Abstract
In this randomized phase Ib trial, we tested combining the E39 peptide vaccine with a vaccine created from E39′, an attenuated version of E39.
Patients with breast or ovarian cancer, who were disease-free after standard of care therapy, were enrolled and randomized to one of three arms. Arm EE received six E39 inoculations; arm EE′ received three E39 inoculations followed by three E39′; and arm E′E received three E39′ inoculations, followed by three E39. Within each arm, the first five patients received 500mcg of peptide and the remainder received 1000mcg. Patients were followed for toxicity, and immune responses were measured. This initial analysis after completion of the primary vaccination series has confirmed the safety of both vaccines. Immune analyses suggest incorporating the attenuated version of the peptide improves immune responses and that sequencing of E39 followed by E39′ might produce the optimal immune response.
Keywords: folate binding protein, breast cancer, ovarian cancer, peptide vaccine, attenuated vaccine, activation induced cell death
1 Introduction
With the recent success of immunotherapy, specifically checkpoint blockade, in the treatment of advanced cancers, there is renewed interest in exploring a broad range of immunotherapy strategies [1]. While checkpoint inhibitors have shown success in patients with metastatic cancer of various disease sites, the toxicity associated with these medications may limit their application in early stage disease. Additionally, the effectiveness of these therapies may be limited to a relatively small percentage of patients with a pre-existing immune response to their tumors [2, 3]. A successful cancer vaccine, on the other hand, can harness the power of the immune system to generate a de novo immune response to a tumor associated antigen found on a patient’s tumor with minimal or no systemic toxicity. Vaccines could therefore be used safely in the adjuvant setting. To this end, our group has developed a number of peptide vaccines targeting the HER2 antigen, which have been demonstrated to be safe, immunogenic, and have shown promising clinical efficacy in certain subgroups of patients [4–6].
More recently, our group has begun investigating vaccines targeting other common tumor associated antigens. Folate binding protein (FBP), also known as folate receptor alpha, is expressed at high levels on malignant cells and low levels on normal cells, lowering the risk of undesired toxicities [7]. Additionally, FBP expression has been shown to correlate with aggressive biology in ovarian and breast cancer [8, 9]. The E39 (FBP 191–199: EIWTHSYKV) peptide is an immunogenic epitope derived from the FBP protein, which can be administered with an immunoadjuvant (granulocyte macrophage colony stimulating factor [GM-CSF]) as a peptide vaccine (GALE-301). A phase I/IIa trial vaccinating patients with ovarian and endometrial cancer in the adjuvant setting has shown that it is safe, elicits a strong in vivo immune response and has promising clinical efficacy [10].
Historically, cancer vaccines have been evaluated in the setting of metastatic disease, with mostly disappointing results [11]. This is, at least in part, due to specific challenges of treating metastatic disease, such as large disease burden and the immunosuppressive tumor microenvironment [12, 13]. Consequently, our group has focused on administering peptide vaccines in the adjuvant, minimal disease setting [4–6, 10]. Even patients rendered disease-free after completion of standard therapies, however, will have some immune tolerance to their tumor and the associated antigens. Peptides used in vaccines must therefore be highly immunogenic to overcome this initial tolerance. Despite this prerequisite, repeated stimulation with such a highly immunogenic peptide may elicit its own type of immune tolerance manifested by some combination of T cell exhaustion, activation inducted cell death of specific cytotoxic T lymphocyte (CTL) populations, and induction of regulatory T cells [14, 15]. One proposed strategy to mitigate this risk of over-stimulation is to use an attenuated version of the immunogenic peptide in combination with the wild-type peptide. We have recently published pre-clinical work in which we engineered an attenuated version of the E39 peptide, termed E39′ (EIWTFSTKV, GALE-302). Compared with E39, this peptide has 2 amino acid substitutions at positions 5 and 7. This work showed that E39′, alone and in combination with E39, induced effective E39-specific CTL proliferation. In fact, E39′ induced more effective in vitro CTL activity than repeated stimulation with E39, likely through improved selection of effector CTL population [15]. While this pre-clinical work suggested the promise of such a strategy, questions remained regarding the effectiveness, dosing, and sequencing of attenuated peptides as part of a broader vaccination strategy.
In this randomized, phase Ib trial, we enrolled patients with breast or ovarian cancer who were rendered disease-free by standard of care therapies and randomized them to receive E39 or one of two sequences combining of E39 and E39′. Here, we present initial toxicity and immunologic response data for all patients after completion of the primary vaccination series (PVS).
2 Materials and Methods
2.1 Patient Characteristics and Clinical Protocols
The trial is a prospective, randomized, non-blinded, single-center phase Ib study being conducted under BB-IND #12391 (E39) and BB-IND#15305 (E39′). The trial protocol was reviewed and approved by the University of Texas MD Anderson Cancer Center Institutional Review Board prior to study initiation. Eligible patients had a diagnosis of breast or ovarian cancer, were disease-free after standard of care therapies, and were surgically or naturally post-menopausal. Patients were excluded for: active immunosuppressive therapy to include ongoing chemotherapy, steroids or methotrexate; poor performance status (ECOG >2); evidence of end-organ dysfunction; a history of autoimmune disease; or involvement in other experimental protocols, except with permission of the principal investigator of the other study. Eligible patients were screened, counseled, and consented prior to enrollment. The E39 and E39′ vaccines are HLA-A2 restricted, so enrolled patients were screened for HLA status and HLA-A2 negative patients were excluded. HLA-A2 positive patients were stratified based on cancer diagnosis (breast or ovarian) and randomized by computer tables to one of three arms for the PVS.
