Abstract
Helper versus cytotoxic T lineage decision in the thymus has been studied as a model for silencing of alternative lineage genes. While the transcription factor RUNX3 is required for the initiation of Cd4 silencing in developing CD8 T cells, it is unknown how silencing of Cd4 and other helper T lineage genes is maintained. We show that the histone methyltransferase G9a is necessary for silencing of helper T lineage genes in proliferating mouse CD8 T cells. Despite normal initial Cd4 downregulation, G9a-deficient CD8 T cells de-repress Cd4 and other helper lineage genes during repeated division in lymphopenia or in response to tumor Ag. However, G9a was dispensable for continued silencing of those genes in CD8 T cells that respond to infection by L. monocytogenes. These results demonstrate that G9a facilitates maintenance of cellular identity of CD8 T cells during cell division, which is further reinforced by inflammatory signals.
Introduction
During a binary fate decision, genes related to the opposing lineage are heritably silenced (1, 2). This silencing is achieved through the constitutive activity of transcription factors involved in the lineage determining process or by recruitment of epigenetic machinery in a locus-specific manner, presumably by those lineage-specific transcription factors. The differentiation of the common thymocyte precursor to the helper or the cytotoxic T cell lineage in the thymus has been studied to understand the requirements for transcription factors and epigenetic gene regulation for stable lineage decisions (1-9). CD4+ CD8+ double positive (DP) thymocytes are subjected to positive selection of rearranged TCRαβ by self-peptides presented on MHC class II or I (MHC-II or -I), and differentiate into cells in the helper or cytotoxic T lineages, respectively. MHC-I-selected thymocytes express the transcription factor RUNX3 that establishes the silencing of helper T lineage genes, including Cd4 and Zbtb7b (10-14).
However, it is poorly understood how helper T lineage-associated genes are heritably silenced in mature cytotoxic T cells. During thymocyte development, Cd4 is transiently repressed by RUNX1, an orthologue of RUNX3, in CD4− CD8− double negative (DN) thymocytes via direct binding to the silencer cis-element in the locus (11). This repression is subsequently reversed upon selection of a successfully rearranged Tcrb locus (15). Cd4 is expressed uniformly in DP thymocytes that give rise to helper and cytotoxic T lineage cells after positive selection. While Cd4 continues to be expressed in helper lineage T cells, CD8+ cytotoxic T cells terminate Cd4 transcription by upregulating RUNX3, which binds the identical cis-element as RUNX1 (11). Deletion of the silencer element or disrupting RUNX binding sites in the silencer results in continued Cd4 expression in CD8 T cells (16, 17). However, deletion of the silencer or Runx3 in differentiated CD8 T cells does not reactivate Cd4, indicating that the initial repression but not maintenance of Cd4 silencing requires RUNX3 (16, 18). RUNX3 may therefore recruit epigenetic modifications to the Cd4 locus and loci encoding helper-lineage genes, which are maintained independently of RUNX3. Since the Cd4 locus is only reversibly repressed in DN thymocytes but irreversibly silenced in CD8 T cells, we hypothesized that the irreversible silencing is mediated by epigenetic modifiers that specifically interact with RUNX3 but not RUNX1.
In this study, we identified the histone methyltransferase (HMT) G9a as an epigenetic modifier that preferentially interacts with RUNX3 to RUNX1 and is necessary for continued silencing of helper lineage genes in dividing CD8 T cells under non-inflammatory conditions. G9a-deficiency resulted in de-repression of several genes, which are otherwise expressed only in CD4 T cells, while it was compensated for by the inflammatory cytokine IL-12. These results suggest that G9a and inflammatory cues cooperatively maintain the identity of CD8 T cells during their division.
Materials and methods
Mice
C57BL/6N (B6N) and B6-CD45.1 mice were purchased from Charles River. OT-I mice (19) were purchased from Taconic. Cd4-cre (10) and CD8-cre (E8I-cre) (20) mice were obtained from D. Littman (New York University). Ehmt2-flox mice were previously described (21). All mice were generated in or have been backcrossed more than 8 times to B6. Unless otherwise specified, littermate cre+ Ehmt2+/+ or cre– Ehmt2F/F were used as control. All mice were maintained in the specific pathogen-free facility at Washington University School of Medicine. All experiments were conducted following a protocol approved by the Washington University Animal Studies Committee.
Co-immunoprecipitation
1200M and AKR1 cell lines were transduced with MSCV-based retrovirus as described (22). For interactome analyses, 1200M cells in which endogenous Runx1 expression had been knocked down (22), were transduced with FLAG-HA-tagged RUNX1 or RUNX3. RUNX1- and RUNX3-interacting proteins in nuclear extract were immunoprecipitated with anti-FLAG beads (M2, Sigma), eluted with 3xFLAG peptide (GenScript), and analyzed by mass spectrometry at the Taplin Mass Spectrometry Facility at Harvard University. For analytical immunoprecipitation, nuclear proteins were extracted from AKR1 cells that were transduced with RUNX1, RUNX3, RUNX1 and FLAG-tagged G9a retrovirus (FLAG-G9a), or RUNX3 and FLAG-G9a. Immune complexes containing FLAG-tagged protein were precipitated with anti-FLAG, followed by immunoblotting using anti-FLAG and anti-pan-RUNX Abs (12).
