Abstract
Background
The purpose of this study is to clarify the source distribution patterns of magnetoencephalographic (MEG) spikes correlated with postsurgical seizure-free outcome in pediatric patients with focal cortical dysplasia (FCD).
Methods
Thirty-two patients with pathologically-confirmed FCD were divided into seizure-free and -persistent groups according to their surgical outcomes based on Engel's classification. In each patient, presurgical MEG was retrospectively reviewed. Dipole sources of MEG spikes were calculated according to a single dipole model. We obtained the following quantitative indices for evaluating dipole distribution: maximum distance over all pairs of dipoles, standard deviation of the distances between each dipole and the mean coordinate of all dipoles, average nearest neighbor distance, the rate of dipoles located within 10 mm, 20 mm, 30 mm from the mean coordinate, and the rate of dipoles included in the resection. These indices were compared between the two patient groups.
Results
Average nearest neighbor distance was significantly smaller in the seizure-free group compared to the seizure-persistent group (p=0.008). The rate of dipoles located within 10 mm, 20 mm, 30 mm from the mean coordinate were significantly higher in the seizure-free group (p=0.001, 0.001, 0.005, respectively). The maximum distance, standard deviation and the resection rate of dipoles did not show a significant difference between the two groups.
Conclusions
A spatially-restricted dipole distribution of MEG spikes is correlated with postsurgical seizure-free outcomes in patients with FCD. The distribution can be assessed by quantitative indices that are clinically useful in the presurgical evaluation of these patients.
Keywords: Focal cortical dysplasia, epilepsy surgery, magnetoencephalography, source localization, equivalent current dipole
Introduction
Focal cortical dysplasia (FCD) is a major cause of medically-intractable epilepsy in the pediatric population but may be amenable to surgical treatment1, 2. Previous studies have shown that complete removal of the anatomical/electrophysiological abnormality is an important prognostic factor of postsurgical seizure freedom3, 4. Therefore, investigation of epileptic discharges, including interictal spikes, is critical for planning epilepsy surgery in pediatric patients with FCD.
Magnetoencephalography (MEG) is a non-invasive tool that records neuromagnetic fields from the brain, and is useful for localizing epileptic discharges in presurgical evaluation. Previous studies have investigated interictal MEG spikes by using a single dipole model in FCD patients, showing the intrinsic epileptogenicity of the lesion as compared with MRI and intracranial EEG5–9. Several researchers observed dipole distribution in a restricted cortical area, which is called a 'dipole cluster'10, 11. They suggested that removal of the cluster is correlated with a favorable surgical outcome10, 11, however, postsurgical seizure-freedom is not always achieved. In the past studies, the dipole cluster was subjectively detected by visual inspection of dipole distribution maps8, 9, or by applying predefined criteria, such as 'six or more spike sources with 1 cm or less between adjacent sources'10, 11. The basis of these criteria is still unclear: no studies have revealed how closely the dipoles should be located for determining the dipole cluster that is clinically relevant. Revisiting the concept of dipole cluster beyond the subjective, predefined criteria is necessary for better planning of epilepsy surgery.
The purpose of this study is to objectively and quantitatively reveal the dipole distribution that is useful for estimating postsurgical outcomes. We investigate 1) the spatial patterns of dipole distribution by using numerical indices, and 2) the relation of these indices with the surgical outcome in patients with FCD. We hypothesize that these indices showing a spatially-restricted dipole distribution are correlated with postsurgical seizure freedom and provide the basis of clinically-relevant dipole cluster.
Patients and Methods
Patients
We retrospectively studied 32 pediatric patients (18 males, 14 females, age 1–18, mean 11 years old) who underwent an MEG as a part of clinical evaluation and subsequent epilepsy surgery in 2003–2016. All patients had a histopathological diagnosis of FCD. There were 11 patients diagnosed according to the classification proposed by Palmini et al.12 (Patients 4–7, 9, 18, 21–25), and 11 patients with the International League Against Epilepsy classification13 (Patients 8, 10–13, 15–16, 26, 28–29, 32). The pathology reports of 10 patients (Patients 1–3, 14, 17, 19–20, 27, 30–31) only indicated FCD without further stratification. We included patients with isolated FCD and with associated principal lesions, such as encephalomalacia, infarction and cyst, therefore, the patients were not characterized by a single pathology. Six patients had previous surgery, which showed cortical dysplasia/malformation without further classification (Patients 15, 17, 25, 27 and 32) and ganglion cell tumor (Patient 20) in pathology.
Patients were post-operatively followed to 12–92 months (mean 32 months). Surgical outcomes were evaluated by Engel's classification at the time of last follow-up14, and we divided the patients into two groups: Seizure-free (Class Ia outcome, 16 patients) and seizure-persistent (other outcomes, 16 patients). All aspects of the study were approved by the institutional review board and were performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients and their guardians. Table 1 gives an overview of the clinical profiles of the patients.
Table 1.
