Abstract
The extent of sex differences in childhood language development is unclear. We conducted a systematic literature review synthesizing results from studies examining sex differences in brain structure and function relevant to language development during childhood. We searched PubMed and Scopus databases, and this returned a total of 46 published studies meeting criteria for inclusion that directly examined sex differences in brain development relevant to language function in children. The results indicate that: (a) sex differences in brain structure or function do not necessarily lead to differences in language task performance; (b) evidence for sex differences in brain and language development are limited; (c) when present, sex differences often interact with a variety of factors such as age and task. Overall, the magnitude of sexual dimorphism of brain developmental trajectories associated with language is not as significant as previously thought. Sex differences were found, however, in studies employing tighter age ranges. This suggests that sex differences may be more prominent during certain developmental stages but are negligible in other stages, likely due to different rates of maturation between the sexes. More research is needed to improve our understanding of how sex differences may arise due to the influence of sex hormones and developmental stages, and how these differences may lead to differences in various language task performance. These studies are expected to provide normative information that may be used in studies examining neurodevelopmental disorders that frequently affect more males than females, and also often affect language development.
Keywords: sex differences, children, brain, language, development, laterality
1 INTRODUCTION
For decades, sex differences in language development has been a topic of interest for researchers and the general public alike. Reports of sex differences in speech and language abilities of boys and girls date back to the late 1950s. An influential paper by Anastasi et al. (1980) claimed that girls are superior to boys in language abilities from childhood to adulthood. Other studies reported similar findings (e.g., Denno, 1982; Halpern, 2013; Maccoby et al., 1966; Maccoby & Jacklin, 1974). The view that girls exhibit superior language abilities has been generally accepted in both the scientific and non-scientific community. However, this position has been challenged by claims that male and female brains exhibit similar characteristics (Joel et al., 2015; Joel & Fausto-Sterling, 2016) and meta-syntheses pooling results from hundreds of studies that have shown that there are negligible differences in behavioral performance on most cognitive tasks including language between the sexes (Hyde, 2005, 2016; Lindberg et al., 2010; Zell et al., 2015). These opposing views are in line with the inconsistent findings reported to date in the literature on factors affecting sexual dimorphism in language development. A thorough examination of the magnitude, consistency, and developmental trajectories of brain structure and function associated with sex differences in language abilities is warranted.
A widespread network of brain structures is involved in language processing. Most commonly reported regions include cortical areas in the left hemisphere such as the inferior frontal gyrus and auditory regions such as the planum temporale and superior temporal gyrus (for a meta-analysis see Vigneau et al., 2005). These structures are thought to form part of a loop that facilitates semantic and phonological processing. Other work highlighted the importance of subcortical structures such as the basal ganglia and cerebellum in language processing. The basal ganglia critically support the initiation of speech sequence production (Bohland et al., 2010), while the cerebellum interfaces between cortical motor and sensory areas via the thalamus to play a role in speech error correction when there is a mismatch between the expected and actual self-initiated sensory (auditory, somatosensory) feedback (Kotz et al., 2009). Further, the corpus callosum is relevant for lateralization for cortical structure and function (Hinkley et al., 2016). Lesions to the corpus callosum impair processing of syntactic information in the left hemisphere and prosodic information in the right hemisphere (see Friederici, 2011). These studies were mostly based on research on adults and hence the significance of these language related regions during childhood development, and possible differences between the sexes, is not clear.
Prior to the 1920s, studies of sex differences in the brain were restricted to postmortem examinations, but the advent of neuroimaging provided researchers with a sophisticated means by which to evaluate the brain in vivo. Normal brain development is characterized by an inverted-U shape curve of growth in gray matter volume/density, and a general increase in white matter that plateaus at around the 3rd and 4th decade of life (Giedd et al., 2009). Between the time a child is born and their second birthday, cortical thickness of the brain increases to 97% and surface area of the brain increases to 69% of its adult value (Lyall et al., 2015). Typically, sensory and motor regions mature earlier, with those involving higher order executive functioning maturing later (Gogtay et al., 2004). Aspects of brain development continue well into adulthood (Lenroot & Giedd, 2006).
The brain undergoes rapid growth and change during the critical period for speech and language development that occurs until approximately age 3. Early vocal learning begins even prior to birth (Locke, 1993a) and infants then attend to prosodic elements of speech at first, presumably governed by the right hemisphere. Further, at 20 months, the two hemispheres show differential activation to unknown (right) vs. known words (left) (Mills et al., 1993). During the first 2 years of life, children move from pre-linguistic communication to intentional communication consisting of short 2-3-word sentences. Matsuzawa et al. (2001) did not find sex differences in brain development in infants and young children, however the sample size was small. Reiss et al. (1996) did find larger volume in boys compared to girls as young as 5 years of age. By 5 years of age, children are likely speaking with adult-like grammar and communicating easily with most people (https://www.nidcd.nih.gov/health/speech-and-language). Speech and language continues to flourish during these initial 5 years of life, and it is a crucial period for studying neurolinguistic development. A recent systematic review provided a comprehensive summary of all functional neuroimaging studies of language in children to date (Weiss-Croft & Bladeweg, 2015). This review highlighted four main findings. First, brain activity in regions supporting semantic processing increased with age. Second, brain activity in sensory and motor regions increased, while activation in higher order cognitive regions decreased with age. Third, brain activity in the posterior cingulate cortex and precuneus attenuated with age. Fourth, results showed that language lateralization is established by 5 years of age. From this it is clear that the brain areas associated with language undergo significant changes throughout the course of development; however, these changes may not necessarily occur in the same manner in boys and girls.
