Abstract
OBJECTIVE
In order to understand the role of depressive symptoms in preclinical Alzheimer’s disease (AD) it is essential to define their temporal relationship to AD proteinopathies in cognitively normal (CN) older adults. The study objective was to examine associations of brain amyloid-beta (Aβ) and longitudinal measures of depression and depressive-symptom clusters in a CN sample.
METHOD
Two hundred seventy community-dwelling, CN elderly underwent baseline Pittsburgh compound B (PiB)-PET measures of cortical aggregate Aβ and annual Geriatric Depression Scale-30 (GDS) assessments, calculated as total GDS scores and mean scores for three GDS item clusters (Apathy-Anhedonia, Dysphoria and Anxiety-Concentration), over 1-5 years (mean 3.8). We evaluated continuous PiB as a predictor of GDS or each GDS cluster across time, in separate mixed-effects models with backward elimination. Initial predictors included PiB, age, sex, Hollingshead and AMNART scores, APOEε4, depression history and their interactions with time.
RESULTS
Higher PiB predicted accelerated rates of increase in GDS over time, adjusting for depression history. Higher PiB also predicted steeper rates of increase for Anxiety-Concentration scores, adjusting for depression history and the AMNART-time interaction. In a post-hoc model estimating anxiety scores without concentration disturbance items, the PiB-time interaction remained significant.
CONCLUSIONS
Higher Aβ burden was associated with increasing anxious-depressive symptoms over time in CN older people. Prior depression history was related to higher but not worsening symptoms. These results suggest a direct or indirect association of elevated Aβ with worsening anxious-depressive symptoms and support the hypothesis that emerging neuropsychiatric symptoms represent an early manifestation of preclinical AD.
Keywords: Depression, anxiety, amyloid, preclinical Alzheimer’s disease
INTRODUCTION
Alzheimer’s disease (AD) begins with a long ‘preclinical’ phase defined by the accumulation of brain deposits of fibrillar amyloid and pathological tau, a process spanning more than a decade before the onset of mild cognitive impairment (MCI).(1–3) Increasingly, observational studies have implicated depression and other neuropsychiatric symptoms (NPS) as predictors of AD progression during this long preclinical period. Cognitively normal (CN) older people with NPS, particularly depression-related symptoms and anxiety, have been found to be twice as likely to develop amnestic-MCI, a prodromal phase of AD dementia, compared to those without these symptoms, over 3-6 years, in multiple epidemiological cohorts.(3–6)
Alternative, and possibly complementary, models propose that depressive symptoms may be causal factors accelerating AD progression or they may be stigmata of AD, even as early as ‘preclinical’ stages.(7) It is also possible that these roles may differ by symptom or symptom-clusters or across time and disease stages. Understanding these mechanisms is important to accurately classify and treat CN individuals at high risk for early AD progression and to differentiate these individuals from older adults with psychiatric symptoms distinct from AD-related processes.
A small number of studies have investigated the relationship of in vivo markers of amyloidosis to syndromal depression or continuous measures of depression-related symptoms using cerebrospinal fluid (CSF) AD biomarker analyses and positron emission tomography (PET) imaging modalities in samples comprised of CN older people. In cross-sectional analyses, Pomara and colleagues found that CSF amyloid-beta (Aβ)42 levels were reduced, as in AD, in cognitively intact elderly with late-life depression but not in those without depression, and further, lower levels of Aβ42 were inversely related to Hamilton-Depression scores across the whole sample.(8) CSF total tau and phosphorylated tau levels did not differ across depressed and non-depressed groups. More recently, Babulal and colleagues found no cross-sectional association of CSF AD biomarkers with continuous scores of mood disturbance assessed by the Profile of Mood States-short form (POMS-SF) in a CN community-dwelling sample.(9) Notably, higher baseline CSF tau/Aβ42 ratio, but not other CSF markers, predicted one year increases in anxiety and total mood disturbance scores measured by the POMS-SF and in total Neuropsychiatric Inventory Questionnaire (NPI-Q) scores suggesting a dynamic relationship between AD-specific markers and changes in emotional tone. In related findings, mean cortical binding of fibrillar amyloid determined by Pittsburgh-Compound B (PiB)-PET imaging was not associated with depression or other NPS measures at baseline but was positively associated with 15-item Geriatric Depression Scale (GDS-15) change scores over 1 year. Similarly, Harrington and colleagues classified a large, non-depressed, CN sample into high or low Aβ groups using PET radioligands and found that the high Aβ group was 4.5-fold more likely than the low Aβ group to develop high categorical depression, defined by the GDS-15, after 54 months, in unadjusted analyses.(10)
Together, these recent and select findings suggest that CN older individuals with biomarker evidence of amyloidosis (principally high fibrillar brain Aβ) are more likely to experience rising depressive symptoms over time. At the same time, these preliminary observations raise more pointed questions as to the quality, severity and time course of depressive symptoms that are most characteristic of preclinical AD and the specificity of their associations with AD molecular markers.
