Abstract
A critical factor in the transmission and pathogenesis of Toxoplasma gondii is the ability to convert from an acute disease-causing, proliferative stage (tachyzoite), to a chronic, dormant stage (bradyzoite). The conversion of the tachyzoite-containing parasitophorous vacuole membrane into the less permeable bradyzoite cyst wall allows the parasite to persist for years within the host to maximize transmissibility to both primary (felids) and secondary (virtually all other warm-blooded vertebrates) hosts. This review presents our current understanding of the latent stage, including the factors that are important in bradyzoite induction and maintenance. Also discussed are the recent studies that have begun to unravel the mechanisms behind stage switching.
Keywords: Toxoplasma, Toxoplasmosis, Differentiation, Encystation, Tachyzoite, Bradyzoite, Latency, Gene regulation, Epigenetics, Immunity
Introduction
Toxoplasma gondii is an obligate intracellular protozoan parasite capable of infecting warm-blooded vertebrates worldwide [1]. Although the parasite cannot survive outside the host cell, it has a significantly high transmissibility. It is estimated that approximately one-third of the human population is infected with Toxoplasma [2]. It is one of the most widespread parasites due to its large range of host species and ability to transmit between hosts through diverse routes [3].
Toxoplasma belongs to the phylum Apicomplexa, which includes other important pathogens such as Plasmodium, Eimeria, Babesia, Neospora, Sarcocystis, and Cryptosporidium [4–6]. Upon initial infection of an immunocompetent host with Toxoplasma, an often asymptomatic acute infection is followed by establishment of chronic infection, which is marked by the formation of intracellular tissue cysts. These cysts can persist within the host tissues throughout the life of the host, maintained in the quiescent state by its immune system. Upon immune suppression, the bradyzoites in the cyst will transition back into proliferating tachyzoites and can lead to devastating tissue destruction. Reactivated toxoplasmosis is a major concern in HIV-infected individuals and other immunocompromised patients [7]. In most cases, due to the proclivity of the latent bradyzoite to reside in central nervous system tissues, encephalitis is usually the main cause of death after reactivation of infection [8]. In addition, Toxoplasma tachyzoites are capable of crossing the placental blood barrier to be transmitted vertically from mother to fetus. Vertical transmission can occur if an immunologically naïve woman becomes infected with the parasite during her pregnancy, and can cause serious congenital birth defects or spontaneous abortion [6, 9]. Ocular toxoplasmosis is also a common manifestation of Toxoplasma, in which the retina of the eye may be destroyed in both healthy and immunocompromised individuals [10]. Ocular toxoplasmosis was traditionally thought to stem from reactivation of a congenital infection, but acquired infection is now also appreciated to be a main cause of this disease [11], particularly in South America. Toxoplasma has also been linked with water borne outbreaks in humans and deaths in marine wildlife due to contamination of water with oocysts [12–14], which has led the parasite to being classified as a Category B Biodefense pathogen by NIAID.
A crucial event for parasite transmission and pathogenesis is the stage conversion between the actively replicating tachyzoites and the encysted bradyzoites. Conversion to a dormant tissue cyst augments parasite persistence inside the host, which, in turn, increases the chances of transmission through predation. Bradyzoites, therefore, provide Toxoplasma with extraordinary flexibility with respect to transmission, allowing the parasite to bypass its sexual stage to disseminate [3]. Of clinical relevance, the bradyzoite cysts appear to be refractory to current available drug treatments, giving rise to chronic infection that presently cannot be eradicated.
Characteristics of the bradyzoite cyst
Tissue cysts are intracellular structures that may develop in any visceral organ; however, they exhibit tropism for neural and muscle tissues. In vivo, cysts are most commonly detected in the brain, the eye, and in both skeletal and cardiac muscles [8]. Within the central nervous system (CNS), cysts have been detected in neurons, astrocytes, and microglia [15–17], although recent studies show that parasites primarily interact with neurons in mice [18, 19].
The size of immature cysts has been estimated to be about 5 μm with at least two parasites inside, indicating that cyst wall formation is an early event in bradyzoite development [20–23]. Furthermore, using bioluminescence imaging technology in combination with parasites expressing luciferase under the control of a bradyzoite-specific gene promoter, it was demonstrated that in vivo formation of bradyzoites commences as early as 1 day post-infection [24]. As the cyst matures, changes in its size may be observed due to bradyzoite replication within the cyst [25]. Mature tissue cyst size and shape is variable; on average, a mature brain cyst is spherical, about 10–70 μm in diameter and the number of parasites within the cyst may reach the thousands. In muscle tissues, cysts are generally more elongated in length and may reach a size up to 100 μm [8].
A number of major ultrastructural differences have been observed between the tachyzoite and bradyzoite stages. Bradyzoites are more slender than tachyzoites and have a pronounced crescent shape [26]. Bradyzoites contain a nucleus located at their posterior end and have electron dense rhoptry organelles and numerous micronemes [8, 16, 27]. Furthermore, bradyzoites contain amylopectin granules that stain red with periodic acid Schiff (PAS) [8]. It has been reported that in vitro differentiated bradyzoites may lose the parasite’s apicoplast, a non-photosynthetic plastid-like organelle [22, 28]. Mature bradyzoites represent a growth-arrested stage of the parasite with a uniform 1 N content nuclear DNA [29]. Although this stage is generally considered to be quiescent, it may still be motile. In vitro bradyzoites have been observed exiting from an existing cyst to invade a new host cell and establish a new cyst [22]. This feature could explain the clusters of cysts that have been observed in vivo as well as the increase in cyst burdens in chronically infected mice. Recently, a novel method for isolating tissue cysts from brains of infected mice has been developed and has permitted a detailed study of in vivo cyst characteristics [30]. Analysis of these enriched cysts by immunostaining for a parasite surface protein found that clusters of parasites within a subset of cysts were undergoing cell division in an asynchronous manner, providing some of the first direct evidence that tissue cysts are more dynamic than previously believed [30].
Transformation of the parasitophorous vacuole membrane into the cyst wall
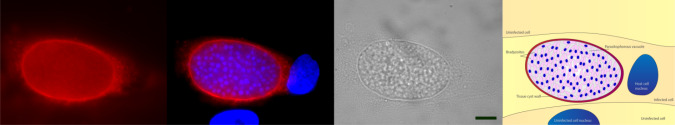
The formation of the cyst wall and the matrix material provide a physical barrier to host immune factors. The cyst wall is visible as a reinforcement of the parasitophorous vacuole membrane (PVM) that occurs beneath this membrane [16]. Typically, the PVM that contains the cyst is elastic and thin (< 0.5 μm), consisting of chitin or similar polysaccharides and glycoproteins that can be detected using various fluorescently labeled lectins [31–33]. The agglutinin from Dolichos biflorus (DBA) that recognizes α-linked N-acetyl galactosamine has high affinity for the bradyzoite cyst wall structure and is regularly used to detect cysts in tissue culture (Fig. 1). Currently, only a few components of the bradyzoite cyst wall have been identified and their molecular functions remain unclear. CST1, a glycoprotein recognized by DBA, localizes in the cyst wall under the PVM [33, 34]. CST1 was recently identified as being an O-glycosylated protein with a mucin domain, previously annotated as SRS44 and disruption of the gene results in impaired cyst formation and extremely fragile cysts [28]. Dense granule proteins (GRA proteins) have also been found in the bradyzoite cyst wall [34]. At the bradyzoite stage, GRA5 is predominantly localized to the modified PVM rather than the granular material that precipitates underneath this membrane [23, 34]. The other GRA proteins that have been observed in the cyst wall so far are GRA1, GRA2, GRA3, GRA6, and GRA7 [23, 34–36]. MAG1 (Matrix antigen 1) is another cyst wall and matrix-specific antigen [37]. Notably, MAG1 is also detected inside the parasitophorous vacuole of the tachyzoite stage but is present in significantly lower abundance, suggesting that it has an important role in the formation of the bradyzoite cyst wall [34, 38]. Recently, three novel cyst wall proteins were identified: a bradyzoite pseudokinase (BPK1), a microneme adhesive repeat domain-containing protein 4 (MCP4), and a proteophosphoglycan [39–41], but the functions of these proteins have yet to be resolved. Further characterization of the cyst wall and its components are required to understand the molecular mechanisms of tissue cyst development.
Fig. 1.
5-day-old, in vitro bradyzoite cyst induced under alkaline conditions, stained with fluorophore-linked lectin from Dolichos biflorus (red), which binds to the polysaccharide-rich cyst wall. Blue: 4′,6-diamidino-2-phenylindole, DAPI (nucleus). Scale bar 10 µm. Labeled schematic depicts the stained tissue cyst in the infected host cell
Identification of genes contributing to bradyzoite development
Bradyzoites express certain genes exclusive to this stage of the life cycle. The findings that bradyzoites express specific antigens such as surface antigen 4 (SAG4); bradyzoite surface antigen, BSR4; surface antigen 2/2D (SAG2/2D), matrix antigen 1 (MAG1); 116-kDa cyst wall antigen (CST1); bradyzoite antigens 1–5 (BAG 1/5), specific metabolic enzyme isoforms (lactate dehydrogenase 2, LDH2; enolase 2, ENO1) [42–51] have led to extensive research focused on describing the function of bradyzoite-specific genes.
Initial attempts tried to identify genes that are specifically expressed or induced during Toxoplasma stage conversion. In an early study, a cDNA library was constructed from mRNA isolated from brain cysts in chronically infected mice. Screening of this expression library in bacteria with a pool of polyclonal antibodies against bradyzoite antigens led to the identification of several bradyzoite-related genes such as mag1 [37], bag1 [45], and ldh2 [46, 47]. Parallel studies using material from in vitro differentiated bradyzoites confirmed the presence of the bradyzoite marker BAG1 [43] as well as other proteins, such as SAG4 [52]. Expressed sequence tag (EST) libraries from both in vitro- and in vivo-prepared bradyzoites have also been used for the detection of numerous stage-specific genes [53]. Another approach employed a subtractive cDNA library to identify genes that were exclusively expressed in bradyzoites, many of which are involved in crucial cellular processes such as metabolism, protein folding, and DNA repair [49]. Promoter trapping screens have also been effective for the identification of bradyzoite-specific genes [32, 54, 55]. These screens have led to the detection of several bradyzoite gene loci, for example the bradyzoite surface antigen bsr4 (p36) [55]. More recently, deep RNA sequencing has allowed for a more global, non-biased approach to determining the patterns of gene expression in tachyzoites versus bradyzoites. Analysis of the total RNA from forebrains from both acute and chronically infected mice identified 547 parasite genes significantly regulated between the two stages of disease. In addition to previously reported alterations to metabolic gene expression, this approach also identified many novel stage-specific markers. From these results, it appears that the parasites downregulate one set of sag-related surface markers in the tachyzoite to upregulate another set of similar genes in the bradyzoite. A large proportion (~ 50%) of the significantly upregulated genes in the bradyzoite were hypothetical genes, hinting at a number of parasite-specific processes that could be targeted with inhibitors as therapies against chronic toxoplasmosis [56].
Improvements in the tools facilitating molecular genetics analyses in Toxoplasma have allowed the construction of an increasing number of bradyzoite-specific gene knockouts. The construction of the bag1 knockout strain was one of the first attempts to disrupt a bradyzoite-specific gene [57, 58]. Other knockouts that have been described include the membrane proton-ATPase (PMA1) [59], the bradyzoite surface antigen SRS9 [60], the dense granule proteins GRA4 and GRA6 [61], the genetic locus ROP4/7 [61], the plant-like Apetala-2 transcription factors AP2XI-4, AP2IX-9, AP2IX-4, AP2IV-4 and AP2IV-3 [62–66], and the bradyzoite secreted pseudokinase 1 (BPK1) [39]. In all knockouts made to date, the production of cyst-like structures was observed in vitro, suggesting that individually these genes are not required for initiating bradyzoite switching. Nevertheless, defects in cyst morphology, cyst number, in vivo cyst formation, and transmission of infection indicate that many of these genes contribute significantly to cyst development.
In one study, chemical mutagenesis was used to identify tachyzoite to bradyzoite interconversion defective (Tbd−) mutants [67]. Other approaches applied insertional mutagenesis and reverse genetic screens [41, 68–70]. Finally, another study employed a signature-tagged insertional mutagenesis screen designed to isolate generally avirulent parasites. These clones were subsequently screened for reduced in vivo cyst formation to identify genes required only for pathogenesis [71]. Despite differences in the type of mutagenesis performed and the methods of selection of defective parasites, this work from different groups has collectively identified factors that may contribute to bradyzoite differentiation. However, most of the candidates have yet to be verified in independent studies as having a clear role in cyst formation. Moreover, some study designs may have isolated mutants resistant to in vitro differentiation stresses, and the genes may have more to do with stress resistance than differentiation. Despite these caveats, the genes and their associated bradyzoite phenotypes that have been identified from these screens are discussed in Table 1.
Table 1.
