Abstract
Non-receptor Src family kinases (SFKs), mostly notably c-Src and c-Yes, are recently shown to be expressed by Sertoli and/or germ cells in adult rat testes. Studies have shown that SFKs are involved in modulating the cell cytoskeletal function, and involved in endocytic vesicle-mediated protein endocytosis, transcytosis and/or recycling as well as intracellular protein degradation events. Furthermore, a knockdown to SFKs, in particular c-Yes, has shown to induce defects in spermatid polarity. These findings, coupled with emerging evidence in the field, thus prompt us to critically evaluate these findings to put forth a developing concept regarding the role of SFKs and cell polarity, which will become a basis to design experiments for future investigations.
Keywords: Src family kinases, c-Src, c-Yes, testis, spermatogenesis, spermatids, Sertoli cells, cell polarity
Introduction
In a typical cross-section of an adult mammalian testis, cell polarity across the seminiferous epithelium is readily notable (Figure 1) [1]. For instance, Sertoli cell nuclei are located mostly near the basement membrane of the tunica propria. Furthermore, peritubular myoid cells are restrictively localized to the myoid cell layer behind the acellular zone of the tunica propria namely the basement membrane (also known as basal lamina) and the type I collagen layer (Figure 1). On the other hand, elongating/elongated spermatids are mostly found in the adluminal compartment until these cells are line-up near the tubule lumen, with their heads all point to the basement membrane and their tails to the tubule lumen (Figure 1). This morphological setting of cell polarity is being used to support spermatogenesis. It is envisioned that the robust cellular output of spermatozoa in the tens of millions daily in the seminiferous tubules from an adult male (including rodents and humans) is analogous to a car manufacturing plant. This requires highly orchestrated alignment of developing germ cells (i.e., vehicles) that move through the assembly line (conferred by actin and/or microtubule (MT)-based tracks) with well aligned machineries (e.g., endocytic vesicles aided by GTPases) to support the assembly of different components (e.g., various integral membrane proteins, adaptor proteins) onto the developing germ cells, and the removal of some necessary wastes (e.g., residual bodies). Nonetheless, the concept of cell polarity in particular the involving molecules and the signaling pathways that confer cell polarity in the testis have not been carefully investigated. For instance, it was first reported in 2008 that the Par (partitioning defective)-based polarity complex and its binding partners, namely Par3, Par6 and Cdc42, were expressed by Sertoli and/or germ cells in the testis, and these proteins are involved in apical-basal polarity of developing spermatids and cell adhesion function [2]. Thereafter, the other two known polarity protein complex modules that work in concert with the Par-based polarity complex to support cell polarity [3, 4], namely the Scribble- [5] and the Crumbs-based [6] complexes, were also identified in the testis with their functions partially characterized [7, 8]. Furthermore, many of the planar cell polarity (PCP) proteins necessary to modulate PCP including Van Gogh-like 2 (Vangl 2) were later shown to be expressed in the testis, also with defined functions and possibly interacting with other polarity proteins (e.g., Scribble) to modulate spermatogenesis [9]. Since the subject regarding the role of polarity proteins and PCP proteins in testis functions has recently been reviewed [8, 10, 11], in order to avoid redundancy, we focus herein on a rapidly developing field on the involvement of Src family kinases (SFK) in regulating cell polarity in the testis. However, there are emerging evidence that SFKs are also involved in PCP in the testis, most notably spermatids, so that some brief discussions on PCP are provided wherever appropriate, such as noted in Table 1. Since this is a rapidly developing field, but studies from other epithelia are of importance to reproductive biologists, we seek to summarize these findings and to provide a thoughtful basis for future investigation.
Figure 1. A schematic drawing that illustrates the general morphological features of Sertoli and germ cells in the epithelium of the seminiferous tubule.

The blood-testis barrier (BTB) physically divides the seminiferous epithelium into the adluminal (apical) compartment and the basal compartment, which in turn lays on the basement membrane of the tunica propria. Undifferentiated and differentiated spermatogonia are found in the basal compartment. Type B spermatogonia differentiate into preleptotene spermatocytes, which are the germ cells that are being transported across BTB while transforming to leptotene spermatocytes to enter the adluminal compartment to form zygotene and pachytene spermatocytes to prepare for meiosis I/II. The most notable anchoring junction in the testis is the actin-rich adherens junction (AJ) type called ectoplasmic specialization (ES), which is typified by the presence of an array of actin filament bundles found in the Sertoli cell near the plasma membrane and these bundles are sandwiched between the cisternae of endoplasmic reticulum and the apposing Sertoli cell-cell or Sertoli-spermatid plasma membranes. ES is either found at the Sertoli cell-cell interface called basal ES. The basal ES together with the tight junction (TJ) and gap junction all utilize F-actin for attachment. These actin-based junctions together with the intermediate filament-based desmosome constitute the BTB. At the Sertoli cell-spermatid (step 8–19 spermatids in the rat testis) interface, the ES is designated apical ES. The ES is an important structure to support Sertoli cell adhesion at the BTB and also spermatid adhesion. However, the ES is also an important cellular structure to support and Sertoli cell and spermatid polarity, and also spermatid PCP (planar cell polarity) during spermatogenesis.
Table 1.