To approach these questions, we investigated the relationship of brain Aβ burden, determined by PiB-PET, to longitudinal measures of depression in a cohort of CN older adults, comprised of individuals with a wide range of Aβ values, including a subset with high Aβ burden consistent with preclinical AD. We hypothesized that higher Aβ would predict greater depression scores, even in a subclinical range, and after adjustment for other potential confounders including depression history. Building on prior work that defined latent factors of subclinical depressive symptoms within the same cohort, we also examined associations of Aβ and depressive symptom clusters over time in this CN sample.
MATERIALS AND METHODS
Participants
Data were derived from the Harvard Aging Brain Study (HABS), an observational study of older adult volunteers, aimed at defining neurobiological and clinical changes in early AD. Two hundred and seventy participants completed study visits over 5 years (mean number of visits 3.8, range 1-5). Participants were English-speaking, community-dwelling men and women, ages 62-90, who were cognitively normal and were free from active, major psychiatric disorders at the time of enrollment.(11) A history of past or current depression adequately treated with standard antidepressant medication (selective serotonin reuptake inhibitors or dual serotonin-norepinephrine reuptake inhibitors, bupropion, mirtazapine, trazodone or nortriptyline) was allowed. At screening, all participants scored below cutoff for mild depression, defined by scores of 11 or greater on the 30-item Geriatric Depression Scale (GDS).(12) Cognitively normal status was defined by Clinical Dementia Rating(13) global score 0 and education-adjusted normal performance for the Wechsler Logical Memory subtest(14) and the Mini-Mental State Examination (MMSE).(15) The Partners Human Research Committee approved this study and all participants provided written informed consent.
Clinical measures
Baseline clinical assessments relevant to these analyses included the MMSE, the American National Reading Test(16) intelligence quotient (AMNART), a measure of premorbid intelligence (greater score indicates greater intelligence) and a Hollingshead score, calculated according to primary occupation and educational attainment (range 11–73 in the sample; higher score indicates lower socioeconomic status).(17) Participants were classified by genotype as APOEε4 allele carriers or non-carriers. Self-reported depression and treatment were elicited at baseline via history obtained by a study physician, followed by medical record review if necessary. Depression history was defined as a dichotomous variable in which participants with any self-reported depression, including past or current diagnoses, were classified together and compared to no depression history.
Depression was quantified at baseline and annually using the 30-item GDS (item range 0-1; total range 0-30; higher score indicates greater depression).(12) In addition to calculating a total score for each time point, we also calculated an average score corresponding to each of three clusters of GDS items. These aggregate GDS items, the Anxiety-Concentration, Apathy-Anhedonia and Dysphoria clusters, were previously defined by principal component analysis using a HABS sample that was nearly identical to the current baseline sample (Supplement; Methods).(11)
All depression data were acquired in a blinded fashion with regard to other assessments and procedures.
PiB-PET data
Fibrillar amyloid burden was measured using PiB-PET according to established protocols at the Massachusetts General Hospital PET facility.(18–21) PiB distribution volume ratio (DVR) was calculated per methods established in prior studies that include a single region representing the aggregate cortical areas at risk for amyloid burden, across the frontal, lateral temporal and lateral and medial parietal lobes.(21, 22) Analyses used aggregate PiB DVR as a continuous measure. Participants and investigators were blinded to all PiB data. PiB DVR data across the full range of values were analyzed.
Statistical Analyses
Unadjusted associations between baseline GDS total score, the GDS cluster scores and PiB were tested using Pearson correlations. Relations among categorical predictors were evaluated using chi-square tests and differences in mean relations between categorical predictors versus baseline numerical variables were assessed with Satterthwaite t-tests.
Mixed random and fixed effects longitudinal analyses were run across time in the study for each of four dependent variables (GDS total score and average scores for the Anxiety-Concentration, Dysphoria and Apathy-Anhedonia clusters) in separate analyses, employing a backward elimination algorithm (p<0.05 cut off) on an initial pool of fixed predictors and variances/covariances of random terms. During backward elimination, by convention, nonsignificant terms are retained in the model if higher order terms subsuming them, e.g. interactions, are still in the model. The time predictor was the linear component of years in the study (preliminary graphs did not suggest curvilinearity). Fixed terms were baseline PiB DVR, age at baseline, history of depression (yes/no), AMNART, Hollingshead score, sex, APOEε4 carrier status (yes/no) and the interaction of each of these predictors with time in study. Antidepressant use was not included as a predictor as this substantially overlapped with depression history. Random terms were intercepts and linear slopes across time per participant, initially allowing for a correlation between them. Percent variance accounted for in the dependent variable by fixed and random predictors was computed.
SAS Version 9.4 (SAS, Cary, NC), SPSS 23 (IBM, Armonk, NY) and R v3.3.2 statistical software were used.