Genes associated with bradyzoite development or maintenance in Toxoplasma
| Name | ToxoDB ID | Function | References |
|---|---|---|---|
| CST1 | TGME49_264660 (SRS44) | Cyst wall glycoprotein, recognized by Dolichos biflorus lectin | [28, 33, 34] |
| MAG1 | TGME49_270240 | Cyst wall and matrix protein, bradyzoite-specific marker | [37] |
| BPK1 | TGME49_253330 | Cyst wall protein. In Δbpk1 strain cysts are smaller and more sensitive to pepsin-acid treatment; Δbpk1 strain has reduced ability to cause oral infection | [39] |
| MCP4 | TGME49_208730 | Cyst wall protein | [40] |
| PPG1 | TGME49_297520 | Serine/proline-rich proteophosphoglycan (GU182879), this gene has a role in cyst wall formation | [41] |
| SAG4 (or SRS35B) | TGME49_280570 | SAG-family-related surface protein of bradyzoite | [52] |
| BSR4 (or SRS16C) | TGME49_320180 | SAG-family-related surface protein of bradyzoite | [55] |
| SAG2C (or SRS49D) | TGME49_207160 | SAG-family-related surface protein of bradyzoite | [188, 189] |
| SAG2D (or SRS49C) | TGME49_207150 | SAG-family-related surface protein of bradyzoite | [188, 189] |
| SAG2Y (or SRS49A) | TGME49_207130 | SAG-family-related surface protein of bradyzoite. The transcript is upregulated in bradyzoites. Δsag2y forms cysts both in vitro and in vivo but demonstrates a defect in persistence in vivo | [190] |
| SAG2X (or SRS49B) | TGME49_207140 | SAG-family-related surface protein of bradyzoite. The transcript is upregulated in bradyzoites. Δsag2x forms cysts both in vitro and in vivo but demonstrates a defect in persistence in vivo | [190] |
| SRS9 (or SRS16B) | TGME49_320190 | SAG-family-related surface protein of bradyzoite | [60] |
| BRP1 | TGME49_314250 |
Bradyzoite rhoptry protein 1, localized in rhoptries of bradyzoites and found to be secreted in PV of in vitro bradyzoites Δbrp1 strain develops and establishes both acute and chronic infection normally |
[191] |
| HSP60 | TGME49_247550 | Mitochondrial heat shock protein, member of mitochondria chaperone family. HSP60 transcript is highly increased in bradyzoites. The protein is localized in two vesicular bodies distinct from the mitochondrion in encysted bradyzoites | [50] |
| hsp30/BAG1 (BAG1) | TGME49_259020 | Small heat shock protein, bradyzoite-specific marker, not necessary for cyst formation | [43, 45] |
| LDH2 | TGME49_291040 | Lactate dehydrogenase 2 is a glycolytic enzyme upregulated in bradyzoites. Δldh2 parasites form fewer brain cysts | [46, 47, 74] |
| ENO1 | TGME49_268860 | Bradyzoite-specific Enolase 1, functions in glycolysis | [73] |
| PMA1 | TGME49_252640 | P type ATPase, upregulated in bradyzoite stage. The protein is localized in the membrane of mature bradyzoites purified from brain tissue cysts of chronically infected mice. The Δpma-1 strain has reduced ability to form bradyzoites in response to a number of different in vitro triggers, but is still capable of establishing chronic infection in mice | [51, 59] |
| AP2XII-6 | TGME49_249190 | Putative transcription factor. The insertional mutant on this locus is defective in upregulating bradyzoite markers during in vitro induction | [82] |
| AP2XI-4 | TGME49_315760 | Putative transcription factor. Δap2xi-4 parasites are unable to express many genes including some bradyzoite markers during pH stress differentiation, and have defects in cyst formation in mice | [63] |
| AP2IX-9 | TGME49_306620 | Transcription factor that represses bradyzoite formation. Δap2ix-9 parasites have enhanced ability to form cysts in vitro. Overexpression of AP2IX-9 inhibits bradyzoite formation | [62, 65] |
| AP2IX-4 | TGME49_288950 | Cell cycle-regulated transcription factor that represses expression of bradyzoite genes. Δap2ix-4 parasites have reduced ability to form cysts under alkaline stress in vitro | [64] |
| AP2IV-3 | TGME49_ 318610 | Transcription factor that is induced during bradyzoite development and promotes expression of bradyzoite-specific genes. Overexpression of TgAP2IV-3 enhances bradyzoite formation in vitro | [65] |
| AP2IV-4 | TGME49_318470 | Cell cycle-regulated transcription factor that represses bradyzoite-specific transcripts during tachyzoite development. Parasites lacking this factor do not form cysts in mouse brain tissue | [66] |
| GRA1 | TGME49_270250 | Dense granule protein. In the cyst, GRA1 is localized in the cyst wall and the cyst matrix. Unknown function | [23, 34, 35] |
| GRA3 | TGME49_227280 | Dense granule protein. In the cyst, GRA3 is localized in the cyst wall. Unknown function | [23, 34, 35] |
| GRA5 | TGME49_286450 | Dense granule protein. In the cyst, GRA5 is localized at the cyst wall membrane. Unknown function | [23, 34, 42, 192] |
| GRA6 | TGME49_275440 | Dense granule protein. In the cyst, GRA6 is localized in the cyst wall. Unknown function | [23, 34, 35] |
| GRA7 | TGME49_203310 | Dense granule protein. In the cyst, GRA6 is localized in the cyst wall. Unknown function | [36] |
| Splicing factor, arginine/serine rich | TGME49_213635 | Putative splicing factor. The insertional mutant on this locus is defective in bradyzoite differentiation in vitro | [82] |
| OWP1 | TGME49_204420 | Annotated as putative oocyst wall protein. The insertional mutant on this locus is defective in bradyzoite differentiation in vitro | [82] |
| Replication factor A protein 3 | TGME49_238110 | Function unknown. The insertional mutant on this locus is defective in bradyzoite differentiation in vitro | [82] |
| PUS1 | TGME49_202640 | Pseudouridine synthase homolog. Two insertional mutants on this locus are defective in tachyzoite-to-bradyzoite differentiation in vitro. Infection with Δpus-1 parasites results in higher mortality and increased cyst burden | [70] |
| ZFP1 | TGME49_218362 | Nucleolar CCHC motif zinc-finger protein 1. Both the insertional mutant and the Δzfp1 strain have impaired differentiation in vitro and fail to upregulate bradyzoite-specific transcripts | [69] |
| Tg-ncRNA-1 | TGME49_238110 | Non-coding RNA 1. Insertional mutants on this locus show differentiation defects and reduced cyst loads | [71, 193] |
| TgRRS1 | TGME49_320450 | Ribosome biogenesis regulatory protein 1 that it localized to the nucleolus. Two insertional mutants in tgrrs1 are defective in bradyzoite formation in vitro | [194] |
| DNA primase subunit, large subunit | TGME49_297840 | Function unknown. The insertional mutant on this locus is defective in bradyzoite differentiation in vitro | [82] |
| TBC domain-containing protein | TGME49_285730 | Function unknown. The insertional mutant on this locus is defective in bradyzoite differentiation in vitro | [82] |
| HECT-domain (ubiquitin-transferase) domain-containing protein | TGME49_270580 | Function unknown. The insertional mutant on this locus is defective in bradyzoite differentiation in vitro | [82] |
| Supt5 | TGME49_233300 | Rho-GAP domain-containing protein, transcription elongation factor. The insertional mutant on this locus is defective in bradyzoite differentiation in vitro | [82] |
| TgSRCAP | TGME49_280800 | SWI/SNF-like factor, which is upregulated during bradyzoite differentiation | [184] |
| TgRSC8 | TGME49_286920 | SWI/SNF-like factor. An insertional mutant with reduced protein levels of TgRSC8 expresses lower levels of some bradyzoite-specific transcripts during in vitro induction | [41, 185] |
| eIF2-α | TGME49_286420 | Homologue of the alpha subunit of the eukaryotic initiation factor 2. eIF2-α is highly phosphorylated in bradyzoites. Important factor in regulation of bradyzoites differentiation and maintenance as well as in reactivation of bradyzoites to tachyzoites | [87, 155, 156, 195] |
| HDAC3 | TGME49_227290 | Histone deacetylase 3, the protein is highly enriched in promoters of silent genes. Chemical inhibition of HDAC3-induced bradyzoite differentiation in vitro | [172, 174] |
| GCN5a | TGME49_254555 | Histone lysine acetyltransferase A. Transcriptional analysis of Δgcn5a parasites demonstrates a role for TgGCN5a in upregulating bradyzoite transcripts in vitro | [172, 173] |
Further investigation is necessary to describe the molecular mechanisms of Toxoplasma differentiation. Individual studies of the genes implicated in bradyzoite development have been informative, but the complete picture of this developmental pathway is still elusive. Nevertheless, the advent of techniques such as mRNA deep sequencing will allow full-scale expression profiling to compare both the tachyzoite and bradyzoite developmental stages and will refine our current understanding of this clinically relevant developmental pathway. Importantly, new in vitro models need to be pursued to delineate the phenomenon of stress-induced and spontaneous differentiation, and candidate genes need to be supported by in vivo data.
Metabolism in the bradyzoite stage
As the parasites transform from replicating tachyzoites to latent bradyzoites, changes in parasite metabolism have been observed. It may be assumed that these changes allow the parasites to remain dormant for long periods of time and are mediated by upregulation of bradyzoite-specific forms of various metabolic enzymes, particularly those involved in dealing with oxygen radical metabolism and carbohydrate metabolism. The formation of the amylopectin storage granules upon differentiation to bradyzoites indicates that the parasites modulate carbohydrate metabolism during stage switching. Tight regulation of amylopectin levels is required for maintaining chronic infection in mice [30]. Site-directed mutagenesis of an enzyme responsible for amylopectin digestion, the Toxoplasma glycogen phosphorylase (TgGP), was performed to determine the importance of amylopectin metabolism in both tachyzoite and bradyzoite stages. Mutation of a phosphorylated serine to inactivate (S25A) TgGP led to excessive accumulation of amylopectin. Conversely, mutation of this residue to a phosphoserine-mimetic (S25E) caused amylopectin deficiency. Although both mutant lines were capable of differentiation in vitro, they both displayed significantly reduced cyst burdens in the brains of chronically infected mice compared to the parental line, indicating that maintaining the appropriate levels of amylopectin is necessary for establishing a persistent chronic infection [30, 72].
It also appears that bradyzoites rely on anaerobic glycolysis for generation of ATP, since they do not retain a functional TCA cycle [48]. Further evidence for changes in carbohydrate metabolism comes from differential regulation of bradyzoite-specific isoforms of lactate dehydrogenase (LDH2), glucose-6-phosphate dehydrogenase (G6-PI), and enolase-1 (ENO1), all of which display different stability and enzymatic properties compared to their tachyzoite counterparts [48, 73]. The bradyzoite-specific LDH2 isoform is hypothesized to be important for establishing chronic infection in the host, since mice infected with LDH2-knockdown parasites displayed much lower numbers of brain cysts compared to the parental line [74].
Persistence of chronic Toxoplasma infection also relies on continued autophagy and turnover of old organelles or cellular components. Cathepsin protease L is not required for tachyzoite replication or the initial stages of bradyzoite development, but is critical for viability of the mature tissue cyst. Genetic or chemical ablation of cathepsin protease activity caused an accumulation of undigested autophagosomes and loss of bradyzoite viability, indicating that the parasites must recycle proteins and organelles to persist [75].
The long-lived latent cysts must also be capable of dealing with exposure to various radicals and reactive metabolic byproducts; consequently, enzymes that cope with this stress are upregulated during bradyzoite formation, e.g., thioredoxin and glutathione peroxidase [53].
Experimental models of cyst formation
Strain differences
Genetic analysis of Toxoplasma isolates from Europe and North America has identified three predominant lineages of the parasite designated Types I, II, and III, which resulted from a single genetic cross approximately 10,000 years ago [76]. The three strains differ in their growth rate, virulence, their ability to traverse or to pass through epithelial layers (transmigration), and their capacity to form cysts [77, 78].
Type I strains generally replicate faster and, consequently, are more virulent in mice. A Type I strain isolated in 1939, designated “RH” after the patient from which it was isolated [79], is the most common laboratory strain in use. Today’s RH strain has a rapid replication rate and is highly amenable to genetic manipulation, making it a favored tool for the study of tachyzoite biology. However, RH parasites are limiting in the study of conversion from tachyzoite to bradyzoite. Although it has been reported that, under stress conditions, RH tachyzoites will upregulate specific bradyzoite genes and, in some reports, produce bradyzoite-specific proteins or cyst wall components [80–82], the parasites are largely incapable of forming mature bradyzoite cysts either in vitro (in cell culture) or in vivo (during mouse infection). However, low-passage type I strains are capable of switching to bradyzoites [83], which means that extensive passage in vitro is associated with the loss of developmental competency [84]. Type II and Type III strains replicate at a slower rate, readily convert to bradyzoites, and are hypovirulent in mice, but these characteristics change with prolonged in vitro passage. Differences in the developmental capacity among these strains are influenced by adaptation to in vitro growth in HFF cells.
Genome sequence data from parasite lines of all three types have been collected and can be accessed at the Toxoplasma genome database (http://www.toxodb.org). This genome information has proven essential in dissection of the mechanisms and pathways for conversion from tachyzoite to bradyzoite forms.
Stress-induced bradyzoite formation
The early stages of the transition from tachyzoite to bradyzoite may be studied using in vitro culture and conditions shown to induce the conversion. These methods have been used to examine potential pathways associated with the early stages of bradyzoite development. Different kinds of stress applied to proliferating tachyzoites have been demonstrated to induce cyst formation.