Patient profile
| Patient | Age/Sex | Diagnosis | Scalp EEG (Interictal/Ictal) |
MEG | MRI | Resection | Pathology | Surgical outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 13/M | L TLE | LT/LT | LT | Arachnoid cyst+CD in LT(ant) | LT(ant) | CD | Free |
| 2 | 9/F | L TLE | LT/L hemi | LT | Abnormal T2 signal in LT(inf) | LT(inf) | Low-grade glioma+CD | Free |
| 3 | 11/F | R FLE | BiF/RF | RF | CD in RF(mes) | RF(mes) | CD | Free |
| 4 | 11/M | R FLE | RF/Diffuse | RF | CD in RF(mes) | RF(mes) | Palmini IIB | Free |
| 5 | 18/F | R TLE | RT/RT | RT | CD in RT(mes) | RT(ant+mes) | Palmini IA | Free |
| 6 | 11/M | L TLE | LT/LF,LT | LT | L MTS, CD in LT | LT(ant) | MTS+Palmini IIA | Free |
| 7 | 10/M | L TLE | LT/LT,LC,LP | LT | L MTS, L hemi atrophy | LT(ant) | MTS+Palmini IIA | Free |
| 8 | 13/M | Multifocal | RF,RP/RF | RF,RT | Dysmorphic cortex in RT(pos), PMG in R hemi | R functional hemispherectomy | ILAE IIId | Free |
| 9 | 16/F | L TLE | LT/LT | LT | Heterotopia, dysmorphic T2 hyperintense in LT,LO | LT(ant+pos) | Palmini IIA | Free |
| 10 | 13/F | L TLE | LT/LT | LT | Blurring in LT(ant) | LT(ant) | ILAE IIa | Free |
| 11 | 9/M | R OLE | RO/RO | RO | Encephalomalacia in RO | RO | ILAE IIId | Free |
| 12 | 7/F | R TLE | RF,RT/RF,RT | RT | CD in RT(inf) | RT(inf) | Ganglioglioma+ILAE IIIb | Free |
| 13 | 7/M | R FLE | RF,RT/RF,RT | RF | CD in RF(preF) | RF(preF) | ILAE IIb | Free |
| 14 | 5/M | R FLE | RF,RC,RT/RF,RC,RT | RF,RP | normal | RF+RP | CD | Free |
| 15 | 2/F | L TLE | LF,LT/LF,LT | LF,LT | Previous resection, residual CD in LT(sup) | LT(sup) | ILAE IIa | Free |
| 16 | 3/F | L FLE | LF/Diffuse | LF | CD in LF(preF) | LF(preF) | ILAE IIb | Free |
| 17 | 12/M | L TLE | LT,LP/LT,LC,LP | LT | Previous resection | LT+LP | CD | Persistent |
| 18 | 14/M | R FLE | RF,RC/BiF,RC | RF,RT | CD in RF(preC) | RF(preC) | Palmini IA | Persistent |
| 19 | 16/F | L TLE | LT/LT | LT | L MTS | LT(ant) | MTS+CD | Persistent |
| 20 | 15/M | L TLE | LT/Diffuse | LT,LP | Previous resection in LF(preC) | LT(ant) | CD | Persistent |
| 21 | 15/M | R FLE | RF/RF | RF | CD in RF(sup) | RF(sup) | Palmini IIB | Persistent |
| 22 | 15/F | R TLE | RT/RF,RT | RT | normal | LT(ant) | Palmini IA | Persistent |
| 23 | 12/M | R TLE | RT/RF,RC,RT | RT | T2 hyperintense in RT(ant) | RT(ant) | Palmini IIA | Persistent |
| 24 | 15/M | L TLE | LF,LT/LF,LT | LT | normal | LT(ant) | Palmini IA | Persistent |
| 25 | 17/F | L TLE | LT,LP/LT,LP | LT,LP | Previous resection in LT | LT(pos) | Palmini IA | Persistent |
| 26 | 8/F | R FLE | RF,RC/BiF,BiC,BiP | RF | Lesion in RF(mes) | RF(mes) | ILAE IIb | Persistent |
| 27 | 11/M | L TLE | BiF,BiT/LC | BiF,BiT | Previous resection in LT(pos),LO | LT(ant) | CD | Persistent |
| 28 | 12/M | L TLE | LT/LT | LF,LT,LP | Previous infarction in LF,LT | LF+LT(ant) | ILAE IIId | Persistent |
| 29 | 6/F | R FLE | RF,RT/Diffuse | RF,RT,RP | CD in RF(inf) | RF(inf) | ILAE IIb | Persistent |
| 30 | 1/F | R FLE | R hemi/R hemi | R hemi | Cortical malformation in RF | R functional hemi spherectomy | CD | Persistent |
| 31 | 17/M | L TLE | LT/LT | LT | Small cystic foci in L hippocampal region | LT(inf) | Glioneuronal tumor+CD | Persistent |
| 32 | 12/M | L FLE | BiF,LT/LF,LT | BiF,LT | Previous resection in LT(ant), blurring in LT(inf) | LF (pre)+LT (inf) | ILAE IIa | Persistent |
M: Male, F: Female, L: Left, R: Right, Bi: Bilateral, FLE: Frontal lobe epilepsy, TLE: Temporal lobe epilepsy, L(R)F: Left(Right) frontal, L(R)T: Left(Right) temporal, L(R)C: Left(Right) central, L(R)P: Left(Right) parietal, L(R)O: Left(Right) occipital, hemi: hemisphere, CD: Cortical dysplasia, MTS: Mesial temporal sclerosis, PMG: Polymicrogyria, ant: anterior, pos: posterior, sup: superior, inf: inferior, mes: mesial, preF: prefrontal, preC: precentral, Free: Seizure-free, Persistent: Seizure-persistent, also shown by the gray background.
MEG recording
MEG was recorded with a 306-channel, whole-head MEG system (Elekta-Neuromag, Helsinki, Finland). The sampling frequency was 600 Hz (Patients 1–8, 17–27) or 1000 Hz (Patients 9–16, 28–32) with a band-pass filter of 0.1–200 Hz. We recorded spontaneous activity for 50–60 min in each patient. Patients were recorded in supine position and instructed to rest or sleep. Antiepileptic medications were maintained without tapering and no sedation was performed at the time of MEG study. We collected scalp EEG simultaneously with MEG by using a 70-channel electrode cap. The EEG findings are shown in Table 1. The data were low-pass filtered at 40 Hz for the analysis. The details of the MEG recording have previously been described15.