Wallentin (2009) published a critical review of literature on sex differences in language processing. He noted that most studies reporting sex differences on the basis of p values were only marginally significant. Studies with small numbers of subjects were more likely to report differences than those with large numbers of subject which implies that many of the significant findings were actually false positives. Moreover, large variation in the size or shape of certain brain structures raises doubts over the legitimacy of reports of sex differences and could indicate some may be spurious results. Overall, Wallentin (2009) concluded there was no convincing evidence for sex differences in language areas of the brain. However, this paper focused almost exclusively on studies of adults, and therefore we have a limited understanding of sex differences in language abilities in children and adolescents.
Since Wallentin’s review, there have been a number of studies that have been published examining childhood development on the topic of sex differences in brain structure and function in relation to language ability. Here, we aim to provide a review of these studies, focused on sex differences in brain structure and function relevant to language. We sought to investigate the consistency of any sex differences, and how they change over time with respect to various language functions. First, we sought to establish whether there are measurable sex differences in brain structure as they relate to language development. It was expected that any such differences would be highly dependent on factors such as age, brain region, and methods used to quantify structural measures. Second, we sought to examine how sex differences in brain function are associated with behavioral performance of language tasks. We anticipated that sex differences in the functional organization of the brain would be associated with corresponding differences in behavioral performance. This article discusses the implications of the results, placing them in the context of the broader scientific literature.
2 MATERIAL AND METHODS
2.1 Protocol
A systematic literature search was conducted using the PRISMA guidelines (http://www.prisma-statement.org) which describes robust guidelines for conducting meta-analyses and systematic reviews. Eligibility criteria are detailed below and in subsequent sections. The review considered any study published that directly investigated sex differences in language tasks in typical childhood development. The last date searched was 04/07/2017 (MM/DD/YYYY). We also considered studies focusing on structural and functional brain development. There was no restriction on the publication date.
2.2 Information sources
Online searches for articles were conducted using Scopus (https://www.scopus.com/) and PubMed (http://www.ncbi.nlm.nih.gov/pubmed). To ensure that our systematic review did not miss any items, we cross-checked the results against bibliographies in the articles. We identified relevant articles by searching for items that contained all of the words ‘child, ‘language’, ‘growth’ or ‘development’, ‘sex’ or ‘gender’ and either ‘MRI or magnetic resonance imaging, or fMRI or functional magnetic resonance imaging or EEG or electroencephalography or DTI or diffusion tensor imaging’ in the article title, abstract, or keywords (Scopus) and in all fields (PubMed). Notably, a child was considered any individual under the age of 18.
According to the APA Dictionary of Psychology (APA, 2015), whereas gender refers to the psychological, behavioral, social, and cultural aspects of being male and female, sex refers to the biological aspects of being male or female. In the context of neuroimaging research, many papers erroneously use these terms interchangeably. Here, we explicitly use the term sex differences to reflect the fact that we are primarily interested in biological differences rather than gender differences. This search returned a total of 126 items (PubMed) and 150 items (Scopus). Of these results, 86 items were common items from both databases while 40 (PubMed) and 64 (Scopus) articles were unique. In total the search identified 190 unique articles via PubMed and Scopus. A manual search of Google Scholar was also conducted. This search was designed to identify studies that were not detected by the database search (e.g. did not contain all the necessary search terms) but would still be relevant for inclusion in the systematic review. This yielded 20 additional articles.
2.3 Study selection
The 190 unique articles were manually inspected to assess their eligibility for inclusion in the systematic review. We excluded articles if they: (a) did not explicitly compare differences between males and females (i.e., the study statistically controlled for or ignored the effect of sex); (b) only included adults (i.e., only subjects over the age of 18 included); (c) focused on a disordered population (i.e., autism, dyslexia, or schizophrenia); (d) did not report empirical data (i.e., were reviews, systematic reviews, or meta-analyses); (e) did not report data on either brain structure or function. Based on these criteria, we excluded a total of 151 studies leaving a total of 39 studies.
Upon closer examination of the 39 full texts, we discovered that 13 did not meet the criteria for inclusion. Of these, seven focused on populations that may not be considered typically developing. Respectively, the studies tested children with auditory processing disorder (Bauer et al., 2009), children who were left handed (Szflarski et al., 2012), and those who lived in impoverished conditions (Tarullo et al., 2017). Others compared development in monolingual and bilingual children (Mohades et al., 2015) and early and late talkers (Preston et al., 2010). One paper was a review (Giedd et al., 2006) while another controlled for sex (Urger et al., 2015). The remaining six of the thirteen papers not meeting the criteria were excluded for miscellaneous reasons. Specifically, PubMed and Scopus both returned one entry with the same title and year of publication but a different sequence of authors. Respectively, the entries were listed as Gurholt et al. (2003) and Wilke et al., (2003). The discrepancy in authorship created the initial impression that the articles were different, but closer inspection revealed the contents of the articles were identical. Since one of the articles was already excluded, we removed the other from the list as well. A second focused on metabolites (Lebel et al., 2016). A third examined associations between intelligence, genes, and cortical thickness (Brouwer et al., 2014). A fourth examined the effect of diet on the brain (Li et al., 2010). A fifth, Guadalupe et al. (2015), was a synthesis of a large dataset from multiple cohorts in which only one sample contained children from the age of 17 years. Finally, one study did not provide adequate detail about the examination of sex (Xiaojuan et al., 2008).
