Abstract
Objective
Some atypical antipsychotics are associated with metabolic side effects, which are risk factors for gestational diabetes. The study aim was to examine the risk of gestational diabetes associated with continuation during pregnancy compared to discontinuation of aripiprazole, ziprasidone, quetiapine, risperidone, or olanzapine.
Methods
Non-diabetic pregnant women with a live-born infant in Medicaid (2000–2010) who had ≥ 1 antipsychotic dispensing during the 3-months before pregnancy were included. For each antipsychotic, women with ≥ 2 dispensings (continuers) were compared to women with no dispensing during the first half of pregnancy (discontinuers). A generalized linear model and propensity score stratification was used to obtain absolute and relative risks of gestational diabetes, adjusting for confounders.
Results
Among 1,543,334 pregnancies, the number of baseline antipsychotic users was 1,924 for aripiprazole, 673 for ziprasidone, 4,533 for quetiapine, 1,824 for risperidone, and 1,425 for olanzapine. Continuers generally had higher comorbidity and longer baseline antipsychotic use. The crude risk of gestational diabetes for continuers vs. discontinuers, respectively, was 4.8% vs.4.5% for aripiprazole, 4.2% vs. 3.8% for ziprasidone, 7.1% vs. 4.1% for quetiapine, 6.4% vs. 4.1% for risperidone, and 12.0% vs. 4.7% for olanzapine. The adjusted relative risks were 0.82 (0.50–1.33) for aripiprazole, 0.76 (0.29–2.00) for ziprasidone, 1.28 (1.01–1.62) for quetiapine, 1.09 (0.70–1.70) for risperidone, and 1.61 (1.13–2.29) for olanzapine.
Conclusion
Compared to women who discontinued before the start of pregnancy, those who continued olanzapine or quetiapine had an increased risk of gestational diabetes that may be explained by the metabolic effects associated with the treatment.
INTRODUCTION
Gestational diabetes mellitus is a complication of pregnancy, defined as carbohydrate intolerance with onset or recognition during pregnancy.1 It can lead to adverse pregnancy outcomes such as preeclampsia, cesarean delivery, neonatal hypoglycemia, and macrosomia.2 The estimated prevalence of gestational diabetes in the United States (US) ranged between 4.6% and 9.2% in 2010.3 Up to 50% of women with gestational diabetes develop type 2 diabetes mellitus in the decades following pregnancy,4 a risk over seven times higher than that in women without gestational diabetes.5 Gestational diabetes shares many risk factors with type 2 diabetes mellitus including older age, non-white race, and obesity.2,6
There is a well-recognized association between treatment with some atypical antipsychotic medications and metabolic side effects including weight gain and diabetes in the general population.7–11 The US Food and Drug Administration (FDA) required all manufacturers of atypical antipsychotics to add a warning for risk of hyperglycemia and diabetes to their labels in 2003. However, the metabolic safety of antipsychotics for pregnant women, whom are already predisposed to insulin resistance,12 is not fully understood. A small number of studies and case reports suggested an increased risk of gestational diabetes for antipsychotic users during pregnancy,13–16 but recent studies did not find any association.17,18 Furthermore, while there are differences in the severity of metabolic side effects between antipsychotics19 and biochemical evidence explaining such differences exists,20 information on the comparative risk of gestational diabetes is scarce.21
Psychiatric disorders treated with antipsychotics, such as bipolar disorder, are often recognized during the reproductive age range22 and have a significant impact on the health and wellness of patients around the time of pregnancy.23 Despite limited safety information regarding their use in pregnancy, an increasing number of women at this age are treated with antipsychotics in the US.24–26 While for some women treatment continuation during pregnancy is necessary to prevent the sequelae of untreated mental illness,4 for others clinicians must weigh the risks and benefits of continuing treatment during pregnancy and may consider discontinuation or a switch to an alternative treatment. Understanding the potential risk of developing gestational diabetes, and how this risk may vary by the type of antipsychotic utilized, is an important consideration for patients and clinicians weighing these risks. Previous studies compared users of antipsychotics with non-users to assess the risk of gestational diabetes associated with the drug.15–18 Such studies are prone to confounding by indication, as women who do not take antipsychotics are different from women who require antipsychotic treatment around the time of pregnancy in many ways that might affect the risk of gestational diabetes, such as having a healthier life style and diet patterns. In a nationwide cohort of pregnant women who were all treated with antipsychotics prior to the start of pregnancy, we therefore compared the risk of gestational diabetes between women who continued antipsychotic treatment during pregnancy and those who discontinued before the start of pregnancy.
METHODS
Data Source and Study Population
The Medicaid Analytic eXtract (MAX) is a person-level nationwide claims database, which contains information on demographics, hospitalizations, outpatient visits, and pharmacy dispensing records. We created a cohort of pregnant women linked to their live-born infants from MAX (2000–2010),27 which has been successfully used in recent studies of medication safety in pregnancy.28–30 Women were required to have continuous Medicaid coverage from 3 months before the last menstrual period to one month after delivery and not have other insurance benefits, which may lead to incomplete ascertainment of claims.
The study cohort consisted of women who filled a prescription for one of the five most frequently used atypical antipsychotics (hereafter referred to as antipsychotics) namely aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone during the 3 months before the last menstrual period. Women with pre-existing diabetes were excluded since they are not at risk for developing gestational diabetes. To identify these women, we modified an algorithm developed and validated by Andrade et al31 (see Figure S1 for a detailed description of the algorithm).
Outcome definition
Based on the algorithm by Andrade et al,31 we classified as gestational diabetes cases those women who (1) had two or more diagnosis codes for any diabetes between 141 days after last menstrual period and delivery; and (2) who had a glucose tolerance test or a gestational diabetes diagnosis in the same time frame. The original algorithm had a positive predictive value of 88% in detecting gestational diabetes cases in claims data. We compared the results with or without considering metformin as an antidiabetic medication because it is sometimes used to treat polycystic ovary syndrome. The results were identical, so metformin was included.