RESULTS
Demographic, clinical and imaging data are shown in Table 1 and Supplement Figure 1. The mean GDS score at baseline was 2.8 for the whole sample, 3.7 for participants with a history of depression. Average scores for items corresponding to the Apathy-Anhedonia and the Anxiety-Concentration clusters were greater than for the Dysphoria cluster (means 0.161, 0.129 and 0.030 respectively, where these are equivalent to the mean proportion of items for the cluster which were endorsed by participants (Supplement; Methods)). At baseline, Anxiety-Concentration scores were significantly but weakly correlated with the other cluster scores (for Apathy-Anhedonia, r=0.2, p<0.0001; for Dysphoria r=0.2, p=0.0003) whereas, the other clusters were marginally related to each other (for Apathy-Anhedonia and Dysphoria r=0.1, p=0.09). At baseline, PiB was not significantly correlated with GDS total score (r=0.08, p=0.2), Anxiety-Concentration score (r=0.03, p=0.6), Dysphoria score (r= −0.01, p=0.9) or Apathy-Anhedonia score (r=0.1, p=0.06).
Table 1.
Demographic, clinical and imaging data for study participants at baseline
| Group | N | Mean or N (%) | p-value* | Range (observed) | SD | |
|---|---|---|---|---|---|---|
| Age (years) | Total | 270 | 73.6 | 63, 90 | 6.1 | |
| No Depression | 73.7 | 0.5 | ||||
| Depression | 72.8 | |||||
| Sex (female) | Total | 270 | 158 (58.5) | |||
| No Depression | 135 (57) | 0.4 | ||||
| Depression | 23 (66) | |||||
| Hollingshead score | Total | 270 | 27.6 | 11,73 | 15.1 | |
| No Depression | 27.4 | 0.9 | ||||
| Depression | 27.8 | |||||
| AMNART | Total | 269 | 120.6 | 78, 132 | 9.3 | |
| No Depression | 120.4 | 0.07 | ||||
| Depression | 123.7 | |||||
| MMSE | Total | 270 | 29.0 | 25,30 | 1.1 | |
| No Depression | 29.1 | 0.4 | ||||
| Depression | 29.0 | |||||
| APOEε4 carrier status (positive) | Total | 256 | 75 (29.3) | |||
| No Depression | 62 (28) | 0.2 | ||||
| Depression | 13 (39) | |||||
| Amyloid-β (Cortical PiB DVR) | Total | 270 | 1.170 | 0.947, 1.820 | 0.166 | |
| No Depression | 1.16 | 0.01 | ||||
| Depression | 1.24 | |||||
| GDS (possible range 0-30) | Total | 270 | 2.8 | 0,10 | 2.6 | |
| Anxiety-Concentration cluster (possible range 0-31) | Total | 270 | 0.129 | 0, 0.778 | 0.156 | |
| Apathy-Anhedonia cluster (possible range 0-31) | Total | 270 | 0.161 | 0, 0.857 | 0.174 | |
| Dysphoria cluster (possible range 0-31) | Total | 270 | 0.030 | 0, 0.667 | 0.101 | |
| History of depression (yes) | Total | 270 | 35 (13.0) | |||
| Any antidepressant use | Total | 270 | 36 (13.3) | |||
| No Depression | 19/235 (8.1) | <0.001 | ||||
| Depression | 17/35 (48.6) | |||||
| SSRI/SNRI use | Total | 270 | 26 (9.6) | |||
| No Depression | 11/235 (4.7) | <0.001 | ||||
| Depression | 15/35 (43) |
Abbreviations: AMNART (American National Adult Reading Test score), MMSE (Mini-Mental State Examination, GDS (Geriatric Depression Scale-30 item). APOEε4 (Apolipoprotein E ε4), PiB DVR (Pittsburgh Compound B distribution volume ratio), SSRI/SNRI (selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor).
Mann-Whitney test or chi-square test.
Thirty-five participants, 13% of the sample, reported a history of depression (7% of participants reported active depression within 2 years of enrollment (current depression) and 6% reported no active depression within 2 years of enrollment (past depression). Participants with and without a history of depression did not differ proportionally across sex or APOEε4 categories (Table 1). Compared to no depression history, participants with a history of depression had significantly higher mean baseline PiB (Table 1). More specifically, a significant difference in mean PiB was found between those with no depression history compared to those with current depression (1.16 vs. 1.28; p=0.006) but not compared to those with past depression (1.16 vs. 1.22; p=0.2).
Longitudinal Analyses
In the final model for GDS, participants with a history of depression had a higher adjusted mean GDS score across time compared to those without self-reported depression (Table 2). The interaction of PiB with time was also a significant predictor in this model such that higher baseline PiB was associated with steeper increases in GDS scores over time (Figure 1). Neither APOEε4, nor its interaction with time, was associated with higher GDS and no other fixed terms were significant. Significant random terms were an uncorrelated intercept and linear slope across time. In a post-hoc model, we repeated the final model, testing for possible effect modification based on APOEε4 status. Terms for the multiplicative interactions of PiB-APOEε4 and PiB-APOEε4-time were added as predictors to the final model and were not significantly associated with GDS.