After excluding these 13 studies, 26 studies from the database search remained. Twenty additional studies were identified from Google Scholar. This resulted in a total of 46 studies. Figure 1 depicts a visual representation of the search process.
Figure 1.
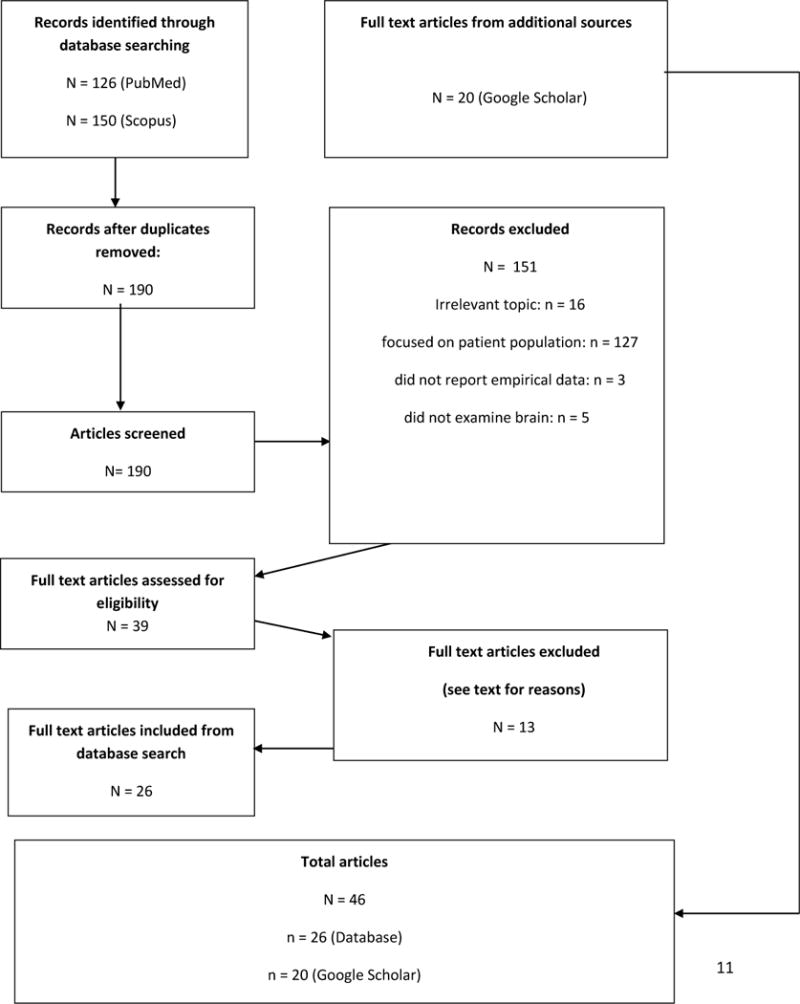
Diagrammatic representation of the systematic review process
2.4 Study collation
Important details of each study that was deemed fit for inclusion in the systematic review were recorded. These include information regarding whether they were identified via database search or Google Scholar, participant demographics, cognitive/linguistic tests, neuroimaging methodologies, whether or not there was explicit mention of correction for multiple comparisons and main findings and are presented in Table 1.
Table 1.
| Measure | Age in years | Left Inferior frontal gyrus (IFG) | Superior temporal gyrus (STG) | Association fibers | Corpus callosum | Basal Ganglia |
|---|---|---|---|---|---|---|
| Structure normalized to whole Brain Volume (mm/cm3) | 0 - 0.1 | Holland et al., 2014 **, Choe et al., 2013 | ||||
| “ “ | 4-18 | Blanton et al., 2004 * | Vadlamudi et al., 2006 | Tanaka-Arakawa 2015 ** | Caviness et al., 1996, Geidd et al., 1996 *, Sowell et al., 2002 * | |
| Sowell et al., 2002 * | ||||||
|
| ||||||
| Structure Not-Normalized to Brain Volume (mm/cm3) | 0 - 18 | Vadlamudi et al., 2006 | Tanaka-Arakawa 2015 ** | Sowell et al., 2002 * | ||
|
| ||||||
| Gray Matter Volume | 3 - 27 | Wilke et al., 2007 ** | Sowell et al., 2002 * | DeBellis et al., 2001 ** |
Wilke et al., 2007 ** Lenroot et al., 2007 ** |
|
| Wilke et al., 2007 ** | ||||||
| Lenroot et al., 2007 ** | ||||||
| Variability in GMV | 4-20 | Lange et al 1997 ** | Lange et al., 1997 ** | |||
|
| ||||||
| Area mm2 | 1.5 – 2.5 | Pujol et al., 1993 * | ||||
|
| ||||||
| Laterality L>R Covariance, laterality | 3 - 18 | Zeilinski et al., 2010 ** | Pries et al., 1999 * | |||
|
| ||||||
| Myelin Water Fraction | 0.2-5.5 | Denoi et al., 2015 ** | Denoi et al., 2015 ** | Dean et al., 2013* | ||
| “ | 0 - 8 | Su et al., 2008 | Su et al., 2008 | Su et al., 2008 | ||
|
| ||||||
| FA | 5 - 26 | Schmithorst et al., 2008 ** | Wang et al., 2012 | Schmithorst et al., 2008 ** | ||
| Lebel et al., 2009 ** | ||||||
| Qiu et al., 2011 * | ||||||
| Asato et al., 2010 ** | ||||||
|
| ||||||
| MD, AD, RD | 5 - 18 | Schmithorst et al., 2008 ** | Wang et al., 2012 | Wang et al., 2012 | ||
| >FA, <MD, AD, RD | 8 - 9 | Seunarine et al., 2016 * | ||||
| Asato et al., 2010 ** | ||||||
| >FA, <MD, AD, RD | 10 - 14 | Seunarine et al., 2016 * | ||||
3 RESULTS AND DISCUSSION
In total, structural MRI was the dominant methodology, with 26 studies reporting structural MRI studies. Substantially fewer studies investigated brain function using functional MRI (11 studies), EEG (6 studies), MEG (2 studies) and fNIRS (1 study). The age ranges used in individual studies varied widely from 2 to 90 days (Holland et al., 2014) to groups that included children as well as adults as old as 67 years (Szflarski et al., 2006). Likewise, the sample sizes varied significantly from 12 (6M and 6F) (Molfese et al., 1978) to 508 (284M 224F) (Hanlon et al., 1999). The hypothesis tested (e.g., sex differences, sex by age differences, sex differences modulated by a behavioral performance measure, etc.) also varied substantially between studies making direct comparisons between more than a few studies difficult.
3.1 Sex differences in brain and language development
Overall, we found inconsistent evidence for sexual dimorphism in language and brain development. Evidence for significant sex differences in brain structure and function is limited, and research to date related to this topic has resulted in conflicting reports. Furthermore, when sex differences in the brain were reported, this did not necessarily result in measurable differences in language ability. From this work, it appears that if sex differences do indeed exist, they are dependent on several heterogeneous factors. This review is based on a total of 46 studies. This number is considerably fewer than the number of brain-based studies examining sex differences in adults