Abstract
Recent research has identified the lateral habenula (LHb) as a brain region playing an important role in the production of stressful and anxiogenic states. Additionally, norepinephrine (NE) has long been known to be involved in arousal, stress and anxiety, and NE projections to the LHb have been identified emanating from the locus coeruleus (LC). The current research was devised to test the hypothesis that NE release within the LHb contributes to the occurrence of anxiogenic behaviors. Male rats were implanted with bilateral guide cannula aimed at the LHb and subsequently treated with intracranial (IC) infusions of the selective α2 adrenergic autoreceptor agonist, dexmedetomidine (DEX) (0, 0.5, 1.0 μg/side), prior to assessment of ambulatory and anxiogenic behavior in tests of spontaneous locomotion, open field behavior, and acoustic startle-response. Results demonstrated that DEX administration significantly reduced the overall locomotor behavior of subjects at both doses indicating that infusion of even small doses of this α2 agonist into the LHb can have profound effects on the subjects’ general levels of alertness and activity. DEX was also found to attenuate anxiety as evidenced by a reduction in the magnitude of a startle-response to an acoustic 110 dB stimulus. Taken together, these results identify a role for NE release within the LHb in both arousal and anxiety.
Keywords: norepinephrine, lateral habenula, anxiety, arousal, open field, startle-response
1. Introduction
Recent research has identified a role for the lateral habenula (LHb) in the production of negative (anxiogenic) affective states [1–4]. For example, LHb neurons are active when anticipated rewards are withheld, in the presence of punishing events [4–9], and during the behavioral response to anxiogenic or stressful stimuli [5,10–14]. Gill et al. [15] have shown that inactivation of the LHb with GABA receptor agonists attenuates anxiogenic behaviors as seen by increased time spent in the open arms of an elevated plus maze and reduced burying time in a test of defensive burying. Deep brain stimulation of the rat LHb has been reported to decrease self-administration of sucrose, while lesions of the LHb produces the opposite effect [5]. In related work, Matsumoto and Hikosaka [6] have demonstrated that weak electrical stimulation of the LHb strongly inhibits midbrain dopamine (DA) neurons, indicating that the LHb may be responsible for hindering reward-related signals. This attenuation of reward signaling now appears to be a consequence of LHb glutamatergic projections to the rostromedial tegmental nucleus (RMTg) that act to inhibit midbrain DA cell firing via GABAergic innervation of the ventral tegmental area (VTA) [1,16]. Consistent with this finding is a report demonstrating that when rodents are exposed to aversive stimuli, such as foot shock, there is an increase in LHb stimulation of the RMTg, and that optogenetic activation of this pathway leads to behavioral avoidance [17]. Recent research has also indicated that the LHb contributes to the anxiogenic response that occurs with cocaine administration [12]. In an attempt to identify the neurochemical substrates responsible for the behavioral effects of LHb activation, our laboratory has previously focused on the roles of DA and serotonin (5-HT) within the LHb as related to the encoding of anxiogenic events [18,19], but there is reason to believe that norepinephrine (NE) may also play a role [20–22].
NE has long been known to modulate the behavioral response to arousing, stressful, and anxiogenic stimuli [22–28]. Acute stress activates the NE system [29–31] with transmission increasing in response to both internal and environmental stressors, and stimulation of central NE systems has been shown to contribute to stress-induced activation of the HPA axis [32]. Consistent with these findings are reports that suppression of NE activity hinders the ability of mammals to effectively respond to challenging environmental situations [33,34]. The source of NE for much of this work has been the locus coeruleus (LC), which contains the largest number and highest density of noradrenergic cell bodies in the brain [24,33,35–38] and sends projections to a multitude of brain regions including the LHb [39–41]. Increased levels of stress and anxiety have been correlated with both heightened LC activity [23,42–44] and increased tyrosine hydroxylase expression in LC neurons [31,45]. Furthermore, stimulation of the LC has been shown to increase fearful and anxiety-like behavior in both humans and monkeys [24,39] and neuronal firing within the LC increases following the presentation of threatening, aversive, and stressful stimuli [23,46,47]. Conversely, inhibition of LC-NE neurons during stressful events attenuates the expression of anxiogenic behaviors [48].
In the current study, we examined the effects of reducing NE release within the LHb via intracranial administration of a highly selective α2 adreno-autoreceptor agonist (that has been shown to reduce NE release [49]) on three well-established behavioral tests that are sensitive to changes in the arousal and/or anxiogenic states of the subjects: a test of spontaneous locomotion [50–54], examination of the latency to enter the center regions of an open field [55–59], and the subjects’ response in an acoustic startle test [60–71]. Given NE’s known involvement in the experience of stress and anxiety, coupled with evidence that the LHb plays a role in these aversive states and that the LHb receives NE projections from the LC, we hypothesized that NE release within the LHb contributes to a state of heightened arousal and anxiety. The current study describes tests of this hypothesis.
2. Materials and Methods
2.1 Subjects
Subjects were 101 male Sprague-Dawley rats (250–300 g) obtained from Charles River Laboratories (Hollister, CA, USA). The rats were pair-housed within a temperature-controlled (22°) vivarium that was maintained on a reverse 12-hour light/dark cycle (lights on from 8:00 pm to 8:00 am). All animals were provided ad libitum access to food and water throughout the entire course of the experiment. To acclimate the subjects to their new environment, and to gentle them prior to behavioral testing, each subject was handled daily for 2 min beginning 7 days prior to surgery. An initial open field pilot study was conducted on 16 subjects. Subsequent subjects were tested in the open field, locomotor, and startle-response paradigms. All experimental protocols were reviewed and approved by the University of California at Santa Barbara’s Institutional Animal Care and Use Committee (IACUC) and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Surgery
Each subject underwent stereotaxic surgery during deep anesthesia induced by an intramuscular injection of ketamine/xylazine (56.25 and 7.5 mg/kg, respectively). Immediately prior to surgery animals received subcutaneous injections of Meloxicam (2 mg/kg), a non-opiate analgesic, and Buprenorphine (0.05 mg/kg), an opioid partial agonist, to reduce post-operative pain. Subjects were then fitted with bilateral intracranial (IC) guide cannula (22-gauge, 5 mm, Plastics One) stereotaxically aimed 2 mm above the LHb using the following coordinates: lateral inclination of 11°; AP −3.4; ML ±1.5; DV −3.2 mm relative to bregma. These coordinates were originally derived from Jhou et al. [14] and have been confirmed in previous studies in our laboratory [18]. Cannula were secured to the skull using dental acrylic and four stainless steel screws. While still anesthetized, each animal received a 3.0 mL subcutaneous (SC) injection of 0.9% physiological saline for hydration. Subjects were returned to their appropriate home cages after regaining consciousness and were given single daily SC injections of Meloxicam for two days post-surgery. All animals were allowed to recover for at least 7 additional days before behavioral testing was initiated.