The most commonly used method to induce bradyzoite cysts for in vitro culture is continuous alkaline treatment of parasite-infected host cells by adjusting the growth medium to pH 8.0–8.2 [80]. A brief alkaline treatment of extracellular tachyzoites prior to invasion has also been demonstrated to increase frequency of cyst development [85]. A number of other methods that involve adjustments to the culture medium or culture conditions to trigger stress-induced bradyzoite formation include various forms of nutrient deprivation through arginine starvation [86], axenic incubation [49], and depletion of pyrimidine in uracil phosphoribosyltransferase (UPRT)-deficient parasites grown in 0.03% CO2 [22, 81].
Bradyzoite formation may also be enhanced using heat shock as an exogenous stress [80]. Soete et al. [80] observed bradyzoite formation after heat shocking host cells at 43 °C before allowing parasites to invade at 37 °C and applying an additional heat shock to the infected cells.
Chemical treatment of the parasites and host cells has also been used to apply stress and trigger cyst formation. Tunicamycin treatment of parasite-infected cells causes endoplasmic reticulum (ER) stress and subsequent bradyzoite development [87]. Chemical stress may also be applied using sodium arsenite [80]. Chemical inhibition of calcium-induced egress with the herbicide fluridone has also been demonstrated to result in a larger proportion of bradyzoite cysts in culture [88].
Compounds that inhibit mitochondrial function and subsequently induce oxidative stress (i.e., sodium nitroprusside or atovaquone) will also trigger bradyzoite development [89, 90]. Interferon-gamma (IFN-γ) treatment of tachyzoite infected fibroblasts will not induce bradyzoite development [80], but, when murine macrophages are infected with Toxoplasma and subsequently treated with IFN-γ, bradyzoite development commences, likely as a result of nitric oxide production from the activated macrophage [91].
Cyclic adenosine monophosphate (cAMP)-mediated signaling regulates a number of parasite processes including differentiation from tachyzoite to bradyzoite [92]. Using an engineered optogenic Toxoplasma strain in which cAMP levels could be controlled by photo-activation, Hartmann et al. [92] demonstrated that a minimum level of intra-parasitic cAMP was required for initiation of bradyzoite formation, but increased levels of cAMP impaired the process, indicating that cAMP-responsive factors are involved in the differentiation signaling pathway. The importance for tightly regulated cAMP levels in parasite differentiation is underscored by a recent study on a cAMP-dependent protein kinase A homologue, TgPKA3. Knockout of the TgPKA3 catalytic subunit led to a growth defect in tachyzoites and an increase in spontaneous cyst formation, in both Type I and Type II backgrounds. The role of TgPKA3 in cAMP levels was confirmed by rescue of the aberrant growth and differentiation phenotypes with the compound 3-isobutyl-1-methylxanthine, which restores cAMP levels in the parasite [93].
Host cell determinants and spontaneous bradyzoite development
Factors in the host cell may also act as determinants of cyst development, reviewed in [94]. Toxoplasma tachyzoites replicate rapidly and require sufficient levels of nutrients that are scavenged from the host cell to support this proliferation. If host cell metabolic conditions limit the supply of essential nutrients to the parasites, it will slow the replication rate and favor differentiation to the bradyzoite stage [91, 95]. This has been demonstrated in in vitro parasite culture when removal of essential nutrients such as arginine or lipoprotein (a source of cholesterol) from the culture medium favors bradyzoite differentiation [86, 96]. The host cell glycolytic flux can also influence the rate of parasite initiation of differentiation. Dividing host cells in high glucose conditions have an enhanced rate of glycolysis. This generates higher levels of lactate which was shown to favor tachyzoite replication and to inhibit conversion to bradyzoites [97]. Treatment of human host cells with a trisubstituted pyrrole called “Compound 1” prior to tachyzoite infection slowed parasite replication and led to the upregulation of bradyzoite-specific gene expression [98]. Although compound 1 was originally developed as an inhibitor of cyclic guanosine monophosphate (cGMP) kinase [99, 100], it was found to exert its effect through host cell factors [98]. The same study also demonstrated that pre-stressing host cells with alkaline pH before parasite infection was capable of enhancing cyst formation [98].
While cellular stress is a strong mediator of tachyzoite-to-bradyzoite conversion, other factors may play a role in initiating the development of cysts. Tachyzoites of cystogenic Type II and Type III strains will spontaneously convert to bradyzoites in in vitro culture, particularly when host cells have been inoculated with a low multiplicity of infection (MOI) or if egressed tachyzoites are continuously removed from the culture [101]. Although Toxoplasma tachyzoites are capable of infecting any nucleated cell from a warm-blooded animal, certain cell types are more conducive to cyst formation than others [102]. Transcriptomic analysis of parasite gene expression during infection of a number of different cell types indicated greater bradyzoite-specific gene expression in certain cell types such as neurons or muscle cells, even in the absence of extrinsic stress. These data suggest that a subset of cell types is more conducive to bradyzoite formation [103]. The cell cycle stage of host cells that are more permissive to bradyzoite formation may be a strong influence. Terminally differentiated mouse myotubes (skeletal muscle fibres) restricted tachyzoite replication and promoted bradyzoite differentiation compared to their dividing myoblast progenitors [104]. Neurons are in a similar state of terminal differentiation to skeletal muscle cells and it is tempting to speculate that this explains the enrichment of tissue cysts in the CNS and muscle tissue in infected animals.
Host immunity factors and tissue cysts
Since the initiation of cyst development has been largely studied in the context of stress, it has been proposed that in vivo stress applied by immune system factors during host infection is crucial in triggering tachyzoites to transform into bradyzoites [105]. The host immune system can effectively control Toxoplasma infection (for a recent review, see [106]), but it appears that rather than specific immune effectors actively inducing chronic infection, a functional immune response maintains parasite in the latent bradyzoite state, and that a dysfunctional immune response simply allows acute infection to go unchecked.
Cell-mediated immunity is vital for the restriction of Toxoplasma and the development of chronic infection in an immunocompetent host (reviewed in [106, 107]). Increased susceptibility to Toxoplasma has been observed in patients with impaired T-cells as well as in mice that lack B-cells, CD8+, or CD4+ T-cells [108–110]. CD4+ cells, which can activate CD8+ cells, have been proven to be necessary for the maintenance of CD8+ functions during the chronic stage of infection [111, 112].
Notably, it appears that highly virulent Toxoplasma strains have evolved strategies to inhibit the functions of CD8+ cells. A recent study showed that CD8+-mediated immunity is seriously compromised during infection by the RH strain [113]. The kinase activity of ROP18 in the virulent Type I strain was found to target the host activating transcription factor 6β (ATF6-β) for degradation [114], which resulted in downregulation of CD8+ cell-mediated immune responses. Although RH parasites are capable of developing tissue cysts, infection never progresses to the chronic stage, because the host immune system is rapidly overwhelmed by ROP18 function and tachyzoite proliferation leads to systemic infection, organ failure, and death.
The major product of activated CD8+ [115], CD4+ T-cells [116] as well as of non-T and non-NK cells [117–119] is IFN-γ, a cytokine that is well documented to be a key mediator of Toxoplasma infection. In contrast to wild-type mice that survive and develop chronic infection, IFN-γ knockout (KO) mice succumb to infection with avirulent Toxoplasma strains [120]. Moreover, chronic parasite infection does not develop in mice that have been treated with monoclonal antibodies against IFN-γ [115, 121]. Depending on the host species and the cell type, IFN-γ has been proven to activate a variety of effector mechanisms that are required for resistance during chronic infection.
After Toxoplasma infection, IFN-γ induces the formation of reactive oxygen and nitric oxide (NO) metabolites in macrophages, microglia, and astrocyte cells of mice and humans [122–124]. It appears that the NO by-product is a key factor for suppressing parasite replication and inducing bradyzoite development [89, 125–127], similar to in vitro induction of bradyzoites with sodium nitroprusside, as previously discussed. However, NO is not an essential factor for in vivo formation of bradyzoites. Tissue cysts could develop in inducible nitric oxide synthase KO mice [120] as well as in mice that had been treated with aminoguanidine, the inhibitor of the enzyme that produces NO [128].
An IFN-γ-mediated tryptophan degradation response has been observed in human brain endothelial cells, HFF cells, and macrophages infected with Toxoplasma [129–131]. In addition, IFN-γ-treatment of Toxoplasma-infected cells results in upregulation of indoleamine 2,3-dioxygenase 1 and 2 (IDO1 and IDO2), which are host enzymes that are responsible for degradation of tryptophan [132–134]. Since Toxoplasma is auxotrophic for tryptophan, upregulation of IDO1 and IDO2 restricts availability of this essential amino acid and thus inhibits parasite replication [135]. Parasite replication may be restored with the addition of exogenous tryptophan [136]. In vivo inhibition of IDO1 and IDO2 with a chemical inhibitor did not affect the levels of IFN-γ produced after Toxoplasma challenge, but treatment of chronically infected mice with IDO inhibitors led to reactivation of the disease and increased Toxoplasma cyst burden, demonstrating an important role for IDO during latent infection [134]. Notably, IDO has also recently been shown to be involved in host immunity against Trypanosoma cruzi and malaria [137, 138].
The host immune effector Tumor Necrosis Factor α (TNFα) has also been shown to play an important role in establishing chronic Toxoplasma infection [139, 140]. IFN-γ may function in synergy with TNFα, since treatment of Toxoplasma-infected murine macrophages induces the expression of bradyzoite markers [89]. Interleukin 6 (IL-6) is another inflammatory cytokine that causes the increase of cyst numbers produced in vitro in HFF cells [141]. It also appears that control of both acute and chronic stages of infection depends on IL-12, a cytokine that drives the production of IFN-γ [115, 142, 143].
In addition to host cell background influencing spontaneous bradyzoite conversion, the particular in vivo distribution of cysts in brain and muscle tissues may be facilitated by specific local immune responses [144, 145] or potentially ineffective immune responses within these tissues [146]. Alternatively, organ or cell-type-specific factors that trigger high levels of stage conversion and tissue cyst formation may contribute to enhance parasite persistence in these tissues. As previously mentioned, treatment of host cells with Compound 1 modulates host cell factors, making them more permissive to bradyzoite formation [98]. Treatment of host cells with Compound 1 prior to infection with Toxoplasma induced the upregulation of the human cell division autoantigen-1 gene (CDA-1). The increased expression levels of CDA-1 affected parasite replication, arresting the parasite cell cycle and inducing bradyzoite gene expression, directly implicating host cell factors in the differentiation process [98]. Furthermore, it was recently noted that the metabolic state of a cell, as well as non-immune secreted metabolites, could designate host cells as either permissive or resistant to bradyzoite development [97, 147].
Although the host immune responses play critical roles in elimination of tachyzoites and contribute to host environmental changes that foster tachyzoite to bradyzoite conversion and maintenance of tissue cysts, it remains unclear how these signaling pathways compare to induction methods currently used for in vitro differentiation models. The growing convenience of RNA sequencing will be valuable for comparing the transcriptional responses of parasites during the early stages of bradyzoite development during in vivo infection and the commonly used in vitro induction models. Pinpointing the commonalities within the different models will identify the core signaling molecules that drive the transition to the chronic stage.
Reactivation of infection: bradyzoites to tachyzoites
The tissue cysts remain largely quiescent for the life of a host, but tachyzoites can re-emerge and cause toxoplasmosis in immunocompromised patients. The reactivation mechanisms have not been studied extensively, but it is generally assumed that abrogation of the host immune response results in bradyzoite-to-tachyzoite conversion [105]. Human immunodeficiency virus (HIV) patients with reduced numbers of CD4+ cells acquire toxoplasmosis [7, 148]. A similar course of infection occurs in chronically infected mice that lack CD4+ cells [109], further supporting that cell-mediated immunity is important for suppressing bradyzoite reactivation. In addition, it has been demonstrated that in vivo depletion of CD8+ and CD4+ cells in chronically infected mice results in reactivation of Toxoplasma infection [149]. Suppressing IFN-γ production also facilitates bradyzoite reactivation during chronic infection in mice [150–152]. In long-term murine astrocyte cultures of brain-derived Toxoplasma cysts, the presence of IFN-γ is necessary to suppress reactivation. Removal of IFN-γ from the culture, 120 day post-infection, resulted in cyst rupture and the appearance of cells heavily infected with tachyzoites predominating in the culture [153]. One current hypothesis of the mechanism that links IFN-γ with suppression of reactivation is that IFN-γ may stimulate continuous anti-parasitic activity in the astrocytes, causing cell cycle arrest, which has been shown to be associated with bradyzoite differentiation [29]. A direct role of the host immune system in repression of reactivation remains elusive, since, even in healthy mice, tissue cysts can occasionally rupture, releasing invasive parasites that infect new cells [17].
