Abstract
HIV-associated neurocognitive disorders affecting greater than 30% of patients are caused by HIV-1 infection of the CNS, and in part, include neurotoxic effects of the viral transactivator of transcription, Tat protein. In addition to increasing the risk for becoming HIV infected, cocaine abuse enhances the neuropathogenic impacts of HIV-1. To investigate the outcome of Tat and cocaine interference in the hippocampal neuronal network, microelectrode arrays were employed to develop a systematic framework to assess the rank-cross-correlation coefficient in cultured hippocampal neurons. Tat and cocaine differentially disturbed neuronal spiking rates, amplitude, synchronous activity and oscillations within the hippocampal neuronal network via potentiation of inhibitory neurotransmission. The Tat-mediated impairment of neuronal spiking was reversible by removal of Tat, which restored neuronal activity. The presence of astrocytes co-cultured with neuronal networks diminished the effects of Tat and cocaine on neuron function suggesting a role for astrocytes in stabilizing neuronal behavior and increasing neuronal spontaneous activities such as bursting amplitude, frequency and wave propagation rate. Taken together, our studies indicate that the HIV protein Tat and cocaine impair hippocampal neuronal network functioning and that the presence of astrocytes alleviates network dysfunction pointing to a newly discovered pathway through which ionic homeostasis is maintained by neuron-glial crosstalk in the CNS.
Keywords: Microelectrode arrays (MEAs), HIV-1 Tat, GABAA-Receptor, Hippocampal Neuron-Astrocyte Co-Culture
INTRODUCTION
Infection of the brain by human immunodeficiency virus-1 (HIV-1) may lead to neurocognitive disorders. Even with successful antiretroviral treatment, neurocognitive deficits remain one of the major comorbidities among HIV+ patients and the primary cause of HIV− associated neurological disorders (HAND) (Bilgrami et al, 2013). Although HIV-1 does not directly infect neurons, it infects astrocytes and microglia. The HIV-1 protein, Tat, is released from infected cells and induces a secondary pathological effect upon uptake by uninfected cells including neurons. Tat reduces neuronal excitability through damage to dendritic spines (Fitting et al., 2010) and affects synapto-dendritic stability, leading to behavioral deficits (Hahn et al., 2015). Tat also induces cell death and synapse loss through NMDA (N-methyl-D-aspartate) receptor activation (Shin et al., 2012, Krogh et al., 2014, Zou et al., 2015). Tat affects the dopaminergic system, particularly in the hippocampus (Silvers et al., 2007; Jacobs et al., 2013; Yuan et al., 2015; Schier et al., 2017). Tat decreases GABAergic (gamma-aminobutyric acid-ergic) neurotransmission efficacy through opioid receptors (Xu et al., 2016). Tat-mediated NMDA activation also results in NMDAR1 (NMDA receptor 1) overexpression, eventually leading to increased extracellular glutamate and apoptosis (Eugenin et al., 2003). Along with in vitro studies, increasing evidence from in vivo studies suggests Tat-induced neuronal injury and death (Fitting et al, 2010). In fact, Tat facilitates glutamate or NMDA-induced calcium flux (Haughey et al., 2001). Through possibly related mechanisms, Tat increases intracellular calcium levels and mitochondrial calcium uptake and induces reactive oxygen species production (Kruman et al., 1998). Over 20% of HIV+/AIDS patients use addictive drugs [(United Nations Office on Drugs and Crime, 2012)]. The interaction of HIV-1 and drug abuse contributes to the emergence and more rapid progression of the HAND symptoms. Several studies have shown that besides increasing the risk of HIV-1 transmission, cocaine abuse enhances the pathogenic impact of HIV-1 on the nervous system (Yang et al., 2010; Yao et al., 2012; Bush et al., 2012, Dahal et al., 2014; Wayman et al., 2015). Combined Tat and cocaine impair blood brain barrier integrity (Ghandi et al., 2010) and elevate permeability and susceptibility of cells to damage in different brain regions including the basal ganglia and cerebral cortex (Nath et al., 2001). Tat potentiates cocaine-induced psycho-stimulation (Paris et al., 2014). Cocaine and Tat also lead to impaired neuronal autophagy and contribute to accelerated apoptosis in the rat hippocampus (De Simone et al., 2016).
To investigate Tat interference in the hippocampal neuronal network, we employed microelectrode arrays (MEA) to develop a systematic framework to assess the rank-cross-correlation coefficient in cultured hippocampal neurons in the presence of Tat and cocaine. Being a copula-based correlation coefficient (Mohseni Ahooyi et al., 2015), the rank correlation is highly sensitive to nonlinearity and can accurately capture nonlinear correlations among recording electrodes. Using this approach, we proposed an algorithm to connect a directed causal link to an electrode where the activity is initiated to a subsequently activated electrode. Performing this analysis for all pairs of array electrodes, the outcome will be a time-varying directed causal network, which dynamically switches between different modes throughout the neuronal spiking activity. Using the estimated network, we introduced a method to compute the average network-wide wave propagation rate (WPR), offering another useful measure to study network behavior.
We applied this data based network analysis method to process MEA recordings from neuronal cultures undergoing Tat and cocaine treatments and to assess the electrophysiological activity and neuronal network properties. We show that Tat alters neuronal spike frequency, amplitude and WPR. Our data show that Tat’s effects are reversible, as Tat removal allows partial restoration of neuronal activity. In co-culture studies, we show the protective role of astrocytes against Tat and cocaine. Finally, we studied the effect of Tat in the presence of agonists of excitatory receptors and antagonists of inhibitory receptors. MEA recordings suggest that Tat reduces the excitability of hippocampal neurons through activation of GABAA receptors and partial deactivation of glutamate receptors.
MATERIALS AND METHODS
Primary Neuronal and Astrocyte Cultures
Hippocampal neurons were dissected and cultured from E18 rat embryos as described previously [Shekarabi, 2005]. Briefly, the tissues were treated with 0.25% trypsin for 18 mins at 37°C. They were then washed with Neurobasal media (Invitrogen, USA) containing 2% fetal calf serum, 2 mM glutamine and 100 U/ml penicillin and 100 U/ml streptomycin (Invitrogen, USA) and then dissociated into warm complete Neurobasal medium supplemented with 2% B27 (Invitrogen, USA). After 24 hours, the media replaced with complete media without serum. For MEA preparation, standard MEA60s (Multichannel Systems, Germany) with 60 titanium nitrate electrodes were sequentially coated with 20 g/ml of poly-D-Lysine (Sigma, USA) and 10 g/ml of laminine (Invitrogen, USA). Approximately 5×105 neurons were plated onto each MEA and maintained for 25 DIV (days in vitro) at 37°C and 5% CO2 prior to any treatments. Astrocytes were derived from the rat P3 neonatal pups. After dissection, the neonatal cortices were digested in 0.25% trypsin for 30 minutes. The heterogeneous cell mixture was plated on poly-D-lysine coated plates and maintained for 3 weeks with FBS-containing media in order to eliminate neurons. For mixed neuron-astrocyte co-cultures, 5×105 of E18 rat hippocampal neurons were co-cultured with astrocytes at a ratio of 1:1. Cells were cultured on MEA60 electrodes according to the above procedure and the cultures were maintained for 25 days prior to the treatments.
Tat and cocaine co-treatments
The cultures were treated daily with recombinant Tat (rTat) at 100 ng/ml (full length, 101 amino acids), Immunodiagnostics, MA, USA). Tat bioactivity was verified by the LTR-luciferase assay (data not shown). In separate experiments, neurons were transduced with adenovirus (Ad) particles expressing Tat 101 (Mukerjee et al., 2008) or Ad-Null viruses at an MOI of 1. Transduced neurons were allowed to express Tat protein for 72 hours prior to MEA recordings. Cocaine (Sigma-Aldrich) was applied daily at concentrations of 2 μM, 4 μM and 8 μM in the absence or presence of Tat.
MEA Recordings
MEA recordings of cultured neurons were performed according to the following timeline. First, the MEA activity was recorded before rTat treatments (day 0). Immediately thereafter, the first rTat treatment (100 ng/ml) was applied and 24 hours post-treatment, the activity was recorded (day 1). The same steps were repeated to obtain recordings at 48 hours post-treatment (day 2). The cultures were then allowed to grow without any rTat treatment for another two days prior to recordings on day 4. Following this recording, rTat was removed by washing the cultures twice with warm 37°C neurobasal medium and then fresh complete medium was added. Post-treatment Tat removal recordings were performed at 24 hours (day 6) and 72 hours (day 8).