2.3 Drug infusions
The selective α2 adrenergic autoreceptor agonist, dexmedetomidine (DEX) (Sigma-Aldrich), was dissolved in a vehicle solution of aCSF for bilateral IC infusion into the LHb. The drug (0, 0.5, or 1.0 μg) was administered over a 120 s duration in volumes of 0.25 μL/side delivered via a 10 μL Hamilton syringe seated in a motorized syringe pump (KD Scientific, Holliston, MA). Drug infusions were accomplished by inserting a 28-gauge internal cannula into the implanted guide cannula that projected 2 mm beyond the tip of the guide. The internal cannula was connected to the drug-filled Hamilton syringe by PE20 tubing and was left in place for 1 min post-infusion to allow for diffusion of the drug away from the injection site.
2.4 Apparatus
2.4.1 Open field apparatus
The open field apparatus was a 4-foot (120 cm) squared enclosure constructed of 40 cm high wood walls. The floor of the apparatus was subdivided into 16 equal-sized square regions (30 cm x 30 cm) with a total of 12 squares around the periphery and 4 in the middle of the apparatus. A digital camera affixed to the ceiling directly above the enclosure detected and recorded the locations of each subject in real time throughout the course of behavioral testing. The camera was interfaced with a desktop computer running Any-Maze software (Stoelting Co., Wood Dale, IL) that allowed for storage and analysis of the recorded behavioral tracking data.
2.4.2 Locomotor apparatus
The spontaneous locomotor activity of subjects was measured in 12 identical Plexiglas chambers each measuring 20 cm L x 40 cm W x 20 cm H (Kinder Scientific, San Diego, CA). A series of infrared photodetector-emitter pairs were embedded in the walls 8 cm above the floor of the chamber, with 15 pairs evenly spaced along the long axis and 7 along the narrow axis of the apparatus. Any movement within the chamber produced interruptions in the photobeams that were then recorded by a desktop computer running custom software (Kinder Scientific). Data were compiled as the total distance (cm) that a subject traveled per unit time (5-min bins).
2.4.3 Acoustic startle-response (ASR) apparatus
The ASR of subjects was measured in four startle boxes each measuring 28 cm L x 30 cm W x 29 cm H (SR-LAB, San Diego Instruments, San Diego, CA) each equipped with a Plexiglas cylinder (8.9 cm diameter) in which the animal was placed for testing. The cylinder was mounted on a Plexiglas base and each apparatus was housed in a ventilated sound-attenuated chamber. A high frequency speaker located 24 cm above the animal transmitted all acoustic signals. Background noise (produced by the ventilation fan) was 70 dB. Movement of subjects within the cylinder were transduced by a piezoelectric accelerometer mounted below the cylinder, rectified, digitized and recorded by a desktop computer running SR-LAB software. At the onset of each acoustic stimulus, 200 1-ms readings were recorded to obtain the subject’s startle amplitude.
2.5 Procedures
2.5.1 Open field procedure
A total of 61 subjects were individually tested during a single trial in the open field. 10 min prior to behavioral testing each subject was administered a bilateral intra-LHb infusion of DEX (0, 0.5 or 1.0 μg/injection/side; n = 25, 19 and 17, respectively). Subjects were then individually placed into one corner of the open field after which movements, locations, and entry into each square region were recorded via the overhead digital camera over a single 10 min session. An entry was counted when all four of the animal’s paws were within the same square. Subjects were returned to their home cages after completion of the test session.
2.5.2 Locomotor procedure
One week after completion of the open field test, a subset of the same subjects (n = 52) was tested for spontaneous ambulatory behavior after treatment with the same dose of DEX that they had previously received in the open field. Each subject was placed individually into an assigned chamber and allowed to acclimate to the apparatus for 30 min. Subjects were then removed, administered their respective bilateral IC injection of DEX (0, 0.5, or 1.0 μg/side; n = 19, 18 and 15, respectively), then immediately returned to the same chamber for an additional 30 min. Distance traveled by each subject was recorded during both the habituation and test segments of the session.
2.5.3 Startle-response procedure
The startle-response test was conducted one week after completion of the locomotor test in a further subset of the subjects who had been tested in both the open field and locomotor assays (n = 48). The procedures employed were those followed by previous investigators using the same apparatus [72,73]. 10 min after receiving an intra-LHb infusion of DEX (0, 0.5 or 1.0 μg/side; n = 17, 18 and 13, respectively), each subject was individually placed into the Plexiglas cylinder of the startle apparatus where, after a 5-min habituation period, they were presented with one of four acoustic signals [no acoustic stimulus (70 dB background noise; st0), 74 dB/20 ms (st74), 90 dB/20 ms (st90), or 110dB/40 ms (st110)] in a semi-random order over the course of the trial. St0 and st110 trials were presented 10 times each, and st74 and st90 trials were presented 5 times each with the average inter-trial interval being 15 s (range of 10–20 s). Startle amplitude for each trial was defined as the maximum response recorded during the first 200 ms after each stimulus presentation. The data for startle amplitude were averaged across trials for each different stimulus. Subjects were returned to their home cages after completion of the test session.
2.6 Histology
Upon completion of behavioral testing, subjects were euthanized with an overdose of sodium pentobarbital and phenytoin sodium solution (Euthasol). Intracardiac perfusions were performed with 200 mL of phosphate buffer saline (PBS) immediately followed by 200 mL of 4% paraformaldehyde (PFA) in PBS. Brains were post-fixed in 4% PFA overnight at 4° C before being cryoprotected in a 30% sucrose solution. The tissue was then frozen and sliced into 40 μm coronal sections then nissl-stained using a 0.25% thionin solution. Inclusion of subjects in the data analysis required strict histological conformation that bilateral cannula were both accurately placed directly above the LHb as confirmed by magnified visual inspection conducted by an individual (AE) blind to the treatment condition and group assignment of each animal.
