Abstract
Background & aims
Indirect comparisons suggest that alternate-day fasting (ADF) may produce greater improvements in body composition, fat distribution, and/or the adipokine profile compared to daily calorie restriction (CR), but this has not been tested directly. In a pre-planned secondary analysis of a randomized controlled trial, we compared changes in the VAT:SAT ratio, FFM:total mass ratio, and the adipokine profile between ADF and CR.
Methods
Overweight and obese participants (n = 100) were randomized to 1) ADF (alternating every 24-h between consuming 25% or 125% of energy needs); 2) CR (consuming 75% of needs every day); or 3) control (consuming 100% of needs every day) for 24 wk.
Results
The VAT:SAT ratio did not change in any group. The FFM:total mass ratio increased in both ADF (0.03 ± 0.00) and CR (0.03 ± 0.01) compared to the control group (P < 0.01), with no differences between the intervention groups. Circulating leptin decreased in both the ADF group (−18 ± 6%) and CR group (−31 ± 10%) relative to the control group (P < 0.05), with no differences between the intervention groups. Circulating levels of adiponectin, resistin, IL-6, and TNF-α did not change in either intervention group relative to the control group.
Conclusion
ADF and CR similarly improve the FFM:total mass ratio and reduce leptin after a 24-wk intervention.
Keywords: calorie restriction, alternate day fasting, body composition, visceral adipose tissue, adipokine, obese adult
INTRODUCTION
Most people who attempt to lose weight by reducing energy intake (EI) choose an approach called ‘daily calorie restriction’ (CR), which reduces EI by a small amount (e.g. 25%) each day. However, there has been recent interest in an alternative approach called ‘alternate-day fasting’ (ADF) (1). ADF repeatedly alternates between a 24-h period of intense (e.g. 75%) energy restriction (ER), and a 24-h period of no restriction or slight energy surplus. We recently reported that ADF and CR reduced body weight similarly after 24 wk of intervention (2). What has yet to be determined, however, is whether ADF or CR produce greater improvements in body composition, fat distribution, and/or the adipokine profile.
To date, ADF and CR have only been compared indirectly regarding their effects on body composition and fat distribution: systematic reviews have compared trials that included an ADF arm but not a CR arm against trials that included a CR arm but not an ADF arm (3,4). These comparisons, along with considerations of mechanistic evidence, suggest that ADF may preferentially reduce visceral adipose tissue (VAT) and preserve fat-free mass (FFM) compared to CR (3–7). In a systematic review performed by our research team, among moderate-duration (13–30 wk) interventions, ADF and similar diets (i.e. other diets that incorporate an intermittent fasting schedule) produced similar weight loss but greater waist circumference reduction compared to CR diets (3). This may have been due to the intense ER that occurs every other day with ADF, which could trigger a lipolytic response that is especially potent in visceral adipocytes (5). Indeed, a meta-analysis of weight loss trials found that the percent change in VAT versus subcutaneous adipose tissue (SAT) correlated positively with the degree of ER independently of weight loss during the first 12 wk of treatment (6). Another systematic review performed by our research team found that FFM accounted for ~25% of the weight that was lost with CR, but only ~10% of the weight that was lost with ADF and similar diets (4). Also, in diet-induced obese mice, ADF increased FFM in relation to both CR and an ad libitum control diet (7).
There is also evidence to suggest that ADF could modulate circulating adipokines to a greater extent compared to CR (3,8). The adipokine profile is known to change in response to weight loss, but it can also change in response to ER per se (8). For example, circulating concentrations of adipocyte-produced adipokines (other than adiponectin) tend to decrease in response to ER independently of weight loss, while circulating concentrations of stroma-vascular-fraction-produced adipokines tend to increase or not change (8). ADF and CR could therefore differentially affect the adipokines that are especially responsive to ER per se. Furthermore, if ADF were to preferentially reduce VAT compared to CR (3), this would be expected to have a concomitant effect on the adipokine profile as well.
In this preplanned secondary analysis, we compared changes in VAT:SAT ratio, FFM:total mass ratio, and the adipokine profile of overweight and obese men and women randomly assigned to an ADF group, a CR group, or a non-intervention control group for 24 wk. We hypothesized that the ADF group would experience a greater reduction in the VAT:SAT ratio, a greater increase in the FFM:total mass ratio, and a greater adipokine response compared to the CR group.
METHODS AND MATERIALS
Participants
Study methods were already published in the main paper (2). Briefly, overweight and obese [25 ≤ body mass index (BMI) < 40] men and women aged 18–65 y were recruited with the use of flyers. Participants were excluded if they smoked, self-reported to exercising more than 3 h/wk, were diabetic, had a history of cardiovascular disease, were pregnant or had an irregular menstrual cycle, had excessive body weight fluctuation (> 4 kg) immediately prior to trial enrollment, or were contraindicated to magnetic resonance imaging (for measurement of VAT). Individuals were also excluded if they took medications known to affect metabolism or blood lipids. The study protocol was approved by the Office for the Protection of Research Subjects at the University of Illinois, Chicago. Written consent was provided by all participants in the trial.