Recent findings of members of SFK that modulate cell polarity and/or PCP function
| Member of SFK | Detection method | Cell/tissue type | PCP-related SFK activities | Ref |
|---|---|---|---|---|
| c-Src | Src 2–17 antibody, SFK inhibitors SU6656 and Bosutinib | Mouse auditory sensory epithelium | 1. Relevant to Ptk7-mediated epithelial PCP regulation in vivo 2. Altered Src signaling may affect the cortical cytoskeleton |
[49] |
| Kras-Src biosensor, SFK inhibitor PP1 | Bovine aortic endothelial cells | 1. It plays an important role in regulating the shear stress-induced cell polarity 2. It regulates the polarized distribution of FAK 3. High shear stress-induced Src polarity localization is mainly mediated by actin cytoskeleton |
[50] | |
| Src RNAi (siRNA), SFK inhibitors PP2 and SU6656 | Osteoclasts | Phosphorylates FGD6 (guanine exchange factor) to form complexes containing IQGAP1, ARHGAP10, Talin-1/2, or Filamin A to regulate cell polarity and membrane recycling | [51] | |
| thermosensitive v-src mutant, mAb to avian Src (EC10), Src rabbit pAb | MDCK cells | 1. Impacts on cortactin distribution and controls cortical actin dynamics 2. Promotes microtubule-dependent fusion of macropinosomes to the apical recycling endosome |
[52] | |
| SFK inhibitor SU6656, SYF fibroblasts deficient of Src, Yes, and Fyn, rescue assay by re-expressing Src | RAT2 fibroblasts | Integrin/FAK/Src/p190RhoGAP pathway regulates nuclear reorientation required for cell polarization | [53] | |
| Src-2 antibody, Src pY416 antibody (2101) | FAK (R454) Knock-in mouse embryo fibroblasts (MEFs) | Reduces c-Src Y416 phosphorylation in R454 MEFs that exhibited enhanced focal adhesion formation, decreased migration, and defects in cell polarity | [54] | |
| Src RNAi (shRNA) Src antibody 2102P SFK inhibitor PP2 |
Acinar lung cancer cell | Regulates p190A RhoGAP phosphorylation, inhibiting RhoA-ROCK1 signalling and inducing polarity orientation | [55] | |
| Src RNAi (siRNA), Src antibody 36D10, SFK inhibitors PP1, PP2 and Dasatinib | Primary cultured spheroids of human colorectal adenocarcinoma | Critical for polarity switching of cancer tissue-originated spheroid | [56] | |
| Src RNAi (siRNA), SFK inhibitor PP2 | Multiple cancer cell lines | CD133/integrin/Src/Akt/GSK3ß/ß-catenin signaling induced by polarized cell migration is required for maintenance of cancer stem cell properties | [57] | |
| Src42A, Src64B (mammalian Src orthologs) | Src mutants |
Drosophila ectodermal cells |
It requires for STAT92E (STAT transcription factor) apical localization and its interaction with Baz (a homolog of PAR3) | [58] |
| Src42A | Src mutants, genetic screen | Drosophila eye discs and larval wing imaginal disc | A key modulator of tumor invasion and tumor cell migration | [59] |
| c-Yes | c-Yes RNAi (siRNA) | Sertoli cells, testis | Its knockdown causes a disruption of the Sertoli cell tight junction-permeability barrier function, germ cell loss from the seminiferous epithelium, and also defects in spermatid polarity | [60] |
| c-Yes mouse monoclonal (sc-8403) | Pancreatic epithelial cells | It co-localizes with occludin, Hippo pathway element YAP (c-yes associated protein) and TEAD (TEA-dependent) to regulate cell proliferation through the coordination of PCP with apical-basal polarity events | [61] | |
| Fyn | SFK inhibitor Bosutinib, Fyn-null oocytes | Oocyte | Leads to defects in cortical actin organization and polarity, and the polarized association of Par-3 with the cortex overlying the meiotic spindle is completely disrupted | [62] |
| Fyn antibody (sc-434), SFK inhibitor PP2 | Hippocampal neurons | It is associated with raft-localized and is involved in neurite outgrowth and polarity formation | [63] | |
| Lyn | Lyn RNAi (siRNA and shRNA), SFK inhibitor PP2, competition experiments using blocking peptide | Primary human neutrophils, neutrophil-like differentiated HL-60 cells | 1. It is activated and recruited to leading edge of polarized neutrophils in response to chemo-attractants 2. Its depletion impairs β2-integrin and Rap1 activation/translocation and recruitment of CrkL-C3G complex at the leading edge |
[64] |
| Lyn RNAi (siRNA) Bcr-Abl/Lyn inhibitor Bafetinib |
Multiple cancer cells | It functions as a key modulator of SNAI family protein localization and stability through control of the Vav-Rac1-PAK1 (Vav-Rac1-p21-activated kinase) pathway, promoting EMT | [65] | |
| MDCK cells expressing tetracycline-inducible human Lyn | MDCK cells | 1. Lyn mainly localizes to the plasma membrane in polarized MDCK cells and to endomembranes in non-polarized MDCK cells 2. Calcium-dependent cell-cell interactions recruit Lyn from endomembranes to the plasma membrane, and loss of cell-cell interactions relocates Lyn from the plasma membrane to endomembranes through Rab11-mediated recycling |
[66] | |
| SFKs | SFK inhibitor PP2 | Vascular endothelium | This treatment leads to phosphorylation of a small pool of VE-cadherin on Y658 that binds to polarity protein LGN (Y658) which is the p120-catenin binding site assumed to be responsible for VE-cadherin internalization) | [67] |
Src family kinases (SFKs) and cell polarity in the testis
Introduction
Src family of non-receptor protein kinases (SFKs) are widely expressed by virtually all mammalian cells, consisting of at least nine members: Src, Yes, Fyn, Lck, Hck, Fgr, Lyn, Blk and Yrk [12, 13]. SFKs are known to play a prominent role in various cellular events, regulating numerous cell functions including cell migration, cell proliferation, cell differentiation, cell cytoskeletons, cell survival, apoptosis, calcium signaling, androgen signaling and spermatogenesis; and since SFKs are oncogenes so that they are also involved in pathogenesis of diseases, such as tumorigenesis, HIV-1 infection and Alzheimer’s disease [12, 14–21]. In short SFKs are involved in virtually all aspect of cellular function in epithelia and endothelia. Interestingly, all SFK members share some common structural features along their polypeptide sequences which have recently been reviewed [18] and summarized in Figure 2. However, the involvement of SFKs in cell polarity is an emerging concept. In the testis, while many of the SFK members including c-Src, c-Yes, Fyn, Lyn, Lck and Hck have been identified, the two best studied SFK members are c-Src and c-Yes [18, 22–25]. As such, much of the discussion herein is focused on these two SKF members.