Exposure definition
We defined the exposure during the first 140 days of pregnancy (Figure S1). ‘Continuers’ were defined as women with two or more additional dispensings for the same drug they used before pregnancy during the first 140 days. ‘Discontinuers’ were defined as women without any antipsychotic dispensing during the first 140 days of pregnancy. In dose-response analyses, we also included women with only one dispensing during the first 140 days to estimate the relationship at lower doses. We excluded women with dispensings for a different drug during pregnancy than the one received before the start of pregnancy and women who were dispensed more than one type of antipsychotic during the first 140 days of pregnancy. As a result, the five exposure groups were mutually exclusive. In addition, we combined the users of individual drugs to form three ‘risk-stratified groups’, based on the drugs’ weight gain potential and risk of diabetes outside of pregnancy.7 Aripiprazole and ziprasidone were in the low-risk group, quetiapine and risperidone in the medium-risk group, and olanzapine constituted the high-risk group.
Covariates
Covariates for confounding adjustment were assessed from 3 months before to 3 months after the last menstrual period. The covariates were selected based on clinical plausibility as confounders or proxies of confounders for the association between antipsychotic continuation and gestational diabetes, and included demographics (age, race, Medicaid eligibility type), psychiatric diagnoses (anxiety disorder, attention-deficit hyperactivity disorder (ADHD), bipolar disorder, depression, schizophrenia or other psychoses, other psychiatric disorders), comorbidity (pain disorders, hypertension, obesity, dyslipidemia), other medication use (anticonvulsants, antidepressants, anxiolytics, benzodiazepines, mood stabilizers (other than antipsychotics), opioids, other hypnotics, stimulants, antihypertensives), history of gestational diabetes, and the duration of antipsychotic treatment received in the 3 months before the last menstrual period. We quantified the number of different generics received and the number of emergency department visits during the 3 months prior to the last menstrual period to capture health services utilization as a general marker of the extent of comorbid illness.
Analyses
Analyses by individual drugs
The analyses were done separately for each of the five antipsychotics. We first examined the characteristics of the continuers and discontinuers of each antipsychotic. The unadjusted risk differences per 100 women (RD100) and relative risks with corresponding 95% confidence intervals (CI) were estimated using generalized linear regression models with identity (for RD100) or log link (for relative risk). Propensity score stratification was used to adjust for confounding.32 The propensity score was the predicted probability of continuing the treatment as opposed to discontinuing after last menstrual period, estimated by logistic regression with all covariates mentioned above. After trimming patients in the non-overlapping parts of the propensity score distribution,33 we created 50 strata based on the distribution of the propensity score among continuers. Weighted generalized linear models were used to estimate adjusted RD100 and relative risk along with 95% CIs, weighting the discontinuers based on the distribution of the continuers across the strata. To address potential residual confounding, we added covariates with a standardized difference that remained > 0.1 after propensity score weighting to the outcome model, and examined if this changed the interpretation of the result from the model without additional covariates.
We explored dose-response relationships between the risk of gestational diabetes and the cumulative dose of each antipsychotic over the 140-day exposure window. Restricted cubic splines were used to allow for non-linear relationships, adjusting for known or suspected risk factors of gestational diabetes including age, non-white race, obesity, diagnosis of schizophrenia or bipolar disorder, and the duration of antipsychotic use during the 3-month baseline period.34
Analyses by risk-stratified drug group
Several additional analyses were conducted at the risk-stratified drug group level due to the small number of patients in the drug specific analyses. First, we restricted the analyses to women with a recorded diagnosis of the approved indications for antipsychotics (schizophrenia, bipolar disorder, or depression) with a rationale that some antipsychotics are used off-label for non-psychiatric conditions such as insomnia at different doses35 and the different usage of these drugs may be associated with different baseline risks of gestational diabetes. Second, we extended the baseline period to 6 or 12 months before the last menstrual period in the subsets of women who had Medicaid eligibility during this time to assess whether a longer baseline period allowed for better confounding control. For the same reason, we used the high-dimensional propensity score algorithm to empirically identify 50 additional covariates that may serve as proxies of unmeasured confounders and used them in the propensity score model alongside the pre-defined covariates.36
Assessing the impact of missing obesity information
Because obesity is one of the most important risk factors for gestational diabetes but is incompletely captured in claims data, we conducted a bias analysis to examine the extent to which adjustment for confounding by unmeasured or poorly measured obesity would change the observed associations.37 The prevalence estimate of overweight or obesity was obtained from the Massachusetts General Hospital registry for pregnant women with psychiatric illness.38 Informed by the literature, we assumed that overweight or obese women have 4 times the risk of gestational diabetes compared to non-obese women,39 and examined the potential bias over a range of obesity prevalence differences (0 to 25%) between continuers and discontinuers.
All analyses were performed using R (R Core Team, 2016) and SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Among 1,543,334 linked pregnancies in MAX, we identified 1,924 women who met our inclusion criteria with a filled prescription for aripiprazole during the 3 months prior to the last menstrual period, 673 for ziprasidone, 4,533 for quetiapine, 1,824 for risperidone, and 1,425 for olanzapine. In the first exclusion step, the proportions excluded with pre-existing diabetes were 4.9%, 6.5%, 4.6%, 5.3%, and 4.5%, respectively (Figure S2). Depending on the drug, 19.7% to 34.0% continued treatment during the first half of pregnancy (Table 1). Continuers were generally older, had more psychiatric diagnoses and medication use, were more likely to have obesity diagnoses, and had used antipsychotics for a longer duration before the last menstrual period (Table S1). After propensity score weighting, most patient characteristics were well balanced between continuers and discontinuers, except for a few important covariates such as obesity and bipolar disorders which remained slightly unbalanced between the olanzapine continuers and discontinuers (Table 1, Table S2).
Table 1.
