Abstract
Maintenance of the regulatory T (Treg) cell pool is essential for peripheral tolerance and prevention of autoimmunity. Integrins, heterodimeric transmembrane proteins consisting of α and β subunits that mediate cell-to-cell and cell-to-extracellular matrix interactions, play an important role in facilitating Treg cell contact-mediated suppression. Here we show that integrin activation plays an essential, previously unappreciated role in maintaining murine Treg cell function. Treg cell-specific loss of talin, a β integrin-binding protein, or expression of talin(L325R), a mutant that selectively abrogates integrin activation, resulted in lethal systemic autoimmunity. This dysfunction could be attributed, in part, to a global dysregulation of the Treg cell transcriptome. Activation of integrin α4β1 led to increased suppressive capacity of the Treg cell pool, suggesting that modulating integrin activation on Treg cells may be a useful therapeutic strategy for autoimmune and inflammatory disorders. Taken together, these results reveal a critical role for integrin-mediated signals in controlling peripheral tolerance by virtue of maintaining Treg cell function.
INTRODUCTION
Integrins are heterodimeric transmembrane proteins made up of α and β subunits that mediate cell-to-cell and cell-to-extracellular matrix interactions. The regulation of the affinity of integrins for their extracellular ligands is involved in a multitude of signaling pathways that control cellular survival, proliferation and differentiation. Thus, mutations or genetic deficiencies in integrins or major components of the integrin signaling pathway can lead to defective organ development, immunodeficiency, cancer and autoimmune disease (1).
The affinity of integrins for extracellular ligands is tightly regulated and critical for normal hematopoietic cell adhesion and function (2). Binding of the large cytoskeletal protein talin to the β integrin cytoplasmic domain is a key final step in inducing conformational changes in the integrin that confer high affinity (integrin activation) (3, 4). Talin is essential for inside-out integrin activation, which regulates the affinity of integrins in accordance with changes to the extracellular environment sensed by the cell (5), and for outside-in integrin signaling, which is initiated by the binding of extracellular ligands (6). In addition to facilitating integrin activation, talin functions to link integrins to the actin cytoskeleton (7) and recruits numerous other integrin-associated proteins (2).
In T cells, integrin signaling is essential for facilitating trafficking throughout the body during homeostasis and infection. For instance, integrin α4β1 (VLA-4) facilitates cell trafficking to sites of inflammation and integrin αLβ2 (LFA-1) facilitates trafficking to lymph node. Importantly, LFA-1, along with talin, is recruited to the immunological synapse (IS) that forms between T cells and antigen presenting cells (APCs) and is thought to stabilize this cell-to-cell interaction to facilitate T cell activation (8). Using a CD4Cre conditional mouse model in which talin was deleted in all T cells, Huttenlocher and colleagues previously showed that talin is required for T cell-APC contacts, contact-mediated T cell proliferation, and polarization of stable F-actin to the IS (9). We utilized the same mouse model to demonstrate that T-cell specific deletion of talin resulted in spontaneous T cell activation that appeared to be due, in part, to defects in Treg cell function, homeostasis and survival, indicating that there may be a specific requirement for talin-mediated integrin signaling in Treg cells (10). However, because this model results in loss of talin in all CD4+ and CD8+ T cells during thymic development, we were unable to exclude the possibility that talin-dependent functions in non-Treg T cells might be involved in the maintenance of immune homeostasis.
Regulatory T (Treg) cells are a distinct subset of CD4+ T cells that maintain immune homeostasis (11). Deficiency in the number or function of Treg cells results in inadequate immune suppression, which can result in autoimmune and inflammatory disorders (12–14). The role of talin in integrin activation may thus represent a previously unexplored mechanism contributing to Treg cell function, identity and homeostasis. The integrin LFA-1 is required for contact-dependent suppressive mechanisms of Treg cells, such as the downregulation of co-stimulatory molecules on dendritic cells through expression of CTLA-4 (15–17), cytolysis through the production of granzyme B in Treg cells (18), conversion of ATP to adenosine through the expression of both CD39 and CD73 on the surface of Treg cells (19) and clustering around activated dendritic cells to provide a physical barrier to prevent activation of naïve T cells (20). Whether integrins play a role in Treg cell homeostasis beyond simply facilitating adhesion to mediate these contact-dependent suppressive mechanisms, however, remains largely unexplored.
Here we sought to investigate whether integrin activation is required to maintain Treg cell identity and function. Treg cell-specific deletion of talin resulted in spontaneous lethal autoimmunity, demonstrating an essential role for talin in maintaining Treg cell homeostasis and function. Mice harboring Treg cells expressing a mutant form of talin, talin(L325R), a mutant that selectively abrogates integrin activation (21–23), developed immune-mediated pathology resembling that observed in mice with talin-deficient Treg cells, indicating that talin is required in Treg cells owing to its role in integrin activation. Conversely, activation of β1 integrins led to increased expression of IL-2Rα on Treg cells, and increased the suppressive capacity of talin-deficient Treg cells. Together these findings suggest that talin and integrin activation play a critical role in controlling Treg cell-mediated peripheral tolerance and raise the possibility that activating integrins in Treg cells may be a useful therapeutic strategy in the treatment of immune-mediated disorders.
MATERIALS AND METHODS
Mice
All mice were bred and housed in specific pathogen-free conditions prior to use. Tln1fl/fl, Tln1L325R/fl, Foxp3GFP, and Foxp3Cre mice have been described previously (22, 24–26). In some experiments, mice were injected intraperitoneally with β1aAb (9EG7, BioXCell) or an isotype control (Rat IgG2a, BioXCell) in PBS at 2mg/kg, every 5 days for 4–6 weeks.
Antibodies and flow cytometry
Murine antibodies against the following proteins were obtained from Biolegend: CD4 (RM4-5), CD8 (53-6.7), Foxp3 (150D), CD44 (1M7), CD62L (MEL-14), IFNγ (XMG1.2), TNFα (MP6-XT22), IL-17A (TC11-18H10.1), IL-2Rα (PC61), CD103 (2E7), CTLA4 (UC10-4B9), CD39 (24DMS1), CD73 (TY/11.8), GITR (DTA-1), GFP (FM264G), and Ki67 (16AB). For intracellular detection of cytokines, splenocytes were stimulated ex vivo with PMA (Sigma) and ionomycin (Sigma) in the presence of Brefeldin A (Sigma) for 4 hours at 37°C; cells were fixed and permeabilized with the Foxp3 Transcription Factor Fixation/Permeabilization kit (eBioscience) prior to intracellular staining. All samples were analyzed on an Accuri C6 or LSRFortessa (BD Biosciences).
Isolation of T cells from liver and lung
Mice were euthanized with CO2 and perfused through the left ventricle of the heart with a heparin (Sigma) solution in PBS to remove all blood. Lungs were treated with 1.3mM EDTA (Fisher), digested with 75U/mL collagenase solution (Sigma), and T cells were isolated with a Percoll (Fisher) gradient. Livers were mechanically disassociated and T cells were isolated using a Percoll (Fisher) gradient.
