Abstract
Conventional antidepressant medications, which act on monoaminergic systems, display significant limitations, including a time lag of weeks to months and low rates of therapeutic efficacy. GLYX-13 is a novel glutamatergic compound that acts as an NMDA modulator with glycine-like partial agonist properties; like the NMDA receptor antagonist ketamine produces rapid antidepressant actions in depressed patients and in preclinical rodent models. However, the mechanisms underlying the antidepressant actions of GLYX-13 have not been characterized. Here, we use a combination of neutralizing antibody, mutant mouse, and pharmacological approaches to test the role of BDNF-TrkB signaling in the actions of GLYX-13. The results demonstrate that the antidepressant effects of GLYX-13 are blocked by intra-mPFC infusion of an anti-BDNF neutralizing antibody or in mice with a knock-in of the BDNF Val66Met allele, which blocks the processing and activity dependent release of BDNF. We also demonstrate that pharmacological inhibitors of BDNF-TrkB signaling or of L-type voltage dependent Ca2+ channels (VDCCs) block the antidepressant behavioral actions of GLYX-13. Finally, we examined the role of the Rho GTPase proteins by injecting a selective inhibitor into the mPFC and found that activation of Rac1 but not RhoA is involved in the antidepressant effects of GLYX-13. Together, these findings indicate that enhanced release of BDNF through exocytosis caused by activation of VDCCs and subsequent TrkB-Rac1 signaling is required for the rapid and sustained antidepressant effects of GLYX-13.
Introduction
Major depressive disorder (MDD) is a chronic, debilitating illness that affects approximately 17 % of the population and is one of the leading causes of disability among all medical illnesses (1). Currently, MDD is treated with monoaminergic agents, but large-scale clinical trials (i.e., STAR*D) show that these drugs can take weeks to months to produce a therapeutic response, have limited efficacy, and low rates of remission (2–4). On the other hand, there is growing evidence that the glutamatergic system plays an important role in the pathophysiology and treatment of MDD (5–7). Importantly, clinical findings demonstrate that glutamatergic agents, notably ketamine, a dissociative anesthetic that blocks N-methyl-D-aspartate (NMDA) receptor channel activity, causes rapid (within hours) and long-lasting (7 to 10 days) antidepressant effects (8,9). However, use of ketamine is associated with cognitive impairment and psychotomimetic symptoms (10–12), stimulating studies to develop alternative glutamatergic approaches for the treatment of MDD.
One such agent is GLYX-13 (also known as Rapastinel), a novel allosteric modulator of the NMDA receptor with glycine-like partial agonist properties (13,14). Recent clinical results show that GLYX-13 rapidly decreases depressive symptoms within hours and these effects are sustained for up to 7 days (15). Importantly, GLYX-13 does not produce the dissociative and psychotomimetic side effects caused by ketamine. Rodent studies report that GLYX-13 injection also produces rapid antidepressant effects that last for at least 1 week, and increases synaptic number and function in the medial prefrontal cortex (mPFC) (16,17). These findings indicate that GLYX-13, like ketamine rapidly stimulates neuroplasticity-signaling that result in long-lasting structural alterations that underlie the antidepressant behavioral responses (17).
The structural as well as behavioral actions of ketamine result in part from increased brain derived neurotrophic factor (BDNF) signaling (18–20). The antidepressant actions of ketamine are blocked in BDNF deletion mutant mice, as well as in mice with a knock-in of the BDNF Met allele, a functional polymorphism found in humans that blocks the processing and activity dependent release of BDNF (18,19,21). Studies in primary neuronal cultures provide direct evidence that ketamine increases BDNF release in an activity dependent manner that also requires L-type voltage dependent calcium channel (VDCC) activation (20). Together, these findings indicate that the actions of ketamine require rapid, activity dependent release of BDNF.
Despite this progress on ketamine, the molecular mechanisms underlying the antidepressant actions of GLYX-13, notably the role of BDNF signaling have not been determined. The current study addresses this question using a combination of approaches, including an anti-BDNF neutralizing antibody, BDNF Val66Met knock-in mice, and pharmacological inhibitors of BDNF-tropomyosin-related kinase B (TrkB) receptor signaling and VDCCs. In addition, we examine the role of downstream BDNF-TrkB pathways involved in synapse formation, namely activation of Rho GTPase signaling that controls BDNF dependent synaptic plasticity during structural long term potentiation (22).
Material and Methods
Animals and drug administration
Male Sprague-Dawley rats, C57BL/6J mice, or mutant BDNF Val66Met knock-in mice were used. GLYX-13 were dissolved in saline and injected through tail vein. Verapamil was injected 30 min prior to injection of GLYX-13. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committees.
Surgical and infusion procedures
Guide cannula were implanted into mPFC bilaterally. A function-blocking anti-BDNF antibody, K252a, NSC 23766, Y-27632 or BDNF were infused bilaterally.
Behavior studies
The FST, NSFT and FUST was carried out as previously described for rat and mouse (23–25). In FST, each animal was placed in the swim cylinders for a 10 min period and videotaped. Data were analyzed by scoring the immobility time. In NSFT, animals were food-deprived overnight and placed in an open field with a small amount of food in the center. The latency to feed was measured. In FUST, each animal was exposed to a cotton-tipped applicator infused with water or fresh urine from females of the same strain for 5 min and the time spent sniffing the cotton-tipped applicator was measured.
Primary cortical culture and BDNF analysis
Primary cortical culture and measurement of BDNF was performed as previously described (20). Cortical neuron dissected from E18 embryos were treated 3 nM GLYX-13 and the effects of anti-BDNF antibody on mTOR signaling and verapamil on BDNF release were examined using western blot and ELISA assay.
Western Blot
The phosphorylation level of mTOR, Erk, TrkB, and PAK and expression of postsynaptic proteins were evaluated in western blot following previous report (23). Total levels of the respective protein or GAPDH (Cell Signaling #5174, 1:1000) were used for loading control.
Immunohistochemistry
Rats were perfused transcardially. Brains were cut in a cryostat for coronal section. The number of c-Fos-positive cells was counted in the regions near the injection sites in the mPFC from two sections per animal after 3-3′-diaminobenzidine (DAB) staining.
Statistics
Data for TrkB and pTrkB western blots and dose response experiments were analyzed using one-way ANOVA. Data for FUST were analyzed using repeated-measure three-way ANOVA with time spent sniffing water or urine as a repeated measure. According to the interaction of three factors (drug, microinjection/genotype, and water/urine), post hoc analysis using two-way ANOVA followed by Tukey’s multiple comparison test or paired t-test were performed. Data for NSFT were analyzed using Kaplan–Meier survival analysis followed by the Mantel–Cox log-rank test. When a main effect was observed, treatment effect was analyzed using the Bonfferoni multiple comparison test. For other experiments, normality was analyzed with the D’Agostino-Pearson test. Depending on the experiment, the results were analyzed using two-way ANOVA followed by Tukey’s or Duncan’s multiple comparison test or Kursukal-Wallis test followed by Dunn’s multiple comparison test.