Selected patient characteristics comparing continuers to discontinuers of each atypical antipsychotic medication, weighted by propensity score
| Group Na | Aripiprazole | Ziprasidone | Quetiapine | Risperidone | Olanzapine | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cont. 416 % |
Disc.1421 % |
Cont. 140 % |
Disc. 431 % |
Cont. 1542 % |
Disc. 2951 % |
Cont. 343 % |
Disc. 1449 % |
Cont. 375 % |
Disc. 978 % |
|
| Age and race | ||||||||||
| Mean age (SD)b | 24.8 (7.2) | 24.4 (6.7) | 25.0 (6.4) | 25.0 (5.4) | 26.8 (6.4) | 26.5 (6.3) | 25.3 (7.4) | 25.5 (7.5) | 28.5 (6.9) | 27.1 (6.4) |
| White | 66.6 | 68.6 | 67.9 | 68.2 | 72.4 | 72.5 | 49.3 | 50.3 | 51.2 | 52.6 |
| Black | 19.7 | 19.2 | 20.7 | 21.0 | 15.4 | 16.0 | 30.3 | 30.9 | 24.5 | 23.4 |
| Other race | 13.7 | 12.3 | 11.4 | 10.8 | 12.1 | 11.5 | 20.4 | 18.8 | 24.3 | 24.1 |
| Mental health diagnosis | ||||||||||
| ADHD | 9.4 | 9.2 | 10.7 | 10.0 | 8.3 | 8.3 | 14.9 | 15.0 | 4.5 | 5.3 |
| Bipolar disorder | 44.5 | 45.0 | 44.3 | 45.2 | 35.9 | 35.3 | 25.1 | 25.6 | 31.7 | 41.9 |
| Schizophrenia/Other psychoses | 10.1 | 10.7 | 17.1 | 17.8 | 7.4 | 8.2 | 17.8 | 18.7 | 22.9 | 24.1 |
| Depression | 39.2 | 38.0 | 41.4 | 39.8 | 42.9 | 43.7 | 44.3 | 45.6 | 38.7 | 39.3 |
| Anxiety disorder | 24.8 | 25.9 | 22.1 | 22.6 | 28.7 | 29.5 | 19.8 | 19.3 | 20.8 | 24.1 |
| Comorbidity and other psychotropic use | ||||||||||
| Prior GDMc | 4.8 | 4.7 | 2.1 | 1.6 | 4.9 | 5.2 | 3.2 | 2.8 | 2.9 | 1.5 |
| Obesity | 5.3 | 4.6 | 3.6 | 4.5 | 2.3 | 2.2 | 2.3 | 1.7 | 2.4 | 0.7 |
| Antidepressants | 71.6 | 71.8 | 73.6 | 72.4 | 75.6 | 75.5 | 72.6 | 72.2 | 70.7 | 71.2 |
| Benzodiazepines | 33.7 | 35.2 | 39.3 | 40.5 | 39.4 | 38.9 | 23.6 | 24.7 | 29.1 | 31.3 |
| Mood stabilizersd | 29.6 | 28.9 | 31.4 | 35.4 | 31.8 | 31.3 | 28.6 | 32.1 | 21.3 | 26.5 |
| Opioids | 35.8 | 38.1 | 40.7 | 40.3 | 46.7 | 47.1 | 27.4 | 27.7 | 32.8 | 34.1 |
| Antipsychotic use in 90 days before LMP | ||||||||||
| Exposed ≤30 days | 20.2 | 20.0 | 18.6 | 17.3 | 19.3 | 19.3 | 27.7 | 28.0 | 20.5 | 21.0 |
| Exposed >30 days, ≤60 days | 30.8 | 31.2 | 23.6 | 25.8 | 20.2 | 20.4 | 28.9 | 29.9 | 25.9 | 23.6 |
| Exposed >60 days | 49.0 | 48.8 | 57.9 | 56.9 | 60.5 | 60.2 | 43.4 | 42.1 | 53.6 | 55.4 |
Number of subject in each group after trimming and stratification
Age was categorized in propensity score models but presented with the mean in this table for simplicity
The presence of a diagnosis of GDM in a previous pregnancy was assessed based on all available data before the start of the index pregnancy
Mood stabilizers: Lithium, Carbamazepine, divalproex, lamotrigine, oxcarbazepine, topiramate, valproic acid, valproate sodium
Bolded cells: Absolute Standardized difference > 0.1 after propensity score weighting.