Treg cell suppression assays
CD4+CD25− conventional T (Tconv) cells were isolated from spleens of wild-type mice using the CD4+ T cell negative isolation kit (Miltenyi Biotec) with a biotin-conjugated anti-CD25 (PC61, Biolegend) antibody included to deplete pre-activated cells. Tconv cells were then labeled with CFSE as previously described (27). Wild-type or talin-deficient CD4+YFP+ Treg cells were sorted with a FACS Aria 2 (BD Biosciences). Antigen-presenting cells were isolated from spleens of wild-type mice and depleted of CD3+ T cells using CD3 microbeads (Miltenyi Biotec). CSFE-labeled Tconv cells were co-cultured with antigen presenting cells (1:3 ratio) and Treg cells (32:1, 16:1, 8:1, 4:1, 2:1 and 1:1 ratios) in the presence of 250ng/mL soluble anti-CD3 (2C11) for 96 hours at 37°C. Percentage suppression was calculated as: [(divided Tconv cells without Treg cells) – (divided Tconv cells with Treg cells for a given experimental condition)]/(divided Tconv cells without Treg cells) * 100.
Histology
Organs were fixed overnight at 25°C in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Images were acquired on a Nikon Eclipse TE300 microscope.
RNA Sequencing
Total RNA from FACS-sorted YFP+ Treg cells isolated from Tln1wt/wtFoxp3Cre/wt and Tln1fl/flFoxp3Cre/wt from 1–2 mice was extracted using TRIzol reagent (Life Technologies) according to the manufacterer’s protocol. cDNA libraries were prepared using TruSeq non-Stranded Total RNA Sample Prep Kit (Illumina) according to manufacturer’s instructions. cDNA libraries were sequenced with a HiSeq2500 (Illumina). FASTQ files were processed with kallisto 0.42.4 with following commands: kallisto quant -b 8 –i kallisto_GRCm38.rel79.cdna.all.idx -l 200 -s 20 -t 4. Differential expressed genes were identified by identifying genes that had an absolute log2 fold change > 1 and q-value < 0.05. Log2 fold change was calculated by log2(TPM1+1/TPM2+1) and q-value was calculated by sleuth using the default setting for two-condition comparisons. Genes with less than 1 TPM standard deviation across samples were removed before PCA analysis. PCA was performed using R.
Statistics
An unpaired Student’s t-test (two-tailed) was used for statistical evaluation of the data between two groups, using a statistical software package (Graph Pad Prism). P values are denoted in figures by * P<0.05, **P<0.01, *** P<0.005.
Study Approval
All animal studies were approved by the Institutional Animal Care and Use Guidelines of the University of California, San Diego.
RESULTS
Spontaneous lethal inflammation in mice with a Treg cell-specific deletion of talin
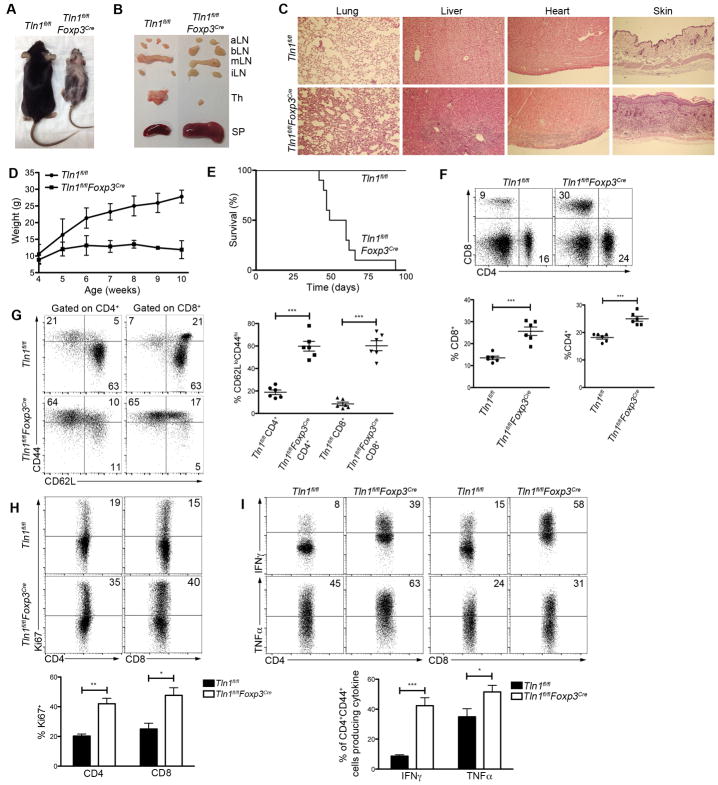
To investigate the possibility that talin plays a role in Treg cell function, we generated mice in which talin was deleted selectively in Treg cells, using a previously described Foxp3Cre mouse strain (26). Strikingly, male Tln1fl/flFoxp3Cre hemizygous mice developed systemic autoimmunity that manifested as runting, failure to thrive, dermatitis, lymphadenopathy, splenomegaly, and lymphocytic infiltration into multiple organs, ultimately resulting in death by 2–3 months of age (Fig. 1A–E). These mice exhibited increased percentages of CD4+ and CD8+ T cells that displayed an activated (CD62LloCD44hi) phenotype, were highly proliferative based on Ki67 expression, and were capable of high levels of IFNγ and TNFα production (Fig. 1F–I).
Figure 1. Spontaneous lethal inflammation in mice with a Treg cell-specific deletion of talin.
(A and B) Morphology (A) or organs (B) (spleen, lymph nodes (aortic, brachial, mesenteric, inguinal), and thymi) of male Tln1fl/fl or Tln1fl/flFoxp3Cre mice. (C) H&E stains of lung, liver, heart, and skin tissue from Tln1fl/fl or Tln1fl/flFoxp3Cre mice. (D and E) Body weight from 4–10 weeks of age (D) and survival (E) of Tln1fl/fl or Tln1fl/flFoxp3Cre mice; n=10. (F–H) Percentages of splenic CD4+ or CD8+ T cells (F) expressing CD44, CD62L (G), or Ki-67 (H) from Tln1fl/fl or Tln1fl/flFoxp3Cre mice; displayed cells gated on CD4+ or CD8+ events; n=6. (I) IFNγ and TNFα expression by splenic CD4+ (left) and CD8+ (right) T cells from Tln1fl/fl or Tln1fl/flFoxp3Cre mice; displayed cells gated on CD4+CD44hi or CD8+CD44hi events; n=6. Data are mean ± SEM and representative of at least 2 independent experiments. *, P < 0.05; **, P <0.01, *** P <0.005, unpaired Student’s t-test.