2.2 Vaccine and Vaccination Series
The E39 and E39′ peptides were produced commercially by an FDA-compliant production facility in good manufacturing practices for patient use. The peptide was purified to >95% before use. Sterility, endotoxin (limulus amebocyte lysate test), and general safety testing were performed. In addition, the manufacturer performed purity/stability testing periodically. Single dose vials were tested for bacterial and fungal contaminants prior to use. The single dose vials were stored in the investigational pharmacy.
The bulk peptide was reconstituted in saline at a concentration of 500mcg/0.5mL. For the low dose patients, 500mcg of peptide was mixed with 250mcg of GM-CSF (at a concentration of 250mcg/1.0mL). The total volume (1.5mL) from the combination of peptide and immunoadjuvant was administered intradermally in three 0.5mL inoculums at three different sites within 5cm of each other, ensuring these sites drained to the same nodal basin. For the high dose patients, two doses of 500mcg were mixed with GM-CSF (125mcg/0.5mL each). The total volume (2mL) from the combination was administered intradermally in four 0.5mL inoculums at four different sites within 5cm of each other.
Patients in each arm of the trial received six inoculations (V1–V6) of their respective peptide + GM-CSF as the PVS, with one injection given every 3–4 weeks (Figure 1). The EE arm received six inoculations of E39; the EE′ arm received three inoculations of E39 followed by three of E39′; and the E′E arm received three inoculations of E39′, followed by three of E39. Within each arm, the first five patients received the low dose (500mcg) dose of peptide and the latter five patients received the high dose (1000mcg) (Figure 2).
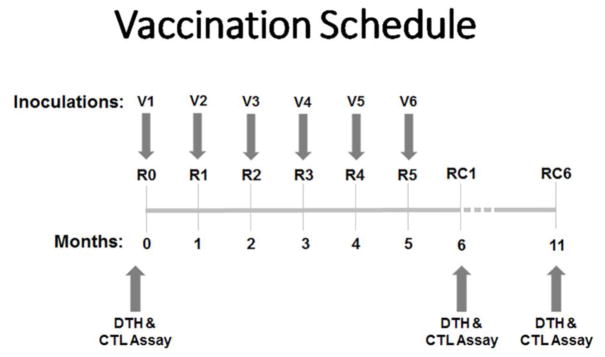
Figure 1.
Vaccination Schedule. Patients received 6 inoculations (V1–V6), one every 3–4 weeks. Immunologic assay and DTH measurement was performed prior to the start of the primary vaccine series, then 1 and 6 months after the last inoculation (R0, RC1, RC6).
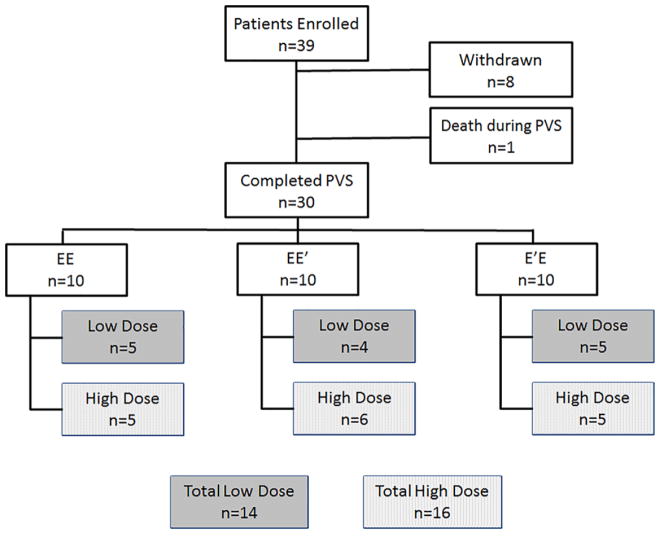
Figure 2.
Consort Diagram through the Primary Vaccine Series. A total of 39 patients were enrolled, 30 of which completed the primary vaccine series (PVS). There were 10 patients in each arm. One low dose patient in the EE′ arm did not finish the PVS and was replaced by a high dose patient so a total of 14 patients received the low dose (5 EE, 4 EE′, 5 E′E) and 16 received the high dose (5 EE, 6 EE′, 5 E′E).
2.3 Primary and Secondary Endpoints
The primary immunologic objective of the study was to determine which primary vaccination strategy maximizes E39-specific immunity as determined by the generation of E39-specific CTL. The primary clinical objective was to assess safety.
2.4 Immune Monitoring
Patients were assessed for evidence of in vivo immunologic response by evaluation of both the local reaction to each inoculation and a delayed-type hypersensitivity (DTH) reaction. The local reaction to each inoculation was assessed 48–72 hours after injection; the sensitive ballpoint-pen method was used to measure the reaction in two dimensions and the orthogonal mean was determined [16]. The DTH reaction was assessed at three time points (R0: pre-PVS; RC1: one month post-PVS; and RC6: six months post-PVS) (Figure 1). Briefly, to determine the DTH, 100mcg of E39 (without GM-CSF) as well as a parallel control (equivalent volume of sterile saline) were injected intradermally at a site on the anterior thigh opposite the side of the vaccination site; the response was measured in two dimensions at 48–72 hours post injection and the orthogonal mean was determined.