Results
Antidepressant actions of GLYX-13 are blocked by Anti-BDNF antibody infusion into mPFC
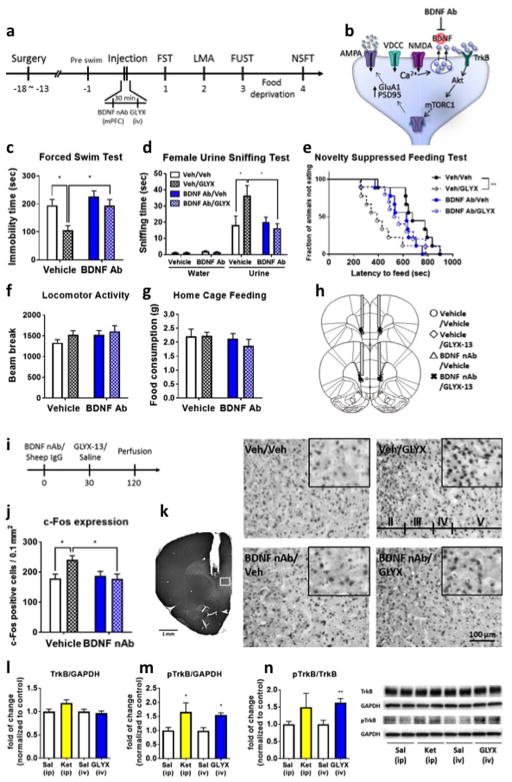
Previous studies demonstrate that infusion of GLYX-13 into the mPFC is sufficient to produce rapid antidepressant responses similar to systemic GLYX-13 administration, providing the rationale for targeting mPFC (26). Rats were infused with a BDNF neutralizing Ab (nAb) (0.5 μg/side) 30 min before GLYX-13 (3 mg/kg i.v.) injection and behavioral testing started 24 hr later (Fig. 1a). In vehicle infused rats, GLYX-13 produced a significant antidepressant effect in the FST (day 1, Fig. 1c) and NSFT (day 4, Fig. 1e), but no effect on locomotor activity or home cage feeding (Fig. 1f, and g). In the female urine sniffing test, a measure of motivation and reward in males, GLYX-13 administration produced a significant increase in time sniffing female urine, but not water (day 3, Fig. 1d). Infusion of the BDNF nAb prior to GLYX-13 injection significantly blocked the behavioral action of GLYX-13 in these three tests of antidepressant activity; BDNF nAb alone had no effect on these three behaviors or on locomotor activity or home cage feeding (Fig 1c–g). We also show that GLYX-13 increases c-Fos immunolabelling, a marker of neuronal activity, in infralimbic (IL) PFC, replicating a previous report (17), and infusion of the BDNF nAb, but not control IgG, blocks the induction of Fos+ neurons (Fig 1j,k). To determine if BDNF is required for the sustained antidepressant effects of GLYX-13, we tested the effects of BDNF nAb infusion 24 hr after GLYX-13 administration. The results demonstrate that infusion of BDNF nAb into the mPFC at this time point had no effect on the antidepressant effects of GLYX-13 in the FST or NSFT (supplementary Fig. 1a–e).
Figure 1. Intra-mPFC infusion of BDNF nAb blocks the antidepressant effects of GLYX-13 in rats.
(a) Rats were implanted with bilateral cannula in the medial prefrontal cortex (mPFC) and allowed to recover for approximately 2 weeks. The anti-brain derived neurotrophic factor (BDNF) nAb (0.5 μg/side) was infused into the mPFC 30 min prior to administration of vehicle or GLYX-13 (3 mg/kg, i.v.). Twenty-four hour after GLYX-13 administration behavioral studies were initiated and conducted over the next 4 days (c–g). (b) Diagram showing postsynaptic signaling. Significant effects of GLYX-13 were observed in vehicle infused rats and were blocked by anti-BDNF nAb in (c) the forced swim test (FST) (interaction: F1,33 = 1.88, P > 0.05, effect of GLYX-13: F1,33 = 8.76, P < 0.01, effect of BDNF nAb: F1,33 = 8.40, P < 0.01); (d) the female urine sniffing test (FUST) (Three-way ANOVA; effect of GLYX-13 x effect of BDNF nAb x water/urine: F1,1 = 5.29, P < 0.05, Two-way ANOVA; effect of GLYX-13 x effect of BDNF nAb: F1,33 = 5.77, P < 0.05, effect of GLYX-13: F1,33 = 2.42, P > 0.05, effect of BDNF nAb: F1,33 = 3.88, P > 0.05); and (e) the novelty suppressed feeding test (NSFT) (Mantel–Cox log-rank test: Chi square = 8.871, P < 0.05). No significant effects were seen in (f) locomotor activity (interaction: F1,33 = 0.234, P > 0.05, effect of GLYX-13: F1,33 = 1.55, P > 0.05, effect of BDNF nAb: F1,33 = 1.52, P > 0.05) or (g) home cage feeding (interaction: F1,33 = 0.398, P > 0.05, effect of GLYX-13: F1,33 = 1.12, P > 0.05, effect of BDNF nAb: F1,33 = 0.334, P > 0.05). The results are shown as mean ± S.E.M. n = 9 (Veh/Veh), 9 (Veh/GLYX), 9 (BDNF nAb/Veh), 10 (BDNF Ab/GLYX). (h) Location of cannula placements in the mPFC. (i) Control IgG or anti-BDNF nAb was microinfused 30 min before i.v. injection of saline or GLYX-13. Rats were perfused 90 min after i.v. injection and tissues were processed for c-Fos immunoreactivity. (j) Intra-mPFC infusion of anti-BDNF nAb attenuates GLYX-13-induced c-Fos expression in the mPFC (interaction: F1,20 = 6.22, P < 0.05, P > 0.05, effect of GLYX-13: F1,33 = 3.14, P > 0.05, effect of BDNF nAb: F1,33 = 3.53, P > 0.05). n = 6/group (k) Representative images showing c-Fos expression in the mPFC. *p < 0.05 and **p < 0.01 Tukey’s, Dunn’s or Bonfferoni’s multiple comparison test, following significant results of two-way ANOVA, Kruskal-Wallis or Mantel–Cox log-rank test. Rats were administered vehicle (i.v. or i.p.), GLYX-13 (3 mg/kg, i.v.) or ketamine (10 mg/kg, i.p.) and PFC dissections were collected 1 hr later. Levels of phosphorylated TrkB (l) and total TrkB receptor (m) in crude synaptosomal preparations was determined by western blot analysis. Levels of GAPDH were determined to control for protein levels and results are the ratio of each protein divided by GAPDH. (n) The ratio of pTrkB/TrkB is also calculated. The results are shown as mean ± S.E.M. n = 5/group. *p < 0.05 compared with the control group (two tailed unpaired Student’s t-test).
BDNF binds as a dimer to the TrkB tyrosine kinase receptor, resulting in autophosphorylation and activation of downstream pathways (27). To evaluate the effect of GLYX-13 on the activity of TrkB, we analyzed levels of the phosphorylated and activated form of the TrkB receptor by western blot analysis. We compared the effects of GLYX-13 (3 mg/kg, i.v.) with ketamine (10 mg/kg, i.p.), each with a separate vehicle (i.v. or i.p.) and mPFC was dissected 1 hr after drug administration. A single dose of GLYX-13 rapidly and significantly increased levels of phospho-TrkB without effecting total TrkB demonstrating enhanced BDNF-TrkB signaling (Fig. 1l–n). This induction of phospho-TrkB was not observed 24 hr later, indicating that the effect was transient (supplementary Fig. 1f–h) and consistent with the finding that the infusion of BDNF nAb at this time point had no effect on behavior (supplementary Fig. 1).