3. Results
3.1 Open field test
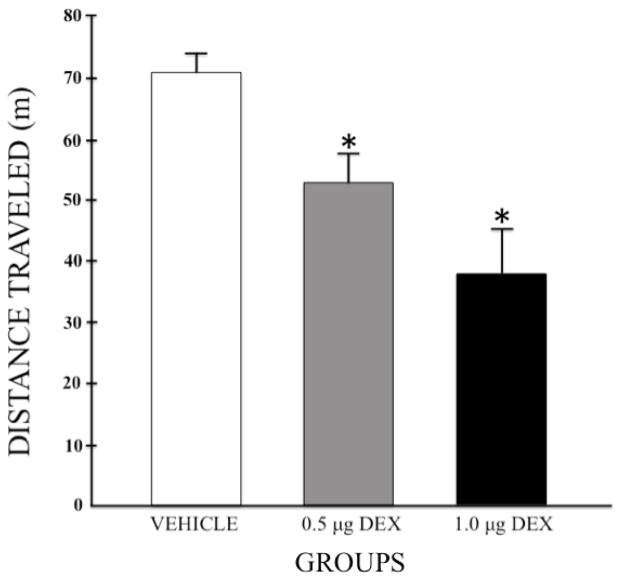
The mean (+SEM) total distance that each group traveled in the open field is depicted in figure 1. Group sizes were n = 25, 19, and 17 for the vehicle, low dose, and high dose groups, respectively. A one-way independent group Analysis of Variance (ANOVA) computed on these data revealed a significant main effect of Group (F (2,58) = 12.565; p < 0.001) and post-hoc Least Significant Difference (LSD) tests confirmed that pretreatment with either dose of DEX decreased the total distance that subjects traveled in the open field compared to subjects pretreated with vehicle (p = 0.006 and p < 0.001 for 0.5 and 1.0 μg/side, respectively). Additionally, subjects treated with the high dose of DEX (1.0 μg/side) were less active than subjects treated with the low dose (0.5 μg/side) of the drug (p = 0.045).
Figure 1.
Mean (+SEM) total distance traveled in the open field for each group following pretreatment with bilateral intra-LHb infusions of DEX (0, 0.5, or 1.0 μg/side). Group sizes were n = 25, 19, and 17 for the vehicle, low dose, and high dose groups, respectively. * p < 0.05 when compared to the vehicle control group.
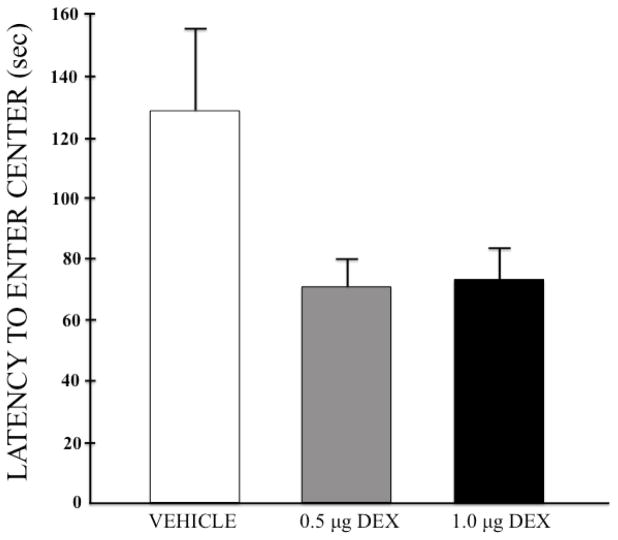
Figure 2 depicts the group mean (+SEM) latencies to enter the center squares of the apparatus, which was examined to assess the animals’ anxiogenic state. A one-way ANOVA narrowly failed to reach significance (F (2,58) = 2.907, p = 0.063), although the data clearly identify a trend toward faster entries into the center of the apparatus for both DEX doses. To test this possibility more directly, another measure of anxiety was implemented (i.e. the startle-response test), the results of which are described in section 3.4 below.
Figure 2.
Mean (+SEM) latencies to first enter the center of the open field for each group after pretreatment with bilateral intra-LHb infusions of DEX (0, 0.5, or 1.0 μg/side).
3.2 Locomotor test
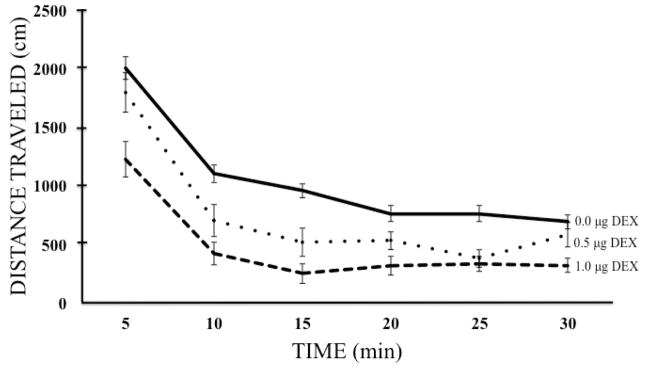
Assessment of spontaneous locomotor activity was used to determine whether intra-LHb administration of DEX produced any non-specific motoric effects. Note that a two-factor (Group x Time) ANOVA computed on the first 30-min of baseline activity (data not shown) confirmed the expected decrease in activity over time as the animals habituated to the apparatus (a main effect of Time; F (5, 245) = 183.91, p < 0.001), however, there were no observed Group differences nor any Group x Time interaction prior to drug administration (F (2,49) = 0.373, p = 0.690 and F (10,245) = 1.011, p = 0.435, respectively). The effects of intra-LHb DEX infusions on spontaneous locomotor behavior are depicted in figure 3. A second two-factor ANOVA (Group x Time) revealed significant Group differences in locomotor activity (F (2, 49) = 16.53, p < 0.001) post-DEX administration and LSD post-hoc tests confirmed that both drug doses (p = 0.003 and p < 0.001 for 0.5 and 1.0 μg/side, respectively) significantly reduced the locomotor activity of subjects compared to vehicle-pretreated controls. Group sizes were n = 19, 18, and 15 for the vehicle, low dose, and high dose groups, respectively. Additionally, the locomotor activity of subjects treated with the high dose of DEX was found to be significantly less than that of subjects treated with the low dose (p = 0.009). These data, therefore, confirm the effects of treatment on ambulatory behavior previously observed in the open field. As in the habituation portion of the test session, there was again a significant and expected main effect of Time as subjects further reduced their activity as the session progressed (F (5, 245) = 111.34, p < 0.001). Analysis of locomotor activity post-DEX administration additionally revealed a significant Group x Time interaction (F (10, 245) = 2.180, p = 0.020) indicating that groups decreased their responding at different rates over the course of the testing session.
Figure 3.
Mean (± SEM) total distance traveled (in cm) by each group of subjects during a 30-min locomotor activity test after intra-LHb infusions of DEX (0, 0.5, or 1.0 μg/side). Group sizes were n = 19, 18, and 15 for the vehicle, low dose, and high dose groups, respectively.