Table 2.
Longitudinal mixed effects model for GDS total scores, measured annually, showing fixed effects predictors retained in final model
| Model: R2=0.04 for fixed effects, p<0.0001; R2=0.82 including random terms, p<0.0001 | ||||
|---|---|---|---|---|
| Predictor | Regression Coefficient | 95% Confidence Interval | Standard Error | p value |
| PiB DVR interacting with time | 0.65 | 0.02, 1.28 | 0.32 | 0.04 |
| Depression history (yes) | 1.36 | 0.35, 2.35 | 0.50 | 0.008 |
| PiB | 1.6 | −0.49, 3.74 | 1.08 | 0.13 |
| Years in study (time) | −0.56 | −1.31, 0.20 | 0.38 | 0.15 |
Abbreviations: GDS (Geriatric Depression Scale-30 item), PiB DVR (Pittsburgh Compound B distribution volume ratio). The regression coefficient is the unstandardized partial regression coefficient. For binary predictors, this is equivalent to the difference in adjusted means between the two groups.
Figure 1. Values predicted from the fixed effects of the best-fitting model for Geriatric Depression Scale (GDS) total scores, as a function of PiB and depression history.
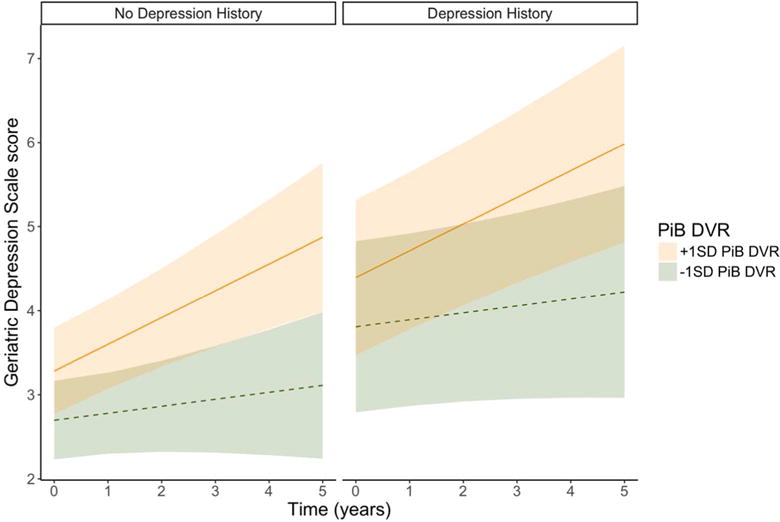
GDS trajectories corresponding to PiB values 1 standard deviation (SD) above (solid line) and 1 SD below (dotted line) the mean value of PiB (1.17) are shown with 95% confidence limits. Model predictions by history of depression are shown separately. Depression history is related to vertical elevation of lines across the whole span of the study, whereas PiB is related to the slope of the lines; higher PiB is associated with a steeper upward slope.
As in the model for GDS total score, final predictors of Anxiety-Concentration scores included a main effect of depression history and the PiB-time interaction with effects in the same direction as before (Table 3). Lower AMNART IQ was also associated with steeper increases in Anxiety-Concentration scores across time (Table 3). No other fixed terms were significant. Among random terms, only the intercept showed significant variance and was retained in the final model.
Table 3.
Longitudinal mixed effects model for Anxiety-Concentration scores, measured annually, showing fixed effect predictors retained in final model
| Model: R2=0.03 for fixed effects, p<0.0001; R2=0.71 including random terms, p<0.0001. | ||||
|---|---|---|---|---|
| Predictor | Regression Coefficient | 95% Confidence Interval | Standard Error | p value |
| PiB DVR interacting with time | 0.04 | 0.007, 0.067 | 0.015 | 0.015 |
| Depression history (yes) | 0.056 | 0.003, 0.107, | 0.026 | 0.04 |
| PiB | 0.056 | −0.059, 0.172 | 0.059 | 0.34 |
| AMNART interacting with time | −0.0007 | −0.001, −0.00002 | 0.0003 | 0.045 |
| Years in study (time) | 0.043 | −0.041, 0.127 | 0.042 | 0.31 |
| AMNART | 0.0002 | −0.002, 0.002 | 0.001 | 0.81 |
Abbreviations: PiB DVR (Pittsburgh Compound B distribution volume ratio), AMNART (American National Adult Reading Test). The regression coefficient is the unstandardized partial regression coefficient. For binary predictors, this is equivalent to the difference in adjusted means between the two groups.
To examine the possibility that the relationship of PiB to Anxiety-Concentration scores was specifically attributable to two GDS items related to concentration (“Is your mind as clear as it used to be?” and “Do you have trouble concentrating?”), we calculated an average Anxiety-only score by excluding these two items. In a secondary model analyzing Anxiety-only scores, effects were virtually the same as before (for the PiB-time interaction, p=0.03; for depression history, p=0.02; for fixed effects, R2=0.02; R2=0.7 including random terms, p<0.0001), except that the AMNART-time interaction term was no longer predictive in this model.