Antidepressant Effects of GLYX-13 are Blocked in BDNF Val66Met Knock-in Mice
We have reported that the antidepressant actions of ketamine are blocked in BDNF Met knock-in mice (18), a human polymorphism that blocks the processing and activity dependent release of mature BDNF (28). Here, the behavioral actions of GLYX-13 in BDNF Val66Met knock-in mice were evaluated. In studies to determine the effective dose of GLYX-13 in mice we found that 1 and 3 mg/kg (tail vein, i.v.) were effective in both the FST and NSFT (Supplementary Fig. 2b,e); however, 3 mg/kg of GLYX-13 resulted in a small but significant increase in locomotor activity 48 hours after dosing (Supplementary Fig. 2c). The 1 mg/kg dose had no effect on locomotor activity at either 24 or 48 hour after dosing, so this dose was used for further studies (Supplementary Fig. 2c, d)
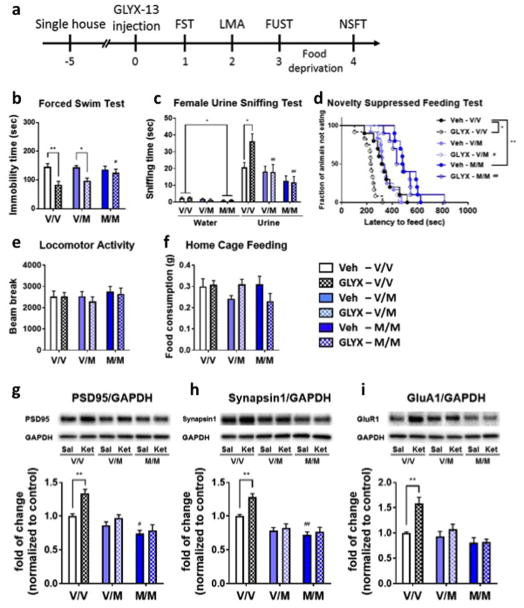
Wild type (WT) Val/Val littermates and BDNF Val/Met and Met/Met knock-in mice were tested (Fig. 2a). There were no differences in baseline levels of immobility in the FST (Fig. 2b), but Met/Met mice showed reduced water sniffing time in the FUST and increased latency to feed in NSFT compared to Val/Val mice (Fig. 2c,d) suggesting increased anxiety in Met/Met, consistent with previous results (28). In WT mice, we observed significant antidepressant effects of GLYX-13 in all of three tests for antidepressant activity with no effects on locomotor activity or home cage feeding (Fig. 2b–f). In Val/Met mice, GLYX-13 produced a significant effect in the FST, but no significant effects in the FUST or NSFT (Fig. 2b–d). In Met/Met mice, there were no significant effects of GLYX-13 in FST, FUST, or NSFT (Fig. 2b–d). These findings indicate that the behavioral actions of GLYX-13 require processing and activity dependent release of mature BDNF.
Figure 2. Antidepressant actions of GLYX-13 are attenuated in BDNF Val66Met knock-in mice (a–f).
(a) Experimental timeline for behavioral testing starting 1 day after i.v. injection of either saline or GLYX-13 (1 mg/kg, i.v.). Significant effects of GLYX-13 were observed in Val/Val mice and were partially or completely blocked in Val/Met and Met/Met mice in (b) the FST (Kruskal-Wallis statistics = 20.2, P < 0.01). Significant effects of GLYX-13 in Val/Val mice were blocked in both of Val/Met and Met/Met mice in (c) the FUST (Three-way ANOVA; effect of GLYX-13 x effect of genotype x water/urine: F1,1 = 3.20, P < 0.05, Two-way ANOVA; effect of GLYX-13 x effect of genotype: F2,58 = 3.24, P < 0.05, effect of GLYX-13: F1,58 = 2.54, P > 0.05, effect of genotype: F2,58 = 9.94, P < 0.01); and (d) the NSFT (Mantel–Cox log-rank test: Chi square = 79.45, P < 0.0001). No significant effects were seen in (e) locomotor activity (interaction: F2,58 = 0.138, P > 0.05, effect of GLYX-13: F1,58 = 0.356, P > 0.05, effect of genotype: F2,58 = 0.733, P > 0.05) or (f) home cage feeding (interaction: F2,58 = 3.37, P < 0.05, effect of GLYX-13: F1,58 = 0.00229, P > 0.05, effect of genotype: F2,58 = 0.831, P > 0.05). The results are shown as mean ± S.E.M. n = 10 (V/V veh), 12 (V/V GLYX), 12 (V/M Veh), 10 (V/M GLYX), 10 (M/M GLYX), 10 (M/M GLYX). Enhancement of synaptic proteins by GLYX-13 was attenuated in BDNF Val66Met knock-in mice. Significant effects of GLYX-13 on expression levels of synaptic proteins including PSD95, synapsin1 and GluR1 in Val/Val mice were blocked in both of Val/Met and Met/Met mice (g–i). (g) interaction: F2,30 = 3.92, P < 0.05, effect of GLYX-13: F1,30 = 13.1, P > 0.01, effect of genotype: F2,30 = 27.4, P < 0.001. (h) interaction: F2,30 = 3.65, P < 0.05, effect of GLYX-13: F1,30 = 8.70, P > 0.01, effect of genotype: F2,30 = 35.7, P < 0.001. (i) interaction: F2,30 = 5.47, P < 0.01, effect of GLYX-13: F1,30 = 11.4, P > 0.01, effect of genotype: F2,30 = 14.5, P < 0.001. The results are shown as mean ± S.E.M. n = 6/group. *p < 0.05 and **p < 0.01 Tukey’s, Dunn’s or Bonfferoni’s multiple comparison test, following significant results of two-way ANOVA, Kruskal-Wallis or Mantel–Cox log-rank test.
Previous studies demonstrate that GLYX-13 increases synaptic number and function, including increased levels of synaptic proteins in the mPFC (17). Here we found that in vehicle treated mice, basal level of PSD95 and synapsin1 were significantly decreased in Met/Met mice compared to Val/Val mice (Fig. 2g,h). Administration of GLYX-13 increased levels of PSD95, synapsin1 and GluR1 in WT mice, but these effects were blocked in Val/Met and Met/Met mice (Fig. 2g–i). The results indicate that processing and release of mature BDNF is required for the synaptic actions of GLYX-13.