Application of receptors agonists and antagonists
Agonists of excitatory and antagonist of inhibitory receptors were applied at the following concentrations and neuronal activities were recorded before and after the application of drugs to the control- and Tat-treated groups. Drugs included L-glutamic acid (10 μm) (Sigma-Aldrich, USA), glycine (1 μm) (Sigma-Aldrich), bicuculline (25 μm), CGP-54626 hydrochloride (5 μm), carbamoylcholine chloride (10 μm), and L-(−)-Norepinephrine (10 μm) (Cayman chemicals, Ann Arbor, MI). All drugs were added 15 minutes before the recordings. To evaluate the effect of agonists for both ionotropic and metabotropic, the cultures were also co-treated with L-glutamate and the NMDA receptor co-activator, glycine.
Cell Viability MTT Assay
To measure neuronal viability by the MTT assay, cells were plated on a 96-well plate (105 cells/well) and after two weeks were transduced with Ad-Null and Ad-Tat, MOI 3. After 72 hours post transduction, cells were incubated 4 hours at 37°C with 20 μL MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) solution (5 mg/ml). After removal of the media and suspension in DMSO, the absorbance was measure at 560 nm using a microplate reader (Fisher Scientific, USA).
Fluorescent Microscopy
Intracellular Tat was detected in Ad-Tat transduced cultures using immunocytochemistry. Neurons were transduced with Ad-Tat and Ad-Null on 14 DIV with MOI 3. After 72 hours, the cells were fixed and permeabilized using −20°C cooled acetone (Sigma, USA). Following blocking with (1%) BSA in PBST, the neurons were labeled with mouse monoclonal β3 tubulin and rabbit polyclonal Tat antibodies (Abcam, USA). Alexa Fluor®secondary antibodies (ThermoFisher Scientific, OR) and VECTASHIELD medium (Vector Laboratories) were used for labeling and mounting, respectively. Images were prepared via Leica fluorescent microscope (Leica Microsystems, IL).
Data Acquisition and Pre-Processing
All MEA recordings were performed with a frequency of 2 kHz and recorded on an MEA60 system (Multichannel systems, Germany). The msd raw data were transformed to the ASCII format and transferred into the MATLAB® (Mathworks) environment for further processing and analysis using our custom codes developed methods. To increase the signal to noise ratio, we used Fast Fourier Transform (FFT) to determine the frequency content of signals from the individual channels. Based on the resulting spectrum, we filtered the data with 6th-order Butterworth filter with a sampling frequency of 2 kHz and a cut-off frequency of 60 Hz. The filtered data from all channels was obtained for all experimental conditions, and based on these data, the connectivity and wave propagation rates were determined as described below.
MEA Data Analysis: Learning Functional Connectivity Using the Cross rank-Correlation
We calculated the cross-correlation of two time series at each time point over a time interval of Δ. Cross-correlation calculates the degree of association between a random variable Xi and a lagged copy of another random variable Xj. Once calculated, it is possible to confirm whether the maximum of the absolute cross-correlation function exceeds some pre-specified threshold . If this condition is confirmed, it indicates that there is a causal (direct or indirect) correlation between two recordings. To determine the direction of causality, the time at which this maximum is met (τ′) is calculated. If τ′ ≥ 0 then (Xj → Xi), otherwise Xi → Xj. As τ′ ≥ 0, we conclude Xj → Xi.
Conventional linear cross correlation is defined as follows:
where Cov and Var denote the covariance and variance function, respectively. Although using this coefficient is a common practice, it has limitations; linear cross-correlation, as suggested by its name, is only capable of estimating the linear association between two variables. When two variables are nonlinearly dependent, linear cross-correlation fails to give a reliable measure since it approaches zero as nonlinearity increases. In this case, nonlinearity is treated as non-dependence, which renders the method unreliable for complex and nonlinear systems. To overcome this major limitation, we proposed the application of Spearman’s cross rank-correlation, which is defined as the linear correlation of the rank-transformed variables:
where Yi is the rank transformation of Xi and calculated as:
where 1{.} is the indicator function, n is the number of samples and χi,k denotes the k-th sample of Xi. The linear and rank correlations are of two random variables are close when the underlying association is linear. It is also clear that the existence of randomness (e.g. noise or disturbance) lowers the correlation. On the other hand, the lower row reveals the weakness of linear rank correlation as, unlike its rank counterpart, linear correlation interprets nonlinearity as uncorrelated.
This simple fact can have profound implications on the linear correlation concept as opposed to rank correlation. Assume that at some point τ′ within the horizon Δ, linear cross- correlation reaches some maximum while cross rank-correlation meets its maximum at τ″, , consequently this change in the calculation method may lead to a significant discrepancy between the estimated direction of causality and the wave propagation speed. Returning to our example, since the linear correlation cannot capture the nonlinear dependence at time point τ″ = 41 s, it mistakenly detects the peak correlation at τ′ = 17 s.
Built using this rationale, we propose replacing the linear- with cross rank-correlation. Adopting this approach, we can then take all possible pairs of (with m denoting the number of recording electrodes) and calculate at the moving horizon time steps lΔ. Note that is called the rank autocorrelation coefficient which always has its maximum at τ = 0 as this always holds. The result will give a fully directed correlation network which estimates the functional connectivity of the neuronal culture in the interval . Where Δ is a design parameter and is a function of the neuronal activity and MEA sampling rate. Once the neuronal functional connectivity is determined for each interval, the network-wide average wave propagation rate (WPR) is calculated as:
where Li,j is the euclidean distance of the electrodes (Xi, Xj).
Experimental Design and Statistical Analysis
For each set of experiments, neurons were dissociated from the hippocampi of n≥12 rat E18 embryos. For each experimental condition, we employed two MEA replicates. Each MEA gives rise 60 simultaneous high throughput recording data. The significance was assessed through the student’s t test. The bar graphs represent mean ± StDev.
RESULTS
HIV-1 Tat attenuates neuronal network activity
We developed a computational method to assess the pathological effects of Tat protein and cocaine on a neuronal network established from hippocampal rat neurons cultured onto MEAs. We cultured these neurons for 25 days to allow for the formation of synapses. The cultures were then transduced with Ad-Null and Ad-Tat. Seventy-Two hours after Ad-Tat transduction, the network neuronal activity showed a significant suppression when compared to the pretransduced cultures (Fig. 1A). This extensive attenuation is population-wide as captured by the MEA network-wide recordings. The raster plots of the individual spikes revealed that within 60 seconds of recording from 60 MEA electrodes for Ad-Null (comment) and Tat-transduced cultures, a significant reduction in overall network activity was observed in Tat-transduced cultures (Fig. 1B). This latter effect is efficiently measurable through the WPR whereby, Tat caused a 3-fold decrease in the normal WPR compared to a near zero value (Fig. 1C). Tat also increased the average distance between spikes in the spike train. The probability density analysis of the detected spikes showed that this measure was increased 5-fold in the presence of Tat (20 ms) as opposed to the pre-treatment (4 ms) (Fig. 1D). The network-wide average auto-correlograms of Fig. 1E illustrated that Tat altered the normal self-similarity patterns (top) of the neuronal LFP waves (bottom). This effect can be caused both by lower intensity of the neuronal firing and higher randomness in the firing times. The frequency content analysis of the LFP wave further revealed that Tat suppresses neuronal oscillations particularly in the range of 0–5 Hz (Fig. 1F). Signal analysis revealed that the expression of Tat almost completely attenuated normal neuronal spiking by reducing the spike amplitude and firing frequency compared to the pre-treatment condition and Ad-Null transduced (control) cultures (Fig. 1G and 1H). Furthermore, Tat appears to reduce the number of correlated electrodes through the course of recordings while it caused a decrease in the average number of normal connections between the MEA electrodes is reduced by a factor of two when Tat is applied (Fig. 1I). Microscopic examinations revealed no detectable structural abnormalities of neuronal processes resulting from the Ad-Tat treatments (Fig. 1.J).