In the model for Dysphoria scores, history of depression was associated with a higher adjusted mean for Dysphoria scores across time compared to no depression history. No other fixed terms were significant except for a positive linear effect of time indicating greater Dysphoria scores over time (for depression history, p<0.0001; for time, p=0.003; for fixed effects, R2=0.04; R2=0.72 including random terms, p<0.0001). Significant random terms were an uncorrelated intercept and linear slope across time.
For the final model for Apathy-Anhedonia scores, significant predictors included an interaction of age with time (older age raised the trajectory of Apathy-Anhedonia scores), an interaction of Hollingshead score with time (lower socioeconomic status lowered the trajectory of symptoms across time) and a main effect of AMNART (higher cognitive reserve was associated with more Apathy-Anhedonia scores across time; Table 4). Among random terms, only the intercept showed significant variance and was retained in the final model.
Table 4.
Longitudinal mixed effects model for Apathy-Anhedonia scores, measured annually, showing fixed effect predictors retained in final model
| Model: R2=0.05 for fixed effects, p<0.0001; R2=0.75 including random terms, p<0.0001. | ||||
|---|---|---|---|---|
| Predictor | Regression Coefficient | 95% Confidence Interval | Standard Error | p value |
| Age interacting with time | 0.001 | 0.0001, 0.002 | 0.0005 | 0.03 |
| Hollingshead interacting with time | −0.0005 | −0.0008, −0.00008, | 0.0002 | 0.02 |
| AMNART | 0.003 | 0.0008, 0.006 | 0.001 | 0.009 |
| Age | 0.002 | −0.002, 0.005 | 0.002 | 0.39 |
| Hollingshead | 0.0007 | −0.0008, 0.002 | 0.0008 | 0.37 |
| Years in study (time) | −0.06 | −0.13, 0.017 | 0.04 | 0.13 |
Abbreviations: AMNART (American National Adult Reading Test intelligence quotient)
Residuals from predictions of the random and fixed terms for all final models reasonably conformed to assumptions of normality and homogeneity of variance. For the Dysphoria Model, however, residuals from the fixed term predictions alone were somewhat positively skewed because of the floor effect of zero values in the distribution of dysphoria scores.
DISCUSSION
We examined the relationship of brain Aβ burden to longitudinal measures of depression in a community-based sample of CN older people, and found that higher baseline amyloidosis was associated with worsening depressive symptoms over time. Higher brain Aβ was associated with increasing anxious-depressive symptoms rather than symptoms related to dysphoria or apathy-anhedonia in this sample, suggesting that this particular dimension of depressive symptoms may be most useful as an early, dynamic marker in preclinical AD.
Our results are consistent with recent findings from two other clinical-imaging cohort studies. Washington University Alzheimer’s Disease Research Center investigators reported that CN participants with high baseline Aβ burden had greater GDS-15 change scores over 1 year compared to those with low Aβ burden, adjusting for age, sex and education.(9) Similarly, the Australian Imaging, Biomarkers and Lifestyle (AIBL) research group found that CN participants with high Aβ were over 4 times more likely to develop categorical depression (defined by the standard GDS-15 cutoff) at 54 months compared to those with low Aβ, in unadjusted analyses.(10) As in the HABS cohort, these samples included some participants with a depression history and/or antidepressant medication use, and also similar to HABS, GDS scores at baseline were generally low(9) or in a subclinical range.(10) No significant differences in GDS or other mood disturbance measures were found between high versus low Aβ groups at baseline in either study.
While significant cross-sectional associations of brain Aβ and depression were not found in these CN cohorts using Aβ-specific PET ligands, Yasuno and colleagues reported an age and education adjusted association of PiB-PET-derived Aβ measures and low range GDS-15 scores among CN older individuals with elevated PiB retention (only values within the highest two tertiles for the sample were analyzed).(23) This sample excluded individuals with a depression history or antidepressant use, reducing the likelihood of reverse causation and providing indirect support for high Aβ as a factor that precedes subclinical depressive symptoms.