Patients were assessed for evidence of ex vivo immunologic response by quantification of E39-specific CTL using a dextramer assay as previously described performed on blood obtained at the same time points as the DTH (R0, RC1, RC6) [17,18]. Briefly, fresh PBMC was isolated and stained with the following antibodies: CD8 APC-H7 (BD Biosciences), CD3 PE Cy7 (BD Biosciences), E39-APC-conjugated dextramer (Immudex), and the following pacific blue conjugated lineage antibodies: CD4 (BD Biosciences), CD14 (BD Biosciences), CD16 (BD Biosciences), CD19 (Biolegend), and CD56 (BDBiosciences). Cells were then analyzed on a LSRFortessa Aanlyzer (BD Biosciences; 18-color, 5-laser: 355nm, 405nm, 488nm, 561nm and 640nm). The frequency of E39-specific CD8+ T cells was determined as the percentage of cells that were alive, lineage-, CD3+ CD8+ and E39-dextramer+.
2.5 Toxicity
Patients were monitored post-inoculation for an hour and re-examined 48–72 hours after each inoculation for local and systemic toxicity. Additionally, patients were questioned about any associated toxicity at each follow up visit. The graded toxicity scale (NCI Common Terminology Criteria for Adverse Events, v4.03) was utilized to assess and grade local and systemic toxicities.
2.6 Statistical Analysis
Clinicopathologic data were compared between groups. Median and range were used to summarize continuous data, and the groups were compared using a Mann-Whitney U test. Chi squared or Fischer exact test were used to compare categorical variables between groups. Local reaction and DTH data are presented as orthogonal means ± standard errors; CTL data are presented as percentage of cells± standard errors. Groups at specific time points were compared using student’s t test, while changes in values over time were analyzed using ANOVA. Statistical analyses were performed using SPSS version 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). Statistical significance was considered achieved if p<0.05.
3 Results
3.1 Patient Characteristics and Clinical Protocols
A total of 39 patients were enrolled (12 EE, 14 EE′, 13 E′E), including 35 breast cancer and four ovarian cancer patients. Eight patients withdrew prior to completion of the PVS (none due to complications of therapy) and 1 patient died during the PVS (unrelated to treatment). Thirty patients completed the PVS (10 EE, 10 EE′, 10 E′E), 27 with breast and three ovarian cancer. In the EE′ arm, one of the low dose patients did not complete the PVS and was replaced with a high dose patient, so a total of 14 patients received the low dose (5 EE, 4 EE′, 5E′E) and 16 received the high dose (5 EE, 6 EE′, 5 E′E) (Figure 2). Clinicopathologic features by study arms and by dosing group are shown in tables 1 and 2 respectively.
Table 1.
Demographics By Trial Arm.
| EE | EE′ | E′E | p-value | |
|---|---|---|---|---|
| N=10 | N=10 | N=10 | ||
| Median Age (yrs) | 53 | 60 | 52 | 0.26 |
| Range | 41–68 | 50–77 | 42–83 | |
| Breast | n=10 | n=8 | n=9 | |
| T Stage < T2 | 80.0% | 62.5% | 55.6% | 0.59 |
| Grade 3 | 40.0% | 25.0% | 44.4% | 0.78 |
| Node Positive | 60.0% | 37.5% | 66.7% | 0.56 |
| Hormone Receptor+ | 60.0% | 87.5% | 55.6% | 0.35 |
| HER2+ | 30.0% | 12.5% | 11.1% | 0.57 |
| Ovarian | n=0 | n=2 | n=1 |
Table 2.
Demographics by dose.
| Low Dose (500mcg) | High Dose (1000mcg) | p-value | |
|---|---|---|---|
| n=14 | n=16 | ||
| Median Age (yrs) | 54 | 57 | 0.80 |
| Range | 42–83 | 41–82 | |
| Breast | n=13 | n=14 | |
| T Stage < T2 | 61.5% | 71.4% | 0.69 |
| High Grade | 38.5% | 35.7% | 0.98 |
| Node Positive | 69.2% | 42.9% | 0.25 |
| Hormone Receptor+ | 69.2% | 64.3% | 0.99 |
| HER2+ | 0.0% | 35.7% | 0.04 |
| Ovarian | n=1 | n=2 |
3.2 Toxicity
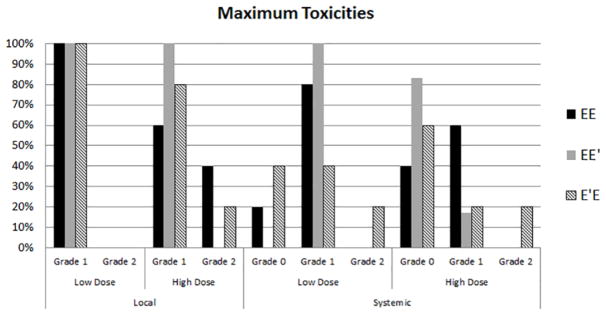
Local and systemic toxicities were mild for all patients with no toxicities > grade 2. The most common local toxicities were injection site erythema, induration and pruritus. The most common systemic toxicities were mild flu-like symptoms of fatigue and malaise. There were no significant differences in systemic or local toxicity between the arms. When comparing dosing groups, there were also no significant differences in local or systemic toxicity (Figure 3).
Figure 3.
