat map is colored by the log10 normalized counts with the highest expression in red and the lowest expression in white.
Figure 4.
High expression of the IgG metagene is associated with better clinical outcome in triple negative breast cancer (TNBC). Kaplan–Meier analysis of survival outcomes in TNBC in women with high vs. low expression of the IgG metagene, comprising 16 IgG genes and CXCL8. (A) Disease-free survival; (B) overall survival.
Table 5.
Multivariate analysis of expression level of IgG genes and survival outcomes in TNBC patients (after adjustment for for tumor size, grade, age and lymph node status).
| Hazard ratio | 95% confidence interval | pValue | |
|---|---|---|---|
| Disease-free survival (DFS) | |||
| IGKC (every 1 unit increase of Nanostring count) | 0.58 | (0.39, 0.88) | 0.0103* |
| IGHM (every 1 unit increase of Nanostring count) | 0.60 | (0.42, 0.86) | 0.0055* |
| IGHG1 (every 1 unit increase of Nanostring count) | 0.66 | (0.44, 0.99) | 0.0437* |
| Overall survival (OS) | |||
| IGKC (every 1 unit increase of Nanostring count) | 0.66 | (0.41, 1.06) | 0.0854 |
| IGHM (every 1 unit increase of Nanostring count) | 0.65 | (0.40, 1.04) | 0.0707 |
| IGHG1 (every 1 unit increase of Nanostring count) | 0.64 | (0.42, 0.97) | 0.0340* |
*Statistically significant.
Intratumoral CD38+ Plasma Cell Density and IgG Gene Expression Add Significant Prognostic Power to Classical Clinicopathological Parameters
To test the prognostic power of the B cell/plasma cell-related measures reported here, we evaluated the impact of incorporating their effects into survival outcome analysis with a panel of traditional clinicopathological features (patient age, tumor grade, tumor size, and lymph node status). As shown in Table 6, the additional assessment of intratumoral CD38+ plasma cell density with clinicopathological features significantly increased the prognostic value for both DFS (ΔLRχ2 = 17.28, p = 1.71E−08) and OS (ΔLRχ2 = 10.03, p = 6.32E−08), compared to clinicopathological features alone. Of the three genes tested (IGKC, IGHM, and IGHG1), IGHG1 conferred the highest added prognostic value for both DFS (ΔLRχ2 = 21.99, p = 1.88E−09) and OS (ΔLRχ2 = 16.23, p = 3.47E−09). Adding expression level of IgG metagene (Table S7 in Supplementary Material) also increased the prognostic value compared to clinicopathological features alone (DFS: ΔLRχ2 = 7.74, p = 1.43E−06; OS: ΔLRχ2 = 6.12, p = 3.89E−07). The best combination was achieved by integrating intratumoral CD38+ plasma cell density and IGHG1 which conferred the best added prognostic value for DFS (ΔLRχ2 = 27.38, p = 5.22E−10) and OS (ΔLRχ2 = 21.29, p = 1.03E−08).
Table 6.
Table showing the change in the log-likelihood of the models with added prognostic terms.
| Variables | Disease-free survival |
Overall survival |
||
|---|---|---|---|---|
| ΔLRχ2 | pValue | ΔLRχ2 | pValue | |
| CP + iCD38 PC vs. CP | 17.28 | 1.71E−08* | 10.03 | 6.32E−08* |
| CP + IGKC vs. CP | 18.45 | 9.91E−09* | 14.26 | 8.74E−09* |
| CP + IGHM vs. CP | 15.44 | 4.04E−08* | 15.14 | 5.78E−09* |
| CP + IGHG1 vs. CP | 21.99 | 1.88E−09* | 16.23 | 3.47E−09* |
| CP + IgG genes vs. CP | 7.74 | 1.43E−06* | 6.12 | 3.89E−07* |
| CP + iCD38 PC + IGHG1 vs. CP | 27.38 | 5.22E−10* | 21.29 | 1.03E−08* |
Statistical significance of the change was determined by a likelihood ratio test.