Behavioral Actions of GLYX-13 are Blocked by Inhibition of L-type VDCC
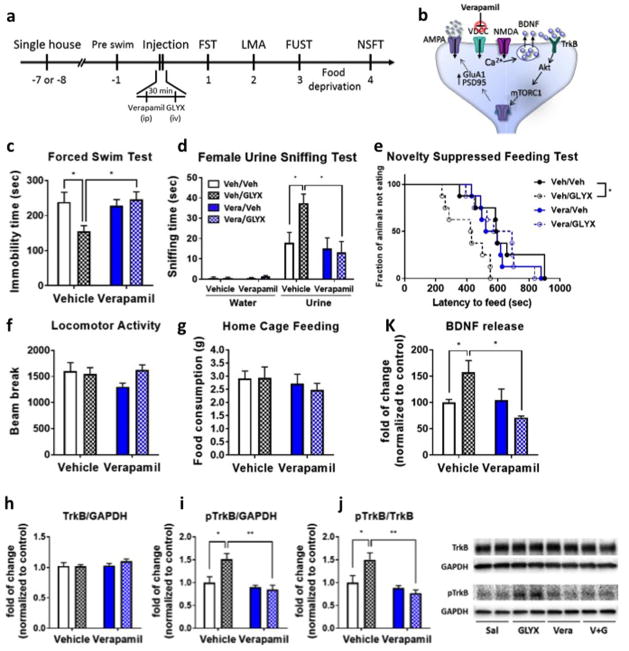
Our previous studies have demonstrated that the antidepressant behavioral actions of ketamine are blocked by pretreatment with a selective L-type VDCC antagonist (i.e., verapamil or nifedipine) (19). This is consistent with evidence that depolarization-induced release of BDNF in cultured neurons requires activation of VDCC (29). Here we tested the effects of a verapamil (10 mg/kg i.p.) on the behavioral effects of GLYX-13 in rats (3 mg/kg i.v.) (Fig. 3a). This dose of verapamil was chosen based on pharmacokinetic studies demonstrating effective blood and brain concentrations (30). GLYX-13 produced significant antidepressant responses in the FST, NSFT and FUST, and these effects were completely blocked by pretreatment with verapamil (Fig. 3c–g). Verapamil administration alone had no effect on any of the antidepressant behaviors, or on locomotor activity or home cage feeding (Fig. 3c–g).
Figure 3. L-type voltage dependent calcium channel (VDCC) antagonist, verapamil, blocks the antidepressant effects of GLYX-13 in rats.
(a) Experimental timeline for behavioral testing starting 1 day after i.p. injection of either saline or verapamil (10 mg/kg) and i.v. injection of either saline or GLYX-13 (3 mg/kg). (b) Diagram showing postsynaptic signaling. Significant effects of GLYX-13 were observed and were blocked by verapamil in (c) the FST (interaction: F1,28 = 5.53, P < 0.05, effect of GLYX-13: F1, 28 = 2.37, P > 0.05, effect of verapamil: F1,28 = 3.51, P > 0.05); (d) the FUST (Three-way ANOVA; effect of GLYX-13 x effect of verapamil x water/urine: F1,1 = 4.45, P < 0.05, Two-way ANOVA; effect of GLYX-13 x effect of verapamil: F1,28 = 4.54, P < 0.05, effect of GLYX-13: F1,28 = 3.01, P > 0.05, effect of verapamil: F1,28 = 7.16, P < 0.05); and (e) the NSFT (Mantel–Cox log-rank test:: Chi square = 14.37, P < 0.05). No significant effects were seen in (f) locomotor activity (Kruskal-Wallis statistics = 5.19, P > 0.05) or (g) home cage feeding (interaction: F1,28 = 0.168, P > 0.05, effect of GLYX-13: F1,28 = 0.940, P > 0.05, effect of verapamil: F1,28 = 0.113, P > 0.05). The results are shown as mean ± S.E.M. n = 8/group. *p < 0.05 Tukey’s, Dunn’s or Bonfferoni’s multiple comparison test, following significant results of two-way ANOVA, Kruskal-Wallis or Mantel–Cox log-rank test. (h) Pretreatment with verapamil (10 μM) blocked GLYX-13-induced BDNF release (n = 6 (Veh/Veh), 5 (Veh/GLYX), 4 (Vera/Veh), 4 (Vera/GLYX); interaction: F1, 17 = 6.97, P < 0.05, effect of GLYX-13: F1, 17 = 0.515, P > 0.05, effect of verapamil: F1,17 = 5.70, P < 0.05). *p < 0.05 Duncan’s multiple comparison test, following significant results of two-way ANOVA. Significant effects of GLYX-13 on phosphorylation level of TrkB were blocked by verapamil pretreatment. (i) TrkB/GAPDH (interaction: F1,20 = 0.883, P > 0.05, effect of GLYX-13: F1,20 = 0.701, P > 0.05, effect of verapamil: F1,20 = 1.27, P > 0.05) (j) pTrkB/GAPDH (interaction: F1,20 = 7.06, P < 0.05, effect of GLYX-13: F1,20 = 4.74, P < 0.05, effect of verapamil: F1,20 = 13.01, P < 0.01) (k) pTrkB/TrkB (interaction: F1,20 = 6.78, P < 0.05, effect of GLYX-13: F1,20 = 2.67, P > 0.05, effect of verapamil: F1,20 = 13.00, P < 0.01). The results are shown as mean ± S.E.M. n = 6/group. *p < 0.05 and **p < 0.01 Tukey’s multiple comparison test, following significant results of two-way ANOVA.
To test the effects of verapamil on GLYX-13-induced BDNF-TrkB signaling, we analyzed levels of phospho-TrkB. A single dose of GLYX-13 (3 mg/kg, i.v.) increased levels of phospho-TrkB 1 hr after drug administration, and this effect was completely blocked by pretreatment of verapamil (Fig. 3h–j). These findings demonstrate that VDCC activation is essential to GLYX-13 enhancement of BDNF-TrkB signaling. To directly examine the influence of GLYX-13 on BDNF release and effects of verapamil, we utilized primary neuronal cultures. The results demonstrate that incubation of primary neuronal cultures with GLYX-13 (3 nM) increases BDNF release into the culture media (Figure 3k), similar to a previous report (Lepack et al., 2016); in addition, preincubation with verapamil (10 μM) completely blocked the GLYX-13-induction of BDNF release (Fig. 3k and supplementary Fig. 3). Blockade of GLYX-13 induction of BDNF release was also observed at lower concentrations of verapamil (0.1 μM, Supplementary figure 3).
Antidepressant Effects of GLYX-13 are Blocked by Inhibition of BDNF-TrkB Signaling
To directly test the requirement for TrkB activation we determined the effects of pretreatment with a selective TrkB receptor antagonist (K252a) on the antidepressant actions of GLYX-13. Rats were infused with K252a (25 pmol/side) into the mPFC 30 min before administration of GLYX-13 (3 mg/kg i.v.), and were tested 24 hr later (Fig. 4a). In vehicle infused rats, GLYX-13 produced significant antidepressant effects in the FST, FUST and NSFT, and these responses were blocked by K252a infusion (Fig. 4c–g). K252a microinjection alone had no effect on behavior compared to vehicle (Fig. 4c–g).
Figure 4. TrkB is activated by and required for the antidepressant actions of GLYX-13.