Cont: Continuers; Disc: Discontinuers; SD: standard deviation; ADHD: attention-deficit hyperactivity disorder; GDM: gestational diabetes; LMP: last menstrual period
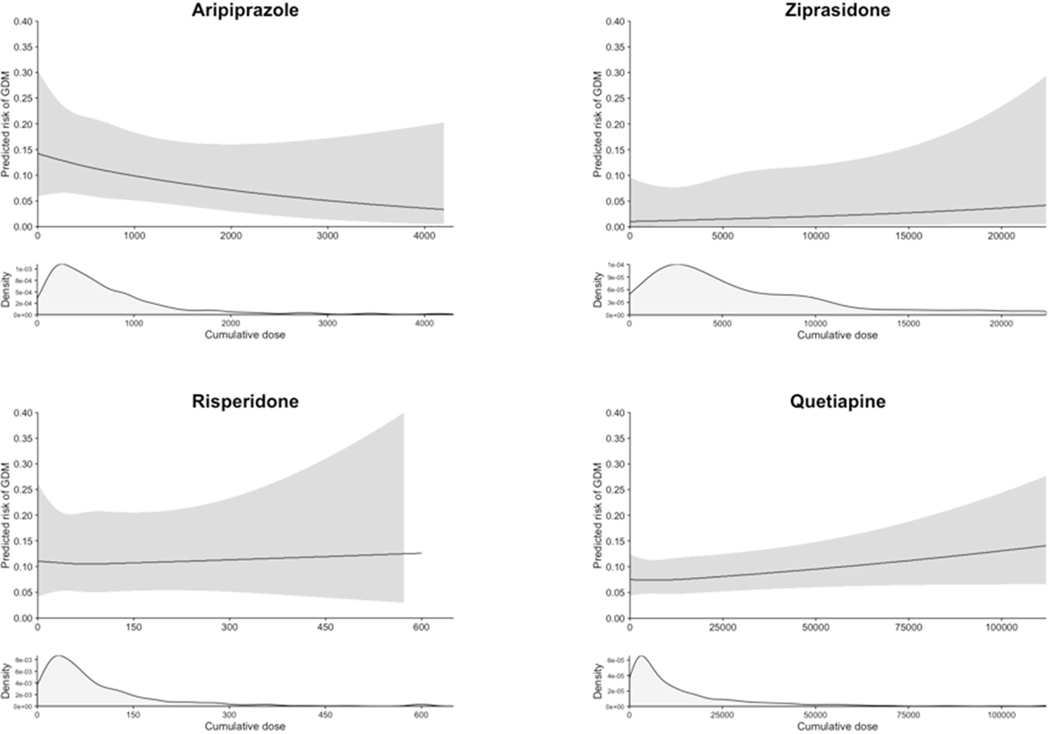
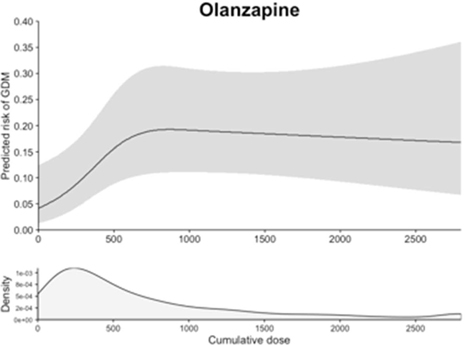
The absolute risk of gestational diabetes ranged from 4.2% to 12.0% in continuers and from 3.8% to 4.7% in discontinuers (Table 2 and Figure 1), depending on the drug considered. The unadjusted relative risk of gestational diabetes associated with continuing the medication during the first 140 days of pregnancy was 1.06 (95% CI 0.65–1.72) for aripiprazole, 1.12 (0.48–2.61) for ziprasidone, 1.75 (1.36–2.24) for quetiapine, 1.56 (0.98–2.49) for risperidone, and 2.55 (1.73–3.74) for olanzapine (Table 2). There was evidence for an elevated risk of gestational diabetes after confounding adjustment for olanzapine (adjusted relative risk=1.61, 1.13–2.29) and quetiapine (1.28, 1.01–1.62), but not for aripiprazole (0.82, 0.50–1.33), ziprasidone (0.76, 0.29–2.00), and risperidone (1.09, 0.70–1.70). In dose-response analysis, the risk increased with increasing cumulative dose of olanzapine until about 700 mg and plateaued thereafter (Figure 2). No clear trend was seen for other antipsychotics considering the width of the confidence band.
Table 2.
Unadjusted and adjusted risk of gestational diabetes, comparing continuers to discontinuers of each antipsychotic medication or group
| Na | Case (n) | Unadjusted | Adjusted | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk | RD100 | (95% CI) | RR | (95% CI) | Risk | RD100 | (95% CI) | RR | (95% CI) | ||||
| Aripiprazole | Continuer | 419 | 20 | 4.8% | 0.3 | (−2.0, 2.6) | 1.06 | (0.65, 1.72) | 4.6% | −1.0 | (−3.4, 1.3) | 0.82 | (0.50, 1.33) |
| Discontinuer | 1505 | 68 | 4.5% | 5.6% | |||||||||
| Ziprasidone | Continuer | 167 | * | 4.2% | 0.4 | (−3.0, 3.9) | 1.12 | (0.48, 2.61) | 3.6% | −1.1 | (−4.8, 2.6) | 0.76 | (0.29, 2.00) |
| Discontinuer | 506 | 19 | 3.8% | 4.7% | |||||||||
| Quetiapine | Continuer | 1543 | 110 | 7.1% | 3.1 | (1.6, 4.5) | 1.75 | (1.36, 2.24) | 7.1% | 1.6 | (0.0, 3.1) | 1.28 | (1.01, 1.62) |
| Discontinuer | 2990 | 122 | 4.1% | 5.6% | |||||||||
| Risperidone | Continuer | 359 | 23 | 6.4% | 2.3 | (−0.4, 5.0) | 1.56 | (0.98, 2.49) | 6.7% | 0.6 | (−2.4, 3.5) | 1.09 | (0.70, 1.70) |
| Discontinuer | 1465 | 60 | 4.1% | 6.1% | |||||||||
| Olanzapine | Continuer | 384 | 46 | 12.0% | 7.3 | (3.8, 10.8) | 2.55 | (1.73, 3.74) | 11.7% | 4.4 | (0.8, 8.1) | 1.61 | (1.13, 2.29) |
| Discontinuer | 1041 | 49 | 4.7% | 7.3% | |||||||||
| Low risk group | Continuer | 586 | 27 | 4.6% | 0.3 | (−1.6, 2.2) | 1.07 | (0.70, 1.62) | 4.5% | −0.4 | (−2.4, 1.5) | 0.91 | (0.60, 1.39) |
| Discontinuer | 2011 | 87 | 4.3% | 4.9% | |||||||||
| Medium risk group | Continuer | 1902 | 133 | 7.0% | 2.9 | (1.6, 4.2) | 1.71 | (1.38, 2.13) | 7.0% | 1.9 | (0.6, 3.2) | 1.37 | (1.12, 1.69) |
| Discontinuer | 4455 | 182 | 4.1% | 5.1% | |||||||||
| High risk groupb | Continuer | 384 | 46 | 12.0% | 7.3 | (3.8, 10.8) | 2.55 | (1.73, 3.74) | 11.7% | 4.4 | (0.8, 8.1) | 1.61 | (1.13, 2.29) |
| Discontinuer | 1041 | 49 | 4.7% | 7.3% | |||||||||
Number of subject in each group before trimming and stratification
High risk group is the same as the olanzapine users
Cell size < 11
RD100: Risk difference per 100 pregnancies; RR: relative risks; CI: confidence interval
Figure 1. Absolute risks of gestational diabetes for unadjusted and adjusted analyses.