Molecular mechanisms
Translational control—downregulation of global translation and preferential translation of transcription factors
Given that virtually any type of cellular stress can induce bradyzoite differentiation, and can do so independently of the host cell, attention has been directed to the study of stress response pathways in the parasite to illuminate the mechanisms driving stage conversion. A common response to cellular stress in all eukaryotic organisms is the control of global translation via phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α). Depending on the type of stress the cell is experiencing, one of a number of specific kinases is activated to phosphorylate eIF2α, leading to global downregulation of translation with concurrent, preferential translation of a subset of mRNAs that code for factors required to deal with the stress insult [154]. Recent evidence points to translational control via the Toxoplasma homologue of eIF2α, TgIF2α, as a contributing factor in regulating the transition of tachyzoite to bradyzoite, maintenance of the dormant bradyzoite, as well as the reactivation of bradyzoites into proliferating tachyzoites, reviewed in [155]. When Toxoplasma tachyzoites are subjected to different cellular stresses that are known to induce bradyzoite development, TgIF2α becomes phosphorylated [156]. In contrast to intracellular replicating tachyzoites, TgIF2α is phosphorylated to a high degree in mature bradyzoites [87]. Dephosphorylation of mammalian eIF2α is specifically inhibited by treatment with salubrinal or guanabenz [157, 158]. These compounds also inhibit dephosphorylation of TgIF2α and treatment of tachyzoites with either salubrinal or guanabenz triggers bradyzoite differentiation in vitro [87, 159]. Furthermore, cultured bradyzoite cysts treated with salubrinal or guanabenz were no longer able to reactivate into replicating tachyzoites [159].
Once TgIF2α has been phosphorylated, translation is dramatically downregulated [87]. The decrease in protein synthesis conserves resources and allows the parasite to reprogram its genome to respond accordingly to the stress, which, in the case of Toxoplasma, may include switching to the latent bradyzoite stage [3]. Discerning which mRNAs are preferentially translated, while TgIF2α is phosphorylated, would be of great use in understanding the precise sequence of events and the essential components required for the transition from tachyzoite to bradyzoite. Polysome profiling has been used to identify the mRNAs that are undergoing translation at a specific time point. We have developed a polysome fractionation protocol for Toxoplasma combined with microarray analyses, which allowed the identification of a subset of mRNAs that are suggested to undergo preferential translation in parasites experiencing ER stress as a result of tunicamycin treatment [160]. A number of these preferentially translated mRNAs encode proteins containing plant-like AP2 domains, which have been identified as the major family of transcription factors in Apicomplexa [161]. This set of preferentially translated transcription factors are prime candidates for the main effectors upregulating bradyzoite-specific genes and the transition to the dormant cysts.
Transcriptional regulation of bradyzoite-specific genes
Once the switch to cyst development has been triggered, upregulation of bradyzoite-specific genes has been observed with a concurrent downregulation of tachyzoite-specific genes. A number of studies using different techniques have sought to identify the genome-wide changes in gene expression during the bradyzoite developmental program [40, 53, 56, 162, 163]. Cascades of gene expression are observed as the parasites transition from one stage to the next, indicating that transcriptional regulation plays an important role in the regulation of stage switching. A number of these studies also report distinct functional groups of genes that are differentially regulated, with surface antigens, metabolic proteins, and secreted proteins distinguished with significant differences in expression levels [40, 162].
The initial results from genome sequencing of Toxoplasma and related apicomplexan parasites suggested a surprisingly small number of putative transcription factors, prompting focus on epigenetic means for gene regulation [164]. However, a growing body of evidence indicates that gene expression is regulated by the conventional eukaryotic mechanisms. Much of the conventional general transcriptional machinery appears to be conserved in Toxoplasma [165]. There also appears to be specific DNA motifs for recruitment of factors controlling stage-specific gene expression. The promoters of 16 bradyzoite-specific genes were mapped to high resolution, which led to the identification of cis-acting elements that appear to be specific to genes induced during differentiation. These elements from the two bradyzoite-specific genes bag1 and B-NTPase were inserted into the constitutive DHFR-TS promoter and converted it into a promoter that enhanced expression of a reporter under bradyzoite-induction conditions [83].
To identify DNA-associated factors that could function as repressors of bradyzoite gene expression during tachyzoite proliferation, pull-downs using the bradyzoite-specific promoter ENO1 as bait were performed. This study identified different regulatory factors such as an RNA helicase, kinases, phosphatases, and heat shock proteins, as well as a number of hypothetical proteins containing domains with high homology to known chromatin-association factors. One of the proteins pulled down was an FK506-binding protein (FK506BP) homologue called Toxoplasma nuclear factor 3 (TgNF3). TgNF3 displays a nuclear localization in tachyzoites, but was found to be predominantly cytoplasmic in bradyzoites. TgNF3 was shown to associate with both nucleosomes and free histones to repress ENO1 and 18S ribosomal RNA genes [166].
The characterization of the ApiAP2 family of plant-like transcription factors in Toxoplasma and related apicomplexan parasites [161] has prompted efforts to identify the specific AP2 factors that may be responsible for coordinating the response to the different signals that trigger bradyzoite differentiation (reviewed in [167]). Collation of the various gene expression analyses that have been performed on differentiating parasites has identified a handful of AP2 factors that are specifically upregulated during bradyzoite development and are dampened once the mature tissue cyst is formed [65, 168]. Genetic disruption of these AP2 factors is revealing unexpected complexity in the stage transition from tachyzoite to bradyzoite, which appears to be mediated by interplay between both activators and repressors of bradyzoite genes.
At least two of these AP2 factors, AP2IV-3 and AP2XI-4, function as activators of bradyzoite genes [63, 65]. Deletion of AP2XI-4 in both Type I and Type II backgrounds generated mutant lines that were unable to initiate expression of bradyzoite marker genes during stress [63]. The Type II mutant also displayed a significant defect in cyst formation during in vivo infection in mice [63]. AP2IV-3 is also induced early during bradyzoite development to promote expression of bradyzoite genes [65]. Knockout of AP2IV-3 reduced the rate of cyst formation in response to alkaline stress, while inducible overexpression of this factor increased the rate of cyst formation, even in the absence of extrinsic stress. Gene expression analysis of the AP2IV-3 overexpressing parasites confirmed upregulation of a subset of known alkaline stress-responsive genes [65].
Conversely, another AP2 factor that is upregulated early during bradyzoite development AP2IX-9 acts as a repressor of bradyzoite formation. AP2IX-9 knockout parasites have a higher rate of spontaneous cyst conversion in normal growth medium and overexpression of AP2IX-9 decreased the rate of differentiation under alkaline stress [62, 65]. Both AP2IV-3 and AP2IX-9 appear to regulate the same target genes and were found to directly associate with the chromatin in at least one promoter region (bag1); however, further studies are required to understand how the action of these antagonistic factors culminates in a decision to either continue replication as a tachyzoite, or commit to bradyzoite development.
Adding to the complexity of tissue cyst development at the molecular level is findings on two other AP2 factors that are cell cycle regulated in tachyzoites. Although not essential for tachyzoite replication, AP2IX-4 knockout mutants displayed a defect in cyst formation in vitro and in vivo [64]. Localization of AP2IX-4 protein revealed that it is cell cycle regulated and expressed only in dividing parasites, both tachyzoites and bradyzoites. Gene expression analysis reveals that AP2IX-4 operates as a repressor of bradyzoite genes during alkaline stress, consistent with AP2IX-4 being present in dividing parasites [64]. Similarly, another cell cycle-regulated factor that peaks at the S/M stage in tachyzoites, AP2IV-4, was also identified as a repressor of bradyzoite genes when gene expression analysis on the knockout parasites revealed many bradyzoite-specific transcripts to be upregulated [66]. Although this mutant line displayed a comparable cyst formation rate to the parental line under alkaline stress in vitro, it was completely deficient in tissue cyst formation during mouse infection, suggesting that the aberrant expression of bradyzoite proteins early during infection primed the host immune response for efficient clearing of the parasite load before chronic infection could be established [66]. Collectively, these studies argue against a single master regulator of bradyzoite differentiation and suggest that multiple transcription factors are involved in fine-tuning gene expression at multiple checkpoints during the transition to latency.
The cell cycle and bradyzoite formation
The cyclic regulation of many of the regulators involved in bradyzoite formation suggests that this differentiation process is intimately linked to the parasite cell cycle. Early experiments showed that when the cell cycle is blocked, differentiation does not proceed [169]. Subsequent studies have attempted to elucidate the point in the cell cycle in which the tachyzoite switches to the bradyzoite developmental program and which factors play a role at this transition point. Gene Set Enrichment Analysis (GSEA) on a number of independent gene expression data sets from differentiation experiments provides strong evidence that bradyzoite formation and cell cycle regulation are coupled. Genes associated with the S/M phase of the cell cycle were more highly enriched in bradyzoites, while genes corresponding to the G1 phase were more enriched in tachyzoites [170, 171]. Cell cycle comparison between replicating and differentiating tachyzoites identified an enrichment of parasites in late S and M stages when bradyzoite development was triggered [95]. Concurrent with this observation, microarray analysis at different points in the tachyzoite replication cycle found that the levels of a number of bradyzoite-specific transcripts peaked at the late mitotic phase of the cell cycle [168]. This was observed in unstressed parasite populations, suggesting that even under normal conditions, certain factors that are capable of initiating bradyzoite development are present, but the master signal for the transition is either absent or overpowered by other regulators that drive tachyzoite replication. This evidence supports a model whereby tachyzoites pass through a point in the cell cycle in which they are poised for development into a bradyzoite. If the signal for bradyzoite development is present, the gene expression program for cyst formation proceeds, but, in the absence of such stress signals, tachyzoite replication continues.
Changes in the epigenetic landscape for tachyzoite versus bradyzoite genes
A series of studies have demonstrated that epigenetics and histone post-translational modifications (PTMs) play a vital role in protozoan parasite biology [63]. More specifically, Toxoplasma epigenetics has been linked with the regulation of genes expressed during oxidative stress responses, cell cycle, and DNA repair mechanisms, as well as during tachyzoite-to-bradyzoite stage conversion [172–177].
Mining of the Toxoplasma genome has identified a wealth of factors homologous to the chromatin remodeling machinery of other well-characterized eukaryotes [164, 176]. In addition, chromatin immunoprecipitation coupled to microarray chip (ChIP-chip) experiments have been utilized to determine that H3K4 tri-methylation and H3K9 acetylation are markers of active promoters in Toxoplasma [178]. The first evidence that linked epigenetic regulation with bradyzoite development was the observation that nucleosomes present in inactive bradyzoite-specific promoters are hypoacetylated during the tachyzoite stage but become acetylated under bradyzoite differentiation conditions [172, 179]. The converse was shown for tachyzoite-specific genes, whereas constitutively expressed genes were hyperacetylated in both developmental stages [172]. The differential association of histone deacetylase TgHDAC3 and histone acetyltransferase TgGCN5a at stage-specific promoters was also observed using ChIP assays [172]. Specifically, TgGCN5a is highly enriched at the promoter regions of transcribed genes [172, 173, 180], while TgHDAC3 is found at promoter regions of bradyzoite-specific genes that are silent during tachyzoite replication [172]. Moreover, analysis of the transcriptional profile of a TgGCN5a KO strain suggested a role for TgGCN5a in triggering bradyzoite gene expression in response to alkaline stress. TgGCN5a KO parasites under alkaline treatment did not upregulate bag1 or ldh2, and these parasites were deficient in stress recovery [173]. Toxoplasma also expresses a second GCN5 histone acetyltransferase homologue, TgGCN5b, which appears to be crucial during tachyzoite replication, but a role for this enzyme in mediating the transition to bradyzoite cyst has yet to be determined [29]. The involvement of specific HDACs in differentiation was revealed through the use of the compound FR235222, which specifically inhibits TgHDAC3 [174]. Treatment of Toxoplasma-infected cells with FR235222 induced bradyzoite differentiation [174]. In addition, comparisons of the transcriptional profiles of tachyzoites in the presence or absence of apicidin, another histone deacetylase inhibitor, suggested that the tachyzoite-to-bradyzoite conversion is regulated in part through histone acetylation and deacetylation modifications [181]. While these studies support the role of histone modifying enzymes in bradyzoite differentiation, it should be noted that “histone” modifying enzymes have numerous other substrates whose activity could be regulated by acetylation [182, 183].
Chemical inhibitors have also been used to determine the role of methyltransferases in Toxoplasma stage differentiation. Specifically, pre-treatment of extracellular tachyzoites with AMI-I, an inhibitor of the arginine methyltransferase TgCARM1, induced cyst formation [172]. ChIP assays were used to confirm that AMI-I treatment resulted in a reduction of H3R17 methylation in the nucleosomes at promoter regions of tachyzoite-specific genes, suggesting an important function of the H3R17 mark for tachyzoites [172]. However, methylation of H3R17 can also be observed in in vitro bradyzoites, pointing to a key function for TgCARM1 in both stages. A significant gene duplication event and subsequent divergence in the SET-containing domain histone lysine methyltransferases has been reported in Toxoplasma [175]. TgSET8 was shown to be enriched in heterochromatic regions such as silenced rRNA genes, satellite repeats, and telomeric sites, with the repressive methylation marks H3K9 and H4K20 [175]. Notably, increased H4K20me1 levels have been detected in bradyzoite containing cysts purified from mice [175], indicating that TgSET8 may facilitate the maintenance of the chronic stage inside the host.