*Statistically significant; CP, clinicopathological parameters (patient age, tumor grade, tumor size, and lymph node status); iCD38 PC, intratumoral CD38+ plasma cell density; IgG genes, expression level of a panel of IgG genes (Table S7 in Supplementary Material); LR, likelihood ratio.
Discussion
While the significance of tumor-infiltrating T cells in breast cancer is well accepted, this is the first study to our knowledge, to provide evidence for a critical role of both B cells and plasma cells in TNBC. Here, we demonstrated that intratumoral CD38+ plasma cell density is an independent and incremental prognostic marker, even after adjusting for patient age, tumor grade, tumor size, lymph node status, and the density of tumor-infiltrating CD3+ T cells and CD20+ B cells. We also report that higher expression level of a panel of IgG genes also predicted better clinical outcome in TNBC patients, supporting a previous publication (44).
Much of the work on B cells and plasma cells in breast cancer has been at the molecular level. Some studies provide evidence that high expression of a B cell/plasma cell metagene is associated with favorable prognosis in breast cancers (43–46); others propose that expression level of IGKC alone has equivalent predictive and prognostic value (46). In our hand, expression of IGHG1, and not IGKC, offered the most significant increases in prognostic value compared to classical clinicopathological parameters alone. Intriguingly, expression level of a subset of the 60-gene B cell/plasma cell metagene is associated with worse prognosis in various cancer types that are reported (43, 67, 68), including the finding that expression of IGHG1 may be linked with tumor cell proliferation and immune evasion in pancreatic, lung, and breast cancer cell lines (69–72). These data may imply that B cells or plasma cells could assume pro-tumoral roles under certain conditions; however, the factors driving the emergence of this putative pathologic phenotype and the roles played by B cells and plasma cells in these circumstances have yet to be revealed. Of note, studying the IGHG1 expression may also be valuable if the setting allowed molecular testing such as quantitative polymerase chain reaction (Table 6). We have performed a parallel set of immunohistochemical stains using CD138 for plasma cells in our cohort. CD38 expression in the plasma cells moderately correlated with CD138 in our patient cohort (R = 0.39, p < 0.0001). Although CD138 has been proposed to also be a plasma cells marker (73), it is widely known that many types of tumor cells can express CD138 including breast cancer (54–57). Since we also observed CD138 strong staining in tumor cells of our TNBC cohort, we did not pursue it further.
The limited cellular level studies on the roles of B cells and plasma cells in breast cancer have generated similar discordant conclusions: Mohammed et al. used IHC staining to show that a high density of CD38+ lymphocytes predicted worse prognosis, while the density of CD20+ B cells did not significantly affect outcome in primary invasive ductal breast cancer (41). It is possible that in this tumor type, as in esophageal and gastric cancers (74), that immunosuppressive IL10-expressing plasma cells were present and inhibited the anti-tumor T cell response. In this study, we have no evidence of such a pathological role of plasma cells in TNBC. Thus, as suggested by the molecular data, distinct immune mechanisms may be operating in different cancer subtypes and under specific sets of conditions.
The importance of immune parameters in determining prognosis and treatment response across all cancer types has been recognized in several attempts to incorporate their measurement into routine clinical practice (52, 75). However, these attempts have not been successful; moreover both approaches exclude mention of B cells or plasma cells, likely due to the relative lack of data and integrated studies in this area. Our data argue for a re-appraisal of these guidelines and for more widespread investigation of the functional and prognostic roles of these cell types and their gene products.
In summary, our results demonstrate that the density of CD38+ plasma cells within TNBC tumors has a significant impact on DFS rate. The prognostic value of plasma cell density is independent of clinicopathological parameters, and of the densities of tumor-infiltrating T cells and B cells. In addition, expression level of the IgG gene, IGHG1 provided high prognostic value in TNBC for both OS and DFS, representing an easily measurable molecular prognostic marker. The important role of the humoral immune system warrants further studies and may be potentially utilized in routine diagnostic work in addition to its inclusion in cancer immunotherapy.