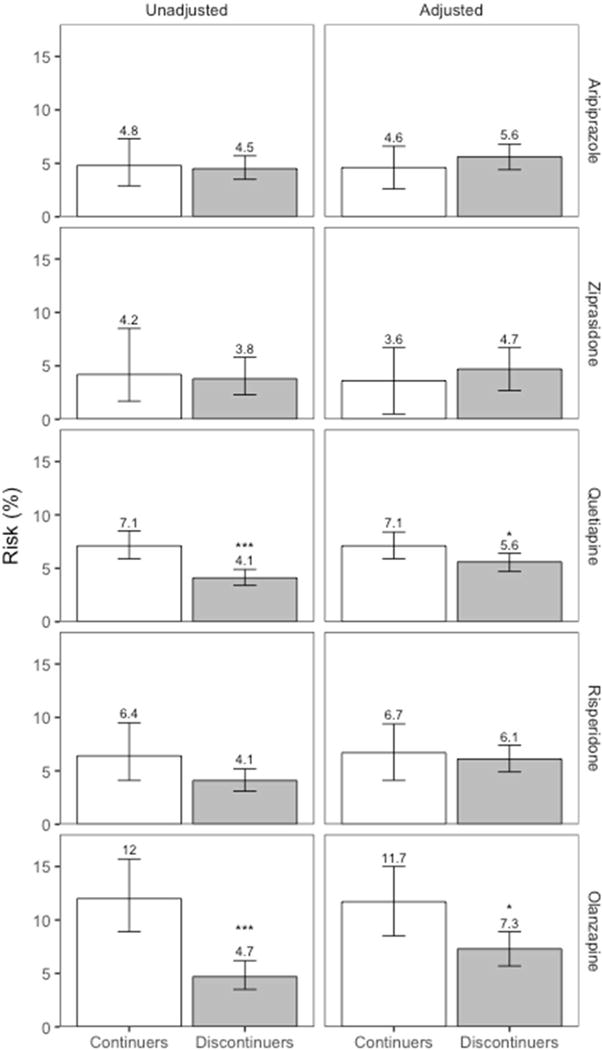
The numbers on top of each bar indicates the unadjusted and adjusted absolute risks.
*: p < 0.05, ***: p < 0.0001
Figure 2. Dose-response analyses between the cumulative dose of antipsychotic exposure during the first 20 weeks of pregnancy and the risk of gestational diabetes.
Upper panels: Restricted cubic spline curves with 3 knots at the 25th, 50th, and 75th percentiles of the cumulative dose (mg) during the first 20 weeks of pregnancy (LMP to 140 days after LMP), adjusting for age, race, obesity, diagnosis of schizophrenia or bipolar disorder, and the duration of treatment during the 3 months prior to LMP.
Lower panels: Density curve showing the distribution of cumulative dose among the users of each antipsychotic medication who had one or more prescription dispensed during the first 20 weeks of pregnancy.
To stabilize the dose-response curve at the extreme ranges, the maximum possible cumulative dose during the 140 days of exposure window was limited to the daily maximum dose multiplied by 140 days for each antipsychotic (mg). To convert to a daily dose, the cumulative dose can be divided by the duration of the exposure window (140 days).
LMP: last menstrual period; GDM: gestational diabetes
The adjusted relative risks in the low-(aripiprazole, ziprasidone), medium-(risperidone, quetiapine), and high-risk (olanzapine) group were 0.91 (0.60–1.39), 1.37 (1.12–1.69), and 1.61 (1.13–2.29), respectively. Additional group-level analyses results are presented in Figure 3 (Table S3). Across the different analyses, the risk of gestational diabetes seemed to be elevated in continuers compared to discontinuers in the high- and the medium-risk group, but not in the low risk group. However, the effects are less precisely estimated in some of the analyses due to the reduced study size.
Figure 3. Forest plot of the results from additional analyses based on the risk-stratified groups.
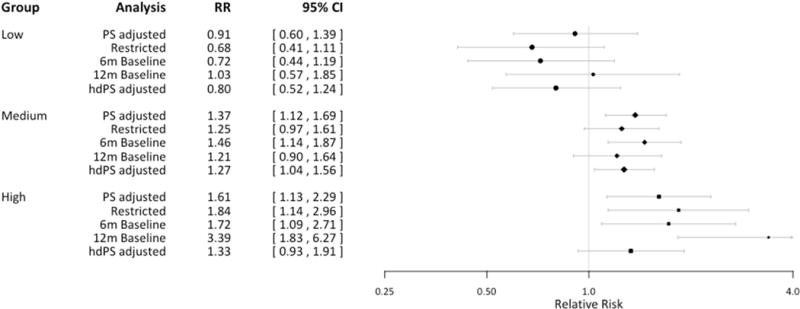
PS: Propensity score; hdPS: high-dimensional propensity score; RR: relative risk
PS adjusted: adjusted RR from PS stratification in the main analysis
Restricted: analysis restricted to women with diagnosis of schizophrenia, bipolar disorder, or depression
6m or 12m baseline: results from extending 3-month baseline to either 6 months or 12 months
hdPS adjusted: stratification adjustment using 50 additional confounder proxy variables in PS estimation
The bias analyses illustrated that with an overall obesity prevalence of 62% among atypical antipsychotic users observed in the Massachusetts General Hospital registry, the absolute difference in the prevalence of obesity between continuers and discontinuers would have to be more than 25% for the observed relative risk of 1.61 in olanzapine users to be completely attributable to residual confounding (solid line in Figure 4A, Table S4). To put this into context, a 25% difference would mean that 80% of continuers and 55% of discontinuers would be obese patients, with all other covariates balanced between the two groups. In quetiapine users, the difference would have to be greater than 20% (solid line in Figure 4B, Table S4). If, in contrast, there were more obese women among discontinuers than among continuers, the obesity-adjusted relative risks would be higher than what we observed for both drugs. If we assume the relative risks to be closer to the lower bounds of each confidence interval, however, confounding due to smaller differences in obesity could explain the increased relative risk.
