Abstract
Immune tolerance is a vital component of immunity, as persistent activation of immune cells causes significant tissue damage and loss of tolerance leads to autoimmunity. Likewise, unwanted immune responses can occur in inherited disorders, such as hemophilia and Pompe disease, in which patients lack any expression of protein, during treatment with enzyme replacement therapy, or gene therapy. While the liver has long been known as being tolerogenic, it was only recently appreciated in the last decade that liver directed adeno-associated virus (AAV) gene therapy can induce systemic tolerance to a transgene. In this review, we look at the mechanisms behind liver induced tolerance, discuss different factors influencing successful tolerance induction with AAV, and applications where AAV mediated tolerance may be helpful.
Introduction
The basis of gene therapy is the delivery of a functional copy of the disease causing gene that is missing or deficient in patients. Viruses are commonly used in the field of gene therapy for many reasons: 1) abundance, 2) easily manipulated, and 3) they have naturally evolved to deliver their genetic payload to target cells or tissues. Within the field of gene therapy, adenovirus, adeno-associated virus, retrovirus, and lentivirus have enjoyed the most success. The choice of viral vector system is based on several factors: widespread versus localized transduction, long-term vs short-term transgene expression, packaging capacity, and immunogenicity. Of all the viral based vector systems used for gene therapy, adeno-associated virus (AAV) has become one of the most commonly used today.
AAV is a parvovirus that contains a single stranded DNA genome of ~4.7kb. AAV is unable to replicate without a helper virus and is non-pathogenic in hosts, including humans. Recombinant AAV (rAAV) retains the inverted terminal repeats (ITRs) of the wild-type genome allowing rAAV to have a packaging capacity of up to 5kb. rAAV vectors have several important properties that make them well suited for gene therapy. They can infect non-dividing cells, vector genomes are minimally integrative and are maintained episomally, come in a wide array of serotypes with a specific tropism for a certain tissue, and importantly, have low immunogenicity [1–3]. Nevertheless, there remains a risk for immune responses. For example, when treating an inherited disorder in which there is no protein expression, the therapeutic protein can be seen as non-self and may trigger a T and B cell mediated immune response [4].
It is widely accepted that AAV liver directed gene therapy can harness the tolerogenic nature of the liver and induce systemic immunological tolerance to transgene products [5–8]. Tolerance is defined as the failure of the body to mount an immune response to an antigen whether it be to ‘self’ or a foreign protein. Regulatory T cells (Tregs) are known to play a crucial role in the induction and maintenance of tolerance. Tregs suppress immune responses in the periphery through a number of mechanisms including direct and indirect suppression of antigen presenting cells, B lymphocytes, and T effector cells (Fig. 1) [9–14]. By leveraging this unique ability to induce immune tolerance to transgene products, it is possible to develop lasting treatments for a multitude of diseases (for a more complete listing of diseases which have been treated using gene therapy see the article by Roncarolo, et al. within this issue).
Figure 1. Mechanisms involved in the induction of tolerance via AAV directed gene therapy.
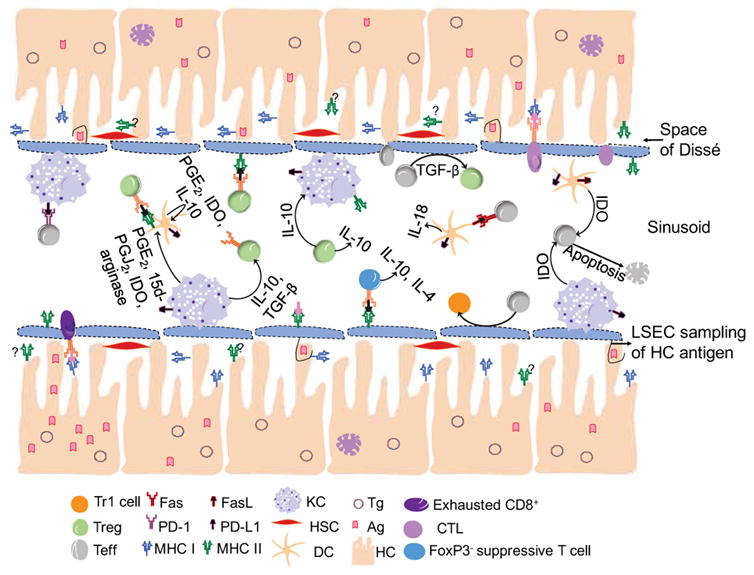
The induction of tolerance within the liver relies on the integrity of the tolerogenic environment of the liver. The maintenance of this tolerogenic environment, as well as the induction of systemic tolerance, is the result of a cellular orchestration within the liver. Tg=Transgene, Ag=Antigen, KC=Kupffer cell, HSC=Hepatic Stellate Cell, DC=Dendritic Cell, HC=Hepatocyte
Multiple tissues have been investigated for inducing transgene tolerance including hematopoietic stem cells, thymus, muscle, and liver (for a more complete review see [15]). This review will primarily focus on AAV gene transfer to the liver, the current understanding of specific cellular interactions between resident liver and immune cells, and mechanisms of tolerance induction. We will highlight key factors to consider for successful and durable tolerance induction and provide an overview of pre-clinical data supporting AAV mediated tolerance induction in several different disease models, as well as discuss potential limitations for translation into humans. Finally, we will discuss a novel application of AAV gene therapy, using transgene tolerance induction to treat an autoimmune disease.
Immune tolerance and Tregs
A deleterious immune response to an AAV delivered transgene is a potential complication associated with long-term correction of disease. This response becomes more prominent in cases where the therapeutic gene being delivered is completely absent, not just mutated [4]. In this case, the transgene product could be recognized as non-self and trigger the activation of both humoral and cell-mediated immune responses which rely heavily on the activation of CD4+ T cells for optimal effectiveness. In the case of humoral responses, CD4+ T cells provide help for the maturation of B lymphocytes and production of high affinity antibodies specific for the transgene product [14]. In the case of cell-mediated responses, CD4+ T cells are not directly required for the activation of CD8+ responses, although, the speed and strength of the CD8+ T cells activation, as well as the formation of a CD8+ memory T cell response, is in fact dependent on a CD4+ T cell help.
One approach for mitigating such adverse responses is through the induction of tolerance driven by transgene specific Tregs. Tregs are a unique subset of CD4+ T cells expressing Forkhead box P3 (FoxP3) that help maintain immune homeostasis, and for this review will be defined as CD4+CD25+FoxP3+ T cells. In addition to preventing excessive inflammatory damage to tissues, Tregs suppress self-reactive T cells that have escaped thymic selection and are considered one of the most important regulators of peripheral tolerance. Tregs are capable of suppressing other cell types in both a contact–dependent and –independent manner, through the ligation of cell surface antigens such as: cytotoxic T lymphocyte antigen 4 (CTLA-4), lymphocyte activation gene 3 (LAG-3), CD18, Neuropilin-1 (Nrp-1), lymphocyte function-associated antigen 1 (LFA-1), and CD39, or by releasing anti-inflammatory cytokines IL-10 and TGF-β, as well as perforins and granzymes [16]. As such, Tregs have the capacity to prevent immune responses in both an antigen-specific and non-specific fashion [17].
Tregs are divided into two broad categories: 1) natural Tregs (nTregs) which are developed in the thymus and 2) inducible Tregs (iTregs) which are developed from peripheral CD4+ T effector cells. While functionally similar, iTregs are considered to be more plastic showing transient FoxP3+ expression, while nTregs have stable FoxP3+ expression [18]. Phenotypic differentiation of nTregs from iTregs can be difficult. Both Helios and Neuropilin-1 expression have been suggested as markers of nTregs [19, 20], although others have argued that they are not suitable for distinguishing the two populations [21–23].
Tregs are not the only regulatory cells found within the body. Type1 regulatory (Tr1) cells are suppressive T cells that are also found within the liver. Tr1 cells are characterized as being CD3+CD4+CD25−FoxP3−LAG3+CD49b+ as well as producing IL-10 and TGF-β [24–26]. Though no conclusive link has yet been established, the fact that Tr1 cells are found within the liver and produce two cytokines common in the tolerogenic liver suggests that these cells play a role in the induction of tolerance following AAV hepatic gene transfer [27]. While there are other immune regulatory cells such as Th3 cells, CD8+Tregs, and regulatory B cells, their roles in tolerance following AAV hepatic gene transfer are outside the scope of this review.
AAV Induced Liver Tolerance
There is substantial evidence demonstrating that AAV hepatic gene therapy induces antigen specific Tregs that modulate immune tolerance [6, 8, 14, 28–31]. To date, most studies supporting liver induced tolerance have utilized mouse models, especially when focusing on the mechanisms behind the induction of tolerance. Unfortunately, the genetic variances between strains of mice make understanding the process more challenging. For example, C57BL/6 mice suppress cytotoxic lymphocyte (CTL) responses to systemic AAV delivery, however the same is not true for BALB/c mice which show impaired tolerance induction and are susceptible to the clearance of transduced hepatocytes through a CTL-mediated mechanism [32]. Studies conducted in dogs and non-human primates (NHP) show that AAV mediated liver induced tolerance is more complex and is dependent not only on the transgene, but on whether a species-specific transgene is used [33–38]. While such results are encouraging, it is currently unknown if this is true in humans.