Study design
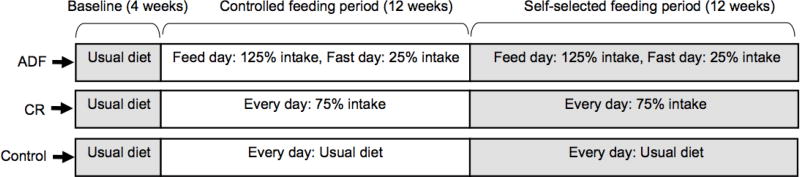
As reported in the main paper (2), the trial consisted of a 4-wk baseline run-in period, followed by a 24-wk weight loss intervention period, followed by a 24-wk weight maintenance intervention period. However, the secondary analyses reported in this paper pertain only to the baseline period and weight loss intervention period, in order to avoid the confounding effects of weight regain on outcomes of interest. After completing the baseline period, participants were randomized (stratified by age, BMI, and sex) to an ADF group (n = 34), a CR group (n = 35), or a non-intervention control group (n = 31) (Figure 1). Participants in the intervention groups received a controlled diet for the first 12 wk of the intervention. They then self-selected their meals based on their diet group assignment for the final 12 wk of the intervention. Participants in the control group consumed their usual diet throughout the entire trial and visited the research center at the same frequency as the intervention groups.
Figure 1.
Study design. Participants maintained their usual diets during a 4-wk baseline run-in period to ensure body weight stability. They were then stratified by age, sex; and BMI; and randomly assigned to an ADF group, a CR group, or a non-intervention control group. Participants in the intervention groups received a controlled diet for the first 12 wk of intervention. They then self-selected their diets based on their individual daily calorie goals for the final 12 wk of intervention. Participants in the control group consumed their usual diet throughout the entire trial.
Baseline
The baseline period lasted 4 wk in order to stabilize participants’ body weights, and to determine individual energy requirements. The energy deficit required during the intervention was calculated from total energy expenditure (TEE) assessed during a 14-d period by doubly-labeled water as previously described (2). This procedure was repeated at wk 24 as part of a calculation of achieved ER (described below).
Controlled feeding period (Week 0–12)
Participants in the intervention groups received a diet based on the guidelines from the American Heart Association (9), with approximately 30% of energy deriving from fat, 55% of energy deriving from carbohydrate, and 15% of energy deriving from protein (Table 1). All meals were provided as a 3-d rotating menu for the first 12 wk of intervention. The energy content of the meals was formulated to produce an average 25% ER over a 48-h period. Participants in the CR group consumed 75% of energy needs every day, while participants in the ADF group repeatedly alternated between consuming 25% of energy needs over 24-h (‘fast day’) and consuming 125% of energy needs over 24-h (‘feast day’). Fast day meals were consumed between times 1200 h and 1400 h in order to standardize the duration of complete food abstention among participants in the ADF group. The intervention groups collected their meals at the beginning of each week in rolling insulated coolers, and all meals were consumed outside of the research center. Participants were permitted to consume calorie-free beverages such as black coffee, black tea, and diet sodas during the controlled feeding period. Additionally, participants were encouraged to consume plenty of water throughout the trial.
Table 1.
Nutrient composition of the provided diets during the controlled feeding period
| ADF | CR | ||
|---|---|---|---|
| Nutrientsa,b | Fast day | Feed day | Every day |
| Energy (kcal) | 500 | 2500 | 1500 |
| Fat (g) | 14 (25%)c | 72 (26%)c | 42 (25%)c |
| Saturated fat (g) | 5 | 39 | 22 |
| Monounsaturated fat (g) | 5 | 18 | 11 |
| Polyunsaturated fat (g) | 4 | 15 | 9 |
| Trans fat (g) | 0 | 0 | 0 |
| Cholesterol (mg) | 32 | 163 | 96 |
| Protein (g) | 20 (16%)c | 88 (14%)c | 53 (14%)c |
| Carbohydrate (g) | 74 (59%)c | 375 (60%)c | 229 (61%)c |
| Fiber (g) | 11 | 31 | 22 |
Values are for an example individual with a daily total energy expenditure of 2000 kcal. Each participant received an individualized nutrient prescription based on his or her total energy expenditure.
No differences between groups for any nutrient when diets are matched for total energy over 48 h.
Percent of total energy.
Self-selected feeding period (Week 12–24)
For the final 12 wk of intervention, participants received weekly one-on-one dietary counseling sessions to learn how to maintain the ADF- or CR regimen on their own at home. The EI prescriptions for this period were the same as during the controlled feeding period, but no food was provided. Individualized meal plans were developed for each intervention-group participant. These plans included menus, food lists, and portion sizes that were consistent with the participant’s food preferences and target energy levels. Participants were also taught how to make general healthy food choices by choosing low-fat meat and dairy options, as well as increasing fruit and vegetable intake.
Energy restriction and physical activity
Data for energy intake (EI), ER, and physical activity were reported previously (2), and are reported here again to serve as process evaluations. EI was estimated using doubly-labeled water. At baseline, this was assumed to be equal to TEE, due to the stable body weight of the participants. EI during the course of the intervention was calculated based on TEE at wk 24 after correction for changes in body energy stores [for weight loss, 1 g of fat mass (FM) = 9.3 kcal, and 1 g of fat-free mass (FFM) = 1.1 kcal] (10). ER was calculated by comparing actual EI during the intervention period to prescribed EI:
All participants were instructed to not change their baseline physical activity habits throughout the trial. Physical activity was assessed for 7 consecutive days at baseline and wk 24 with a validated (11) multi-sensor activity monitor (SenseWear Armband Mini; BodyMedia Inc) as described previously (2).
Body composition and fat distribution
Body weight was measured weekly after an overnight fast while participants wore underwear and a hospital gown. FM and FFM were assessed at baseline and wk 24 by dual energy X-ray absorptiometry (DEXA) (QDR 4500W, Hologic Inc. Arlington, MA). SAT and VAT were assessed at baseline and wk 24 by MRI as described previously (2).