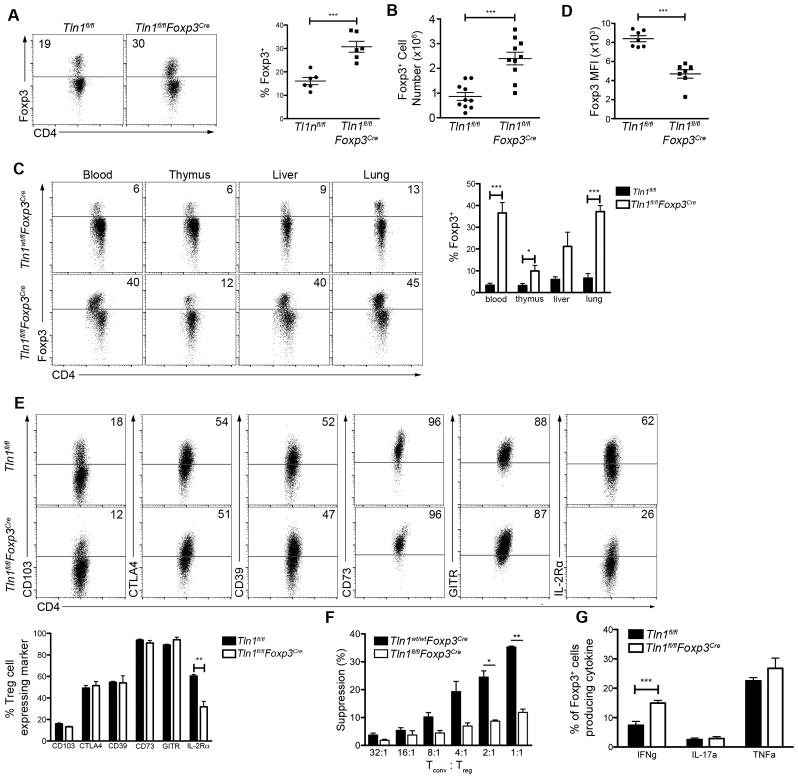
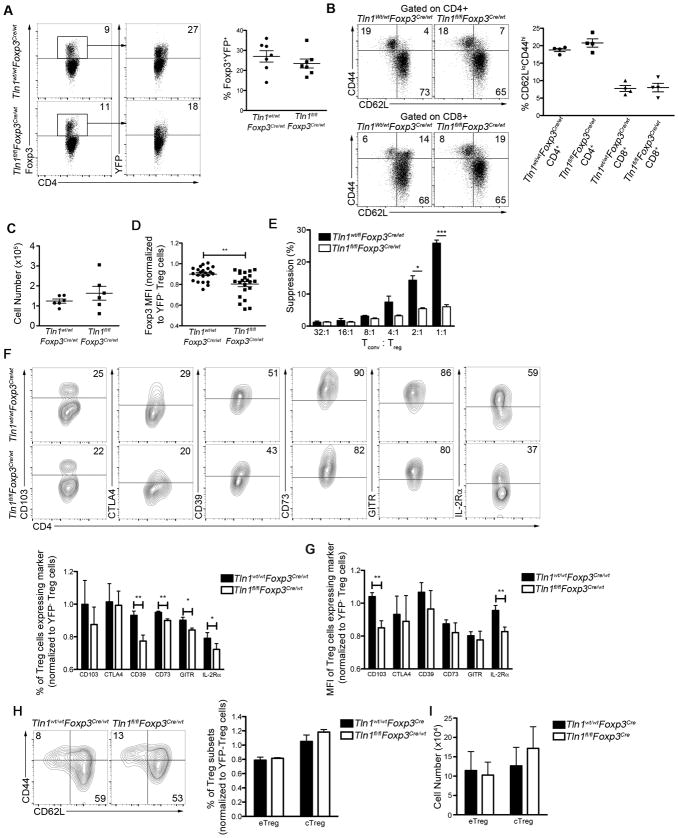
Autoimmune and inflammatory disorders can result from either a deficiency in the number or function of Treg cells. However, male Tln1fl/flFoxp3Cre mice harbored elevated numbers of Foxp3+ cells in the spleen (Fig. 2A, 2B), suggesting that deletion of talin did not decrease the size of the Treg cell population. As integrin signaling is required for T cell trafficking throughout the body, we hypothesized that deletion of talin might prevent Treg cells from homing to organs, resulting in a numerical deficiency that could account for the severe autoimmunity observed in these mice. However, Treg cells were readily detectable in the thymus, blood, liver and lung (Fig. 2C).
Figure 2. Talin deficiency decreases Treg cell suppressive capacity.
(A–B) Percentages (n=6) (A) and numbers (n=10) (B) of splenic CD4+ T cells from Tln1fl/fl or Tln1fl/flFoxp3Cre mice expressing Foxp3. (C) Foxp3+ cells as a percentage of CD4+ T cells within tissues (blood, thymus, liver, lung and lymph node) derived from Tln1fl/fl or Tln1fl/flFoxp3Cre mice; n=6. Mice were perfused prior to isolation of cells from liver and lung. (D) Mean fluorescence intensity of Foxp3 from talin-deficient and wild-type Treg cells; n=7. (E) Expression of putative suppressor molecules on Treg cells from Tln1fl/fl or Tln1fl/flFoxp3Cre mice; displayed cells gated on CD4+Foxp3+ cells; n=6. (F) Suppression by sorted YFP+ Treg cells from Foxp3Cre or Tln1fl/flFoxp3Cre mice at decreasing Tconv:Treg cell ratios, measured at 72 hours. (G) Percentages of splenic Treg CD4+YFP+ cells expressing IFNγ, IL-17A and TNFα; n=6. Data are mean ± SEM and representative of at least 2 independent experiments. *, P < 0.05; **, P <0.01, *** P <0.005, unpaired Student’s t-test.
We next assessed the phenotype and suppressive function of talin-deficient Treg cells in male Tln1fl/flFoxp3Cre mice. Notably, talin-deficient Treg cells expressed less Foxp3 on a per cell basis than wild-type Treg cells (Fig. 2D). Analysis of the expression of putative Treg cell suppressive molecules revealed that with the exception of IL-2Rα, other suppressive molecules including CD103, CTLA4, CD39, CD73, and GITR remained at wild-type levels (Fig. 2E). Nonetheless, despite normal expression of most suppressive molecules, talin-deficient Treg cells isolated from male Tln1fl/flFoxp3Cre animals exhibited significantly impaired suppressive capacity in vitro (Fig. 2F). In addition, talin-deficient Treg cells produced significantly higher levels of IFNγ compared to wild-type Treg cells (Fig. 2G), suggesting the possibility that a subset of Foxp3+ Treg cells may have acquired an inflammatory phenotype as a consequence of talin deficiency. Taken together, these results reveal that expression of talin is essential for Treg cells to maintain immune homeostasis by facilitating their suppressive function.