Ethics Statement
The Centralized Institutional Review Board of SingHealth provided ethical approval for the use of patient materials in this study (CIRB Ref: 2013/664/F and 2015/2199). In Singapore, a retrospective study is exempted for informed consent.
Author Contributions
PT and JI conceived and directed the study. PT, JY, and JI supervised the research. JY interpreted the data and performed biostatistical analysis. JL constructed TMAs, performed IHC, prepared samples for NanoString, and collated data. BL performed bioinformatics analysis. HL and WL performed biostatistical analysis. NC and JI performed immunohistochemical scoring. CO constructed TMAs, performed IHC, and collated data. TP and AT contributed to the scientific content of the study. JY and JI drafted the manuscript with the assistance and final approval of all authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Timothy Tay Kwang Yong for critical review of this manuscript.
Footnotes
Funding. This research was jointly supported by A*STAR Biomedical Research Council and by Singapore National Research Foundation under the Stratified Medicine Program administered by the Singapore Ministry of Health’s National Medical Research Council (SMPO201302), and partially funded by Transition Award from the Singapore National Medical Research Council (NMRC/TA/0041/2015) and SingHealth Duke-NUS Pathology Academic Clinical Program Budding Clinician-Scientist grant (ACP PATH BCS 14 001).
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01209/full#supplementary-material.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol (2009) 23(1):123–33. 10.1038/modpathol.2009.145 [DOI] [PubMed] [Google Scholar]
- 3.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA (2015) 313(2):165–73. 10.1001/jama.2014.17322 [DOI] [PubMed] [Google Scholar]
- 4.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med (2010) 363(20):1938–48. 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 5.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res (2007) 13(15 Pt 1):4429–34. 10.1158/1078-0432.CCR-06-3045 [DOI] [PubMed] [Google Scholar]
- 6.Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer (2009) 9(1):29–33. 10.3816/CBC.2009.n.005 [DOI] [PubMed] [Google Scholar]
- 7.Thike AA, Yong-Zheng Chong L, Cheok PY, Li HH, Wai-Cheong Yip G, Huat Bay B, et al. Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod Pathol (2014) 27(3):352–60. 10.1038/modpathol.2013.145 [DOI] [PubMed] [Google Scholar]
- 8.Cheng CL, Thike AA, Tan SY, Chua PJ, Bay BH, Tan PH. Expression of FGFR1 is an independent prognostic factor in triple-negative breast cancer. Breast Cancer Res Treat (2015) 151(1):99–111. 10.1007/s10549-015-3371-x [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto H, Koo SL, Dent R, Tan PH, Iqbal J. Role of inflammatory infiltrates in triple negative breast cancer. J Clin Pathol (2015) 68(7):506–10. 10.1136/jclinpath-2015-202944 [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol (2001) 166(5):2961–9. 10.4049/jimmunol.166.5.2961 [DOI] [PubMed] [Google Scholar]
- 11.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol (2015) 33(9):983–91. 10.1200/JCO.2014.58.1967 [DOI] [PubMed] [Google Scholar]
- 12.Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat (2012) 132(3):793–805. 10.1007/s10549-011-1554-7 [DOI] [PubMed] [Google Scholar]
- 13.Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol (2015) 26(7):1518. 10.1093/annonc/mdv241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res (2015) 17(1):124. 10.1186/s13058-015-0632-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol (2014) 25(8):1544–50. 10.1093/annonc/mdu112 [DOI] [PubMed] [Google Scholar]