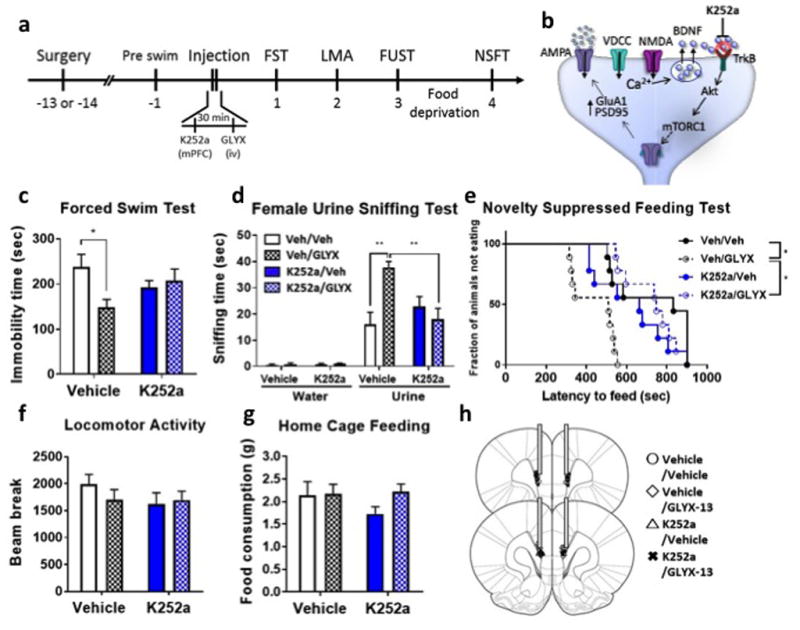
Intra-mPFC infusion of K252a blocked the antidepressant effects of GLYX-13 in rats. (a) Experimental timeline for behavioral testing starting 1 day after intra-mPFC infusion of either saline or K252a (25 pmol/side) and i.v. injection of either saline or GLYX-13 (3 mg/kg). (b) Diagram showing postsynaptic signaling. Significant effects of GLYX-13 were observed and were blocked by K252a in (c) the FST (interaction: F1,32 = 5.49, P < 0.05, effect of GLYX-13: F1,32 = 2.97, P > 0.05, effect of K252a: F1,32 = 0.0899, P > 0.05); (d) the FUST (Three-way ANOVA; effect of GLYX-13 x effect of K252a x water/urine: F1,1 = 13.19, P < 0.05, Two-way ANOVA; effect of GLYX-13 x effect of K252a: F1,32 = 12.41, P < 0.01, effect of GLYX-13: F1,32 = 4.98, P < 0.05, effect of K252a: F1,32 = 2.90, P > 0.05); and (e) the NSFT (Mantel–Cox log-rank test: Chi square = 22.16, P < 0.0001). No significant effects were seen in (f) locomotor activity (interaction: F1,32 = 0.954, P > 0.05, effect of GLYX-13: F1,32 = 1.06, P > 0.05, effect of K252a: F1,32 = 0.323, P > 0.05) or (g) home cage feeding (interaction: F1,32 = 1.11, P > 0.05, effect of GLYX-13: F1,32 = 0.647, P > 0.05, effect of K252a: F1,32 = 1.46, P > 0.05). The results are shown as mean ± S.E.M. n = 9/group. *p < 0.05 and **p < 0.01 Tukey’s, Dunn’s or Bonfferoni’s multiple comparison test, following significant results of two-way ANOVA, Kruskal-Wallis or Mantel–Cox log-rank test.
We also tested the role of BDNF-TrkB in the signaling actions of GLYX-13 in primary cortical cultured neurons. GLYX-13 increases phosphorylated levels of mTOR and ERK, and these effects were blocked by pretreatment of anti-BDNF antibody (Supplementary Fig. 4a,b). Together the results demonstrate a requirement for BDNF-TrkB receptor activation in the signaling as well as behavioral actions of GLYX-13.
Rho GTPase is Required for the Antidepressant Behavioral Actions of GLYX-13
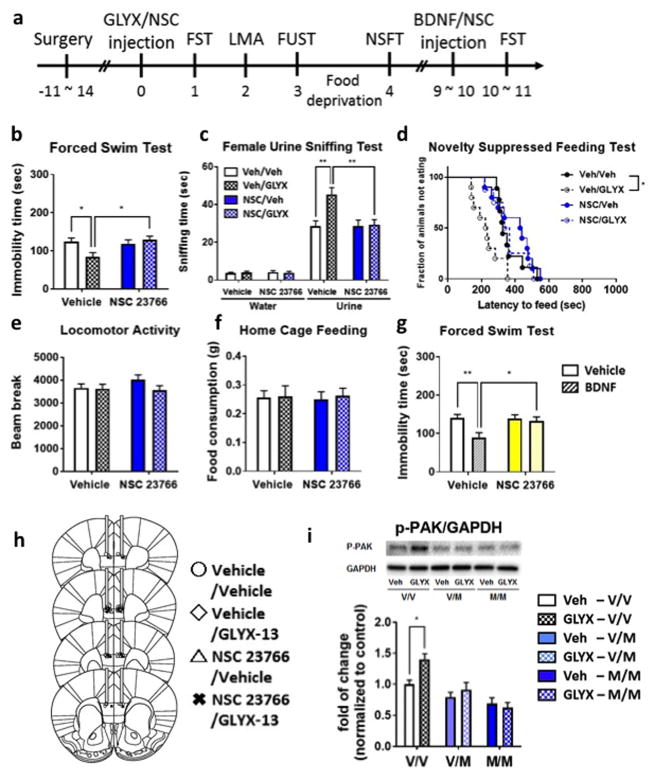
Recent evidence demonstrates that Rho GTPases Rac1 and RhoA mediate BDNF-dependent synaptic structural plasticity (22). To determine if the antidepressant effects of GLYX-13 require activation of Rho GTPase signaling, mice were infused with selective inhibitors of Rac1 (NSC 23766; 3.76 nmol/side) or ROCK (Rho-associated protein kinase), a major downstream signaling kinase of RhoA (Y-27632; 8 nmol/side, bilateral) into mPFC just after GLYX-13 (1 mg/kg, i.v.); behavioral analysis was conducted starting 24 hr later (Fig. 5a, supplementary Fig. 5a). The dose and timing of Rho GTPase inhibitor infusions are based on previous reports (31–33). In vehicle (saline) infused mice, GLYX-13 produced a significant antidepressant effect in the FST, FUST and NSFT (Fig. 5b–d, supplementary Fig. 5b–d). Moreover, the Rac1 selective inhibitor NSC 23766 significantly blocked the behavioral action of GLYX-13 in FST, FUST and NSFT (Fig. 5b–d), but alone had no effects on any behaviors tested. In contrast, infusions of Y-27632 failed to block the effects of GLYX-13 in these three tests for antidepressant activity (Supplementary Fig. 5b–d).
Figure 5. Intra-mPFC infusion of Rac1 antagonist blocks the antidepressant effects of GLYX-13 in mice.