Figure 4. The potential effect of obesity as an unmeasured confounder on the observed relative risk among the users of olanzapine or quetiapine.
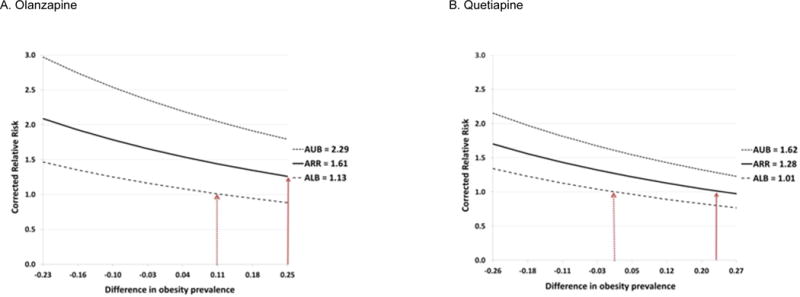
ARR = apparent relative risk before adjusting for obesity, ALB = apparent lower bound, AUB = apparent upper bound, PC1 = obesity prevalence among the exposed, PC0 = obesity prevalence among the unexposed, and RRCD = strength of association between obesity and the risk of gestational diabetes. Difference in the obesity prevalence (x-axis) is calculated as PC1 - PC0
Dotted lines above and below the solid line correspond to the upper and lower bound of the confidence interval for the point estimate, respectively. The solid red arrows show that > 25% difference in obesity prevalence for olanzapine users or > 20% difference in obesity prevalence for quetiapine users are required to explain the observed effect (ARR) by confounding only. The dotted red arrows show that when we take the uncertainty of the estimated ARR into account, smaller difference in obesity prevalence can account for the observed effect.
DISCUSSION
In pregnant women who were treated with an atypical antipsychotic before the start of pregnancy, continuation of treatment through the first half of pregnancy was associated with a moderate increased risk of gestational diabetes for olanzapine and quetiapine. We did not observe evidence of an increased risk for aripiprazole, ziprasidone, or risperidone continuers. Multiple analyses consistently showed stronger association with olanzapine. In addition, there was evidence of a cumulative dose-response relationship for olanzapine.
As for any observational study, it is important to consider alternative explanations for these findings. The main concern for this study is potential residual confounding by factors not captured comprehensively in our data, in particular obesity. We demonstrated through formal bias analyses that the imbalance in obesity prevalence between continuers and discontinuers would have to be very high (i.e., 20–25%) after accounting for all other covariates to fully explain the observed risk. Although this possibility cannot be excluded, it seems unlikely given that all women were treated before the start of pregnancy and we accounted for a broad range of proxy variables. The prevalence of pre-existing diabetes and risk factors tended to be higher for ziprasidone and lower for olanzapine, which suggests that antipsychotic prescribing may be somewhat selective with respect to such factors. Alternatively, this finding may be explained by a depletion of the most susceptible women, who could have developed diabetes after initiation of olanzapine and before their last menstrual period and were excluded from our cohort, leaving on treatment those with a lower baseline risk. Despite the fewer documented baseline risk factors for gestational diabetes, olanzapine continuers had the highest absolute risk of gestational diabetes compared to women who continued on drugs that are less likely to cause significant weight gain. Our findings are consistent with prior knowledge that olanzapine induces the most weight gain among the five study drugs,7,21 which provides a plausible mechanism for the observed elevation in risk in women continued on this medication. While quetiapine was associated with a small increased risk of gestational diabetes in this study, risperidone which is similarly associated with modest weight gain21 was not. Use of low-dose quetiapine for insomnia is well known, and women without psychiatric disorder who used quetiapine for insomnia before the start of pregnancy may be more likely to discontinue after becoming pregnant. While this could have resulted in confounding in the main quetiapine analysis, restricting the analysis to women with a mental disorder diagnosis showed almost identical results (data not shown). Further research is needed to confirm this finding.
A few studies have investigated the association between antipsychotic exposure during pregnancy and the risk of gestational diabetes, and even fewer considered specific drugs. Using Swedish National registries, Reis and Kallen reported an increase in the risk of gestational diabetes (odds ratio = 1.78, 1.04–3.01) among women who self-reported any antipsychotic use in early pregnancy16 and Boden et al concluded that mothers who used olanzapine or clozapine during pregnancy had a higher risk of gestational diabetes (odds ratio = 1.94, 0.97–3.91) compared to those who do not.15 Vigod et al. did not find an increased risk in a high-dimensional propensity score-matched cohort in Canada, either for all antipsychotics considered (relative risk = 1.10, 0.77–1.57) or for 166 olanzapine users only.17 Unlike our study, all three studies had nonusers as a reference group who are less comparable in terms of health status and disease severity than discontinuers, and they did not exclude women with pre-existing diabetes. Higher relative risks in Swedish studies may be partly due to the fact that only a small number of confounders were adjusted for. The absolute risk among the unexposed women was higher in Vigod et al than the discontinuers in our study (6.2% vs. 4 to 5%), which implies significant difference in baseline risk among the two populations and potentially why we observed different results. In a recent publication,18 prevalent users of antipsychotics in early pregnancy were compared with women with psychiatric conditions but not treated with antipsychotics to increase comparability of groups, under the assumption that the other psychotropic medications do not affect the risk of gestational diabetes. But the sample size was insufficient to consider individual antipsychotics and specific antipsychotics may have differential effects on the risk of gestational diabetes.