Activated integrins in Treg cells are required to maintain peripheral tolerance
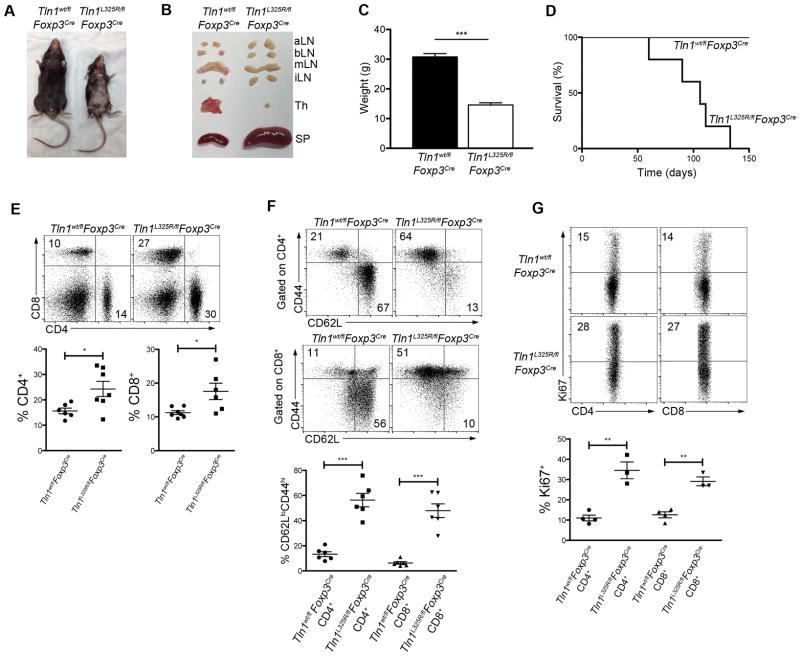
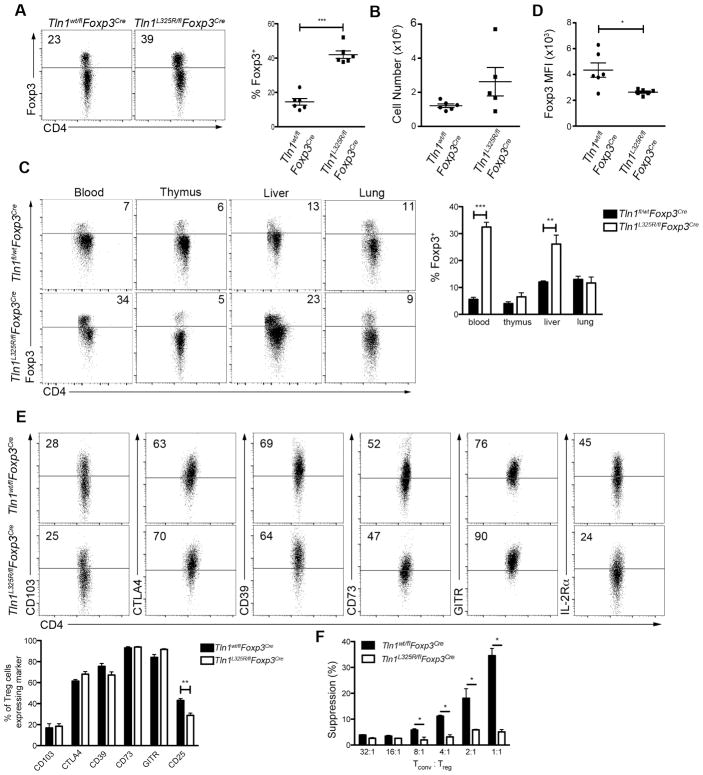
We next sought to investigate the mechanisms by which talin influences Treg cell function. Talin has many binding partners and exerts multiple functions, including binding to and activating integrins, linking integrins to the cytoskeleton, and recruiting signaling molecules such as phosphatidylinositol phosphate kinase (28) and TIAM1 (29) to focal adhesions. The talin(L325R) mutation has been previously shown to inhibit the capacity of talin to activate integrins without disrupting the binding of talin to integrins or affecting the interaction of talin with other known binding partners (21–23, 30). The mutation in talin blocks the conformational change that leads to integrin activation, as it specifically blocks the interaction between the talin head and the membrane proximal region of the β-integrin cytoplasmic tail, but does not disrupt the interaction between the talin head and the distal regions of the β-integrin cytoplasmic tail (21). We therefore generated Tln1L325R/flFoxp3Cre hemizygous male mice to selectively abolish the capacity of talin to activate integrins in Treg cells. Treg cells from Tln1L325R/flFoxp3Cre male animals express half the wild-type level of talin, all of which is the mutant form, and were compared to Tln1wt/flFoxp3Cre control animals, which express half the wild-type level of talin, all of which is in the wild-type form. Importantly, Tln1wt/flFoxp3Cre mice exhibited no evidence of systemic disease. By contrast, Tln1L325R/flFoxp3Cre mice developed systemic autoimmunity resembling that exhibited by male hemizygous Tln1fl/flFoxp3Cre mice (Fig. 3A–C). This inflammation resulted in death of these animals at 3–4 months of ages (Fig. 3D). These mice exhibited increased numbers of activated, proliferating CD4+ and CD8+ T cells (Fig. 3E–G). Moreover, like talin-deficient Treg cells, talin(L325R) Treg cells were not numerically deficient (Fig. 4A, 4B), and were detectable at high numbers in the blood, thymus and peripheral organs (Fig. 4C). Additionally, Treg cells isolated from Tln1L325R/flFoxp3Cre mice exhibited lower Foxp3 expression on a per cell basis (Fig. 4D). With respect to suppressive molecules, talin(L325R) Treg cells were impaired only in IL-2Rα expression (Fig. 4E), similar to talin-deficient Treg cells. Moreover, talin(L325R) Treg cells isolated from Tln1L325R/flFoxp3Cre mice exhibited impaired in vitro suppressive capacity compared to control Treg cell counterparts (Fig. 4F), similar to talin-deficient Treg cells. Together these data indicate that integrin activation, controlled by talin, plays a critical role in mediating Treg cell suppressive function and immune homeostasis.
Figure 3. Expression of mutant talin(L325R) in Treg cells results in lethal systemic inflammation.
(A and B) Morphology (A) or organs (B), spleen, lymph nodes (aortic, brachial, mesenteric, inguinal), and thymi) of male Tln1wt/flFoxp3Cre or Tln1L325R/flFoxp3Cre mice. (C) Body weight of Tln1wt/flFoxp3Cre or Tln1L325R/flFoxp3Cre mice at 8 weeks of age; n=10. (D) Survival of Tln1wt/flFoxp3Cre or Tln1L325R/flFoxp3Cre; n=5. (E) Percentages of splenic CD4+and CD8+ T cells; n=6. (F) Expression of activation markers CD44 and CD62L from splenic CD4+and CD8+ T cells; n=6. (G) Expression of proliferation marker Ki-67 from splenic CD4+and CD8+ T cells; n=3. Data are mean ± SEM and representative of at least 2 independent experiments. *, P < 0.05; **, P <0.01, *** P <0.005, unpaired Student’s t-test.
Figure 4. Integrin activation is critical for Treg cell homeostasis and function.
(A–B) Percentage (A) and by number (B) of splenic CD4+ T cells from Tln1wt/flFoxp3Cre or Tln1L325R/flFoxp3Cre mice expressing intracellular Foxp3; n=6. (C) Foxp3+ cells as a percentage of CD4+ T cells in various tissues (blood, thymus, liver, lung, and lymph node) from Tln1wt/flFoxp3Cre or Tln1L325R/flFoxp3Cre mice; n=6. Mice were perfused prior to isolation of cells from liver and lung. (D) MFI of Foxp3 from CD4+Foxp3+ Treg cells; n=6. (E) Expression of putative suppressor molecules on Treg cells from Tln1wt/flFoxp3Cre or Tln1L325R/flFoxp3Cre mice; displayed cells gated on CD4+Foxp3+; n=4. (F) Suppression by sorted YFP+ Treg cells from Tlnwt/flFoxp3Cre and Tln1L325R/flFoxp3Cre mice at decreasing Tconv:Treg cell ratios, measured at 72 hours. Data are mean ± SEM and representative of at least 2 independent experiments. *, P < 0.05; **, P <0.01, *** P <0.005, unpaired Student’s t-test.
