Abstract
MicroRNA-155 (miR-155) is a small non-coding RNA critical for regulation of inflammation as well as innate and adaptive immune responses. MiR-155 has been shown to be dysregulated in both donor and recipient immune cells during acute graft versus host disease (aGVHD). We previously reported that miR-155 is upregulated in donor T cells of mice and humans with aGVHD and that mice receiving miR-155 deficient (miR155−/−) splenocytes had markedly reduced aGVHD. However, molecular mechanisms by which miR-155 modulates T cell function in aGVHD have not been fully investigated. We identify that miR-155 expression in both donor CD8+ T cells and conventional CD4+ CD25- T cells is pivotal for aGVHD pathogenesis. Using murine aGVHD transplant experiments, we show that miR-155 strongly impacts alloreactive T cell expansion through multiple distinct mechanisms: modulating proliferation in CD8+ donor T cells and promoting exhaustion in donor CD4+ T cells in both the spleen and colon. Additionally, miR-155 drives a pro-inflammatory Th1 phenotype in donor T cells in these two sites, and miR-155−/− donor T cells are polarized towards an IL-4-producing Th2 phenotype. We further demonstrate that miR-155 expression in donor T cells regulates CCR5 and CXCR4 chemokine-dependent migration. Notably, we show that miR-155 expression is crucial for donor T cell infiltration into multiple target organs. These findings provide further understanding of the role of miR-155 in modulating aGVHD through T cell expansion, effector cytokine production, and migration.
Introduction
Acute graft-versus-host disease (aGVHD) is a frequent complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT), with 30-75% of allo-HSCT recipients developing aGVHD (1, 2). The pathogenesis of aGVHD involves a complex cascade of humoral and cellular interactions in which donor T cells target HLA mismatched host tissues, causing tissue injury through secretion of pro-inflammatory cytokines and direct cytotoxicity (3, 4). Even with current immunosuppressive treatments, aGVHD remains a significant cause of morbidity and mortality in HSCT patients, limiting its application as a safe curative therapy for hematologic malignancies and other disorders (5). Thus, novel therapeutic strategies are needed to prevent and cure aGVHD. In order to achieve this goal, the pathogenesis of aGVHD needs to be further elucidated.
MicroRNAs (miRs) are small non-coding RNAs of about 18-24 nucleotides in length that regulate gene expression within the adaptive immune response, including T cell and dendritic cell (DC) differentiation, proliferation, apoptosis, and effector functions (6, 7). Several studies have shown the importance of miRs such as miR-155, miR-146a and b, miR-142, miR-181a, miR-34a, and miR-100 in aGVHD (8–11). Early work identified miR-155 as a critical regulator of inflammation as well as innate and adaptive immune responses (12, 13). In particular, miR-155 is required for normal function of B and T lymphocytes and is up-regulated upon B and T cell activation (13–19). Mice deficient for miR-155 (miR-155−/−) are viable but immunodeficient, exhibiting T cells with attenuated IFN-γ and TNF-α release in response to antigen stimulation (13, 15). Moreover, CD4+ T cells lacking miR-155 expression exhibit bias towards Th2 differentiation, as evidenced by the high levels of interleukins (IL) IL-4 and IL-10 and low levels of IFN-γ and TNF-α (13, 15). MiR-155 has been shown to be dysregulated in both donor and recipient immune cells during aGVHD. One study found that miR-155 is upregulated in activated dendritic cells (DCs) and that miR-155−/− transplant recipients demonstrated decreased GVHD through reduced DC migration and inflammasome activation (20). Previously, we have reported that miR-155 is upregulated in donor T cells of mice and humans with aGVHD. Mice receiving miR-155−/− splenocytes demonstrate significantly reduced aGVHD, while recipients receiving miR-155 overexpressing splenocytes developed rapid and fatal aGVHD (21). These findings suggest that targeting miR-155 ex vivo in donor lymphocytes could be a strategy to mitigate clinical aGVHD. However, these experiments were performed using whole splenocytes and/or unfractionated T cells and therefore did not define the specific T cell subset(s) that are crucial in mediating miR-155 dependent effects on aGVHD. Furthermore, the mechanisms by which miR-155 regulates donor T cell function in aGVHD have not been elucidated.
In this work, using both CD4- and CD8-dependent murine models of aGVHD, we show that miR-155 expression in conventional CD4+ CD25- is critical for miR-155 mediated aGVHD development. Similarly, miR-155 expression in donor CD8+ T cells significantly impacts overall survival and clinical aGVHD severity. Using miR-155−/− murine donors, we have identified multiple distinct mechanisms by which miR-155 modulates donor T cells during aGVHD. First, we show that miR-155 expression in donor T cells is necessary for T cell expansion (modulating proliferation in CD8+ donor T cells and promoting exhaustion in donor CD4+ T cells in both the spleen and colon) during aGVHD. Second, miR-155 expression in donor T cells drives a pro-inflammatory Th1 response with miR-155−/− donor T cells secreting remarkably lower Th1 cytokines TNF-α and IFN-γ while producing significantly higher IL-4 in both the spleen and colon. Lastly, we show that miR-155 expression in donor T cells regulates CCR5 and CXCR4 chemokine-dependent migration and that miR-155 expression is crucial for donor T cell infiltration in end target organs. These findings show that miR-155 modulates aGVHD through T cell expansion, effector function, and migration and provide further understanding into the role of miR-155 in aGVHD.
Methods
Mice
C57BL/6 (B6, H2b), C57BL/6-Tg(CAG-EGFP)1Osb/J (B6 GFP, H2b), Cg-miR-155tm1.1Rsky/j (miR-155−/−, H2b), B6D2F1 (F1, H2b/d), BALB/c (H2d), and C3.SW-H2b/SnJ (H2b) mice were purchased from Jackson ImmunoResearch Laboratories (Bar Harbor, ME). GFP miR-155−/− mice were generated by crossing C57BL/6-Tg(CAG-EGFP)1Osb/J mice with Cg-miR-155tm1.1Rsky/j mice for at least 5 generations to yield GFP+ miR-155−/− progeny. Progeny were confirmed as GFP+ and miR-155−/− by standard DNA PCR using the following primers publically available by Jackson ImmunoResearch Laboratories: GFP Transgene F 5′- AAG TTC ATC TGC ACC ACC G -3′; GFP Transgene R 5′- TCC TTG AAG AAG ATG GTG CG -3′; miR-155−/− Wildtype F 5′- GTG CTG CAA ACC AGG AAG G -3′; miR-155−/− Wildtype R 5′- CTG GTT GAA TCA TTG AAG ATG G -3′; miR-155−/− Mutant 5′- CGG CAA ACG ACT GTC CTG GCC G -3′. All mice were bred and maintained in an OSU animal care facility. For all experiments, mice used were between 8 and 12 wk of age. All animal studies were conducted in accordance with the rules and regulations of the Institutional Animal Care and Use Committee at OSU.