We provide further biomarker evidence for depression-related symptoms as outcomes of AD pathological changes at the preclinical stage and, in this case, independent of previously diagnosed depression. Regardless of depression history, higher Aβ burden predicted de novo or rising depression-spectrum symptoms in the near term that may lead to clinical depression over a longer period of years. Notably, 7% of the HABS sample reported a current diagnosis of depression and the unadjusted mean PiB value was significantly higher in these participants compared to those with no depression history. This points to Aβ-related brain changes as a possible etiological basis for clinical depression in a subset of these participants. Early expansion of Aβ, interacting with tau, within medial temporal lobe structures such as the entorhinal cortex, might affect activity in functionally-coupled limbic and neocortical regions, resulting in changes in emotional regulation.(24) The emergence of these symptoms in individuals with high Aβ may also coincide with progressive Aβ deposition and local neurodegeneration within subcortical structures and circuits involved in emotional responses, such as anxiety.(25, 26)
Theoretical constructs of depression and other NPS in neurodegenerative disorders, along with instruments for their measurement, continue to evolve.(27–29) In line with recent consensus criteria, anxiety may be symptom of emotional dysregulation during preclinical AD that could anticipate syndromal depression or other changes in emotion, temperament and behavior, as encompassed in the newly defined Mild Behavioral Impairment construct.(29)
NPS may be most useful as clinical or prognostic markers in CN older individuals with evidence of other biological risk factors or sentinels of decline.(30) Holmes and colleagues found higher anxiety-subscale scores, assessed by the Hospital Anxiety and Depression Scale (HADS), in APOEε4 carriers compared to non-carriers, specifically within the subgroup of CN older people with high Aβ.(31) No main effect of Aβ group with anxiety was reported. In a subset of the HABS cohort, we have previously reported that greater Aβ burden was associated with higher self-reported loneliness, a novel NPS measured by the 3-item UCLA-loneliness scale, after adjustment for HADS-anxiety scores and other demographic and psychosocial factors. This effect was also found to be stronger in APOEε4 carriers versus non-carriers.(32)
We found no main effect of APOEε4 in models for GDS total score or anxiety-concentration scores, and in a post-hoc model, no interaction effect of APOEε4 and Aβ on longitudinal GDS scores. Similarly, Locke and colleagues found no direct effect of APOEε4 carrier status on longitudinal depression scores in over six-hundred CN adults ages 21-86, followed for nearly 8 years.(33) Given the low endorsement of depressive symptoms in our cohort and the confounded relationship of Aβ and APOEε4, this post-hoc analysis may have lacked sufficient power to detect a true relationship between the Aβ-APOEε4 interaction and low range GDS scores in these analyses.
Low educational attainment and wealth are established risk factors for greater depressive symptom burden in community-dwelling older people., (34, 35) whereas, in our models, cognitive reserve and socioeconomic status did not predict total GDS scores or anxiety-only scores. This lack of effect may be attributable to the relatively high education and socioeconomic status of the sample that diminished the influence of these factors. In other results, we found associations of higher cognitive reserve and socioeconomic status with greater apathy-anhedonia scores, controlling for age and time, which were in the opposite direction to expectations.(35, 36) These results most likely reflect a survivor effect specific to this CN sample.
It is important to acknowledge that the fixed effects in these models, such as the PiB-time interaction and depression history, account for a small percent of the variance for depression scores over time and that the magnitude of this effect also appears to be small. At the same time, it is plausible that the strength of these relationships were attenuated by antidepressant medication use. While these findings suggest that Aβ may play a role in the pathogenesis of certain forms of late-life depression this observed relationship may be limited and/or indirect. Individuals with the highest levels of Aβ are also likely to harbor tau accumulation and neurodegenerative brain changes which may mediate the reported relationship between higher Aβ and rising depression scores.(37) If not directly biologically determined, these symptoms might also be a psychological reaction to other subtle cognitive or somatic changes and stresses occurring in late-stage preclinical AD. Finally, as multiple and heterogeneous factors may impact depression within an individual and across a sample, psychosocial factors and pathogenic processes such as vascular disease or stress-related mechanisms may prove to be stronger predictors of late-life depressive symptoms than Aβ. Additional studies in both clinically depressed and non-clinical samples are needed to determine if depressive-spectrum symptoms have sufficient specificity and power to meaningfully inform preclinical AD assessment in routine screening or in select groups.
There are limitations to this study. The relationships observed in these analyses were likely to be impacted by study exclusion criteria, including restricted GDS scores at screening. For example, dysphoric symptoms were infrequently endorsed in this sample, limiting our ability to detect associations with Aβ. Except for mild remitted depression, individuals with major psychiatric disorders and active medical and neurological conditions were excluded from the HABS cohort, thereby focusing and limiting the external validity of these findings to older persons with relatively good mental and brain health. Sample size limited our ability to analyze potential moderating effects of antidepressant medications and, among participants with a depression history, relevant characteristics such as recency of depressive episode and age of onset were not analyzed. Finally, the backward elimination method is sometimes criticized for being too liberal given that multiple runs produce multiple p values. For each dependent variable, however, significant effects found for PiB and other predictors of interest in the final model were also significant, or marginally so, in the initial full predictor set. Removal of extraneous terms mostly served to produce parsimonious models.