An interesting family of chromatin remodelers that has not been studied extensively, but has been linked to epigenetic regulation of stage conversion, is the SWI/SNF ATP-dependent nucleosome remodeling complexes. Intriguingly, the Toxoplasma genome has 17 predicted members of this family [176]. The mRNA from one member of this family, TgSRCAP (Snf2-Related CBP Activator Protein), is upregulated during in vitro bradyzoite stage conversion [184]. As previously mentioned, another member of this family, TgRSC8, was identified in an insertional mutagenesis screen designed to select for mutants defective in bradyzoite formation [41, 185].
Changing nucleosome composition is an additional mechanism that may be used to control gene expression during stage conversion in Toxoplasma. In addition to the canonical histones, H2A and H2B, the Toxoplasma genome encodes a number of histone H2 variants, denoted H2AX, H2AZ, and H2Bv [164]. H2Bv forms dimers with H2A.Z on the promoter regions of active genes [186]. Notably, the incidence of H2A.Z changes in relation to the transcriptional status of a gene; preliminary data showed that H2A.Z is positioned within the coding region of silent bradyzoite-specific genes and within promoter regions but not coding regions of actively expressed genes [187]. In contrast, H2AX is enriched at promoters of repressed genes as well as within silent genomic regions in tachyzoites [186]. Furthermore, the expression levels of H2AX are increased during in vitro bradyzoite formation, suggesting that H2AX may facilitate global gene repression during the latent bradyzoite stage [186].
Multiple approaches have demonstrated the importance of epigenetic regulation during the transition from tachyzoite to bradyzoite. Many of the fundamentals of epigenetic regulatory mechanisms appear to be well preserved in parasites. However, there is a surprisingly high diversity within the proteins that constitute the chromatin remodeling machinery in Toxoplasma. PTM-histone mapping studies [187] as well as characterization of “species-specific” and “stage-specific” epigenetic modifications of Toxoplasma will assist in elucidating the complete mechanism of the complex developmental pathway driving bradyzoite formation.
Conclusion and future directions
The ability of Toxoplasma to form latent cysts and persist in the host for many years is the key to the parasite’s successful dissemination throughout a large proportion of the human population and numerous animal hosts worldwide.
We are beginning to understand the early stages of the transition from tachyzoite to bradyzoite induced by stress, but further research is required to flesh out the mechanisms involved. Importantly, these mechanisms need to be compared to newer models of in vitro differentiation that do not require the application of exogenous stress (i.e., spontaneous differentiation). Recent advances have identified a number of AP2 and chromatin remodeling factors that are responsible for controlling the gene expression program as the parasites switch from tachyzoite to bradyzoite, but how they are engaged and how they interplay remains largely unresolved. Many elements are at work in this complex, multifactorial process, and advances in the molecular tools for cystogenic Toxoplasma strains will help to address remaining questions.
An area that has been largely neglected to date is the process of reactivation and how the parasite re-enters the proliferative tachyzoite cycle after dormancy. Phosphorylation of TgIF2α appears to play a crucial role in perpetuating quiescence of the bradyzoites within the cyst and tantalizing new studies demonstrate that pharmacological interference with translational control can impede tachyzoite growth and the reactivation of infection [159].
Ultimately, we will require a detailed understanding of the signaling processes required for bradyzoite formation to interrupt the tachyzoite–bradyzoite transition. Definition of these pathways may enable us to chemically target central mediators of the differentiation and latency processes, and allow development of novel therapies for the individuals at risk of toxoplasmosis.
Acknowledgements
The authors thank Dr. Michael White for his careful and critical review of our manuscript. Research in the laboratories of Drs. Sullivan and Kim is supported by Grants from the National Institutes of Health: AI116496 and AI124723 (WJS), R01AI087625 (KK), and AI092801 (to KK and WJS). This manuscript is dedicated to the memory of Dr. Zoi Tampaki who was a dedicated scientist, a valued colleague, and a beloved friend. We miss you every day.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hutchison WM, Dunachie JF, Siim JC, Work K. Life cycle of Toxoplasma gondii. BMJ. 1969;4:806. doi: 10.1136/bmj.4.5686.806-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan WJ, Jr, Jeffers V. Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol Rev. 2012;36:717–733. doi: 10.1111/j.1574-6976.2011.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luft BJ, Remington JS. AIDS commentary, Toxoplasmic encephalitis. J Infect Dis. 1988;157:1–6. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- 5.McLeod R, Mack D, Brown C. Toxoplasma gondii—new advances in cellular and molecular biology. Exp Parasitol. 1991;72:109–121. doi: 10.1016/0014-4894(91)90129-K. [DOI] [PubMed] [Google Scholar]
- 6.Wong SY, Remington JS. Biology of Toxoplasma gondii. AIDS. 1993;7:299–316. doi: 10.1097/00002030-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. J Am Med Assoc. 1984;252:913–917. doi: 10.1001/jama.1984.03350070031018. [DOI] [PubMed] [Google Scholar]
- 8.Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28:1019–1024. doi: 10.1016/S0020-7519(98)00023-X. [DOI] [PubMed] [Google Scholar]
- 9.Jones J, Lopez A, Wilson M. Congenital toxoplasmosis. Am Fam Physician. 2003;67:2131–2138. [PubMed] [Google Scholar]
- 10.Wallace GR, Stanford MR. Immunity and Toxoplasma retinochoroiditis. Clin Exp Immunol. 2008;153:309–315. doi: 10.1111/j.1365-2249.2008.03692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasconcelos-Santos DV. Ocular manifestations of systemic disease: toxoplasmosis. Curr Opin Ophthalmol. 2012;23:543–550. doi: 10.1097/ICU.0b013e328358bae5. [DOI] [PubMed] [Google Scholar]
- 12.Bowie WR, King AS, Werker DH, Isaac-Renton JL, Bell A, Eng SB, Marion SA. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet. 1997;350:173–177. doi: 10.1016/S0140-6736(96)11105-3. [DOI] [PubMed] [Google Scholar]
- 13.Aramini JJ, Stephen C, Dubey JP, Engelstoft C, Schwantje H, Ribble CS. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiol Infect. 1999;122:305–315. doi: 10.1017/S0950268899002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MA, Gardner IA, Kreuder C, Paradies DM, Worcester KR, Jessup DA, Dodd E, Harris MD, Ames JA, Packham AE, Conrad PA. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) Int J Parasitol. 2002;32:997–1006. doi: 10.1016/S0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson DJ, Hutchison WM. The host-parasite relationship of Toxoplasma gondii in the brains of chronically infected mice. Virchows Archiv A Pathol Anat Histopathol. 1987;411:39–43. doi: 10.1007/BF00734512. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson DJ, Hutchison WM. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 1987;73:483–491. doi: 10.1007/BF00535321. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson DJ, Hutchison WM, Pettersen E. Tissue cyst rupture in mice chronically infected with Toxoplasma gondii. An immunocytochemical and ultrastructural study. Parasitol Res. 1989;75:599–603. doi: 10.1007/BF00930955. [DOI] [PubMed] [Google Scholar]
- 18.Cabral CM, Tuladhar S, Dietrich HK, Nguyen E, MacDonald WR, Trivedi T, Devineni A, Koshy AA. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog. 2016;12:e1005447. doi: 10.1371/journal.ppat.1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koshy AA, Dietrich HK, Christian DA, Melehani JH, Shastri AJ, Hunter CA, Boothroyd JC. Toxoplasma co-opts host cells it does not invade. PLoS Pathog. 2012;8:e1002825. doi: 10.1371/journal.ppat.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K, Weiss LM. Toxoplasma gondii: the model apicomplexan. Int J Parasitol. 2004;34:423–432. doi: 10.1016/j.ijpara.2003.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soete M, Dubremetz JF. Toxoplasma gondii: kinetics of stage-specific protein expression during tachyzoite-bradyzoite conversion in vitro. Curr Top Microbiol Immunol. 1996;219:76–80. [PubMed] [Google Scholar]
- 22.Dzierszinski F, Nishi M, Ouko L, Roos DS. Dynamics of Toxoplasma gondii differentiation. Eukaryot Cell. 2004;3:992–1003. doi: 10.1128/EC.3.4.992-1003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane A, Soete M, Dubremetz JF, Smith JE. Toxoplasma gondii: appearance of specific markers during the development of tissue cysts in vitro. Parasitol Res. 1996;82:340–346. doi: 10.1007/s004360050123. [DOI] [PubMed] [Google Scholar]
- 24.Di Cristina M, Marocco D, Galizi R, Proietti C, Spaccapelo R, Crisanti A. Temporal and spatial distribution of Toxoplasma gondii differentiation into Bradyzoites and tissue cyst formation in vivo. Infect Immun. 2008;76:3491–3501. doi: 10.1128/IAI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Waaij D. Formation, growth and multiplication of Toxoplasma gondii cysts in mouse brain. Trop Geogr Med. 1959;11:345–360. [Google Scholar]
- 26.Mehlhorn H, Frenkel JK. Ultrastructural comparison of cysts and zoites of Toxoplasma gondii, Sarcocystis muris, and Hammondia hammondi in skeletal muscle of mice. J Parasitol. 1980;66:59–67. doi: 10.2307/3280590. [DOI] [PubMed] [Google Scholar]
- 27.Weiss LM, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci J Virtual Libr. 2000;5:D391–D405. doi: 10.2741/A521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomita T, Bzik DJ, Ma YF, Fox BA, Markillie LM, Taylor RC, Kim K, Weiss LM. The Toxoplasma gondii cyst wall protein CST1 is critical for cyst wall integrity and promotes bradyzoite persistence. PLoS Pathog. 2013;9:e1003823. doi: 10.1371/journal.ppat.1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Dixon SE, Ting LM, Liu TK, Jeffers V, Croken MM, Calloway M, Cannella D, Hakimi MA, Kim K, Sullivan WJ., Jr Lysine acetyltransferase GCN5b interacts with AP2 factors and is required for Toxoplasma gondii proliferation. PLoS Pathog. 2014;10:e1003830. doi: 10.1371/journal.ppat.1003830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts E, Zhao Y, Dhara A, Eller B, Patwardhan A, Sinai AP (2015) Novel approaches reveal that Toxoplasma gondii bradyzoites within tissue cysts are dynamic and replicating entities in vivo. mBio 6:e01155–e01215 [DOI] [PMC free article] [PubMed]
- 31.Wang T, Gao JM, Yi SQ, Geng GQ, Gao XJ, Shen JL, Lu FL, Wen YZ, Hide G, Lun ZR. Toxoplasma gondii infection in the peritoneal macrophages of rats treated with glucocorticoids. Parasitol Res. 2014;113:351–358. doi: 10.1007/s00436-013-3661-3. [DOI] [PubMed] [Google Scholar]
- 32.Knoll LJ, Boothroyd JC. Molecular biology’s lessons about toxoplasma development: stage-specific homologs. Parasitol Today. 1998;14:490–493. doi: 10.1016/S0169-4758(98)01347-7. [DOI] [PubMed] [Google Scholar]
- 33.Zhang YW, Halonen SK, Ma YF, Wittner M, Weiss LM. Initial characterization of CST1, a Toxoplasma gondii cyst wall glycoprotein. Infect Immun. 2001;69:501–507. doi: 10.1128/IAI.69.1.501-507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson DJ. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int J Parasitol. 2004;34:347–360. doi: 10.1016/j.ijpara.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 35.Torpier G, Charif H, Darcy F, Liu J, Darde ML, Capron A. Toxoplasma gondii: differential location of antigens secreted from encysted bradyzoites. Exp Parasitol. 1993;77:13–22. doi: 10.1006/expr.1993.1056. [DOI] [PubMed] [Google Scholar]
- 36.Lemgruber L, Lupetti P, Martins-Duarte ES, De Souza W, Vommaro RC. The organization of the wall filaments and characterization of the matrix structures of Toxoplasma gondii cyst form. Cell Microbiol. 2011;13:1920–1932. doi: 10.1111/j.1462-5822.2011.01681.x. [DOI] [PubMed] [Google Scholar]
- 37.Parmley SF, Yang S, Harth G, Sibley LD, Sucharczuk A, Remington JS. Molecular characterization of a 65-kilodalton Toxoplasma gondii antigen expressed abundantly in the matrix of tissue cysts. Mol Biochem Parasitol. 1994;66:283–296. doi: 10.1016/0166-6851(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 38.Parmley S, Slifer T, Araujo F. Protective effects of immunization with a recombinant cyst antigen in mouse models of infection with Toxoplasma gondii tissue cysts. J Infect Dis. 2002;185(Suppl 1):S90–S95. doi: 10.1086/338464. [DOI] [PubMed] [Google Scholar]