Flow cytometry
The following mAbs were purchased from Biolegend: APC conjugated anti-CD62L (MEL-14); APC-Cy7 conjugated CD45.2 (104); FITC conjugated anti-CD62L (MEL-14), -Vα2 (B20.1); Pacific Blue conjugated anti-CD44 (IM7); PE conjugated anti-Vβ5 (MR9-4), -IFN-γ (XMG1.2); PE-Cy7 conjugated anti-CD8a (53.6.7); PerCP-Cy5.5 conjugated anti-CD4 (GK1.5), -CD90.1 (OX-7). Cells were analyzed with an LSR II or an LSR Fortessa or sorted with a FACS Aria II (BD). Dead cells were excluded by staining with DAPI (Sigma) or Aqua Live/Dead (Life Technologies). Data were analyzed on FlowJo software (TreeStar).
T cell transfer, tumor inoculation, and L. monocytogenes (Lm) infection
Naive CD8 cells from OT-I TCR transgenic mice were sorted by flow cytometry as Vα2+ Vβ5+ CD62L+ CD44lo/– CD4– CD8+ cells. 2 × 105 cells were transferred i.v. to Tcrb–/–Tcrd–/– mice. PBMCs and splenocytes were isolated from the recipient mice 4 weeks after transfer and analyzed for surface marker and gene expression. For experiments with transplanted tumors, 1 × 106 E.G7-OVA (ATCC #CRL-2113) cells were injected s.c. in the flank of B6-CD45.1 mice. 5 days later, 1 × 106 OT-I cells were transferred i.v. to the tumor bearing mice followed by analysis of T cells collected from the draining lymph node 7 days later. For Lminfection, 5 × 103 OT-I cells were transferred i.v. into B6-CD45.1 mice, which were infected i.v. with 2 × 104 CFU of Lm expressing OVA (Lm-OVA) on the next day as described (23).
Quantitative RT-PCR
Total RNA was purified using Trizol (Life Technologies) and reverse-transcribed using qScript (QuantaBio). Gene expression was quantitated using a Luminaris SYBR green reagent (Thermo Fisher) and a Roche LightCycler 480. Primer sequences are listed in Supplemental Table 1.
RNA-sequencing (RNA-seq)
RNA-seq was done essentially as described previously (24) using total RNA extracted from 5×104 Ehmt2−/− and Ehmt2+/+ Vα2+ CD8+ T cells purified from Tcrb−/−Tcrd−/− recipient mice 4 weeks after transfer. Sequenced tags were mapped to the mouse genome mm9 using Tophat (25) with default setting, followed by transcript assembly and estimation of expression levels using Cufflinks (26-29) on Galaxy (https://usegalaxy.org/).
Chromatin immunoprecipitation (ChIP)
Mono nucleosomes were prepared from cultured CD8 T cells by micrococcal nuclease digestion as described (30). H3K9me3-modified nucleosomes were immunoprecipitated using anti-H3K9me3 (Abcam 8898) conjugated with Protein G magnetic beads (Life Technologies). For genome-wide analysis, purified DNA from precipitated nucleosomes was sequenced with a HiSeq 2500 sequencer (Illumina) with a 50-bp single end read option as described (23).
Statistical Analysis
All statistics were performed using Graphpad Prism (version 7.0) using non-parametric two-tailed student T-tests for comparing 2 groups. Multiple groups were analyzed using two-tailed ANOVA. All other statistics were performed as described in the manuscript. Statistical analyses are shown with the mean±SD. p-values smaller than 0.05 were considered significant.