Talin influences Treg cell phenotype and function
Because the inflammatory environment within diseased male Tln1fl/flFoxp3Cre hemizygous mice may have altered Treg cell phenotype and function, we next examined female Tln1fl/flFoxp3Cre/wt heterozygous mice which harbor both Foxp3Cre talin-deficient (YFP+) and Foxp3wt talin-sufficient (YFP−) Treg cell populations as a result of random X chromosome inactivation (Fig. 5A) (26, 31). Owing to the presence of wild-type Treg cells, these female heterozygous mice appeared healthy without evidence of systemic T lymphocyte activation (Fig. 5B), enabling us to examine talin-deficient Treg cells in the absence of inflammation. Like their counterparts in diseased male mice, peripheral Foxp3Cre talin-deficient (YFP+) Treg cells derived from the spleens of healthy female heterozygous mice were present in numbers comparable to control Treg cells (Fig. 5C). To exclude the possibility that Cre expression may adversely reduce Foxp3 expression, we normalized the Foxp3 mean fluorescence intensity (MFI) of talin-deficient Treg cells to the Foxp3 MFI of talin-sufficient Treg cells isolated from the same individual animals (32).
Figure 5. Talin controls the suppressive function of peripheral Treg cells.
(A) Percentages of talin-deficient Foxp3+ and YFP+ Treg cells from Tln1wt/flFoxp3Cre/wt or Tln1fl/flFoxp3Cre/wt mice, displayed cells gated on CD4+ events; n=7. (B) Percentages of splenic CD4+ (top) and CD8+ (bottom) T cells expressing CD44 and/or CD62L from female Tln1wt/wtFoxp3Cre/wt and Tln1fl/flFoxp3Cre/wt heterozygous mice; n=4. (C) Absolute number of Foxp3+YFP+ cells isolated from Tln1wt/wtFoxp3Cre/wt and Tln1fl/flFoxp3Cre/wt heterozygous mice; n=6. (D) Foxp3 MFI of Foxp3+YFP+ Treg cells from Tln1wt/wtFoxp3Cre/wt and Tln1fl/flFoxp3Cre/wt heterozygous mice, normalized to the Foxp3 MFI of Foxp3+YFP− wild-type Treg cells from each individual animal; n=22. (E) Suppression by sorted YFP+ Treg cells from Tln1wt/wtFoxp3Cre/wt or Tln1fl/flFoxp3Cre/wt mice at decreasing Tconv:Treg cell ratios, measured at 72 hours. Expression (F) and MFI (G) of putative suppressive molecules by splenic Treg cells from Tln1wt/wtFoxp3Cre/wt or Tln1fl/flFoxp3Cre/wt mice; displayed cells gated on CD4+Foxp3+YFP+ cells; n=5. (H and I) Percentage (H) and absolute number (I) of eTreg (CD62LloCD44hi) and cTreg (CD62LhiCD44lo) in Tln1fl/flFoxp3Cre/wt and control mice; n=5. Data are mean ± SEM and representative of at least 2 independent experiments. *, P < 0.05; **, P <0.01, *** P <0.005, unpaired Student’s t-test.
Consistent with our observations in inflamed Tln1fl/flFoxp3Cre male mice, we observed a slight reduction in Foxp3 expression on a per cell basis in talin-deficient Treg cells isolated from uninflamed female mice (Fig. 5D). In addition, consistent with our hypothesis that talin is required for Treg cell function, talin-deficient Treg cells were unable to suppress T cell proliferation in vitro (Fig. 5E). In contrast to our observations in inflamed male mice, however, talin-deficient Treg cells isolated from female animals exhibited impaired expression of many putative suppressive molecules, including CD103, CD39, CD73, GITR and IL-2Rα (Fig. 5F, 5G). These data indicate that the inflammatory environment within male Tln1fl/flFoxp3Cre mice alters the phenotype of talin-deficient Treg cells. However, because talin-deficient Treg cells isolated from inflammatory environments were able to maintain expression of each of these suppressive markers, the reduced suppressive capacity we observed in talin-deficient Treg cells was not likely due to the reduced expression of CD103, CD39, CD73, and GITR.
Notably, only talin-deficient Treg cells isolated from the spleen, but not thymi, of healthy female Tln1fl/flFoxp3Cre/wt mice exhibited these defects. Talin-deficient Treg cells isolated from the thymus were present at normal numbers, expressed comparable amounts of Foxp3 to wild-type Treg cells, and did not exhibit defects in expression of any suppressive molecules, indicating that talin is dispensable for Treg cell development in the thymus (Supplemental Fig. 1). Taken together, examination of uninflamed Tln1fl/flFoxp3Cre/wt female mice revealed that talin is required for maintaining high expression of many important Treg cell suppressive molecules in the periphery.
To further explore how integrin signaling may mediate Treg cell suppression, we investigated if talin is required for Treg cell differentiation. Recent work has suggested that Treg cells can be subdivided into central Treg (cTreg) cells and effector (eTreg) cells, based on phenotypic and functional characteristics (33). cTregs, characterized by high expression of CD62L and low expression of CD44, express high levels of the chemokine receptor CCR7, allowing localization and trafficking to lymphoid tissues. cTreg cells are thought to depend on IL-2 signaling for survival, and as such, express high levels of IL-2Rα. eTreg cells are also capable of responding to IL-2 signaling, but are thought to depend more highly on ICOS signaling for survival. eTreg cells are concentrated in peripheral organs, express low levels of CD62L and high levels of CD44, and are thought to be more potently suppressive than cTreg cells. Both populations can be detected in the spleen (11). Our previous work, in which talin was deleted in all T cells revealed a numerical defect in cTreg cells (10), and as such we hypothesized that integrin signaling may be required to maintain the cTreg cell population. However, based on the expression of CD62L and CD44, deletion of talin did not affect the percentage or numbers of cTreg or eTreg cells in the spleen (Fig. 5H, 5I). Thus, integrin signaling is most likely dispensable for Treg cell differentiation. Additionally, talin-deficient cTreg cells expressed significantly less IL-2Rα than control cTreg cells, while talin-deficient eTreg cells express significantly less GITR than control eTreg cells, indicating that talin is required for both subsets (Supplemental Fig. 2).