or the number of studies examining behavioral studies of sex differences in children. As such, the interpretation of the results can be only as strong as the studies included within the review. In the sections below, we summarize our findings on sex differences under two main themes: 1) sex differences in brain structure supporting language and 2) sex differences in brain function relevant to performing various language tasks. Where applicable, we also discuss the relationship between brain-based sex differences and behavioral performance on language tasks. We conclude the review by focusing on emerging themes, highlighting limitations of review studies, and suggesting areas for future research.
3.2 Sex differences in brain structure
The studies examining sex differences in brain structure in children employed a variety of measures, including cortical area (Pujol et al., 1993), gray and white matter volume (Blanton et al., 2004; Caviness et al., 1996; Choe et al., 2013; De Bellis et al., 2001; Giedd et al., 1997; Holland et al., 2014; Lee et al., 2015; Lenroot et al., 2007; Sowell et al., 2004; Valdalumi et al., 2006; Wilke et al., 2007), myelination (Su et al., 2008), fractional anisotropy/diffusivity (Lebel et al., 2009; Mohades et al., 2015; Schmithorst et al., 2008, Seunarine et al., 2016), myelin water fraction (Dean et al., 2015; Deoni et al., 2015) and laterality (Lebel et al., 2009; Qiu et al., 2011). Generally, whether or not sex differences were found depended largely on the specific brain measures and regions examined, and interactions with age (trajectories and how age was modelled). Most of the above studies collected some measure of language function or proxy for language function such as verbal IQ, however these were largely acquired to ensure that study participants exhibited typical range of functioning based on age-based norms, or to use as a covariate of no interest (to control for their effects on brain measures). Namely, these measures were not used to specifically examine the relationship between structure and language ability (some notable exceptions are Dean et al., 2015; Lebel et al., 2009; Lee et al., 2015; Qiu et al., 2011). This makes it somewhat difficult to establish a robust relationship between brain structure and performance on language tasks. While sex differences in brain structure were reported, whether such structural differences are associated with corresponding sex differences in behavioral performance of language tasks remain unclear. Reviewed studies that examined specific brain structures relevant to examining possible sex differences supporting language function are discussed in more detail below. Table 1 provides a summary of the different methods used to study sex differences in children, categorized by the main brain structures reviewed below (Table 1).
3.2.1 Inferior frontal gyrus (IFG)
The inferior frontal gyrus (IFG) comprises cortical areas rostral to ventral premotor cortex, and typically include Brodmann areas 44, 45, 47. The left IFG in particular overlaps with Broca’s area and supports functions critical to speech and language, including analysis of semantic and syntactic relations (Skeide & Friederici, 2016) and planning and production of speech articulation (Hickok & Poeppel, 2007). Several studies have reported sex differences in the IFG. In a study examining age and “gender” effects during normal cortical maturation, Blanton et al. (2004) examined gray and white matter volume in 21 boys and 25 girls between 6 to 17 years old. The authors found significantly greater left IFG gray matter volume in boys relative to girls, and age related left IFG white matter volume increases in boys. Continued modification of IFG during normal development was observed in boys. The significance of this finding is unclear, as the neuroanatomical results and their relationship to language functioning was not explored. Nevertheless, the authors speculated that the age associated re-organization of the left IFG in males may be what makes this region more sensitive to abnormality, which could render males to be more susceptible to developmental disorders affecting language development.