aGVHD murine models
Mice were transplanted under standard protocols approved by the University committee on Use and Care of Laboratory Animals at OSU. Only age- and sex-matched mice were used for transplant experiments. Briefly, 8- to 12-week female F1 mice were irradiated with 1100 cGy administered in 2 fractions to minimize toxicity. T cell depleted bone marrow (BM) cells (10×106) plus 20×106 total splenocytes from GFP+ B6 WT (WT) or GFP+ miR-155 deficient (Cg-miR-155tm1.1Rsky/j, from now on named miR-155−/−) donors were administered via tail vein injection after the radiation (Sup. Fig. 1). The parent to F1 model of aGVHD was chosen because it is a full or major MHC-mismatched model, where aGVHD develops in response to class I, II MHC molecules, and is dependent on mainly CD4+ cells, although CD8+ cells could elicit additional pathology.(22) For CD4+ subset experiments, this protocol was modified; and instead of splenocytes, untouched CD4+ Tconv cells (1×106) or whole T cells depleted of Treg (2×106) from B6 WT or miR-155−/− deficient mice were injected into lethally irradiated F1 (CD4+ Tconv, Treg depleted T cells) recipients. T cells were purified by negative selection using Miltenyi LS columns (Miltenyi Biotec, Auburn, CA) and determined to be have >95% purity. Transplants dominated by CD8+ T cell response involved utilizing C3.SW-H2b/SnJ recipient mice, resulting in a major MHC matched, minor histocompatibility mismatched model. For these experiments, 10×106 T cell depleted bone marrow alone or in conjunction with 20×106 whole splenocytes or 15×106 CD8+ T cells from B6 WT or miR-155−/− donors was injected into lethally irradiated (1100cGy) C3.SW-H2b/SnJ recipients. For experiments in which tissues were collected for fluorescence stereomicroscopy, a second MHC-mismatched aGVHD murine model was performed at the University of Minnesota using university approved animal protocols. Briefly, BALB/c recipients were lethally irradiated with 600 cGy on day −1 and infused on day 0 with 10 × 106 B6 BM cells and 2 × 106 CD25-depleted purified T cells obtained from GFP+ B6 or miR-155−/− mice. Stereomicroscopic imaging was performed as previously described (23) with a Retiga Exi color camera and QCapture software (Qimaging, Burnaby, BC) mounted onto a Leica MZFLIII stereomicroscope using a GFP2 or a GFP/dsRED-bandpass filter and a 1.0× transfer lens (Leica Microsystems, Bannockburn, IL).
Clinical and histologic assessment of aGVHD
Recipient mice were weighed 2-4 times a week and monitored daily for clinical signs of aGVHD and survival. GVHD scores were performed using a system modified from Cooke et al. (24). Briefly, this scoring system incorporates 5 clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity. Individual mice were ear tagged and graded (in a scale from 0 to 3) twice a week. Mice who reached an aGVHD score of more than or equal to 7 were very sick and were euthanized and their tissues harvested. GVHD was also assessed by detailed histopathology analysis of liver and gut tissues using a previously reported scoring system with a range of 0 (absence of signs of GVHD) to 4 (maximal GVHD damage).(25)
Flow cytometry analysis
On days 10 and 21, cohorts of mice were euthanized and splenocytes, whole liver, and lamina propria of the colon were harvested for flow cytometric analysis. Hepatic tissue and colonic lamina propria were digested into a single cell suspension using a commercial mouse liver dissociation kit (Miltenyi Biotec) and mouse lamina propria tissue dissociation kit (Miltenyi Biotec). To select only the donor T cells, a specific gating strategy was used (Sup. Fig. 1). A complete list of antibodies used is listed in Supplemental Table 1. For cytokine evaluation, splenocytes or cells from colonic lamina propria were incubated for 5 hours with eBioscience Cell Stimulation Cocktail (plus protein transport inhibitors) (Thermo Fisher Scientific) for T cell stimulation and protein transport inhibition. Cells were then stained with surface antibodies, permeabilized, fixed, stained with intracellular antibodies and analyzed within 24 hours. Analysis was performed with a FACS LSRII cytometer; FACSDiva software (BD Pharmingen) data analysis was performed using FlowJo (Tree Star).
Migration assay
Splenic T cells were isolated from mice on day 10 post bone marrow transplant for transwell migration assays. Cells (5×105) were washed, placed in a transwell migration chamber (pore size, 5 μm; Costar 3421; Corning) and allowed to migrate for 4 h at 37˚C. For CCR5-dependent chemotaxis, cells were rested for 4 h prior to placement in migration chambers. CCR5 ligands CCL3 (macrophage inflammatory protein-1α; MIP-1α; 100 ng/μL; PeproTech, Rocky Hill, NJ) and CCL5 (RANTES; 100 ng/μL) or CXCR4 ligand CXCL12 (stromal cell-derived factor-1α; SDF-1α; 100 ng/μL) were placed in the lower chamber to induce CCR5- or CXCR4-depedent chemotaxis, respectively. Lower chambers with medium only served as a control for spontaneous migration. The number of cells that migrate across the membrane towards the ligands were quantified using Count Bright Absolute Counting Beads (Thermo Fisher) per manufacturer’s instructions. The migration index (MI) was calculated using the following formula: MI = cells per μL that migrate towards ligand/cells per μL that spontaneously migrate in the absence of ligand.
Statistical analysis
Survival data were analyzed using Kaplan-Meier and log-rank test methods. Differences between continuous variables at a single time point were analyzed using t tests. Differences between groups over time were analyzed using a two-way ANOVA. All tests were two-sided, and statistical significance was declared for p < 0.05. All analyses were performed using GraphPad Prism 6.0.
Results
MiR-155 is indispensable for CD4+ Tconv and CD8+ T cell-mediated aGVHD
Since miR-155 is involved in the regulation of several T cell subsets (CD4+, CD8+ and Treg), our first goal was to identify the T lymphocyte population that is required for miR-155 dependent effects on aGVHD modulation. To evaluate the contribution of miR-155 expression in CD4+ CD25- Tconv cells to aGVHD pathogenesis, we used the well-established CD4-dependent major histocompatibility mismatch model C57BL/6 into B6D2F1. Mice that received miR-155−/− CD4+ Tconv cells showed improved overall survival (Fig. 1A) as well as significantly reduced clinical scores when compared to recipients of WT CD4+ Tconv cells (Fig. 1B). Histopathologically, recipients of WT CD4+ Tconv cells had moderate amounts of mixed inflammatory infiltrate in the periportal regions of the liver and within the lamina propria of the intestines at the time of euthanasia (arrows) which was markedly reduced in the miR-155−/− CD4+ Tconv cell recipients (Fig. 1C). Accordingly, recipients of miR-155−/− CD4+ Tconv cells had significantly lower pathology scores for both the liver and the gastrointestinal (GI) tract (Fig. 1D) when compared to WT recipients. Furthermore, the pathology scores in tissues from mice receiving miR-155−/− CD4+ Tconv cells were comparable to those of mice that received T cell-depleted bone marrow only. These findings demonstrate that miR-155 expression in donor CD4+ Tconv cells is indispensable for aGVHD development.
Figure 1. MiR-155 is indispensable for CD4+ Tconv cell-mediated aGVHD.
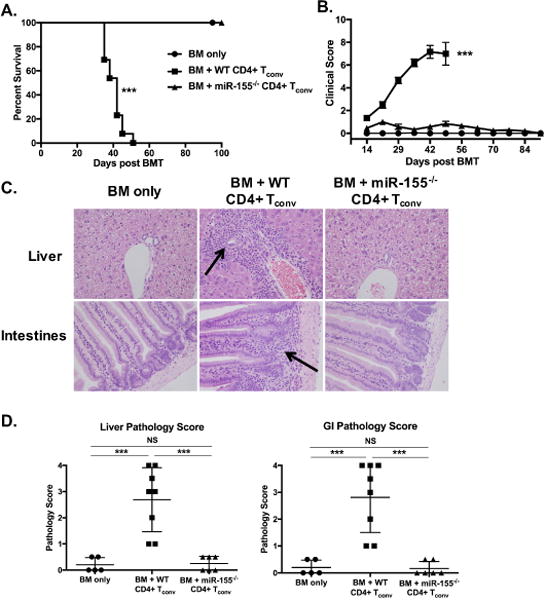
B6D2F1 recipient mice were lethally irradiated (1100cGy) and transplanted with 10×106 T cell-depleted B6 WT BM cells plus 1×106 CD4+ Tconv cells from GFP expressing miR-155−/− or GFP-B6 WT donors. (A) Kaplan-Meier curve of overall survival, with the median survival of recipients of BM + WT naïve CD4+ Tconv cells (n=13) of 42 days as compared to 100% survival of all recipient mice of miR-155−/− CD4+ Tconv cells (n=13) on day 100. (B) Clinical scores. Data for survival and clinical score is a combination of two independent experiments with n=5-7 mice per group. (C) Representative histopathologic images from recipient mice at time of euthanasia. Arrows highlight areas of tissue inflammation. All images at 400× magnification and stained with H&E. (D) Pathology scores for liver and gastrointestinal tract (GI) from recipient mice at time of euthanasia. Data expressed as mean ± SD. One of two independent experiments shown. For all panels, NS p>0.05, * p<0.05, ** p<0.01, and *** p<0.001.