Results and Discussion
G9a forms a complex with RUNX3
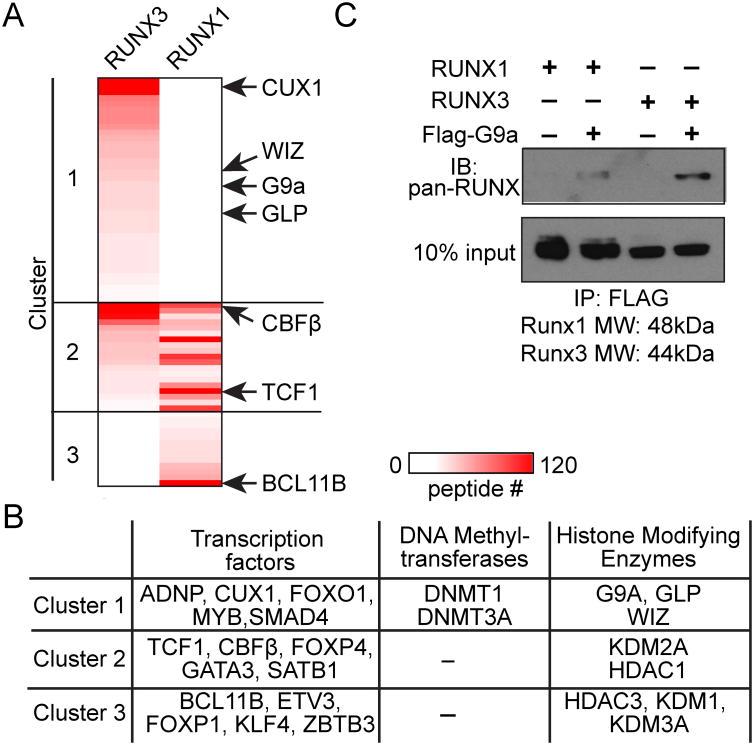
To identify candidate epigenetic modifiers that are recruited by RUNX3, we analyzed RUNX1- and RUNX3-intractomes in 1200M thymoma cells, which have active Cd4 silencing machinery (15). Among 71 DNA-binding proteins and epigenetic modifiers identified in RUNX1- or RUNX3-interactomes, all three components of the G9a HMT complex, G9a, GLP and WIZ as well as a known G9a-interacting transcription factor CUX1 (31) were found predominantly in the RUNX3-interactome (Fig. 1A, 1B). Preferential interaction between G9a and RUNX3 compared to RUNX1 was confirmed by co-immunoprecipitation against FLAG-G9a and immunoblotting with anti-pan-RUNX Ab that detects both RUNX1 and RUNX3 (Fig. 1C). These data show that G9a and RUNX3 form a complex, which may deliver the HMT activity to Cd4 and other helper lineage-related genes repressed by RUNX3 in developing CD8 T cells.
Figure 1. G9a preferentially forms a complex with RUNX3 to RUNX1.
(A) A heat map showing the 71 DNA-interacting proteins that were co-immunoprecipitated with only RUNX3 (cluster 1), RUNX1 and RUNX3 (cluster 2), or only RUNX1 (cluster 3) from transduced 1200M cells. (B) A list of the transcription factors, DNA methyltransferases, and histone modifying enzymes in (A). (C) Immunoblotting (IB) for RUNX proteins co-immunoprecipitated with FLAG-G9a from lysates of AKR1 cells transduced with RUNX1, RUNX3 or FLAG-G9a. Blots are representative of 2 experiments.
G9a is required for silencing of helper lineage-associated genes in proliferating CD8 T cells in vivo under non-inflammatory conditions
To define the role of G9a in CD8 T cells, we conditionally inactivated Ehmt2, encoding G9a, at the DP stage of thymocyte development using Cd4-cre. Ehmt2 mRNA was barely detectable in CD8+ mature thymocytes from Ehmt2F/F Cd4-cre mice (data not shown)(referred to as Ehmt2−/− CD8 T cells hereafter). As previously reported using pLck-cre (32), numbers and frequencies of total thymocytes and CD4+ and CD8+ splenic T cells were comparable between Ehmt2−/− and control Ehmt2+/+ mice (data not shown). Different from the reported phenotype in Runx3−/− thymocytes (11, 14), CD4 was normally downregulated in mature CD8 thymocytes, and was not expressed in splenic naive or memory CD8 T cells under steady state conditions (data not shown).
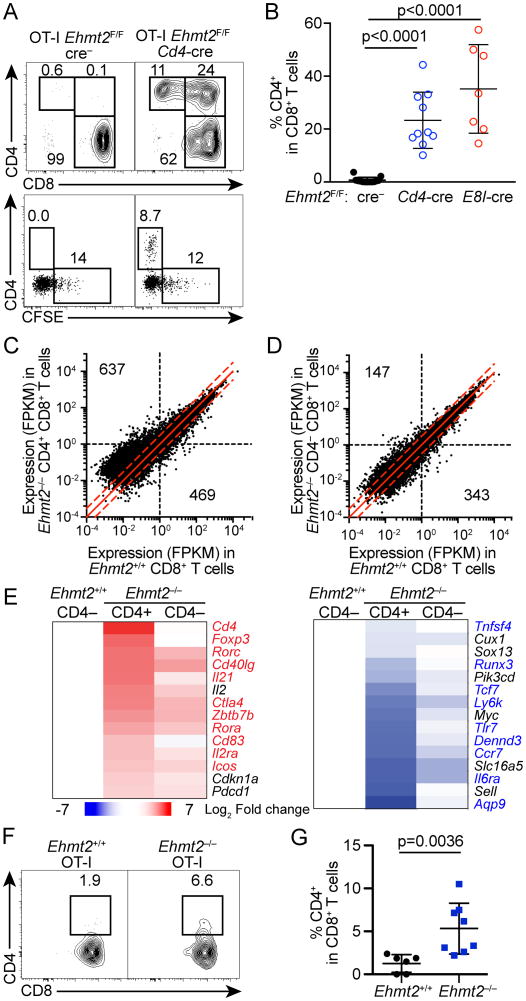
To determine whether G9a is required for maintaining Cd4 silencing during cell division, we adoptively transferred Ehmt2−/− or control Ehmt2+/+ naïve CD4– CD8+ T cells expressing the OT-I TCR transgene into Tcrb−/−Tcrd−/− mice, in which donor-derived CD8 T cells divide under non-inflammatory conditions. By four weeks after transfer, both Ehmt2−/− CD8 T cells expanded at similar rates if not faster than control Ehmt2+/+ cells as determined by CFSE, and repopulated in the recipients' peripheral blood (Fig. 2A, data not shown). While Ehmt2+/+ CD8 T cells remained CD4-negative, approximately 30% of Ehmt2–/– CD8 T cells that had diluted CFSE beyond the limit of detection upregulated CD4 (Fig. 2A, 2B). Since a similar result was observed with transferred Vα2+ CD8 T cells from Ehmt2F/F CD8-cre OT-I mice, in which Ehmt2 was deleted after positive selection (Fig. 2B), it is unlikely that Cd4 de-repression is secondary to deregulated thymocyte selection in the absence of G9a.