- 16.Yeong J, Thike AA, Lim JC, Lee B, Li H, Wong SC, et al. Higher densities of Foxp3+ regulatory T cells are associated with better prognosis in triple-negative breast cancer. Breast Cancer Res Treat (2017) 163(1):21–35. 10.1007/s10549-017-4161-4 [DOI] [PubMed] [Google Scholar]
- 17.Bottai G, Raschioni C, Losurdo A, Di Tommaso L, Tinterri C, Torrisi R, et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res (2016) 18(1):121. 10.1186/s13058-016-0783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hainaut P, Plymoth A. Targeting the hallmarks of cancer: towards a rational approach to next-generation cancer therapy. Curr Opin Oncol (2013) 25(1):50–1. 10.1097/CCO.0b013e32835b651e [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 20.Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut (2017) 66(2):342–51. 10.1136/gutjnl-2015-310814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer (2016) 4(1):40. 10.1186/s40425-016-0145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen JS, Nelson BH. Tumor-infiltrating B cells and T cells: working together to promote patient survival. Oncoimmunology (2012) 1(9):1623–5. 10.4161/onci.21650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet (2009) 373(9668):1033–40. 10.1016/S0140-6736(09)60251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eccles SA. Monoclonal antibodies targeting cancer: ‘magic bullets’ or just the trigger? Breast Cancer Res (2000) 3(2):86. 10.1186/bcr276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer (2012) 12(4):298–306. 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 26.Madorsky Rowdo FP, Baron A, Urrutia M, Mordoh J. Immunotherapy in cancer: a combat between tumors and the immune system; you win some, you lose some. Front Immunol (2015) 6:127. 10.3389/fimmu.2015.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrio MM, Levy EM, Mordoh J. Editorial: “cancer immunotherapy: lights and shadows”. Front Immunol (2015) 6:350. 10.3389/fimmu.2015.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer (2012) 12(4):278–87. 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 29.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol (2010) 185(9):4977–82. 10.4049/jimmunol.1001323 [DOI] [PubMed] [Google Scholar]
- 30.Marsigliante S, Biscozzo L, Marra A, Nicolardi G, Leo G, Lobreglio GB, et al. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett (1999) 139(1):33–41. 10.1016/S0304-3835(98)00379-6 [DOI] [PubMed] [Google Scholar]
- 31.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother (2003) 52(12):715–38. 10.1007/s00262-003-0409-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res (1992) 12(5):1463–6. [PubMed] [Google Scholar]
- 33.Lee AH, Happerfield LC, Bobrow LG, Millis RR. Angiogenesis and inflammation in ductal carcinoma in situ of the breast. J Pathol (1997) 181(2):200–6. [DOI] [PubMed] [Google Scholar]
- 34.Miligy I, Mohan P, Gaber A, Aleskandarany MA, Nolan CC, Diez-Rodriguez M, et al. Prognostic significance of tumour infiltrating B lymphocytes in breast ductal carcinoma in situ. Histopathology (2017) 71(2):258–68. 10.1111/his.13217 [DOI] [PubMed] [Google Scholar]
- 35.Brown JR, Wimberly H, Lannin DR, Nixon C, Rimm DL, Bossuyt V. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res (2014) 20(23):5995–6005. 10.1158/1078-0432.CCR-14-1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol (2008) 180(1):361–71. 10.4049/jimmunol.180.1.361 [DOI] [PubMed] [Google Scholar]
- 37.Shimabukuro-Vornhagen A, Schlößer HA, Gryschok L, Malcher J, Wennhold K, Garcia-Marquez M, et al. Characterization of tumor-associated B-cell subsets in patients with colorectal cancer. Oncotarget (2014) 5(13):4651–64. 10.18632/oncotarget.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroeger DR, Milne K, Nelson BH. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res (2016) 22(12):3005–15. 10.1158/1078-0432.CCR-15-2762 [DOI] [PubMed] [Google Scholar]