In a larger study of 98 boys and 102 girls between 5 to 19 years, Wilke et al. (2007) reported greater gray matter volume in the left IFG for girls compared to boys, but this was not associated with differences in verbal IQ as an indicator for language abilities. Another study measuring the degree of myelination in boys and girls from birth to 8 years of age in the left IFG found no sex differences (Su et al., 2008). The studies reviewed in this section together suggest that boys and girls have different developmental trajectories of the left IFG, particularly during school-age and adolescence.
3.2.2 Superior temporal gyrus (STG)
The horizontal plane of the superior temporal gyrus (STG) houses the primary and secondary auditory cortices, critical regions that support speech and language processing. Auditory regions in the STG have been examined for potential sex differences using a variety of measures, including cortical volume. Lange et al. (1997) identified greater variability in the overall brain structural volume of the left STG in pubertal males relative to females; this pattern was not found in pre-pubertal subjects. Such variability may suggest poorer performance on language tasks in pubertal boys as compared to pubertal girls, but given that no tests of language were performed in the study, this is difficult to verify (for a more general review on the influence of puberty on sex differences in structural brain development see Herting & Sowell, 2017). In another study where STG was examined in children and adolescents, Wilke et al. (2007) observed greater pSTG gray matter volume in boys relative to girls between 5 and 18 years of age. Girls on the other hand showed greater gray matter volume in the left IFG. No sex by age interaction was found in this study. In addition, sex differences in white matter volume of the pSTG were not found. Similarly, Su et al (2008) found no sex difference in the degree of myelination in Wernicke’s area, located in the posterior part of the STG (Su et al., 2008; see also Deoni et al., 2015).
In terms of structural asymmetry, one study reported greater leftward asymmetry of the planum temporale in females than males aged between 3 and 14 years (Preis et al., 1999), although the authors caution that unpublished data using a larger sample found no such difference. In line with this work, Vadlamudi et al. (2006) did not find any sex differences in the asymmetry of the planum temporale in children between 4 to 16 years.
3.2.3 Corpus callosum (CC)
The corpus callosum is the major commissural fiber bundle that inter-connects the two cerebral hemispheres. Sub regions in the corpus callosum connect speech relevant areas between the two hemispheres. More specifically, the anterior third of the corpus callosum connects the bilateral the prefrontal, premotor, and supplementary motor areas; the anterior midbody connects the motor areas; the posterior midbody connects the posterior parietal lobes; the isthmus connects the posterior parietal and superior temporal lobes; the splenium connects the occipital and inferior temporal lobes (Hofer & Frahm, 2006). The main function of the corpus callosum is to integrate information between the left and right hemispheres, but it is also involved in sensory processing, memory, and attention (Giedd et al., 1999). It also plays a crucial role in language lateralization (Hinkley et al., 2016). As such, examining the structure of the corpus callosum could provide important glimpses into patterns of brain laterality and its effects on language development.
Based on research in children and adults that examined structural connectivity measures, female brains have been reported to be better optimized for inter-hemispheric connectivity. Males on the other hand exhibited greater intra-hemispheric connectivity (Ingalhalikar et al., 2014; see also Gur & Gur, 2017). Intra-hemispheric connectivity to a large extent is supported by the structural integrity of the corpus callosum that is necessary to perform semantic and phonology judgment tasks that require bilateral neural coordination (Baxter et al., 2003; Bitan et al., 2010; Burman et al., 2008).
Other studies examining sex differences in callosal morphology in young children (Giedd et al., 1999) and adolescents (De Bellis et al., 2001; Pujol et al., 1993; Reiss et al., 1996) failed to find convincing evidence for sex differences in either area or growth rates. Later work using larger samples reported mixed findings with respect to sex differences when considering interactions with age. For example, De Bellis et al. (2001), even when adjusting for total cerebral volume, found no age-related differences in the area of the corpus callosum between the sexes. It is worth noting however that when considering the interaction with Tanner stages (a scale of physical development including external primary and secondary sex characteristics; Marshall & Tanner, 1969, 1970), rather than age, the interaction was significant.
Size differences in sub-regions of the corpus callosum may reflect differences in structural connectivity between the two hemispheres that support language task performance. Tanaka-Arakawa and colleagues (2015) studied the development of the corpus callosum via MRI images acquired from subjects ranging in age from one month to 25 years. The corpus callosum was segmented into 7 subregions in the mid-sagittal plane, and the area measure of each sub region was computed: rostrum, genu, rostral body, anterior midbody, posterior midbody, isthmus, and splenium. Whole brain size was also calculated in order to provide size ratios for the subregions relative to the whole brain. Although there were no sex differences or sex by age interactions in the absolute size of the corpus callosum, the ratio of total corpus callosum to the whole brain was significantly higher in females than males, as were each ratio of genu, posterior midbody, splenium to the whole brain volume.
Similarly, using myelin water fraction, a surrogate measure of myelin content, Dean et al. (2015) modelled non-linear growth rates in the corpus callosum in 108 children aged between 2.5 months and 5.5 years. Using a longitudinal design, the authors found significant correlation between myelin water fraction with motor and cognition measures across the sexes. The overall trajectories of myelin water fraction did not differ between the sexes, however the growth rate of development was higher in males than females, while the amplitude of developmental trajectories was higher in females relative to males.