Figure 2. G9a is required to maintain silencing of helper lineage genes in CD8 T cells during lymphopenia- or tumor Ag-driven proliferation.
(A, B) CD4 expression and CFSE dilution of CD8 T cells in PBMC of Tcrb–/–Tcrd–/– mice that received Ehmt2–/– or Ehmt2+/+ OT-I T cells 4 weeks prior to the analysis. Data are pooled from 3 experiments in which one donor of each genotype was transferred into 2-3 recipients. (C, D) RNA-seq analysis of CD4+ CD8+ Ehmt2–/–, CD4– CD8+ Ehmt2–/– and CD4– CD8+ Ehmt2+/+ OT-I T cells harvested from Tcrb–/–Tcrd–/– mice 4 weeks after transfer. Quantification of genes with ≥1 FPKM in Ehmt2–/– or Ehmt2+/+ samples and >2-fold difference in expression is indicated for each genotype. Dashed red lines: 2-fold change between genotypes. (E) Heat maps showing genes differentially expressed between CD4+ CD8+ or CD4– CD8+ Ehmt2–/– and control Ehmt2+/+ CD8 T cells. Values represent the log2 fold change of the mean of 2-4 mice compared to Ehmt2+/+ CD8 T cells. Highlighted genes represent genes differentially expressed between CD4 and CD8 memory T cells from ImmGen datasets. (F, G) Expression of CD4 of OT-I T cells in the lymph node draining transplanted E.G7-OVA tumors. n=6-8 in 2 experiments.
To determine whether Ehmt2−/− CD8 T cells de-repressed additional helper lineage genes, global gene expression in CD4+ CD8+ and CD4– CD8+ Ehmt2–/– T cells as well as control Ehmt2+/+ CD8+ T cells 4 weeks after transfer was profiled by RNA-seq. Approximately 1,100 genes were differentially expressed by greater than 2-fold with the majority (637 genes) being upregulated in Ehmt2–/– CD8 T cells compared to Ehmt2+/+ CD8 T cells (Fig. 2C, 2D). Among the genes that were differentially expressed between CD4 and CD8 memory T cells in the Immgen datasets (>1.8-fold difference), 92 genes that are more highly expressed in CD4 T cells, including Cd4, Foxp3, Cd40lg, Rorc, Rora, Zbtb7b and Il21, were de-repressed in the Ehmt2–/– CD4+ CD8+ T cells (Fig. 2E). We also observed downregulation of 18 genes that are more highly expressed in CD8 T cells in the absence of G9a (Fig. 2E), suggesting that G9a also contributes to turning-on genes in CD8 T cells directly or indirectly. A similar change in gene expression, albeit to lesser extent, was also observed in Ehmt2–/– CD4– CD8+ T cells in which we have confirmed Ehmt2 deletion was also nearly complete (Fig. 2E, data not shown). In Ehmt2–/– CD8 T cells, the level of total H3K9me2 was substantially reduced whereas that of H3K9me3 was unchanged (Supplemental Fig. 1A). In addition, we did not observe a reduction in H3K9me3 deposition near transcriptional start sites of the genes that were upregulated in CD4+ CD8+ Ehmt2–/– T cells compared to Ehmt2+/+ CD8 T cells (Supplemental Fig. 1B). Cd4 upregulation was also observed in Ehmt2–/– OT-I T cells in response to E.G7-OVA tumor cells transplanted to WT mice (Fig. 2F, 2G). These results suggest that de-repression of helper-lineage genes in proliferating Ehmt2–/– CD8 T cells occurs also in lymphocyte-repleted mice although it may not continue once they slow down or stop division, such as memory CD8 T cells under steady-state conditions. Collectively, these data indicate that the G9a is required for continued silencing of a subset of helper lineage-associated genes in dividing CD8 T cells, which appears independent of H3K9me3. Since the G9a/GLP complex recruits PRC2 to its repressive target loci in embryonic stem cells (33), these helper lineage-associated genes may be kept silenced through the G9a-mediated recruitment of PRC2 activity.