3.3 Startle-response test
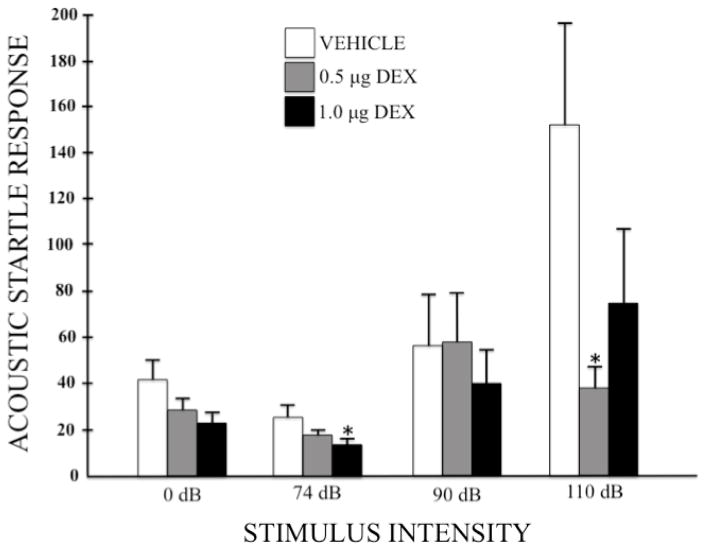
Given the trend toward faster center-square entries of DEX-treated subjects in the open field test (suggestive of a possible reduction in anxiety; see figure 2), another index of anxiogenic state (i.e. the acoustic startle-response (ASR) test) was utilized to examine the effects of DEX (0, 0.5, 1.0 μg/side) administration into the LHb. Figure 4 depicts the group mean (+SEM) performance in this test across different intensities of acoustic stimuli. A two-factor ANOVA (Group x Stimulus Intensity) revealed a significant main effect of Stimulus Intensity (F (3, 135) = 9.751, p < 0.001) demonstrating, as expected, an increase in responsiveness to the acoustic stimulus as the intensity of that stimulus increased; no significant main effect of Group (F (2, 45) = 2.857, p = 0.068); but a significant Group x Stimulus Intensity interaction (F (6, 135) = 2.859, p = 0.012), indicating that the impact of increasing the acoustic stimulus differed across groups. To identify the source of these effects, a series of pre-planned one-tailed Bonferonni-protected independent-group t-tests were then computed to compare each DEX-treated group to the vehicle control group at each stimulus intensity. No differences were observed at the 0 dB intensity, although the high dose of DEX suppressed responding at near significant levels (t(28) = 2.034, p = 0.052). As the intensity of the acoustic stimulus increased from sub-threshold (74 dB) to near threshold (90 dB) to supra-threshold (110 dB) levels, the responding of the vehicle group dramatically increased (as expected), an effect altered by pretreatment with DEX. When compared to vehicle, the high dose of DEX produced a statistically reliable reduction in startle response at the low 74 dB level (t(28) = 2.151, p = 0.04) but, due to increased variability in responding, not at higher intensities. In contrast, the low dose of DEX had no effect on the startle response at the baseline, low or near threshold levels, but reliably reduced startle responding to the 110 dB stimulus (t(33) = 2.717, p = 0.01).
Figure 4.
Mean (+SEM) startle response in arbitrary units to acoustic stimuli of 0, 74, 90, and 110 dB after pretreatment with intra-LHb infusions of DEX (0, 0.5, or 1.0 μg/side). Group sizes were n = 17, 18, and 13 for the vehicle, low dose, and high dose groups, respectively. * p < 0.05 when compared to the vehicle control group.
3.4 Histology
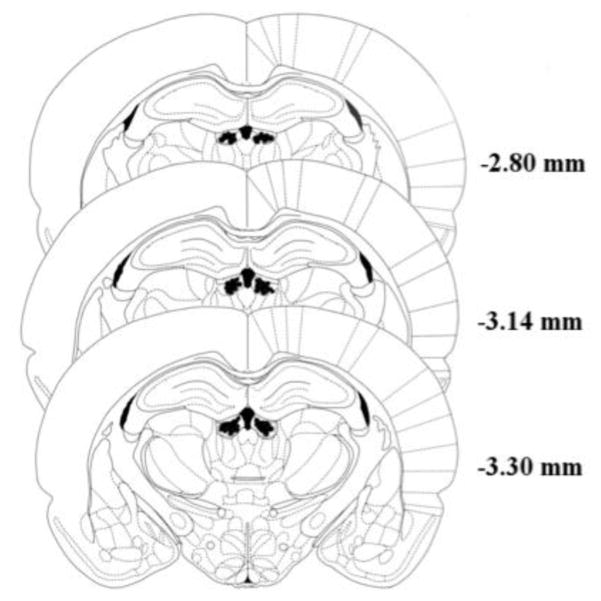
Figure 5 shows the bilateral cannula placement in the target LHb region (figure adapted from the atlas of Paxinos and Watson [74]). To ensure that our treatments were clearly restricted to the LHb, subjects were removed from data analysis if they displayed evidence of necrosis (n = 18) or if one of their bilateral cannula lay external to the target region (n = 22). Of the 22 missed targets, the majority (n=12) had cannula located within the third ventricle. Such placements would necessarily produce widespread distribution of the drug and hence raise the risk of nonspecific DEX effects. Indeed, when the low and high doses of this subgroup are combined (n = 9) they exhibited a significant reduction in ambulatory behavior (distance traveled) in both the locomotor test and open field when compared to the vehicle animals with LHb cannula (independent samples t-test, p < 0.001). This, of course, underscores the need to remove these subjects from the data analyses. Additionally, and of particular interest to the current study, the animals with “missed” (i.e, ventricular) cannula did not exhibit differences from vehicle controls in either measure of anxiety – i.e., latency to enter the center regions of the open field or the acoustic startle response test (p>.05). Similarly of the 10 subjects with missed cannula located in brain regions adjacent to the LHb, six were administered either the high or low dose of DEX and those animals, either individually or as a group, did not exhibit evidence of changes in either ambulatory or anxiogenic responses compared to vehicle controls.
Figure 5.
Histological confirmation of bilateral cannula placements within the lateral habenula. Shaded regions on either side of the third ventricle represent areas in which intracranial cannula tips were successfully localized to the target region. Figure adapted from [74]. Numbers represent the distance of each coronal slice from bregma.
4. Discussion
The present study provides preliminary evidence for a role for LHb NE in the production of both sedative and anxiogenic behavioral states in male rats. Administration of the low dose of the selective α2 adrenergic autoreceptor agonist, dexmedetomidine (DEX), produced both sedative effects (in the open field and locomotor activity tests) and anxiolytic effects (in the startle-response test). These findings confirm and extend previous research demonstrating a role for NE involvement in arousal [25,26,75] as well as in the production of stressful and anxiogenic states [22–24] but now identify the LHb, which previously has been implicated in the neurobiology of stress and anxiety [1–4], as a contributing source to the noradrenergic modulation of these states.