To evaluate whether miR-155 expression in CD8+ T cells is necessary for aGVHD development, we used a CD8-dependent minor histocompatibility mismatch model, B6 into C3.SW-H2b/SnJ that exhibits lethality only when donor splenocytes or whole T cells are transplanted into recipient mice (26–28). Mice that received miR-155−/− splenocytes showed improved overall survival (Fig. 2A) as well as significantly reduced clinical scores compared to recipients of WT splenocytes (Fig. 2B). We also performed a CD8+ fractionation experiment with the B6 into C3.SW-H2b/SnJ model. In concordance with transplanting whole splenocytes, mice that received miR-155−/− CD8+ T cells showed significantly reduced clinical scores and body weight loss when compared to recipients of WT CD8+ T cells (Fig. 2C and D). Overall, this data demonstrates that miR-155 expression in CD8+ donor T cells is instrumental in aGVHD pathogenesis.
Figure 2. MiR-155 expression in donor CD8+ T cells is important for aGVHD.
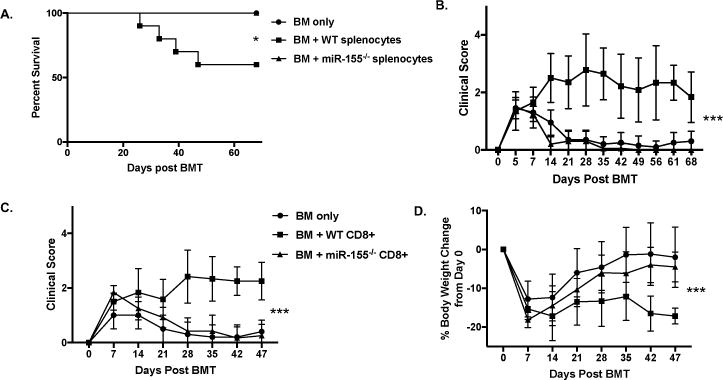
C3.SW-H2b/SnJ recipient mice were lethally irradiated (1100cGy) and transplanted with 10×106 T cell-depleted B6 WT BM cells alone or with 20×106 splenocytes or 15×106 CD8+ T cells from GFP+ miR-155−/− or GFP+ B6 WT donors. N=5-10 for all groups with data from one of two independent experiments shown. (A) Kaplan-Meier curve of overall survival, with the 60% survival of recipients of BM + WT splenocytes (n=10) as compared to 100% survival of all recipient mice of miR-155−/− splenocytes (n=10) on day 68. (B) Clinical scores of BM only and BM + splenocyte recipient mice every 7 days starting on day of transplant. (C) Clinical scores of BM only and BM + CD8+ T cell recipient mice every 7 days starting on day of transplant. (D) Percent change in body weight compared to starting body weight of BM only and BM + CD8+ T cell recipient mice every 7 days starting on day of transplant. P values <0.05 were considered significant using a two-way ANOVA. P values <0.05 were considered significant using a two-way ANOVA, with statistical significance achieved only when comparing B6 WT vs. miR-155−/− donors. For all panels, NS p>0.05, * p<0.05, ** p<0.01, and *** p<0.001.
To further validate our findings, we performed a B6 in to F1 transplant utilizing WT or miR-155−/− donor T cells with or without CD4+ CD25+ Treg depletion. As expected, mice that received Treg depleted WT T cells demonstrated accelerated clinical and pathological aGVHD (Sup. Fig. 2A–C) compared to recipients of WT T cells alone. On the other hand, recipients of miR-155−/− T cells alone as well as miR-155−/− Treg depleted T cells showed similarly improved survival and significantly reduced histopathological scores of target tissues (Sup. Fig. 2D). This data shows that recipients of miR-155−/− T cells demonstrate such a potent decrease in aGVHD that a Treg depletion alone is not able to overcome it and supports the findings that miR-155 expression in Tconv CD4+ CD8+cells is central to aGVHD pathogenesis.
MiR-155 expression is important for donor T cell expansion and exhaustion
Since donor T cell expansion is one of the initiating steps in aGVHD pathogenesis, we sought to investigate the impact of miR-155 on donor T cell proliferation. Lethally irradiated F1 mice that received T cell depleted BM cells along with either WT or miR-155−/− splenocytes were euthanized on days 4, 10 and 21 post-transplant and cell proliferation was measured using Ki67 staining. We confirm our previous findings and show that there was no difference in proliferation at the very early time-point (day 4) post-transplant (Sup. Fig. 3). However, by day 10 and 21 there was significantly reduced proliferation (as evidenced by Ki67 positivity) and absolute numbers of miR-155−/− donor CD8+ T cells compared to WT (Fig. 3A). Similarly, we saw reduced miR-155−/− donor CD4+ T cell numbers compared to WT (Fig. 3B). Surprisingly, miR-155−/− donor CD4+ T cells showed either no difference (day 10) or increased in Ki67 positivity (day 21) compared to WT.
Figure 3. MiR-155 expression in donor T cells is important for their expansion.
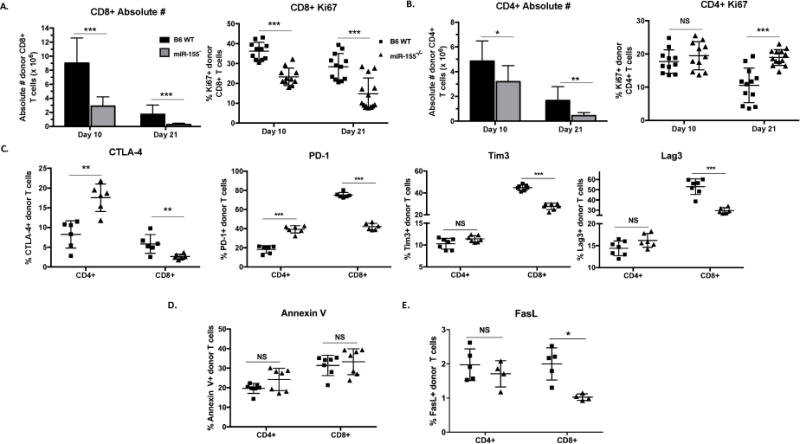
B6D2F1 mice were lethally irradiated with 1100cGy and transplanted with 10×106 B6 WT T cell depleted bone marrow cells and either 20×106 GFP+ B6 WT or GPF+ miR-155−/−. On days 10 and 21, cohorts of mice were euthanized and splenocytes harvested for flow cytometric analysis. N=4-7 mice in each group per experiment for each time point. P values <0.05 were considered significant using Student’s t-test with Holm-Sidak method. For all panels, NS p>0.05, * p<0.05, ** p<0.01, and *** p<0.001. Data expressed as mean ± SD. (A) Absolute numbers and percentage of Ki67+ donor CD8+ T cells on days 10 and 21 post-transplant with data combined from two independent experiments. (B) Absolute numbers and percentage of Ki67+ donor CD4+ T cells on days 10 and 21 post-transplant with data combined from two independent experiments. (C) Percentage of CTLA-4+, PD-1+, Tim3+ or Lag3+ donor T cells on day 10 post-transplant with one of two independent experiments shown. (D) Percentage of donor CD8+ and CD4+ T cells positive for Annexin V on days 10 post-transplant with one of two independent experiments shown. (E) Percentage of FasL+ donor CD8+ and CD4+ on day 10 post-transplant with one of two independent experiments shown.