G9a is dispensable for silencing of helper lineage genes in the presence of strong TCR or IL-12R signals
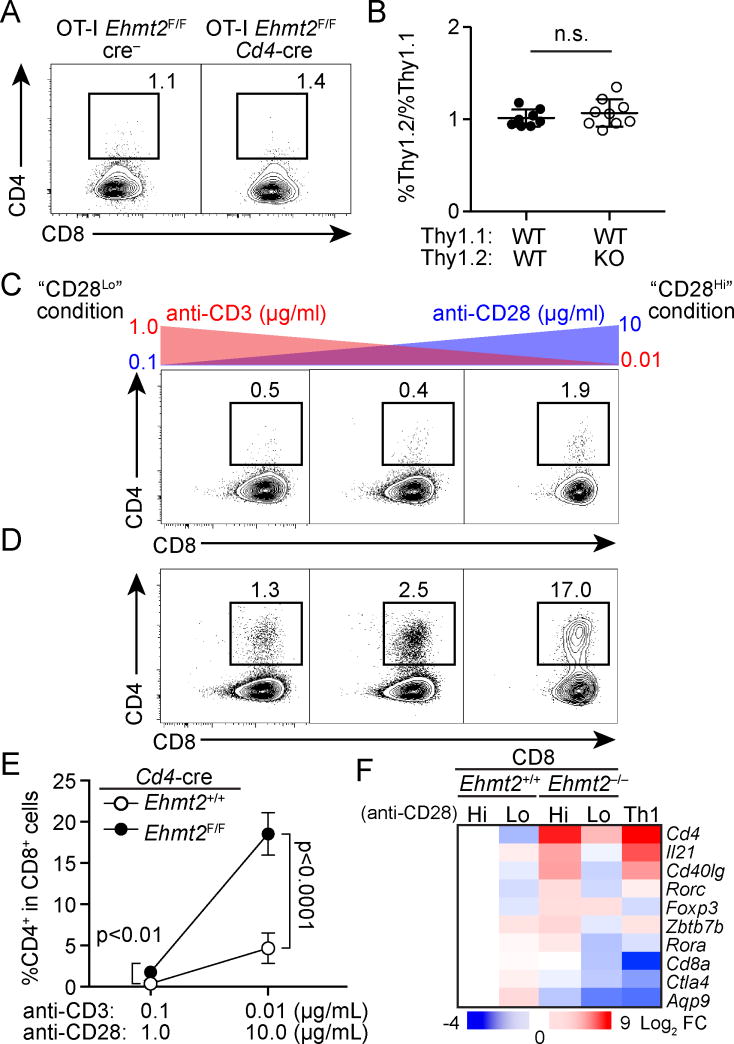
To determine whether G9a is required for continued Cd4 silencing in dividing CD8 T cells in response to infection, Ehmt2–/– or Ehmt2+/+ OT-I T cells (Thy1.2/CD45.2) mixed with internal control congenic OT-I T cells (Thy1.1/CD45.2) were transferred into CD45.1 WT mice, which were subsequently infected with Lm-OVA. In contrast to lymphopenia- and tumor-driven proliferation, Ehmt2–/– OT-I T cells remained CD4-negative (Fig. 3A). In addition, we observed comparable expansion and the ability to produce IFN-γ of Ehmt2–/– and Ehmt2+/+ OT-I T cells relative to control OT-I T cells (Fig. 3B, data not shown). These results indicate that G9a is dispensable for CD8 T cells under inflammatory conditions to maintain Cd4 silencing and express IFN-γ, which is also RUNX3-dependent (34).
Figure 3. Increased TCR signaling compensates for G9a-deficiency in silencing of helper lineage genes in CD8 T cells.
(A, B) CD4 and CD8 expression of Ehmt2–/– and Ehmt2+/+ OT-I T cells (Thy1.2/CD45.2) that were co-transferred as a 1:1 mixture with Thy1.1/CD45.2 OT-I T cells into CD45.1 mice 4 days after Lm-OVA infection. (B) The ratios of Thy1.2+ to Thy1.1+ donor cells 4 days after infection. Data points represent individual recipient in three experiments, in which cells from one donor per genotype were transferred into 3 recipients. (C, D) Expression of CD4 and CD8 on Ehmt2+/+ (C) or Ehmt2–/– (D) T cells cultured in the presence of indicated concentrations of anti-CD3 and anti-CD28 Abs. Data are representative of 3 experiments (n=2 per genotype per experiment). (E) Percentage of CD4+ cells in cultured Ehmt2–/– or Ehmt2+/+ CD8 T cells shown by mean±SD. (F) qPCR analysis of gene expression in Ehmt2–/– and Ehmt2+/+ CD8 T cells cultured in either CD28Hi or CD28Lo condition. Ehmt2+/+ Th1 CD4 T cells were used as control (Th1).