Figure 2. A schematic drawing that illustrates the various function domains that are conserved among members of the SFK, and the conversion between the active and inactive state of SFKs.
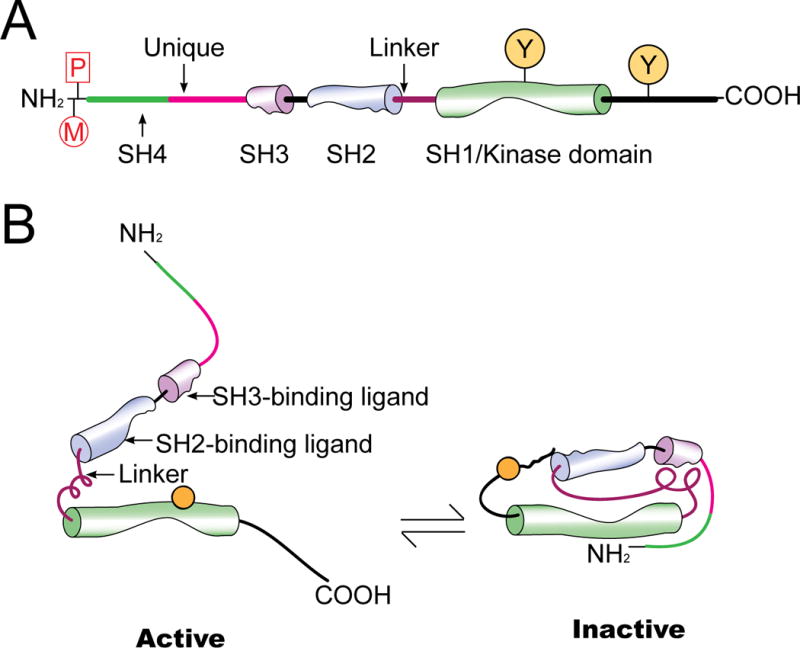
Members of SFK (e.g., c-Src, c-Yes) consist of 4 SH (src-homology) domains: SH4 at the N-terminal region, followed by SH3 and SH2, and then a SH1 domain containing the catalytic kinase domain which is linked to the SH2 domain via a short polyproline type II helix called SH2-SH1 linker. In the SH4 domain of c-Src and c-Yes, there is a penultimate glycine (Gly2) residue co-translationally myristoylated (M) to be used for membrane targeting. Also, a cysteine residue (Cys3) near the N-terminus of c-Yes is post-translationally palmitoylated (P) to assist subcellular trafficking of a SFK protein (e.g., facilitating membrane association of c-Yes following palmitoylation). (B) Under normal physiological conditions, SFK is auto-inhibited through intramolecular interactions wherein the SH2 domain binds to the inhibitory phosphotyrosine at the C-terminal tail, while the SH3 domain interacts with the SH2-SH1 polyproline linker so that these two protein interacting motifs block the kinase domain and stabilize SFK in an inactive conformation (right). SFKs are activated through binding of a ligand to the SH2 and SH3 domain, or dephosphorylation of the inhibitory phosphotyrosine by protein tyrosine phosphatases. This leads to a conformational change which allows phosphorylation of the stimulatory tyrosine in the activation loop and to confer intrinsic kinase activity in kinase domain (SH1) (left).