- 39.Buchholz KR, Bowyer PW, Boothroyd JC. Bradyzoite pseudokinase 1 is crucial for efficient oral infectivity of the Toxoplasma gondii tissue cyst. Eukaryot Cell. 2013;12:399–410. doi: 10.1128/EC.00343-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchholz KR, Fritz HM, Chen X, Durbin-Johnson B, Rocke DM, Ferguson DJ, Conrad PA, Boothroyd JC. Identification of tissue cyst wall components by transcriptome analysis of in vivo and in vitro Toxoplasma gondii bradyzoites. Eukaryot Cell. 2011;10:1637–1647. doi: 10.1128/EC.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craver MP, Rooney PJ, Knoll LJ. Isolation of Toxoplasma gondii development mutants identifies a potential proteophosphogylcan that enhances cyst wall formation. Mol Biochem Parasitol. 2010;169:120–123. doi: 10.1016/j.molbiopara.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomavo S, Fortier B, Soete M, Ansel C, Camus D, Dubremetz JF. Characterization of bradyzoite-specific antigens of Toxoplasma gondii. Infect Immun. 1991;59:3750–3753. doi: 10.1128/iai.59.10.3750-3753.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohne W, Gross U, Ferguson DJ, Heesemann J. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 44.Bohne W, Parmley SF, Yang S, Gross U. Bradyzoite-specific genes. Curr Top Microbiol Immunol. 1996;219:81–91. doi: 10.1007/978-3-642-51014-4_9. [DOI] [PubMed] [Google Scholar]
- 45.Parmley SF, Weiss LM, Yang S. Cloning of a bradyzoite-specific gene of Toxoplasma gondii encoding a cytoplasmic antigen. Mol Biochem Parasitol. 1995;73:253–257. doi: 10.1016/0166-6851(95)00100-F. [DOI] [PubMed] [Google Scholar]
- 46.Yang S, Parmley SF. A bradyzoite stage-specifically expressed gene of Toxoplasma gondii encodes a polypeptide homologous to lactate dehydrogenase. Mol Biochem Parasitol. 1995;73:291–294. doi: 10.1016/0166-6851(95)00124-J. [DOI] [PubMed] [Google Scholar]
- 47.Yang S, Parmley SF. Toxoplasma gondii expresses two distinct lactate dehydrogenase homologous genes during its life cycle in intermediate hosts. Gene. 1997;184:1–12. doi: 10.1016/S0378-1119(96)00566-5. [DOI] [PubMed] [Google Scholar]
- 48.Denton H, Roberts CW, Alexander J, Thong KW, Coombs GH. Enzymes of energy metabolism in the bradyzoites and tachyzoites of Toxoplasma gondii. FEMS Microbiol Lett. 1996;137:103–108. doi: 10.1111/j.1574-6968.1996.tb08090.x. [DOI] [PubMed] [Google Scholar]
- 49.Yahiaoui B, Dzierszinski F, Bernigaud A, Slomianny C, Camus D, Tomavo S. Isolation and characterization of a subtractive library enriched for developmentally regulated transcripts expressed during encystation of Toxoplasma gondii. Mol Biochem Parasitol. 1999;99:223–235. doi: 10.1016/S0166-6851(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 50.Toursel C, Dzierszinski F, Bernigaud A, Mortuaire M, Tomavo S. Molecular cloning, organellar targeting and developmental expression of mitochondrial chaperone HSP60 in Toxoplasma gondii. Mol Biochem Parasitol. 2000;111:319–332. doi: 10.1016/S0166-6851(00)00324-8. [DOI] [PubMed] [Google Scholar]
- 51.Holpert M, Luder CG, Gross U, Bohne W. Bradyzoite-specific expression of a P-type ATPase in Toxoplasma gondii. Mol Biochem Parasitol. 2001;112:293–296. doi: 10.1016/S0166-6851(00)00361-3. [DOI] [PubMed] [Google Scholar]
- 52.Odberg-Ferragut C, Soete M, Engels A, Samyn B, Loyens A, Van Beeumen J, Camus D, Dubremetz JF. Molecular cloning of the Toxoplasma gondii sag4 gene encoding an 18 kDa bradyzoite specific surface protein. Mol Biochem Parasitol. 1996;82:237–244. doi: 10.1016/0166-6851(96)02740-5. [DOI] [PubMed] [Google Scholar]
- 53.Manger ID, Hehl A, Parmley S, Sibley LD, Marra M, Hillier L, Waterston R, Boothroyd JC. Expressed sequence tag analysis of the bradyzoite stage of Toxoplasma gondii: identification of developmentally regulated genes. Infect Immun. 1998;66:1632–1637. doi: 10.1128/iai.66.4.1632-1637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohne W, Wirsing A, Gross U. Bradyzoite-specific gene expression in Toxoplasma gondii requires minimal genomic elements. Mol Biochem Parasitol. 1997;85:89–98. doi: 10.1016/S0166-6851(96)02814-9. [DOI] [PubMed] [Google Scholar]
- 55.Knoll LJ, Boothroyd JC. Isolation of developmentally regulated genes from Toxoplasma gondii by a gene trap with the positive and negative selectable marker hypoxanthine–xanthine–guanine phosphoribosyltransferase. Mol Cell Biol. 1998;18:807–814. doi: 10.1128/MCB.18.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pittman KJ, Aliota MT, Knoll LJ. Dual transcriptional profiling of mice and Toxoplasma gondii during acute and chronic infection. BMC Genom. 2014;15:806. doi: 10.1186/1471-2164-15-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang YW, Kim K, Ma YF, Wittner M, Tanowitz HB, Weiss LM. Disruption of the Toxoplasma gondii bradyzoite-specific gene BAG1 decreases in vivo cyst formation. Mol Microbiol. 1999;31:691–701. doi: 10.1046/j.1365-2958.1999.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bohne W, Hunter CA, White MW, Ferguson DJ, Gross U, Roos DS. Targeted disruption of the bradyzoite-specific gene BAG1 does not prevent tissue cyst formation in Toxoplasma gondii. Mol Biochem Parasitol. 1998;92:291–301. doi: 10.1016/S0166-6851(97)00236-3. [DOI] [PubMed] [Google Scholar]
- 59.Holpert M, Gross U, Bohne W. Disruption of the bradyzoite-specific P-type (H+)-ATPase PMA1 in Toxoplasma gondii leads to decreased bradyzoite differentiation after stress stimuli but does not interfere with mature tissue cyst formation. Mol Biochem Parasitol. 2006;146:129–133. doi: 10.1016/j.molbiopara.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Kim SK, Fouts AE, Boothroyd JC. Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. J Immunol. 2007;178:5154–5165. doi: 10.4049/jimmunol.178.8.5154. [DOI] [PubMed] [Google Scholar]
- 61.Fox BA, Falla A, Rommereim LM, Tomita T, Gigley JP, Mercier C, Cesbron-Delauw MF, Weiss LM, Bzik DJ. Type II Toxoplasma gondii KU80 knockout strains enable functional analysis of genes required for cyst development and latent infection. Eukaryot Cell. 2011;10:1193–1206. doi: 10.1128/EC.00297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radke JB, Lucas O, De Silva EK, Ma Y, Sullivan WJ, Jr, Weiss LM, Llinas M, White MW. ApiAP2 transcription factor restricts development of the Toxoplasma tissue cyst. Proc Natl Acad Sci USA. 2013;110:6871–6876. doi: 10.1073/pnas.1300059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker R, Gissot M, Croken MM, Huot L, Hot D, Kim K, Tomavo S. The Toxoplasma nuclear factor TgAP2XI-4 controls bradyzoite gene expression and cyst formation. Mol Microbiol. 2013;87:641–655. doi: 10.1111/mmi.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang S, Holmes MJ, Radke JB, Hong DP, Liu TK, White MW, Sullivan WJ. Toxoplasma gondii AP2IX-4 regulates gene expression during bradyzoite development. mSphere. 2017;2:e00054–e00017. doi: 10.1128/mSphere.00054-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong DP, Radke JB, White MW. Opposing transcriptional mechanisms regulate toxoplasma development. mSphere. 2017;2:e00347–e00416. doi: 10.1128/mSphere.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radke JB, Worth D, Hong DP, Huang S, Sullivan WJ, Wilson EH, White M. Transcriptional repression by ApiAP2 factors is central to chronic toxoplasmosis. bioRxiv. 2017 doi: 10.1101/100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh U, Brewer JL, Boothroyd JC. Genetic analysis of tachyzoite to bradyzoite differentiation mutants in Toxoplasma gondii reveals a hierarchy of gene induction. Mol Microbiol. 2002;44:721–733. doi: 10.1046/j.1365-2958.2002.02903.x. [DOI] [PubMed] [Google Scholar]
- 68.Matrajt M, Donald RG, Singh U, Roos DS. Identification and characterization of differentiation mutants in the protozoan parasite Toxoplasma gondii. Mol Microbiol. 2002;44:735–747. doi: 10.1046/j.1365-2958.2002.02904.x. [DOI] [PubMed] [Google Scholar]
- 69.Vanchinathan P, Brewer JL, Harb OS, Boothroyd JC, Singh U. Disruption of a locus encoding a nucleolar zinc finger protein decreases tachyzoite-to-bradyzoite differentiation in Toxoplasma gondii. Infect Immun. 2005;73:6680–6688. doi: 10.1128/IAI.73.10.6680-6688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson MZ, Brewer J, Singh U, Boothroyd JC. A pseudouridine synthase homologue is critical to cellular differentiation in Toxoplasma gondii. Eukaryot Cell. 2009;8:398–409. doi: 10.1128/EC.00329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frankel MB, Mordue DG, Knoll LJ. Discovery of parasite virulence genes reveals a unique regulator of chromosome condensation 1 ortholog critical for efficient nuclear trafficking. Proc Natl Acad Sci USA. 2007;104:10181–10186. doi: 10.1073/pnas.0701893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugi T, Tu V, Ma Y, Tomita T, Weiss LM. Toxoplasma gondii requires glycogen phosphorylase for balancing amylopectin storage and for efficient production of brain cysts. mBio. 2017;8:e01289–e01317. doi: 10.1128/mBio.01289-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dzierszinski F, Mortuaire M, Dendouga N, Popescu O, Tomavo S. Differential expression of two plant-like enolases with distinct enzymatic and antigenic properties during stage conversion of the protozoan parasite Toxoplasma gondii. J Mol Biol. 2001;309:1017–1027. doi: 10.1006/jmbi.2001.4730. [DOI] [PubMed] [Google Scholar]
- 74.Al-Anouti F, Tomavo S, Parmley S, Ananvoranich S. The expression of lactate dehydrogenase is important for the cell cycle of Toxoplasma gondii. J Biol Chem. 2004;279:52300–52311. doi: 10.1074/jbc.M409175200. [DOI] [PubMed] [Google Scholar]
- 75.Di Cristina M, Dou Z, Lunghi M, Kannan G, Huynh MH, McGovern OL, Schultz TL, Schultz AJ, Miller AJ, Hayes BM, van der Linden W, Emiliani C, Bogyo M, Besteiro S, Coppens I, Carruthers VB. Toxoplasma depends on lysosomal consumption of autophagosomes for persistent infection. Nat Microbiol. 2017;2:17096. doi: 10.1038/nmicrobiol.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su C, Evans D, Cole RH, Kissinger JC, Ajioka JW, Sibley LD. Recent expansion of Toxoplasma through enhanced oral transmission. Science. 2003;299:414–416. doi: 10.1126/science.1078035. [DOI] [PubMed] [Google Scholar]
- 77.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 78.Barragan A, Sibley LD. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 2003;11:426–430. doi: 10.1016/S0966-842X(03)00205-1. [DOI] [PubMed] [Google Scholar]
- 79.Sabin AB. Isolation of a filtrable, transmissible agent with “Neurolyti” properties from Toxoplasma-infected tissues. Science. 1938;88:189–191. doi: 10.1126/science.88.2278.189. [DOI] [PubMed] [Google Scholar]
- 80.Soete M, Camus D, Dubremetz JF. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–370. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 81.Bohne W, Roos DS. Stage-specific expression of a selectable marker in Toxoplasma gondii permits selective inhibition of either tachyzoites or bradyzoites. Mol Biochem Parasitol. 1997;88:115–126. doi: 10.1016/S0166-6851(97)00087-X. [DOI] [PubMed] [Google Scholar]
- 82.Lescault PJ, Thompson AB, Patil V, Lirussi D, Burton A, Margarit J, Bond J, Matrajt M. Genomic data reveal Toxoplasma gondii differentiation mutants are also impaired with respect to switching into a novel extracellular tachyzoite state. PLoS One. 2010;5:e14463. doi: 10.1371/journal.pone.0014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Behnke MS, Radke JB, Smith AT, Sullivan WJ, Jr, White MW. The transcription of bradyzoite genes in Toxoplasma gondii is controlled by autonomous promoter elements. Mol Microbiol. 2008;68:1502–1518. doi: 10.1111/j.1365-2958.2008.06249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frenkel JK, Dubey JP, Hoff RL. Loss of stages after continuous passage of Toxoplasma gondii and Besnoitia jellisoni. J Protozool. 1976;23:421–424. doi: 10.1111/j.1550-7408.1976.tb03799.x. [DOI] [PubMed] [Google Scholar]
- 85.Weiss LM, Ma YF, Takvorian PM, Tanowitz HB, Wittner M. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infect Immun. 1998;66:3295–3302. doi: 10.1128/iai.66.7.3295-3302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fox BA, Gigley JP, Bzik DJ. Toxoplasma gondii lacks the enzymes required for de novo arginine biosynthesis and arginine starvation triggers cyst formation. Int J Parasitol. 2004;34:323–331. doi: 10.1016/j.ijpara.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Narasimhan J, Joyce BR, Naguleswaran A, Smith AT, Livingston MR, Dixon SE, Coppens I, Wek RC, Sullivan WJ., Jr Translation regulation by eukaryotic initiation factor-2 kinases in the development of latent cysts in Toxoplasma gondii. J Biol Chem. 2008;283:16591–16601. doi: 10.1074/jbc.M800681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomavo S, Boothroyd JC. Interconnection between organellar functions, development and drug resistance in the protozoan parasite, Toxoplasma gondii. Int J Parasitol. 1995;25:1293–1299. doi: 10.1016/0020-7519(95)00066-B. [DOI] [PubMed] [Google Scholar]