DEX, an imidazole compound [76], is the active isomer of the α2 agonist medetomidine [77–79] and has been shown to have potent hypnotic [80,81] as well as antinociceptive [82,83] properties. It has one of the highest ratios of α2/α1 receptor selectivity of any α2 agonist [79], being eight times more selective for the α2 receptor than clonidine [76]. DEX administration onto isolated, superfused slices of rat LC dose-dependently decreases both NE release and cell firing rate [35]. DEX has been shown to have dose-dependent hypnotic effects when microinjected into the LC at doses of 0.3–333.3 μg [84] and in our own preliminary studies (not described here), microinjection of 10.0, 5.0, or 2.0 μg/side of DEX into the LHb produced moderate to heavy sedation in subjects. The current experiment therefore utilized much lower doses (0.5 and 1.0 μg/side) of DEX in an attempt to examine its putative anxiolytic effects independent (if possible) of potentially confounding sedative effects.
To assess the presence of sedative effects resulting from reducing NE release within the LHb, we examined both open field and spontaneous locomotor behaviors. The measurement of spontaneous locomotor behavior serves as an index of general levels of arousal and ambulatory capacity in rats [50–54,85]. Here, intra-LHb administration of DEX significantly and dose-dependently decreased the overall activity level of treated subjects (Fig 4) suggesting that NE released within the LHb is likely involved in producing enhanced states of general arousal. A DEX-induced reduction in locomotion is consistent with the well-characterized sedative properties of the drug resulting from α2 adrenoreceptor stimulation. Indeed, DEX has been shown to produce dose-dependent sedative effects in rats when administered systemically [78,86,87] as well as into the LC [81,84,88]. While the sedative properties of DEX have been linked to inhibition of LC cell firing [80], the significant decline in locomotor activity observed in this experiment demonstrates, for the first time, an LHb role in the arousing and stimulatory actions of the NE system.
Data from the open field test confirm the results obtained with the locomotor activity test. Once again, intra-LHb administration of DEX produced reliable dose-dependent decreases in the total distance that animals traveled during the test session (Fig 2). This test, however, is also sensitive to the subjects’ anxiogenic state. When rats are placed in an unfamiliar open field they will typically express “thigmotaxis” – an inherent defensive behavior whereby the animals remain along the walls of the apparatus showing reluctance to enter the center regions of the open field [55,56,89,90]. This has obvious adaptive benefits in that avoiding open spaces in the wild would reduce the animals’ exposure to predators. As rats become more familiar with an open field, they will begin to spend more time exploring it, quantifiable by less time spent near the edges and increased center time [55]. Previous research has demonstrated that acute administration of anxiolytic drugs, such as diazepam, increases the amount of time that rats will spend in the center regions of the open field [91] and latencies to enter the center of the exposed field similarly decrease with declining levels of anxiety [55,57]. Conversely, restraint stress and acute administration of anxiogenic substances has been shown to increase the latency of rats to enter the center of an open field [58,92,93]. In the present study, the open field behavior of subjects pretreated with DEX was consistent with that of animals treated with other anxiolytics, as reflected in the fact that treated subjects exhibited near significant reductions in their latencies to enter the center of the open field compared to vehicle controls (p = 0.063). Note that the observed sedative effects of DEX cannot explain the anxiolytic actions of the drug since subjects in a sedative state would have been expected to take longer to enter the center of the open field – rather than what was observed (i.e., shorter entry latency). Hence we conclude that NE release in the LHb independently has both arousal and anxiogenic actions.
To further test the possibility that intra-LHb DEX had anxiolytic effects, we examined the behavior of animals in an acoustic startle-response (ASR) test. The acoustic startle-response (ASR) is a “flinch-like” reflex involving the rapid contraction of body muscles in response to a high amplitude acoustic stimulus [60]. The ASR of rats is amplified during anxious states [60,64–69], an effect that has also been reported in a variety of species including mice, rats, cats, monkeys, and humans [63]. Startle-inducing acoustic stimuli have therefore served as an index of a subject’s anxiogenic state [61,62] in that anxiogenic drugs have been shown to increase acoustic and fear-potentiated startle responses in rats [70,94–97], while anxiolytic drugs do the opposite [64,68,70,71,94,98–101]. In these studies, ASRs have typically been elicited with single noise bursts of intensities ranging from 95–110 dB [102] while acoustic stimuli with intensities below this range are generally considered sub-threshold for eliciting a startle response. Thus, in the current study, the 0 and 74 dB stimuli were below the threshold for typical elicitation of an ASR, the 90 dB stimulus approached the threshold, while the 110 dB stimulus was expected to reliably provoke an ASR. This pattern of responding was confirmed in the response of the vehicle control group (see Fig 5), which exhibited baseline responding to the 0 and 74 dB stimuli, increased slightly at the 90 dB intensity, and exhibited a strong ASR at the 110 dB level. Note that at the threshold 90 dB level, all three groups increased their responsiveness to the acoustic stimulus relative to the sub-threshold stimuli thereby demonstrating that the DEX-treated subjects were capable of responding despite the acknowledged sedative impact of the drug. While the ASR of vehicle-pretreated subjects dramatically increased in response to the 110 dB tone, this responsiveness was reliably prevented by pretreatment with the low 0.5 μg/side dose of DEX. If this significant difference in the ASR to 110 dB was due to the sedative effects of the drug, once again one would expect that subjects administered 0.5 μg/side of DEX to have shown significantly reduced response rates relative to vehicle controls during trials of acoustic intensities lower than 110 dB – which was not the case. These data are therefore consistent with the results reported after systemic administration of the α2 adrenoreceptor antagonist yohimbine, which has been shown to enhance the amplitude of ASR in rats [95,96]. The current findings, therefore, suggest that NE release within the LHb may be a contributing factor to the production of anxiogenic states.
In contrast to the response pattern observed with the low dose of DEX, the high 1.0 μg/side dose of the drug – which produced the greatest reduction in ambulatory behavior in both the open field and the locomotor activity tests, and hence had the strongest sedative impact – reliably reduced responding even at sub-threshold levels. Indeed, an examination of the response patterns depicted in figure 5 clearly shows that the high DEX group exhibited the weakest acoustic startle responding at all intensity levels making it difficult to distinguish whether its behavioral impact in this test was due to a reduction in anxiety or an enhanced level of sedation.
In conclusion, when taken together, the results of the open field, locomotor, and acoustic startle-response tests support the assertion that intra-LHb application of the α2 adrenoreceptor agonist, dexmedetomidine, can reliably produce both sedative and anxiolytic effects. Thus, given that NE pathways have long been implicated in the production of arousal [25,26,103–105] and anxiety [23,24] and have been shown to project from the LC to the LHb [40,41], the current data suggest that reductions in NE release within the lateral habenula play a significant role in the production of these dual behavioral states.
HIGHLIGHTS.