SFKs and spermatid polarity in the testis – their role on Sertoli cell cytoskeletal function
The first indication that SFKs may be involved in cell polarity in the testis was the earlier study of c-Yes when this protein was first found to be localized restrictively to the basal region of the seminiferous epithelium, consistent with its localization at the basal ectoplasmic specialization (basal ES)/blood-testis barrier (BTB) (see Figure 1) [26]. Furthermore, c-Yes was also restrictively expressed and localized at the apical ES at the Sertoli cell-spermatid interface in stage V–VIII tubules [26]. This pattern of localization illustrates that the c-Yes protein is localized in a polarized fashion. Furthermore, the localization of c-Yes across the seminiferous epithelium is almost superimposable to F-actin [26], implicating c-Yes may be playing a role in modulating F-actin dynamics in the testis. This speculation is reached based on studies showing that all three polarity protein modules, including the Par-, the Scribble- and the Crumbs-based polarity complexes in the testis exert their regulatory effects via the actin-based cytoskeleton [2, 5, 6]. Furthermore, the use of a selective c-Yes inhibitor, SU6656, was found to induce Sertoli cell TJ-permeability barrier disruption due to defects in the organization of the actin microfilaments across the cell cytosol in which treatment of Sertoli cells with SU6656 led to extensive actin filament truncation [26]. Collectively, these findings support the notion that c-Yes, a non-receptor protein tyrosine kinase, may be involved in cell polarity in the testis through its effects on the organization of actin-based cytoskeleton. However, the proof that c-Yes is indeed involved in spermatid polarity was not known until a report published in subsequent years that a knockdown of c-Yes in the testis in vivo led to defects in spermatid polarity [27]. Histological analysis of testes following c-Yes knockdown by as much as 75% have shown that many step 19 spermatids failed to undergo c-Yes knockdown-induced spermiation, and considerably number of these spermatids displayed obvious defects in polarity [27] (Figure 3). For instance, in control testes, spermatids are aligned almost perpendicular to the basement membrane with their heads point to the basal region of the epithelium and their tails to the tubule lumen. However, after c-Yes knockdown by RNAi, many elongated spermatid heads deviated by ~90–180° from their intended orientation [27] (Figure 3). More important, the spatial expression of polarity protein Par6 in c-Yes silenced testes was grossly mis-localized [27]. For instance, Par6 that was localized at the tip of spermatid heads in normal testes moved away from the site, instead, Par6 was localized to the convex (dorsal) side of spermatid heads and no longer tightly associated with spermatids [27]. These findings thus provide the first direct proof that c-Yes is playing a role in conferring spermatid polarity. Since c-Yes is a non-receptor protein tyrosine kinase, it remains unknown if c-Yes plays a role in activating or inactivating the Par-, the Scribble- and/or the Crumbs-based protein complexes through changes in the phosphorylation status of any of these proteins. However, studies in Drosophila and other mammalian tissues and cells (e.g., epithelial stem cells) including humans have shown that the Crumbs/CRB-Hippo/Warts/LATS kinase signaling cascade can phosphorylate and inhibit YAP/TAZ (Yes-associated protein, a family of transcriptional co-activators that serve as a sensor for cell polarity, involving in cell proliferation and suppressing apoptotic genes), whereas integrins and SKFs can phosphorylate and activate YAP (Yes-associated protein)/TAZ [28–30]. Thus, it is likely that there is an intimate relationship between c-Yes and the Crumbs-based polarity complex in which c-Yes may modulate the Crumbs-Hippo signaling pathway through phosphorylation. In this context, it is of interest to note that a knockdown of Crumbs homolog-3 (CRB3) in the testis by RNAi was found to cause defects in spermatid polarity [6], but the involvement of c-Yes or c-Src in this event has not been examined.
Figure 3. Defects in spermatid polarity following c-Yes knockdown by RNAi.
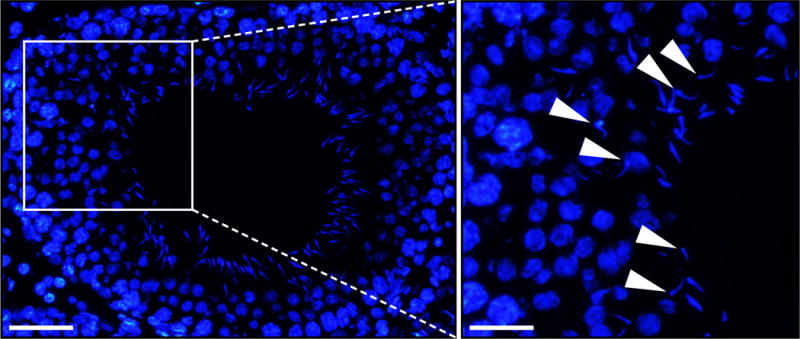
Testes from adult rats received a transfection mixture containing 100 siRNA duplexes specific to c-Yes on day 1 and day 3, i.e., 2 transfections for RNAi to knockdown c-Yes by >70% as described [27]. Control testes received the same amount of non-targeting negative control siRNA duplexes. On day 5, rats were euthanized and testes were removed to freeze in liquid nitrogen. Frozen sections were obtained and cell nuclei stained for DAPI (4′,6-diamidino-2-phenylindole). As noted herein, a knockdown of c-Yes, but not in control testes received the negative non-targeting control siRNA duplexes, caused extensive defects in spermatid polarity in which the head of spermatids no longer pointed towards the basement membrane, but deviating by 90° to 180° from the intended orientation (annotated by white arrowheads). Scale bar, 80 μm and 40 μm in the left and right panel.
c-Yes and c-Src have differential function on Sertoli cell endocytic vesicle-mediated protein trafficking
For apico-basal polarity, such as those noted in mammalian cells and tissues in various organs including developing spermatids and in Sertoli cells in the seminiferous epithelium, accumulating evidence in the field based on studies in other epithelia has shown that it is supported by endocytic vesicle-mediated protein trafficking [31–34]. For instance, due to the presence of different sets of proteins in the apical vs. the basal region of a mammalian cell or tissue, this creates the apico-basal polarity. Indeed, the anchorage of spermatid heads into the Sertoli cell epithelium requires the support of adhesion protein complexes at the Sertoli cell-spermatid interface, so that the acrosomal region of spermatid heads can be aligned by pointing towards the basement membrane (Figures 1, 3). Additionally, intracellular trafficking that transports proteins to the spermatid head is considerably different from those being transported to the tail region [35–37], thereby supporting apico-basal polarity during spermiogenesis. Furthermore, the transport of residual bodies and phagosomes into the base of the seminiferous epithelium for their lysosomal degradation by Sertoli cells at stage VIII–IX of the epithelial cycle [38] also requires the tight coordination of lysosomal enzymes to be synthesized and be transported to the site for degradation of unwanted proteins and/or organelles vs. their storage for subsequent usage during fertilization [39, 40].