3.2.4 Association fibers
In addition to the corpus callosum, other major white matter tracts including the dorsal and ventral auditory pathways play an important role in language development (Brauer et al., 2013; Friederici & Gierhan, 2013). Diffusion tensor imaging (DTI) is a structural MRI technique that has often been applied to examine the microstructural development of white matter in children. In one study that examined diffusion metrics derived from DTI scans in children 8-16 years, Seunarine et al. (2016) found that, in general, girls exhibit advanced development in widespread areas including the corpus callosum. Compared to boys, girls showed decreased mean diffusivity (MD), axial and radial diffusivity, and increased fractional anisotropy (FA). MD quantifies the magnitude of diffusion in any given direction, while FA reflects the degree of diffusion in a preferential direction; it reflects white matter coherence. In general, FA increases and MD decreases during neurodevelopment, reflecting increasing white matter integrity. The sex differences observed were greatest in a narrow age range (8-9) but converged at 10-14 years (Seunarine et al., 2016). In addition, boys showed a steeper slope of development of these diffusion metrics in the ages that were examined, whereas girls did not show age related changes. Similar sex differences in the rates of changes in diffusion metrics were also reported by others (Clayden et al., 2012; Schmithorst et al., 2008).
There is a lack of evidence supporting sex differences in the arcuate fasciculus, the bundle of fibers connecting the inferior frontal gyrus and the superior temporal gyrus. Two studies report a distinct lack of sex differences in the laterality of the arcuate fasciculus in children between 5 - 13 years (Lebel & Beaulieu, 2009) and 7- 23 years (Qiu et al., 2011). While leftward lateralization is associated with higher reading scores (particularly in first graders), and girls scored better than boys on a reading task (Qiu et al., 2011), this does not appear to be related to the extent of lateralization of the arcuate fasciculus. Fractional anisotropy (FA) values in the arcuate fasciculus do not differ between boys and girls (Seunarine et al., 2016) but girls between 5 and 18 appear to show greater mean diffusivity (MD) in the right arcuate fasciculus as they mature (Schmithorst et al., 2008).
There is conflicting evidence regarding white matter diffusivity characteristics of the superior longitudinal fasciculus (SLF), a branch of which is the arcuate fasciculus, mentioned above. The SLF connects the frontal and temporal lobes and is crucial for speech and language production. One study found adolescent boys to have higher axial diffusivity (AD), a measure that generally goes hand in hand with MD, in the right inferior and superior longitudinal fasciculus (Bava et al., 2011). Greater AD along the fiber projections of the SLF may suggest maturation of the SLF evidenced by more restricted diffusion. However, another study of adolescents (Wang et al., 2012) reported that girls have higher AD while boys have higher FA in the same structure. Results also showed that girls, but not boys, exhibit positive correlations between FA and verbal IQ in the left cortico-spinal tract and superior longitudinal fasciculus. There are also conflicting reports of MD in the SLF. Seunarine et al. (2016) found that MD was greater in males than females 8-16 years of age, and in contrast Wang et al. (2012) reported MD was greater in females than males 13-17 years old. One study suggests there is earlier development in all language related tracts in females than males except for the right SLF (Asato et al., 2010; see also Wang et al., 2012). Overall, there are conflicting reports of sex differences in the superior longitudinal fasciculus in children and adolescents. These can be attributed to differences in the age range of the samples and indicates that there are actually negligible differences between the sexes in brain structure associated with language ability.
3.2.5 Basal ganglia (BG)
The basal ganglia comprise a group of subcortical structures implicated in speech production, due to their major role in the initiation, execution, sequencing, and timing of movements including speech production (Price, 2010; 2012). Greater activity in the BG is associated with faster (left dorsal putamen) and more accurate (left caudate) phonological processing (Tettamanti et al., 2005) as well as detecting syntactical anomalies (left caudate; Moro et al., 2001). Generally, girls are reported to have larger subcortical gray matter volume than age-matched boys, although there are region specific trends across basal ganglia and thalamus subregions (reviewed in Herting et al., 2014), even when corrected for total brain volume (Caviness et al., 1996; Choe et al., 2013; Giedd et al., 1997; Lange et al., 1997; Neufang et al., 2009; Sowell et al., 2002; Wilke et al., 2007). This difference is partially attributable to varying levels of sex hormones: when testosterone levels are low, caudate volume increases, but when levels increase during adolescence, caudate volume decreases (Herting et al., 2014). This study also found boys to have larger left thalamus volumes than girls. Although the caudate is associated with language ability, the extent to which this relationship is associated with sex differences has not yet been directly examined. Maturation of the basal ganglia occurs earlier in females than males. For example, girls achieve peak caudate size between 7.5 to 10.5 years of age which is ~2.5 (Giedd et al., 1997) to 3.5 (Lenroot et al., 2007) years earlier than boys. Interestingly, aberrant caudate volume is reported in a variety of developmental disorders affecting speech and language such as stuttering (e.g. Foundas et al., 2013; Sowman et al., 2017), which is significantly more prevalent in boys than girls. Overall, there is some evidence that sex differences reported in the BG may be related to different rates of maturation between boys and girls, rather than inherent sex differences.