As T cell expansion is a coordinated balance between active proliferation, exhaustion, and apoptosis, we asked whether miR-155 expression in donor T cells impacts T cell exhaustion and apoptosis. Lethally irradiated F1 mice that received T cell depleted BM cells along with either WT or miR-155−/− splenocytes were euthanized on day 10 post-transplant and co-inhibitory receptor expression on the donor T cells was analyzed by flow cytometry.
Co-inhibitory receptors such as Tim3, Lag3, and PD-1, along with CTLA-4 are important for defining T cell exhaustion, a state which occurs when T cells are exposed to prolonged and persistent inflammatory or antigenic signals (29, 30). We focused on CTLA-4 as it is a known target of miR-155 and a potent inhibitory signal for actively proliferating T cells (18, 31, 32) and also evaluated three other co-inhibitory receptors, PD-1, Tim3, and Lag3. In the spleen, miR-155−/− donor CD4+ T cells expressed significantly higher CTLA-4 and PD-1 (but not Tim3 or Lag3), while miR-155−/− donor CD8+ T cells demonstrated significantly lower expression of all four inhibitory receptors when compared to B6 WT (Fig. 3C).
We then evaluated markers of apoptosis and found that there were no significant differences in Annexin V positivity between miR-155−/− and WT donor CD4+ and CD8+ T cells (Fig. 3D). FasL expression was very low on donor T cells with no significant difference between WT and miR-155−/− donor CD4+ T cells, while miR-155−/− donor CD8+ T cells expressed slightly lower FasL compared to WT (Fig. 3E).
As T cells in the spleen and other end target organs can demonstrate differences in tolerance status, we sought to investigate the expression of co-inhibitory markers on donor T cells in the liver and in the colonic lamina propria. Strikingly, miR-155−/− donor CD4+ T cells demonstrate significantly higher expression of all exhaustion markers analyzed – CTLA-4, PD-1, Tim3 and Lag3 in the colon but not the liver (Fig. 4A and B). Consistent with spleen, miR-155−/− CD8+ donor T cells express lower exhaustion markers compared to WT in the lamina propria and liver (Fig. 4A and B). Taken together, our data demonstrates that miR-155 modulates donor T cell expansion by affecting two distinct mechanisms: proliferation in CD8+ T cells and co-inhibitory receptor expression in CD4+ T cells.
Figure 4. MiR-155 expression in donor T cells is important for modulating their exhaustion status in end target organs.
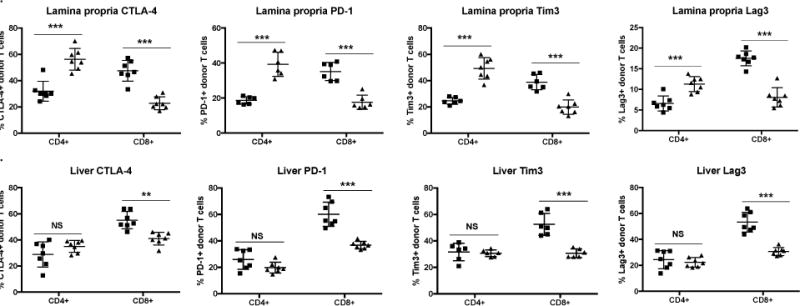
B6D2F1 mice were lethally irradiated with 1100cGy and transplanted with 10×106 B6 WT T cell depleted bone marrow cells and either 20×106 GFP+ B6 WT or GPF+ miR-155−/−. On day 10, a cohort of mice was euthanized and liver as well as colonic lamina propria were harvested for flow cytometric analysis. N= 5-7 mice in each group for each time point. P values <0.05 were considered significant using Student’s t-test with Holm-Sidak method. For all panels, NS p>0.05, * p<0.05, ** p<0.01, and *** p<0.001. Data expressed as mean ± SD. (A) Percentage of CTLA-4+, PD-1+, Tim3+, or Lag3+ donor CD8+ and CD4+ T cells on day 10 post-transplant from colonic lamina propria. (B) Percentage of CTLA-4+, PD-1+, Tim3+, or Lag3+ donor CD8+ and CD4+ T cells on day 10 post-transplant from liver.
MiR-155 expression in donor T cells drives a pro-inflammatory Th1 phenotype
As we saw a decrease in absolute T cell numbers with no significant difference in apoptosis, we sought to investigate the effector functions of these lymphocytes. MiR-155 has been shown to be important for dictating T cell polarization towards a pro-inflammatory Th1 phenotype in other diseases (33–35), therefore, we hypothesized that miR-155 promotes T cell polarization towards a pro-inflammatory phenotype in murine models of aGVHD. To test this hypothesis, we harvested splenocytes and colonic lamina propria lymphocytes 10 and 21 days post-transplant for evaluation of cytokine production. MiR-155−/− donor T cells produced dramatically lower pro-inflammatory Th1 cytokines IFNγ and TNFα (Fig. 5A and B) while producing significantly higher Th2 cytokine IL-4 (Fig. 5C and D) in both the spleen and the colon. These findings support our hypothesis that miR-155 plays a pivotal role in aGVHD pathogenesis by skewing effector cytokine production towards a more pro-inflammatory pathogenic Th1 phenotype.
Figure 5. MiR-155 expression in donor T cells drives a pro-inflammatory Th1 phenotype.
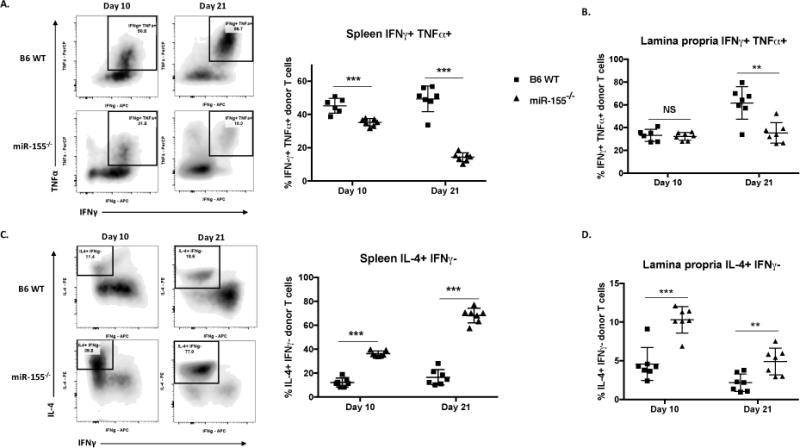
B6D2F1 mice were lethally irradiated with 1100cGy and transplanted with 10×106 B6 WT T cell depleted bone marrow cells and either 20×106 GFP+ B6 WT or GPF+ miR-155−/−. On days 10 and 21, a cohort of mice was euthanized and splenocytes and colonic lamina propria cells harvested for flow cytometric analysis. Splenocytes were plated in RPMI+20% FBS media and incubated for 5 hours with 81nM PMA, 1.34μM ionomycin, 10.6μM brefeldin A, and 2μM monensin, then stained and analyzed within 24 hours. N=4-7 mice in each group per experiment, one of two independent experiments shown for spleen. P values <0.05 were considered significant using Student’s t-test with Holm-Sidak method. For all panels, NS p>0.05, * p<0.05, ** p<0.01, and *** p<0.001. Data expressed as mean ± SD. (A) Representative scatter plots and percentages of IFNγ positive, TNFα positive donor T cells from the spleen on days 10 and 21 post-transplant. (B) Percentages of IFNγ positive, TNFα positive donor T cells from colonic lamina propria on days 10 and 21 post-transplant. (C) Representative scatter plots and percentages of IL-4 positive, IFNγ negative donor T cells from the spleen on days 10 and 21 post-transplant. (D) Representative scatter plots and percentages of IL-4 positive, IFNγ negative donor T cells from colonic lamina propria on days 10 and 21 post-transplant.