Toxicity Percentage of patients in each group experiencing maximum toxicities of each grade. There were no grade 3 or higher toxicities experienced by any patient. There were no significant differences in maximum toxicities between groups based on dosing or vaccine sequence.
3.3 Immune Response
3.3.1 Local reaction
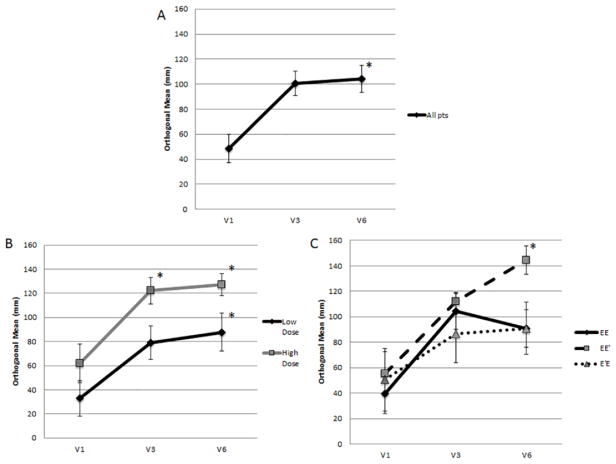
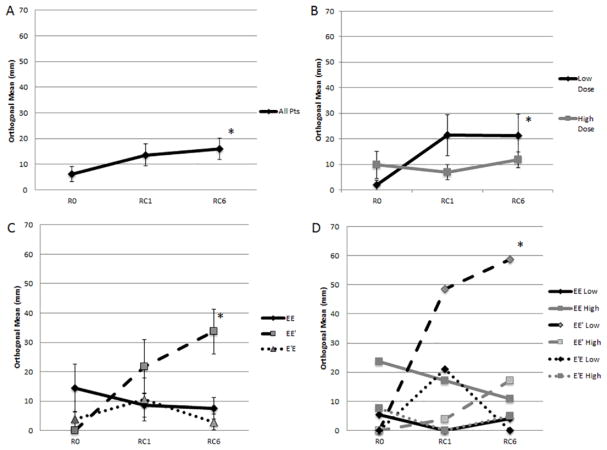
In the entire cohort of patients, the local reaction assessed after the last inoculation of the PVS was significantly greater than the local reaction after the first inoculation (V6: 104.3±10.7mm vs V1: 48.4±11.4mm, p=0.001; Figure 4A). Analyzing patients based on dosing, both low dose and high dose groups, showed significant increase at V6 vs V1 (low dose: 87.8±15.8 vs. 32.9±14.8mm, p=0.02; high dose: 127.3±9.2mm vs. 62.0±16.2mm, p=0.008; Figure 4B). The high dose group showed consistently larger local reactions than the low dose group, with the difference being significant at V3 (V1: p=0.2, V3: p=0.02; V6: p=0.07). Comparing the study arms individually, all groups showed an increase at V6 vs V1, but the EE′ group showed the largest increase, which reached statistical significance (EE: p=0.07, EE′: p=0.002, E′E: p=0.14; Figure 4C).
Figure 4.
Local reaction after first (V1), third (V3) and sixth (V6) inoculations. Figure 4A: All patients. The local reaction to the last inoculation of the PVS was significantly greater than the first (V6: 104.3±10.7mm vs V1: 48.4±11.4mm, p=0.001). Figure 4B: By dosing group. Both high dose and low dose groups showed significant increase at V6 vs V1 (low dose: 87.8±15.8 vs. 32.9±14.8mm, p=0.02; high dose: 127.3±9.2mm vs. 62.0±16.2mm, p=0.008). The high dose group showed consistently larger local reactions than the low dose group, with the difference being significant at V3 (V1: p=0.2, V3: p=0.02; V6: p=0.07). Figure 4C: By study arm. All groups showed an increase at V6 vs V1, but only the EE′ group showed a statistically significant increase (EE: p=0.07, EE′: p=0.002, E′E: p=0.14). *=p<0.05.
3.3.2 Delayed Type Hypersensitivity
In the analysis of all patients, there was a significant increase in DTH over the course of the trial (p=0.04; Figure 5A). This was driven by the low dose group, which had a significant increase in their DTH response through the trial (R0:1.9±1.9mm; RC1: 21.4±8.1mm; RC6:21.3±8.2mm, p=0.03). There was no statistical change in the DTH response over time in the high dose group. (Figure 5B). Comparing DTH response by treatment arms, the EE′ arm had the greatest response, with a significant increase over time compared to the EE and E′E arms (<0.001; Figure 5C). Within the EE′ arm, the low dose patients showed the largest increase in DTH, with a significant increase from R0 (0±0mm) to RC1 (48.5±14.5mm, p=0.01) and RC6 (58.8± 7.7mm, p<0.001) (Figure 5D).
Figure 5.
DTH at baseline (R0), 1 month (RC1), and 6 months (RC6) post-PVS. Figure 5A: DTH in all patients. There was a significant increase in DTH over the course of the trial (p=0.04). Figure 5B: DTH by dosing group. The low dose group had a significant increase in their DTH response through the trial (p=0.03). Figure 5C: DTH by study arm. The EE′ arm had the greatest response, with a significant increase over time compared to the EE and E′E arms (<0.001). Figure 5D: DTH by study arm AND dosing group. Within the EE′ arm, the low dose patients showed the largest increase in DTH, with a significant increase from R0 (0±0mm) to RC1 (48.5±14.5mm, p=0.01) and RC6 (58.8± 7.7mm, p<0.001). *=p<0.05.