Distinct dependency of silencing of helper lineage genes on G9a expressed in CD8 T cells between inflammatory and non-inflammatory conditions suggests that cell extrinsic signals through TCR, co-stimulatory molecules or cytokine receptors engage compensatory pathways that reinforce gene silencing. To define such cell-extrinsic determinants, we first cultured naive polyclonal CD8 T cells with varying concentrations of anti-CD3 and anti-CD28 Abs and determined whether distinct intensities of signaling through TCR or CD28 alter Cd4 de-repression in Ehmt2–/– CD8 T cells. As seen in the lymphopenic condition, a substantial fraction of Ehmt2–/– CD8 T cells de-repressed CD4 when they were cultured with low anti-CD3 and high anti-CD28 Ab concentrations (“CD28Hi” condition), whereas CD4 was barely expressed in Ehmt2+/+ CD8 T cells (Fig. 3C-E). Ehmt2–/– CD8 T cells also upregulated additional helper lineage-related genes, such as Il21 and Rorc (Fig. 3F). In contrast, when Ehmt2–/– CD8 T cells were stimulated with high anti-CD3 and low anti-CD28 Ab concentrations (“CD28Lo” condition), de-repression of the helper lineage genes was markedly reduced (Fig. 3D-F).
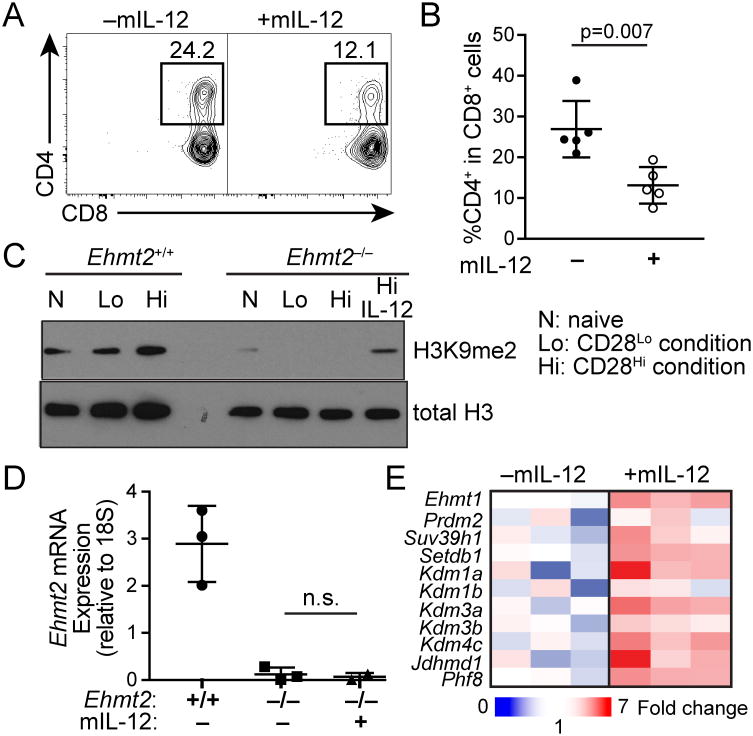
Furthermore, Cd4 de-repression in Ehmt2–/– CD8 T cells cultured in the CD28Hi condition was significantly inhibited by the cytokine IL-12 (Fig. 4A, 4B). We detected elevated H3K9me2 in Ehmt2–/– CD8 T cells cultured in the presence of IL-12 compared to those without IL-12 (Fig. 4C, 4D). The IL-12 treatment upregulated the H3K9me3 demethylase Kdm4c as well as GLP/Ehmt1 by 3-fold (Fig 4E), thus possibly maintaining H3K9me2-dependent gene regulation by increasing demethylation of H3K9me3 by KDM4C or by elevating residual HMT activity of GLP. These results suggest that the inflammation-dependent compensation may reinforce stable lineage-specific gene expression signature in CD8 T cells that proliferate in response to infection.
Figure 4. Signals through IL-12 receptor compensate for G9a-deficiency in repression of helper lineage genes in CD8 T cells.