MiR-155 expression in donor T cells promotes chemokine-dependent migration
We have previously shown that allo-activated miR-155−/− T cells express less surface CCR5 and CXCR4 chemokine receptors based on mean fluorescent intensity (21). To determine whether miR-155−/− T cells display a difference in chemokine directed migration compared to their WT counterpart, we performed ex-vivo transwell migration assays. Donor T cells were isolated on day 10 from mice receiving WT or miR-155−/− splenocytes and transwell migration assays towards CCR5 and CXCR4 ligands were performed. miR-155−/− T cells exhibit significantly impaired migration towards CCR5 ligands (Fig. 6A) and CXCR4 ligand (Fig. 6B) when compared to WT T cells revealing another facet of miR-155 mediated modulation of T cell function.
Figure 6. MiR-155 expression in donor T cells promotes chemokine-dependent migration.
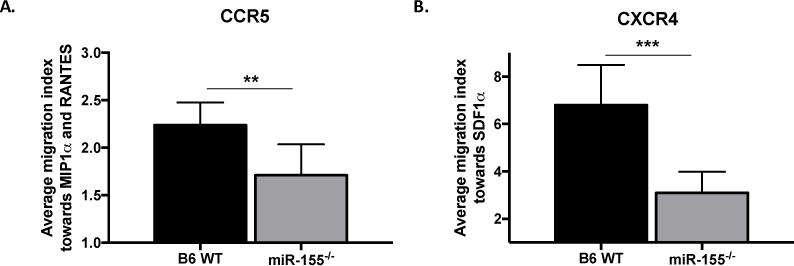
B6 WT and miR-155−/− T cells were isolated from splenocytes harvested on day 10 post bone marrow transplant. Ex vivo transwell migration assays towards CCR5 ligands CCL3 (MIP-1α) (100 ng/μL) and CCL5 (RANTES) (100 ng/μL) and CXCR4 ligand CXCL12 (SDF-1α) (100 ng/μL) were performed. N=4-7 mice per group per experiment. For transwell migration assay, samples were pooled from 1-3 mice with n=2-3, each run in triplicate. (A) Average migration index towards CCR5 ligands MIP-1α and RANTES. (B) Average migration index towards CXCR4 ligand SDF-1α. Data representative of 2 – 4 experiments. Data expressed as mean ± SD. For all panels, NS p>0.05, * p<0.05, ** p<0.01, and *** p<0.001.
MiR-155 expression in donor T cells is important for donor T cell infiltration into end target organs
To better understand how miR-155 modulates aGVHD pathogenesis in vivo, we sought to detect donor T cells in end target organs of mice at different time points during aGVHD disease development. Using a major MHC mismatch model B6 into BALB/c (36), GFP+ B6 WT or miR-155−/− T cells were injected into lethally irradiated BALB/c donors. On days 7, 14, and 21 after allogeneic bone marrow transplantation, mice that received miR-155−/− donor splenocytes had lesser T cells infiltration in end target organs, including mesenteric lymph nodes, liver, lung, skin, and spleen when compared to mice receiving B6 WT splenocytes (Fig. 7A). To quantitate T cell infiltration into end target organs (colon and liver), we used the B6 into F1 model and enumerated absolute number of donor T cells on days 10 and 21 post-transplant. In concordance with our fluorescence stereomicroscopy (Fig. 7A) and splenic donor T cell numbers (Fig. 3A and B), there were significantly less donor CD4+ and CD8+ T cells isolated from the liver and colonic lamina propria of mice receiving miR-155−/− splenocytes at both day 10 and 21 post-transplant when compared to mice receiving B6 WT splenocytes (Fig. 7B and C). These findings clearly show that miR-155 impacts donor T cell migration into end target organs.
Figure 7. MiR-155 expression in donor T cells is pivotal for donor T cell infiltration into target organs.
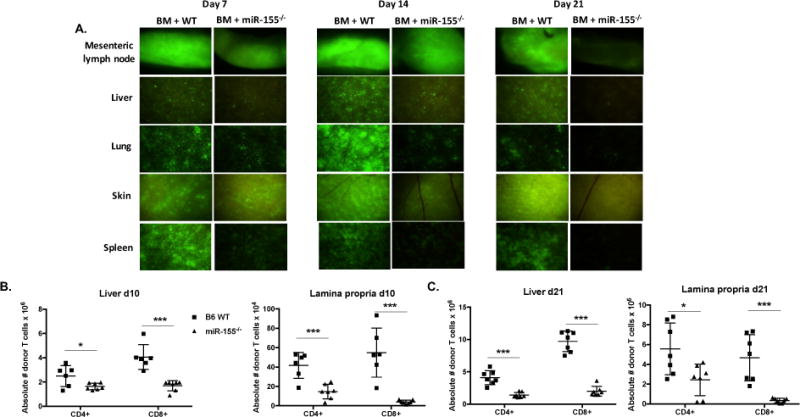
(A) BALB/c mice were lethally irradiated with 700cGy and transplanted with 10×106 B6 WT T cell depleted bone marrow cells and either 2×106 GFP+ B6 WT or GPF+ miR-155−/− CD25-depleted purified T cells. On days 7, 14, and 21, cohorts of mice were euthanized, and tissues taken for GFP (FITC) fluorescence stereomicroscopy. Zoom factors from 3.5× to 10× were used for imaging (3.5× for mesenteric lymph node; 7.0× for liver, spleen, and skin; and 10.0× for lung). N=3 mice per group per time point, with one representative tissue section shown. B6D2F1 mice were lethally irradiated with 1100cGy and transplanted with 10×106 B6 WT T cell depleted bone marrow cells and either 20×106 GFP+ B6 WT or GPF+ miR-155−/− splenocytes. On days 10 and 21, cohorts of mice were euthanized and both the whole liver and colon were harvested. N=6-7 mice in each group for each time point. Single cell suspensions were made of whole liver and colonic lamina propria and were then quantified by flow cytometry using CountBright Absolute Counting Beads (Thermo Fisher Scientific (B) Absolute numbers of donor CD4+ and CD8+ T cells in the liver and colonic lamina propria day 10 post-transplant. (C) Absolute numbers of donor T cells in the liver and colonic lamina propria day 21 post-transplant. For all panels, NS p>0.05, * p<0.05, ** p<0.01, and *** p<0.001.