Figure 1. Tat mediated impairments of neuronal activity.
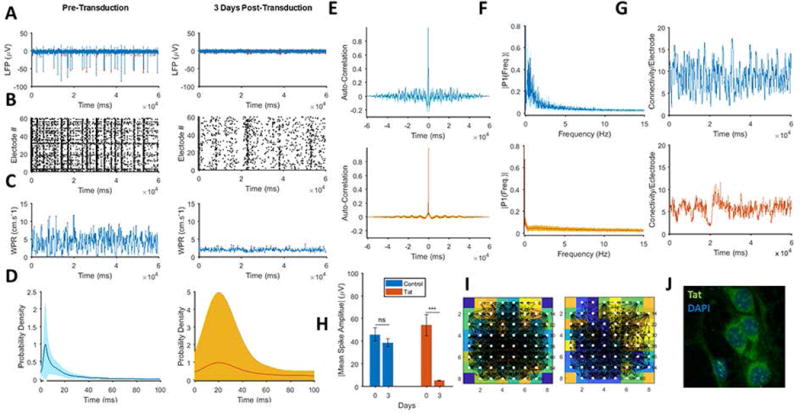
A. Adenovirus-mediated Tat expression attenuates neuronal firing amplitude (left) to near-silence low-amplitude spikes (right). B. Raster plots indicate significant alterations of neuronal firing in all electrodes following Tat transduction (left) vs. 3 days post-transduction of Ad-Tat (right). Tat results in fewer numbers of bursts and fewer numbers of spikes per burst. C. Wave propagation rate (WPR) of pre- (left) and post-Tat-transduction (right) cultures shows three-fold reduction of peak WPR in presence of Tat. D. Tat transduction increases average inter-spike intervals from 4 ms (left) to 20 ms (right). E. Autocorrelation shows changes in self-repeating patterns of the LFP wave between pre- (top) and post-Tat-transduction (bottom). As illustrated by these panels, Tat increases the randomness and reduces the periodicity of the neuronal activity oscillations. F. Fast Fourier Transformation (FFT) of LFP time-domain displays Tat-induced suppression of low frequency components of the neuronal activity. G. The average degree of network wide connectivity, which indicates wave similarity among all different pairwise selections of electrodes, shows a significant decrease by a factor of two under the Tat-transduction (bottom). H. Tat expression (red) significantly decreases average spike amplitudes in comparison to the control, which were transduced with Ad-Null. I. Functional connectivity architecture was derived for the pre- (left) and post-Tat treatments (right) at a sample spike propagation moment, to show cross rank correlation-based network, displays an altered connectivity map with fewer links. J. Tat expression in the hippocampal neurons transduced with Ad-Tat. Quantified data show mean ± StDev, as determined by student t-test, ***= p<0.001.
In order to investigate the ability of exogenous Tat protein to suppress neuronal spiking, 100 ng/ml rTat was applied to cultures which resulted in a significant attenuation of the neuronal activity both in the firing rate and amplitude of the neuronal spikes of the wave propagation rates within 24 h post-treatment (Fig. 2A–2C and 2E–2F). rTat also increased the intervals between the spikes from 4 ms to 20 ms (Fig. 2D, left and middle panels) and altered the 0–5 Hz frequency content of the LFP waves (Fig. 2H, top and middle panels). Additional rTat treatment of the cells for one more day did not further suppress the neuronal firing rate when compared to results from the first day, but altered the noise (random oscillations) in the recordings. We also observed that rTat continued its suppressive effect until day 4 post-treatment, even though no further rTat was applied to the cultures. On the second day of rTat treatment, the neuronal signals seemed to completely lose their self-similarity, which may be a sign of randomness or an increase in the spontaneous and non-synchronous activity of neurons. Surprisingly, the autocorrelation plots continued to regress to their initial state as rTat was removed, suggesting a partial restoration in the neuronal oscillatory behavior. Interestingly, the rTat removal led to a partial restoration of the neuronal activity to the observed levels on day 1, when recorded on days 6 and 8. This restoration occurred in the neuronal bursting (Fig. 2B), wave propagation rate (Figs. 2C and 2G), average inter-spike intervals (Fig. 2D), firing frequency (Fig. 2E) and firing amplitude (Fig. 2F). However, the frequency content of the LFP wave did not show much improvement compared to the rTat application (Fig. 2H). After removing of rTat from the culture media, this trend continued to a point where the neuronal firing rate was doubled on day 8 in the Tat-treated group. The amplitudes of the recorded activities did not show any significant restoration compared to the initial condition on day 0. Based on this experiment, it was concluded that the detrimental effects of the rTat on the neuronal firing rate, amplitude, synchronous activity and oscillations is partially restored upon removal of rTat, while the amplitudes remained at a much lower average magnitude compared to their pretreatment state.
Figure 2. Effect of addition and wash out of soluble recombinant Tat (rTat) on restoration of neuronal activity.
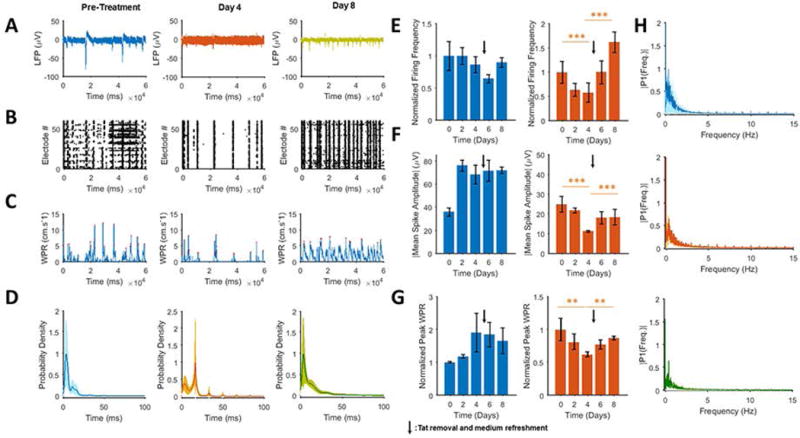
A. Extracellular action potential recordings of pre-treatment (left), after two 100 ng/day applications of rTat (middle) and four days post-rTat removal (right) shows that rTat attenuates neuronal spiking, both in its frequency and amplitude. rTat removal restores the firing frequency but only partially restores the amplitude. B. Raster plots shows that 48 hours of rTat treatment (middle) reduces the number of bursts by two fold. Highly synchronized bursts were restored throughout the network after rTat removal (right). C. Calculation of WPRs, corresponding to the LFP recordings, demonstrates restoration of the network-wide activity; pretreatment (left), after rTat (middle) and after rTat removal (right). D. Probability density peak of the inter-spike distributions shows a remarkable shift from 4 ms (pretreatment) to 20 ms (rTat treatment) and a rebound to 4 ms after rTat removal. E. Time variations of the normalized firing frequency of rTat-treated neurons compared to the control culture (heat inactivated Tat-treated). The firing rebound is apparent from day 4 onward for the rTat groups. F. Time variations of the normalized absolute peak firing amplitudes of the rTat (right) vs. control (left). Similar to the firing frequency, the spike amplitudes undergo restoration upon rTat removal. G. Normalized mean peak WPR also shows a reduction and partial restoration upon application and removal of rTat (right), while the control group follows an increasing and decreasing pattern (left) H. Frequency content spectrum of the LFP signal for pretreatment (top), rTat applied (middle) and rTat removed (bottom) groups indicate the lowered power of 0–5 Hz components in presence of rTat, however removal of rTat does not improve this parameter. Quantified data show mean ± StDev; as determined bystudent t-test, **= p<0.01, ***= p<0.001.