Reducing NE release in the LHb altered open field, locomotor and startle behaviors
Reductions in LHB NE release had anxiolytic effects in the acoustic startle test
Reductions in LHB NE release produced decreases in ambulatory behavior
NE release in the LHb is associated with states of arousal and anxiety
Acknowledgments
The authors wish to thank Dr. Kerisa Shelton for her mentorship in preparation for this project and Brian James for his assistance in the collection of data. This work was funded by a research grant from the National Institute of Drug Abuse (DA03370) awarded to AE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lecca S, Meye FJ, Mameli M. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur J Neurosci. 2014;39:1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- 3.Meye FJ, Lecca S, Valentinova K, Mameli M. Synaptic and cellular profile of neurons in the lateral habenula. Front Hum Neurosci. 2013;7:860. doi: 10.3389/fnhum.2013.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 5.Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, Ben-Tzion M, Yadid G. Electrical stimulation of the lateral habenula produces an inhibitory effect on sucrose self-administration. Neuropharmacology. 2011;60:381–387. doi: 10.1016/j.neuropharm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shumake J, Ilango A, Scheich H, Wetzel W, Ohl FW. Differential neuromodulation of acquisition and retrieval of avoidance learning by the lateral habenula and ventral tegmental area. J Neurosci. 2010;30:5876–5883. doi: 10.1523/JNEUROSCI.3604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland RJ, Nakajima S. Self-stimulation of the habenular complex in the rat. J Comp Physiol Psychol. 1981;95:781–791. doi: 10.1037/h0077833. [DOI] [PubMed] [Google Scholar]
- 10.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bromberg-Martin ES, Hikosaka O. Lateral habenula neurons signal errors in the prediction of reward information. Nat Neurosci. 2011;14:1209–1216. doi: 10.1038/nn.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, Ben-Tzion M, Ami-Ad L, Yaka R, Yadid G. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology. 2010;59:452–459. doi: 10.1016/j.neuropharm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Good CH, Wang H, Chen Y-H, Mejias-Aponte CA, Hoffman AF, Lupica CR. Dopamine D4 receptor excitation of lateral habenula neurons via multiple cellular mechanisms. J Neurosci. 2013;33:16853–16864. doi: 10.1523/JNEUROSCI.1844-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jhou TC, Good CH, Rowley CS, Xu S-P, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill MJ, Ghee SM, Harper SM, See RE. Inactivation of the lateral habenula reduces anxiogenic behavior and cocaine seeking under conditions of heightened stress. Pharmacol Biochem Behav. 2013;111:24–29. doi: 10.1016/j.pbb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shelton K, Bogyo K, Schick T, Ettenberg A. Pharmacological modulation of lateral habenular dopamine D2 receptors alters the anxiogenic response to cocaine in a runway model of drug self-administration. Behav Brain Res. 2016;310:42–50. doi: 10.1016/j.bbr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein AK, Purvis EM, Ayala K, Collins L, Jrug J, Mayes M, Ettenberg A. Activation of 5-HT1B receptors in the Lateral Habenula attenuates the anxiogenic effects of cocaine. 2017 doi: 10.1016/j.bbr.2018.04.014. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Schank JR, Liles LC, Weinshenker D. Norepinephrine signaling through β-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry. 2008;63:1007–1012. doi: 10.1016/j.biopsych.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratt JA. The neuroanatomical basis of anxiety. Pharmacol Ther. 1992;55:149–181. doi: 10.1016/0163-7258(92)90014-Q. [DOI] [PubMed] [Google Scholar]
- 24.Redmond DE, Huang YH. II. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci. 1979;25:2149–2162. doi: 10.1016/0024-3205(79)90087-0. [DOI] [PubMed] [Google Scholar]
- 25.Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berridge CW, Schmeichel BE, España RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev. 2012;16:187–197. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis M, Redmond DE, Baraban JM. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 1979;65:111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- 28.Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuro-Psychopharmacology Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/S0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 30.Svensson TH. Peripheral, autonomic regulation of locus coeruleus noradrenergic neurons in brain: putative implications for psychiatry and psychopharmacology. Psychopharmacology (Berl) 1987;92:1–7. doi: 10.1007/BF00215471. http://www.ncbi.nlm.nih.gov/pubmed/3110818. [DOI] [PubMed] [Google Scholar]
- 31.Page M, Akaoka H, Aston-Jones G, Valentino R. Bladder distension activates noradrenergic locus coeruleus neurons by an excitatory animo acid mechanism. Neuroscience. 1992;51:555–563. doi: 10.1016/0306-4522(92)90295-D. [DOI] [PubMed] [Google Scholar]
- 32.Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol. 1995;16:89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs BL. Single unit activity of locus coeruleus neurons in behaving animals. Prog Neurobiol. 1986;27:183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- 34.Weiss JM, Simson PG. Neurochemical basis of stress-induced depression. Psychopharmacol Bull. 1985;21:447–457. http://www.ncbi.nlm.nih.gov/pubmed/4041059. [PubMed] [Google Scholar]
- 35.Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. BJA Br J Anaesth. 1993;71:447–449. doi: 10.1093/bja/71.3.447. [DOI] [PubMed] [Google Scholar]
- 36.Grant SJ, Huang YH, Eugene Redmond D. Behavior of monkeys during opiate withdrawal and locus coeruleus stimulation. Pharmacol Biochem Behav. 1988;30:13–19. doi: 10.1016/0091-3057(88)90419-4. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Hunt S, Sah P. Norepinephrine and corticotropin-releasing hormone: partners in the neural circuits that underpin stress and anxiety. Neuron. 2015;87:468–470. doi: 10.1016/j.neuron.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Swanson LW. The locus coeruleus: a cytoarchitectonic, golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- 39.Redmond DE, Huang YH, Snyder DR, Maas JW. Behavioral effects of stimulation of the nucleus locus coeruleus in the stump-tailed monkey Macaca arctoides. Brain Res. 1976;116:502–510. doi: 10.1016/0006-8993(76)90498-4. [DOI] [PubMed] [Google Scholar]