Glucose, insulin, adipokines, and IGF-1
Blood samples were taken at baseline, wk 12, and wk 24 following an overnight fast. In the ADF group, blood samples were collected on feast days to provide within-group consistency. Participants refrained from physical exercise for 48 h prior to sample collection. Blood was centrifuged for 10 min at 1000 g and 4°C to separate plasma from red blood cells, and was stored at −80°C until analyzed. Glucose (intra-assay coefficient of variation: 1.5%) was quantified using the glucose oxidase procedure (Beckman Autoanalyser II; Beckman Coulter Inc.; Fullerton, CA). Insulin (intra-assay coefficient of variation: 3.1%) was quantified using an electrochemiluminescence assay. Insulin resistance (IR) was calculated using the Homeostasis Model Assessment (HOMA) method (12) by applying the following formula: HOMA-IR = insulin (µIU/mL) × glucose (mg/dL)/405. Participants with HOMA-IR values > 2.73 were considered to be ‘insulin resistant’ (13). Adiponectin (intra-assay coefficient of variation: 3.4%), leptin (3.0%), resistin (5.3%), IGF-1 (4.3%), IL-6 (1.6%), and TNF-α (4.2%) were quantified using high-sensitivity ELISA kits (R&D Systems, Minneapolis, MN).
Statistical analysis
Analyses were performed only on the participants who completed the weight loss intervention period. Data are presented as mean ± SEM. Significance for all statistical tests was accepted at P < 0.05. SPSS (v. 20; SPSS Inc, Chicago, IL) was used for analysis. Normality was assessed by the Kolmogorov-Smirnov test, and no variables were observed to meet the criteria for non-normality. Baseline differences among the ADF, CR, and control groups were assessed by a one-way ANOVA. Post hoc comparisons among groups were performed by Tukey’s tests as appropriate. A repeated-measures ANOVA assessed variables measured at baseline, 12 wk, and 24 wk. Post hoc sensitivity analyses were also performed. Diet soda has been shown to affect metabolism and related hormones (14,15), so all analyses were re-run, with participants’ data being excluded if they self-reported to consuming ≥ 1 serving/d (240 mL/d). Also, there is evidence that insulin resistance modulates the metabolic response to ADF, and at the same time is benefitted by such an intervention (16). Therefore, linear mixed effects models examined whether pre-intervention insulin resistance (HOMA-IR) modified the effects of the diets on circulating adipokine concentrations by testing a 3-way interaction (group × time × insulin resistance). In each model, a participant-specific random effect was included to account for correlation with participant over time. HOMA-IR was analyzed continuously in this model.
RESULTS
Participant dropouts and baseline characteristics
After 24 wk of treatment, n = 9 dropped out of the ADF group, n = 6 dropped out of the CR group, and n = 6 dropped out of the control group. The number of completers in each group was as follows: ADF (n = 25), CR (n = 29), and control (n = 25). Insulin resistance (HOMA-IR > 2.73) at baseline was observed in 52%, 41%, and 40% of participants in the ADF, CR, and control groups, respectively. Participant baseline characteristics did not differ significantly among groups (Table 2); however, VAT was different between the intervention groups (ADF: 1.9 ± 0.2 kg; CR: 2.3 ± 0.2 kg). An independent-samples T test was performed to compare the two groups directly, and the difference was not significant (P = 0.17).
Table 2.
Baseline characteristics of study completers
| Characteristics | ADF | CR | Control | P value |
|---|---|---|---|---|
| n | 25 | 29 | 25 | |
| Age (y) | 46 ± 2 | 44 ± 2 | 44 ± 2 | 0.87 |
| Sex (F/M) | 22/3 | 23/6 | 21/4 | |
| Body weight (kg) | 94 ± 3 | 100 ± 3 | 91 ± 3 | 0.12 |
| Height (cm) | 166 ± 2 | 168 ± 2 | 164 ± 2 | 0.34 |
| BMI (kg/m2) | 34 ± 1 | 35 ± 1 | 34 ± 1 | 0.31 |
| FM (kg) | 38 ± 1 | 40 ± 1 | 35 ± 2 | 0.11 |
| FFM (kg) | 54 ± 2 | 58 ± 2 | 54 ± 2 | 0.38 |
| VAT (kg) | 1.9 ± 0.2 | 2.3 ± 0.2 | 2.0 ± 0.3 | 0.43 |
| SAT (kg) | 8.4 ± 0.4 | 8.7 ± 0.4 | 8.0 ± 0.7 | 0.56 |
| Glucose (mg/mL) | 91.4 ± 2.4 | 91.7 ± 2.6 | 88.6 ± 1.8 | 0.61 |
| Insulin (µIU/mL) | 17.6 ± 3.0 | 19.1 ± 3.3 | 15.9 ± 2.2 | 0.76 |
| HOMA-IR | 4.20 ± 0.81 | 4.43 ± 0.79 | 3.48 ± 0.49 | 0.66 |
| Adiponectin (ng/mL) | 4321 ± 589 | 4051 ± 373 | 3521 ± 451 | 0.53 |
| Leptin (ng/mL) | 66.2 ± 6.0 | 73.1 ± 10.2 | 53.3 ± 6.1 | 0.17 |
| Resistin (ng/mL) | 13.5 ± 1.9 | 13.7 ± 0.9 | 12.2 ± 1.2 | 0.88 |
| TNF-α (pg/mL) | 1.49 ± 0.15 | 1.83 ± 0.19 | 1.81 ± 0.19 | 0.18 |
| IGF-1 (ng/mL) | 84.1 ± 4.5 | 102.3 ± 7.6 | 101.5 ± 11.7 | 0.16 |
| IL-6 (pg/mL) | 2.15 ± 0.31 | 2.12 ± 0.21 | 2.10 ± 0.25 | 0.41 |
Abbreviations: F, female; M, male. Data are presented as mean ± SEM. P values among groups at baseline are provided from one-way ANOVA. There were no differences among groups for any parameter.