In all patients, anatomical MRI data were acquired with magnetization-prepared rapid acquisition gradient-echo sequences (MPRAGE; TE=3.37 ms, TR=2000 ms, voxel size=1×1×1 mm) with a high-resolution 3T scanner (TIM TRIO, Siemens AG, Erlangen, Germany). Post-surgical MRI was also obtained with MPRAGE, T1- or T2-axial/coronal/sagittal sequences.
MEG analysis
We visually examined MEG data and identified interictal spikes. Equivalent current dipoles (ECDs) were calculated at the peak of each spike using a single-dipole model without selecting a region of interest (i.e., all 306 sensors were used for the analysis). ECDs with goodness of fit >70 % and dipole moment <500 nAm were considered adequate as spike sources. The mean coordinate of all ECDs was obtained in each patient. Then we calculated the following indices for evaluating the spatial distribution of ECDs: (1) maximum distance: the largest distance over all pairs of ECDs; (2) SDD: standard deviation of the distances between each ECD and the mean coordinate; (3) average nearest neighbor distance: mean distance between each ECD and its nearest ECD; (4)–(6) within 10 mm, within 20 mm and within 30 mm: the rate of ECDs located within 10 mm, 20 mm and 30 mm from the mean coordinate, respectively; (7) resection rate: the rate of ECDs located in the resection. We coregistered the postsurgical MRI to the presurgical images and visually determined whether each ECD was removed or not.
Statistics
We compared all seven indices as defined above between the seizure-free and - persistent groups by using Mann-Whitney tests. Since the numbers of ECDs are different according to the number of spikes recorded in each patient, we tested the correlations between the numbers of ECDs and indices (1)–(7) by means of Spearman's correlation coefficient for understanding its effect on these indices. We also investigated the correlations between (1)–(6) and the resection rate to test whether the ECD distribution affects the removal of ECDs. P value<0.01 was considered significant.
Results
Table 2 summarizes the results of each patient. The average nearest neighbor distance was significantly smaller in seizure-free group than in seizure-persistent group (p=0.008). The seizure-free group also showed larger values of within 10 mm, within 20 mm, within 30 mm (p=0.001, 0.001, 0.005). We did not find any significant difference in other indices between these patients groups.
Table 2.
Results
| Patient | Number of ECDs |
Mean coordinates (x, y, z) |
Maximum distance |
SD | Nearest | Within 10 mm | Within 20 mm | Within 30 mm | Resection rate |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | −44.1, 17.6, 19.2 | 40.1 | 6.2 | 3.1 | 0.75 | 0.98 | 1.00 | 0.95 |
| 2 | 28 | −47.4, −6.5, 38.3 | 60.1 | 12.2 | 7.7 | 0.32 | 0.61 | 0.86 | 0.61 |
| 3 | 59 | 22.7, 63.5, 60.7 | 34.4 | 5.4 | 2.9 | 0.75 | 0.98 | 1.00 | 0.88 |
| 4 | 47 | 35.9, 34.1, 62.6 | 111.6 | 20.3 | 7.7 | 0.32 | 0.74 | 0.89 | 0.00 |
| 5 | 40 | 31.8, 10.5, 21.4 | 82.8 | 15.1 | 7.7 | 0.23 | 0.50 | 0.80 | 0.70 |
| 6 | 16 | −44.7, 2.1, 47.8 | 68.8 | 15.1 | 10.7 | 0.19 | 0.81 | 0.94 | 0.50 |
| 7 | 21 | −38.3, − 11.1, 39.1 | 95.6 | 19.3 | 10.9 | 0.00 | 0.14 | 0.52 | 0.43 |