The liver has long been known as the organ responsible for detoxifying the body of foreign antigens as well as metabolizing many drugs and nutrients. Roughly 30% of the body’s total blood volume passes through the liver each minute [39]. The liver’s anatomical location results in roughly 80% of the blood being delivered directly from the intestines via the portal vein and 20% coming from arteries [40]. Blood traveling from the intestines inundates the liver with nutrients, bacterial products from the gut, gram-negative bacteria (and lipopolysaccharides (LPS) found on their cell walls), and cellular debris. Yet, these potentially immunogenic antigens fail to elicit immune responses in the liver [41, 42] as the liver has developed a means to locally regulate adaptive immunity.
The ability of the liver to induce tolerance was first recognized in 1969 by Calne et al. where they demonstrated that porcine liver allo-transplantation was capable of preventing rejection of a secondary transplanted organ from the same donor [43]. This concept has been extended to show that reduced organ rejection is seen when cells from the organ donor are injected into the recipients liver prior to transplantation [44]. Given this unique characteristic of the liver, it is an ideal target for tolerance induction via AAV. Studies in animal models show that tolerance is dependent on classical antigen presenting cells (APCs) within the liver [45] and that restricted expression to hepatocytes enhances tolerance [5]. However, the precise mechanisms of tolerance induction via liver directed AAV gene therapy is still unclear.
The tolerogenic effects produced by the liver are controlled, in a large part, by resident APCs. Liver APCs consist of both non-conventional and conventional types. In contrast to conventional APCs, like dendritic cells (DCs), the non-conventional hepatic APCs consist of Kupffer cells (KCs), liver sinusoidal endothelial cells (LSEC), hepatocytes, and hepatic stellate cells that express low-level of major histocompatibility complex (MHC)-I/II and co-stimulatory molecules during steady state [42, 46]. Although the composition of T lymphocytes found within the liver is much different compared to lymph nodes, spleen, and peripheral blood, they also play a role in the induction and maintenance of tolerance.
Kupffer Cells
KCs are the largest population of resident macrophages representing between 80%–90% of all macrophages found in the body [41, 47] and ~35% of the non-parenchymal cells within the liver. KCs are responsible for the phagocytosis of particulate material, elimination of cellular debris, and clearance of pathogens from the liver. However, the life span of these cells remains unclear. Studies on this topic are conflicting with some reporting these cells are replaced as often as every 1–2 weeks [47], while others show KCs to be long-lived lasting 3 months to 1 year [48].
Unlike other macrophages in the body, KCs do not actively patrol the parenchyma scavenging for pathogens. Instead, KCs remain stationary, attached to the luminal surface of LSECs and act as an immune sentinel as they are exposed to the circulating blood. Their function is to detect, bind, and phagocytose pathogens and other foreign materials, as well as release cytokines and chemokines, thereby alerting the rest of the immune system to the presence of harmful materials [39, 41, 47]. At steady state, KCs are known to exist in an anti-inflammatory, M2 activation state. This may be due, in part, to the fact that under physiological conditions, KCs are exposed to a constant supply of LPS in the range of 100 pg/mL to 1 ng/mL [49]. While it may seem counter intuitive, constant LPS exposure promotes KCs to produce high amounts of IL-10, TGF-β, and arachidonic acid metabolite prostaglandin E2 (PGE2), all of which promote an anti-inflammatory macrophage state [50–54].
In many cases, hepatic gene transfer results in the expression of a secreted transgene. KCs play an important role in the induction of tolerance to soluble antigens [55]. Recent studies have shown that KCs can drive expansion of CD4+CD25+FoxP3+Treg cells in the liver via IL-10 [27, 45, 56]. They also showed that KCs were capable of further expanding Tregs by converting antigen-specific Teff into Tregs [56]. The reports went on to show that this phenomenon was only seen when KCs possessed a tolerogenic signature, which is marked by program death ligand-1 (PD-L1) expression and IL-10 production. Whereas, the loss in IL-10 resulted in a failure to convert immunogenic Teff into Tregs [56]. Others have also shown that the secretion of IL-10 is crucial for tolerance induction to hepatocyte expressed antigens [29]. It was found that the administration of AAV to the liver resulted in the production of IL-10 by KCs in response to the transgene and not the capsid. Furthermore, the authors demonstrated that IL-10 production by KCs was critical for Tregs, and the maintenance of tolerance [27, 29]. These findings are paramount as most AAV tolerance induction protocols rely on hepatocytes being the cell transduced.
While KC derived IL-10 may play a major role in tolerance induction within the liver, this is not the only mechanism. At homeostasis, KCs express low amounts of both MHC I and MHC II [51]. This low MHC expression reduces the ability of the KCs to present antigen and provide signal 1 to the T cells. This incomplete activation results in either anergy or Treg induction [51]. Additionally, KCs are known to express high amounts of PGE2, 15-deoxyΔ12,14prostaglandin J2 (15d-PGJ2), indoleamine 2,3-dioxygenase (IDO), and arginase [57–59]. All of which are also capable of inhibiting the induction of antigen specific T cell activation by dendritic cells [29, 55, 60]. Lastly, KCs have been shown to express the immune suppressive protein, PD-L1, which once bound to its receptor PD-1, induces anergy or cell death in PD-1 positive Teffs, thus promoting the expansion of Tregs [56, 61].
Liver Sinusoidal Endothelial Cells
LSECs are the most prominent non-parenchymal cells of the liver, strategically positioned in the liver, lining the sinusoid creating a physical barrier between the space of Dissè and the intraluminal space [46, 62]. LSECs are the most efficient endocytic cells in the body and are responsible for the transportation of molecules from the bloodstream to hepatocyte surfaces via transcytosis [63–65]. Interestingly, LSECs have been shown to directly interact with hepatocytes, thereby acquiring hepatocyte-derived antigens and inducing Tregs [66]. This sampling of hepatocyte antigens by LSECs may prove to be an important and/or crucial step in the induction of tolerance via liver directed AAV gene therapy.
LSEC are one of the first cells within the liver to contact lymphocytes and play a role in lymphocyte recruitment. It has been shown that LSECs can present secreted antigens to CD4+ T cells in such a manner that results in a tolerogenic state and not immunity [67]. Recently, Merlin et al. used a lentivirus vector to deliver clotting factor VIII (FVIII) to LSEC which resulted in the induction of tolerance in a hemophilia A mouse model [68]. The tolerogenic nature of LSECs is mediated through a verity of mechanisms. Due to high local levels of IL-10, MHC and co-stimulatory molecules CD80/CD86 expression is maintained at very low levels which is inefficient for priming of T cells [69, 70]. LSECs are also capable of inducing cytokine production in T cells, but due to the lack of IL-12 priming, these CD4+ T cells turn in to IL-10/IL-4 producing cells [49], which were shown to have suppressive effects even though they lacked the classical Treg markers, FoxP3 and CD25 [71]. More recently, Xu et al. have shown that LSECs are responsible for inducing tolerance to auto-reactive CD4+ cells by converting them into FoxP3−LAG+CD49b− Tr1 cells [72]. This suggests that LSEC may be responsible for the induction of Tr1 like cells as opposed to Tregs. Others have shown that LSEC are in fact capable of inducing FoxP3+Tregs through the conversion of FoxP3− non-Tregs into FoxP3+Treg cells in a TGF-β dependent manner [66]. It was also found that the LSEC are in fact the most efficient cells for making this conversion when compared to DCs and KCs [66]. These findings show LSEC can induce two different regulatory cell subsets that both contribute to immunological tolerance, but further studies are needed to fully elucidate the conditions that result in each cell type, and the exact role LSECs play in the induction and maintenance of tolerance induced by liver directed AAV gene therapy.
Dendritic Cells
Under steady-state conditions, there are more DCs found within the mouse liver than any other parenchymal organ [73]. DCs in the liver are found predominately in the perivascular region, portal space, beneath the Glisson’s capsule, and scattered throughout the parenchyma [74]. Unlike other APCs in the liver, DCs are highly motile with migration rates from the liver being ~105 DCs per hour [75]. In the liver, DCs patrol the tissue and engulf antigens. However, DCs within the liver, similar to KCs, are not as efficient at presenting antigens when compared to DCs from other tissues [76–78].
In steady-state conditions, hepatic DCs have an immature (iDC) phenotype and are poor activators of Teff cells and tend to be more tolerogenic. This preference for tolerance over immunity by iDCs is due in part to their low expression of MHC II on their cell surfaces. Further, while iDCs do express the co-stimulatory molecules CD40 and CD80/86, they are at such low levels they are nearly impossible to detect. Hepatic DCs also secrete PGE2, an ant-inflammatory prostaglandin that results in the up-regulation of IDO in DCs as well as enhances their IL-10 production, resulting in an increased capacity to induce Treg [79, 80]. High levels of IL-10 and low levels of IL-12 result in an antigen-independent hypo-responsiveness that shifts Th1 cells to a Th2 phenotype, thereby reducing the likelihood of immunity and promoting development of Tregs [81, 82]. IL-10 is also responsible for the expansion of Tregs as the ligation of IL-10 to its receptor on a T cell overrides co-stimulatory signals and prevents the recruitment of phosphatidylinositol 3-kinase, which has been shown to be necessary for the formation of Teff cells [83, 84].