- 40.Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc B Biol Sci. 2009;364:1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottesfeld Z. Origin and distribution of noradrenergic innervation in the habenula: a neurochemical study. Brain Res. 1983;275:299–304. doi: 10.1016/0006-8993(83)90990-3. [DOI] [PubMed] [Google Scholar]
- 42.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol. 2000;405:397–406. doi: 10.1016/S0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- 44.Van Bockstaele EJ, Valentino RJ. Neuropeptide regulation of the locus coeruleus and opiate-induced plasticity of stress responses. In: Eiden LE, editor. Advances in Pharmacology. Vol. 68. Elsevier Inc; San Diego: 2013. pp. 405–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melia KR, Duman RS. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci. 1991;88:8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J Neurosci. 1987;7:2837–2843. doi: 10.1523/JNEUROSCI.07-09-02837.1987. http://www.ncbi.nlm.nih.gov/pubmed/3625275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grant SJ, Redmond DE. Neuronal activity of the locus ceruleus in awake Macaca arctoides. Exp Neurol. 1984;84:701–708. doi: 10.1016/0014-4886(84)90217-6. [DOI] [PubMed] [Google Scholar]
- 48.McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87:605–620. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starke K, Göthert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. http://www.ncbi.nlm.nih.gov/pubmed/2568648. [DOI] [PubMed] [Google Scholar]
- 50.Kelley AE. Chapter 19: Locomotor activity and exploration. In: van Haaren F, editor. Methods in behavioral pharmacology: Techniques in the behavioral and neural sciences. Vol. 10. Elsevier Science; 1993. pp. 499–518. [DOI] [Google Scholar]
- 51.Robbins TW. A critique of the methods available for the measurement of sponteneous motor activity. In: Iverson LL, Iverson SD, Snyder SH, editors. Handbook of Psychopharmacology: Principles of Behavioral Pharmacology. Vol. 7. Plenum Press; New York: 1977. pp. 37–82. [DOI] [Google Scholar]
- 52.Perrault G, Morel E, Sanger DJ, Zivkovic B. The interaction between zolpidem and β-CMC: a clue to the identification of receptor sites involved in the sedative effect of zolpidem. Eur J Pharmacol. 1988;156:189–196. doi: 10.1016/0014-2999(88)90321-4. [DOI] [PubMed] [Google Scholar]
- 53.McElroy JF, Fleming RL, Feldman RS. A comparison between chlordiazepoxide and CL 218,872--a synthetic nonbenzodiazepine ligand for benzodiazepine receptors on spontaneous locomotor activity in rats. Psychopharmacology (Berl) 1985;85:224–226. doi: 10.1007/BF00428419. http://www.ncbi.nlm.nih.gov/pubmed/2861621. [DOI] [PubMed] [Google Scholar]
- 54.Sanger DJ, Morel E, Perrault G. Comparison of the pharmacological profiles of the hypnotic drugs, zaleplon and zolpidem. Eur J Pharmacol. 1996;313:35–42. doi: 10.1016/0014-2999(96)00510-9. [DOI] [PubMed] [Google Scholar]
- 55.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- 56.Walsh RN, Cummins RA. The open-field test: A critical review. Psychol Bull. 1976;83:482–504. doi: 10.1037//0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- 57.Schmitt U, Hiemke C. Strain differences in open-field and elevated plus-maze behavior of rats without and with pretest handling. Pharmacol Biochem Behav. 1998;59:807–811. doi: 10.1016/S0091-3057(97)00502-9. [DOI] [PubMed] [Google Scholar]
- 58.Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. http://www.ncbi.nlm.nih.gov/pubmed/1584846. [DOI] [PubMed] [Google Scholar]
- 59.Yang XM, Gorman AL, Dunn AJ. The involvement of central noradrenergic systems and corticotropin-releasing factor in defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1990;255:1064–1070. http://www.ncbi.nlm.nih.gov/pubmed/2262892. [PubMed] [Google Scholar]
- 60.Inagaki H, Kiyokawa Y, Tamogami S, Watanabe H, Takeuchi Y, Mori Y. Identification of a pheromone that increases anxiety in rats. Proc Natl Acad Sci. 2014;111:18751–18756. doi: 10.1073/pnas.1414710112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaltwasser MT. Startle-inducing acoustic stimuli evoke ultrasonic vocalization in the rat. Physiol Behav. 1990;48:13–17. doi: 10.1016/0031-9384(90)90253-Z. [DOI] [PubMed] [Google Scholar]
- 62.Theresia Kaltwasser M. Acoustic startle induced ultrasonic vocalization in the rat: a novel animal model of anxiety? Behav Brain Res. 1991;43:133–137. doi: 10.1016/S0166-4328(05)80063-4. [DOI] [PubMed] [Google Scholar]
- 63.Curzon P, Zhang M, Radek RJ, Fox GB. The behavioral assessment of sensorimotor processes in the mouse: acoustic startle, sensory gating, locomotor activity, rotarod, and beam walking. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. CRC Press; Boca Raton: 2009. http://www.ncbi.nlm.nih.gov/pubmed/21204341. [PubMed] [Google Scholar]
- 64.Davis M. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behav Neurosci. 1986;100:814–824. doi: 10.1037//0735-7044.100.6.814. [DOI] [PubMed] [Google Scholar]
- 65.Inagaki H, Kiyokawa Y, Takeuchi Y, Mori Y. The alarm pheromone in male rats as a unique anxiety model: psychopharmacological evidence using anxiolytics. Pharmacol Biochem Behav. 2010;94:575–579. doi: 10.1016/j.pbb.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 66.Miles L, Davis M, Walker D. Phasic and sustained fear are pharmacologically dissociable in rats. Neuropsychopharmacology. 2011;36:1563–1574. doi: 10.1038/npp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harris AC, Gewirtz JC. Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 2004;171:140–147. doi: 10.1007/s00213-003-1573-0. [DOI] [PubMed] [Google Scholar]
- 68.Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 69.Brown JS, Kalish HI, Farber IE. Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. J Exp Psychol. 1951;41:317–328. doi: 10.1037/h0060166. http://www.ncbi.nlm.nih.gov/pubmed/14861383. [DOI] [PubMed] [Google Scholar]
- 70.Yeomans J. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev. 2002;26:1–11. doi: 10.1016/S0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]
- 71.Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. http://www.ncbi.nlm.nih.gov/pubmed/9577241. [DOI] [PubMed] [Google Scholar]
- 72.Geyer MA, Swerdlow NR. Current Protocols in Neuroscience. John Wiley & Sons, Inc; Hoboken, NJ: 1998. Measurement of Startle Response, Prepulse Inhibition, and Habituation; pp. 8.7.1–8.7.15. [DOI] [PubMed] [Google Scholar]
- 73.Swerdlow N. Discrepant findings of clozapine effects on prepulse inhibition of startle: is it the route or the rat? Neuropsychopharmacology. 1998;18:50–56. doi: 10.1016/S0893-133X(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 74.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic; New York: 2005. [Google Scholar]