| 8 | 18 | 31.5, −18.1, 51.2 | 109.9 | 33.2 | 10.6 | 0.00 | 0.50 | 0.78 | 0.17 |
| 9 | 108 | −38.8, 3.2, 40.6 | 120.7 | 19.7 | 5.3 | 0.06 | 0.47 | 0.84 | 0.49 |
| 10 | 57 | −43.6, 13.0, 21.6 | 49.4 | 8.5 | 4.4 | 0.49 | 0.88 | 0.98 | 0.77 |
| 11 | 34 | 19.7, −53.9, 64.7 | 54.6 | 11.0 | 6.8 | 0.18 | 0.71 | 0.94 | 0.88 |
| 12 | 23 | 42.7, −10.1, 44.0 | 41.0 | 7.7 | 6.8 | 0.39 | 0.91 | 1.00 | 0.35 |
| 13 | 65 | 16.5, 50.8, 52.6 | 95.1 | 21.8 | 6.0 | 0.11 | 0.52 | 0.83 | 0.49 |
| 14 | 33 | 52.5, −9.5, 77.1 | 80.7 | 13.3 | 7.1 | 0.36 | 0.82 | 0.88 | 0.79 |
| 15 | 59 | −38.7, 6.1, 46.5 | 64.6 | 11.1 | 4.6 | 0.32 | 0.83 | 0.92 | 0.32 |
| 16 | 207 | −38.4, 45.3, 63.1 | 44.1 | 6.4 | 2.3 | 0.65 | 0.97 | 1.00 | 0.79 |
| 17 | 21 | −28.0, −1.8, 69.2 | 102.6 | 28.4 | 12.4 | 0.05 | 0.10 | 0.33 | 0.19 |
| 18 | 19 | 26.8, 29.8, 91.9 | 94.8 | 22.3 | 8.0 | 0.05 | 0.47 | 0.84 | 0.32 |
| 19 | 14 | −41.2, 9.4, 33.3 | 70.8 | 16.4 | 11.5 | 0.00 | 0.21 | 0.64 | 0.50 |
| 20 | 26 | −40.6, 0.5, 50.7 | 79.4 | 17.6 | 12.0 | 0.04 | 0.38 | 0.69 | 0.46 |
| 21 | 23 | 15.8, 34.3, 103.4 | 59.9 | 11.8 | 6.7 | 0.17 | 0.61 | 0.96 | 0.48 |
| 22 | 29 | 45.4, 5.1, 32.8 | 51.1 | 9.7 | 7.4 | 0.14 | 0.72 | 0.97 | 0.55 |
| 23 | 82 | 49.6, 2.8, 44.7 | 86.8 | 11.4 | 5.6 | 0.26 | 0.78 | 0.95 | 0.23 |
| 24 | 28 | −36.7, −3.6, 39.6 | 79.0 | 14.9 | 8.3 | 0.29 | 0.75 | 0.93 | 0.43 |
| 25 | 21 | −28.8, − 15.1, 74.1 | 98.7 | 21.5 | 15.2 | 0.05 | 0.48 | 0.86 | 0.57 |
| 26 | 13 | 26.2, 36.7, 63.1 | 81.6 | 16.3 | 14.5 | 0.08 | 0.46 | 0.62 | 0.15 |
| 27 | 123 | −12.8, 18.3, 60.9 | 125.7 | 47.0 | 3.7 | 0.00 | 0.00 | 0.02 | 0.03 |
| 28 | 75 | −39.6, −9.0, 64.9 | 84.1 | 15.1 | 6.5 | 0.11 | 0.49 | 0.73 | 0.44 |
| 29 | 14 | 38.1, 19.7, 58.5 | 68.4 | 15.1 | 12.2 | 0.00 | 0.36 | 0.71 | 0.00 |
| 30 | 112 | 36.6 −19.9, 52.9 | 87.5 | 21.7 | 4.9 | 0.02 | 0.22 | 0.63 | 0.34 |
| 31 | 5 | −45.5, − 23.0, 38.5 | 50.7 | 15.7 | 17.5 | 0.20 | 0.80 | 0.80 | 0.80 |
| 32 | 8 | −8.7, 21.9, 68.0 | 99.3 | 32.2 | 13.5 | 0.00 | 0.00 | 0.00 | 0.25 |
ECDs: Equivalent current dipoles, SD: Standard deviation of the distances between each ECD and the mean coordinate, Nearest: Average nearest neighbor distance of ECDs, Within 10 mm, 20 mm, 30 mm: Rate of ECDs within 10 mm, 20 mm, 30 mm from the mean coordinate. Patients in the seizure-persistent group are shown by the gray background
The average nearest neighbor distance was correlated with the number of ECDs (Rs=−0.88, p<0.001). There were also correlations seen in the maximum distance (Rs=− 0.62, p<0.001), SDD (Rs=−0.59, p<0.001), within 10 mm (Rs=0.54, p<0.001), within 20 mm (Rs=0.55, p=0.001) and within 30 mm (Rs=0.53, p=0.002) with the resection rate.
Fig. 1 shows the typical patterns of dipole distribution in seizure-free and - persistent groups. Fig. 2 plots the rate of seizure-free patients to within 10 mm, within 20 mm, within 30 mm (Fig. 2-upper) and the average nearest neighbor distance (Fig. 2-lower). The trend shows more dipoles within 10 mm, 20 mm, 30 mm from the mean coordinate result in a higher rate of seizure-free patients, and larger average nearest neighbor distances result in a lower seizure-free rate.
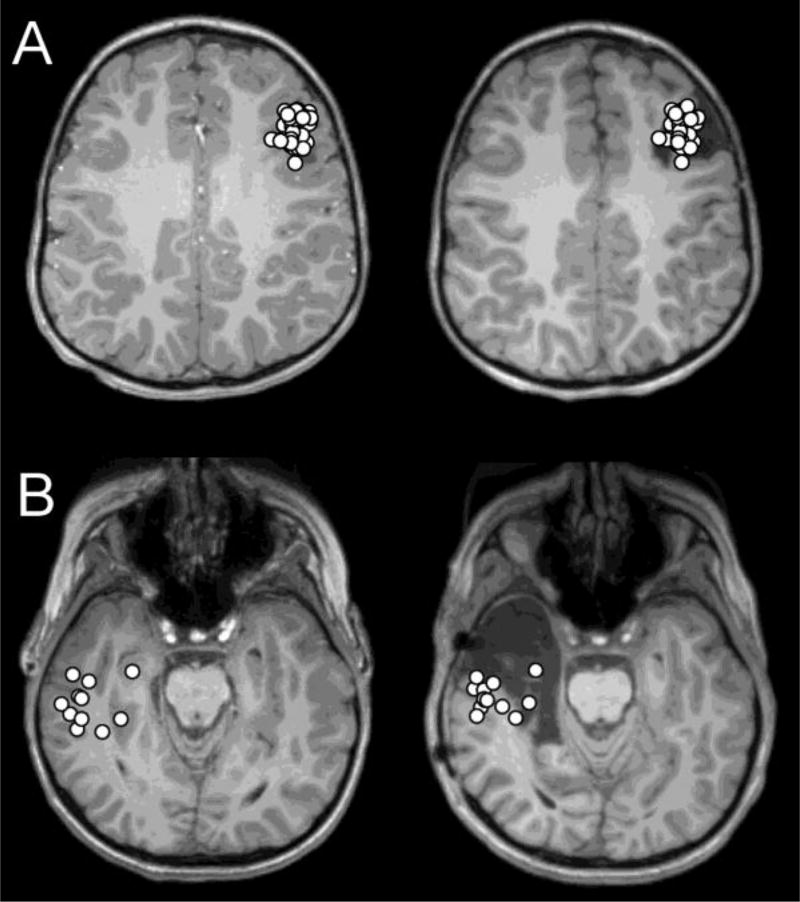
Figure 1.