Earlier reports that examined the role of c-Src and c-Yes, two members of the non-receptor SFK, in endocytic vesicle-mediated intracellular trafficking of proteins have shown that these two protein kinases are playing differential role in this cellular events without much redundancy [18, 41, 42]. For instance, c-Yes is monopalmitoylated and once it is synthesized in mammalian cells, the newly produced c-Yes protein is transported from the Golgi pool of caveolin to be near the plasma membrane. Once c-Yes arrives its destination, it is being used to mediate endosome trafficking, possibly involving in protein transcytosis and/or recycling. On the other hand, c-Src, unlike c-Yes, is nonpalmitoylated and it is shuffled between plasma membrane and late endosomes/lysosomes in the mammalian cell. This thus suggests that c-Src may support endosome-mediated degradation intracellularly [41–43]. To further support this possibility, c-Yes knockout mice, although fertile, were shown to display defects in protein transcytosis/recycling [42, 44]. However, c-Src knockout mice were infertile [45], and VEGF (vascular endothelial growth factor) that was earlier shown to promote blood-brain barrier leakage [46] no longer capable of compromising the blood-brain barrier in these mice [45] due to defects in intracellular endosome-mediated protein degradation. In a recent report based on the use of different biochemical assays to track the fate of cell surface labeled proteins using biotin, such as sulfo-NHS-SS-biotin, by monitoring protein endocytosis and recycling vs. protein degradation and phagocytosis. c-Yes and c-Src were found to play differential role in endocytic vesicle-mediated protein trafficking [47]. For instance, it was shown that c-Yes promoted endocytosed and biotinylated BTB integral membrane proteins to the pathways of transcytosis and/or recycling [47]. However, c-Src promoted endosome-mediated protein degradation such as facilitating the removal of residual bodies via the formation of phagosomes. These observations are physiologically significant since they illustrate that in the testis, these two SFK members are likely working in concert to determine the fate of endocytosed proteins at the Sertoli cell BTB such that they can either be recycled to form “new” TJ ultrastructures to support the assembly of “new” BTB behind the preleptotene under transport at the immunological barrier in stage VIII tubules by c-Yes (Figure 4), or promoted by c-Src to undergo intracellular degradation such that those from the “old” BTB site above the preleptotene spermatocytes at the immunological barrier can support the eventual breakdown of the “old” BTB ultrastructures to facilitate preleptotene spermatocyte transport (Figure 4). The combined efforts of c-Yes and c-Src thus provide a novel mechanism to support the timely breakdown and reassembly of the BTB ultrastructures surrounding the preleptotene spermatocytes under transport at the BTB. While the underlying molecular mechanism(s) by which c-Src and c-Yes modulate the BTB ultrastructures during preleptotene spermatocyte transport is not known, it is likely that c-Src or c-Yes mediates its effects via changes in the phosphorylation status of BTB components. These include the integral membrane proteins (e.g., occludin, CAR, JAMs, claudins) and/or their adaptors (e.g., ZO-1, ZO-2) at the Sertoli cell BTB, and actin regulatory proteins, such as actin bundling proteins palladin and Eps8 (and also for barbed end capping) and branched actin nucleation protein Arp3, actin nucleation protein formin 1. These changes in turn modulate protein endocytosis, transcytosis, recycling and/or degradation.
Figure 4. A model illustrating the role of c-Src and c-Yes to support germ cell transport and polarity.
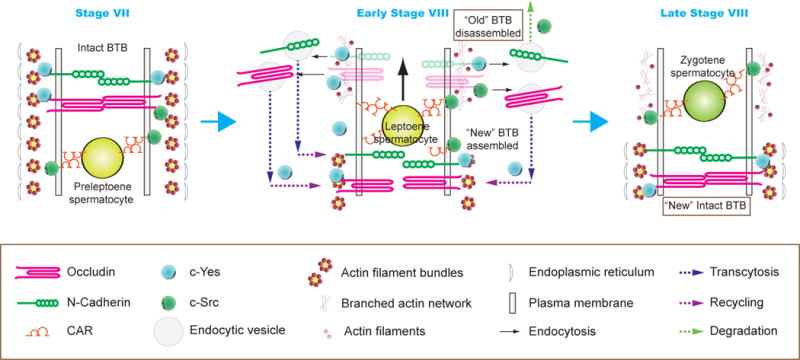
In this hypothetical model, the BTB integrity is maintained by SFKs such as c-Yes and c-Src such as at stage VII of the epithelial cycle (left panel). For instance, it was shown that c-Src associates with CAR [48], whereas c-Yes associates with occludin and N-cadherin [26]. However, at stage VIII of the epithelial cycle, these two SFKs promote protein endocytosis in which c-Yes targets the endocytosed proteins (e.g., occludin, N-cadherin, CAR) to be recycled to the base of the preleptotene spermatocyte to support the reassembly of a “new” BTB before the “old” BTB is disassembled. At the same time, c-Src promotes the endocytosed proteins to undergo endosome-mediated protein degradation as recently reported [47] (middle panel). As such, the BTB integrity is maintained as noted in the right panel. As noted in Figure 3, SFKs (e.g., c-Yes) also promotes germ cell polarity which may also facilitate the directional transport of preleptotene spermatocytes across the BTB as noted herein.