- 91.Bohne W, Heesemann J, Gross U. Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages. Infect Immun. 1993;61:1141–1145. doi: 10.1128/iai.61.3.1141-1145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hartmann A, Arroyo-Olarte RD, Imkeller K, Hegemann P, Lucius R, Gupta N. Optogenetic modulation of an adenylate cyclase in Toxoplasma gondii demonstrates a requirement of the parasite cAMP for host-cell invasion and stage differentiation. J Biol Chem. 2013;288:13705–13717. doi: 10.1074/jbc.M113.465583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sugi T, Ma YF, Tomita T, Murakoshi F, Eaton MS, Yakubu R, Han B, Tu V, Kato K, Kawazu S, Gupta N, Suvorova ES, White MW, Kim K, Weiss LM. Toxoplasma gondii cyclic AMP-dependent protein kinase subunit 3 is involved in the switch from tachyzoite to bradyzoite development. mBio. 2016;7:e00755–e00816. doi: 10.1128/mBio.00755-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luder CGK, Rahman T. Impact of the host on Toxoplasma stage differentiation. Microb Cell. 2017;4:203–211. doi: 10.15698/mic2017.07.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Radke JR, Guerini MN, Jerome M, White MW. A change in the premitotic period of the cell cycle is associated with bradyzoite differentiation in Toxoplasma gondii. Mol Biochem Parasitol. 2003;131:119–127. doi: 10.1016/S0166-6851(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 96.Ihara F, Nishikawa Y. Starvation of low-density lipoprotein-derived cholesterol induces bradyzoite conversion in Toxoplasma gondii. Parasit Vectors. 2014;7:248. doi: 10.1186/1756-3305-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weilhammer DR, Iavarone AT, Villegas EN, Brooks GA, Sinai AP, Sha WC. Host metabolism regulates growth and differentiation of Toxoplasma gondii. Int J Parasitol. 2012;42:947–959. doi: 10.1016/j.ijpara.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Radke JR, Donald RG, Eibs A, Jerome ME, Behnke MS, Liberator P, White MW. Changes in the expression of human cell division autoantigen-1 influence Toxoplasma gondii growth and development. PLoS Pathog. 2006;2:e105. doi: 10.1371/journal.ppat.0020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gurnett AM, Liberator PA, Dulski PM, Salowe SP, Donald RG, Anderson JW, Wiltsie J, Diaz CA, Harris G, Chang B, Darkin-Rattray SJ, Nare B, Crumley T, Blum PS, Misura AS, Tamas T, Sardana MK, Yuan J, Biftu T, Schmatz DM. Purification and molecular characterization of cGMP-dependent protein kinase from Apicomplexan parasites. A novel chemotherapeutic target. J Biol Chem. 2002;277:15913–15922. doi: 10.1074/jbc.M108393200. [DOI] [PubMed] [Google Scholar]
- 100.Donald RG, Zhong T, Wiersma H, Nare B, Yao D, Lee A, Allocco J, Liberator PA. Anticoccidial kinase inhibitors: identification of protein kinase targets secondary to cGMP-dependent protein kinase. Mol Biochem Parasitol. 2006;149:86–98. doi: 10.1016/j.molbiopara.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 101.McHugh TD, Gbewonyo A, Johnson JD, Holliman RE, Butcher PD. Development of an in vitro model of Toxoplasma gondii cyst formation. FEMS Microbiol Lett. 1993;114:325–332. doi: 10.1111/j.1574-6968.1993.tb06593.x. [DOI] [PubMed] [Google Scholar]
- 102.da Silva Ferreira, Mda F, Barbosa HS, Gross U, Luder CG. Stress-related and spontaneous stage differentiation of Toxoplasma gondii. Mol BioSyst. 2008;4:824–834. doi: 10.1039/b800520f. [DOI] [PubMed] [Google Scholar]
- 103.Swierzy IJ, Handel U, Kaever A, Jarek M, Scharfe M, Schluter D, Luder CGK. Divergent co-transcriptomes of different host cells infected with Toxoplasma gondii reveal cell type-specific host-parasite interactions. Sci Rep. 2017;7:7229. doi: 10.1038/s41598-017-07838-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swierzy IJ, Luder CG. Withdrawal of skeletal muscle cells from cell cycle progression triggers differentiation of Toxoplasma gondii towards the bradyzoite stage. Cell Microbiol. 2015;17:2–17. doi: 10.1111/cmi.12342. [DOI] [PubMed] [Google Scholar]
- 105.Gross U, Bohne W, Soete M, Dubremetz JF. Developmental differentiation between tachyzoites and bradyzoites of Toxoplasma gondii. Parasitol Today. 1996;12:30–33. doi: 10.1016/0169-4758(96)80642-9. [DOI] [PubMed] [Google Scholar]
- 106.Dupont CD, Christian DA, Hunter CA. Immune response and immunopathology during toxoplasmosis. Semin Immunopathol. 2012;34:793–813. doi: 10.1007/s00281-012-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma. TNF-alpha, and inducible nitric oxide synthase. J Immunol. 2000;164:2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 109.Johnson LL, Sayles PC. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infect Immun. 2002;70:185–191. doi: 10.1128/IAI.70.1.185-191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Denkers EY, Sher A. Role of natural killer and NK1+ T-cells in regulating cell-mediated immunity during Toxoplasma gondii infection. Biochem Soc Trans. 1997;25:699–703. doi: 10.1042/bst0250699. [DOI] [PubMed] [Google Scholar]
- 111.Lutjen S, Soltek S, Virna S, Deckert M, Schluter D. Organ- and disease-stage-specific regulation of Toxoplasma gondii-specific CD8-T-cell responses by CD4 T cells. Infect Immun. 2006;74:5790–5801. doi: 10.1128/IAI.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Combe CL, Curiel TJ, Moretto MM, Khan IA. NK cells help to induce CD8(+)-T-cell immunity against Toxoplasma gondii in the absence of CD4(+) T cells. Infect Immun. 2005;73:4913–4921. doi: 10.1128/IAI.73.8.4913-4921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tait ED, Jordan KA, Dupont CD, Harris TH, Gregg B, Wilson EH, Pepper M, Dzierszinski F, Roos DS, Hunter CA. Virulence of Toxoplasma gondii is associated with distinct dendritic cell responses and reduced numbers of activated CD8+ T cells. J Immunol. 2010;185:1502–1512. doi: 10.4049/jimmunol.0903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamamoto M, Ma JS, Mueller C, Kamiyama N, Saiga H, Kubo E, Kimura T, Okamoto T, Okuyama M, Kayama H, Nagamune K, Takashima S, Matsuura Y, Soldati-Favre D, Takeda K. ATF6beta is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med. 2011;208:1533–1546. doi: 10.1084/jem.20101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 116.Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 117.Suzuki Y, Claflin J, Wang X, Lengi A, Kikuchi T. Microglia and macrophages as innate producers of interferon-gamma in the brain following infection with Toxoplasma gondii. Int J Parasitol. 2005;35:83–90. doi: 10.1016/j.ijpara.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 118.Kang H, Suzuki Y. Requirement of non-T cells that produce gamma interferon for prevention of reactivation of Toxoplasma gondii infection in the brain. Infect Immun. 2001;69:2920–2927. doi: 10.1128/IAI.69.5.2920-2927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X, Suzuki Y. Microglia produce IFN-gamma independently from T cells during acute toxoplasmosis in the brain. J Interf Cytokine Res. 2007;27:599–605. doi: 10.1089/jir.2006.0157. [DOI] [PubMed] [Google Scholar]
- 120.Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, Gazzinelli RT, Sher A. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 121.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 122.Langermans JA, van der Hulst ME, Nibbering PH, van Furth R. Endogenous tumor necrosis factor alpha is required for enhanced antimicrobial activity against Toxoplasma gondii and Listeria monocytogenes in recombinant gamma interferon-treated mice. Infect Immun. 1992;60:5107–5112. doi: 10.1128/iai.60.12.5107-5112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chao CC, Anderson WR, Hu S, Gekker G, Martella A, Peterson PK. Activated microglia inhibit multiplication of Toxoplasma gondii via a nitric oxide mechanism. Clin Immunol Immunopathol. 1993;67:178–183. doi: 10.1006/clin.1993.1062. [DOI] [PubMed] [Google Scholar]
- 124.Peterson PK, Gekker G, Hu S, Chao CC. Human astrocytes inhibit intracellular multiplication of Toxoplasma gondii by a nitric oxide-mediated mechanism. J Infect Dis. 1995;171:516–518. doi: 10.1093/infdis/171.2.516. [DOI] [PubMed] [Google Scholar]
- 125.Adams LB, Hibbs JB, Jr, Taintor RR, Krahenbuhl JL. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from l-arginine. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- 126.Luder CG, Algner M, Lang C, Bleicher N, Gross U. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int J Parasitol. 2003;33:833–844. doi: 10.1016/S0020-7519(03)00092-4. [DOI] [PubMed] [Google Scholar]
- 127.Ibrahim HM, Bannai H, Xuan X, Nishikawa Y. Toxoplasma gondii cyclophilin 18-mediated production of nitric oxide induces Bradyzoite conversion in a CCR5-dependent manner. Infect Immun. 2009;77:3686–3695. doi: 10.1128/IAI.00361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hayashi S, Chan CC, Gazzinelli R, Roberge FG. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996;156:1476–1481. [PubMed] [Google Scholar]
- 129.Daubener W, Spors B, Hucke C, Adam R, Stins M, Kim KS, Schroten H. Restriction of Toxoplasma gondii growth in human brain microvascular endothelial cells by activation of indoleamine 2,3-dioxygenase. Infect Immun. 2001;69:6527–6531. doi: 10.1128/IAI.69.10.6527-6531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gupta SL, Carlin JM, Pyati P, Dai W, Pfefferkorn ER, Murphy MJ., Jr Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect Immun. 1994;62:2277–2284. doi: 10.1128/iai.62.6.2277-2284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daubener W, Gutsche M, Nockemann S, MacKenzie C, Seghrouchni S, Hadding U. Protamine enhances the activity of human recombinant interferon-gamma. J Interf Cytokine Res. 1996;16:531–536. doi: 10.1089/jir.1996.16.531. [DOI] [PubMed] [Google Scholar]
- 132.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murray HW, Szuro-Sudol A, Wellner D, Oca MJ, Granger AM, Libby DM, Rothermel CD, Rubin BY. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989;57:845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Divanovic S, Sawtell NM, Trompette A, Warning JI, Dias A, Cooper AM, Yap GS, Arditi M, Shimada K, Duhadaway JB, Prendergast GC, Basaraba RJ, Mellor AL, Munn DH, Aliberti J, Karp CL. Opposing biological functions of tryptophan catabolizing enzymes during intracellular infection. J Infect Dis. 2012;205:152–161. doi: 10.1093/infdis/jir621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sibley LD, Messina M, Niesman IR. Stable DNA transformation in the obligate intracellular parasite Toxoplasma gondii by complementation of tryptophan auxotrophy. Proc Natl Acad Sci USA. 1994;91:5508–5512. doi: 10.1073/pnas.91.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schmitz JL, Carlin JM, Borden EC, Byrne GI. Beta interferon inhibits Toxoplasma gondii growth in human monocyte-derived macrophages. Infect Immun. 1989;57:3254–3256. doi: 10.1128/iai.57.10.3254-3256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Knubel CP, Martinez FF, Fretes RE, Diaz Lujan C, Theumer MG, Cervi L, Motran CC. Indoleamine 2,3-dioxigenase (IDO) is critical for host resistance against Trypanosoma cruzi. FASEB J. 2010;24:2689–2701. doi: 10.1096/fj.09-150920. [DOI] [PubMed] [Google Scholar]
- 138.Tetsutani K, To H, Torii M, Hisaeda H, Himeno K. Malaria parasite induces tryptophan-related immune suppression in mice. Parasitology. 2007;134:923–930. doi: 10.1017/S0031182007002326. [DOI] [PubMed] [Google Scholar]
- 139.Yap GS, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]
- 140.Schluter D, Kwok LY, Lutjen S, Soltek S, Hoffmann S, Korner H, Deckert M. Both lymphotoxin-alpha and TNF are crucial for control of Toxoplasma gondii in the central nervous system. J Immunol. 2003;170:6172–6182. doi: 10.4049/jimmunol.170.12.6172. [DOI] [PubMed] [Google Scholar]
- 141.Weiss LM, Laplace D, Takvorian PM, Tanowitz HB, Cali A, Wittner M. A cell culture system for study of the development of Toxoplasma gondii bradyzoites. J Eukaryot Microbiol. 1995;42:150–157. doi: 10.1111/j.1550-7408.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 142.Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Streilein JW. Ocular immune privilege in the immunosuppressive intraocular microenvironment. Ocular Immunol Inflamm. 1995;3:139–144. doi: 10.3109/09273949509085042. [DOI] [PubMed] [Google Scholar]