(a) Mice were implanted with bilateral cannula in the mPFC and allowed to recover for approximately 2 weeks. NSC 23766 (3.76 nmol/side) was infused into the mPFC just after administration of vehicle or GLYX-13 (1 mg/kg, i.v.). Twenty-four hour after GLYX-13 administration behavioral studies were initiated and conducted over the next 4 days (b–f). Significant effects of GLYX-13 were observed and were blocked by NSC 23766 in (b) the FST (interaction: F1,33 = 5.91, P < 0.05, effect of GLYX-13: F1,33 = 2.09, P > 0.05, effect of NSC 23766: F1,33 = 3.62, P > 0.05); (c) the FUST (Three-way ANOVA; effect of GLYX-13 x effect of NSC 23766 x water/urine: F1,1 = 5.68, P < 0.05, Two-way ANOVA; effect of GLYX-13 x effect of NSC 23766: F1,33 = 6.12, P < 0.05, effect of GLYX-13: F1,33 = 7.49, P < 0.01, effect of NSC 23766: F1,33 = 6.18, P < 0.05); and (d) the NSFT (Mantel–Cox log-rank test: Chi square = 15.93, P < 0.01). No significant effects were seen in (e) locomotor activity (interaction: F1,33 = 1.15, P > 0.05, effect of GLYX-13: F1,33 = 1.41, P > 0.05, effect of NSC 23766: F1,33 = 0.52, P > 0.05) or (f) home cage feeding (interaction: F1,33 = 0.0182, P > 0.05, effect of GLYX-13: F1,33 = 0.00262, P > 0.05, effect of NSC 23766: F1,33 = 0.0805, P > 0.05). (g) On day 9 or 10, NSC 23766 (3.76 nmol/side) and BDNF (100 ng/side) were infused into the mPFC. Twenty-four hour after injection FST was conducted (interaction: F1,32 = 4.28, P < 0.05, effect of BDNF: F1,32 = 7.48, P < 0.05, effect of NSC 23766: F1,32 = 4.06, P > 0.05). The results are shown as mean ± S.E.M. n = 9 (Veh/Veh), 10 (Veh/GLYX), 10 (NSC/Veh), 8 (NSC/GLYX). (h) Location of cannula placements in the mPFC. (i) Phosphorylation level of p21 (RAC1) activated kinase 1 (PAK-1) in crude synaptosomal preparations of BDNF Val66Met knock-in mice was determined by western blot analysis. Levels of GAPDH were determined to control for protein levels and results are the ratio of each protein divided by GAPDH. The results are shown as mean ± S.E.M. n =6 per each group.
To directly test the requirement for Rho GTPase signaling in the antidepressant actions of BDNF, the influence of the Rac1 and ROCK inhibitors on BDNF-mediated behavior in the FST were determined (Fig. 5a, supplementary Fig. 5a). We used the same animals used for the GLYX-13 studies, and counterbalanced the groups so that half of the vehicle treated animals were included in the second vehicle infusion group, and the other half for the BDNF infusion group, and half of the GLYX-13 treated animals for vehicle infusion group, and the other half for the BDNF infusion group (Table. 1). We confirmed there was no significant difference between the immobility of the vehicle infused group and that of BDNF infused group in the first FST (supplementary Fig. 6). BDNF infusion into the mPFC resulted in significant antidepressant effects in the FST, as previously reported in hippocampus (34); moreover, infusion of the Rac1 inhibitor NSC 23766 but not Y-27632 completely blocked the antidepressant response to BDNF (Fig. 5g, supplementary Fig. 5g).
Table 1.
Counterbalance of mice used for studies of Rac1 and RhoA-ROCK in the actions of GLYX-13 and BDNF.
The left hand experimental design shows the mice (total of 37) used for studies of the Rac1 inhibitor, NSC23766 administered before GLYX-13 (first injection) and then after counterbalancing the same mice for studies of BDNF (second injection). The right hand experimental design shows the mice (total of 36) used for studies of the RhoA-ROCK inhibitor, Y-27632 administered before GLYX-13 (first injection) and then after counterbalancing the same mice for studies of BDNF (second injection).
Caption: Drug history for each animal group in NSC 23766 and Y-27632 studies.
Legend: Drug treatment for each animal in NSC 23766 (left) and Y-27632 (right) studies. Animals were injected saline or GLYX-13 via tail vein and saline, NSC 23766, or Y-27632 into mPFC on day 0. On day 9–11, the same animals were injected saline, BDNF, NSC 23766, or Y-27632 into mPFC. To avoid any possible problems resulting from prior GLYX-13 exposure, drug treatment in second injection was counterbalanced.
| Animal No. | Drug (first injection) | Drug (second injection) | Animal No. | Drug (first injection) | Drug (second injection) | ||||
|---|---|---|---|---|---|---|---|---|---|
| iv | mPFC | mPFC | mPFC | iv | mPFC | mPFC | mPFC | ||
| 1 | Saline | Saline | BDNF | Saline | 1 | Saline | Saline | Saline | Saline |
| 2 | Saline | Saline | BDNF | Saline | 2 | Saline | Saline | Saline | Saline |
| 3 | Saline | Saline | Saline | Saline | 3 | Saline | Y-27632 | BDNF | Y-27632 |
| 4 | Saline | Saline | Saline | Saline | 4 | Saline | Y-27632 | BDNF | Y-27632 |
| 5 | GLYX-13 | Saline | BDNF | Saline | 5 | GLYX-13 | Saline | BDNF | Saline |
| 6 | GLYX-13 | Saline | BDNF | Saline | 6 | GLYX-13 | Saline | Saline | Saline |
| 7 | GLYX-13 | Saline | BDNF | Saline | 7 | GLYX-13 | Y-27632 | Saline | Y-27632 |
| 8 | GLYX-13 | Saline | Saline | Saline | 8 | GLYX-13 | Y-27632 | Saline | Y-27632 |
| 9 | GLYX-13 | Saline | Saline | Saline | 9 | GLYX-13 | Y-27632 | BDNF | Y-27632 |
| 10 | Saline | NSC23766 | Saline | NSC23766 | 10 | Saline | Saline | Saline | Saline |
| 11 | Saline | NSC23766 | Saline | NSC23766 | 11 | Saline | Saline | BDNF | Saline |
| 12 | Saline | NSC23766 | BDNF | NSC23766 | 12 | GLYX-13 | Y-27632 | Dead | |
| 13 | Saline | NSC23766 | BDNF | NSC23766 | 13 | Saline | Y-27632 | Saline | Y-27632 |
| 14 | Saline | NSC23766 | BDNF | NSC23766 | 14 | GLYX-13 | Saline | BDNF | Saline |
| 15 | GLYX-13 | NSC23766 | Saline | NSC23766 | 15 | GLYX-13 | Saline | BDNF | Saline |
| 16 | GLYX-13 | NSC23766 | Saline | NSC23766 | 16 | GLYX-13 | Y-27632 | BDNF | Y-27632 |
| 17 | GLYX-13 | NSC23766 | BDNF | NSC23766 | 17 | Saline | Y-27632 | BDNF | Y-27632 |
| 18 | GLYX-13 | NSC23766 | BDNF | NSC23766 | 18 | Saline | Saline | BDNF | Saline |
| 19 | Saline | NSC23766 | BDNF | NSC23766 | 19 | Saline | Saline | Saline | Saline |
| 20 | Saline | NSC23766 | BDNF | NSC23766 | 20 | Saline | Saline | BDNF | Saline |
| 21 | Saline | NSC23766 | Saline | NSC23766 | 21 | Saline | Y-27632 | Saline | Y-27632 |
| 22 | Saline | Saline | BDNF | Saline | 22 | Saline | Y-27632 | BDNF | Y-27632 |
| 23 | Saline | Saline | BDNF | Saline | 23 | Saline | Y-27632 | Saline | Y-27632 |
| 24 | Saline | Saline | Saline | Saline | 24 | GLYX-13 | Saline | Saline | Saline |
| 25 | GLYX-13 | NSC23766 | Saline | NSC23766 | 25 | GLYX-13 | Saline | Saline | Saline |
| 26 | GLYX-13 | NSC23766 | BDNF | NSC23766 | 26 | GLYX-13 | Saline | BDNF | Saline |
| 27 | GLYX-13 | NSC23766 | Saline | NSC23766 | 27 | GLYX-13 | Y-27632 | Saline | Y-27632 |
| 28 | GLYX-13 | Saline | Saline | Saline | 28 | GLYX-13 | Y-27632 | BDNF | Y-27632 |
| 29 | GLYX-13 | Saline | Saline | Saline | 29 | GLYX-13 | Y-27632 | Saline | Y-27632 |
| 30 | GLYX-13 | Saline | BDNF | Saline | 30 | Saline | Saline | BDNF | Saline |
| 31 | Saline | NSC23766 | Saline | NSC23766 | 31 | Saline | Saline | Saline | Saline |
| 32 | Saline | NSC23766 | Saline | NSC23766 | 32 | Saline | Y-27632 | BDNF | Y-27632 |
| 33 | Saline | Saline | Saline | Saline | 33 | Saline | Y-27632 | Saline | Y-27632 |
| 34 | Saline | Saline | BDNF | Saline | 34 | GLYX-13 | Saline | Saline | Saline |
| 35 | GLYX-13 | Saline | Saline | Saline | 35 | GLYX-13 | Y-27632 | Saline | Y-27632 |
| 36 | GLYX-13 | Saline | Dead | 36 | GLYX-13 | Y-27632 | BDNF | Y-27632 | |
| 37 | GLYX-13 | NSC23766 | BDNF | NSC23766 | |||||
We also examined downstream effectors of Rac1 by measuring the phosphorylation levels of p21-activated kinase (PAK). For these studies we examined synaptosomal fractions of PFC from BDNF Val66Met knock-in mice to avoid difficulties with microdissections that would be required for analysis of samples from the inhibitor infusion studies. GLYX-13 administration increased phospho-PAK levels in WT Val/Val, but not in Val/Met or Met/Met mice (Fig. 5i). These data indicate that Rac1-PAK signaling is stimulated by GLYX-13 and that this is mediated by BDNF-TrkB signaling.