Combined effect of Tat and Cocaine on the neuronal network
The Tat-induced impairments were further investigated in the presence of cocaine. Hippocampal neuronal cultures were recorded before and after application of 2 μM, 4 μM and 8 μM of cocaine to the Ad-Null and Ad-Tat groups. Following the recordings, we analyzed the rank autocorrelations of the signals for different experimental conditions. Both Tat and cocaine treatments abolished the average autocorrelation effect of the MEA signals, indicating that there was a strong Tat and cocaine-induced impairment in neuronal firing in terms of the self-correlation behavior (Fig. 3A and 3B). As expected, this negative effect is more significant with higher dosages of cocaine as well as in the presence of Tat which further attenuated the neuronal spiking (Fig. 3B). To study the effect of Tat and cocaine on the neuronal network functional connectivity and conduction velocities of the wave propagations, we employed our proposed approach to calculate directed neuronal network within the subsequent 100 ms intervals over the recording horizon for each of the 10 conditions (Fig. 3B). The neuronal cultures displayed highly connected functional networks during each firing and had very sparse functional networks in the refractory periods. Using the estimated networks, we calculated the WPR at each of the 100ms intervals over the recording horizon. The averaged WPR for the Ad-Null and Ad-Tat treated cultures at different dosages of cocaine were then calculated (Fig. 3C). Each peak on the pretreatment curves corresponds to one burst. Interestingly, Ad-Tat decreased the conduction velocities approximately 50% compared to the pretreatment condition (data not shown). Higher dosages of cocaine had the opposite effect on Ad-Null transduced cultures and increased the averaged WPR in these cultures (Fig. 2C). Higher cocaine concentrations alleviated the conduction velocities of Tat treated cultures further at higher concentrations of cocaine. These experiments showed a dose dependent increase in the peak wave propagation rate in Ad-Tat transduced cultures.
Figure 3. Dose response effect of cocaine on the spiking activity.

A. Increasing the cocaine concentration up to 8 μM (from left to right) in the Ad-Null-expressing group increases the spiking activity and amplitude of the firing. B. Increasing the cocaine concentration up to 8 μM (from left to right) in the Ad-Tat-expressing group decreases the spiking activity and amplitude of the firing. C. Normalized peak WPR shows a decrease at a cocaine concentration of 2 μM and then rises constantly as the concentration increases (top). Higher concentrations of cocaine decrease WPR in the Ad-Tat-expressing group. Quantified data show mean ± StDev;as determined by student t-test, **= p<0.01, ***= p<0.001.
Tat expression alters firing properties neurotransmitter receptors
Tat-induced impairments of neuronal spiking strongly suggested that Tat may directly or indirectly induce changes in the activities of neurotransmitters receptors or the number of their represented synapses within the network. To examine this possibility, we performed experiments aimed at studying predominantly expressed neuronal excitatory and inhibitory receptors in the hippocampus including glutamate receptors, GABA (A and B), norepinephrine and acetylcholine receptors.
To investigate the effect of glutamate receptors, agonists of ionotropic and metabotropic glutamate receptors (including NMDA receptor), L-glutamate and glycine, were added to cultures already transduced by Ad-Null or Ad-Tat for 3 days. While the Ad-Null transduced cells underwent a normal bursting activity, after 15 minutes post-L-glutamate and glycine treatments, they displayed an elevated number of bursts, with the firing amplitude not significantly affected (Figs. 4A and 4B). However, unlike Ad-Null transduced cultures, activation of the glutamate receptors did not have a significant effect on restoring the amplitude or frequency of bursts in Ad-Tat transduced neurons (Figs. 4C and 4D). In the latter, the detected spontaneous bursting (Fig. 4C) occurred with limited frequency and amplitude compared to the pre-treatment (Figs. 4C and 4D, left panels). As suggested by Fig. 4E, in both Ad-Null (left panel) and Tat-expressing (right panel) cultures, the application of glutamate and glycine does not lead to a significant increase in firing amplitude. The normalized firing frequency of both groups showed an increase with higher ratio in the Tat expressing cultures (Fig. 4F). This is primarily due to the negligible activity of the Tat expressing neurons before applying glutamate and glycine. The same trend was observed for the WPR, as glutamate receptors were activated and the WPR was increased in Ad-Ad-Null and Ad-Tat-treated cultures (Fig. 4G, right panel).
Figure 4. Activation of glutamate receptors does not avert Tat-induced silencing of neuronal activity.
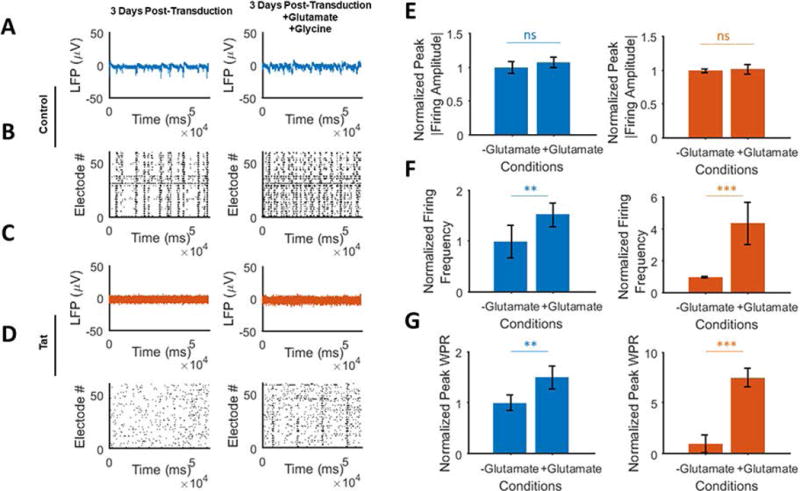
A. Extracellular action potential recordings of, 3 days post-transduction before (left) and after (right) glutamate and glycine additions in Ad-null expressing cultures. B. Raster plots of the detected spikes are shown (bottom row) that there is no attenuation of the activity associated with Ad-Null and a significant increase in the number of bursts as a result of GluRs activation. C. MEA recordings are shown for cultures after transduction with Ad-Tat before (left) and after (right) glutamate and glycine additions. D. Spikes raster plots of the glutamate receptor inhibitor treated Tat expressing neurons show the total disruption of the spiking activity (right) compared to the non-treated cultures (left). E. No significant changes were detected following GluR receptor activation in the amplitude of firings in both Ad-Null (left) and Ad-Tat (right) groups. F. A 4-fold increase in the spike frequencies of both groups, particularly in Ad-Tat group is shown. G. Normalized peak WPRs derived from multi-dimensional signal processing of 60 MEA electrodes show a significant increase in Tat expressing neurons (right), suggesting that GluR activation facilitates the signals propagation in presence of Tat. Quantified data show mean ± StDev;as determine by student t-test, n.s. not significant, **= p<0.01, ***= p<0.001.
To assess the effect of adrenergic receptors agonists, norepinephrine (NE), a potent agonist of adrenergic receptors (Griffith et al., 2008), was applied to the control and Tat expressing cultures to examine the neuronal network activity following inhibition of α and β adrenergic receptors. In Ad-Null transduced cultures, NE induced an increase in spontaneous firing activity throughout the network with lower amplitude bursts compared to the pre-treatment (Figs. 5A and 5B). However, the application of norepinephrine only led to a limited increase in the Tat-expressing neurons in terms of the bursting amplitude and frequency compared to pretransduction (Figs. 5C and 5D). NE application to Ad-Null-expressing cultures decreased the firing amplitude, increased the firing frequency over two fold and reduced WPR (Figs. 5E–5G). On the other hand, Tat-expressing neurons displayed significant increases in the mean peak absolute firing amplitude, frequency and WPR as a result of NE when compared to the pre-NE condition. However, when normalized with respect to the control, the level of activity restoration was not significant.
Figure 5. Activation of norepinephrine (NE) in the presence of Tat.