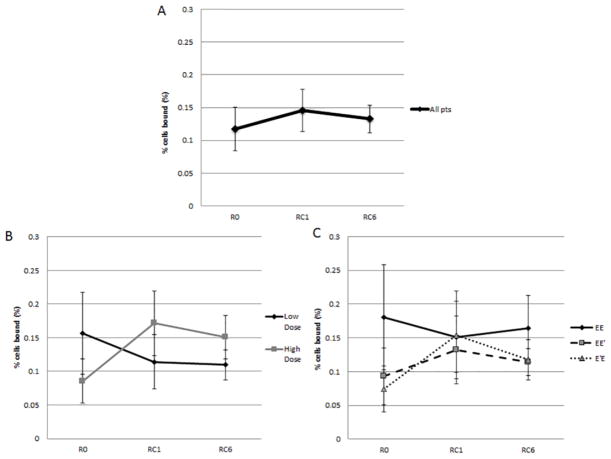
3.3.3 E39-specific T cells
When evaluating all patients, regardless of arm of the trial or dose administered, there was no change in levels of E39-specific CTLs across the trial (R:0.11±0.03%; RC1: 0.15±0.03%; RC6:0.13±0.02%, p=0.70) (Figure 6A). When evaluating by high versus low dose, however, there was a near doubling of E39-CTL between the R0 (0.086±0.033%) and RC1 (0.171±0.048%, p=0.16) for patients who received the higher dose (Figure 6B). When evaluating by treatment arms, both the EE′ (0.09±0.04% to 0.13±0.05%; p=0.57) and E′E (0.08±0.03% to 0.15±0.07%; p=0.32) groups experienced an increase in E39-specific CTL with the increase in the E′E group again representing nearly a doubling in antigen-specific CTL (Figure 6C).
Figure 6.
CTL at baseline (R0), 1 month (RC1) and 6 months (RC6) post-PVS. Figure 6A: All patients CTL. There was no difference in levels of E39-specific CTLs (R:0.11±0.03%; RC1: 0.15±0.03%; RC6:0.13±0.02%, p=0.70). Figure 6B: CTL by dosing group. The high dose group had a non-significant increase in of E39-CTL between the R0 and RC1 (p=0.16), but a slight decrease at RC6. The low dose group had no significant change in CTL. Figure 6C: CTL by study arm. The EE′ (0.09±0.04% to 0.13±0.05%; p=0.57) and E′E (0.08±0.03% to 0.15±0.07%; p=0.32) groups experienced an increase in E39-specific CTL with the increase in the E′E group nearly a doubling in antigen-specific CTL.
4 Discussion
This phase Ib trial represents the first study to compare treatment with a peptide vaccine alone and in combination with an attenuated version. We have shown that E39 administered alone or sequentially with E39′ is safe and well tolerated. Although none of the 3 arms showed a significant increase in the number of E39-specific CTL, it is interesting to note that the two strategies utilizing both the wild-type and attenuated peptides did stimulate an increase in the number of E39-CTL after the PVS, while a PVS consisting only of the wild-type peptide did not. In addition, the in vivo responses, as assessed by both local reaction and DTH, were stronger in those patients receiving both E39 and E39′, with the DTH and local reaction data both favoring the EE′ arm. Taken together, the data suggest that the best strategy for vaccination is to administer a PVS consisting of three inoculations of the wild-type peptide (E39) followed by three inoculations of the attenuated peptide (E39′).
This study has investigated a vaccine strategy targeting FPB, which represents a nearly ideal TAA for immunotherapy. FBP is expressed in 80–90% of ovarian cancers and 40% of breast cancers, to include up to 80% of triple negative breast cancers, but has very low expression in normal tissue [7–9, 19]. Moreover, FBP expression is associated with particularly poor prognosis in both ovarian and breast cancer [8, 9]. The E39 peptide is an immunogenic epitope of FBP, while the E39′ peptide was developed by altering two specific amino acids in the E39 sequence (histidine at position 5 changed to phenylalanine; tyrosine at position 7 changed to threonine). These amino acid substitutions alter the interaction between the peptide-HLA-A2 complex and the T cell receptor resulting in an attenuate version of the peptide. Our preclinical work suggests this attenuated peptide elicits an effective CTL response, perhaps even avoiding the activation-induced cell death induced by repeated stimulation with the wild-type peptide [15].
While the E39 peptide was previously shown to be safe and well-tolerated in a trial enrolling patients with ovarian and endometrial cancer, the safety of E39′ inoculations in humans has not been previously demonstrated [10]. In this study, patients receiving E39′ as a component of their PVS experienced only grade 1 and 2 toxicities confirming the safety of this attenuated peptide. As seen in our previous study evaluating the E39 peptide vaccine, as well as our studies evaluating HER2-derived peptide vaccines, most of the toxicity experienced was skin reactions, to include erythema and pruritus, and mild flu-like symptoms [4–6, 10]. In several of our previous randomized, controlled trials, control patients have received GM-CSF alone, and we have seen similar rates of systemic toxicity between vaccine and control arms, implying that the toxicity from the peptide vaccines is related to the GM-CSF, not the peptide itself [4, 5]. Therefore, although this trial did not have a GM-CSF only arm, it is reasonable to suggest that the toxicity seen is attributable to the adjuvant.