Energy restriction, physical activity, and body weight
EI and ER data were available for n = 16 participants in the ADF group, n = 21 participants in the CR group, and n = 11 participants in the control group. For these participants, the three groups had similar EI (ADF: 2663 ± 121 kcal/d; CR: 2931 ± 143 kcal/d; control: 2698 ± 183 kcal/d) at baseline. Average percent ER during the intervention was similar between the intervention groups (ADF: 21 ± 4%; CR: 24 ± 4%, P = 0.65), and greater than in the control group (P = 0.001). The three groups had similar physical activity at baseline (ADF: 7422 ± 699 steps/d; CR: 6895 ± 563 steps/d; control: 7363 ± 833 steps/d). At end of study, there was a substantial numerical increase in average physical activity in the CR group (1696 ± 972 steps/d), but not the ADF group (489 ± 642 steps/d) or control group (−415 ± 555 steps/d). These changes did not reach statistical significance within- or between groups as a result of large inter-individual variability. There was a diet-by-time interaction (P < 0.01) for weight loss, with post-hoc testing revealing that the ADF group and CR group lost more weight (ADF: −7.3 ± 0.9%; CR: −7.7 ± 1.0%) than the control group but a similar amount of weight as each other (Table 3).
Table 3.
Effects of Alternate Day Fasting or Daily Calorie Restriction on Glucose, Insulin, Adipokines, and IGF-1
| Alternate day fasting | Daily calorie restriction | Control | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Baseline | Wk 12 | Wk 24 | Baseline | Wk 12 | Wk 24 | Baseline | Wk 12 | Wk 24 | Diet | Time | Diet × time |
|
| Glucose (mg/mL) | 91.4 ± 2.4 | 90.5 ± 2.0 | 91.4 ± 1.5 | 91.7 ± 2.6 | 89.4 ± 1.8 | 96.9 ± 2.5 | 88.6 ± 1.8 | 88.4 ± 1.7 | 93.8 ± 2.4 | 0.63 | < 0.01a,b | 0.14 |
| Insulin (µIU/mL) | 17.6 ± 3.0 | 12.7 ± 1.6 | 10.2 ± 1.2 | 19.1 ± 3.3 | 11.9 ± 1.2 | 14.7 ± 2.1 | 15.9 ± 2.2 | 15.2 ± 1.9 | 16.5 ± 2.7 | 0.64 | < 0.05 | < 0.05c |
| HOMA-IR | 4.20 ± 0.81 | 2.81 ± 0.33 | 2.32 ± 0.29 | 4.43 ± 0.79 | 2.60 ± 0.26 | 3.64 ± 0.58 | 3.48 ± 0.49 | 3.32 ± 0.43 | 3.98 ± 0.76 | 0.70 | < 0.05 | < 0.05d |
| Adiponectin (ng/mL) | 4321 ± 589 | 4567 ± 630 | 5285 ± 620 | 4051 ± 373 | 4575 ± 380 | 4730 ± 376 | 3684 ± 600 | 3521 ± 541 | 4228 ± 612 | 0.41 | < 0.05a,e | 0.66 |
| Leptin (ng/mL) | 66.2 ± 6.0 | 58.7 ± 8.5 | 54.6 ± 6.2 | 73.1 ± 10.2 | 45.8 ± 6.0 | 50.1 ± 7.0 | 53.3 ± 6.1 | 50.5 ± 7.1 | 63.0 ± 8.5 | 0.89 | < 0.01 | < 0.01c |
| Resistin (ng/mL) | 13.5 ± 1.9 | 9.1 ± 1.0 | 10.6 ± 0.9 | 13.7 ± 0.9 | 10.5 ± 1.0 | 11.8 ± 1.0 | 12.2 ± 1.2 | 10.7 ± 0.8 | 11.2 ± 1.0 | 0.77 | < 0.001f | 0.37 |
| TNF-α (pg/mL) | 1.49 ± 0.15 | 1.68 ± 0.22 | 1.56 ± 0.11 | 1.83 ± 0.19 | 1.99 ± 0.21 | 1.96 ± 0.21 | 1.81 ± 0.19 | 1.51 ± 0.14 | 1.54 ± 0.24 | 0.27 | 0.91 | 0.08 |
| IGF-1 (ng/mL) | 84.1 ± 4.5 | 86.3 ± 4.9 | 87.3 ± 4.9 | 102.3 ± 7.6 | 107.3 ± 6.2 | 113.4 ± 10.4 | 101.5 ± 11.7 | 95.0 ± 10.0 | 87.5 ± 8.4 | 0.07 | 0.99 | 0.22 |
| IL-6 (pg/mL) | 2.15 ± 0.31 | 2.75 ± 0.35 | 2.30 ± 0.29 | 2.12 ± 0.21 | 2.59 ± 0.30 | 2.48 ± 0.44 | 2.10 ± 0.25 | 2.31 ± 0.23 | 2.87 ± 0.37 | 0.99 | < 0.05e | 0.45 |
Data are presented as mean ± SEM. P values are provided from repeated-measures ANOVA.
Value at wk 24 greater than value at baseline.