CONCLUSION
Higher brain Aβ burden was associated with increasing anxious-depressive symptoms over time in CN older adults. Prior depression history was related to higher but not worsening symptoms. These results suggest a direct or indirect association of elevated Aβ with worsening anxious-depressive symptoms and provide support for the hypothesis that emerging NPS represent an early manifestation of preclinical AD. Further longitudinal follow-up is necessary to determine whether these escalating depressive symptoms give rise to clinical depression and/or MCI and dementia stages of AD over an extended period of years.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Aging (NIA) R03 AG045080, R01 AG027435, K24 AG035007, the Harvard Medical School Department of Psychiatry Dupont-Warren Fellowship and Livingston Award, the Rogers Family Foundation, the Massachusetts Alzheimer’s Disease Research Center (P50 AG005134) and the Harvard Aging Brain Study (P01 AGO36694, R01 AG037497).
Footnotes
DISCLOSURES N. Donovan has received salary support from Eisai Inc. and Eli Lilly and Company. Her spouse is employed by Alkermes, PLC.
J. Locascio has no disclosures to report
G. Marshall has received salary support from Eisai Inc. and Eli Lilly and Company and consulting fees from Halloran and GliaCure Inc.
J. Gatchel has no disclosures to report.
Bernard J. Hanseeuw has no disclosures to report.
D. Rentz has served as a paid consultant for Eli Lilly, Janssen Alzheimer Immunotherapy, Biogen Idek, Lundbeck Pharmaceuticals and sits on the Scientific Advisory Board for Neurotrack.
K. Johnson has served as paid consultant for Abbvie, AZtherapies, Biogen, Bristol-Myers Squibb, GE Healthcare, Genentech, Isis Pharmaceuticals Inc, Janssen Alzheimer’s Immunotherapy, Piramal, Siemens Medical Solutions, Merck, Novartis, Roche, Lundbeck, and Genzyme. He is a site principal investigator co-investigator for Lilly/Avid, Biogen, Eisai, Janssen Alzheimer Immunotherapy, Merck, and Navidea clinical trials. He has spoken at symposia sponsored by Janssen Alzheimer’s Immunotherapy, GEHC, Lundbeck, and Pfizer.
R. Sperling has served as a paid consultant for Abbvie, Biogen, Bracket, Genentech, Lundbeck, Roche, and Sanofi. She has served as a co-investigator for Avid, Eli Lilly, and Janssen Alzheimer Immunotherapy clinical trials. She has spoken at symposia sponsored by Eli Lilly, Biogen, and Janssen Alzheimer Immunotherapy.
None of these relationships are related to the content of the manuscript.
(Corresponding “Spaghetti plots” of raw longitudinal data for GDS are shown in Supplement Figure 2).
References
- 1.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Boeve BF, Ivnik RJ, Petersen RC, Pankratz VS, Rocca WA. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63:435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 4.Steenland K, Karnes C, Seals R, Carnevale C, Hermida A, Levey A. Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. J Alzheimers Dis. 2012;31:265–275. doi: 10.3233/JAD-2012-111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJ, Pankratz VS, Boeve BF, Sochor O, Tangalos EG, Petersen RC, Rocca WA. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171:572–581. doi: 10.1176/appi.ajp.2014.13060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donovan NJ, Amariglio RE, Zoller AS, Rudel RK, Gomez-Isla T, Blacker D, Hyman BT, Locascio JJ, Johnson KA, Sperling RA, Marshall GA, Rentz DM. Subjective Cognitive Concerns and Neuropsychiatric Predictors of Progression to the Early Clinical Stages of Alzheimer Disease. Am J Geriatr Psychiatry. 2014;22:1642–1651. doi: 10.1016/j.jagp.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters ME, Lyketsos CG. Beyond memory: a focus on the other neuropsychiatric symptoms of dementia. Am J Geriatr Psychiatry. 2015;23:115–118. doi: 10.1016/j.jagp.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomara N, Bruno D, Sarreal AS, Hernando RT, Nierenberg J, Petkova E, Sidtis JJ, Wisniewski TM, Mehta PD, Pratico D, Zetterberg H, Blennow K. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry. 2012;169:523–530. doi: 10.1176/appi.ajp.2011.11081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babulal GM, Ghoshal N, Head D, Vernon EK, Holtzman DM, Benzinger TL, Fagan AM, Morris JC, Roe CM. Mood Changes in Cognitively Normal Older Adults are Linked to Alzheimer Disease Biomarker Levels. Am J Geriatr Psychiatry. 2016;24:1095–1104. doi: 10.1016/j.jagp.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington KD, Gould E, Lim YY, Ames D, Pietrzak RH, Rembach A, Rainey-Smith S, Martins RN, Salvado O, Villemagne VL, Rowe CC, Masters CL, Maruff P, Group AR Amyloid burden and incident depressive symptoms in cognitively normal older adults. Int J Geriatr Psychiatry. 2016 doi: 10.1002/gps.4489. [DOI] [PubMed] [Google Scholar]