While the toxicity profile remained minimal with the addition of the attenuated peptide vaccine, the ultimate goal of this therapy is to develop a strong peptide-specific immune response. Indeed, the in vivo immune response to the combination of the two peptides was strong. Though all patients that finished the PVS showed a significant increase in both local reaction and DTH over time, the dose and sequence of the peptides influenced the magnitude of response. The low dose of the peptides led to a greater response in DTH, while the high dose showed increase local reaction. The local reaction increase in the high dose group is not unexpected as this is a measurement of direct and immediate immune response to each inoculation of peptide and GM-CSF, so a higher dose of peptide is expected to show a larger reaction. The DTH results, however, are more telling of a meaningful immune response to the target peptide. DTH responses rely on immune memory and circulating immune cells as this test is done without immunoadjuvant and at time points and locations remote from the actual inoculations. Additionally, DTH has been previously validated as a measure of in vivo immune response and shown to correlate with clinical outcomes [6, 20].
Interestingly, the indication from these DTH results, that a lower dose of peptide induces a more meaningful immune response, is in contrast with our recently published results from the phase I/IIb trial of the E39 peptide vaccine in patients with endometrial and ovarian cancer where patients receiving a higher vaccine dose demonstrated better clinical outcome [10]. This difference may be related to the differences in patient populations. While the vast majority of the patients in the endometrial/ovarian cancer trial are expected to have high expression of FBP, 90% of the patients in our current trial have breast cancer and only about 40% of breast cancer patients are expected to have previous FBP exposure. The higher expression of FBP the endometrial cancer group may have caused deletion of the high avidity E39-CTL, leaving the lower avidity CTL to expand in response to the higher E39 dose. Additionally, the amount and potency of immunosuppressive chemotherapy received as standard of care is markedly different between these patient populations, with breast cancer patients receiving less chemotherapy (less than 70% of breast cancer patients in the current trial received chemotherapy) and less aggressive regimens when chemotherapy is used, leaving a more functional immune system that may be more responsive to lower doses of peptide. Thus, in this trial of mostly breast cancer patients with less exposure to FBP and having received less cytotoxic chemotherapy, it appears that a lower dose of peptide may be the preferred dose.
In addition to using a lower dose of peptide, sequencing the attenuated peptide after the wild-type peptide (EE′ dosing scheme) improves the DTH response. Preclinical work by our group suggests that, over time, repeated exposure to an immunogenic peptide can cause selection of T cells with low affinity T cell receptors, which specialize more in cytokine expression and have less cytotoxic function [15]. This leads to high levels of IFNγ and IL-2 expression, which may in turn lead to exhaustion of the effector CTLs and induction of regulatory T cells [15, 21–23]. Indeed, patients in the EE group, receiving repeated doses of E39, showed an initial increase in local reaction but the local reaction in this group waned at the end of the PVS. Additionally, this group showed no increase in DTH after the PVS. This may represent a clinical manifestation of the detrimental T cell selection identified in pre-clinical work. Theoretically, substituting an attenuated version of this peptide at some time during the vaccine series may stimulate a more effective and functional CTL response to a peptide vaccine. In this trial, the EE′ group showed larger local reactions and DTH responses compared with the other arms, indicating that this sequence of fully immunogenic peptide to start the response, followed by attenuated peptide in the second half of the PVS, induced the most meaningful immune responses in this trial.
One confounding aspect of the immune analyses performed as part of this study is the CTL response. Specifically, looking at the entire study population, there was no increase in the levels of E39-specific CTLs present in the peripheral blood. However, when evaluating by treatment arms, there was an increase in E39-specific CTL for both the EE′ (0.09% to 0.13%; p=0.57) and E′E (0.08% to 0.15%; p=0.32) groups, with the increase in the E′E group representing nearly a doubling in antigen-specific CTL. While this difference was not significant, it is likely that the number of patients in each of the groups was too small to show a statistically significant difference. Differences in the FBP expression rates between the arms, which were not directly assessed in this trial, could also contribute to the differences in E39-specific CTLs seen at baseline. Additionally, the dextramer assay serves to measure only the gross number of E39-specific CTLs and does not inform regarding the expansion and retraction of specific clonal subpopulations. As demonstrated in our pre-clinical work, the theoretical advantage of an attenuated peptide vaccine comes from selection of a more effective group of CTLs rather than a broad expansion in the number of CTLs, so the most effective vaccine therapy could very well cause a decrease in total number of CTLs, while simultaneously leading to expansion of memory effector T cells. Ultimately, the near doubling in the specific T cell response does suggest there was some impact from vaccination and it is also notable that the increase was greatest in the low dose groups and groups receiving the attenuated version of the peptide, consistent with the DTH data.
This trial is continuing with patients receiving booster inoculations, which, as we have previously shown, effectively extend immune responses to cancer vaccines [24]. In this particular trial, given the use of two vaccines, there is an additional randomization step when selecting patients for booster inoculation with either E39 or E39′. All patients are being assessed for significant residual immunity after the PVS, defined as a doubling of their E39-CTL levels. Once this is determined, patients will be stratified by presence of significant residual immunity and then randomized to receive a single booster inoculation of either E39 or E39′ followed by short-term (1 mo) and long-term (6 mos) assessments of both in vitro and in vivo immune responses.