(A) Dipole distribution of a patient in the seizure-free group (Patient 16), projected on the presurgical (Left) and postsurgical (Right) MRI. Most dipoles are tightly clustered (average nearest neighbor distance=2.3 mm, within 10 mm=0.65) and located within the resection. (B) Dipole distribution of a patient in the seizure-persistent group (Patient 23), projected on the presurgical (Left) and postsurgical (Right) MRI. Most dipoles are loosely clustered (average nearest neighbor distance=5.6 mm, within 10 mm=0.26) and located outside the resection.
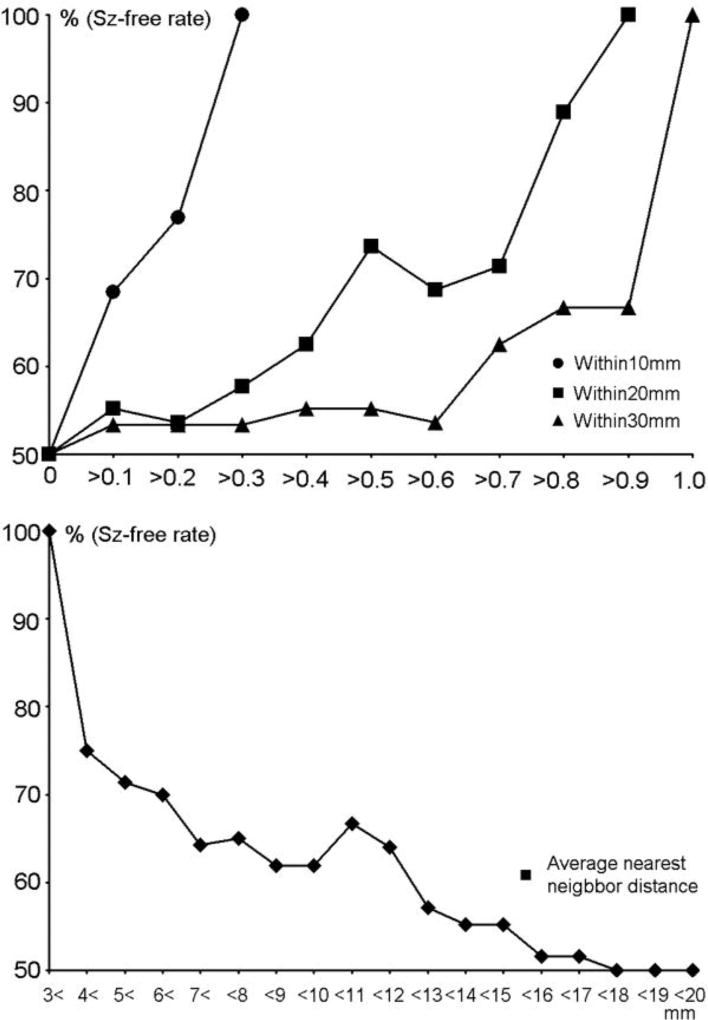
Figure 2.
(Upper) The rate of postsurgical seizure-free patients (%) is plotted corresponding to the threshold of within 10 mm (circle), within 20 mm (square), within 30 mm (triangle). The trend shows that larger values of within 10 mm, within 20 mm and within 30 mm result in a higher rate of seizure-free patients. (Lower) The rate of postsurgical seizure-free patients (%) is plotted corresponding to the threshold of average nearest neighbor distance. The trend shows that larger average nearest neighbor distances result in a lower seizure-free rate.
Discussion
In this study, we investigated the spatial patterns of dipole source distribution obtained from interictal epileptiform discharges recorded using MEG in patients with FCD. The results suggest that a spatially-restricted dipole distribution is correlated with seizure-free surgical outcome, as shown by the significantly smaller average nearest neighbor distance and the higher rate of dipoles located within 10 mm, 20 mm and 30 mm from the mean coordinate in the seizure-free group compared to the seizure-persistent group of patients.
Previous MEG studies have observed a pattern of dipole distribution in a restricted cortical area, which is described as a 'dipole cluster' in patients with epilepsy16–20. Dipole clusters are considered to guide a resection volume and predict a good outcome in epilepsy surgery21. However, these studies predefined the criteria to determine a dipole cluster variably, as described by 'six or more spike sources with ≤1 cm between adjacent sources'10, 11, 16, 18, 22–28, '10 or more ECDs located contiguously within neighboring gyri'29, '10 or more spike sources with 15 mm or less between adjacent sources'19, or 'at least 5 dipoles within a 1-cm2 region'21, 30. No studies have demonstrated objective and quantitative basis of these criteria. Moreover, there is evidence suggesting that FCD represents specific features in MEG spikes and their source distribution, as compared with astrocytic inclusions27 or other lesions31. The patterns of dipole clusters may be useful for diagnosing FCD distinctive from other etiologies when they are defined based on quantitative observation. Recent studies classified the dipole cluster into 'tight' and 'loose' clusters at a lobar/sublobar level and suggested that tight clusters are characteristic in FCD patients20, 32. Our results may be informative for quantitatively determining the criteria of a dipole cluster that is clinically relevant in patients with FCD by using numerical indices of dipole distribution. More specifically, the mean values of average nearest neighbor distance were 6.5 mm and 10.0 mm in seizure-free and -persistent groups, respectively. The criteria of dipole clusters, such as '≤1 cm between adjacent sources' and '15 mm or less between adjacent sources', include most of the dipole distributions seen in both of our patient groups, and may not be useful for estimating postsurgical outcomes. In fact, the rate of seizure-free patients is only 62 % and 55 % at the threshold of 10 mm and 15 mm for average nearest neighbor distance in our patients (Fig. 2-lower). Considering the average nearest neighbor distance was correlated with the number of dipoles, the distance between dipoles may not be appropriate for the criteria of dipole cluster. Alternatively, all patients who had 30 %, 90 % and 100 % of dipoles within 10 mm, 20 mm and 30 mm from the mean coordinate became seizure-free after surgery (Fig. 2), suggesting that these indices provide a strong basis of clinically-relevant dipole cluster and a good indicator for estimating postsurgical outcome.