Our study has several strengths. The study population arises from the nation-wide Medicaid program that is representative of close to 50% of all pregnancies in the US.40 Moreover Medicaid finances 80% of all antipsychotic prescriptions and 36% of all treatment cost for gestational diabetes in the US.41,42 We used automated dispensing records to define exposure, which is free of recall bias, and a validated outcome definition. This study is one of the largest studies conducted in pregnant women taking antipsychotic and we were able to investigate individual drug effects rather than a drug class effect.
The study is not without limitations, however. Residual confounding due to unmeasured or poorly measured factors such as life style factors is possible. But comparing continuers to discontinuers rather than to non-users alleviates this concern because discontinuers are likely to be more similar to continuers than non-users. In addition to conducting a formal bias analysis, we showed that adjusting for a large number of empirically identified confounders that may serve as proxies for unmeasured or poorly measured confounders does not change the findings. We could not fully adjust for the duration of antipsychotic exposure, which may have extended many years before recording in our database. However, adjusting for the treatment duration during the year before pregnancy provided consistent results. We do not have information on the reasons for discontinuation, which may be associated with the disease severity or indication for antipsychotic use not recorded in our data. However, disease severity seems unlikely to explain the observed associations since we only observed an increased risk for selected antipsychotics. A pharmacy dispensing record does not guarantee the actual intake of the drug. By requiring at least two prescriptions during the first 20 weeks of pregnancy, we were more confident that the continuers in our study actually took the medication.
CONCLUSION
In a large cohort of women without pre-existing diabetes who were treated with antipsychotics before pregnancy, we observed an increased risk of gestational diabetes among women who continue to use olanzapine or quetiapine during the first 20 weeks of pregnancy compared to those who discontinue. There was a positive dose-response relationship between the use of olanzapine and gestational diabetes risk. We did not find a difference in the risk of gestational diabetes comparing continuers to discontinuers of aripiprazole, ziprasidone, and risperidone. Further studies are needed to understand the potential effect on gestational diabetes risk of switching antipsychotic agents during pregnancy. Such information would aid treatment decisions in women for whom treatment discontinuation is not an option. In conclusion, while the risk of gestational diabetes is an important consideration in selection of a drug, other dimensions of antipsychotic treatment including the benefit of continuing a specific treatment and the risk of efficacy loss due to changes in treatment should be taken into account in decision making for pregnant women.
Supplementary Material
Acknowledgments
Park had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Park, Hernandez-Diaz, Bateman, J.M. Cohen, Desai, Patorno, Huybrechts.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Park, Hernandez-Diaz, Bateman, Huybrechts.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Park, Hernandez-Diaz, Bateman, Desai, Glynn, Huybrechts.
Obtained funding: Huybrechts, Hernández-Díaz.
Administrative, technical, or material support: Hernández-Díaz, Mogun, Huybrechts.
Study supervision: Hernández-Díaz, Bateman, Huybrechts.
Conflict of Interest Disclosures: This study was supported by a grant from the National Institute of Mental Health (R01MH100216). YP was supported by the Pharmacoepidemiology Program at Harvard TH Chan School of Public Health, partially funded by Pfizer, Takeda, Bayer and Asisa. KFH was supported by a career development grant (K01MH099141) from the National Institute of Mental Health. RJD is Principal Investigator of a research grant from Merck to Brigham and Women’s Hospital for unrelated work. LSC has received research support from Alkermes, AstraZeneca, Bristol-Myers Squibb/Otsuka, Forest/Actavis, Ortho-McNeil Janssen, and Sunovion Pharmaceuticals, Inc. for the National Pregnancy Registry for Atypical Antipsychotics and received other research support for unrelated work from Cephalon, Inc., the National Institute on Aging, the National Institute of Mental Health, and Takeda/Lundbeck. SHD has consulted for Boehringer-Ingelheim and UCB, and was the epidemiologist for the North American Antiepileptic Drugs pregnancy registry and advisor for the National Pregnancy Registry for Atypical Antipsychotics, both of which are funded by multiple companies. YP and BTB consulted for Optum for unrelated projects. KFH, BTB, and SHD are investigators on grants to the Brigham and Women’s Hospital from Lilly and Pfizer and BTB on grants from Baxalta, unrelated to the topic of this manuscript.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
YP is currently a postdoctoral researcher at IBM Research Cambridge
All the work in this manuscript was conducted while the author was at Brigham and Women’s Hospital as a research trainee.
This manuscript has not been published before and is not under consideration by other journals. Some parts of the result were presented at International Conference on Pharmacoepidemiology & Therapeutic Risk Management in Dublin, Ireland in August, 2016.