5 Conclusion
This initial analysis of the ongoing phase Ib trial of E39 and E39′ in breast and ovarian cancer patients has confirmed the safety of both epitopes administered as part of a vaccine. Immune analyses suggest that incorporation of the attenuated version of the peptide into the vaccination strategy will optimize both in vivo and in vitro immune responses. While these early analyses suggest that the E39 followed by E39′ sequencing might produce the optimal immune response, additional analyses following completion of the booster inoculations are required to confirm this.
Highlights.
FBP-derived peptide vaccine E39 elicits a strong in vivo immune response.
Highly immunogenic vaccines may overstimulate the immune system.
E39′ (attenuated E39) is being tested in a phase Ib trial.
E39 and E39′ are safe with local and systemic toxicities < grade 2.
Sequencing E39 followed by E39′ may elicit optimal immune response.
Acknowledgments
Funding
This work was supported by grants from the NCI (Cancer Center Support Grant P30CA016672 to MD Anderson Cancer Center) and by awards from the Nancy Owens Memorial Foundation (EAM), Pink Ribbons Project (EAM) and the Jeanne F. Shelby Scholarship Fund (EAM). Study drug and partial funding was provided by Galena Biopharma, Inc.
Abbreviations
- FBP
Folate Binding Protein
- GM-CSF
granulocyte macrophage colony stimulating factor
- CTL
cytotoxic T lymphocyte
- PVS
Primary Vaccine Series
- DTH
delayed-type hypersensitivity
- R0
assessment pre-PVS
- RC1
assessment 1 month post-PVS
- RC6
assessment 6 months post-PVS
- V1–6
Vaccinations 1–6
Footnotes
Ethics approval and consent to participate:
Written informed consent was obtained from all study participants. Prospective participants were provided with a copy of the consent form to read. The research nurse coordinator or PI then explained the study and reviewed the consent form with each patient. Patients were given ample time to ask and have all questions answered prior to signing the consent form. An initial consent was obtained in order to review the patient’s records, draw a blood sample, and examine their surgical specimens and determine eligibility. Once a patient was determined to be eligible, the process of informed consent was repeated and an additional consent was obtained for full participation in the trial. The protocol was reviewed and approved by the MD Anderson Cancer Center IRB.
Consent for publication:
No individual person’s data is being published.
Availability of data and material:
Data for this trial is still being accrued for final analysis. Data will not be made publicly available until all final data has been analyzed.
Competing interests:
Dr. George Peoples is a consultant to Galena Biopharma and has partial inventor rights for the E39 and E39′ vaccines.
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of San Antonio Military Medical Center, the U.S. Army Medical Department, U.S. Air Force Medical Department, the Department of the Army, the Department of Air Force, Department of Defense or the U.S. Government.
Authors’ Contributions:
TJV contributed to data analysis, drafting and critical revision of the manuscript.
JKL contributed to patient accrual and critical revision of the manuscript.
NQ contributed to acquisition of data, and critical revision of the manuscript.
AVP contributed to acquisition of data, and critical revision of the manuscript.
GA contributed to acquisition of data, and critical revision of the manuscript.
DFH contributed to data analysis and drafting of the manuscript.
DOJ contributed to data analysis and critical revision of the manuscript.
KMP contributed to data analysis and critical revision of the manuscript.
JMG contributed to data analysis and critical revision of the manuscript.
JSB contributed to data analysis and critical revision of the manuscript.
GTC contributed to data analysis, drafting and critical revision of the manuscript.
GEP contributed to the study conception and design, acquisition of data, data analysis, drafting and critical revision of this manuscript.
EAM contributed to the study conception and design, patient accrual, acquisition of data, data analysis, drafting and critical revision of this manuscript.
Trial Registration: NCT02019524
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Timothy J. Vreeland, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, 1400 Pressler St, Unit 1484, Houston, TX 77030.
Jennifer K. Litton, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1155 Pressler St, Unit 1354, Houston TX 77030.
Na Qiao, Department of Breast Surgical Oncology, The University of Texas MD Anderson Cancer Center, 1400 Pressler St, Unit 1434, Houston, TX 77030.
Anne V. Philips, Department of Breast Surgical Oncology, The University of Texas MD Anderson Cancer Center, 1400 Pressler St, Unit 1434, Houston, TX 77030.
Gheath Alatrash, Department of Stem Cell Transplantation and Cellular Therapy, The University of MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 423, Houston TX, 77030.
Diane F. Hale, Department of Surgery, San Antonio Military Medical Center, 3551 Roger Brooke Dr, San Antonio TX, 78234.
Doreen O. Jackson, Department of Surgery, San Antonio Military Medical Center, 3551 Roger Brooke Dr, San Antonio TX, 78234.
Kaitlin M. Peace, Department of Surgery, San Antonio Military Medical Center, 3551 Roger Brooke Dr, San Antonio TX, 78234.
Julia M. Greene, Department of Surgery, San Antonio Military Medical Center, 3551 Roger Brooke Dr, San Antonio TX, 78234.
John S Berry, Department of Surgery, Womack Army Medical Center, 2817 Reilly Rd, Fort Bragg NC, 28310.
Guy T. Clifton, Department of Surgery, San Antonio Military Medical Center 3551 Roger Brooke Dr, San Antonio TX, 78234.
George E Peoples, Department of Surgery, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Rd, Bethesda MD, 20814.