Several researchers have demonstrated that surgical removal of dipole cluster may predict favorable surgical outcomes in patients with epilepsy, by investigating whether the dipole cluster was completely or partially removed17, 21, 33–36. A few studies suggested a higher rate of dipole removal is correlated with favorable surgical outcomes37, 38, while Kim et al.39 reported no statistical relationship between these two measurements. For FCD patients, Widjaja et al.10 found that the complete removal of a dipole cluster had a higher rate of achieving Engel's Class I outcome than partial removal; however Wilenius et al.11 did not find a significant correlation between the resection rate of dipoles and seizure-free outcomes in patients with dipole clusters.
Our results failed to show a relationship between resection rate and postsurgical seizure-freedom. Mislocalization of MEG spike sources may occur due to spike propagation40, leading to a low rate of dipole resection in some patients of our seizure-free group. On the other hand, the resection rate was correlated with the dipole distribution pattern as indicated by maximum distance, SDD, within 10 mm, within 20 mm and within 30 mm, suggesting that a restricted dipole distribution correlates with a higher resection rate. The results also differ depending on other factors, such as the existence of MRI-visible lesions39; thus, the significance of dipole removal is still unclear.
We did not consider the size of the patient's brain in our analysis, although it is different depending on the patient's age. The brain size affects the correlation between the dipole distribution and anatomical regions. For example, a certain distance, such as 30 mm, may cross multiple gyri in younger patients, while it may be included in a single gyrus in older patients. Applying an anatomical atlas to the source space would be useful for counting the numbers of dipoles at a lobar or sublobar level and evaluating the dipole distribution correlated with the anatomical regions. Similarly, the brain size may be a factor affecting the resection rate, however, our patients did not show a significant difference in the resection rate between seizure-free and -persistent groups even without patients under 5 years of age (Patients 15, 16 and 30, P>0.01). There are only a small number of young pediatric patients who show considerably small size of the brain in our study. Further investigations of such patients will clarify the effect of brain size on the clinical relevance of dipole distribution.
This study has several limitations. First, the study design is retrospective, and a prospective study is necessary for controlling the confounding factors. Second, we did not consider the pathological classification of FCD, since the pathology findings were obtained by different criteria. Moreover, our study included patients with different types of FCD pathology, such as isolated and associated with principal lesions. Previous studies have suggested an impact of FCD type in the clinical outcome26, 32. Third, we investigated the dipole distribution regardless of its location. Bilateral or distant dipole locations may reflect multiple dipole clusters29, in which a single mean coordinate is not feasible for analysis. Nakajima et al.28 reported the different patterns of dipole distribution in FCD patients depending on the lesion location at the bottom of the sulcus or gyral surface. Further studies are necessary for addressing these issues.
In conclusion, investigating the distribution patterns of spike dipole sources is informative for understanding the clinical usefulness of MEG in patients with FCD. Dipole source distribution in a restricted area suggests favorable outcomes of epilepsy surgery.
Acknowledgments
This work was supported by National Institutes of Health (S10RR014978, R01NS069696-01A1) and NIBIB (P41EB015896).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- 1.Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:369–87. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476–87. doi: 10.1002/ana.410370410. https://doi.org/10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- 3.Krsek P, Maton B, Jayakar P, Dean P, Korman B, Rey G, et al. Incomplete resection of focal cortical dysplasia is the main predictor of poor postsurgical outcome. Neurology. 2009;72:217–23. doi: 10.1212/01.wnl.0000334365.22854.d3. https://doi.org/10.1212/01.wnl.0000334365.22854.d3. [DOI] [PubMed] [Google Scholar]
- 4.Rowland NC, Englot DJ, Cage TA, Sughrue ME, Barbaro NM, Chang EF. A meta-analysis of predictors of seizure freedom in the surgical management of focal cortical dysplasia. J Neurosurg. 2012;116:1035–41. doi: 10.3171/2012.1.JNS111105. https://doi.org/10.3171/2012.1.JNS111105. [DOI] [PubMed] [Google Scholar]
- 5.Ishibashi H, Simos PG, Wheless JW, Baumgartner JE, Kim HL, Castillo EM, et al. Localization of ictal and interictal bursting epileptogenic activity in focal cortical dysplasia: agreement of magnetoencephalography and electrocorticography. Neurol Res. 2002;24:525–30. doi: 10.1179/016164102101200483. https://doi.org/10.1179/016164102101200483. [DOI] [PubMed] [Google Scholar]
- 6.Otsubo H, Iida K, Oishi M, Okuda C, Ochi A, Pang E, et al. Neurophysiologic findings of neuronal migration disorders: intrinsic epileptogenicity of focal cortical dysplasia on electroencephalography, Electrocorticography, and magnetoencephalography. J Child Neurol. 2005;20:357–63. doi: 10.1177/08830738050200041501. https://doi.org/10.1177/08830738050200041501. [DOI] [PubMed] [Google Scholar]