Concluding remarks and future perspectives
As briefly discussed herein, the involvement of SFKs in cell polarity, in particular spermatid polarity during spermiogenesis, is an emerging concept in the field. These findings should be carefully evaluated and expanded in future studies. However, there are many unanswered open questions. For instance, would the loss of SFKs, such as either c-Yes or c-Src, in Sertoli cells in the testis by using RNAi for its knockdown vs. its specific knockout (KO) lead to changes in the expression of polarity proteins (e.g., the Par-, the Scribble- or the Crumbs-based polarity complexes or any of their component proteins)? Besides the involvement of actin-binding and regulatory proteins, what role does MT- and/or MT-binding and regulatory proteins in SFK-mediated spermatid polarity? It is expected that many of these questions will be answered in the years to come.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (NSFC Grant 31371176, 81571426 and 81170554), Science Technology Foundation of Zhejiang Province (No. 2014F10033), Innovation discipline of Laboratory Animal Genetic Engineering (No. 201604), and National Institutes of Health (R01 HD056034 to C.Y.C. and U54 HD029990 Project 5 to C.Y.C.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Nothing to declare
References
- 1.Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–53. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–62. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–30. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Campanale JP, Sun TY, Montell DJ. Development and dynamics of cell polarity at a glance. J Cell Sci. 2017;130:1201–7. doi: 10.1242/jcs.188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su WH, Wong EWP, Mruk DD, Cheng CY. The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis. Endocrinology. 2012;153:6041–53. doi: 10.1210/en.2012-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Y, Lui WY, Lee WM, Cheng CY. Polarity protein Crumbs homolog-3 (CRB3) regulates ectoplasmic specialization dynamics through its action on F-actin organization in Sertoli cells. Scientific reports. 2016;6:28589. doi: 10.1038/srep28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Gao Y, Chen H, Jesus T, Tang E, Li N, et al. Cell polarity, cell adhesion, and spermatogenesis: role of cytoskeletons. F1000Research. 2017;6:1565. doi: 10.12688/f1000research.11421.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen Q, Mruk D, Tang EI, Wong CKC, Lui WY, Lee WM, et al. Cell polarity and cytoskeletons-lesson from the testis. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Mruk DD, Lee WM, Cheng CY. Planar cell polarity (PCP) protein Vangl2 regulates ectoplasmic specialization dynamics via its effects on actin microfilaments in the testes of male rats. Endocrinology. 2016;157:2140–59. doi: 10.1210/en.2015-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Mruk DD, Lui WY, Wong CKC, Lee WM, Cheng CY. Cell polarity and planar cell polarity (PCP) in spermatogenesis. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen Q, Tang EI, Gao Y, Jesus TT, Chu DS, Lee WM, et al. Signaling pathways regulating blood-tissue barriers – lesson from the testis. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2017 doi: 10.1016/jbbamem201704020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espada J, Martin-Perez J. An update on Src family of nonreceptor tyrosine kinases biology. Int Rev Cell Mol Biol. 2017;331:83–122. doi: 10.1016/bs.ircmb.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Roskoski RJ. Src protein-tyrosine kinase structure, mechanism, and small molecular inhibitors. Pharmacol Res. 2015;94:9–25. doi: 10.1016/j.phrs.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Kim CG, Lee H, Gupta N, Ramachandran S, Kaushik I, Srivastava S, et al. Role of Forkhead Box Class O proteins in cancer progression and metastasis. Semin Cancer Biol. 2017 doi: 10.1016/jsemcancer201707007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nygaard HB. Targeting Fyn kinase in Alzheimer’s disease. Biological psychiatry. 2017 doi: 10.1016/j.biopsych.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coiras M, Ambrosioni J, Cervantes F, Miró JM, Alcamí J. Tyrosine kinase inhibitors: potential use and safety considerations in HIV-1 infection. Expert Opinion on Drug Safety. 2017;16:547–59. doi: 10.1080/14740338.2017.1313224. [DOI] [PubMed] [Google Scholar]
- 17.Castoria G, Auricchio F, Migliaccio A. Extranuclear partners of androgen receptor: at the crossroads of proliferation, migration, and neuritogenesis. FASEB J. 2017;31:1289–300. doi: 10.1096/fj.201601047R. [DOI] [PubMed] [Google Scholar]
- 18.Xiao X, Mruk DD, Cheng FL, Cheng CY. c-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv Exp Med Biol. 2012;763:295–317. doi: 10.1007/978-1-4614-4711-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anguita E, Villalobo A. Src-family tyrosine kinases and the Ca2+ signal. Biochim Biophys Acta. 2017;1864:915–32. doi: 10.1016/j.bbamcr.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Engen JR, Wales TE, Hochrein JM, Meyn MA, 3rd, Banu Ozkan S, Bahar I, et al. Structure and dynamic regulation of Src-family kinases. Cell Mol Life Sci. 2008;65:3058–73. doi: 10.1007/s00018-008-8122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chojnacka K, Mruk DD. The Src non-receptor tyrosine kinase paradigm: New insights into mammalian Sertoli cell biology. Mol Cell Endocrinol. 2015;415:133–42. doi: 10.1016/j.mce.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Lawson C, Goupil S, Leclerc P. Increased activity of the human sperm tyrosine kinase SRC by the cAMP-dependent pathway in the presence of calcium. Biol Reprod. 2008;79:657–66. doi: 10.1095/biolreprod.108.070367. [DOI] [PubMed] [Google Scholar]