3.3 Sex differences in brain function
Sex differences found in language-relevant brain structures as reviewed in the previous section may not necessarily be associated with sex differences in language task performance measured at the behavioral level, or differences in brain activity patterns in the same brain areas. Namely, it is possible that sex differences in language task performance, and patterns of brain activity associated with language processing may exist in the absence of structural differences and vice versa. In the following sections, we review studies that have reported brain functional differences between the sexes observed during performance of various language tasks. Research utilizing functional magnetic resonance imaging (fMRI) typically involved assessing differences in either magnitude of activation (Burman et al., 2008; Plante et al., 2006; Wood et al., 2004) or functional connectivity (Bitan et al., 2010; Burman et al., 2013; Wu et al., 2013), or correlated brain activity patterns, during language tasks. The studies examining magnetoencephalographic (MEG) or electroencephalographic (EEG) components assessed differences in amplitude and latency of brain oscillations acquired while children performed language tasks. We organize the sections below by the various language tasks (or no task, as in resting state fMRI) that were used to examine brain activity. Table 2 provides a summary of the different language tasks used to study sex differences in brain function in children, categorized by the main brain structures that showed functional differences (Table 2).
Table 2.
| Task | Age in years | Inferior frontal gyrus (IFG) | Superior temporal gyrus (STG) | Basal Ganglia (BG) |
|---|---|---|---|---|
| Resting State | ||||
| Node efficiency | 5.6-18.4 | Wu et al., 2013 ** | ||
| Node betweeness | 5.6-18.4 | Wu et al., 2013 ** | ||
| ICA connectivity | 7-12, 13-18 | Sole-Palledis et al 2016 ** | Sole-Palledis et al 2016 ** | Sole-Palledis et al 2016 ** |
| Lateralization | 7-29 | Nielsen et al., 2013 ** | Nielsen et al., 2013 ** | Nielsen et al., 2013 ** |
| Slow Wave activity during sleep | 8.7-19.4 | Ringli et al., 2013 | Ringli et al., 2013 | |
| Coherence | 0 - 16 | Hanlon et al., 1999 ** | ||
|
| ||||
| Comprehension/Speech/non speech Perception | ||||
| Association between intelligence and connectivity | Schmithorst & Holland 2013 | Schmithorst & Holland 2013 | ||
| Word Matching Task, left laterality | 6-9 | Gummadavelli et al., 2013 * | ||
| “ | 10-13 | Gummadavelli et al., 2013 * | ||
| Left laterality | 1-1.45 | Molfese et al., 1990 | ||
| left laterality | 76-103 days | Shucard et al., 1981 | ||
| Listening to consonant vowel consonants left laterality | 3.9 – 4.9 | Molfese and Hess 1978 | ||
| Listening to familiar and unfamiliar voices left laterality | 3.4-4.5 | Yamasaki et al., 2013 * | ||
| 4.5-6 | Yamasaki et al., 2013 * | |||
|
| ||||
| Speech Production | ||||
| Verb generation/naming to description left lateralization or voxelwise activation | 4 – 9 | Yu et al., 2014 * | Yu et al., 2014 * | |
| “ “ | 10 - 18 |
Yu et al., 2014 * Gaillard et al., 2013 Plante et al., 2006 ** Szflarski et al., 2006 ** |
Yu et al., 2014 * Gaillard et al., 2013 Plante et al., 2006** Szflarski et al., 2006 ** |
|
| Oral reading, number of voxels | 6-15 | Wood et al., 2004 * | ||
| Story processing voxelwise activation | 5 - 18 | Plante et al., 2006 ** | Plante et al., 2006 ** | |
|
| ||||
| Orthographic/Phonological Processing | ||||
| effective connectivity, activation | 9 - 15 | Bitan et al,. 2010 *Burman et al., 2008 ** | Bitan et al,. 2010 *Burman et al., 2013 | |
| left laterality | 10 – 10.6 | Spironelli et al., 2010 * | ||
| functional connectivity | “ | Burman et al., 2013 ** | ||
3.3.1 Resting state
Brain activity examined during resting state (when a subject is passively lying in the scanner and letting their mind wander without performing any form of overt or covert task) can provide insights into the degree of interaction among functionally connected regions including language areas such as Broca’s and Wernicke’s areas (Hampson et al., 2002). There is some evidence for sex differences in network connectivity involving language-relevant areas such as the left IFG and left putamen. For example, Wu et al. (2013) showed that boys exhibit higher node efficiency (ability of a node to propagate information to other areas in a network) in the left putamen and left orbitofrontal cortex, while girls exhibit higher node betweenness (a measure of the influence of a region over the flow of information between all other regions within a network) in the triangular portion of the left IFG. These results await confirmation with larger samples of children. Sole-Padulles et al. (2016) reported a resting state fMRI study of 113 children between the ages of 7 and 18 years. Here, they examined the intrinsic connectivity of multiple networks, including those involved in language. While the results showed significant age-related changes in network connectivity from childhood to adolescence in both boys and girls, there were no significant sex differences or age by sex interactions. Thus, this well-powered study failed to find support for the existence of sex differences in intrinsic functional connectivity of the language networks in children. Likewise, using 1011 subjects from multiple publicly available datasets with subjects aged from 7 to 29 years, Nielsen et al. (2013) found no difference in the functional lateralization of resting state connectivity of language areas between males and females. Strong left lateralized networks involving Broca’s and Wernicke’s areas were present in both sexes and this did not differ significantly between the sexes.