Value at wk 24 greater than value at wk 12.
The ADF group and CR group experienced greater reductions over time compared to the control group, but similar reductions compared to each other.
The ADF group experienced a greater reduction over time compared to both the CR group and the control group.
Value at wk 12 greater than value at baseline.
Value at wk 24 lower than value at baseline, but greater than value at wk 12. Value at wk 12 also lower than value at baseline.
Body composition and fat distribution
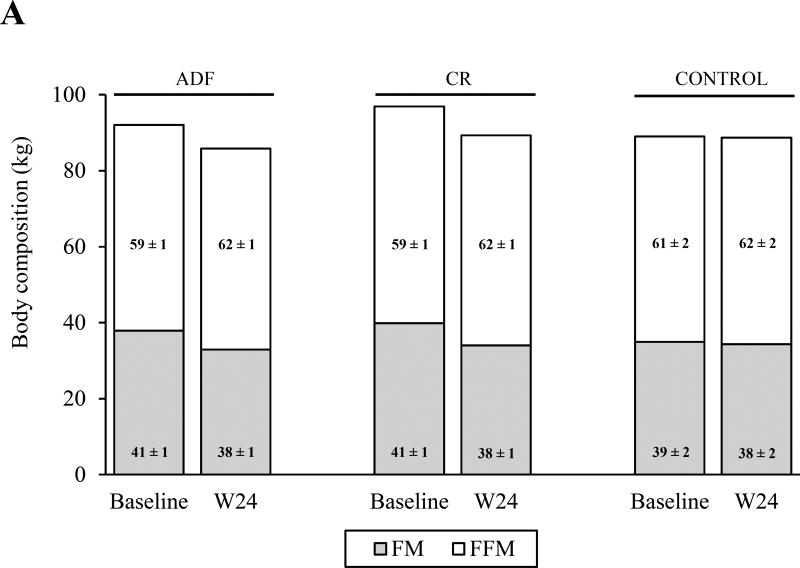
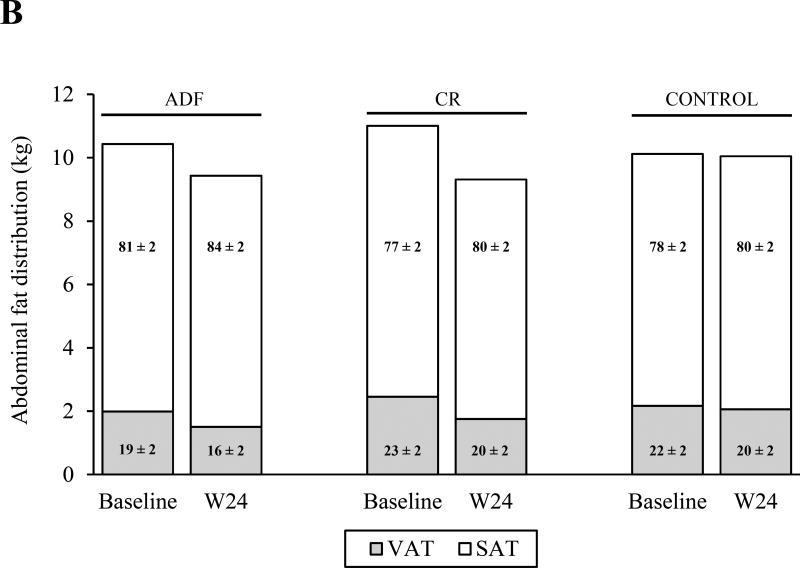
The intervention groups lost more VAT (ADF: −24 ± 4%; CR: −29 ± 5%) and FM (ADF: −10 ± 2%; CR: −15 ± 3%) compared to the control group (P < 0.01 for all comparisons), with no differences between the intervention groups (Figure 2). The CR group also lost more SAT (−12 ± 3%) than the control group (P < 0.05), but not more than the ADF group. VAT was different between the intervention groups at end of study (ADF: 1.4 ± 0.2 kg; CR: 1.6 ± 0.2 kg), so an independent-samples T test was performed to compare the two groups directly. This analysis showed no difference between groups. The VAT:SAT ratio did not change in any group. FFM decreased in both intervention groups (ADF: −1.2 ± 0.4 kg; CR: −1.8 ± 0.8 kg), and increased in the control group (0.3 ± 0.4 kg), but these changes were not significantly different among groups. The FFM:total mass ratio increased in both ADF (0.03 ± 0.00) and CR (0.03 ± 0.01) compared to the control group (P < 0.01), with no differences between the intervention groups.
Figure 2.
Changes in body composition and abdominal fat distribution. The figure shows the change in body composition (measured by DEXA; A) and abdominal fat distribution (measured by MRI; B) from baseline to week 24 (W24). Absolute values are depicted on the y-axis; and percentage values are depicted within the columns, which represent the relative amounts of (A) fat mass (FM) and fat-free mass (FFM) to total mass, and the relative amounts of (B) visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) to total abdominal mass. A, FM decreased in both intervention groups relative to the control group (P < 0.01 for all comparisons) with no differences between the intervention groups. FFM remained unchanged in all groups. The FFM:total mass ratio increased in both intervention groups compared to the control group (P < 0.01), with no differences between the intervention groups B, VAT decreased in both intervention groups relative to the control group (P < 0.05) with no difference between the intervention groups. SAT decreased in the calorie restriction (CR) group relative to the control group (P < 0.05) but did not change in the alternate day fasting (ADF) group relative to the other two groups. The VAT:SAT ratio did not change among the groups. Data are presented as mean ± SEM.