The second mechanism, through which hepatic DCs can induce or maintain tolerance, is through the direct deletion of Teff cells. In addition to a reduced expression of IL-12, tolerogenic DCs within the liver have an increased amount of IL-18 production [85]. This increased production of IL-18 results in the expression of Fas-L (CD95L, CD178) by hepatic dendritic cells. This expression of Fas-L in turn allows for the direct deletion of Teff cells through a Fas (CD95)/Fas-L manner, as Fas is constitutively expressed on Teff cells within the liver [85]. A second mechanism through which hepatic DCs induce Teff cell deletion is through a PD-L1/2/PD-1 mechanism. Within the liver, both PD-L1 and PD-L2 are expressed by DCs [86]. Simultaneously, Teff cells within the liver up-regulate PD-1 in response to constant stimulation. This allows for the deletion of PD-1 expressing Teff cells through the blockade of phosphoinositide 3-kinase (PI3K) [87]. Finally, hepatic DCs can delete Teff cells through the expression of IDO, which is secreted by predominately plasmacytoid DCs within the liver resulting in the degradation of tryptophan around Teff cells. This response is seen in conjunction with the production of toxic metabolites resulting in the apoptosis of Teff cells [88]. All of these factors show a mechanism through which DCs help promote an overall tolerogenic environment within the liver which is paramount to the success of tolerance induction via liver directed AAV gene therapy.
Hepatocytes
Hepatocytes are the most common cell type within the liver comprising ~80% of all cells. Their primary role includes metabolism, detoxification, synthesis and secretion of proteins. While it has been shown that hepatocyte restricted transgene expression enhances tolerance induction, it is unclear if hepatocytes directly or indirectly contribute to Treg induction. Hepatocytes are unique to parenchymal cells as they exhibit antigen-presenting capabilities [89, 90] and circulating T cells can directly interact with hepatocytes through the fenestrations of the LSEC [91]. Under steady state conditions, hepatocytes express low amounts of MHC-I and Intercellular Adhesion Molecule 1 (ICAM-1, CD54). However, given the large size of the hepatocytes and that MHC-I and ICAM-1 are concentrated to the perisinusoidal cell membrane, it is possible for hepatocytes to prime CD8+ T cells [91, 92].
Under steady state conditions, hepatocytes do not have detectable surface expression of MHC-II [93, 94], raising doubt in the long-held belief that the induction of tolerance following liver directed AAV gene therapy is dependent on the transduction of hepatocytes. However, MHC-II can be induced during inflammation, such as in hepatitis [89]. Even though lymphocytes can directly interact with hepatocytes through the fenestrae, it is unclear if hepatocytes directly present antigen to CD4+ T cells. Burghardt et al. found that following expression of an antigen by a hepatocyte, FoxP3+ Tregs were induced in a Notch-dependent pathway [95]. In work by Lüth et al., they suggest a different mechanism where induction of antigen specific Tregs was dependent on the expression of the antigen in the liver and occurred by TGF-β–dependent peripheral conversion from conventional non-Tregs [96]. While the two groups suggest different mechanisms for the induction of Tregs, there is no reason that the two are mutually exclusive. In fact, Burghardt et al. found that TGF-β significantly increased the amount of Tregs produced in their system while Lüth et al. did not look at Notch signaling. Both mechanisms may play a role in the induction of Tregs with one possibly being necessary and one being enhancing [95, 96].
The induction of FoxP3+ Tregs is not the only means through which hepatocytes induce tolerance. In a study using AAV hepatic gene transfer, it was found that Teff cells were directly removed in a Fas/FasL dependent manner [97]. In this work, an AAV8 vector, expressing a nuclear-targeted form of β-gal, nLacZ, was used to transduce the livers of both wild type and Faslpr/lpr mice. It was found that upon AAV8 liver transduction, a significant increase of FasL was seen on hepatocytes [97]. Further, they found that in AAV8 treated mice, there was a large population of Fas expressing lymphocytes found in the liver which were missing from the naïve mice [97]. These studies suggest that AAV hepatic gene transfer can be used to induce tolerance through a multifaceted approach: the induction of antigen-specific Tregs to the transgene as well as the selective deletion of transgene specific Teff cells through a Fas/FasL dependent pathway.
Factors that Impact the Immune Response to the Transgene
AAV liver gene transfer results in the induction of transgene specific Tregs which can play a major role in the suppression of cellular and antibody responses and the induction of tolerance. However, there are several factors which contribute to the induction of these antigen specific Tregs, as well as the maintenance of tolerance.
A threshold level of transgene expression is required for tolerance
In the context of gene replacement therapy, the transgene needs to be expressed above a threshold level to ensure immune tolerance induction, as expression below this threshold can induce adverse immune responses [5, 98]. Kumar, et al., demonstrated in mice that as little as a half-log difference in vector dose resulted in the activation of a transgene-specific CD8+ T cell response and clearance of transduced hepatocytes (unpublished data and [98]). Whereas, in mice receiving a sufficient tolerogenic vector dose, several overlapping pathways were found to be involved in suppressing CD8+ T cell responses, including Treg, FasL, and IL-10 induction [98]. This is supported by a previous study indicating that higher transgene expression levels suppress humoral immunity and is dependent upon both Treg and Fas-FasL mediated killing of CD4+ T effector cells [5].
Another factor to consider is if the transgene product is retained within the cell or secreted. Using the model antigen ovalbumin, Perrin et al. demonstrated that a log increase in vector dose was required to induce a comparable frequency of ova specific Tregs using cytoplasmic bound ova compared to secreted ova [45]. Regrettably, much of the mechanistic studies into AAV induced immune tolerance have utilized secreted transgenes. The data supports induction of both peripheral and central Tregs due, at least in part, to the fact that secreted transgenes can reach the thymus via the circulatory system [27, 31, 45, 99]. However, non-secreted transgene products may be limited to peripheral Treg induction. More studies will be needed to fully determine if this is sufficient to regulate transgene immune responses. In support of this concept, recent work by our group demonstrated that hepatic directed AAV gene therapy can be used to induce tolerance to myelin oligodendrocyte (MOG) protein, a membrane bound protein (discussed below) suggesting that tolerance induction is not dependent on secreted transgene products [100].
AAV serotype selection
In mice, AAV8 has the highest reported natural tropism for murine hepatocytes independent of the delivery route [101, 102]. Although AAV8 also has superior hepatocyte transduction in humans compared to AAV2, it may not be the best serotype for liver directed gene therapy in in the clinical setting. This is supported by data from Lisowski et al. [103] and Vercauteren et al. using mice with chimeric livers comprised of human and murine hepatocytes [104]. Independently, both studies concluded that AAV3 derived from either library selection or an AAV3 variant had the highest affinity for human hepatocytes. One caveat of these studies is that chimeric mice lack other cells and tissues of human origin and may overestimate the specificity of AAV3 for human hepatocytes. Ironically, AAV3 has long been ignored as a candidate serotype due to its extremely poor transduction efficiencies in murine liver, underscoring that what works great in mouse may not always translate to other species, including humans [105].
Following AAV administration, neutralizing antibodies specific to the serotype are formed, preventing vector re-administration of the same and potentially alternative capsid serotypes [106–109]. However, a recent study demonstrated the use of a highly divergent AAV, serotype 5, is permissive to re-administration with AAV1 without the development of cross-reactive neutralizing antibodies [110]. While re-administration has been successfully performed before in non-human primates [111], this is the first study to show successful re-administration despite high titer neutralizing antibodies formed in response to the initial AAV administration. This work is promising as it demonstrates the potential for a secondary treatment is possible without the need for immune suppression. Albeit, it may preclude the use of an optimal liver targeting serotype as the primary or secondary treatment, and will likely require dose optimization for each serotype used.
Engineered and library selected capsids
Altering AAV capsids is a common technique that has been used to improve transduction efficiencies and subsequent expression levels. A seminal study by Zhong et al. found that AAV2 particles are marked for proteasomal degradation through the phosphorylation of tyrosines within the capsid [112]. A subsequent study by the same group showed that when tyrosines at positions 444, 500, and 730 were changed to phenylalanine, a 30-fold increase in transduction was seen, in vivo [113]. More recently, the tyrosine-phenylalanine mutated capsid was shown to result in a reduced CD8+ response, a key limitation of AAV therapy [114]. Alternatively, directed evolution has also been employed to identify capsids with an increased tropism for a target tissue, or to overcome other barriers to gene therapy using capsid library screens [115–119]. The success of this technique is highlighted by the development of AAV2 variants that can withstand significantly higher neutralizing antibody concentrations [108], as well as the development of variants that have enhanced antibody evasion, requiring ~20-fold higher antibody concentrations for neutralization [120]. Such variants would result in higher transduction efficiencies in patients with pre-existing neutralizing antibodies. However, the model may bias for the selection of capsid variants that do not translate well into other species and humans [105].
Specific engineering of the AAV transgene is another technique that is used to improve expression. It has been postulated that the presence of rare codons within a transgene may result in a reduction in protein expression due to translation being slowed. Codon optimization is a technique in which codon-usage frequency in the transgene is matched to tRNA availability in the target tissue and species. This technique has proven to be beneficial in significantly increasing expression levels of FVIII, resulting in phenotypic correction of hemophilia A [121, 122], without leading to toxicity or an unfolded protein response [123]. In some instances, the increased expression levels have been dramatic with a 37-fold increase seen in FVIII expression levels [124], 21-fold increase seen with hFIX [125] and a ~50-fold increase in human ornithine transcarbamylase (hOTC) expression levels in hepatocytes [126]. Recently, codon optimization has been used to correct Crigler-Najjar syndrome in Gunn rats and UGT1A1−/− mice at vector doses that were much lower than when non-optimized transgene was used [127–129]. However, caution must be used as current reports suggest that codon optimization may be tissue dependent [130].