Talin influences key aspects of the Treg cell transcriptional program
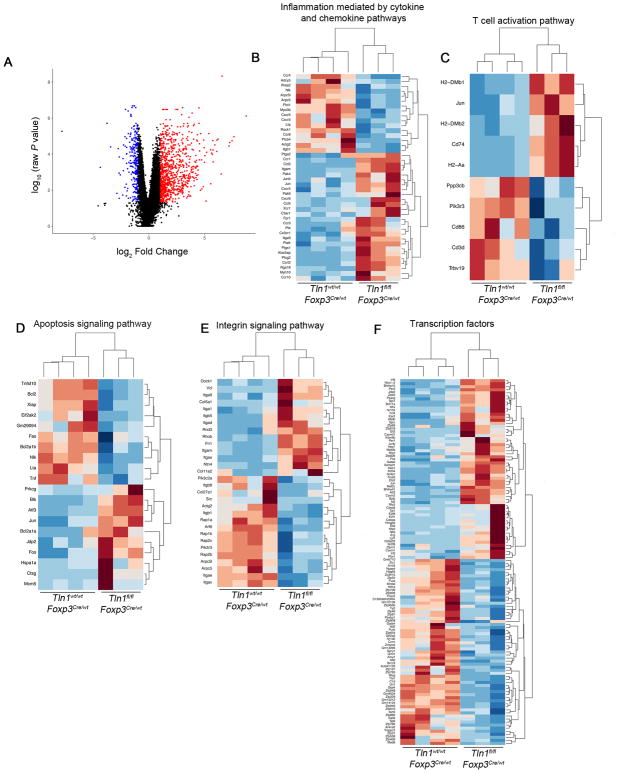
The observation that peripheral but not thymic-derived talin-deficient Treg cells from uninflamed female Tln1fl/flFoxp3Cre/wt mice exhibited reduced suppressive capacity raised the possibility that talin might be required for the maintenance of the Treg cell transcriptional program. To test this hypothesis, we performed RNA-sequencing analysis on YFP+ Treg cells isolated from heterozygous Tln1fl/flFoxp3Cre/wt or Tln1wt/wt Foxp3Cre/wt mice. 1,914 genes (454 upregulated and 1460 downregulated) were differentially expressed (adjusted p value < 0.05) in talin-deficient Treg cells compared to control cells (Fig. 6A, Supplemental Table I). Among these differentially expressed genes were several downregulated transcripts that have been previously reported to influence Treg differentiation and function. These included Treg cell ‘signature’ genes Itgae (CD103) and Nrp1, ‘suppressor’ genes Nt5e (CD73), Il10, and Icos (25, 34–36), and transcriptional regulators such as Id2 (37–39). Gene Ontology (GO) analysis identified differentially expressed transcripts involved in a number of critical Treg cell pathways, including cytokine and chemokine production and signaling, apoptosis, and cell survival (Fig. 6B–D). Moreover, there were significant changes in diverse groups of transcription factors and molecules involved in integrin signaling (Fig. 6E, 6F). Notably, we observed downregulation of multiple integrins including Itgav, Itgae, Itgb1, and Itgb8, indicating that talin may be required for adequate expression of multiple integrin subunits (Fig. 6E). Taken together, these findings suggest that talin is required for the maintenance of diverse aspects of the Treg cell transcriptional program.
Figure 6. Talin modulates multiple aspects of the Treg cell transcriptome.
(A) RNA-seq analysis of mRNA expression in sorted Treg cells isolated from Tlnwt/wtFoxp3Cre/wt (n=4) or Tlnfl/flFoxp3Cre/wt mice (n=3), expressed as log2 normalized counts. Individual points represent genes upregulated (red), downregulated (blue), or not significantly changed (black) in talin-deficient Treg cells (adjusted P-value < 0.05) compared to controls. (B–F) Heatmaps showing fold-change of differentially expressed genes within selected significantly enriched GO categories.
Activation of β integrins enhances Treg cell function and phenotype
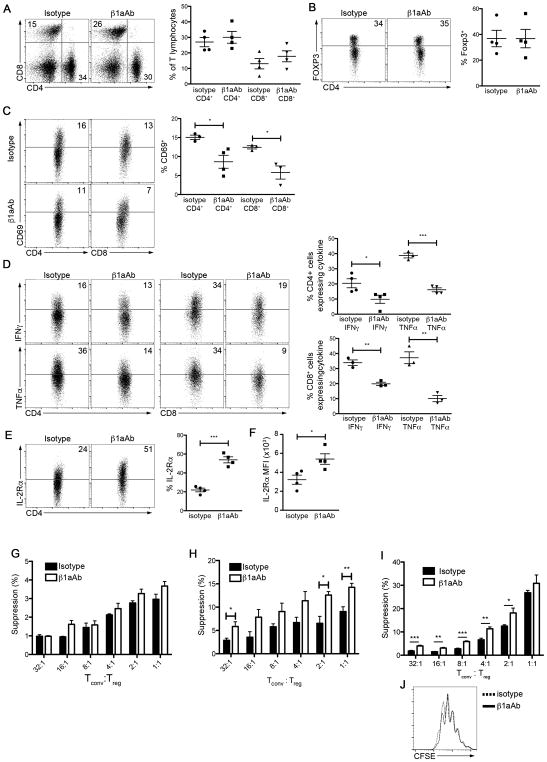
Having observed that disrupting integrin activation resulted in reduced Treg cell function, we sought to test the hypothesis that integrin activation might augment Treg cell function. Integrin-modulating therapy with a β1 integrin-activating antibody (β1aAb) 9EG7, which maintains β1 integrins in their active conformation (40, 41), has been previously shown to exert beneficial effects on cell-matrix interactions in vivo (42). 9EG7 can be used as a reporter of β1 integrin activation and to increase the function of β1 integrins; for example, 9EG7 stimulates and increases the adhesion of multiple cell lines (43, 44), stabilizes the extension of active β1 integrins (45), and increases the maximum ligand binding of β1 integrins by two-fold (46). Treatment of Tln1fl/flFoxp3Cre mice with β1aAb did not alter the proportions of CD4+, CD8+, or Treg cells (Fig. 7A, 7B), and did not prolong the survival of mice (data not shown). Nonetheless, treatment yielded improvement in several immunologic parameters. Percentages of recently activated (CD69+) T lymphocytes and inflammatory cytokine production were reduced in mice treated with β1aAb compared to control-treated mice (Fig. 7C, 7D). Improvements in these parameters were associated with increased expression of IL-2Rα by talin-deficient Treg cells, both on a per cell basis and as a percentage of Foxp3+ cells (Fig. 7E, 7F), thus confirming a role for talin and activated integrins in the maintenance of IL-2Rα expression. However, β1aAb treatment in Tlnfl/flFoxp3Cre mice did not result in improvements to Treg cell suppressive capacity (Fig. 7G). Because Tln1fl/flFoxp3Cre male mice exhibit signs of disease even at weaning at 3 weeks of age, it is likely that Treg cells from these inflamed mice may already be irreversibly impaired.
Figure 7. Integrin-modulating therapy improves Treg cell function and ameliorates inflammation.
(A and B) Percentages of splenic CD4+, CD8+ (A), and Treg cells (B) from male Tln1fl/flFoxp3Cre mice treated with β1aAb (9EG7) or isotype control administered every 5 days for 6 weeks; n=4. (C) Percentages of CD4+ and CD8+ T cells expressing CD69 in β1aAb- or isotype-treated Tln1fl/flFoxp3Cre mice; displayed cells were gated on CD4+ or CD8+ events; n=4. (D) IFNγ and TNFα expression by splenic CD4+ (left) and CD8+ (right) T cells in β1aAb- or isotype-treated Tln1fl/flFoxp3Cre mice; displayed cells were gated on CD4+CD44hi or CD8+CD44hi events; n=4. (E and F) IL-2Rα expression in gated Treg cells from β1aAb- or isotype-treated Tln1fl/flFoxp3Cre mice as a percentage of Foxp3+ cells (E) and on a per cell basis (F); n=4. (G) Suppression by sorted talin-deficent Treg cells from β1aAb- or isotype-treated Tln1fl/flFoxp3Cre mice at decreasing Tconv:Treg cell ratios, measured at 72 hours; cultures were treated daily with β1aAb or isotype control. (H) Suppression by sorted talin-deficent Treg cells from β1aAb- or isotype-treated Tln1fl/flFoxp3Cre/wt mice at decreasing Tconv:Treg cell ratios, measured at 72 hours; cultures were treated daily with β1aAb or isotype control. (I) Suppression by sorted GFP+ Treg cells from β1aAb- or isotype-treated Foxp3GFP mice at decreasing Tconv:Treg cell ratios, measured at 72 hours; cultures were treated daily with β1aAb or isotype control. (J) Proliferation of non-Treg Tconv cells isolated from β1aAb- or isotype-treated Foxp3GFP mice based on CFSE dilution. Data are mean ± SEM and representative of at least 2 independent experiments. *, P < 0.05; **, P <0.01, *** P <0.005, unpaired Student’s t-test.