Glucose, insulin, adipokines, and IGF-1
A time effect was found for glucose (P < 0.01), with post-hoc testing revealing an increase from wk 12 to wk 24 by 5 ± 1%, and from baseline to wk 24 by 4 ± 2% (Table 3). There was a diet-by-time interaction (P < 0.05) for insulin, with post-hoc testing revealing that the ADF group and CR group experienced greater reductions (ADF: −42 ± 12%; CR: −23 ± 10%) than the control group, but similar reductions compared to each other. There was also a diet-by-time interaction (P < 0.05) for HOMA-IR, with post-hoc testing revealing that the ADF group experienced a greater reduction (−45 ± 13%) than the CR group (−18 ± 11%); and also compared to the control group, which experienced an increase (14 ± 17%).
There was a diet-by-time interaction (P < 0.01) for leptin, with post-hoc testing revealing that the ADF group and CR group experienced greater reductions (ADF: −18 ± 6%; CR: −31 ± 10%) than the control group, but similar reductions compared to each other (Table 3). A time effect was found for adiponectin (P < 0.05), with post-hoc testing revealing an increase from baseline to wk 24 by 18 ± 12%, and from wk 12 to wk 24 by 12 ± 11%. A time effect was also found for IL-6 (P < 0.05), with post-hoc testing revealing an increase from baseline to wk 12 by 21 ± 12%. A time effect was also found for resistin (P < 0.001), with post-hoc testing revealing a decrease from baseline to wk 12 by −24 ± 5%, an increase from wk 12 to wk 24 by 11 ± 4%, and a decrease from baseline to wk 24 by −15 ± 8%. No other effects were statistically significant.
Sensitivity analyses
One participant in the CR group reported consuming ≥ 240 mL/d of diet soda, and no participants in the ADF group or control group reported consuming this amount. In a sensitivity analysis, data from this CR participant were excluded, and the results were not materially affected (data not shown).
Pre-intervention insulin resistance did not modify the effects of the diets for any adipokine or IGF-1; none of the 3-way interactions were significant (data not shown).
DISCUSSION
There is considerable interest in identifying weight-loss therapies that preferentially reduce VAT, preserve FFM, or improve the adipokine profile. In this study, contrary to our hypotheses, ADF and CR similarly increased the FFM:total mass ratio and decreased circulating leptin, without affecting the VAT:SAT ratio or other measured adipokines.
Previous indirect comparisons between ADF and CR – systematic reviews have compared trials that included an ADF arm but not a CR arm against trials that included a CR arm but not an ADF arm – suggested that ADF may preferentially reduce VAT and preserve FFM (3,4). When directly compared in the present study, however, ADF and CR were found to similarly affect both the VAT:SAT ratio and the FFM:total mass ratio. The indirect comparisons involved trials that differed among each other with respect to participants’ age, sex, ethnicity, and BMI (3,4). In addition, these trials used different feeding protocols compared to each other, as some trials provided all meals to participants, and others offered only brief dietary counseling at the start of the trial. It is possible that these confounders affected the results of the systematic reviews (3,4).
It has been reported previously that certain adipokines respond to ER independently of weight loss (8). We therefore hypothesized that ADF would produce a greater response than CR on the adipokine profile, as a result of the intense ER that occurs on fast days. However, ADF and CR similarly reduced leptin and did not affect the other adipokines. Leptin is commonly found to be reduced in response to diet-induced weight loss (17). In contrast, whether adiponectin changes during dietary intervention appears to have little relation to the amount of body weight that is lost (17). Interestingly, circulating adiponectin level appears to be more responsive to diet-induced weight loss in men (17), which may explain why this parameter did not change in our mostly female cohort. Resistin has been shown to either decrease (18) or not respond to ADF (19), and similarly inconsistent findings have been observed in response to CR (20,21). TNF-α has been previously reported to decrease in response to ADF (22), but not CR (23), despite similar weight loss between the studies. The reason for these discrepant results is unclear. As for IGF-1, participants in the ADF and CR groups likely did not reduce protein intake by a sufficient amount to modulate this hormone (24).
Fasting insulin decreased more in the ADF group (−42%) than in the CR group (−23%), although the difference was not statistically significant; and HOMA-IR decreased more in the ADF group (−45%) than in the CR group (−18%), and this difference was statistically significant. These differences occurred despite similar changes in body weight, body composition, and fat distribution between the intervention groups. HOMA-IR was also found to decrease more in response to a 5:2 diet (a diet similar to ADF, with a repeating pattern of 5 consecutive feast days followed by 2 consecutive fast days) compared to a CR diet matched for prescribed energy restriction (25). The findings for insulin and HOMA-IR in the present study should be interpreted cautiously, for at least two reasons. First, these findings are from a secondary analysis; and in the primary analysis, which included all participants (including non-completers), insulin and HOMA-IR both changed similarly between the ADF and CR groups (2). Therefore, while it is possible that following an ADF diet may reduce insulin and HOMA-IR more favorably than following a CR diet (assuming that completing 24 wk of study and losing ~7% body weight is a suitable proxy for dietary adherence), prescribing an ADF diet does not appear to reduce these parameters more favorably than prescribing a CR diet. Second, blood was drawn on the day after a fast day in the ADF group for the analyses performed in this study, which increased the duration of complete food abstention prior to blood draw in this group relative to the CR group (and control group). The concentrations of some of our measured parameters may have been affected by the longer duration of complete food abstention (26). In the aforementioned examination of a 5:2 diet, HOMA-IR decreased considerably after 2 consecutive fast days, but then normalized 2 days later (25).