(A, B) Percentages of CD4+ cells in Ehmt2–/– or control Ehmt2+/+ CD8 T cells cultured in the CD28Lo condition with or without mIL-12 (10 ng/ml). Plots are representative of 2 independent experiments (2-3 mice per experiment). (C) Immunoblotting for total H3 and H3K9me2 of lysates from Ehmt2–/– or Ehmt2+/+ CD8 T cells cultured in the CD28Hi or CD28Lo condition. Data are representative of 2 experiments. (D) Relative expression of Ehmt2 in CD8 T cells from Ehmt2–/– and Ehmt2+/+ mice cultured with the CD28Hi condition with or without mIL-12. (E) qPCR analysis of expression of H3K9 HMTs and demethylases in Ehmt2–/– CD8 T cells cultured in the CD28Hi condition with or without mIL-12. n=3.
Our study has demonstrated that G9a is required for maintaining silencing of multiple helper lineage-associated genes, such as Cd4, in dividing CD8 T cells in response to lymphopenia or tumor Ag. In developing CD8 T cells, Cd4 is shut-off by RUNX3 in a G9a-independent manner. However, the continued silenced state, which is independent of RUNX3, is not maintained in the absence of G9a, suggesting that transient RUNX3-dependent recruitment of G9a establishes the heritably silenced states of the locus in cooperation with other factors, such as additional methyltransferases. Alternatively, G9a is constitutively recruited to the Cd4 locus initially by RUNX3 and subsequently by a RUNX3-independent mechanism. All the G9a-dependent repression targets are not RUNX3 targets (18, 34), and Ehmt2–/– CD8 T cells are also able to proliferate and express IFN-γ, which is dependent on RUNX3. Therefore, there are multiple distinct RUNX3- or G9a-containing complexes that regulate gene activation or repression in CD8 T cells, while Cd4 silencing and repression of some of TFH-signature genes (18), including Icos, Cxcr5, and Il21, appear to be dependent on a complex containing both.
The absence of Cd4 de-repression in CD8 T cells responding to Lm-OVA infection may be explained by high levels of Ag and IL-12 both of which compensate for G9a-deficiency in CD8 T cells in vitro. Although the exact mechanism is unknown, our data suggest that IL-12R signaling alters the balance between histone methylation and demethylation to increase the levels of H3K9me2 independent of G9a, possibly facilitating heritable gene silencing in dividing CD8 T cells through the compensatory pathways. CD8 T cells may thus engage multiple epigenetic pathways in a context-dependent manner to shape their gene expression signature.
Supplementary Material
Acknowledgments
The authors thank D. Littman for cell lines, Cd4-cre and CD8-cre mice, C-S. Hsieh and E.M. Oltz for discussion, J. Chaudhuri, P. Collins, M. Holmgren, S. Hsiung, S. Raju, and E. Russler-Germain for technical assistance.
This study was supported by the National Institutes of Health grants R01AI097244, R56AI114593 (to T.E.), by the Leukemia and Lymphoma Society Scholar award (to T.E.) and T32GM007200 (to D.J.V.) and by the Shawn Hu and Angela Zeng Fellowship (to D.J.V.).
Abbreviations used in the manuscript
- ChIP
chromatin immunoprecipitation
- ChIP-seq
ChIP sequencing
- DN
double negative
- DP
double positive
- H3K9me2
dimethylation of Histone 3 lysine 9
- H3K9me3
trimethylation of histone 3 lysine 9
- HMT
histone methyltransferase
- LM
L. monocytogenes
- RNA-seq
RNA sequencing
- SP
single positive
Footnotes
The authors declare no conflict of interest related to this study.
Data from mass spectrometry have been deposited to PRIDE (https://www.ebi.ac.uk/pride/) under identifier PXD007511. Data from RNA-seq and ChIP-seq have been deposited to the National Center for Biotechnology Information Gene Expression Omnibus under accession number GSE101730 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE101730).