- 7.Itabashi H, Jin K, Iwasaki M, Okumura E, Kanno A, Kato K, et al. Electro- and magneto-encephalographic spike source localization of small focal cortical dysplasia in the dorsal peri-rolandic region. Clin Neurophysiol. 2014;125:2358–63. doi: 10.1016/j.clinph.2014.02.028. https://doi.org/10.1016/j.clinph.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Bast T, Oezkan O, Rona S, Stippich C, Seitz A, Rupp A, et al. EEG and MEG source analysis of single and averaged interictal spikes reveals intrinsic epileptogenicity in focal cortical dysplasia. Epilepsia. 2004;45:621–31. doi: 10.1111/j.0013-9580.2004.56503.x. https://doi.org/10.1111/j.0013-9580.2004.56503.x. [DOI] [PubMed] [Google Scholar]
- 9.Morioka T, Nishio S, Ishibashi H, Muraishi M, Hisada K, Shigeto H, et al. Intrinsic epileptogenicity of focal cortical dysplasia as revealed by magnetoencephalography and electrocorticography. Epilepsy Res. 1999;33:177–87. doi: 10.1016/s0920-1211(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 10.Widjaja E, Otsubo H, Raybaud C, Ochi A, Chan D, Rutka JT, et al. Characteristics of MEG and MRI between Taylor’s focal cortical dysplasia (type II) and other cortical dysplasia: surgical outcome after complete resection of MEG spike source and MR lesion in pediatric cortical dysplasia. Epilepsy Res. 2008;82:147–55. doi: 10.1016/j.eplepsyres.2008.07.013. https://doi.org/10.1016/j.eplepsyres.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Wilenius J, Medvedovsky M, Gaily E, Metsähonkala L, Mäkelä JP, Paetau A, et al. Interictal MEG reveals focal cortical dysplasias: special focus on patients with no visible MRI lesions. Epilepsy Res. 2013;105:337–48. doi: 10.1016/j.eplepsyres.2013.02.023. https://doi.org/10.1016/j.eplepsyres.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Palmini A, Najm I, Avanzini G, et al. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–S8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- 13.Blümcke I, Thom M, Aronica E, Babb T, Guerrini R, Foldvary-Schaefer N, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–74. doi: 10.1111/j.1528-1167.2010.02777.x. https://doi.org/10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel J, Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical Treatment of the Epilepsies. New York: Raven; 1987. pp. 553–71. [Google Scholar]
- 15.Papadelis C, Tamilia E, Stufflebeam S, Grant PE, Madsen JR, Pearl PL, et al. Interictal high frequency oscillations detected with simultaneous magnetoencephalography and electroencephalography as biomarker of pediatric epilepsy. J Vis Exp. 2016;6:118. doi: 10.3791/54883. https://doi.org/10.3791/54883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iida K, Otsubo H, Mohamed IS, Okuda C, Ochi A, Weiss SK, et al. Characterizing magnetoencephalographic spike sources in children with tuberous sclerosis complex. Epilepsia. 2005;46:1510–17. doi: 10.1111/j.1528-1167.2005.14005.x. https://doi.org/10.1111/j.1528-1167.2005.14005.x. [DOI] [PubMed] [Google Scholar]
- 17.Almubarak S, Alexopoulos A, Von-Podewils F, Wang ZI, Kakisaka Y, Mosher JC, et al. The correlation of magnetoencephalography to intracranial EEG in localizing the epileptogenic zone: a study of the surgical resection outcome. Epilepsy Res. 2014;108:1581–90. doi: 10.1016/j.eplepsyres.2014.08.016. https://doi.org/10.1016/j.eplepsyres.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Bennett-Back O, Ochi A, Widjaja E, Nambu S, Kamiya A, Go C, et al. Magnetoencephalography helps delineate the extent of the epileptogenic zone for surgical planning in children with intractable epilepsy due to porencephalic cyst/encephalomalacia. J Neurosurg Pediatr. 2014;14:271–8. doi: 10.3171/2014.6.PEDS13415. https://doi.org/10.3171/2014.6.PEDS13415. [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Joo EY, Seo DW, Kim MY, Lee YH, Kwon HC, et al. Accuracy of MEG in localizing irritative zone and seizure onset zone: Quantitative comparison between MEG and intracranial and EEG. Epilepsy Res. 2016;127:291–301. doi: 10.1016/j.eplepsyres.2016.08.013. https://doi.org/10.1016/j.eplepsyres.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Murakami H, Wang ZI, Marashly A, Krishnan B, Prayson RA, Kakisaka Y, et al. Correlating magnetoencephalography to stereo-electroencephalography in patients undergoing epilepsy surgery. Brain. 2016;139:2935–47. doi: 10.1093/brain/aww215. https://doi.org/10.1093/brain/aww215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vadera S, Jehi L, Burgess RC, Shea K, Alexopoulos AV, Mosher J, et al. Correlation between magnetoencephalography-based "clusterectomy" and postoperative seizure freedom. Neurosurg Focus. 2013;34:E9. doi: 10.3171/2013.4.FOCUS1357. https://doi.org/10.3171/2013.4.FOCUS1357. [DOI] [PubMed] [Google Scholar]