An earlier study by our research group found that adiponectin increased after 8 wk of ADF in the most insulin-sensitive (assessed with HOMA-IR) tertile of participants, but did not change in the other 2 tertiles (16). In the present study, insulin sensitivity did not modify the effects of ADF or CR for any adipokine or IGF-1. Although slightly less than half of the participants in the present study could be regarded as ‘insulin resistant’ based on HOMA-IR (13), many of these participants would still meet most definitions of ‘metabolically healthy obesity’ (27). Thus, it is possible that the insulin sensitivity contrasts in the present study were not large enough to modulate the diets’ effects on adipokines or IGF-1; it would be interesting for future studies with larger contrasts to examine this.
It was surprising that IL-6 increased from baseline to wk 12, as many dietary weight loss trials report a decrease over time with such intervention (28). Given that most of our participants were premenopausal women, our results for IL-6 may have been influenced by the timing of the menstrual cycle: some studies have found that circulating IL-6 levels are lower during the follicular phase and/or during an elevation in circulating estradiol (29–31), although other studies have found contradictory results (32–36). The participants in the present study were not asked to record the timing of their menstrual cycle which is a limitation of our work. It remains unknown as to why IL-6 increased in the control group of our trial from baseline to wk 12, and again from wk 12 to wk 24.
It was also surprising that CR participants substantially increased their physical activity at the end of study despite being instructed and reminded not to do so. It is possible that the physical activity measurements were less accurate at end of study compared to baseline, due to participant fatigue with the various demands of the trial. In addition, it cannot be ruled out that a seasonal effect led to greater physical activity in the CR group at end of study, although it is unclear as to why the other groups would not be similarly affected.
Another limitation of this study was not measuring other fat depots such as superficial- and deep subcutaneous adipose tissue, or parameters related to the metabolic health of subcutaneous adipocytes (e.g. secretion of adipokines, responsiveness to lipolytic stimuli). A key strength of this study is that circulating adipokines were measured after 12 wk of controlled feeding, and once again after 12 wk of meal self-selection. This allowed us compare adipokine responses between ADF and CR under both ideal conditions (for adherence) and more real-world conditions. Our sample size is another strength of this study, especially when compared to other dietary weight loss trials that have previously examined our chosen parameters.
In conclusion, we observed that ADF and CR similarly increased the FFM:total mass ratio, decreased circulating leptin, and did not affect the VAT:SAT ratio or our other measured adipokines. Weight loss, rather than the pattern of ER, appeared to be the main driver of these changes; and weight loss greater than 8% may be needed to elicit changes in levels of circulating adipokines other than leptin.
Supplementary Material
Acknowledgments
We would like to thank everyone who participated in this study.
FUNDING SOURCES
This work was supported by the National Institutes of Health (R01HL106228, T32HL007034, F32DK107157). No funding source had direct involvement in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Abbreviations
- ADF
alternate-day fasting
- BMI
body mass index
- CR
calorie restriction
- DEXA
dual energy X-ray absorptiometry
- EI
energy intake
- ER
energy restriction
- FFM
fat-free mass
- FM
fat mass
- SAT
subcutaneous adipose tissue
- TEE
total energy expenditure
- VAT
visceral adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: Clinicaltrials.gov, number NCT00960505
STATEMENT OF AUTHORSHIP
KAV and CMK designed the experiment. JFT, CMK, AB, MK, SB, and KKH assisted with the conduction of the clinical trial. JFT, CMK, AB, JR, and ER performed the laboratory analyses. JFT analyzed the data and wrote the manuscript. All authors assisted with the preparation of the manuscript and have approved the final article.
CONFLICT OF INTEREST
Krista Varady is the author of the book, “The Every Other Day Diet”, published by Hachette Book Group. The other authors declare no competing interests.
References
- 1.Mosley M, Spencer M. The Fast Diet: lose weight, stay healthy, and live longer with the simple secret of intermittent fasting. London: Short Books; 2013. [Google Scholar]
- 2.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern Med. 2017 doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trepanowski JF, Varady KA. Intermittent versus daily calorie restriction in visceral fat loss. In: Watson RR, editor. Nutrition in the prevention and treatment of abdominal obesity. San Diego: Academic Press; 2014. pp. 181–188. [Google Scholar]
- 4.Varady KA. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev. 2011;12:e593–e601. doi: 10.1111/j.1467-789X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 5.Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med. 1995;27:435–438. doi: 10.3109/07853899709002451. [DOI] [PubMed] [Google Scholar]
- 6.Chaston T, Dixon J. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond) 2008;32:619–628. doi: 10.1038/sj.ijo.0803761. [DOI] [PubMed] [Google Scholar]