- 23.Lalancette C, Bordeleau LJ, Faure RL, Leclerc P. Bull testicular haploid germ cells express a messenger encoing for a tuncated form of the protein tyrosine kinase HCK. Mol Reprod Dev. 2006;73:520–30. doi: 10.1002/mrd.20422. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko Y, Nonoguchi K, Fukuyama H, Takano S, Higashitsuji H, Nishiyama H, et al. Presence of alternative 5′ untranslated sequence and identification of cells expression ctk transcripts in the brain and testis. Oncogene. 1995;10:945–52. [PubMed] [Google Scholar]
- 25.Zhao D, Tateyama S, Miyoshi N, Yamaguchi R, Uchida K, Kai K, et al. Human c-yes-1-related proto-oncogene in clincally normal dogs. J Vet Med Sci. 1994;56:177–9. doi: 10.1292/jvms.56.177. [DOI] [PubMed] [Google Scholar]
- 26.Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol. 2011;43:651–65. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab. 2013;304:E145–E59. doi: 10.1152/ajpendo.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elbediwy A, Vincent-Mistiaen ZI, Thompson BJ. YAP and TAZ in epithelial stem cells: A sensor for cell polarity, mechanical forces and tissue damage. Bioessays. 2016;38:644–53. doi: 10.1002/bies.201600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15810–5. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling C, Zheng Y, Fin F, Yu J, Huang J, Hong Y, et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10532–7. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roman-Fernandez A, Bryant DM. Complex polarity: Building multicellular tissues through apical membrane traffic. Traffic. 2016;17:1244–61. doi: 10.1111/tra.12417. [DOI] [PubMed] [Google Scholar]
- 32.Niedergang F, Gasman S, Vitale N, Desnos C, Lamaze C. Meeting after meeting: 20 years of discoveries by the members of the Exocytosis-Endocytosis Club. Biol Cell. 2017;109:339–53. doi: 10.1111/boc.201700026. [DOI] [PubMed] [Google Scholar]
- 33.Calero-Cuenca FJ, Sotillos S. Nuf and Rip11 requirement for polarity determinant recycling during Drosophila development. Small GTPases. 2016:1–8. doi: 10.1080/21541248.2016.1235386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Mockus G, Schweisguth F. Cell polarity and Notch signaling: Linked by the E3 ubiquitin ligase neuralized? BioEssays : news and reviews in molecular. cellular and developmental biology. 2017 doi: 10.1002/bies.201700128. [DOI] [PubMed] [Google Scholar]
- 35.Kierszenbaum AL, Rivkin E, Tres LL. Cytoskeletal track selection during cargo transport in spermatids is relevant to male fertility. Spermatogenesis. 2011;1:221–30. doi: 10.4161/spmg.1.3.18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kierszenbaum AL, Tres LL. The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol. 2004;67:271–84. doi: 10.1679/aohc.67.271. [DOI] [PubMed] [Google Scholar]
- 37.Kierszenbaum AL, Rivkin E, Tres LL. Molecular biology of sperm head shaping. Soc Reprod Fertil Suppl. 2007;65:33–43. [PubMed] [Google Scholar]
- 38.Clermont Y, Morales C, Hermo L. Endocytic activities of Sertoli cells in the rat. Ann NY Acad Sci. 1987;513:1–15. doi: 10.1111/j.1749-6632.1987.tb24994.x. [DOI] [PubMed] [Google Scholar]
- 39.Sutovsky P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: Killing three birds with one stone. Microsc Res Tech. 2003;61:88–102. doi: 10.1002/jemt.10319. [DOI] [PubMed] [Google Scholar]
- 40.Sutovsky P. Sperm proteasome and fertilization. Reproduction. 2011;142:1–14. doi: 10.1530/REP-11-0041. [DOI] [PubMed] [Google Scholar]
- 41.Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, et al. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J Cell Sci. 2009;122:965–75. doi: 10.1242/jcs.034843. [DOI] [PubMed] [Google Scholar]
- 42.Su T, Bryant DM, Luton F, Verges M, Ulrich SM, Hansen KC, et al. A kinase cascade leading to Rab11-FIP5 controls transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2010;12:1143–53. doi: 10.1038/ncb2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dozynkiewicz MA, Jamieson NB, MacPherson I, Grindlay J, van den Berghe PV, von Thun A, et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev Cell. 2012;22:131–45. doi: 10.1016/j.devcel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luton F, Verges M, Vaerman JP, Sudol M, Mostov KE. The SRC family protein tyrosin kinase p62yes controls polymeric IgA transcytosis in vivo. Mol Cell. 1999;4:627–32. doi: 10.1016/s1097-2765(00)80213-0. [DOI] [PubMed] [Google Scholar]