- 22.Torres CV, Fallah A, Ibrahim GM, Cheshier S, Otsubo H, Ochi A, et al. The role of magnetoencephalography in children undergoing hemispherectomy. J Neurosurg Pediatr. 2011;8:575–83. doi: 10.3171/2011.8.PEDS11128. https://doi.org/10.3171/2011.8.PEDS11128. [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Wu T, Wang Y, Liu H, Zou Y, Liu W, et al. Interictal magnetoencephalographic findings related with surgical outcomes in lesional and nonlesional neocortical epilepsy. Seizure. 2011;20:692–700. doi: 10.1016/j.seizure.2011.06.021. https://doi.org/10.1016/j.seizure.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed IS, Gibbs SA, Robert M, Bouthillier A, Leroux JM, Khoa Nguyen D. The utility of magnetoencephalography in the presurgical evaluation of refractory insular epilepsy. Epilepsia. 2013;54:1950–9. doi: 10.1111/epi.12376. https://doi.org/10.1111/epi.12376. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed IS, Otsubo H, Ferrari P, Sharma R, Ochi A, Elliott I, et al. Source localization of interictal spike-locked neuromagnetic oscillations in pediatric neocortical epilepsy. Clin Neurophysiol. 2013;124:1517–27. doi: 10.1016/j.clinph.2013.01.023. https://doi.org/10.1016/j.clinph.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Widjaja E, Shammas A, Vali R, Otsubo H, Ochi A, Snead OC, et al. FDG-PET and magnetoencephalography in presurgical workup of children with localization-related nonlesional epilepsy. Epilepsia. 2013;54:691–9. doi: 10.1111/epi.12114. https://doi.org/10.1111/epi.12114. [DOI] [PubMed] [Google Scholar]
- 27.Alshafai L, Ochi A, Go C, McCoy B, Hawkins C, Otsubo H, et al. Clinical, EEG, MRI, MEG, surgical outcomes of pediatric epilepsy with astrocytic inclusions versus focal cortical dysplasia. Epilepsia. 2014;55:1568–75. doi: 10.1111/epi.12756. https://doi.org/10.1111/epi.12756. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima M, Widjaja E, Baba S, Sato Y, Yoshida R, Tabei M, et al. Remote MEG dipoles in focal cortical dysplasia at bottom of sulcus. Epilepsia. 2016;57:1169–78. doi: 10.1111/epi.13399. https://doi.org/10.1111/epi.13399. [DOI] [PubMed] [Google Scholar]
- 29.Oishi M, Kameyama S, Masuda H, Tohyama J, Kanazawa O, Sasagawa M, et al. Single and multiple clusters of magnetoencephalographic dipoles in neocortical epilepsy: significance in characterizing the epileptogenic zone. Epilepsia. 2006;47:355–64. doi: 10.1111/j.1528-1167.2006.00428.x. https://doi.org/10.1111/j.1528-1167.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 30.Iida K, Otsubo H, Matsumoto Y, Ochi A, Oishi M, Holowka S, et al. Characterizing magnetic spike sources by using magnetoencephalography-guided neuronavigation in epilepsy surgery in pediatric patients. J Neurosurg. 2005;102:187–96. doi: 10.3171/jns.2005.102.2.0187. https://doi.org/10.3171/jns.2005.102.2.0187. [DOI] [PubMed] [Google Scholar]
- 31.Ueda Y, Egawa K, Ito T, Takeuchi F, Nakajima M, Otsuka K, et al. The presence of short and sharp MEG spikes implies focal cortical dysplasia. Epilepsy Res. 2015;114:141–6. doi: 10.1016/j.eplepsyres.2015.04.020. https://doi.org/10.1016/j.eplepsyres.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 32.El Tahry R, Wang ZI, Kakisaka Y, Murakami H, Shibata S, Krishnan B, et al. A single tight MEG cluster may only represent a fragment of type I FCD. Clin Neurophysiol. 2016;127:2570–2. doi: 10.1016/j.clinph.2016.04.007. https://doi.org/10.1016/j.clinph.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Wheless JW, Willmore LJ, Breier JI, Kataki M, Smith JR, King DW, et al. A comparison of magnetoencephalography, MRI, and V-EEG in patients evaluated for epilepsy surgery. Epilepsia. 1999;40:931–41. doi: 10.1111/j.1528-1157.1999.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 34.Pataraia E, Simos PG, Castillo EM, Billingsley RL, Sarkari S, Wheless JW, et al. Does magnetoencephalography add to scalp video-EEG as a diagnostic tool in epilepsy surgery? Neurology. 2004;62:943–8. doi: 10.1212/01.wnl.0000115122.81621.fe. [DOI] [PubMed] [Google Scholar]
- 35.Schneider F, Irene Wang Z, Alexopoulos AV, Almubarak S, Kakisaka Y, et al. Magnetic source imaging and ictal SPECT in MRI-negative neocortical epilepsies: additional value and comparison with intracranial EEG. Epilepsia. 2013;54:359–69. doi: 10.1111/epi.12004. https://doi.org/10.1111/epi.12004. [DOI] [PubMed] [Google Scholar]
- 36.Wang ZI, Alexopoulos AV, Jones SE, Najm IM, Ristic A, Wong C, et al. Linking MRI postprocessing with magnetic source imaging in MRI-negative epilepsy. Ann Neurol. 2014;75:759–70. doi: 10.1002/ana.24169. https://doi.org/10.1002/ana.24169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genow A, Hummel C, Scheler G, Hopfengärtner R, Kaltenhäuser M, Buchfelder M, et al. Epilepsy surgery, resection volume and MSI localization in lesional frontal lobe epilepsy. Neuroimage. 2004;21:444–9. doi: 10.1016/j.neuroimage.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 38.Fischer MJ, Scheler G, Stefan H. Utilization of magnetoencephalography results to obtain favourable outcomes in epilepsy surgery. Brain. 2005;128:153–7. doi: 10.1093/brain/awh333. https://doi.org/10.1093/brain/awh333. [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Kankirawatana P, Killen J, Harrison A, Oh A, Rozzelle C, et al. Magnetic source imaging (MSI) in children with neocortical epilepsy: surgical outcome association with 3D post-resection analysis. Epilepsy Res. 2013;106:164–72. doi: 10.1016/j.eplepsyres.2013.04.004. https://doi.org/10.1016/j.eplepsyres.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka N, Grant PE, Suzuki N, Madsen JR, Bergin AM, Hämäläinen MS, et al. Multimodal imaging of spike propagation: a technical case report. Am J Neuroradiol. 2012;33:E82–4. doi: 10.3174/ajnr.A2701. https://doi.org/10.3174/ajnr.A2701. [DOI] [PMC free article] [PubMed] [Google Scholar]