Discussion
Over the past years several studies have shown the importance of miRs in aGVHD. A small subset of miRs modulate aGVHD pathogenesis by targeting tissue response, such as miR-100 blockade of inflammatory neovascularization within the intestinal tract (8) and miR-34a-induced apoptosis of epithelial cells in the intestines and skin during aGVHD (11). Other miRs play a role in aGVHD pathogenesis by directly affecting immune cells, either intrinsically, such as miR-146a regulation of TNF signaling in donor T cells (10), or extrinsically, such as secreted serum miR-29a which activates dendritic cells through Toll-Like Receptor 7 and 9, resulting in secretion of pro-inflammatory cytokines during aGVHD (9). Other miRs modulate alloreactive T cell responses through multiple processes, such as miR-142, which regulates T cell proliferation, apoptosis, cell cycling, and cytokine secretion (37). Previously, we have reported that miR-155 is upregulated in donor T cells of mice and humans with aGVHD. Mice receiving miR-155 deficient (miR155−/−) splenocytes do not develop aGVHD, while recipients receiving a miR-155 overexpressing T cells developed rapid and fatal aGVHD (21). However, the specific T cell subset(s) that are crucial in mediating miR-155 dependent effects on aGVHD and the mechanisms by which miR-155 regulates donor T cell function in aGVHD have not been fully determined. We sought to investigate the T cell subsets and mechanisms by which miR-155, a versatile microRNA, modulates aGVHD pathogenesis in donor T cells.
Using T cell fractionation experiments (for Tconv CD4+) and miHA mismatch aGVHD mouse models (for CD8+) we show that miR-155 expression is necessary for both CD8+ and Tconv CD4+ mediated aGVHD development. Furthermore, our data supports previous findings that proved miR-155 is dispensable for Treg suppressive activity despite its role to Treg development (38–41). Therapeutically, this provides the potential for ex vivo treatment of T cells with miR-155 antagomiR without compromising Treg suppressive function.
We show that miR-155 strongly impacts alloreactive T cell expansion. Mice receiving miR-155−/− donor cells had lower absolute numbers of donor CD4+ and CD8+ T cells in all three end target organs – spleen, liver, and colon. To investigate the mechanism that contributes to the lower numbers, we evaluated, proliferation, exhaustion, apoptosis, and migration. MiR-155−/− donor CD4+ T cells showed significantly higher expression of co-inhibitory receptors CTLA-4 and PD-1 in the spleen and CTLA-4, PD-1, Tim3, and Lag3 in the colon. In contrast, the lower number of donor CD8+ T was associated with reduced proliferation (lower Ki67 expression), not increased exhaustion.
CTLA-4 is a documented target of miR-155 on CD4+ T cells, with the 3′-untraslated region (3′-UTR) of CTLA-4 transcript containing a binding site for miR-155 (18, 42). It serves as a potent inhibitory signal for CD28:B7 co-stimulation on T cells, leading to reduced proliferation (31, 32, 43). Orencia (abatacept), a CTLA4-Ig fusion protein has shown great promise in preventing aGVHD (44) and clinical trials are ongoing. This suggests that activating multiple co-inhibitory signaling cascades on T cells by targeting miR-155 may serve as a novel therapeutic against aGVHD. In a murine model of experimental autoimmune encephalomyelitis (EAE), deletion of PD-1 in miR-155−/− mice restored susceptibility to EAE, indicating that regulation of PD-1 expression by miR-155 is an important component of T cell mediated inflammation (45). Furthermore, PD-L1 is a predicted target of miR-155 based on transcriptome-wide binding maps (46). As such, further investigation into the interactions between miR-155 and PD-1/PD-L1 inhibitory signaling cascade are warranted. Therefore, we postulate that miR-155 impacts donor T cell expansion via distinct pathways in CD8+ and CD4+ T cells; influencing proliferation in CD8+ T cells and expression of co-inhibitory molecules in CD4+ T cells.
We also clearly demonstrate that miR-155 expression in donor T cells promotes a pro-inflammatory Th1 phenotype. While the observation that miR-155 deficient T cells are polarized towards a Th2 phenotype has been previously described (13, 15), we demonstrate this phenomenon in vivo using a murine model of aGVHD in two separate organs (spleen and colon). MiR-155−/− donor T cells produce significantly more IL-4 and significantly lesser IFNγ and TNF when compared to WT donor T cells. The reduced production of IFNγ and TNFα by miR-155−/− donor T cells could be one of the mechanisms of mitigating aGVHD as neutralization of pro-inflammatory cytokines alone can prevent aGVHD in mouse models of aGVHD (47). The increased production of IL-4 by miR-155−/− donor T cells may ameliorate aGVHD, potentially through modulation antigen-presenting cells (48).
Interestingly, it has been shown that effector memory CD4+ T cells from recipient mice that receive lethal irradiation have the capacity to home to end target organs such as the colon, but demonstrate limited expansion and IFNγ-producing capabilities, resulting in minimal, transient aGVHD (49). Furthermore, given the importance of the GI tract in the priming aGVHD especially early post-transplant (50–52), we believe that the exhausted phenotype of the miR-155−/− donor CD4+ T cells along with the reduced IFNγ and TNFα production by donor T cells in the colon plays an important role in restraining the inflammatory processes required to initiate and sustain aGVHD pathogenesis.
Chemokine receptors, including CCR5, CXCR3, and CXCR6, dictate lymphocyte trafficking to target organs such as liver, GI tract, and skin in aGVHD (26, 53–55) while CXCR4 and its ligand CXCL12 (SDF-1α) are essential for T cell extravasation out of secondary lymphoid organs (56, 57). In fact, Maraviroc, a small molecule CCR5 antagonist shown to prevent lymphocyte chemotaxis, is associated with lower incidence of aGVHD when administered to human patients after allogeneic HSCT, showing that blocking T cell migration is a feasible strategy to prevent aGVHD (53, 58). We establish the critical role of miR-155 in donor T cell migration. We demonstrate that miR-155 expression in donor T cells drives CCR5 and CXCR4 chemokine-dependent migration. Using fluorescence stereomicroscopy, we show reduced miR-155 −/− donor GFP+ T cells in multiple end target organs and validate it further by quantifying absolute number of CD4+ and CD8+ donor T cells in the spleen, liver and colonic lamina propria of recipient mice, showing markedly reduced numbers of miR-155−/− donor T cells compared to mice receiving B6 WT splenocytes. These findings further strengthen our conclusion that miR-155 expression in donor T cells is critical for migration into end target organs.
In summary, we have shown that miR-155 expression in CD4+ CD25- Tconv and CD8+ T cells are essential for miR-155-mediated aGVHD pathogenesis. Additionally, we have identified multiple mechanisms by which miR-155 modulates donor T cells during aGVHD. First, miR-155 is necessary for donor T cell expansion. Reduced CD8+ proliferation and increased CD4+ exhaustion decreases the total number of T cells that can cause direct tissue damage thereby minimizing aGVHD severity. Second, we have shown that miR-155 drives a pro-inflammatory Th1 phenotype in aGVHD, as evidenced by increased TNFα and IFNγ secretion from WT donor T cells, while miR-155−/− donor T cells expectedly display a Th2 IL-4 predominant phenotype. The dramatic reduction in Th1 pro-inflammatory cytokine production along with increased miR-155−/− donor CD4+ T cell exhaustion can severely dampen the inflammatory milieu which might result in inefficient CD8+ T cell priming and proliferation, further mitigating aGVHD. Third, loss of miR-155 in donor T cells significantly impairs end target organ tissue infiltration and chemokine-dependent T cell migration towards CCR5 and CXCR4 ligands. Reduced T cell migration and tissue infiltration has significant implications for aGVHD pathogenesis as lymphocytes must travel to end target organs for target organ damage to occur. If T cell migration is impaired, tissue damage will be minimized and aGVHD clinical signs reduced.
In conclusion, our results provide information about the role of miR-155 in regulating T cell function post-transplant and provide convincing biological rationale to justify investigation of novel antagomiR-155 therapeutics to prevent or minimize aGVHD.