References
- 1.Burstein HJ, Krilov L, Aragon-Ching JB, Baxter NN, Chiorean EG, Chow WA, et al. Clinical Cancer Advances 2017: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1341–67. doi: 10.1200/JCO.2016.71.5292. [DOI] [PubMed] [Google Scholar]
- 2.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vreeland TJ, Clifton GT, Herbert GS, Hale DF, Jackson DO, Berry JS, et al. Gaining ground on a cure through synergy: combining checkpoint inhibitors with cancer vaccines. Expert Rev Clin Immunol. 2016;12:1347–57. doi: 10.1080/1744666X.2016.1202114. [DOI] [PubMed] [Google Scholar]
- 4.Mittendorf EA, Ardavanis A, Litton JK, Shumway NM, Hale DF, Murray JL, et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget. 2016;7:66192–201. doi: 10.18632/oncotarget.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittendorf EA, Ardavanis A, Symanowski J, Murray JL, Shumway NM, Litton JK, et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann Oncol. 2016;27:1241–8. doi: 10.1093/annonc/mdw150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittendorf EA, Clifton GT, Holmes JP, Schneble E, van Echo D, Ponniah S, et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014;25:1735–42. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–401. [PubMed] [Google Scholar]
- 8.Zhang Z, Wang J, Tacha D, Li P, Bremer R, Chen H, et al. Folate Receptor α Associated With Triple-Negative Breast Cancer and Poor Prognosis. Archives Pathology Amp Laboratory Medicine. 2014;138:890–5. doi: 10.5858/arpa.2013-0309-OA. [DOI] [PubMed] [Google Scholar]
- 9.Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74:193–8. doi: 10.1002/(sici)1097-0215(19970422)74:2<193::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Jackson DO, Byrd K, Vreeland TJ, Hale DF, Herbert GS, Greene JM, et al. Interim analysis of a phase I/IIa trial assessing E39+GM-CSF, a folate binding protein vaccine, to prevent recurrence in ovarian and endometrial cancer patients. Oncotarget. 2017;8:15912–23. doi: 10.18632/oncotarget.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hale DF, Clifton GT, Sears AK, Vreeland TJ, Shumway N, Peoples GE, et al. Cancer vaccines: should we be targeting patients with less aggressive disease? Expert Rev Vaccines. 2012;11:721–31. doi: 10.1586/erv.12.39. [DOI] [PubMed] [Google Scholar]
- 13.Hale DF, Vreeland TJ, Peoples GE. Arming the Immune System Through Vaccination to Prevent Cancer Recurrence. Am Soc Clin Oncol Educ Book. 2016;35:e159–67. doi: 10.1200/EDBK_158946. [DOI] [PubMed] [Google Scholar]
- 14.Chhabra A. Mitochondria-centric activation induced cell death of cytolytic T lymphocytes and its implications for cancer immunotherapy. Vaccine. 2010;28:4566–72. doi: 10.1016/j.vaccine.2010.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry JS, Vreeland TJ, Hale DF, Jackson DO, Trappey AF, Greene JM, et al. Evaluation of Attenuated Tumor Antigens and the Implications for Peptide-Based Cancer Vaccine Development. J Cancer. 2017;8:1255–62. doi: 10.7150/jca.16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokal JE. Editorial: Measurement of delayed skin-test responses. N Engl J Med. 1975;293:501–2. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 17.Mittendorf EA, Alatrash G, Qiao N, Wu Y, Sukhumalchandra P, St John LS, et al. Breast cancer cell uptake of the inflammatory mediator neutrophil elastase triggers an anticancer adaptive immune response. Cancer Res. 2012;72:3153–62. doi: 10.1158/0008-5472.CAN-11-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Sukhumalchandra P, Enyenihi AA, St John LS, Hunsucker SA, Mittendorf EA, et al. A novel HLA-A*0201 restricted peptide derived from cathepsin G is an effective immunotherapeutic target in acute myeloid leukemia. Clin Cancer Res. 2013;19:247–57. doi: 10.1158/1078-0432.CCR-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senol S, Ceyran AB, Aydin A, Zemheri E, Ozkanli S, Kösemetin D, et al. Folate receptor α expression and significance in endometrioid endometrium carcinoma and endometrial hyperplasia. Int J Clin Exp Pathol. 2015;8:5633–41. [PMC free article] [PubMed] [Google Scholar]
- 20.Schneble EJ, Berry JS, Trappey FA, Clifton GT, Ponniah S, Mittendorf E, et al. The HER2 peptide nelipepimut-S (E75) vaccine (NeuVax™) in breast cancer patients at risk for recurrence: correlation of immunologic data with clinical response. Immunotherapy. 2014;6:519–31. doi: 10.2217/imt.14.22. [DOI] [PubMed] [Google Scholar]
- 21.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983–8. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 22.Stoycheva D, Deiser K, Stärck L, Nishanth G, Schlüter D, Uckert W, et al. IFN-γ regulates CD8+ memory T cell differentiation and survival in response to weak, but not strong, TCR signals. J Immunol. 2015;194:553–9. doi: 10.4049/jimmunol.1402058. [DOI] [PubMed] [Google Scholar]
- 23.Minn AJ. Interferons and the Immunogenic Effects of Cancer Therapy. Trends Immunol. 2015;36:725–37. doi: 10.1016/j.it.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmes JP, Clifton GT, Patil R, Benavides LC, Gates JD, Stojadinovic A, et al. Use of booster inoculations to sustain the clinical effect of an adjuvant breast cancer vaccine: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2011;117:463–71. doi: 10.1002/cncr.25586. [DOI] [PubMed] [Google Scholar]