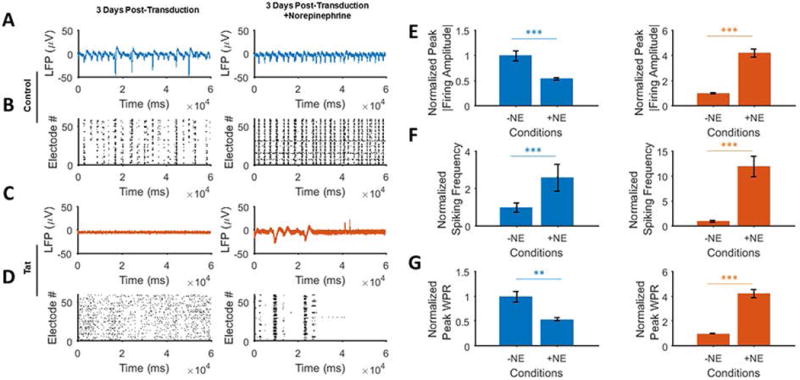
A. EAP recordings before (left) and after (right) application of the norepinephrine receptor agonist, norepinephrine (NE), in Ad-Null transduced cultures 3 days post-transduction. NE increases the spontaneous firing while reducing the firing amplitude. B. Raster plots of the detected spikes show a significant elevation of spike generation and bursting as a result of NE (right) in Ad-Null transduced cells. C. EAP recordings before (left) and after (right) application of norepinephrine in the Tat expressing group, 3 days post-transduction. Application of NE caused some limited firing activity (right) as compared to pre-NE (left). D. Detected spikes raster plots of the Tat expressing neurons show the generation of a few average-sized bursts (right). E. Norepinephrine application shows a reverse effect on the control (left, decrease in amplitude) and Tat expressing groups (right, increase in amplitude). F. There is an apparent increase in the spike frequencies in both groups, particularly the Tat group showed a 12-fold increase. G. Normalized peak WPRs derived from the multi-dimensional signal processing of 60 MEA electrodes show a significant increase in Tat expressing neurons (right), with a relative decrease in the control group (left). Quantified data show mean ± StDev; as determined by student t-test, **= p<0.01, ***= p<0.001.
To evaluate the effect of cholinergic receptors agonist carbachol was used as an agonist of cholinergic receptors (nicotinic acetylcholine receptors (nAChR) and muscarinic acetylcholine receptors (mAChR) (McEvoy et al., 1992). When applied to Ad-Null transduced neurons, carbachol reduced spiking, synchrony and frequency of spontaneous firing but the firing amplitude remained unchanged (Figs. 6A and 6B). However, Tat-induced neuronal silencing was restored in part by the activation of cholinergic receptors compared to the normal firing activity (Figs. 6C and 6D). The normalization compared to the pre-drug-treatment of the control group showed no effect of carbachol on the firing frequency amplitude and WPR, respectively (Figs. 6E–6G). The same parameters showed a large increase when normalized to the silenced activity of the Tat-expressing neurons (Figs. 6E–6G, left panels). However, like the glutamate and adrenergic activation experiments, the restoration was negligible compared to the post-treatment control group.
Figure 6. Activating the acetylcholine receptors in the absence and presence of Tat.
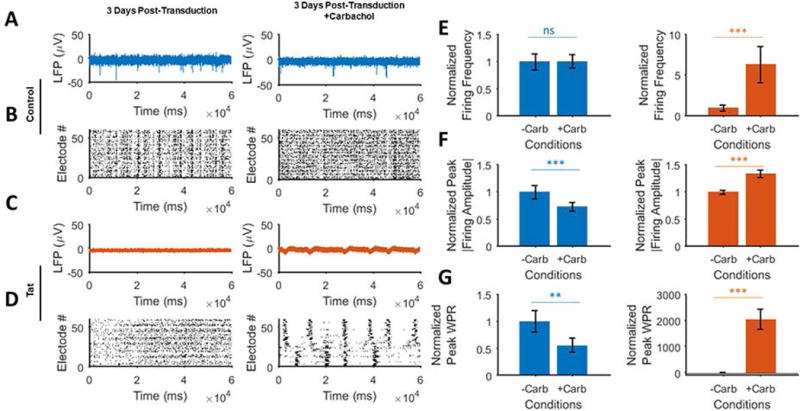
A. Extracellular action potential recordings before (left) and after (right) application of the AChR receptors agonist, carbachol (carbamylcholine) in the control (Ad-Null) group 3 days post-transduction. Carbachol partially increases the spontaneous firing while reducing the firing amplitude. B. Raster plots of the detected spikes show increased asynchronous activity (right) in the control group compared to the baseline activity (left). C. Extracellular action potential recordings before (left) and after (right) application of carbachol in the Tat expressing group 3 days post-transduction. Application of carbachol gives rise to increased firing activity (left) as compared to the pre-carbachol condition (left). D. Detected spikes raster plots of the Tat expressing neurons show the generation of regularly spaced spike trains (right) as opposed to no spontaneous firing (left) before carbachol application. E. Carbachol application shows a negligible effect on the control (left) compared to the Tat expressing groups (right, increased amplitude). F. There is a decrease in the spike frequencies for the control group (left) and increase in the Tat expressing group (right). G. Normalized peak WPRs derived from the electrodes data analyses show a significant increase in Tat expressing neurons (right), with a relative decrease in the control group (right). Quantified data show mean ± StDev as determined by student t-test, n.s. not significant, **= p<0.01, ***= p<0.001.
Lastly, we suppressed GABA receptors, bicuculline and CGP-54626 hydrochloride, as antagonists of GABAA and GABAB receptors, respectively. Furthermore, bicuculline is known as a weak antagonist of inhibitory glycine receptors and a reversible blocker of Ca2+-activated potassium channels (Khawaled et al., 1999). CGP-54626 is also known as a potent and selective antagonist of GABAB receptors (Cruz et al., 2004). The effect of GABAA on the extracellular field potential of Ad-Null-expressing neurons was tested by MEAs in the presence of bicuculline. The relatively long bursts of the pre-treated control neurons (left panel) were shorter in duration and displayed double bursting frequency after bicuculline treatment (Figs. 7A and 7B). Further inhibition of GABAB in Ad-Null-expressing neurons caused lower amplitude, lower frequency and significantly longer bursts. To examine the possibility of Tat-associated enhanced inhibitory behavior, Tat-induced silenced neurons were treated with bicuculline and CGP-54626 (together or separately). Within 15 minutes of bicuculline treatment, neurons generated regularly spaced and medium to high amplitude bursts compared to the pre-bicuculline (Figs. 7C and 7D). The emerging bursts were of higher frequency, lower amplitude and shorter duration and refractory period compared to Ad-Null-expressing cultures. Ad-Tat response to further inhibition of GABAB was also similar to Ad-Null-expressing cultures, but with less robust features. In this case, Tat caused slightly fewer but higher-duration spikes with no significant amplitude change compared to the bicuculline only treated neurons. It is worth mentioning that CGP-54626 resulted in weaker bursts in the Tat group and did not reduce the bursting frequency as low as Ad-Null-expressing cultures (Fig. 7E). In both groups, bicuculline alone or bicuculline and CGP-54626 applications led to a considerable increase in the number of spikes. While bicuculline+CGP-54626 reduced the peak amplitude of the firings compared to bicuculline in Ad-Null-expressing cultures (Fig. 7E, left), this was increased in the Tat-expressing cultures (Fig. 7E, right). Moreover, the average refractory period of the firings reached half of the pre-bicuculline condition. At the same time, the amplitude of firings increased by about 50% (Fig. 7F). Finally, both Ad-Null- and Ad-Tat-expressing cultures displayed significant increases in the WPR, particularly compared to the near-zero WPR in the Tat-expressing neurons (Fig. 7G).
Figure 7. Neuronal network analysis of inhibition of GABA receptors in the presence of Tat.