Supplementary Material
Acknowledgments
This work was supported by NIH grants 1R21HL117707-01R01 (Y.E. and R.G.), T32 5T32CA009338-37 (N.C.Z), HL56067, AI344965, HL11879 (B.R.B.); Leukemia and Lymphoma Society Special Fellow Award (P.R.); Leukemia and Lymphoma Society Scholar Award (R.G.) and American Cancer Society Research Scholar Award (R.G.).
References
- 1.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, Litzow MR, Nieto Y, Savani BN, Schriber JR, Shaughnessy PJ, Wall DA, Carpenter PA. First- and Second-Line Systemic Treatment of Acute Graft-versus-Host Disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150–1163. doi: 10.1016/j.bbmt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeiser R, Socié G, Blazar BR. Pathogenesis of acute graft-versus-host disease: from intestinal microbiota alterations to donor T cell activation. Br J Haematol. 2016;175:191–207. doi: 10.1111/bjh.14295. [DOI] [PubMed] [Google Scholar]
- 4.Paczesny S, Hanauer D, Sun Y, Reddy P. New perspectives on the biology of acute GVHD. Bone Marrow Transplant. 2010;45:1–11. doi: 10.1038/bmt.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji Y, Hocker JD, Gattinoni L. Enhancing adoptive T cell immunotherapy with microRNA therapeutics. Semin Immunol. 2016;28:45–53. doi: 10.1016/j.smim.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikete S, Chu X, Wang L, Bian Y. Endogenous and tumour-derived microRNAs regulate cross-presentation in dendritic cells and consequently cytotoxic T cell function. Cytotechnology. 2016;68:2223–2233. doi: 10.1007/s10616-016-9975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonhardt F, Grundmann S, Behe M, Bluhm F, Dumont RA, Braun F, Fani M, Riesner K, Prinz G, Hechinger AK, Gerlach UV, Dierbach H, Penack O, Schmitt-Graff A, Finke J, Weber WA, Zeiser R. Inflammatory neovascularization during graft-versus-host disease is regulated by v integrin and miR-100. Blood. 2013;121:3307–3318. doi: 10.1182/blood-2012-07-442665. [DOI] [PubMed] [Google Scholar]
- 9.Ranganathan P, Ngankeu A, Zitzer NC, Leoncini P, Yu X, Casadei L, Challagundla K, Reichenbach DK, Garman S, Ruppert AS, Volinia S, Hofstetter J, Efebera YA, Devine SM, Blazar BR, Fabbri M, Garzon R. Serum miR-29a Is Upregulated in Acute Graft-versus-Host Disease and Activates Dendritic Cells through TLR Binding. J Immunol. 2017;198:2500–2512. doi: 10.4049/jimmunol.1601778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stickel N, Prinz G, Pfeifer D, Hasselblatt P, Schmitt-Graeff A, Follo M, Thimme R, Finke J, Duyster J, Salzer U, Zeiser R. MiR-146a regulates the TRAF6/TNF-axis in donor T cells during GVHD. Blood. 2014;124:2586–2595. doi: 10.1182/blood-2014-04-569046. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Romero M, Ratajczak P, Lebœuf C, Belhadj S, Peffault de Latour R, Zhao W-L, Socié G, Janin A. Increased apoptosis is linked to severe acute GVHD in patients with Fanconi anemia. Bone Marrow Transplant. 2013;48:849–853. doi: 10.1038/bmt.2012.237. [DOI] [PubMed] [Google Scholar]
- 12.Tili E, Croce CM, Michaille JJ. miR-155 : On the Crosstalk Between Inflammation and Cancer. Int Rev Immunol. 2009;28:264–284. doi: 10.1080/08830180903093796. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for Normal Immune Function. Science (80-) 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao C, Rajewsky K. MicroRNA Control in the Immune System: Basic Principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Thai T, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the Germinal Center Response by MicroRNA-155. Science (80-) 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 16.Yin Q, Wang X, McBride J, Fewell C, Flemington E. B-cell receptor activation induces BIC/miR-155 expression through a conserved AP-1 element. J Biol Chem. 2008;283:2654–2662. doi: 10.1074/jbc.M708218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haasch D, Chen YW, Reilly RM, Grace Chiou X, Koterski S, Smith ML, Kroeger P, McWeeny K, Halbert DN, Mollison KW, Djuric SW, Trevillyan JM. T cell activation induces a noncoding RNA transcript sensitive to inhibition by immunosuppressant drugs and encoded by the proto-oncogene, BIC. Cell Immunol. 2002;217:78–86. doi: 10.1016/s0008-8749(02)00506-3. [DOI] [PubMed] [Google Scholar]
- 18.Sonkoly E, Janson P, Majuri ML, Savinko T, Fyhrquist N, Eidsmo L, Xu N, Meisgen F, Wei T, Bradley M, Stenvang J, Kauppinen S, Alenius H, Lauerma A, Homey B, Winqvist O, Sthle M, Pivarcsi A. MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J Allergy Clin Immunol. 2010;126:581–589. doi: 10.1016/j.jaci.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 19.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Foxp3-Dependent MicroRNA155 Confers Competitive Fitness to Regulatory T Cells by Targeting SOCS1 Protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Smith BAH, Iype J, Prestipino A, Pfeifer D, Grundmann S, Schmitt-Graeff A, Idzko M, Beck Y, Prinz G, Finke J, Duyster J, Zeiser R. MicroRNA-155-deficient dendritic cells cause less severe GVHD through reduced migration and defective inflammasome activation. Blood. 2015;126:103–112. doi: 10.1182/blood-2014-12-617258. [DOI] [PubMed] [Google Scholar]
- 21.Ranganathan P, Heaphy CEA, Costinean S, Stauffer N, Na C, Hamadani M, Santhanam R, Mao C, Taylor PA, Sandhu S, He G, Shana’ah A, Nuovo GJ, Lagana A, Cascione L, Obad S, Broom O, Kauppinen S, Byrd JC, Caligiuri M, Perrotti D, Hadley GA, Marcucci G, Devine SM, Blazar BR, Croce CM, Garzon R. Regulation of acute graft-versus-host disease by microRNA-155. Blood. 2012;119:4786–97. doi: 10.1182/blood-2011-10-387522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy P, Negrin R, Hill GR. Mouse Models of Bone Marrow Transplantation. Biol Blood Marrow Transplant. 2008;14:129–135. doi: 10.1016/j.bbmt.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor PA, Ehrhardt MJ, Lees CJ, Tolar J, Weigel BJ, Panoskaltsis-Mortari A, Serody JS, Brinkmann V, Blazar BR. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD) Blood. 2007;110:3480–3488. doi: 10.1182/blood-2007-05-087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 25.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Duffner U, Lu B, Hildebrandt GC, Teshima T, Williams DL, Reddy P, Ordemann R, Clouthier SG, Lowler K, Liu C, Gerard C, Cooke KR, Ferrara JLM. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp Hematol. 2003;31:897–902. doi: 10.1016/s0301-472x(03)00198-x. [DOI] [PubMed] [Google Scholar]
- 27.Nabekura T, Shibuya K, Takenaka E, Kai H, Shibata K, Yamashita Y, Harada K, Tahara-Hanaoka S, Honda S-i, Shibuya A. Critical role of DNAX accessory molecule-1 (DNAM-1) in the development of acute graft-versus-host disease in mice. Proc Natl Acad Sci. 2010;107:18593–18598. doi: 10.1073/pnas.1005582107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross DBI. Donor T Cell Outcome and Immune Reconstitution Following Post-Transplant Cyclophosphamide and Regulatory T Cell Therapy in Recipients of MHC Matched Allogeneic Hematopoietic Stem Cell Transplants 2012 [Google Scholar]