Self-complementary AAV (scAAV)
Wild-type AAV and derived vectors have a single-stranded DNA (ssAAV) genome, forming DNA loops at the 5′ and 3′ ends of the inverted terminal repeats (ITR). Since the expression cassette is single-stranded DNA, synthesis of the complementary second strand is needed for mRNA transcription by DNA dependent RNA polymerase. Early studies with AAV2 vectors identified this as a rate limiting step [131]. One approach to overcome this limitation was engineered by mutation of one of the viral ITRs to direct the vector genome to package as double-stranded DNA, forming a self-complementary (scAVV) vector [132]. While the use of scAAV vectors eliminates the need for second strand synthesis and results in higher transgene levels as well as increased transgene expression levels in mice and NHP [125, 133–135], the packaging capacity of scAAV is approximately half (~2.3 kb) of ssAAV which excludes many different therapeutic transgenes. However, it should be noted that increased innate immune responses, via Toll-like receptor-9, have been seen in both muscle and liver in response to the use of scAAV as compared to ssAAV, though the response seen in the liver were directed at the capsid and not the transgene [136–138]. Although there is substantial evidence showing enhanced transgene expression with scAAV2 vectors, new data suggests that the degree of enhancement may be serotype dependent. Therefore, to decrease the activation of innate immunity by TLR9 sensing of the dsDNA of scAAV, one might consider using a ssAAV vector.
Induction of tolerance In Utero and in neonates
Since rAAV genomes persist episomally, most studies have focused on delivery to adult animals, to avoid dilution of vector genomes through hepatocyte expansion that occurs during development. However, certain diseases require early intervention and thus, studies have been conducted to explore the relationship between in utero and neonate AAV gene therapy and tolerance. One benefit to this approach is that it allows for the manipulation of the immune system at an early stage before the immune system has fully matured, thereby taking advantage of a ‘window of opportunity’ to induce tolerance with minimal, if any, side-effects. While this technique is less commonly utilized compared to liver directed AAV, it has enjoyed some success. In mouse and sheep pre-clinical models, it was found that in utero administration of AAV was capable of inducing tolerance to the transgene [139, 140]. However, there are longevity concerns associated with using AAV to induce tolerance in utero or in neonates. The fact that AAV genomes persist in the nucleus in concatemeric episomal forms, and does not generally integrate into the host genome [141], is compounded by AAV’s inability to replicate, suggesting that as the animal or individual grows, the transgene will become increasingly more ‘diluted’ and ineffective over time. This concern was highlighted in a study where FIX expression in sheep was all but undetectable 6 months following in utero gene transfer [140]. With that said, a more recent report has shown renewed promise for AAV in this field. Hinderer et al. used a mucopolysaccharidosis type I model where AAV was administered to newborn dogs and rhesus monkeys which exhibited tolerance to the transgene without the development of neutralizing antibodies [142]. These results may provide a new avenue for in utero and neonatal administration of AAV for inducing tolerance.
Immune response to AAV transgene in other tissues
The liver is by no means the only tissue to which AAV transgenes have been delivered. Skeletal muscle has arguably been one of the most common targets of AAV gene transfer. The route of administration and the dose of the AAV can affect the immunogenicity of the vector and transgene. Intravascular and systemic routes have proved to be less immunogenic than intramuscular administration which can lead to the detection of T cell infiltrates in a dose dependent manner [143–145]. It has also been shown in a preclinical hemophilia B model that the local antigen concentration within the muscle plays a major role in the immunogenicity of the transgene [143]. In many cases, muscle gene transfer is administered in the context of neuromuscular disorders. These disorders are characterized by muscular inflammation which can affect the way the transgene is presented to the immune system thereby resulting in loss of expression instead of the sustained expression commonly seen in healthy muscle tissue [146–148]. However, there is some evidence of tolerance induction following AAV muscle gene transfer. In an rAAV1-AAT clinical trial in humans, Tregs were found in muscle biopsies up to 1 year following gene transfer and were speculated to be responsible for the control of the CD8+ T cell response, as well as the continued expression of the transgene [149]. One caveat is that the AAT protein has immune modulatory properties and thus, may have contributed to local Treg infiltration in muscle. Additionally, in a clinical trial, Tregs were seen following muscle gene therapy for lipoprotein lipase deficiency, though it should be mentioned that an immunosuppressant regimen was also administered along with the AAV which may have affected the gene transfer outcome [150].
The central nervous system (CNS) has traditionally been viewed as an immune privileged organ, protected from inflammation by the blood brain barrier (BBB). However, there is evidence that the CNS is not completely protected from inflammation, as evidenced in autoimmune diseases such as multiple sclerosis [151]. It has been proven that antigen presenting cells as well as T cells are capable of crossing the BBB and infiltrating the ‘protected’ zone [151–154]. Several AAV serotypes have been identified or engineered to cross the BBB providing an opportunity to treat CNS diseases due to long-term expression in widespread areas of the brain, including neurons [155]. As a whole, AAV treatment within the CNS has been well tolerated as is evidenced in many clinical trials (reviewed in [156]). However, it should be noted that in large animal models, immune responses were seen in dogs and NHP when foreign or human proteins were expressed [157, 158]. It is still unclear as to what is the most effective route of administration and vector dose and how these will affect immune responses to transgene following CNS targeted AAV gene therapy.
The eye is another organ that has been a popular target for AAV gene therapy with over a decade of clinical experience [159] focusing on administration to the subretinal space and the vitreous. Much like the liver and CNS, the eye is traditionally considered an immune privileged site. This ‘immune privilege’ is due in part to the blood-retina barrier, reduction in vascularity, and the lack of draining lymphatics. Further, antigens within the eye lead to an altered immune response, Anterior Chamber-Associated Immune Deviation. This ‘less robust’ immune response is characterized by TGF-β, Treg production, and skewing towards Th2 responses, leading to foreign antigen tolerance [160]. However, at least one study has seen inflammation following a dose of 1 × 1012 vg injected subretinally [161]. This finding suggests that, though the eye may be considered a tolerogenic environment, it is possible to deliver enough antigen to overcome immune deviation. The route of administration may also play a role in the immune response to AAV administered to the eye. In NHPs, it has been shown that a vector dose of 109 vg/eye administered intravitreal was safe with no signs of inflammation; whereas, a dose of 1010 vg/eye resulted in signs of inflammation in 78% of animals [162]. Similarly, a clinical study for Leber’s hereditary optic neuropathy showed that inflammation was seen in response to a dose of 1.8 × 1011 vg [146, 163]. These results clearly show that researchers should use caution when delivering AAV to the eye. Both, vector dose and the route of administration must be taken into consideration to avoid inflammation while also opening the possibility of reconsidering the eye as an immune privileged site.
Applications of AAV induced tolerance
Immunological tolerance induction to prevent or eliminate anti-drug antibodies
The need for immunological tolerance in gene therapy stems from historical data showing that patients can develop adaptive immune responses to therapeutic proteins after receiving enzyme replacement therapies. The formation of antibodies presents a challenge in enzyme replacement therapies. In the case of hemophilia, these antibodies are referred to as inhibitors and are responsible for increasing the risk of morbidity and mortality, due to break out bleeds and poor long-term control of hemostasis with bypassing agents. Hemophilia A patients, with factor VIII (FVIII) deficiency, can often successfully eliminate their inhibitors with an immune tolerance induction (ITI) protocol of high-dose and frequency administration of FVIII protein. However, hemophilia B patients with inhibitors often fail ITI due to adverse immune responses to ITI including anaphylaxis and nephrotic syndrome. Thus, the induction of tolerance via liver directed AAV gene therapy may provide an alternative approach for treating inhibitors in hemophilia B patients. To test this hypothesis, we showed that AAV8-F9 liver gene transfer was capable of reversing pre-existing pathogenic antibodies to FIX and resulted in the long-term desensitization of anaphylaxis prone hemophilia B mice, even when protein replacement therapy was resumed [14]. In larger animal models, this finding has also been corroborated. Finn et al. showed that the administration of AAV-8 encoding FVII resulted in an initial increase in inhibitor concentrations followed by the eradication of inhibitors by day 550, in a dog model of hemophilia A [164]. In a dog model of hemophilia B, it was shown that the administration of AAV-8 FIX-Padua, a hyperfunctional form of factor 9, resulted in an initial spike in inhibitor titers at ~15 days following vector administration, followed by a decline in inhibitor titers with a complete eradication seen by day 70 [36]. In both dog studies, tolerance was confirmed by the lack of an increase in inhibitors following a re-challenge with FVII and FIX, respectively.
In a mouse model of Pompe disease, liver directed AAV was successful at preventing antibody production to the transgene, acid alpha-glucosidase (GAA) [165]. Importantly, it was also demonstrated that the antibody response remained absent following several rounds of enzyme replacement therapy [166]. However, in the case of Pompe, the liver induced tolerance is not a sufficient therapy, as protein produced in the liver is unable to adequately cross the blood brain barrier and be taken up by neuronal tissue [167]. In an attempt to circumvent this issue, Doerfler et al. have designed a co-packaged AAV9, capable of transducing both liver and CNS, system for the simultaneous induction of tolerance and production of enzyme in neuronal tissue [168].