Thus, to investigate whether β1aAb treatment might improve suppressive function in talin-deficient Treg cells in the setting of an uninflamed environment, we treated female Tln1fl/flFoxp3Cre/wt mice; Treg cells derived from these mice exhibited a significant increase in their in vitro suppressive capacity (Fig. 7H). However, treatment could not rescue suppression to wild-type levels, suggesting that other integrins may also need to be involved in mediating Treg cell suppressive capacity. To further test the role of with β1 integrin activation in Treg cell function, we treated wild-type animals with β1aAb. β1aAb therapy significantly enhanced the suppressive function of wild-type Treg cells in vitro (Fig. 7I) without altering the proliferation of conventional T cells (Fig. 7J). Taken together, these results raise the possibility that agents that activate integrins in Treg cells may be useful in the treatment of autoimmune disease.
DISCUSSION
Peripheral tolerance mediated by Treg cells is governed by multiple diverse mechanisms, including regulation by transcription factors, microRNAs, cytokines, and TCR signaling. Our findings suggest that talin, through activation of integrins, may be another key mediator of peripheral Treg cell homeostasis and function. Here we have shown that mice with a Treg cell-specific deficiency in talin succumbed to fatal systemic autoimmunity. Furthermore, talin-deficient Treg cells isolated from the periphery were functionally impaired, unable to maintain high expression of IL-2Rα, and exhibited global transcriptional dysregulation.
This work has built on our previous study that identified a specific role for talin in Treg cell homeostasis by using Tln1fl/flCd4Cre mice in which talin is deleted in all T cells (10). In the present study, by using a mouse model with a Treg cell-specific deletion in talin or expression of a mutant form of talin, we have been able to determine that it is talin’s role in integrin activation that is required for Treg cell function. There are two notable differences observed between Tln1fl/flCd4Cre mice, described in our prior study, and Tln1fl/flFoxp3Cre mice, which we used in the current study. First, Tln1fl/flCd4Cre mice do not develop systemic autoimmunity, most likely due to defects in talin-deficient non-Treg CD4+ and CD8+ T cells. It has been previously shown that talin-deficient T cells have defects in contact-dependent proliferation (9), which may explain why they are unable to cause disease. Second, talin-deficient Treg cells were numerically deficient in Tln1fl/flCd4Cre mice, but not in Tln1fl/flFoxp3Cre mice. We hypothesize that this difference may be due to the timing at which the deletion of talin occurs in the two mouse strains. In Tln1fl/flCd4Cre mice, talin is deleted during the double-positive stage of thymic development, while in Tln1fl/flFoxp3Cre/wt mice, talin is deleted only after Foxp3 is expressed by developing thymic Treg cells.
Our data indicate that talin plays a critical role in maintaining Treg cell function and phenotype in the periphery, suggesting a specific role for activated integrins in the maintenance of Treg cell identity after thymic development. Phenotypically, we observed downregulation of CD103, CD39, CD73, and GITR on the surface of peripheral Treg cells, which was apparently masked by the presence of inflammation in diseased male Tln1fl/flFoxp3Cre and Tln1L325R/flFoxp3Cre mice, indicating that the upregulation of these molecules is not capable of rescuing talin-deficient Treg cell function. IL-2Rα expression by talin-deficient Treg cells was reduced in both inflamed and uninflamed mice. This finding is intriguing in light of the fact that IL-2 signaling is a central regulator of Treg cell development, homeostasis and suppressive function (11, 38, 47, 48). Moreover, treatment of inflamed Tln1fl/flFoxp3Cre mice with β1aAb partially rescued IL-2Rα expression, indicating a possible role for direct signaling through β1 integrins in maintaining high IL-2Rα expression on Treg cells. The finding that talin-deficient Treg cells expressed significantly less Il10 at the mRNA level compared to wild-type Treg cells is also notable, as the exact mechanisms which control IL-10 production in Treg cells have not yet fully been elucidated. Our findings suggest the possibility that activation of β1 integrins on Treg cells may be required for these cells to produce IL-10.
It has become increasingly appreciated that in addition to their known role in adhesion in migration, integrins play a role in the reprogramming and function of immune cells. Engagement of both VLA-4 and LFA-1 contributes to T cell activation and differentiation, as well as the production of cytokines (49, 50). Germline deletion of β2 integrins reduced the splenic and thymic Treg cell population size and blunted the suppressive capacity of Treg cells both in vitro and in vivo in an adoptive transfer model of colitis (51). Intriguingly, recent studies revealed that LFA-1 expressed by Treg cells exhibits a stronger intrinsic adhesiveness compared to that expressed by conventional T cells, which can be attributed to reduced calpain levels in Treg cells that effectively slows the recycling of integrins from the cell surface (52, 53). Thus, these prior studies suggest that integrin expression and function may be regulated by different mechanisms in Treg cells compared to conventional T cells. Here we extend these prior findings by demonstrating that defective integrin signaling owing to talin deficiency alters Treg phenotype, function, and transcriptional identity. Moreover, activation of VLA-4 with β1aAb increases the expression of IL-2Rα and the suppressive capacity of Treg cells.
All currently available integrin-targeting therapies for autoimmune disease function to block integrin signaling and are thought to function by preventing trafficking of activated effector T cells to sites of inflammation, thereby ameliorating disease (54). The α4 integrin antagonist natalizumab blocks α4β1 (VLA-4) and α4β7 integrins and is approved for the treatment of inflammatory bowel disease (IBD) and multiple sclerosis (MS); the β7 integrin antagonist vedolizumab blocks α4β7 integrin and is approved for treatment of IBD (54). Our data demonstrate that integrin activation may enhance Treg cell function and reduce inflammation, suggesting that the role of integrins may be more nuanced than previously appreciated. Thus, our findings raise the intriguing possibility that activating integrins on Treg cells may represent a new therapeutic strategy in the treatment of autoimmune and inflammatory disorders.
Supplementary Material
Acknowledgments
This work was supported by NIH grants (DK093507, OD008469, and AI095277 to J.T.C.; HL078784 and HL117061 to B.G.P.; HL31950 to M.H.G.) and a Crohn’s and Colitis Foundation of America Senior Research Award (J.T.C.). J.T.C. is a Howard Hughes Medical Institute Physician-Scientist Early Career Awardee. J.E.K. and P.J.M. were supported by NIH grant T32DK007202.
We thank A. Rudensky for providing Foxp3GFP mice. We thank members of the Chang, Petrich and Ginsberg labs for helpful discussion and critical reading of the manuscript.