- 29.Saha A, O’Connor RS, Thangavelu G, Lovitch SB, Dandamudi DB, Wilson CB, Vincent BG, Tkachev V, Pawlicki JM, Furlan SN, Kean LS, Aoyama K, Taylor PA, Panoskaltsis-Mortari A, Foncea R, Ranganathan P, Devine SM, Burrill JS, Guo L, Sacristan C, Snyder NW, Blair IA, Milone MC, Dustin ML, Riley JL, Bernlohr DA, Murphy WJ, Fife BT, Munn DH, Miller JS, Serody JS, Freeman GJ, Sharpe AH, Turka LA, Blazar BR. Programmed death ligand-1 expression on donor T cells drives graft-versus-host disease lethality. J Clin Invest. 2016;126:2642–2660. doi: 10.1172/JCI85796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walunas TL, Bluestone JA. CTLA-4 regulates tolerance induction and T cell differentiation in vivo. J Immunol. 1998;160:3855–3860. [PubMed] [Google Scholar]
- 32.Chan DV, Gibson HM, Aufiero BM, Wilson AJ, Hafner MS, Mi QS, Wong HK. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes Immun. 2014;15:25–32. doi: 10.1038/gene.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Connell RM, Kahn D, Gibson WSJ, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu JY, Zhang J, Ma JZ, Liang XY, Chen GY, Lu R, Du GF, Zhou G. MicroRNA-155-IFN-γ Feedback Loop in CD4+T Cells of Erosive type Oral Lichen Planus. Sci Rep. 2015;5(16935):1–10. doi: 10.1038/srep16935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Cheng Y, Cui W, Li M, Li B, Guo L. MicroRNA-155 modulates Th1 and Th17 cell differentiation and is associated with multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014;266:56–63. doi: 10.1016/j.jneuroim.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder Ma, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. 2011;4:318–333. doi: 10.1242/dmm.006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Oravecz-Wilson K, Mathewson N, Wang Y, McEachin R, Liu C, Toubai T, Wu J, Rossi C, Braun T, Saunders T, Reddy P. Mature T cell responses are controlled by microRNA-142. J Clin Invest. 2015;125:2825–2840. doi: 10.1172/JCI78753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sánchez-Díaz R, Blanco-Dominguez R, Lasarte S, Tsilingiri K, Martín-Gayo E, Linillos-Pradillo B, de la Fuente H, Sánchez-Madrid F, Nakagawa R, Toribio ML, Martín P. Thymus-Derived Regulatory T Cell Development Is Regulated by C-Type Lectin-Mediated BIC/MicroRNA 155 Expression. Mol Cell Biol. 2017;37:e00341–16. doi: 10.1128/MCB.00341-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahl HF, Fauti T, Ullrich N, Bopp T, Kubach J, Rust W, Labhart P, Alexiadis V, Becker C, Hafner M, Weith A, Lenter MC, Jonuleit H, Schmitt E, Mennerich D. miR-155 inhibition sensitizes CD4+ Th cells for TREG mediated suppression. PLoS One. 2009;4:1–10. doi: 10.1371/journal.pone.0007158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 41.Kohlhaas S, Garden Oa, Scudamore C, Turner M, Okkenhaug K, Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J Immunol. 2009;182:2578–2582. doi: 10.4049/jimmunol.0803162. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Sun E, Li X, Zhang M, Tang Z, He L, Lv K. miR-155 contributes to Df1-induced asthma by increasing the proliferative response of Th cells via CTLA-4 downregulation. Cell Immunol. 2017;314:1–9. doi: 10.1016/j.cellimm.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Harper K, Balzano C, Rouvier E, Mattéi MG, Luciani MF, Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991;147:1037–1044. [PubMed] [Google Scholar]
- 44.Koura DT, Horan JT, Langston AA, Qayed M, Mehta A, Khoury HJ, Harvey RD, Suessmuth Y, Couture C, Carr J, Grizzle A, Johnson HR, Cheeseman JA, Conger JA, Robertson J, Stempora L, Johnson BE, Garrett A, Kirk AD, Larsen CP, Waller EK, Kean LS. Invivo T cell costimulation blockade with abatacept foracute graft-versus-host disease prevention: A first-in-disease trial. Biol Blood Marrow Transplant. 2013;19:1638–1649. doi: 10.1016/j.bbmt.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Braun MY. PD-1 deletion restores susceptibility to experimental autoimmune encephalomyelitis in miR-155-deficient mice. Int Immunol. 2014;26:407–415. doi: 10.1093/intimm/dxu043. [DOI] [PubMed] [Google Scholar]
- 46.Loeb GB, Khan AA, Canner D, Hiatt JB, Shendure J, Darnell RB, Leslie CS, Rudensky AY. Transcriptome-wide miR-155 Binding Map Reveals Widespread Noncanonical MicroRNA Targeting. Mol Cell. 2012;48:760–770. doi: 10.1016/j.molcel.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, Cooke KR, Ferrara JLM. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 48.Foley JE, Mariotti J, Ryan K, Eckhaus M, Fowler DH. Th2 Cell Therapy of Established Acute Graft-Versus-Host Disease Requires IL-4 and IL-10 and Is Abrogated by IL-2 or Host-Type Antigen-Presenting Cells. Biol Blood Marrow Transplant. 2008;14:959–972. doi: 10.1016/j.bbmt.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Juchem KW, Anderson BE, Zhang C, McNiff JM, Demetris AJ, Farber DL, Caton AJ, Shlomchik WD, Shlomchik MJ. A repertoire-independent and cell intrinsic defect in murine GVHD induction by effector memory T cells. Blood. 2011;118:6209–6219. doi: 10.1182/blood-2011-01-330035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, Hamada H, Asakura H, Ishikawa H, Matsushima K. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003;4:154–160. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 51.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JLM. Total Body Irradiation and Acute Graft-Versus-Host Disease: The Role of Gastrointestinal Damage and Inflammatory Cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 52.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. [PubMed] [Google Scholar]
- 53.Moy RH, Huffman AP, Richman LP, Crisalli L, Wang XK, Hoxie JA, Mick R, Emerson SG, Zhang Y, Vonderheide RH, Porter DL, Reshef R. Clinical and immunologic impact of CCR5 blockade in graft-versus-host disease prophylaxis. Blood. 2017;129:906–916. doi: 10.1182/blood-2016-08-735076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer LA, Sale GE, Balogun JI, Li D, Jones D, Molldrem JJ, Storb RF, Ma Q. Chemokine Receptor CCR5 Mediates AlloImmune Responses in Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2010;16:311–319. doi: 10.1016/j.bbmt.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato T, Thorlacius H, Johnston B, Staton TL, Xiang W, Littman DR, Butcher EC. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol. 2005;174:277–283. doi: 10.4049/jimmunol.174.1.277. [DOI] [PubMed] [Google Scholar]
- 56.Girard JP, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–73. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 57.Castor MGM, Pinho V, Teixeira MM. The role of chemokines in mediating graft versus host disease: Opportunities for novel therapeutics. Front Pharmacol. 2012;3:1–13. doi: 10.3389/fphar.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reshef R, Luger SM, Hexner EO, Loren AW, Frey NV, Nasta SD, Goldstein SC, Stadtmauer EA, Smith J, Bailey S, Mick R, Heitjan DF, Emerson SG, Hoxie JA, Vonderheide RH, Porter DL. Blockade of Lymphocyte Chemotaxis in Visceral Graft-versus-Host Disease. N Engl J Med. 2012;367:135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