AAV liver gene immunotherapy for the treatment of autoimmune disorders
Another area that has garnered the attention of liver directed AAV gene therapy is in the treatment of autoimmune diseases. MS is generally considered a CD4+ based autoimmune disease, caused by the breakdown of immune tolerance to specific neuroprotein antigens. Our group has shown that liver directed AAV gene therapy can restore tolerance by inducing antigen specific Tregs that target the autoimmune inflammatory response [100]. In fact, we have found that with the AAV directed hepatocyte expression of the neuroprotein myelin oligodendrocyte glycoprotein (MOG), the onset of experimental autoimmune encephalomyelitis (EAE) can be completely prevented and is robust, capable of resisting a secondary insult [100]. More significantly, we have found that when AAV is given alone, or as part of a combined immunotherapy treatment, it is capable of reversing mild to severe pre-existing EAE disease [100]. This area of research is very exciting as it also opens the door for the discovery of other immune modulating therapies that may improve the efficacy of AAV at inducing tolerance.
Conclusion
Progress in AAV gene therapy has seen several impressive leaps in the last few decades. Preclinical studies in animal disease models have shown sustained therapeutic levels of transgene, even in disease models where the immune system normally mounts a response to the therapeutic protein. Tregs, induced by liver directed AAV gene therapy, are critical for the suppression of transgene immune responses and induction of systemic tolerance. Arguably, one of the more interesting target tissues for therapeutic transgene expression is the liver. Beyond being an ideal protein factory, the liver also modulates immune responses to dietary antigens, which is extended to gene therapy products. Although the precise mechanism is still unclear, immunological tolerance is dependent on multiple cells residing within the liver for antigen presentation and through skewing the local microenvironment towards conversion of CD4+ T effector cells into Treg. A successful protocol for inducing tolerance is dependent on several critical factors. For AAV gene transfer in the liver, animal models have shown that hepatocyte restricted expression and a threshold level of transgene expression is required. In addition, other factors such as serotype selection, vector composition, dosing, and choice of immune modulation also play a role in shaping transgene immune tolerance. The field continues to move forward to develop new strategies to circumvent the anti-AAV humoral and cell mediated immunological barriers that have arisen in clinical trials. With continued safety and efficacy in treating inherited genetic disorders, it may be possible to extend AAV liver gene therapy as an immune modulatory therapy to treat autoimmune disease.
Highlights.
AAV gene therapy can harness the tolerogenic nature of the liver.
Tolerance is dependent on a threshold level of hepatic transgene expression.
Therapy induces antigen specific Tregs that can suppress and reverse existing immune responses.
Acknowledgments
We acknowledge and thank the following for providing funding National Multiple Sclerosis Society (PP2157 and RG 5315-A-1) to BEH, NIH-NIAID (R01AI128074) to BEH, and the Children’s Miracle Network to DMM and GDK.
Footnotes
CONFLICT OF INTEREST
BEH is an inventor on a pending patent related to AAV immunotherapy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakai H, Yant SR, Storm TA, Fuess S, Meuse L, Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo, jt>Journal of virology. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. Journal of virology. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaiss AK, Muruve DA. Immune responses to adeno-associated virus vectors. Curr Gene Ther. 2005;5:323–331. doi: 10.2174/1566523054065039. [DOI] [PubMed] [Google Scholar]
- 4.Cao O, Hoffman BE, Moghimi B, Nayak S, Cooper M, Zhou S, Ertl HC, High KA, Herzog RW. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther. 2009;17:1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, Arruda VR, High KA, Herzog RW. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrzynski E, Herzog RW. Tolerance induction by viral in vivo gene transfer. Clin Med Res. 2005;3:234–240. doi: 10.3121/cmr.3.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobrzynski E, Fitzgerald JC, Cao O, Mingozzi F, Wang L, Herzog RW. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:4592–4597. doi: 10.1073/pnas.0508685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao O, Loduca PA, Herzog RW. Role of regulatory T cells in tolerance to coagulation factors. Journal of thrombosis and haemostasis: JTH. 2009;7(Suppl 1):88–91. doi: 10.1111/j.1538-7836.2009.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Rasko J, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S. Regulatory T cells: history and perspective. Methods in molecular biology (Clifton, NJ) 2011;707:3–17. doi: 10.1007/978-1-61737-979-6_1. [DOI] [PubMed] [Google Scholar]
- 14.Markusic DM, Hoffman BE, Perrin GQ, Nayak S, Wang X, LoDuca PA, High KA, Herzog RW. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med. 2013;5:1698–1709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sack BK, Herzog RW, Terhorst C, Markusic DM. Development of Gene Transfer for Induction of Antigen-specific Tolerance. Molecular therapy. Methods & clinical development. 2014;1:14013. doi: 10.1038/mtm.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.André S, Tough DF, Lacroix-Desmazes S, Kaveri SV, Bayry J. Surveillance of antigen-presenting cells by CD4+ CD25+ regulatory T cells in autoimmunity: immunopathogenesis and therapeutic implications. Am J Pathol. 2009;174:1575–1587. doi: 10.2353/ajpath.2009.080987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caridade M, Graca L, Ribeiro RM. Mechanisms Underlying CD4+ Treg Immune Regulation in the Adult: From Experiments to Models. Front Immunol. 2013;4:378. doi: 10.3389/fimmu.2013.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat Rev Immunol. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197–1209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 20.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. The Journal of experimental medicine. 2012;209:1713–1722. S1711–1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin X, Chen M, Liu Y, Guo Z, He X, Brand D, Zheng SG. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int J Clin Exp Pathol. 2013;6:116–123. [PMC free article] [PubMed] [Google Scholar]
- 22.Elkord E. Helios Should Not Be Cited as a Marker of Human Thymus-Derived Tregs. Commentary: Helios(+) and Helios(−) Cells Coexist within the Natural FOXP3(+) T Regulatory Cell Subset in Humans. Front Immunol. 2016;7:276. doi: 10.3389/fimmu.2016.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szurek E, Cebula A, Wojciech L, Pietrzak M, Rempala G, Kisielow P, Ignatowicz L. Differences in Expression Level of Helios and Neuropilin-1 Do Not Distinguish Thymus-Derived from Extrathymically-Induced CD4+Foxp3+ Regulatory T Cells. PLoS One. 2015;10:e0141161. doi: 10.1371/journal.pone.0141161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng H, Zhang R, Jin B, Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cellular & molecular immunology. 2015;12:566–571. doi: 10.1038/cmi.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 26.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, Di Serio C, Bacchetta R, Andreani M, Brockmann L, Gregori S, Flavell RA, Roncarolo MG. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman BE, Martino AT, Sack BK, Cao O, Liao G, Terhorst C, Herzog RW. Nonredundant Roles of IL-10 and TGF-β in Suppression of Immune Responses to Hepatic AAV-Factor IX Gene Transfer. Molecular Therapy. 2011;19:1263–1272. doi: 10.1038/mt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, Herzog RW. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breous E, Somanathan S, Vandenberghe LH, Wilson JM. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612–621. doi: 10.1002/hep.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martino AT, Nayak S, Hoffman BE, Cooper M, Liao G, Markusic DM, Byrne BJ, Terhorst C, Herzog RW. Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PLoS One. 2009;4:e6376. doi: 10.1371/journal.pone.0006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobrzynski E, Mingozzi F, Liu YL, Bendo E, Cao O, Wang L, Herzog RW. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104:969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- 32.Breous E, Somanathan S, Wilson JM. BALB/c mice show impaired hepatic tolerogenic response following AAV gene transfer to the liver. Mol Ther. 2010;18:766–774. doi: 10.1038/mt.2009.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, Bellinger D, Nichols TC, Arruda VR, Lothrop CD, High KA. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 34.Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD, Powell S, Keller T, McMurray M, Labelle A, Nagy D, Vargas JA, Zhou S, Couto LB, Pierce GF. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- 35.Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, van Ginkel FW, High KA, Lothrop CD. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crudele JM, Finn JD, Siner JI, Martin NB, Niemeyer GP, Zhou S, Mingozzi F, Lothrop CD, Arruda VR. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood. 2015;125:1553–1561. doi: 10.1182/blood-2014-07-588194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, Zhou S, Wright JF, Jiang H, Pierce GF, Arruda VR, High KA. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, Slaughter C, Ng CY, Zhou J, Lozier JN, Mandrell TD, Vanin EF, Nienhuis AW. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- 39.Sheth K, Bankey P. The liver as an immune organ. Current Opinion in Critical Care. 2001;7 doi: 10.1097/00075198-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Crispe IN. Liver antigen-presenting cells. Journal of hepatology. 2011;54:357–365. doi: 10.1016/j.jhep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 42.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 43.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, Davies DA. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 44.Gorczynski RM, Chan Z, Chung S, Cohen Z, Levy G, Sullivan B, Fu XM. Prolongation of rat small bowel or renal allograft survival by pretransplant transfusion and/or by varying the route of allograft venous drainage. Transplantation. 1994;58:816–820. [PubMed] [Google Scholar]