- 39.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med (2014) 189(7):832–44. 10.1164/rccm.201309-1611OC [DOI] [PubMed] [Google Scholar]
- 40.Ito T, Saga S, Nagayoshi S, Imai M, Aoyama A, Yokoi T, et al. Class distribution of immunoglobulin-containing plasma cells in the stroma of medullary carcinoma of breast. Breast Cancer Res Treat (1986) 7(2):97–103. 10.1007/BF01806794 [DOI] [PubMed] [Google Scholar]
- 41.Mohammed ZMA, Going JJ, Edwards J, Elsberger B, McMillan DC. The relationship between lymphocyte subsets and clinico-pathological determinants of survival in patients with primary operable invasive ductal breast cancer. Br J Cancer (2013) 109(6):1676–84. 10.1038/bjc.2013.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei H, Fu P, Yao M, Chen Y, Du L. Breast cancer stem cells phenotype and plasma cell-predominant breast cancer independently indicate poor survival. Pathol Res Pract (2016) 212(4):294–301. 10.1016/j.prp.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 43.Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res (2008) 68(13):5405–13. 10.1158/0008-5472.CAN-07-5206 [DOI] [PubMed] [Google Scholar]
- 44.Hanker LC, Rody A, Holtrich U, Pusztai L, Ruckhaeberle E, Liedtke C, et al. Prognostic evaluation of the B cell/IL-8 metagene in different intrinsic breast cancer subtypes. Breast Cancer Res Treat (2013) 137(2):407–16. 10.1007/s10549-012-2356-2 [DOI] [PubMed] [Google Scholar]
- 45.Alistar A, Chou JW, Nagalla S, Black MA, D’Agostino R, Jr, Miller LD. Dual roles for immune metagenes in breast cancer prognosis and therapy prediction. Genome Med (2014) 6(10):80. 10.1186/s13073-014-0080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt M, Micke P, Gehrmann M, Hengstler JG. Immunoglobulin kappa chain as an immunologic biomarker of prognosis and chemotherapy response in solid tumors. Oncoimmunology (2012) 1(7):1156–8. 10.4161/onci.21653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lakhani S, Ellis I, Schnitt S, Tan P, Van de Vijver M. World Health Organisation classification of tumors of the breast. (Vol. 4). France: International Agency for Research on Cancer; (2012). p. 142–7. [Google Scholar]
- 48.Thike AA, Iqbal J, Cheok PY, Tse GM, Tan PH. Ductal carcinoma in situ associated with triple negative invasive breast cancer: evidence for a precursor-product relationship. J Clin Pathol (2013) 66(8):665–70. 10.1136/jclinpath-2012-201428 [DOI] [PubMed] [Google Scholar]
- 49.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol (2013) 31(31):3997–4013. 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 50.Fan YS, Casas CE, Peng J, Watkins M, Fan L, Chapman J, et al. HER2 FISH classification of equivocal HER2 IHC breast cancers with use of the 2013 ASCO/CAP practice guideline. Breast Cancer Res Treat (2016) 155(3):457–62. 10.1007/s10549-016-3717-z [DOI] [PubMed] [Google Scholar]
- 51.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med (2014) 138(2):241–56. 10.5858/arpa.2013-0953-SA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol (2015) 26(2):259–71. 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature (2014) 515(7528):563–7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu PG, Arber DA, Weiss LM. Expression of T/NK-cell and plasma cell antigens in nonhematopoietic epithelioid neoplasms. An immunohistochemical study of 447 cases. Am J Clin Pathol (2003) 120(1):64–70. 10.1309/48KC17WAU69BTBXQ [DOI] [PubMed] [Google Scholar]
- 55.Mukunyadzi P, Sanderson RD, Fan CY, Smoller BR. The level of syndecan-1 expression is a distinguishing feature in behavior between keratoacanthoma and invasive cutaneous squamous cell carcinoma. Mod Pathol (2002) 15(1):45–9. 10.1038/modpathol.3880488 [DOI] [PubMed] [Google Scholar]
- 56.Bahrami A, Truong LD, Ro JY. Undifferentiated tumor: true identity by immunohistochemistry. Arch Pathol Lab Med (2008) 132(3):326–48. 10.1043/1543-2165(2008)132 [DOI] [PubMed] [Google Scholar]