- 145.Wiendl H, Hohlfeld R, Kieseier BC. Immunobiology of muscle: advances in understanding an immunological microenvironment. Trends Immunol. 2005;26:373–380. doi: 10.1016/j.it.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 146.Jones LA, Alexander J, Roberts CW. Ocular toxoplasmosis: in the storm of the eye. Parasite Immunol. 2006;28:635–642. doi: 10.1111/j.1365-3024.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 147.Mahamed DA, Mills JH, Egan CE, Denkers EY, Bynoe MS. CD73-generated adenosine facilitates Toxoplasma gondii differentiation to long-lived tissue cysts in the central nervous system. Proc Natl Acad Sci USA. 2012;109:16312–16317. doi: 10.1073/pnas.1205589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Israelski DM, Remington JS. Toxoplasmic encephalitis in patients with AIDS. Infect Dis Clin N Am. 1988;2:429–445. [PubMed] [Google Scholar]
- 149.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 150.Suzuki Y, Remington JS. A method for obtaining large numbers of trophozoites of avirulent strains of Toxoplasma gondii using an antibody to interferon-gamma. J Parasitol. 1989;75:174–176. doi: 10.2307/3282964. [DOI] [PubMed] [Google Scholar]
- 151.Suzuki Y, Joh K. Effect of the strain of Toxoplasma gondii on the development of toxoplasmic encephalitis in mice treated with antibody to interferon-gamma. Parasitol Res. 1994;80:125–130. doi: 10.1007/BF00933779. [DOI] [PubMed] [Google Scholar]
- 152.Dunay IR, Chan WC, Haynes RK, Sibley LD. Artemisone and artemiside control acute and reactivated toxoplasmosis in a murine model. Antimicrob Agents Chemother. 2009;53:4450–4456. doi: 10.1128/AAC.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jones TC, Bienz KA, Erb P. In vitro cultivation of Toxoplasma gondii cysts in astrocytes in the presence of gamma interferon. Infect Immun. 1986;51:147–156. doi: 10.1128/iai.51.1.147-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST0340007. [DOI] [PubMed] [Google Scholar]
- 155.Holmes MJ, Augusto LDS, Zhang M, Wek RC, Sullivan WJ., Jr Translational control in the latency of apicomplexan parasites. Trends Parasitol. 2017;33:947–960. doi: 10.1016/j.pt.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sullivan WJ, Jr, Narasimhan J, Bhatti MM, Wek RC. Parasite-specific eIF2 (eukaryotic initiation factor-2) kinase required for stress-induced translation control. Biochem J. 2004;380:523–531. doi: 10.1042/bj20040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 158.Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 159.Konrad C, Queener SF, Wek RC, Sullivan WJ, Jr. (2013) Inhibitors of eIF2alpha dephosphorylation slow replication and stabilize latency in Toxoplasma gondii. Antimicrob Agents Chemother [DOI] [PMC free article] [PubMed]
- 160.Joyce BR, Tampaki Z, Kim K, Wek RC, Sullivan WJ., Jr The unfolded protein response in the protozoan parasite Toxoplasma gondii features translational and transcriptional control. Eukaryot Cell. 2013;12:979–989. doi: 10.1128/EC.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Painter HJ, Campbell TL, Llinas M. The apicomplexan AP2 family: integral factors regulating Plasmodium development. Mol Biochem Parasitol. 2011;176:1–7. doi: 10.1016/j.molbiopara.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cleary MD, Singh U, Blader IJ, Brewer JL, Boothroyd JC. Toxoplasma gondii asexual development: identification of developmentally regulated genes and distinct patterns of gene expression. Eukaryot Cell. 2002;1:329–340. doi: 10.1128/EC.1.3.329-340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Radke JR, Behnke MS, Mackey AJ, Radke JB, Roos DS, White MW. The transcriptome of Toxoplasma gondii. BMC Biol. 2005;3:26. doi: 10.1186/1741-7007-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sullivan WJ, Jr, Hakimi MA. Histone mediated gene activation in Toxoplasma gondii. Mol Biochem Parasitol. 2006;148:109–116. doi: 10.1016/j.molbiopara.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 165.Meissner M, Soldati D. The transcription machinery and the molecular toolbox to control gene expression in Toxoplasma gondii and other protozoan parasites. Microb Infect Inst Pasteur. 2005;7:1376–1384. doi: 10.1016/j.micinf.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 166.Olguin-Lamas A, Madec E, Hovasse A, Werkmeister E, Callebaut I, Slomianny C, Delhaye S, Mouveaux T, Schaeffer-Reiss C, Van Dorsselaer A, Tomavo S. A novel Toxoplasma gondii nuclear factor TgNF3 is a dynamic chromatin-associated component, modulator of nucleolar architecture and parasite virulence. PLoS Pathog. 2011;7:e1001328. doi: 10.1371/journal.ppat.1001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.White MW, Radke JR, Radke JB. Toxoplasma development—turn the switch on or off? Cell Microbiol. 2014;16:466–472. doi: 10.1111/cmi.12267. [DOI] [PubMed] [Google Scholar]
- 168.Behnke MS, Wootton JC, Lehmann MM, Radke JB, Lucas O, Nawas J, Sibley LD, White MW. Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PLoS One. 2010;5:e12354. doi: 10.1371/journal.pone.0012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Radke JR, Striepen B, Guerini MN, Jerome ME, Roos DS, White MW. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol Biochem Parasitol. 2001;115:165–175. doi: 10.1016/S0166-6851(01)00284-5. [DOI] [PubMed] [Google Scholar]
- 170.Croken MM, Ma Y, Markillie LM, Taylor RC, Orr G, Weiss LM, Kim K. Distinct strains of Toxoplasma gondii feature divergent transcriptomes regardless of developmental stage. PLoS One. 2014;9:e111297. doi: 10.1371/journal.pone.0111297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Croken MM, Qiu W, White MW, Kim K. Gene set enrichment analysis (GSEA) of Toxoplasma gondii expression datasets links cell cycle progression and the bradyzoite developmental program. BMC Genom. 2014;15:515. doi: 10.1186/1471-2164-15-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Saksouk N, Bhatti MM, Kieffer S, Smith AT, Musset K, Garin J, Sullivan WJ, Jr, Cesbron-Delauw MF, Hakimi MA. Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol Cell Biol. 2005;25:10301–10314. doi: 10.1128/MCB.25.23.10301-10314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Naguleswaran A, Elias EV, McClintick J, Edenberg HJ, Sullivan WJ., Jr Toxoplasma gondii lysine acetyltransferase GCN5-A functions in the cellular response to alkaline stress and expression of cyst genes. PLoS Pathog. 2010;6:e1001232. doi: 10.1371/journal.ppat.1001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bougdour A, Maubon D, Baldacci P, Ortet P, Bastien O, Bouillon A, Barale JC, Pelloux H, Menard R, Hakimi MA. Drug inhibition of HDAC3 and epigenetic control of differentiation in Apicomplexa parasites. J Exp Med. 2009;206:953–966. doi: 10.1084/jem.20082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sautel CF, Cannella D, Bastien O, Kieffer S, Aldebert D, Garin J, Tardieux I, Belrhali H, Hakimi MA. SET8-mediated methylations of histone H4 lysine 20 mark silent heterochromatic domains in apicomplexan genomes. Mol Cell Biol. 2007;27:5711–5724. doi: 10.1128/MCB.00482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dixon SE, Stilger KL, Elias EV, Naguleswaran A, Sullivan WJ., Jr A decade of epigenetic research in Toxoplasma gondii. Mol Biochem Parasitol. 2010;173:1–9. doi: 10.1016/j.molbiopara.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bougdour A, Braun L, Cannella D, Hakimi MA. Chromatin modifications: implications in the regulation of gene expression in Toxoplasma gondii. Cell Microbiol. 2010;12:413–423. doi: 10.1111/j.1462-5822.2010.01446.x. [DOI] [PubMed] [Google Scholar]
- 178.Gissot M, Kelly KA, Ajioka JW, Greally JM, Kim K. Epigenomic modifications predict active promoters and gene structure in Toxoplasma gondii. PLoS Pathog. 2007;3:e77. doi: 10.1371/journal.ppat.0030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sullivan WJ, Jr, Smith AT, Joyce BR. Understanding mechanisms and the role of differentiation in pathogenesis of Toxoplasma gondii: a review. Mem Inst Oswaldo Cruz. 2009;104:155–161. doi: 10.1590/S0074-02762009000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bhatti MM, Livingston M, Mullapudi N, Sullivan WJ., Jr Pair of unusual GCN5 histone acetyltransferases and ADA2 homologues in the protozoan parasite Toxoplasma gondii. Eukaryot Cell. 2006;5:62–76. doi: 10.1128/EC.5.1.62-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Boyle JP, Rajasekar B, Saeij JP, Ajioka JW, Berriman M, Paulsen I, Roos DS, Sibley LD, White MW, Boothroyd JC. Just one cross appears capable of dramatically altering the population biology of a eukaryotic pathogen like Toxoplasma gondii. Proc Natl Acad Sci USA. 2006;103:10514–10519. doi: 10.1073/pnas.0510319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jeffers V, Sullivan WJ., Jr Lysine acetylation is widespread on proteins of diverse function and localization in the protozoan parasite Toxoplasma gondii. Eukaryot Cell. 2012;11:735–742. doi: 10.1128/EC.00088-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xue B, Jeffers V, Sullivan WJ, Uversky VN. Protein intrinsic disorder in the acetylome of intracellular and extracellular Toxoplasma gondii. Mol BioSyst. 2013;9:645–657. doi: 10.1039/c3mb25517d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sullivan WJ, Jr, Monroy MA, Bohne W, Nallani KC, Chrivia J, Yaciuk P, Smith CK, 2nd, Queener SF. Molecular cloning and characterization of an SRCAP chromatin remodeling homologue in Toxoplasma gondii. Parasitol Res. 2003;90:1–8. doi: 10.1007/s00436-002-0814-1. [DOI] [PubMed] [Google Scholar]
- 185.Rooney PJ, Neal LM, Knoll LJ. Involvement of a Toxoplasma gondii chromatin remodeling complex ortholog in developmental regulation. PLoS One. 2011;6:e19570. doi: 10.1371/journal.pone.0019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dalmasso MC, Onyango DO, Naguleswaran A, Sullivan WJ, Jr, Angel SO. Toxoplasma H2A variants reveal novel insights into nucleosome composition and functions for this histone family. J Mol Biol. 2009;392:33–47. doi: 10.1016/j.jmb.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nardelli SC, Che FY, Silmon de Monerri NC, Xiao H, Nieves E, Madrid-Aliste C, Angel SO, Sullivan WJ, Jr., Angeletti RH, Kim K, Weiss LM (2013) The histone code of Toxoplasma gondii comprises conserved and unique posttranslational modifications. mBio 4:e00922–e01013 [DOI] [PMC free article] [PubMed]
- 188.Lekutis C, Ferguson DJ, Grigg ME, Camps M, Boothroyd JC. Surface antigens of Toxoplasma gondii: variations on a theme. Int J Parasitol. 2001;31:1285–1292. doi: 10.1016/S0020-7519(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 189.Lekutis C, Ferguson DJ, Boothroyd JC. Toxoplasma gondii: identification of a developmentally regulated family of genes related to SAG2. Exp Parasitol. 2000;96:89–96. doi: 10.1006/expr.2000.4556. [DOI] [PubMed] [Google Scholar]
- 190.Saeij JP, Arrizabalaga G, Boothroyd JC. A cluster of four surface antigen genes specifically expressed in bradyzoites, SAG2CDXY, plays an important role in Toxoplasma gondii persistence. Infect Immun. 2008;76:2402–2410. doi: 10.1128/IAI.01494-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schwarz JA, Fouts AE, Cummings CA, Ferguson DJ, Boothroyd JC. A novel rhoptry protein in Toxoplasma gondii bradyzoites and merozoites. Mol Biochem Parasitol. 2005;144:159–166. doi: 10.1016/j.molbiopara.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 192.Lecordier L, Moleon-Borodowsky I, Dubremetz JF, Tourvieille B, Mercier C, Deslee D, Capron A, Cesbron-Delauw MF. Characterization of a dense granule antigen of Toxoplasma gondii (GRA6) associated to the network of the parasitophorous vacuole. Mol Biochem Parasitol. 1995;70:85–94. doi: 10.1016/0166-6851(95)00010-X. [DOI] [PubMed] [Google Scholar]
- 193.Patil V, Lescault PJ, Lirussi D, Thompson AB, Matrajt M. Disruption of the expression of a non-coding RNA significantly impairs cellular differentiation in Toxoplasma gondii. Int J Mol Sci. 2012;14:611–624. doi: 10.3390/ijms14010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Milligan-Myhre KC, Rooney PJ, Knoll LJ. Examination of a virulence mutant uncovers the ribosome biogenesis regulatory protein of Toxoplasma gondii. J Parasitol. 2011;97:1173–1177. doi: 10.1645/GE-2741.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Joyce BR, Queener SF, Wek RC, Sullivan WJ., Jr Phosphorylation of eukaryotic initiation factor-2{alpha} promotes the extracellular survival of obligate intracellular parasite Toxoplasma gondii. Proc Natl Acad Sci USA. 2010;107:17200–17205. doi: 10.1073/pnas.1007610107. [DOI] [PMC free article] [PubMed] [Google Scholar]