- 45.Perrin GQ, Zolotukhin I, Sherman A, Biswas M, de Jong YP, Terhorst C, Davidoff AM, Herzog RW. Dynamics of antigen presentation to transgene product-specific CD4(+) T cells and of Treg induction upon hepatic AAV gene transfer. Molecular therapy. Methods & clinical development. 2016;3:16083. doi: 10.1038/mtm.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horst AK, Neumann K, Diehl L, Tiegs G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cellular & molecular immunology. 2016;13:277–292. doi: 10.1038/cmi.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 48.Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6:718–722. doi: 10.1002/hep.1840060430. [DOI] [PubMed] [Google Scholar]
- 49.Knolle PA, Germann T, Treichel U, Uhrig A, Schmitt E, Hegenbarth S, Lohse AW, Gerken G. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. Journal of immunology (Baltimore, Md: 1950) 1999;162:1401–1407. [PubMed] [Google Scholar]
- 50.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Büschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. Journal of hepatology. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 51.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. Journal of immunology (Baltimore, Md: 1950) 1998;160:5729–5734. [PubMed] [Google Scholar]
- 53.Knolle PA, Uhrig A, Protzer U, Trippler M, Duchmann R, Meyer zum Büschenfelde KH, Gerken G. Interleukin-10 expression is autoregulated at the transcriptional level in human and murine Kupffer cells. Hepatology. 1998;27:93–99. doi: 10.1002/hep.510270116. [DOI] [PubMed] [Google Scholar]
- 54.Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447–455. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ju C, McCoy JP, Chung CJ, Graf ML, Pohl LR. Tolerogenic role of Kupffer cells in allergic reactions. Chem Res Toxicol. 2003;16:1514–1519. doi: 10.1021/tx0341761. [DOI] [PubMed] [Google Scholar]
- 56.Heymann F, Peusquens J, Ludwig-Portugall I, Kohlhepp M, Ergen C, Niemietz P, Martin C, van Rooijen N, Ochando JC, Randolph GJ, Luedde T, Ginhoux F, Kurts C, Trautwein C, Tacke F. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology. 2015;62:279–291. doi: 10.1002/hep.27793. [DOI] [PubMed] [Google Scholar]
- 57.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. Abiraterone and Increased Survival in Metastatic Prostate Cancer. New England Journal of Medicine. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan ML, Wang YD, Tian YF, Lai ZD, Yan LN. Inhibition of allogeneic T-cell response by Kupffer cells expressing indoleamine 2,3-dioxygenase. World J Gastroenterol. 2010;16:636–640. doi: 10.3748/wjg.v16.i5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callery MP, Mangino MJ, Flye MW. Arginine-specific suppression of mixed lymphocyte culture reactivity by Kupffer cells--a basis of portal venous tolerance. Transplantation. 1991;51:1076–1080. doi: 10.1097/00007890-199105000-00028. [DOI] [PubMed] [Google Scholar]
- 60.Wiegard C, Frenzel C, Herkel J, Kallen KJ, Schmitt E, Lohse AW. Murine liver antigen presenting cells control suppressor activity of CD4+CD25+ regulatory T cells. Hepatology. 2005;42:193–199. doi: 10.1002/hep.20756. [DOI] [PubMed] [Google Scholar]
- 61.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karrar A, Broome U, Uzunel M, Qureshi AR, Sumitran-Holgersson S. Human liver sinusoidal endothelial cells induce apoptosis in activated T cells: a role in tolerance induction. Gut. 2007;56:243–252. doi: 10.1136/gut.2006.093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smedsrod B. Clearance function of scavenger endothelial cells. Comparative hepatology. 2004;3(Suppl 1):S22. doi: 10.1186/1476-5926-2-S1-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCourt PA, Hansen B, Svistuonov D, Johansson S, Longati P, Schledzewski K, Kzhyshkowska J, Goerdt S, Smedsrod B. The liver sinusoidal endothelial cell hyaluronan receptor and its homolog, stabilin-1 - Their roles (known and unknown) in endocytosis. Comparative hepatology. 2004;3(Suppl 1):S24. doi: 10.1186/1476-5926-2-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kempka G, Kolb-Bachofen V. Binding, uptake, and transcytosis of ligands for mannose-specific receptors in rat liver: an electron microscopic study. Experimental cell research. 1988;176:38–48. doi: 10.1016/0014-4827(88)90118-8. [DOI] [PubMed] [Google Scholar]
- 66.Carambia A, Freund B, Schwinge D, Heine M, Laschtowitz A, Huber S, Wraith DC, Korn T, Schramm C, Lohse AW, Heeren J, Herkel J. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. Journal of hepatology. 2014;61:594–599. doi: 10.1016/j.jhep.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 67.Knolle PA, Schmitt E, Jin S, Germann T, Duchmann R, Hegenbarth S, Gerken G, Lohse AW. Induction of cytokine production in naive CD4(+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116:1428–1440. doi: 10.1016/s0016-5085(99)70508-1. [DOI] [PubMed] [Google Scholar]
- 68.Merlin S, Cannizzo ES, Borroni E, Bruscaggin V, Schinco P, Tulalamba W, Chuah MK, Arruda VR, VandenDriessche T, Prat M, Valente G, Follenzi A. A Novel Platform for Immune Tolerance Induction in Hemophilia A Mice. Mol Ther. 2017;25:1815–1830. doi: 10.1016/j.ymthe.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knolle PA, Uhrig A, Hegenbarth S, Löser E, Schmitt E, Gerken G, Lohse AW. IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin Exp Immunol. 1998;114:427–433. doi: 10.1046/j.1365-2249.1998.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lohse AW, Knolle PA, Bilo K, Uhrig A, Waldmann C, Ibe M, Schmitt E, Gerken G, Meyer Zum Büschenfelde KH. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology. 1996;110:1175–1181. doi: 10.1053/gast.1996.v110.pm8613007. [DOI] [PubMed] [Google Scholar]
- 71.Kruse N, Neumann K, Schrage A, Derkow K, Schott E, Erben U, Kühl A, Loddenkemper C, Zeitz M, Hamann A, Klugewitz K. Priming of CD4+ T cells by liver sinusoidal endothelial cells induces CD25low forkhead box protein 3- regulatory T cells suppressing autoimmune hepatitis. Hepatology. 2009;50:1904–1913. doi: 10.1002/hep.23191. [DOI] [PubMed] [Google Scholar]
- 72.Xu X, Jin R, Li M, Wang K, Zhang S, Hao J, Sun X, Zhang Y, Wu H, Zhang J, Ge Q. Liver sinusoidal endothelial cells induce tolerance of autoreactive CD4+ recent thymic emigrants. Scientific reports. 2016;6:19861. doi: 10.1038/srep19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steptoe RJ, Patel RK, Subbotin VM, Thomson AW. Comparative analysis of dendritic cell density and total number in commonly transplanted organs: morphometric estimation in normal mice. Transpl Immunol. 2000;8:49–56. doi: 10.1016/s0966-3274(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 74.Woo J, Lu L, Rao AS, Li Y, Subbotin V, Starzl TE, Thomson AW. Isolation, phenotype, and allostimulatory activity of mouse liver dendritic cells. Transplantation. 1994;58:484–491. doi: 10.1097/00007890-199408270-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuno K, Ezaki T, Kudo S, Uehara Y. A life stage of particle-laden rat dendritic cells in vivo: their terminal division, active phagocytosis, and translocation from the liver to the draining lymph. The Journal of experimental medicine. 1996;183:1865–1878. doi: 10.1084/jem.183.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goddard S, Youster J, Morgan E, Adams DH. Interleukin-10 secretion differentiates dendritic cells from human liver and skin. Am J Pathol. 2004;164:511–519. doi: 10.1016/S0002-9440(10)63141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. Journal of immunology (Baltimore, Md: 1950) 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 78.Tokita D, Sumpter TL, Raimondi G, Zahorchak AF, Wang Z, Nakao A, Mazariegos GV, Abe M, Thomson AW. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. Journal of hepatology. 2008;49:1008–1018. doi: 10.1016/j.jhep.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raïch-Regué D, Glancy M, Thomson AW. Regulatory dendritic cell therapy: from rodents to clinical application. Immunol Lett. 2014;161:216–221. doi: 10.1016/j.imlet.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–2676. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, Gonen M, Young JW, DeMatteo RP. Human liver dendritic cells promote T cell hyporesponsiveness. Journal of immunology (Baltimore, Md: 1950) 2009;182:1901–1911. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131–136. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abu-Eid R, Samara RN, Ozbun L, Abdalla MY, Berzofsky JA, Friedman KM, Mkrtichyan M, Khleif SN. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol Res. 2014;2:1080–1089. doi: 10.1158/2326-6066.CIR-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watanabe T, Katsukura H, Shirai Y, Yamori M, Nishi T, Chiba T, Kita T, Wakatsuki Y. A liver tolerates a portal antigen by generating CD11c+ cells, which select Fas ligand+ Th2 cells via apoptosis. Hepatology. 2003;38:403–412. doi: 10.1053/jhep.2003.50343. [DOI] [PubMed] [Google Scholar]