- 57.Lin F, Liu H. Immunohistochemistry in undifferentiated neoplasm/tumor of uncertain origin. Arch Pathol Lab Med (2014) 138(12):1583–610. 10.5858/arpa.2014-0061-RA [DOI] [PubMed] [Google Scholar]
- 58.Pereira B, Chin SF, Rueda OM, Vollan HK, Provenzano E, Bardwell HA, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun (2016) 7:11479. 10.1038/ncomms11479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature (2012) 490(7418):61–70. 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal (2013) 6(269):pl1. 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov (2012) 2(5):401–4. 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res (2009) 11(2):9. 10.1186/bcr2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rody A, Karn T, Liedtke C, Pusztai L, Ruckhaeberle E, Hanker L, et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res (2011) 13(5):R97. 10.1186/bcr3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Z, Gerhold-Ay A, Gebhard S, Boehm D, Solbach C, Lebrecht A, et al. Immunoglobulin kappa C predicts overall survival in node-negative breast cancer. PLoS One (2012) 7(9):e44741. 10.1371/journal.pone.0044741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt M, Hellwig B, Hammad S, Othman A, Lohr M, Chen Z, et al. A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin kappa C as a compatible prognostic marker in human solid tumors. Clin Cancer Res (2012) 18(9):2695–703. 10.1158/1078-0432.CCR-11-2210 [DOI] [PubMed] [Google Scholar]
- 66.Whiteside TL, Ferrone S. For breast cancer prognosis, immunoglobulin kappa chain surfaces to the top. Clin Cancer Res (2012) 18(9):2417–9. 10.1158/1078-0432.CCR-12-0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt M, Micke P, Hengstler JG. IGKC and prognosis in breast cancer-letter. Clin Cancer Res (2013) 19(1):304. 10.1158/1078-0432.CCR-12-2818 [DOI] [PubMed] [Google Scholar]
- 68.Schmidt M, Hengstler JG, von Törne C, Koelbl H, Gehrmann MC. Coordinates in the universe of node-negative breast cancer revisited. Cancer Res (2009) 69(7):2695–8. 10.1158/0008-5472.CAN-08-4013 [DOI] [PubMed] [Google Scholar]
- 69.Zhang C, Lin M, Wu R, Wang X, Yang B, Levine AJ, et al. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci U S A (2011) 108(39):16259–64. 10.1073/pnas.1113884108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Lin D, Peng H, Huang Y, Huang J, Gu J. Cancer-derived immunoglobulin G promotes tumor cell growth and proliferation through inducing production of reactive oxygen species. Cell Death Dis (2013) 4:e945. 10.1038/cddis.2013.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pelegrina LT, Lombardi MG, Fiszman GL, Azar ME, Morgado CC, Sales ME. Immunoglobulin g from breast cancer patients regulates MCF-7 cells migration and MMP-9 activity by stimulating muscarinic acetylcholine receptors. J Clin Immunol (2013) 33(2):427–35. 10.1007/s10875-012-9804-y [DOI] [PubMed] [Google Scholar]
- 72.Jiang C, Huang T, Wang Y, Huang G, Wan X, Gu J. Immunoglobulin G expression in lung cancer and its effects on metastasis. PLoS One (2014) 9(5):e97359. 10.1371/journal.pone.0097359 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.O’Connell FP, Pinkus JL, Pinkus GS. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am J Clin Pathol (2004) 121(2):254–63. 10.1309/617DWB5GNFWXHW4L [DOI] [PubMed] [Google Scholar]
- 74.Fristedt R, Borg D, Hedner C, Berntsson J, Nodin B, Eberhard J, et al. Prognostic impact of tumour-associated B cells and plasma cells in oesophageal and gastric adenocarcinoma. J Gastrointest Oncol (2016) 7(6):848–59. 10.21037/jgo.2016.11.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med (2012) 10(1):1479–5876. 10.1186/1479-5876-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.