Discussion
The results demonstrate that the antidepressant actions of GLYX-13 are mediated by neuronal activation of VDCCs, activity dependent release of BDNF, and stimulation of TrkB signaling, notably new evidence for activation of Rac1 (see Supplementary Fig. 7). These findings are consistent with recent studies demonstrating that structural plasticity of spine synapse formation requires BDNF release and activation of TrkB-RhoGTPase signaling (22,35). Importantly, the results indicate that BDNF is packaged and released from postsynaptic spines and acts in an autocrine cell autonomous fashion to enhance spine maturation and number (35). This work extends recent studies demonstrating that GLYX-13 increases mTORC1 signaling in the mPFC and increases spine synapse number and function (17) (Supplementary Fig. 7). Together the results demonstrate that GLYX-13 causes activity dependent BDNF release that produces rapid and sustained synaptic and behavioral responses in rodent models of antidepressant response. It will be important in future studies to determine if the rapid actions of GLYX-13 in more valid rodent models, such as chronic unpredictable stress (36) also require activity dependent BDNF release.
Previous studies have demonstrated that the actions of ketamine are blocked by infusion of an anti-BDNF neutralizing antibody into the mPFC and in BDNF deletion mutant mice (19,21). Infusion of GLYX-13 into the mPFC is reported to produce an antidepressant response (26), demonstrating that mPFC is a critical target for the actions of GLYX-13 (37,38). Here we show that infusion of a function blocking anti-BDNF antibody into the mPFC also blocks the antidepressant behavioral actions of GLYX-13 in three different paradigms, including a model of behavioral despair (FST), motivation/reward (FUST), and anxiety (NSFT). Together these studies indicate that antidepressant actions of GLYX-13, like ketamine, require extracellular BDNF that is increased as a result of activity dependent release.
To further test this hypothesis we conducted studies using mice with a knockin of the BDNF Met allele, which blocks activity dependent processing and release of BDNF, but does not influence transcript levels (28). BDNF Met/Met mice showed anxiety like behaviors in the FUST and NSFT models, consistent with a previous report (28). Importantly, the results show that the antidepressant actions of GLYX-13 are completely blocked in Met/Met mice, and partially blocked in Val/Met mice in all three tests for antidepressant activity. These findings provide further evidence that activity dependent release of BDNF is required for the actions of GLYX-13, although it is possible that the anxiety phenotype contributes to this blockade. These results may be clinically important as the Met polymorphism is found in approximately 25 percent of Caucasians (39,40), and the antidepressant response to ketamine is significantly reduced in BDNF Met patients, most of which were heterozygous Met carriers by approximately 50 percent compared to Val/Val patients (41). The results of the current study suggest that BDNF Met carriers would also show a reduced response to GLYX-13, a possibility that will be tested in future clinical studies
AMPA receptor-stimulated, activity dependent release of BDNF in cultured neurons is reported to require activation of VDCC, and the subsequent increase in intracellular Ca2+ (29). We have reported that the antidepressant actions of ketamine are blocked by pretreatment with an AMPA receptor antagonist, or with a selective inhibitor of VDCCs (19,23). The actions of GLYX-13 are blocked by pretreatment with an AMPA receptor antagonist, indicating a requirement for neuronal activity (26). In the current study we demonstrate that the antidepressant effects of GLYX-13 are also blocked by pretreatment with the L-type VDCC antagonist verapamil. Together these studies indicate that stimulation of AMPA receptors and VDCCs are required for the antidepressant actions of GLYX-13 via the release of BDNF. This possibility was directly tested in primary cultured neurons, demonstrating that GLYX-13-stimulated release of BDNF is blocked by incubation with low concentrations of verapamil. However, given the actions of verapamil on blood pressure we cannot rule out the possibility that peripheral physiological effects could contribute to the interference with the behavioral actions of GLYX-13.
A recent study has demonstrated that structural synaptic plasticity is mediated by activity dependent release of BDNF and subsequent autocrine stimulation of TrkB signaling (35). In the current study the results show that GLYX-13, as well as ketamine increase phospho-TrkB levels in the mPFC, consistent with evidence of increased BDNF release and activity. A role for TrkB signaling is directly supported by results showing that infusion of a selective TrkB inhibitor into the mPFC blocked the antidepressant behavioral actions of GLYX-13. BDNF-TrkB stimulation regulates multiple downstream pathways, including mTORC1, which is increased by GLYX-13 (17). In the current study we also tested the role of Rho GTPases, which are critical regulators of the actin cytoskeleton and neuronal morphogenesis, including spine-synapse plasticity (42). Activation of Rho GTPases, notably Rac1 and RhoA contribute to BDNF dependent synaptic plasticity (22,35). Here we demonstrate that an inhibitor of Rac1, but not RhoA-ROCK signaling blocks the antidepressant effects for GLYX-13, as well as the effects of direct infusions of BDNF into the mPFC. The results also show that GLYX-13 increases levels of phospho-PAK, a key downstream kinase of Rac1, and this effect is blocked in BDNF Met/Met mice. The results are consistent with previous studies demonstrating a role for Rac1 in BDNF dependent synaptic plasticity (22,43), while the actions of RhoA in synapse formation have been mixed (22,43,44,45). Together, the results demonstrate that activation of Rac1 signaling is essential for antidepressant effects of GLYX-13 and BDNF by enhancing actin polymerization (22,42), and are consistent with our recent report that GLYX-13 increases spine head diameter, number, and function in mPFC pyramidal neurons (17).