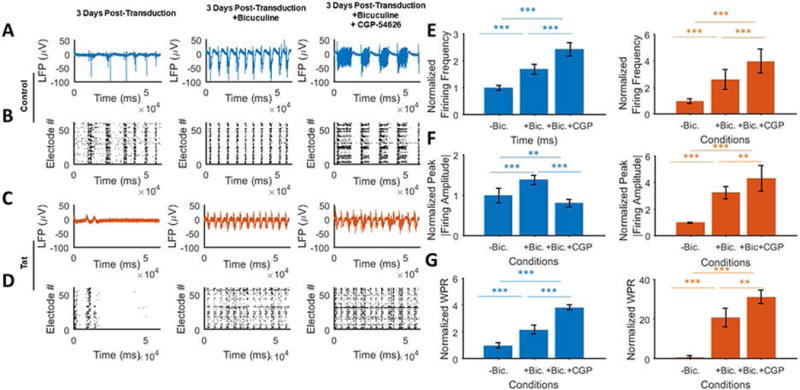
A. Extracellular action potential recordings of cultured neurons in the absence of GABA receptors inhibitors (left) and after applying bicuculline alone (GABAA receptor antagonist) (middle) or with CGP-54626 hydrochloride (GABAB receptor antagonist) (right) in Ad-Null transduced cultures. Inhibition of both GABA receptors leads to an immediate increase in the number and amplitude of the firing activity, and long bursts. B. Raster plots (bottom) of the detected spikes show highly synchronous bursting and long spiking activities induced by GABA inhibition. C. MEA recordings of pre- (left) and post-GABA-receptors inhibition in the presence of Tat expression (middle and right). Similar to the control group, GABA receptor inhibition leads to high amplitude and long bursts. While the firing amplitude is lower than the control group, the rebound activity is higher spontaneous bursting. D. Detected spikes raster plots of the Tat expressing neurons under the GABAA (middle) and GABAA and GABAB receptors inhibition (right). In the latter case, unlike the control groups, which show longer and fewer bursts, GABAB receptor antagonist results in slightly longer bursts without lowering their number. E. Normalized firing frequency of control (left) vs. Tat expressing (right) cultures. Both groups show a relatively proportional increase in the number of spikes when exposed to the antagonists. This response is more significant in the Tat group. F. Normalized peak firing amplitude of the control (left) and Tat expressing (right) groups. While bicuculline increases the absolute amplitude in both cases, the control group experiences a reduction as a result of CGP-54626. G. GABA receptor inhibition significantly increases the wave propagation rates in the neuronal network, with higher folds for the Tat groups. This implies that the lowered neuronal activity in the case of Tat is not primarily due to injury of neuronal processes, metabolic impairments or ion channel down regulation, but is due in large part to the increased inhibitory effect or decreased excitatory effects. Quantified data show mean ± StDev as determined by student t-test, **= p<0.01, ***= p<0.001.
Neuron-astrocyte co-cultures mitigate Tat-mediated Silencing
It has been demonstrated that HIV-1 neurotoxic peptides released by HIV-1 infected glial cells (such as astrocytes) have detrimental effects on the non-HIV infected neighboring neurons (Conant et al., 1998; Nath et al., 1999, Chauhan et al., 2003; Zhou et al., 2004; Fan et al., 2016). To investigate the effects of the presence of astrocytes on Tat expressing neurons, we co-cultured rat prenatal neurons and neonatal astrocytes (Fig. 8A) along with purely neuronal cultures (Fig. 8B) and then transduced them with Ad-Null or Ad-Tat. At 3 days post-transduction, intracellular Tat led to significantly reduced activity in the neurons, but minimally mitigated the activity in neuron-astrocyte co-cultures (Figs. 8C–8F). In summary, in all conditions (Ad-Null, Ad-Tat, Ad-Null+cocaine, Ad-Tat+cocaine), the neuron-astrocyte co-cultures exhibited superior firing activities in terms of bursting frequency and amplitude. Also, Ad-Null- and Ad-Tat-expressing neurons and neuron-astrocytes responded consistently to cocaine (2 μM) by an increased bursting frequency and lowered amplitude (Figs. 8G–8I). In comparison, Ad-Null- and Ad-Tat-expressing neuron-astrocyte co-cultures exhibited higher wave propagation rates compared to the neurons alone under similar conditions (data not shown). These results revealed an increase in the WPR’s of Ad-Null-expressing cultures (with less steep changes in the astrocyte co-cultures) from day 0 to day 3. Unlike neurons, WPR of Tat-expressing neurons did not show any significant decrease induced by Tat, however, like neurons the Tat-expressing co-culture displayed some level of cocaine-induced WPR reduction, while cocaine increases WPR in Ad-Null-expressing co-culture (Fig. 8G). As indicated by the data quantification, in all conditions cocaine application resulted in a firing amplitude reduction, which was negatively affected by the presence of Tat. The effect was much weaker compared to the pure neuron culture. In all conditions, cocaine treatments led to an increase in overall number of spiking (as with the bursting) except Tat-expressing co-cultures which displayed more bursts and fewer total spikes. This is mainly because of the shorter bursts in this group. Tat protein also reduced the spiking activity in neurons but had no significant impact on the co-culture network activity compared to the pre-transduction. The relative robustness of the co-culture to the Tat and cocaine can also be understood by the inter-spike interval analysis. The average interval remained the same (~4 ms) for all co-culture conditions (control and cocaine, Fig. 8J).
Figure 8. The effect of Tat and cocaine on neuron-astrocyte co-cultures.
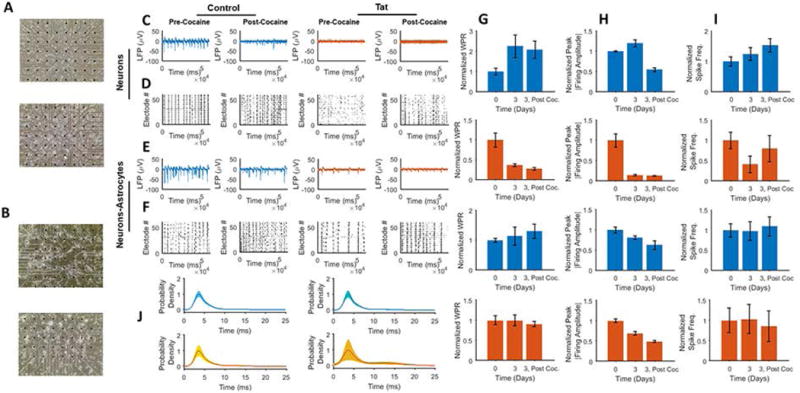
A. MEA neuronal culture 2 DIV (top) and 16 DIV (bottom) B. MEA neuron-astrocyte co-culture 2 DIV (top) and 16 DIV (bottom). C. Extracellular recordings of neuronal culture responses to cocaine (2 μM), from left to right Ad-Null, Ad-Null w/ cocaine, Ad-Tat and Ad-Tat w/ cocaine. In both control and Tat-expressing groups, cocaine increases the number of firings while it decreases the amplitude. D. Raster plots of the detected spikes of neuronal culture treated with from left to right: Ad-Null, Ad-Null w/ cocaine, Ad-Tat and Ad-Tat w/ cocaine. In both control and Tat groups, cocaine leads to increased spontaneous bursting activity. E. Extracellular recordings of neuron-astrocyte co-cultures responding to cocaine (2 μM), from left to right Ad-Null, Ad-Null w/ cocaine, Ad-Tat and Ad-Tat w/ cocaine. In both control and Tat-expressing groups, cocaine increases the number of firings while it decreases the amplitude. F. Raster plots of the detected spikes of neuron-astrocyte co-cultures treated with (from left to right): Ad-Null, Ad-Null w/ cocaine, Ad-Tat and Ad-Tat w/ cocaine. In both control and Tat groups, cocaine leads to increased spontaneous bursting activity. G. Normalized mean peak WPR for (from top to bottom): control neurons, Tat expressing neurons, control neuron-astrocyte co-cultures, Tat expressing neurons with astrocytes. H. Normalized mean peak absolute amplitude for (from top to bottom): control neurons, Tat expressing neurons, control neuron-astrocyte co-culture, Tat expressing neuron with astrocyte co-cultures I. Normalized firing frequency for (from top to bottom): control neurons, Tat expressing neurons, control neuron-astrocyte co-cultures, Tat expressing neurons with astrocyte co culture. J. Inter-spike probability densities for neurons (top) before and after cocaine, and co-cultures (bottom), before and after cocaine. Quantified data show mean ± StDev as determined by student t-test.