References
- 1.Vacchio MS, Bosselut R. What Happens in the Thymus Does Not Stay in the Thymus: How T Cells Recycle the CD4+-CD8+ Lineage Commitment Transcriptional Circuitry To Control Their Function. J Immunol. 2016;196:4848–4856. doi: 10.4049/jimmunol.1600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson CB, Makar KW, Perez-Melgosa M. Epigenetic regulation of T cell fate and function. J Infect Dis. 2002;185(1):S37–45. doi: 10.1086/338001. [DOI] [PubMed] [Google Scholar]
- 3.Bosselut R. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol. 2004;4:529–540. doi: 10.1038/nri1392. [DOI] [PubMed] [Google Scholar]
- 4.He X, Kappes DJ. CD4/CD8 lineage commitment: light at the end of the tunnel? Current opinion in immunology. 2006;18:135–142. doi: 10.1016/j.coi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins A, Littman DR, Taniuchi I. RUNX proteins in transcription factor networks that regulate T-cell lineage choice. Nat Rev Immunol. 2009;9:106–115. doi: 10.1038/nri2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taniuchi I. Transcriptional regulation in helper versus cytotoxic-lineage decision. Current opinion in immunology. 2009;21:127–132. doi: 10.1016/j.coi.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Egawa T. Regulation of CD4 and CD8 coreceptor expression and CD4 versus CD8 lineage decisions. Advances in immunology. 2015;125:1–40. doi: 10.1016/bs.ai.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Issuree PD, Ng CP, Littman DR. Heritable Gene Regulation in the CD4:CD8 T Cell Lineage Choice. Front Immunol. 2017;8:291. doi: 10.3389/fimmu.2017.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 11.Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, Ito Y, Littman DR. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- 12.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setoguchi R, Tachibana M, Naoe Y, Muroi S, Akiyama K, Tezuka C, Okuda T, Taniuchi I. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science. 2008;319:822–825. doi: 10.1126/science.1151844. [DOI] [PubMed] [Google Scholar]
- 14.Woolf E, Xiao C, Fainaru O, Lotem J, Rosen D, Negreanu V, Bernstein Y, Goldenberg D, Brenner O, Berke G, Levanon D, Groner Y. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 16.Zou YR, Sunshine MJ, Taniuchi I, Hatam F, Killeen N, Littman DR. Epigenetic silencing of CD4 in T cells committed to the cytotoxic lineage. Nature genetics. 2001;29:332–336. doi: 10.1038/ng750. [DOI] [PubMed] [Google Scholar]
- 17.Taniuchi I, Sunshine MJ, Festenstein R, Littman DR. Evidence for distinct CD4 silencer functions at different stages of thymocyte differentiation. Molecular cell. 2002;10:1083–1096. doi: 10.1016/s1097-2765(02)00735-9. [DOI] [PubMed] [Google Scholar]
- 18.Shan Q, Zeng Z, Xing S, Li F, Hartwig SM, Gullicksrud JA, Kurup SP, Van Braeckel-Budimir N, Su Y, Martin MD, Varga SM, Taniuchi I, Harty JT, Peng W, Badovinac VP, Xue HH. The transcription factor Runx3 guards cytotoxic CD8(+) effector T cells against deviation towards follicular helper T cell lineage. Nat Immunol. 2017;18:931–939. doi: 10.1038/ni.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 20.Maekawa Y, Minato Y, Ishifune C, Kurihara T, Kitamura A, Kojima H, Yagita H, Sakata-Yanagimoto M, Saito T, Taniuchi I, Chiba S, Sone S, Yasutomo K. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008;9:1140–1147. doi: 10.1038/ni.1649. [DOI] [PubMed] [Google Scholar]
- 21.Tachibana M, Nozaki M, Takeda N, Shinkai Y. Functional dynamics of H3K9 methylation during meiotic prophase progression. The EMBO journal. 2007;26:3346–3359. doi: 10.1038/sj.emboj.7601767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egawa T, Littman DR. Transcription factor AP4 modulates reversible and epigenetic silencing of the Cd4 gene. Proc Natl Acad Sci U S A. 2011;108:14873–14878. doi: 10.1073/pnas.1112293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou C, Pinto AK, Curtis JD, Persaud SP, Cella M, Lin CC, Edelson BT, Allen PM, Colonna M, Pearce EL, Diamond MS, Egawa T. c-Myc-induced transcription factor AP4 is required for host protection mediated by CD8+ T cells. Nat Immunol. 2014;15:884–893. doi: 10.1038/ni.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou C, Verbaro DJ, Tonc E, Holmgren M, Cella M, Colonna M, Bhattacharya D, Egawa T. The Transcription Factor AP4 Mediates Resolution of Chronic Viral Infection through Amplification of Germinal Center B Cell Responses. Immunity. 2016;45:570–582. doi: 10.1016/j.immuni.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts A, Pimentel H, Trapnell C, Pachter L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics. 2011;27:2325–2329. doi: 10.1093/bioinformatics/btr355. [DOI] [PubMed] [Google Scholar]
- 28.Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011;12:R22. doi: 10.1186/gb-2011-12-3-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sellars M, Huh JR, Day K, Issuree PD, Galan C, Gobeil S, Absher D, Green MR, Littman DR. Regulation of DNA methylation dictates Cd4 expression during the development of helper and cytotoxic T cell lineages. Nat Immunol. 2015;16:746–754. doi: 10.1038/ni.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishio H, Walsh MJ. CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription. Proc Natl Acad Sci U S A. 2004;101:11257–11262. doi: 10.1073/pnas.0401343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas LR, Miyashita H, Cobb RM, Pierce S, Tachibana M, Hobeika E, Reth M, Shinkai Y, Oltz EM. Functional analysis of histone methyltransferase g9a in B and T lymphocytes. J Immunol. 2008;181:485–493. doi: 10.4049/jimmunol.181.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mozzetta C, Pontis J, Fritsch L, Robin P, Portoso M, Proux C, Margueron R, Ait-Si-Ali S. The histone H3 lysine 9 methyltransferases G9a and GLP regulate polycomb repressive complex 2-mediated gene silencing. Molecular cell. 2014;53:277–289. doi: 10.1016/j.molcel.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.