Together the results demonstrate that the antidepressant actions of GLYX-13 occur via activity dependent stimulation of L-type VDCC and release of BDNF. Extracellular BDNF then acts in an autocrine fashion to activate TrkB signaling, including Rac1, as well as mTORC1 signaling as reported in our recent study (17) (Supplementary Fig. 7). These findings extend previous work, indicating that the ability of GLYX-13 to increase spine number, maturation, and function is mediated not only by activation of mTORC1 signaling and formation of synaptic proteins (i.e., PSD95, GluA1), but also by activation of Rac1 and enhancement of actin polymerization (Supplementary Fig. 7). It is possible that Rac1 is also activated by Ca2+-CaMKII signaling that occurs via stimulation of NMDA receptors (22), but blockade of Rac1-PAK1 signaling in BDNF Met mice indicates that this effect occurs primarily via BDNF-TrkB signaling (Supplementary Fig. 7). The findings presented here raise the possibility that other rapid acting antidepressants, notably ketamine, also increases spine number and function, in part via stimulation of Rac1.
The current GLYX-13 findings together with previous work on ketamine demonstrate a common signaling pathway underlying the effects of these two rapid acting antidepressant: AMPA and L-type VDCC activity dependent BDNF release and stimulation of TrkB-mTORC1 downstream signaling (17–21) (Supplementary Figure 7); whether ketamine also requires Rac1 signaling is currently being tested. These convergent actions are surprising as GLYX-13 is a glycine site partial agonist and ketamine is an NMDA channel blocker. However, the convergence of these two agents could be explained by different initial cellular targets: GLYX-13 could act directly on postsynaptic NMDA receptors on pyramidal neurons to directly increase Ca2+ signaling and ketamine could block NMDA receptors on tonic firing GABA interneurons to cause a glutamate burst. Studies are currently being conducted to test this hypothesis by cell specific knockdown of NMDA receptor subunits on GABA vs. glutamate neurons in the PFC.
Despite these convergent actions, GLYX-13 lacks the side effect profile of ketamine. This could be related to the cell specific actions of these two agents and the difference in glutamate transmission; ketamine causes a rapid, transient burst of glutamate (30 to 60 min) (46) that coincides temporally with the dissociative and psychotomimetic effects in humans and the locomotor and sensory motor gating deficits in rodents (9,26,47). Preliminary studies demonstrate that GLYX-13 does not cause an increase in extracellular glutamate in the PFC and this could account for the lack of ketamine-like side effects (48). Further studies of the initial cellular targets of GLYX-13 and ketamine as well as characterization of the signaling pathways underlying the antidepressant vs. side effects are needed to address these questions.
Supplementary Material
Acknowledgments
This study was supported by NIMH grants MH045481 (R.S.D.), MH093897 (R.S.D.), the state of Connecticut, Sumitomo Dainippon Pharma (T.K.), and a research grant from Allergan. R.S. Duman has consulted and/or received research support from Naurex, Allergan, Lilly, Forest, Johnson & Johnson, Taisho, Sunovion and Navitor,
Footnotes
Conflict of Interest
The authors have declared there is no conflict of interest to disclose.
References
- 1.Kessler RC, Chiu WC, Demler O, Walters F. Prevalence, severity and co-morbidity of 12 month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;15:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Altamura CA, Mauri MC, Ferrara A, Moro AR, D’Andrea G, Zamberlan F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry. 1993;150:1731–1733. doi: 10.1176/ajp.150.11.1731. [DOI] [PubMed] [Google Scholar]
- 6.Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, et al. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. 1998;37:124–129. doi: 10.1159/000026491. [DOI] [PubMed] [Google Scholar]
- 7.Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR, Jr, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry 2006. 2006;30:1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 8.Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 10.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner D, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 11.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 13.Moskal JR, Kuo AG, Weiss C, Wood PL, O’Connor Hanson A, Kelso S, et al. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005;49:1077–1087. doi: 10.1016/j.neuropharm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XL, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008;55:1238–1250. doi: 10.1016/j.neuropharm.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM GLYX-13 Clinical Study Group. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21:140–149. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- 16.Burgdorf J, Zhang XL, Weiss C, Gross A, Boikess SR, Kroes RA, et al. The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience. 2015;12:202–211. doi: 10.1016/j.neuroscience.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, et al. GLYX-13 Produces Rapid Antidepressant Responses with Key Synaptic and Behavioral Effects Distinct from Ketamine. Neuropsychopharmacology. 2016;42:1231–1242. doi: 10.1038/npp.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014:18. doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242–252. doi: 10.1016/j.neuropharm.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;15:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedrick NG, Harward SC, Hall CE, Murakoshi H, McNamara JO, Yasuda R. Rho GTPase complementation underlies BDNF-dependent homo- and heterosynaptic plasticity. Nature. 2016;538:104–108. doi: 10.1038/nature19784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014;20:531–535. doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutheil S, Ota KT, Wohleb ES, Rasmussen K, Duman RS. High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacology. 2016;41:1874–1887. doi: 10.1038/npp.2015.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandhya VK, Raju R, Verma R, Advani J, Sharma R, Radhakrishnan A, et al. A network map of BDNF/TRKB and BDNF/p75NTR signaling system. J Cell Commun Signal. 2013;7:301–307. doi: 10.1007/s12079-013-0200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29:8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd EL, Abernethy DR. Physiological pharmacokinetics and pharmacodynamics of (+/−)-verapamil in female rats. Biopharm Drug Dispos. 1987;8:285–297. doi: 10.1002/bdd.2510080309. [DOI] [PubMed] [Google Scholar]
- 31.Gao Q, Yao W, Wang J, Yang T, Liu C, Tao Y, et al. Post-training activation of Rac1 in the basolateral amygdala is required for the formation of both short-term and long-term auditory fear memory. Front Mol Neurosci. 2015;8:65. doi: 10.3389/fnmol.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narita M, Takagi M, Aoki K, Kuzumaki N, Suzuki T. Implication of Rho-associated kinase in the elevation of extracellular dopamine levels and its related behaviors induced by methamphetamine in rats. J Neurochem. 2003;86:273–282. doi: 10.1046/j.1471-4159.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 33.Sananbenesi F, Fischer A, Wang X, Schrick C, Neve R, Radulovic J, et al. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci. 2007;10:1012–1019. doi: 10.1038/nn1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan E, et al. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016;538:99–103. doi: 10.1038/nature19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci U S A. 2015;112:8106–8111. doi: 10.1073/pnas.1414728112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarria A, Wohleb ES, Voleti B, Ota KT, Dutheil S, Lepack AE, et al. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis. 2015;82:254–261. doi: 10.1016/j.nbd.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 40.Hosang GM, Shiles C, Tansey KE, McGuffin P, Uher R. Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. BMC Med. 2014;12:7. doi: 10.1186/1741-7015-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72:e27–28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 43.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- 45.Kang MG, Guo Y, Huganir RL. AMPA receptor and GEF-H1/Lfc complex regulates dendritic spine development through RhoA signaling cascade. Proc Natl Acad Sci U S A. 2009;106:3549–3554. doi: 10.1073/pnas.0812861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banerjee P, Donello J, Yoshitake T, Kehr J. Rapastinel (Glyx-13), a Rapid Acting Antidepressant, Does not Increase Extracellular Levels of Dopamine and Glutamate in Rat Medial Prefrontal Cortex. American College of Neuropsychopharmacology, 55th annual meeting.2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.