DISCUSSION
Tat-Mediated Attenuation of Neuronal Activity
Our in vitro MEA studies of Ad-Tat transduced and rTat-treated primary hippocampal neurons show that Tat protein suppresses hippocampal neuronal network activity. Several studies have concluded that Tat protein enhances inhibitory neurotransmission in hippocampal neurons by increasing GABAergic signaling and loss of excitatory synapses (Hargus and Thayer 2013; Shin and Thayer 2013; Kim et al. 2008). However, others have reported that Tat protein induces excitability in neurons in vitro and in vivo using single cell patch clamping (Brailoiu et al. 2008; Xu et al. 2016; Xu and Fitting 2016; Rusnati et al. 1997). Here, we have expanded our studies to compile spiking signals from the hippocampal neuronal network in vitro. Earlier works studying the Tat effects have employed single-cell patch electrodes either on cultures or tissue sections [Song et al., 2003; Brailoiu et al., 2008; Xu et al., 2016]. To the best of our knowledge, this is the first study of a network-wide activity assessment using MEA analyses of hippocampal neurons in the presence of HIV-1 Tat protein and cocaine. Using this technique, MEAs were used to record thousands of neurons in a simultaneous, multi-site and non-invasive manner. The multi-dimensional data were later processed using our proposed method of cross-rank correlation to determine the population-wide effects of Tat protein. These effects are observable in different aspects of neuronal firing including, reducing the numbers of bursts, spikes per burst, firing amplitude, our proposed wave propagation rate measure, and increases in inter-spike intervals and refractory periods. Tat also diminished the self-similarity and oscillatory behavior of the neuronal electrical activities, as captured by auto-correlation spectrums. Similar effects were also been achieved with recombinant HIV-1 Tat protein. The activity of the network was partially restored after removing rTat within the course of 4 days. This restoration is more apparent in the neuronal bursting frequency, inter-spike period and WPR. Although the peak amplitude did not recover completely, compared to the pre-treatment condition, our data implies that Tat’s effects occur rather quickly after application and is progressive over time, and the effect is reversible following its removal. This is consistent with previous studies where the application of rTat protein caused reversible loss of synaptic terminals within 24 hours of the treatment in hippocampal neurons (Shin and Thayer 2013; Kim et al. 2008).
Tat Promotes Neuronal Silencing Primarily by Potentiation of Inhibitory Neurotransmission
Consistent with previous studies which suggested that rat fetal neurons display resistance to HIV-1 Tat toxicity in culture (Aksenova et al. 2009), our MTT viability assay showed no significant loss of neurons in the presence of Tat, suggesting Tat-mediated synaptic impairments rather than neuronal cell death. Tat may affect stability of synaptic proteins. In fact, Tat protein has been linked to the overexpression of proteins (e.g. gephyrin) involved in the inhibitory synapses in rat hippocampal neurons (Hargus et al., 2013) or in the hippocampus of Tat-transgenic mice (Fitting, 2013). In the cortex and striatum, there is evidence of Tat-induced decreases in the GABAergic neurotransmission (Musante et al., 2009; Xu et al., 2016a; 2016b) and increased cortical excitatory activities in mice, rat and human (Musante et al., 2009; Brailoiu et al., 2008). Tat has also been reported to activate the NMDA receptors in human cortex (Magnuson et al., 1995), rat cortex (Haughey et al., 2001) and hippocampus (Haughey et al., 2001; Song et al., 2003, aksenov et al., 2012). Extracellular glutamate levels were shown to increase in the presence of Tat (Eugenin et al., 2003; Gupta et al., 2010). Our observations in the rat hippocampus suggest that Tat interferes in the neuronal communications either through potentiation of the inhibitory, or weakening of the excitatory properties of neurons. The application of agonists of predominantly expressed excitatory receptors (glutamate, adrenergic, cholinergic) and antagonists of GABAergic inhibitory receptors confirmed that Tat potentiates the inhibitory activity of neuronal networks of hippocampal neurons in these cultures. This is supported by minimal effects on excitatory receptors agonists on increasing the firing activity compared to the control groups. In addition, the adrenergic receptors show the maximum response to the activation and glutamate receptors remained almost unresponsive. This observation implies the presence of an excessive inhibitory mechanism that overpowers all the excitatory pathways. Intriguingly, inhibiting the GABA receptors by bicuculline and CGP-54626 to inhibit GABAA and GABAB, respectively, immediately restored the spontaneous firing with a frequency greater than that of bicuculline-treated control cultures, indicating that Tat modulates the neuronal activity through the GABAA receptor. These results are consistent with earlier works suggesting an increase in the number of inhibitory synapses by Tat-mediated gephyrin (a postsynaptic protein involved in organizing GABAA receptor subtypes) overexpression (Hargus et al., 2013; Fitting, 2013). The fact that the frequency of bursting in the bicuculline-treated Tat-expressing neurons is even higher than bicuculline-treated control cultures may indicate the elevated background excitatory activity which is likely to be a result of Tat-mediated glutamate accumulation (Musante et al., 2009; Summa et al. 2010). This may explain the lack of neuronal response in Tat-treated neurons since glutamate already exists at a higher level. The bicuculline-induced restoration also exhibited lower amplitude of firing compared to the control, indicating possible effects of Tat on neuronal bioenergetics. Inhibition of GABAB, on the other hand, did not alter the neuronal excitability in the Tat-expressing group.
Astrocytes exhibit neuroprotective effects against Tat and cocaine
In HIV-infected patients, astrocytes may be infected by HIV-1 (Chauhan et al., 2003). Astrocytes are generally known for their neuroprotective role, however in the case of HIV-1 infection they become sources of HIV-1 associated neurotoxic protein production and release (Conant et al., 1998; Nath et al., 1999, Chauhan et al., 2003; Zhou et al., 2004; Fan et al., 2016). Our data show that the presence of astrocytes increased neuronal spontaneous activities such as bursting amplitude and frequency and WPR. Furthermore, the presence of astrocytes seemed to stabilize the neuronal activity measures when the cultures were treated by cocaine. Astrocytes also prevented the complete silencing of neuronal activity mediated by Tat, which confirms the neuroprotective role of astrocytes compared to the pure neuronal culture. These experiments indicate that astrocytes modulation of ionic homeostasis of surrounding neurons also determines the overall network response to Tat. This observation is supported by findings that astrocytes e enhance synaptic interconnectivity and the formation and stability of excitatory synapses (Clarke et al., 2013; Sloan et al., 2014).
Acknowledgments
This work was supported by grants P01DA037830 and P30MH092177 awarded by the NIH to KK. The authors wish to thank past and present members of the Department of Neuroscience and Center for Neurovirology for their support and sharing of ideas and reagents. We especially thank Dr. George Smith for his critical reading and comments on this work.
Contract grant sponsor: NIDA/NIH; Contract grant number: P01DA037830
Contract grant sponsor: NIMH/NIH; Contract grant number: P30MH092177
Footnotes
Conflict of Interests: The authors declare no competing financial interests.