- 86.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–3159. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 87.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schulz S, Landi A, Garg R, Wilson JA, van Drunen Littel-van den Hurk S. Indolamine 2,3-dioxygenase expression by monocytes and dendritic cell populations in hepatitis C patients. Clin Exp Immunol. 2015;180:484–498. doi: 10.1111/cei.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herkel J, Jagemann B, Wiegard C, Lazaro JF, Lueth S, Kanzler S, Blessing M, Schmitt E, Lohse AW. MHC class II-expressing hepatocytes function as antigen-presenting cells and activate specific CD4 T lymphocyutes. Hepatology. 2003;37:1079–1085. doi: 10.1053/jhep.2003.50191. [DOI] [PubMed] [Google Scholar]
- 90.Arnold B. Parenchymal cells in immune and tolerance induction. Immunol Lett. 2003;89:225–228. doi: 10.1016/s0165-2478(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 91.Warren A, Le Couteur DG, Fraser R, Bowen DG, McCaughan GW, Bertolino P. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology. 2006;44:1182–1190. doi: 10.1002/hep.21378. [DOI] [PubMed] [Google Scholar]
- 92.Bertolino P, Trescol-Biémont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur J Immunol. 1998;28:221–236. doi: 10.1002/(SICI)1521-4141(199801)28:01<221::AID-IMMU221>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 93.Franco A, Barnaba V, Natali P, Balsano C, Musca A, Balsano F. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology. 1988;8:449–454. doi: 10.1002/hep.1840080302. [DOI] [PubMed] [Google Scholar]
- 94.Knolle PA. Staying local-antigen presentation in the liver. Curr Opin Immunol. 2016;40:36–42. doi: 10.1016/j.coi.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 95.Burghardt S, Claass B, Erhardt A, Karimi K, Tiegs G. Hepatocytes induce Foxp3+ regulatory T cells by Notch signaling. J Leukoc Biol. 2014;96:571–577. doi: 10.1189/jlb.2AB0613-342RR. [DOI] [PubMed] [Google Scholar]
- 96.Lüth S, Huber S, Schramm C, Buch T, Zander S, Stadelmann C, Brück W, Wraith DC, Herkel J, Lohse AW. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. 2008;118:3403–3410. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Faust SM, Bell P, Zhu Y, Sanmiguel J, Wilson JM. The role of apoptosis in immune hyporesponsiveness following AAV8 liver gene transfer. Mol Ther. 2013;21:2227–2235. doi: 10.1038/mt.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar SR, Hoffman BE, Terhorst C, de Jong YP, Herzog RW. The Balance between CD8+ T Cell-Mediated Clearance of AAV-Encoded Antigen in the Liver and Tolerance Is Dependent on the Vector Dose. Mol Ther. 2017;25:880–891. doi: 10.1016/j.ymthe.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herzog RW. Complexity of immune responses to AAV transgene products - Example of factor IX. Cellular immunology. 2017 doi: 10.1016/j.cellimm.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keeler GD, Kumar SR, Palaschak B, Silverberg EL, Markusic DM, Jones NT, Hoffman BE. Gene therapy induced antigen-specific Tregs inhibits neuro-inflammation and reverses disease in a mouse model of Multiple Sclerosis. Molecular Therapy. 2017 doi: 10.1016/j.ymthe.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sands MS. AAV-mediated liver-directed gene therapy. Methods in molecular biology (Clifton, NJ) 2011;807:141–157. doi: 10.1007/978-1-61779-370-7_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cooper M, Nayak S, Hoffman BE, Terhorst C, Cao O, Herzog RW. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Human gene therapy. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lisowski L, Dane AP, Chu K, Zhang Y, Cunningham SC, Wilson EM, Nygaard S, Grompe M, Alexander IE, Kay MA. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506:382–386. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vercauteren K, Hoffman BE, Zolotukhin I, Keeler GD, Xiao JW, Basner-Tschakarjan E, High KA, Ertl HC, Rice CM, Srivastava A, de Jong YP, Herzog RW. Superior In vivo Transduction of Human Hepatocytes Using Engineered AAV3 Capsid. Mol Ther. 2016;24:1042–1049. doi: 10.1038/mt.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Markusic DM, Nichols TC, Merricks EP, Palaschak B, Zolotukhin I, Marsic D, Zolotukhin S, Srivastava A, Herzog RW. Evaluation of engineered AAV capsids for hepatic factor IX gene transfer in murine and canine models. J Transl Med. 2017;15:94. doi: 10.1186/s12967-017-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, Kay MA. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. Journal of virology. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Asokan A, Conway JC, Phillips JL, Li C, Hegge J, Sinnott R, Yadav S, DiPrimio N, Nam HJ, Agbandje-McKenna M, McPhee S, Wolff J, Samulski RJ. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nature biotechnology. 2010;28:79–82. doi: 10.1038/nbt.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotech. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- 109.Arruda VR, Xiao W. It’s all about the clothing: capsid domination in the adeno-associated viral vector world. Journal of thrombosis and haemostasis: JTH. 2007;5:12–15. doi: 10.1111/j.1538-7836.2006.02262.x. [DOI] [PubMed] [Google Scholar]
- 110.Majowicz A, Salas D, Zabaleta N, Rodriguez-Garcia E, Gonzalez-Aseguinolaza G, Petry H, Ferreira V. Successful Repeated Hepatic Gene Delivery in Mice and Non-human Primates Achieved by Sequential Administration of AAV5ch and AAV1. Mol Ther. 2017 doi: 10.1016/j.ymthe.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, Cochrane M, Gray E, Tuddenham EG, Davidoff AM. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, Herzog RW, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martino AT, Basner-Tschakarjan E, Markusic DM, Finn JD, Hinderer C, Zhou S, Ostrov DA, Srivastava A, Ertl HC, Terhorst C, High KA, Mingozzi F, Herzog RW. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013;121:2224–2233. doi: 10.1182/blood-2012-10-460733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Büning H, Huber A, Zhang L, Meumann N, Hacker U. Engineering the AAV capsid to optimize vector-host-interactions. Curr Opin Pharmacol. 2015;24:94–104. doi: 10.1016/j.coph.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 116.Marsic D, Govindasamy L, Currlin S, Markusic DM, Tseng YS, Herzog RW, Agbandje-McKenna M, Zolotukhin S. Vector design Tour de Force: integrating combinatorial and rational approaches to derive novel adeno-associated virus variants. Mol Ther. 2014;22:1900–1909. doi: 10.1038/mt.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Excoffon KJ, Koerber JT, Dickey DD, Murtha M, Keshavjee S, Kaspar BK, Zabner J, Schaffer DV. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc Natl Acad Sci U S A. 2009;106:3865–3870. doi: 10.1073/pnas.0813365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koerber JT, Jang JH, Schaffer DV. DNA shuffling of adeno-associated virus yields functionally diverse viral progeny. Mol Ther. 2008;16:1703–1709. doi: 10.1038/mt.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li C, Wu S, Albright B, Hirsch M, Li W, Tseng YS, Agbandje-McKenna M, McPhee S, Asokan A, Samulski RJ. Development of Patient-specific AAV Vectors After Neutralizing Antibody Selection for Enhanced Muscle Gene Transfer. Mol Ther. 2016;24:53–65. doi: 10.1038/mt.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bartel MA, Weinstein JR, Schaffer DV. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene Ther. 2012;19:694–700. doi: 10.1038/gt.2012.20. [DOI] [PubMed] [Google Scholar]
- 121.Sack BK, Merchant S, Markusic DM, Nathwani AC, Davidoff AM, Byrne BJ, Herzog RW. Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS One. 2012;7:e37671. doi: 10.1371/journal.pone.0037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McIntosh J, Lenting PJ, Rosales C, Lee D, Rabbanian S, Raj D, Patel N, Tuddenham EG, Christophe OD, McVey JH, Waddington S, Nienhuis AW, Gray JT, Fagone P, Mingozzi F, Zhou SZ, High KA, Cancio M, Ng CY, Zhou J, Morton CL, Davidoff AM, Nathwani AC. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121:3335–3344. doi: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zolotukhin I, Markusic DM, Palaschak B, Hoffman BE, Srikanthan MA, Herzog RW. Potential for cellular stress response to hepatic factor VIII expression from AAV vector. Molecular therapy. Methods & clinical development. 2016;3:16063. doi: 10.1038/mtm.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Binny C, McIntosh J, Della Peruta M, Kymalainen H, Tuddenham EG, Buckley SM, Waddington SN, McVey JH, Spence Y, Morton CL, Thrasher AJ, Gray JT, Castellino FJ, Tarantal AF, Davidoff AM, Nathwani AC. AAV-mediated gene transfer in the perinatal period results in expression of FVII at levels that protect against fatal spontaneous hemorrhage. Blood. 2012;119:957–966. doi: 10.1182/blood-2011-09-377630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T, Samulski RJ, Monahan PE. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol Ther. 2008;16:280–289. doi: 10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- 126.Bell P, Wang L, Chen SJ, Yu H, Zhu Y, Nayal M, He Z, White J, Lebel-Hagan D, Wilson JM. Effects of Self-Complementarity, Codon Optimization, Transgene, and Dose on Liver Transduction with AAV8. Hum Gene Ther Methods. 2016;27:228–237. doi: 10.1089/hgtb.2016.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ronzitti G, Bortolussi G, van Dijk R, Collaud F, Charles S, Leborgne C, Vidal P, Martin S, Gjata B, Sola MS, van Wittenberghe L, Vignaud A, Veron P, Bosma PJ, Muro AF, Mingozzi F. A translationally optimized AAV-UGT1A1 vector drives safe and long-lasting correction of Crigler-Najjar syndrome. Molecular therapy. Methods & clinical development. 2016;3:16049. doi: 10.1038/mtm.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seppen J, Bakker C, de Jong B, Kunne C, van den Oever K, Vandenberghe K, de Waart R, Twisk J, Bosma P. Adeno-associated virus vector serotypes mediate sustained correction of bilirubin UDP glucuronosyltransferase deficiency in rats. Mol Ther. 2006;13:1085–1092. doi: 10.1016/j.ymthe.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 129.Bortolussi G, Zentillin L, Vaníkova J, Bockor L, Bellarosa C, Mancarella A, Vianello E, Tiribelli C, Giacca M, Vitek L, Muro AF. Life-long correction of hyperbilirubinemia with a neonatal liver-specific AAV-mediated gene transfer in a lethal mouse model of Crigler-Najjar Syndrome. Human gene therapy. 2014;25:844–855. doi: 10.1089/hum.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown HC, Zakas PM, George SN, Parker ET, Spencer HT, Doering CB. 728. Tissue-Directed Transgene Engineering for AAV and Lentivector Gene Therapy Approaches. Molecular Therapy. 2016;24:S287. [Google Scholar]