- 75.Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Curr Biol. 2015;25:1051–1056. doi: 10.1016/j.cub.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 76.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. http://www.ncbi.nlm.nih.gov/pubmed/16369581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mansikka H, Pertovaara A. The role of α2-adrenoceptors of the medullary lateral reticular nucleus in spinal antinociception in rats. Brain Res Bull. 1995;37:633–638. doi: 10.1016/0361-9230(95)00058-M. [DOI] [PubMed] [Google Scholar]
- 78.Savola J-M, Virtanen R. Central α2-adrenoceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medetomidine. Eur J Pharmacol. 1991;195:193–199. doi: 10.1016/0014-2999(91)90535-X. [DOI] [PubMed] [Google Scholar]
- 79.Sinclair MD. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can Vet J = La Rev Vet Can. 2003;44:885–897. http://www.ncbi.nlm.nih.gov/pubmed/14664351. [PMC free article] [PubMed] [Google Scholar]
- 80.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. http://www.ncbi.nlm.nih.gov/pubmed/12552203. [DOI] [PubMed] [Google Scholar]
- 81.Vulliemoz Y, Whittington RA, Virag L. The nitric oxide-cGMP system of the locus coeruleus and the hypnotic action of alpha-2 adrenergic agonists. Brain Res. 1999;849:169–174. doi: 10.1016/S0006-8993(99)02147-2. [DOI] [PubMed] [Google Scholar]
- 82.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–881. doi: 10.1097/00000542-199604000-00015. http://www.ncbi.nlm.nih.gov/pubmed/8638842. [DOI] [PubMed] [Google Scholar]
- 83.Xu M, Wei H, Kontinen VK, Kalso E, Pertovaara A. The dissociation of sedative from spinal antinociceptive effects following administration of a novel alpha-2-adrenoceptor agonist, MPV-2426, in the locus coeruleus in the rat. Acta Anaesthesiol Scand. 2000;44:648–655. doi: 10.1034/j.1399-6576.2000.440604.x. http://www.ncbi.nlm.nih.gov/pubmed/10903011. [DOI] [PubMed] [Google Scholar]
- 84.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–952. doi: 10.1097/00000542-199206000-00013. http://www.ncbi.nlm.nih.gov/pubmed/1350889. [DOI] [PubMed] [Google Scholar]
- 85.Gupta G, Kazmi I, Afzal M, Rahman M, Saleem S, Ashraf MS, Khusroo MJ, Nazeer K, Ahmed S, Mujeeb M, Ahmed Z, Anwar F. Sedative, antiepileptic and antipsychotic effects of Viscum album L. (Loranthaceae) in mice and rats. J Ethnopharmacol. 2012;141:810–816. doi: 10.1016/j.jep.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 86.Garrity AG, Botta S, Lazar SB, Swor E, Vanini G, Baghdoyan HA, Lydic R. Dexmedetomidine-induced sedation does not mimic the neurobehavioral phenotypes of sleep in sprague dawley rat. Sleep. 2015;38:73–84. doi: 10.5665/sleep.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanders RD, Giombini M, Ma D, Ohashi Y, Hossain M, Fujinaga M, Maze M. Dexmedetomidine exerts dose-dependent age-independent antinociception but age-dependent hypnosis in fischer rats. Anesth Analg. 2005;100:1295–1302. doi: 10.1213/01.ANE.0000149595.41576.B3. [DOI] [PubMed] [Google Scholar]
- 88.Pertovaara A, Hämäläinen MM, Kauppila T, Mecke E, Carlson S. Dissociation of the alpha 2-adrenergic antinociception from sedation following microinjection of medetomidine into the locus coeruleus in rats. Pain. 1994;57:207–215. doi: 10.1016/0304-3959(94)90225-9. http://www.ncbi.nlm.nih.gov/pubmed/7916451. [DOI] [PubMed] [Google Scholar]
- 89.Lamprea MR, Cardenas FP, Setem J, Morato S. Thigmotactic responses in an open-field. Brazilian J Med Biol Res. 2008;41:135–140. doi: 10.1590/S0100-879X2008000200010. [DOI] [PubMed] [Google Scholar]
- 90.Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- 91.Hazim AI, Ramanathan S, Parthasarathy S, Muzaimi M, Mansor SM. Anxiolytic-like effects of mitragynine in the open-field and elevated plus-maze tests in rats. J Physiol Sci. 2014;64:161–169. doi: 10.1007/s12576-014-0304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fernandez F, Misilmeri MA, Felger JC, Devine DP. Nociceptin/Orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology. 2004;29:59–71. doi: 10.1038/sj.npp.1300308. [DOI] [PubMed] [Google Scholar]
- 93.Pal R, Gulati K, Chakraborti A, Banerjee B, Ray A. Role of free radicals in stress-induced neurobehavioural changes in rats. Indian J Exp Biol. 2006;44:816–820. http://www.ncbi.nlm.nih.gov/pubmed/17131912. [PubMed] [Google Scholar]
- 94.Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. http://www.ncbi.nlm.nih.gov/pubmed/10463792. [DOI] [PubMed] [Google Scholar]
- 95.Morgan CA, Southwick SM, Grillon C, Davis M, Krystal JH, Charney DS. Yohimbine — facilitated acoustic startle reflex in humans. Psychopharmacology (Berl) 1993;110:342–346. doi: 10.1007/BF02251291. [DOI] [PubMed] [Google Scholar]
- 96.Fendt M, Koch M, Schnitzler H-U. Amygdaloid noradrenaline is involved in the sensitization of the acoustic startle response in rats. Pharmacol Biochem Behav. 1994;48:307–314. doi: 10.1016/0091-3057(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 97.Frankland PW, Josselyn SA, Bradwejn J, Vaccarino FJ, Yeomans JS. Activation of amygdala cholecystokininB receptors potentiates the acoustic startle response in the rat. J Neurosci. 1997;17:1838–47. doi: 10.1523/JNEUROSCI.17-05-01838.1997. http://www.ncbi.nlm.nih.gov/pubmed/9030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chi CC. The effect of amobarbital sodium on conditioned fear as measured by the potentiated startle response in rats. Psychopharmacologia. 1965;7:115–122. doi: 10.1007/BF00403634. [DOI] [PubMed] [Google Scholar]
- 99.Davis M. Diazepam and flurazepam: effects on conditioned fear as measured with the potentiated startle paradigm. Psychopharmacology (Berl) 1979;62:1–7. doi: 10.1007/BF00426027. [DOI] [PubMed] [Google Scholar]
- 100.Davis M. Morphine and naloxone: effects on conditioned near as measured with the potentiated startle paradigm. Eur J Pharmacol. 1979;54:341–347. doi: 10.1016/0014-2999(79)90063-3. [DOI] [PubMed] [Google Scholar]
- 101.Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-V. [DOI] [PubMed] [Google Scholar]
- 102.Li L, Yeomans J. Summation between acoustic and trigeminal stimuli evoking startle. Neuroscience. 1999;90:139–152. doi: 10.1016/S0306-4522(98)00436-9. [DOI] [PubMed] [Google Scholar]
- 103.Smee ML, Weston PF, Skinner D, Day T. Dose-related effects of central noradrenaline stimulation on behavioural arousal in rats. Psychopharmacol Commun. 1975;1:123–130. http://www.ncbi.nlm.nih.gov/pubmed/1223995. [PubMed] [Google Scholar]
- 104.Lidbrink P. The effect of lesions of ascending noradrenaline pathways on sleep and waking in the rat. Brain Res. 1974;74:19–40. doi: 10.1016/0006-8993(74)90109-7. http://www.ncbi.nlm.nih.gov/pubmed/4152613. [DOI] [PubMed] [Google Scholar]
- 105.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]