- 11.Donovan NJ, Hsu DC, Dagley AS, Schultz AP, Amariglio RE, Mormino EC, Okereke OI, Rentz DM, Johnson KA, Sperling RA, Marshall GA. Depressive Symptoms and Biomarkers of Alzheimer’s Disease in Cognitively Normal Older Adults. J Alzheimers Dis. 2015;46:63–73. doi: 10.3233/JAD-142940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 14.Weschler D. WMS-R Weschler Memory Scale Revised Manual. New York: Ths Psychological Corporation, Harcourt Brace Jovanovich, Inc; 1987. [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Paolo AM, Ryan JJ. Generalizability of two methods of estimating premorbid intelligence in the elderly. Arch Clin Neuropsychol. 1992;7:135–143. [PubMed] [Google Scholar]
- 17.Juhn YJ, Beebe TJ, Finnie DM, Sloan J, Wheeler PH, Yawn B, Williams AR. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health. 2011;88:933–944. doi: 10.1007/s11524-011-9572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 20.Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci. 2012;32:16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, Rosand J, Rentz DM, Klunk WE, Mathis CA, Price JC, Dekosky ST, Fischman AJ, Greenberg SM. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 22.Raji CA, Becker JT, Tsopelas ND, Price JC, Mathis CA, Saxton JA, Lopresti BJ, Hoge JA, Ziolko SK, DeKosky ST, Klunk WE. Characterizing regional correlation, laterality and symmetry of amyloid deposition in mild cognitive impairment and Alzheimer’s disease with Pittsburgh Compound B. J Neurosci Methods. 2008;172:277–282. doi: 10.1016/j.jneumeth.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasuno F, Kazui H, Morita N, Kajimoto K, Ihara M, Taguchi A, Yamamoto A, Matsuoka K, Kosaka J, Kudo T, Iida H, Kishimoto T, Nagatsuka K. High amyloid-beta deposition related to depressive symptoms in older individuals with normal cognition: a pilot study. Int J Geriatr Psychiatry. 2016;31:920–928. doi: 10.1002/gps.4409. [DOI] [PubMed] [Google Scholar]
- 24.Leal SL, Noche JA, Murray EA, Yassa MA. Disruption of amygdala-entorhinal-hippocampal network in late-life depression. Hippocampus. 2017;27:464–476. doi: 10.1002/hipo.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loonen AJ, Ivanova SA. Circuits regulating pleasure and happiness in major depression. Med Hypotheses. 2016;87:14–21. doi: 10.1016/j.mehy.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 28.Olin JT, Schneider LS, Katz IR, Meyers BS, Alexopoulos GS, Breitner JC, Bruce ML, Caine ED, Cummings JL, Devanand DP, Krishnan KR, Lyketsos CG, Lyness JM, Rabins PV, Reynolds CF, 3rd, Rovner BW, Steffens DC, Tariot PN, Lebowitz BD. Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geriatr Psychiatry. 2002;10:125–128. [PubMed] [Google Scholar]
- 29.Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, Aguera-Ortiz L, Sweet R, Miller D, Lyketsos CG, Area INSPI Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12:195–202. doi: 10.1016/j.jalz.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR., Jr Proceedings of the Meeting of the International Working G, the American Alzheimer’s Association on “The Preclinical State of AD, July Washington Dc USA. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes SE, Esterlis I, Mazure CM, Lim YY, Ames D, Rainey-Smith S, Martins RN, Salvado O, Dore V, Villemagne VL, Rowe CC, Laws SM, Masters CL, Maruff P, Pietrzak RH, Australian Imaging BLRG. beta-Amyloid, APOE and BDNF Genotype, and Depressive and Anxiety Symptoms in Cognitively Normal Older Women and Men. Am J Geriatr Psychiatry. 2016;24:1191–1195. doi: 10.1016/j.jagp.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Donovan NJ, Okereke OI, Vannini P, Amariglio RE, Rentz DM, Marshall GA, Johnson KA, Sperling RA. Association of Higher Cortical Amyloid Burden With Loneliness in Cognitively Normal Older Adults. JAMA psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Locke DE, Dueck AC, Stonnington CM, Knopman DS, Geda YE, Caselli RJ. Depressive symptoms in healthy apolipoprotein E epsilon4 carriers and noncarriers: a longitudinal study. The Journal of clinical psychiatry. 2013;74:1256–1261. doi: 10.4088/JCP.13m08564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blazer D, Hughes DC, George LK. The epidemiology of depression in an elderly community population. The Gerontologist. 1987;27:281–287. doi: 10.1093/geront/27.3.281. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y. Is old age depressing? Growth trajectories and cohort variations in late-life depression. J Health Soc Behav. 2007;48:16–32. doi: 10.1177/002214650704800102. [DOI] [PubMed] [Google Scholar]
- 36.Tampubolon G, Maharani A. When Did Old Age Stop Being Depressing? Depression Trajectories of Older Americans and Britons 2002–2012. Am J Geriatr Psychiatry. 2017 doi: 10.1016/j.jagp.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, Marshall G, Albers M, Mauro S, Pepin L, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson B, Gomez-Isla T, Hyman B, Vasdev N, Sperling R. Tau PET imaging in aging and early Alzheimer’s disease. Ann Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