- 7.Gotthardt JD, Verpeut JL, Yeomans BL, Yang JA, Yasrebi A, Roepke TA, Bello NT. Intermittent fasting promotes fat loss with lean mass retention, increased hypothalamic norepinephrine content, and increased neuropeptide Y gene expression in diet-induced obese male mice. Endocrinology. 2015;157:679–691. doi: 10.1210/en.2015-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siklova-Vitkova M, Klimcakova E, Polak J, Kovacova Z, Tencerova M, Rossmeislova L, Bajzova M, Langin D, Stich V. Adipose tissue secretion and expression of adipocyte-produced and stromavascular fraction-produced adipokines vary during multiple phases of weight-reducing dietary intervention in obese women. J Clin Endocrinol Metab. 2012;97:E1176–E1181. doi: 10.1210/jc.2011-2380. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge L, DeLany JP, Nguyen T, Howard J, Hadley EC, Redman LM, Ravussin E. Validation study of energy expenditure and intake during calorie restriction using doubly labeled water and changes in body composition. Am J Clin Nutr. 2007;85:73–79. doi: 10.1093/ajcn/85.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med Sci Sports Exerc. 2010;42:2134–2140. doi: 10.1249/MSS.0b013e3181e0b3ff. [DOI] [PubMed] [Google Scholar]
- 12.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care. 2009;32:2184–2186. doi: 10.2337/dc09-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown RJ, Walter M, Rother KI. Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care. 2012;35:959–964. doi: 10.2337/dc11-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoddy KK, Bhutani S, Phillips SA, Varady KA. Effects of different degrees of insulin resistance on endothelial function in obese adults undergoing alternate day fasting. Nutr Healthy Aging. 2016;4:63–71. doi: 10.3233/NHA-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klempel MC, Varady KA. Reliability of leptin, but not adiponectin, as a biomarker for diet-induced weight loss in humans. Nutr Rev. 2011;69:145–154. doi: 10.1111/j.1753-4887.2011.00373.x. [DOI] [PubMed] [Google Scholar]
- 18.Bhutani S, Klempel MC, Berger RA, Varady KA. Improvements in coronary heart disease risk indicators by alternate-day fasting involve adipose tissue modulations. Obesity (Silver Spring) 2010;18:2152–2159. doi: 10.1038/oby.2010.54. [DOI] [PubMed] [Google Scholar]
- 19.Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, Hoddy KK, Calvo Y. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12:146. doi: 10.1186/1475-2891-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Luis D, Aller R, Izaola O, Gonzalez Sagrado M, Bellioo D, Conde R. Effects of a low-fat versus a low-carbohydrate diet on adipocytokines in obese adults. Horm Res. 2007;67:296–300. doi: 10.1159/000099329. [DOI] [PubMed] [Google Scholar]
- 21.Kabrnova-Hlavata K, Hainer V, Gojova M, Hlavaty P, Kopsky V, Nedvidkova J, Kunesová M, Parizková J, Wagenknecht M, Hill M. Calcium intake and the outcome of short-term weight management. Physiol Res. 2008;57:237–245. doi: 10.33549/physiolres.931057. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JB, Summer W, Cutler RG, Martin B, Hyun D-H, Dixit VD, Pearson M, Nassar M, Tellejohan R, Maudsley S. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haluzík M, Haluzík M, Matoulek M, Svačina S, Hilgertová J, Haas T. The influence of short-term fasting on serum leptin levels, and selected hormonal and metabolic parameters in morbidly obese and lean females. Endocr Res. 2001;27:251–260. doi: 10.1081/erc-100107185. [DOI] [PubMed] [Google Scholar]
- 27.Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98:E1610–E1619. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 28.Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ. 2005;172:1199–1209. doi: 10.1503/cmaj.1040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souter I, Janzen C, Martinez-Maza O, Breen EC, Stanczyk F, Chaudhuri G, Nathan L. Serum levels of soluble vascular cell adhesion molecule-1 are decreased in women receiving oral contraceptives compared with normally menstruating women: implications in atherosclerosis. Fertil Steril. 2005;83:1480–1488. doi: 10.1016/j.fertnstert.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 30.Souter I, Huang A, Martinez-Maza O, Breen EC, Decherney AH, Chaudhuri G, Nathan L. Serum levels of soluble vascular cell adhesion molecule-1, tumor necrosis factor-α, and interleukin-6 in in vitro fertilization cycles. Fertil Steril. 2009;91:2012–2019. doi: 10.1016/j.fertnstert.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 31.Chiu K, Arnaud C, Ju J, Mayes D, Bacchetti P, Weitz S, Keller E. Correlation of estradiol, parathyroid hormone, interleukin-6, and soluble interleukin-6 receptor during the normal menstrual cycle. Bone. 2000;26:79–85. doi: 10.1016/s8756-3282(99)00243-4. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien SM, Fitzgerald P, Scully P, Landers A, Scott LV, Dinan TG. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14:84–90. doi: 10.1159/000107423. [DOI] [PubMed] [Google Scholar]
- 33.Al-Harthi L, Wright DJ, Anderson D, Cohen M, Matityahu D, Cohn J, Cu-Unvin S, Burns D, Reichelderfer P, Lewis S. The impact of the ovulatory cycle on cytokine production: evaluation of systemic, cervicovaginal, and salivary compartments. J Interferon Cytokine Res. 2000;20:719–724. doi: 10.1089/10799900050116426. [DOI] [PubMed] [Google Scholar]
- 34.Willis C, Morris J, Danis V, Gallery E. Cytokine production by peripheral blood monocytes during the normal human ovulatory menstrual cycle. Hum Reprod. 2003;18:1173–1178. doi: 10.1093/humrep/deg231. [DOI] [PubMed] [Google Scholar]
- 35.Abrahamsen B, Stilgren L, Rettmer E, Bonnevie-Nielsen V, Beck-Nielsen H. Effects of the natural and artificial menstrual cycle on the production of osteoprotegerin and the bone resorptive cytokines IL-1beta and IL-6. Calcif Tissue Int. 2003;72:18–23. doi: 10.1007/s00223-002-2037-y. [DOI] [PubMed] [Google Scholar]
- 36.Salkeld BD, MacAulay JC, Ball RW, Cannon JG. Modulation of body temperature, interleukin-6 and leptin by oral contraceptive use. Neuroimmunomodulation. 2001;9:319–325. doi: 10.1159/000059389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.