- 131.Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. Journal of virology. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 133.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 134.Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D, Nawathe S, Waddington SN, Bronson R, Jackson S, Donahue RE, High KA, Mingozzi F, Ng CY, Zhou J, Spence Y, McCarville MB, Valentine M, Allay J, Coleman J, Sleep S, Gray JT, Nienhuis AW, Davidoff AM. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Raj D, Davidoff AM, Nathwani AC. Self-complementary adeno-associated viral vectors for gene therapy of hemophilia B: progress and challenges. Expert Rev Hematol. 2011;4:539–549. doi: 10.1586/ehm.11.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martino AT, Suzuki M, Markusic DM, Zolotukhin I, Ryals RC, Moghimi B, Ertl HC, Muruve DA, Lee B, Herzog RW. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu T, Töpfer K, Lin SW, Li H, Bian A, Zhou XY, High KA, Ertl HC. Self-complementary AAVs induce more potent transgene product-specific immune responses compared to a single-stranded genome. Mol Ther. 2012;20:572–579. doi: 10.1038/mt.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sabatino DE, MacKenzie TC, Peranteau W, Edmonson S, Campagnoli C, Liu Y-L, Flake AW, High KA. Persistent Expression of hF.IX After Tolerance Induction by In Utero or Neonatal Administration of AAV-1-F.IX in Hemophilia B Mice. Molecular Therapy. 2007;15:1677–1685. doi: 10.1038/sj.mt.6300219. [DOI] [PubMed] [Google Scholar]
- 140.David AL, McIntosh J, Peebles DM, Cook T, Waddington S, Weisz B, Wigley V, Abi-Nader K, Boyd M, Davidoff AM, Nathwani AC. Recombinant adeno-associated virus-mediated in utero gene transfer gives therapeutic transgene expression in the sheep. Human gene therapy. 2011;22:419–426. doi: 10.1089/hum.2010.007. [DOI] [PubMed] [Google Scholar]
- 141.Penaud-Budloo M, Le Guiner C, Nowrouzi A, Toromanoff A, Cherel Y, Chenuaud P, Schmidt M, von Kalle C, Rolling F, Moullier P, Snyder RO. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. Journal of virology. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hinderer C, Bell P, Louboutin JP, Zhu Y, Yu H, Lin G, Choa R, Gurda BL, Bagel J, O’Donnell P, Sikora T, Ruane T, Wang P, Tarantal AF, Casal ML, Haskins ME, Wilson JM. Neonatal Systemic AAV Induces Tolerance to CNS Gene Therapy in MPS I Dogs and Nonhuman Primates. Mol Ther. 2015;23:1298–1307. doi: 10.1038/mt.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, Bellinger DA, Couto LB, Nichols TC, High KA. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- 144.Toromanoff A, Adjali O, Larcher T, Hill M, Guigand L, Chenuaud P, Deschamps JY, Gauthier O, Blancho G, Vanhove B, Rolling F, Chérel Y, Moullier P, Anegon I, Le Guiner C. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol Ther. 2010;18:151–160. doi: 10.1038/mt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 146.Mingozzi F, High KA. Overcoming the Host Immune Response to Adeno-Associated Virus Gene Delivery Vectors: The Race Between Clearance, Tolerance, Neutralization, and Escape. Annu Rev Virol. 2017;4:511–534. doi: 10.1146/annurev-virology-101416-041936. [DOI] [PubMed] [Google Scholar]
- 147.Flanigan KM, Campbell K, Viollet L, Wang W, Gomez AM, Walker CM, Mendell JR. Anti-dystrophin T cell responses in Duchenne muscular dystrophy: prevalence and a glucocorticoid treatment effect. Hum Gene Ther. 2013;24:797–806. doi: 10.1089/hum.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, Bowles D, Gray S, Li C, Galloway G, Malik V, Coley B, Clark KR, Li J, Xiao X, Samulski J, McPhee SW, Samulski RJ, Walker CM. Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mueller C, Chulay JD, Trapnell BC, Humphries M, Carey B, Sandhaus RA, McElvaney NG, Messina L, Tang Q, Rouhani FN, Campbell-Thompson M, Fu AD, Yachnis A, Knop DR, Ye G-j, Brantly M, Calcedo R, Somanathan S, Richman LP, Vonderheide RH, Hulme MA, Brusko TM, Wilson JM, Flotte TR. Human Treg responses allow sustained recombinant adeno-associated virus–mediated transgene expression. 2013 doi: 10.1172/JCI70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ferreira V, Twisk J, Kwikkers K, Aronica E, Brisson D, Methot J, Petry H, Gaudet D. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther. 2014;25:180–188. doi: 10.1089/hum.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schwartz M, Deczkowska A. Neurological Disease as a Failure of Brain-Immune Crosstalk: The Multiple Faces of Neuroinflammation. Trends Immunol. 2016;37:668–679. doi: 10.1016/j.it.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 152.Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R, Schwaninger M, Engelhardt B, de Vries HE. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta. 2016;1862:461–471. doi: 10.1016/j.bbadis.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 153.Coisne C, Lyck R, Engelhardt B. Live cell imaging techniques to study T cell trafficking across the blood-brain barrier in vitro and in vivo. Fluids Barriers CNS. 2013;10:7. doi: 10.1186/2045-8118-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sagar D, Foss C, El Baz R, Pomper MG, Khan ZK, Jain P. Mechanisms of dendritic cell trafficking across the blood-brain barrier. J Neuroimmune Pharmacol. 2012;7:74–94. doi: 10.1007/s11481-011-9302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, Fyfe J, Moullier P, Colle MA, Barkats M. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hocquemiller M, Giersch L, Audrain M, Parker S, Cartier N. Adeno-Associated Virus-Based Gene Therapy for CNS Diseases. Hum Gene Ther. 2016;27:478–496. doi: 10.1089/hum.2016.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haurigot V, Marco S, Ribera A, Garcia M, Ruzo A, Villacampa P, Ayuso E, Anor S, Andaluz A, Pineda M, Garcia-Fructuoso G, Molas M, Maggioni L, Munoz S, Motas S, Ruberte J, Mingozzi F, Pumarola M, Bosch F. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest. 2013 doi: 10.1172/JCI66778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Samaranch L, Sebastian WS, Kells AP, Salegio EA, Heller G, Bringas JR, Pivirotto P, DeArmond S, Forsayeth J, Bankiewicz KS. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol Ther. 2014;22:329–337. doi: 10.1038/mt.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moore NA, Morral N, Ciulla TA, Bracha P. Gene therapy for inherited retinal and optic nerve degenerations. Expert Opin Biol Ther. 2017:1–13. doi: 10.1080/14712598.2018.1389886. [DOI] [PubMed] [Google Scholar]
- 160.Taylor AW. Ocular immune privilege. Eye. 2009;23 doi: 10.1038/eye.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, Feathers KL, Luong VA, Yzer S, Balaggan K, Viswanathan A, de Ravel TJ, Casteels I, Holder GE, Tyler N, Fitzke FW, Weleber RG, Nardini M, Moore AT, Thompson DA, Petersen-Jones SM, Michaelides M, van den Born LI, Stockman A, Smith AJ, Rubin G, Ali RR. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maclachlan TK, Lukason M, Collins M, Munger R, Isenberger E, Rogers C, Malatos S, Dufresne E, Morris J, Calcedo R, Veres G, Scaria A, Andrews L, Wadsworth S. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol Ther. 2011;19:326–334. doi: 10.1038/mt.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Uretsky S, Sahel J, Galy A, Thomasson N, Honnet G, et al. A recombinant AAV2/2 carrying the wild-type ND4 gene for the treatment of LHON: preliminary results of a first-in-man study and upcoming pivotal efficacy trials. 12th International Symposium on Ocular Pharmacology and Therapeutics; Berlin. 2016. [Google Scholar]
- 164.Finn JD, Ozelo MC, Sabatino DE, Franck HW, Merricks EP, Crudele JM, Zhou S, Kazazian HH, Lillicrap D, Nichols TC, Arruda VR. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116:5842–5848. doi: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A, Brown T, Young SP, Clay TM, Amalfitano A, Chen YT, Koeberl DD. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther. 2005;12:876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 166.Sun B, Bird A, Young SP, Kishnani PS, Chen YT, Koeberl DD. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boustany RM. Lysosomal storage diseases--the horizon expands. Nat Rev Neurol. 2013;9:583–598. doi: 10.1038/nrneurol.2013.163. [DOI] [PubMed] [Google Scholar]
- 168.Doerfler PA, Todd AG, Clément N, Falk DJ, Nayak S, Herzog RW, Byrne BJ. Copackaged AAV9 Vectors Promote Simultaneous Immune Tolerance and Phenotypic Correction of Pompe Disease. Human gene therapy. 2016;27:43–59. doi: 10.1089/hum.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]