References
- Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. D1/NMDA receptors and concurrent methamphetamine+ HIV-1 Tat neurotoxicity. J Neuroimmune Pharmacol. 2012;7:599–608. doi: 10.1007/s11481-012-9362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Adams SM, Mactutus CF, Booze RM. Neuronal survival and resistance to HIV-1 Tat toxicity in the primary culture of rat fetal neurons. Experimental Neurol. 2009;215:253–263. doi: 10.1016/j.expneurol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchetti A, Gullo F, Bruno G, Dossi E, Lecchi M, Wanke E. Exact distinction of excitatory and inhibitory neurons in neural networks: a study with GFP-GAD67 neurons optically and electrophysiologically recognized on multielectrode arrays. Front Neural Circ. 2012;6:63. doi: 10.3389/fncir.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgrami M, O’Keefe P. Neurologic diseases in HIV-infected patients. Handbook of Clinical Neurology. 2013;121:1321–1344. doi: 10.1016/B978-0-7020-4088-7.00090-0. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Brailoiu E, Chang JK, Dun NJ. Excitatory effects of human immunodeficiency virus 1 Tat on cultured rat cerebral cortical neurons. Neuroscience. 2008;151:701–710. doi: 10.1016/j.neuroscience.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Yao H, Guo M, Mori T, Mathias-Costa B, Singh V, Seth P, Wang J, Su TP. Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Current HIV Res. 2012;10:425–428. doi: 10.2174/157016212802138823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, Major EO, Nath A. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278:13512–13519. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nature Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. Bi-directional effects of GABA B receptor agonists on the mesolimbic dopamine system. Nat Neurosc. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Dahal S, Chitti SV, Nair MP, Saxena SK. Interactive effects of cocaine on HIV infection: implication in HIV-associated neurocognitive disorder and neuroAIDS. Front Microbiol. 2014;6:931–931. doi: 10.3389/fmicb.2015.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone FI, Darbinian N, Amini S, Muniswamy M, White MK, Elrod JW, Datta PK, Langford TD, Khalili K. HIV-1 tat and cocaine impair survival of cultured primary neuronal cells via a mitochondrial pathway. J Neuroimmune Pharmacol. 2016;11:58–368. doi: 10.1007/s11481-016-9669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Riedel-Kruse IH, Nawroth JC, Roukes ML, Laurent G, Masmanidis SC. High-resolution three-dimensional extracellular recording of neuronal activity with microfabricated electrode arrays. J Neurophysiol. 2009;101:1671–1678. doi: 10.1152/jn.90992.2008. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Fan Y, He JJ. HIV-1 Tat promotes lysosomal exocytosis in astrocytes and contributes to astrocyte-mediated Tat neurotoxicity. J Biol Chem. 2016;291:22830–22840. doi: 10.1074/jbc.M116.731836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi N, Saiyed ZM, Napuri J, Samikkannu T, Reddy PV, Agudelo M, Khatavkar P, Saxena SK, Nair MP. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: Implications for HIV-1–associated neurocognitive disorder. J Neurovirol. 2010;16:294–305. doi: 10.3109/13550284.2010.499891. [DOI] [PubMed] [Google Scholar]
- Griffith RK. Adrenergic receptors and drugs affecting adrenergic neurotransmission. Foyes principles of medicinal chemistry. 2008;6:392–416. [Google Scholar]
- Gupta S, Knight AG, Gupta S, Knapp PE, Hauser KF, Keller JN, Bruce-Keller AJ. HIV-Tat elicits microglial glutamate release: Role of NAPDH oxidase and the cystine–glutamate antiporter. Neuroscience Lett. 2010;485:233–236. doi: 10.1016/j.neulet.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargus NJ, Thayer SA. Human immunodeficiency virus-1 Tat protein increases the number of inhibitory synapses between hippocampal neurons in culture. J Neurosci. 2013;33:17908–17920. doi: 10.1523/JNEUROSCI.1312-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. Jo Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Jäckel D, Frey U, Fiscella M, Franke F, Hierlemann A. Applicability of independent component analysis on high-density microelectrode array recordings. J Neurophysiol. 2012;108:334–348. doi: 10.1152/jn.01106.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MM, Murray J, Byrd DA, Hurd YL, Morgello S. HIV-related cognitive impairment shows bi-directional association with dopamine receptor DRD1 and DRD2 polymorphisms in substance-dependent and substance-independent populations. J Neurovirol. 2013;19:495–504. doi: 10.1007/s13365-013-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protocols. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kapucu FE, Välkki I, Mikkonen JE, Leone C, Lenk K, Tanskanen JM, Hyttinen JA. Spectral entropy based neuronal network synchronization analysis based on microelectrode array measurements. Front Comput Neurosci. 2016;10:112. doi: 10.3389/fncom.2016.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflügers Archiv:Eur J Physiol. 1999;438:314–321. doi: 10.1007/s004240050915. [DOI] [PubMed] [Google Scholar]
- Krogh KA. Doctoral dissertation. University of Minnesota; 2014. The mechanism of HIV-1 Tat-induced changes in NMDA receptor function. [Google Scholar]
- Leergaard TB. Mapping the connectome: multi-level analysis of brain connectivity. Frontiers E-books. 2012 doi: 10.3389/fninf.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccione A, Gandolfo M, Massobrio P, Novellino A, Martinoia S, Chiappalone M. A novel algorithm for precise identification of spikes in extracellularly recorded neuronal signals. J Neuroscience Methods. 2009;177:241–249. doi: 10.1016/j.jneumeth.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non—N‐methyl‐D‐aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995;37:373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- McEvoy GK, editor. American Hospital Formulary Service – Drug Information 92. Vol. 1992. Bethesda, MD: American Society of Hospital Pharmacists Inc; 1992. p. 1657. Plus Supplements 1992. [Google Scholar]
- Mohseni Ahooyi T, Arbogast JE, Soroush M. Applications of the rolling pin method. 1. An efficient alternative to Bayesian Network modeling and inference. Indust Engin Chem Res. 2014;54:4316–4325. [Google Scholar]
- Mukerjee R, Deshmane SL, Fan S, Del Valle L, White MK, Khalili K, Amini S, Sawaya BE. Involvement of the p53 and p73 transcription factors in neuroAIDS. Cell Cycle. 2008;7:2682–2690. doi: 10.4161/cc.7.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musante V, Summa M, Neri E, Puliti A, Godowicz TT, Severi P, Battaglia G, Raiteri M, Pittaluga A. The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cerebral Cortex. 2009;20:1974–1984. doi: 10.1093/cercor/bhp274. [DOI] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, McLaughlin JP. Effects of conditional central expression of HIV-1 tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice. Neuropsychopharmacology. 2014;39:380. doi: 10.1038/npp.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore VP, Poli D, Godjoski A, Martinoia S, Massobrio P. ToolConnect: A functional connectivity toolbox for In vitro networks. Front Neuroinform. 2016;10:13. doi: 10.3389/fninf.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G. Cytoplasmic permeation pathway of neurotransmitter transporters. Biochemistry. 2011;50:7462–7475. doi: 10.1021/bi200926b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan SA, Barres BA. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Cur Opin Neurobiol. 2014;27:75–81. doi: 10.1016/j.conb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Nath A, Geiger JD, Moore A, Hochman S. Human immunodeficiency virus type 1 Tat protein directly activates neuronal N-methyl-D-aspartate receptors at an allosteric zinc-sensitive site. J Neurovirol. 2003;9:399–403. doi: 10.1080/13550280390201704. [DOI] [PubMed] [Google Scholar]
- Summa M, Severi P, Puliti A, Raiteri M, Pittaluga A. The HIV-1 viral protein Tat modulates glutamate and GABA exocytosis from human and mouse neocortical nerve endings by acting at different binding sites. Retrovirology. 2010;7(S1):P5. doi: 10.1093/cercor/bhp274. [DOI] [PubMed] [Google Scholar]
- Ullo S, Nieus TR, Sona D, Maccione A, Berdondini L, Murino V. Functional connectivity estimation over large networks at cellular resolution based on electrophysiological recordings and structural prior. Front Neuroanat. 2014;8:137. doi: 10.3389/fnana.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) World Drug Report 2012. New York: UNODC; 2012. (United Nations publication, Sales No. E.12.XI.1). [Google Scholar]
- Wayman W, Chen LL, Persons A, Napier T. Cortical consequences of HIV-1 Tat exposure in rats are enhanced by chronic cocaine. Curr HIV Res. 2015;13:80–87. doi: 10.2174/0929867322666150311164504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Fitting S. Inhibition of GABAergic neurotransmission by HIV-1 Tat and opioid treatment in the striatum involves μ-opioid receptors. Front Neurosci. 2016;10:497. doi: 10.3389/fnins.2016.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Hermes DJ, Mackie K, Lichtman AH, Ignatowska-Jankowska BM, Fitting S. Cannabinoids occlude the HIV-1 tat-induced decrease in GABAergic neurotransmission in prefrontal cortex slices. J Neuroimmune Pharmacol. 2016a;11:316–331. doi: 10.1007/s11481-016-9664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yao H, Lu Y, Wang C, Buch S. Cocaine potentiates astrocyte toxicity mediated by human immunodeficiency virus (HIV-1) protein gp120. PLoS One. 2010;5:e13427. doi: 10.1371/journal.pone.0013427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Yang L, Buch S. Platelet-derived growth factor-BB restores Human immunodeficiency Virus Tat–cocaine-mediated impairment of neurogenesis: Role of TRPC1 Channels. J Neurosci. 2012;32:9835–9847. doi: 10.1523/JNEUROSCI.0638-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap PT, Wu G, Shen D. Human brain connectomics: networks, techniques, and applications [life sciences] IEEE Signal Processing Magazine. 2010;27:131–134. [Google Scholar]
- Yuan Y, Huang X, Midde NM, Quizon PM, Sun WL, Zhu J, Zhan CG. Molecular mechanism of HIV-1 Tat interacting with human dopamine transporter. ACS Chem Neurosci. 2015;6:658–665. doi: 10.1021/acschemneuro.5b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BY, Liu Y, Kim Bo, Xiao Y, He J. Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol Cell Neurosci. 2004;27:296–305. doi: 10.1016/j.mcn.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Zou S, Fuss B, Fitting S, Hahn YK, Hauser KF, Knapp PE. Oligodendrocytes are targets of HIV-1 Tat: NMDA and AMPA receptor-mediated effects on survival and development. J Neurosci. 2015;35:11384–11398. doi: 10.1523/JNEUROSCI.4740-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