Footnotes
The raw sequence data presented in this article have been submitted to the National Center for Biotechnology Information Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/); accession number GSE111791
AUTHOR CONTRIBUTIONS
B.G.P., M.H.G., and J.T.C. conceived the project. J.E.K., S.H.K., K.A.R., P.J.M., B.S.B., B.G.P., M.H.G., and J.T.C. designed experiments. J.E.K., S.H.K., K.A.R., Z.H., G.Y., P.J.M., J.N.A., J.M.C., J.L., T.T., J.G.O., and B.S.B performed experiments and data analysis. Y.Z., L.L., and J.D.B. provided critical reagents and advice. J.E.K., B.G.P., M.H.G., and J.T.C. wrote the manuscript.
References
- 1.Zhang Y, Wang H. Integrin signalling and function in immune cells. Immunology. 2012;135:268–275. doi: 10.1111/j.1365-2567.2011.03549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klapholz B, Brown NH. Talin - the master of integrin adhesions. J Cell Sci. 2017;130:2435–2446. doi: 10.1242/jcs.190991. [DOI] [PubMed] [Google Scholar]
- 3.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Critchley DR, Gingras AR. Talin at a glance. J Cell Sci. 2008;121:1345–1347. doi: 10.1242/jcs.018085. [DOI] [PubMed] [Google Scholar]
- 5.Wang JH. Pull and push: talin activation for integrin signaling. Cell Res. 2012;22:1512–1514. doi: 10.1038/cr.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muguruma M, Matsumura S, Fukazawa T. Direct interactions between talin and actin. Biochem Biophys Res Commun. 1990;171:1217–1223. doi: 10.1016/0006-291x(90)90815-5. [DOI] [PubMed] [Google Scholar]
- 8.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 9.Wernimont SA, Wiemer AJ, Bennin DA, Monkley SJ, Ludwig T, Critchley DR, Huttenlocher A. Contact-dependent T cell activation and T cell stopping require talin1. J Immunol. 2011;187:6256–6267. doi: 10.4049/jimmunol.1102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klann JE, Remedios KA, Kim SH, Metz PJ, Lopez J, Mack LA, Zheng Y, Ginsberg MH, Petrich BG, Chang JT. Talin Plays a Critical Role in the Maintenance of the Regulatory T Cell Pool. J Immunol. 2017;198:4639–4651. doi: 10.4049/jimmunol.1601165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liston A, Gray DH. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 12.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 13.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 14.Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T, Verdaguer J, Santamaria P. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 17.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 18.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 22.Haling JR, Monkley SJ, Critchley DR, Petrich BG. Talin-dependent integrin activation is required for fibrin clot retraction by platelets. Blood. 2011;117:1719–1722. doi: 10.1182/blood-2010-09-305433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefanini L, Ye F, Snider AK, Sarabakhsh K, Piatt R, Paul DS, Bergmeier W, Petrich BG. A talin mutant that impairs talin-integrin binding in platelets decelerates alphaIIbbeta3 activation without pathological bleeding. Blood. 2014;123:2722–2731. doi: 10.1182/blood-2013-12-543363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, Tiedt R, Skoda RC, Monkley SJ, Critchley DR, Ginsberg MH. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–3111. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Arsenio J, Kakaradov B, Metz PJ, Kim SH, Yeo GW, Chang JT. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nature immunology. 2014;15:365–372. doi: 10.1038/ni.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- 29.Calderwood DA, I, Campbell D, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–517. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yago T, Petrich BG, Zhang N, Liu Z, Shao B, Ginsberg MH, McEver RP. Blocking neutrophil integrin activation prevents ischemia-reperfusion injury. J Exp Med. 2015;212:1267–1281. doi: 10.1084/jem.20142358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liston A, Lu LF, O’Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. The Journal of experimental medicine. 2008;205:1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franckaert D, Dooley J, Roos E, Floess S, Huehn J, Luche H, Fehling HJ, Liston A, Linterman MA, Schlenner SM. Promiscuous Foxp3-cre activity reveals a differential requirement for CD28 in Foxp3(+) and Foxp3(−) T cells. Immunol Cell Biol. 2015;93:417–423. doi: 10.1038/icb.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, Campbell DJ. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 35.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki M, Miyazaki K, Chen S, Itoi M, Miller M, Lu LF, Varki N, Chang AN, Broide DH, Murre C. Id2 and Id3 maintain the regulatory T cell pool to suppress inflammatory disease. Nature immunology. 2014;15:767–776. doi: 10.1038/ni.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259:40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci U S A. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takada Y, Puzon W. Identification of a regulatory region of integrin beta 1 subunit using activating and inhibiting antibodies. J Biol Chem. 1993;268:17597–17601. [PubMed] [Google Scholar]
- 42.Gerber EE, Gallo EM, Fontana SC, Davis EC, Wigley FM, Huso DL, Dietz HC. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature. 2013;503:126–130. doi: 10.1038/nature12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petridou NI, Skourides PA. A ligand-independent integrin beta1 mechanosensory complex guides spindle orientation. Nat Commun. 2016;7:10899. doi: 10.1038/ncomms10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genova T, Grolez GP, Camillo C, Bernardini M, Bokhobza A, Richard E, Scianna M, Lemonnier L, Valdembri D, Munaron L, Philips MR, Mattot V, Serini G, Prevarskaya N, Gkika D, Pla AF. TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1. J Cell Biol. 2017;216:2107–2130. doi: 10.1083/jcb.201506024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su Y, Xia W, Li J, Walz T, Humphries MJ, Vestweber D, Cabanas C, Lu C, Springer TA. Relating conformation to function in integrin alpha5beta1. Proc Natl Acad Sci U S A. 2016;113:E3872–3881. doi: 10.1073/pnas.1605074113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mould AP, Barton SJ, Askari JA, McEwan PA, Buckley PA, Craig SE, Humphries MJ. Conformational changes in the integrin beta A domain provide a mechanism for signal transduction via hybrid domain movement. J Biol Chem. 2003;278:17028–17035. doi: 10.1074/jbc.M213139200. [DOI] [PubMed] [Google Scholar]
- 47.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim TK, Billard MJ, Wieder ED, McIntyre BW, Komanduri KV. Co-engagement of alpha(4)beta(1) integrin (VLA-4) and CD4 or CD8 is necessary to induce maximal Erk1/2 phosphorylation and cytokine production in human T cells. Hum Immunol. 2010;71:23–28. doi: 10.1016/j.humimm.2009.09.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verma NK, Kelleher D. Not Just an Adhesion Molecule: LFA-1 Contact Tunes the T Lymphocyte Program. J Immunol. 2017;199:1213–1221. doi: 10.4049/jimmunol.1700495. [DOI] [PubMed] [Google Scholar]
- 51.Marski M, Kandula S, Turner JR, Abraham C. CD18 is required for optimal development and function of CD4+CD25+ T regulatory cells. J Immunol. 2005;175:7889–7897. doi: 10.4049/jimmunol.175.12.7889. [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Ganguly A, Mucsi AD, Meng J, Yan J, Detampel P, Munro F, Zhang Z, Wu M, Hari A, Stenner MD, Zheng W, Kubes P, Xia T, Amrein MW, Qi H, Shi Y. Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy. J Exp Med. 2017;214:327–338. doi: 10.1084/jem.20160620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan J, Liu B, Shi Y, Qi H. Class II MHC-independent suppressive adhesion of dendritic cells by regulatory T cells in vivo. J Exp Med. 2017;214:319–326. doi: 10.1084/jem.20160629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016;15:173–183. doi: 10.1038/nrd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.