- 45.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–24. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–38. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao X, Mruk DD, Wong EWP, Lee WM, Han D, Wong CKC, et al. Differential effects of c-Src and c-Yes on the endocytic vesicle-mediated trafficking events at the Sertoli cell blood-testis barrier: an in vitro study. Am J Physiol Endocrinol Metab. 2014;307:E553–E62. doi: 10.1152/ajpendo.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang CQF, Mruk DD, Lee WM, Cheng CY. Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res. 2007;313:1373–92. doi: 10.1016/j.yexcr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andreeva A, Lee J, Lohia M, Wu X, Macara IG, Lu X. PTK7-Src signaling at epithelial cell contacts mediates spatial organization of actomyosin and planar cell polarity. Dev Cell. 2014;29:20–33. doi: 10.1016/j.devcel.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu B, Lu S, Hu YL, Liao X, Ouyang M, Wang Y. RhoA and membrane fluidity mediates the spatially polarized Src/FAK activation in response to shear stress. Sci Rep. 2014;4:7008. doi: 10.1038/srep07008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steenblock C, Heckel T, Czupalla C, Espirito Santo AI, Niehage C, Sztacho M, et al. The Cdc42 guanine nucleotide exchange factor FGD6 coordinates cell polarity and endosomal membrane recycling in osteoclasts. J Biol Chem. 2014;289:18347–59. doi: 10.1074/jbc.M113.504894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mettlen M, Platek A, Van Der Smissen P, Carpentier S, Amyere M, Lanzetti L, et al. Src triggers circular ruffling and macropinocytosis at the apical surface of polarized MDCK cells. Traffic. 2006;7:589–603. doi: 10.1111/j.1600-0854.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 53.Maninova M, Klimova Z, Parsons JT, Weber MJ, Iwanicki MP, Vomastek T. The reorientation of cell nucleus promotes the establishment of front-rear polarity in migrating fibroblasts. J Mol Biol. 2013;425:2039–55. doi: 10.1016/j.jmb.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 54.Lim ST, Chen XL, Tomar A, Miller NL, Yoo J, Schlaepfer DD. Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation. J Biol Chem. 2010;285:21526–36. doi: 10.1074/jbc.M110.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Datta A, Sandilands E, Mostov KE, Bryant DM. Fibroblast-derived HGF drives acinar lung cancer cell polarization through integrin-dependent RhoA-ROCK1 inhibition. Cell Signal. 2017;40:91–8. doi: 10.1016/j.cellsig.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okuyama H, Kondo J, Sato Y, Endo H, Nakajima A, Piulats JM, et al. Dynamic Change of Polarity in Primary Cultured Spheroids of Human Colorectal Adenocarcinoma and Its Role in Metastasis. Am J Pathol. 2016;186:899–911. doi: 10.1016/j.ajpath.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Su YJ, Lin WH, Chang YW, Wei KC, Liang CL, Chen SC, et al. Polarized cell migration induces cancer type-specific CD133/integrin/Src/Akt/GSK3beta/beta-catenin signaling required for maintenance of cancer stem cell properties. Oncotarget. 2015;6:38029–45. doi: 10.18632/oncotarget.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sotillos S, Krahn M, Espinosa-Vazquez JM, Hombria JC. Src kinases mediate the interaction of the apical determinant Bazooka/PAR3 with STAT92E and increase signalling efficiency in Drosophila ectodermal cells. Development. 2013;140:1507–16. doi: 10.1242/dev.092320. [DOI] [PubMed] [Google Scholar]
- 59.Ma X, Shao Y, Zheng H, Li M, Li W, Xue L. Src42A modulates tumor invasion and cell death via Ben/dUev1a-mediated JNK activation in Drosophila. Cell Death Dis. 2013;4:e864. doi: 10.1038/cddis.2013.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab. 2013;304:E145–59. doi: 10.1152/ajpendo.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cravo AS, Carter E, Erkan M, Harvey E, Furutani-Seiki M, Mrsny R. Hippo pathway elements Co-localize with Occludin: A possible sensor system in pancreatic epithelial cells. Tissue Barriers. 2015;3:e1037948. doi: 10.1080/21688370.2015.1037948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo J, McGinnis LK, Kinsey WH. Fyn kinase activity is required for normal organization and functional polarity of the mouse oocyte cortex. Mol Reprod Dev. 2009;76:819–31. doi: 10.1002/mrd.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ko M, Zou K, Minagawa H, Yu W, Gong JS, Yanagisawa K, et al. Cholesterol-mediated neurite outgrowth is differently regulated between cortical and hippocampal neurons. J Biol Chem. 2005;280:42759–65. doi: 10.1074/jbc.M509164200. [DOI] [PubMed] [Google Scholar]
- 64.He Y, Kapoor A, Cook S, Liu S, Xiang Y, Rao CV, et al. The non-receptor tyrosine kinase Lyn controls neutrophil adhesion by recruiting the CrkL-C3G complex and activating Rap1 at the leading edge. J Cell Sci. 2011;124:2153–64. doi: 10.1242/jcs.078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thaper D, Vahid S, Nip KM, Moskalev I, Shan X, Frees S, et al. Targeting Lyn regulates Snail family shuttling and inhibits metastasis. Oncogene. 2017;36:3964–75. doi: 10.1038/onc.2017.5. [DOI] [PubMed] [Google Scholar]
- 66.Morinaga T, Yanase S, Okamoto A, Yamaguchi N. Recruitment of Lyn from endomembranes to the plasma membrane through calcium-dependent cell-cell interactions upon polarization of inducible Lyn-expressing MDCK cells. Sci Rep. 2017;7:493. doi: 10.1038/s41598-017-00538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conway DE, Coon BG, Budatha M, Arsenovic PT, Orsenigo F, Wessel F, et al. VE-Cadherin Phosphorylation Regulates Endothelial Fluid Shear Stress Responses through the Polarity Protein LGN. Curr Biol. 2017;27:2219–25 e5. doi: 10.1016/j.cub.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