References
- 1.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Research and Clinical Practice. 2014;103:341–63. doi: 10.1016/j.diabres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Practice, Bulletins-Obstetrics. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013;122:406–16. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 3.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis. 2014;11:E104. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACOG Committee on Practice Bulletins-Obstetrics. Clinical management guidelines for obstetrician-gynecologists number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001–20. doi: 10.1097/AOG.0b013e31816fd910. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. The Lancet. 2009;373:1773–9. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 6.Reece EA, Leguizamón G, Wiznitzer A. Gestational diabetes: the need for a common ground. The Lancet. 2009;373:1789–97. doi: 10.1016/S0140-6736(09)60515-8. [DOI] [PubMed] [Google Scholar]
- 7.Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PloS one. 2014;9:e94112. doi: 10.1371/journal.pone.0094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobo WV, Cooper WO, Stein CM, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA psychiatry. 2013;70:1067–75. doi: 10.1001/jamapsychiatry.2013.2053. [DOI] [PubMed] [Google Scholar]
- 9.Andrade SE, Lo JC, Roblin D, et al. Antipsychotic medication use among children and risk of diabetes mellitus. Pediatrics. 2011;128:1135–41. doi: 10.1542/peds.2011-0855. [DOI] [PubMed] [Google Scholar]
- 10.Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 11.Regenold W. Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. Journal of Affective Disorders. 2002;70:19–26. doi: 10.1016/s0165-0327(01)00456-6. [DOI] [PubMed] [Google Scholar]
- 12.Buchanan TA, Xiang A, Kjos SL, Watanabe R. What is gestational diabetes? Diabetes care. 2007;30(Suppl 2):S105–11. doi: 10.2337/dc07-s201. [DOI] [PubMed] [Google Scholar]
- 13.Wichman CL. Atypical antipsychotic use in pregnancy: a retrospective review. Archives of women’s mental health. 2009;12:53–7. doi: 10.1007/s00737-008-0044-3. [DOI] [PubMed] [Google Scholar]
- 14.Gentile S. Pregnancy exposure to second-generation antipsychotics and the risk of gestational diabetes. Expert Opin Drug Saf. 2014;13:1583–90. doi: 10.1517/14740338.2014.931368. [DOI] [PubMed] [Google Scholar]
- 15.Boden R, Lundgren M, Brandt L, Reutfors J, Kieler H. Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Archives of general psychiatry. 2012;69:715–21. doi: 10.1001/archgenpsychiatry.2011.1870. [DOI] [PubMed] [Google Scholar]
- 16.Reis M, Kallen B. Maternal use of antipsychotics in early pregnancy and delivery outcome. Journal of clinical psychopharmacology. 2008;28:279–88. doi: 10.1097/JCP.0b013e318172b8d5. [DOI] [PubMed] [Google Scholar]
- 17.Vigod SN, Gomes T, Wilton AS, Taylor VH, Ray JG. Antipsychotic drug use in pregnancy: high dimensional, propensity matched, population based cohort study. Bmj. 2015;350:h2298. doi: 10.1136/bmj.h2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panchaud A, Hernandez-Diaz S, Freeman MP, et al. Use of atypical antipsychotics in pregnancy and maternal gestational diabetes. J Psychiatr Res. 2017;95:84–90. doi: 10.1016/j.jpsychires.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Gentile S. Long-term treatment with atypical antipsychotics and the risk of weight gain : a literature analysis. Drug safety : an international journal of medical toxicology and drug experience. 2006;29:303–19. doi: 10.2165/00002018-200629040-00002. [DOI] [PubMed] [Google Scholar]
- 20.Gentile S. Contributing factors to weight gain during long-term treatment with second-generation antipsychotics. A systematic appraisal and clinical implications. Obes Rev. 2009;10:527–42. doi: 10.1111/j.1467-789X.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 21.Newcomer JW. Second-Generation (Atypical) Antipsychotics and Metabolic Effects. CNS Drugs. 2005;19 doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 22.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 23.Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Archives of general psychiatry. 2008;65:805–15. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toh S, Li Q, Cheetham TC, et al. Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001–2007: a population-based study of 585,615 deliveries. Archives of women’s mental health. 2013;16:149–57. doi: 10.1007/s00737-013-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein RA, Bobo WV, Shelton RC, et al. Increasing use of atypical antipsychotics and anticonvulsants during pregnancy. Pharmacoepidemiology and drug safety. 2013;22:794–801. doi: 10.1002/pds.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park Y, Huybrechts KF, Cohen JM, et al. Antipsychotic Medication Use Among Publicly Insured Pregnant Women in the United States. Psychiatric services. 2017 doi: 10.1176/appi.ps.201600408. appips201600408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to Evaluate Medications in Pregnancy: Design Considerations. PloS one. 2013;8:e67405. doi: 10.1371/journal.pone.0067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. The New England journal of medicine. 2014;370:2397–407. doi: 10.1056/NEJMoa1312828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA: the journal of the American Medical Association. 2015;313:2142–51. doi: 10.1001/jama.2015.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huybrechts KF, Hernandez-Diaz S, Patorno E, et al. Antipsychotic Use in Pregnancy and the Risk for Congenital Malformations. JAMA psychiatry. 2016;73:938–46. doi: 10.1001/jamapsychiatry.2016.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrade SE, Moore Simas TA, Boudreau D, et al. Validation of algorithms to ascertain clinical conditions and medical procedures used during pregnancy. Pharmacoepidemiology and drug safety. 2011;20:1168–76. doi: 10.1002/pds.2217. [DOI] [PubMed] [Google Scholar]
- 32.Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A Propensity-score-based Fine Stratification Approach for Confounding Adjustment When Exposure Is Infrequent. Epidemiology. 2017;28:249–57. doi: 10.1097/EDE.0000000000000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic & clinical pharmacology & toxicology. 2006;98:253–9. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The SAS LGTPHCURV9 Macro. 2011 [Google Scholar]
- 35.Maglione M, Ruelaz MA, Hu J, et al. Comparative Effectiveness Review No 43. Rockville, MD: Agency for Healthcare Research and Quality; Sep, 2011. Off-Label Use of Atypical Antipsychotics: An Update. (Prepared by the Southern California Evidence-based Practice Center under Contract No. HHSA290-2007-10062-1) Available at: http://www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 36.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20:512–22. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiology and drug safety. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 38.Cohen LS, Viguera AC, McInerney KA, et al. Establishment of the National Pregnancy Registry for Atypical Antipsychotics. The Journal of clinical psychiatry. 2015;76:986–9. doi: 10.4088/JCP.14br09418. [DOI] [PubMed] [Google Scholar]
- 39.Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes care. 2007;30:2070–6. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 40.Pandit S. 2012 Maternal and Child Health Update. National Governors Association; 2013. Issue Brief. [Google Scholar]
- 41.Chen Y, Quick WW, Yang W, et al. Cost of gestational diabetes mellitus in the United States in 2007. Popul Health Manag. 2009;12:165–74. doi: 10.1089/pop.2009.12303. [DOI] [PubMed] [Google Scholar]
- 42.Frank RG, Conti RM, Goldman HH. Mental health policy and psychotropic drugs. Milbank Q. 2005;83:271–98. doi: 10.1111/j.1468-0009.2005.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.