Compared to the findings from resting state fMRI, some electrophysiological (EEG) studies have reported evidence of sex differences in language related cortical regions. For instance, in a study that examined mean EEG coherence in 224 girls and 284 boys (2 months-16 years), Hanlon et al. (1999) found sex-specific patterns of timing differences in the development of synchronous EEG coherence peaks. From birth to 6 years, girls showed synchronized EEG coherence peaks in the frontal and left temporal cortical regions associated with language function, while during the same age range boys showed the EEG coherence peaks in visual-spatial processing areas. After the age of 6, boys showed the synchronized peaks in the frontal left temporal language areas (Hanlon et al., 1999). Ringli et al. (2013) recorded over 60 minutes of EEG data during sleep in 11 boys and 11 girls aged between 8.7 and 19.4 years of age. Bilateral slow wave activity, a measure thought to reflect cortical plasticity (Huber et al., 2006; Vyazorskiy, 2009), in language regions was greater for girls compared to boys. Girls showed higher activity in the bilateral temporal regions than boys, while boys had higher activity in the right frontal region compared to girls. The authors suggested that increased slow wave activity may account for previous reports of female superiority on language tasks, but this conclusion is only speculative as they did not actually measure performance on language tasks. Since slow wave activity is thought to be an indicator of maturation, speech and language regions may mature at different rates in males and females.
In summary, when examining resting state activity, there is inconsistent evidence of sexual dimorphism as measured by lateralization, activation, and connectivity of language areas.
3.3.2 Language comprehension and speech perception
Many studies have examined task-based brain activity associated with language comprehension and speech perception. Schmidhorst and Holland (2007) conducted a study in which the relationship between intelligence and brain functional connectivity for narrative comprehension was examined in 151 boys and 152 girls. In girls, increased connectivity between left posterior STG and Wernicke’s areas bilaterally was associated with higher verbal IQ. For boys, functional connectivity between Broca’s area and bilateral auditory regions was associated with higher verbal IQ. An age effect was observed in girls, where a positive correlation with age was observed in the association between intelligence and functional connectivity linking the bilateral auditory areas. These results indicate an increasing inter-hemispheric connectivity of temporal areas supporting narrative comprehension in girls. Due to the large number of subjects and rigorous correction for multiple comparisons, these results provide strong evidence of sex differences in brain function supporting language comprehension in children.
In a study examining sex differences in story listening in pre-school age (3-5 years) children (13M and 17F), Sroka et al. (2015) compared neural activity during passive listening to a story versus listening to a non-speech broadband noise sweep. Both groups showed the expected activity in bilateral auditory cortices, left angular gyrus, and supramarginal gyrus during the passive listening task. Boys showed greater activity in the right anterior cingulate and the superior frontal gyrus than girls even though there was no difference in performance on tests of receptive vocabulary. The authors suggested that, given the involvement of these regions in executive functioning, boys may require greater cognitive resources to achieve similar performance on receptive vocabulary tasks. Interestingly, children with higher vocabulary scores showed increased left-lateralization and greater activity in the bilateral thalamus, hippocampus, and left angular gyrus, which partly mirrors structural studies (Lee et al., 2015; Lee et al., 2009; Sole-Padulles et al., 2016). Although there was stringent correction for multiple comparisons, the small number of subjects in conjunction with the lack of behavioral difference suggests some degree of caution is warranted in interpreting the results.
Dichotic listening tasks are often used to assess speech/language lateralization. In these tasks, different stimuli are presented in each ear simultaneously and subjects are asked to report what is heard. Subjects tend to be more accurate or faster when reporting what is heard in the right ear, a phenomenon also referred to as a right ear advantage ([REA]; see Bryden 1988). In terms of a REA, Hirnstein et al. (2013) reported there was a significant interaction between age and sex, such that the REA emerged earlier in adolescent (10-15 years) girls than adolescent boys. In children (younger than 10) and younger adults (between 16-49 years in this study), however, there were no significant sex differences. Any difference between the sexes in REA was small and was not accompanied by any differences in neural activity as measured with fMRI. The large number of subjects used (>100) and narrow age range examined indicates that when differences do emerge, they are relatively small. Additionally, the study also provides strong evidence that sex differences are negligible in terms of REA in younger children.
In a study examining word recognition, MEG was used to collect high frequency neural oscillation data while boys and girls (age range: 6-13 years) performed a word matching task (Gummadavelli et al., 2013). In this task, a pair of words was presented, one auditorily via speakers, and one visually on screen. If the words were the same (i.e., they matched), then subjects were instructed to do nothing. If the words were different (i.e., they did not match), the subjects were instructed to press a button. Only the data where the stimuli matched were analyzed. Regardless of sex, there were increases in left lateralization of language activation with age in the 70-120 Hz range. Brain activity occurring in this frequency range was localized to Broca’s and Wernicke’s areas and was thought to reflect maturational changes. Boys and girls differed in language laterality with age. Between 6-9 years of age, girls but not boys showed a positive correlation between age and left laterality. Between the 10-13 years of age, more boys (12/20) than girls (5/20) exhibited bilateral activation in Wernicke’s area indicating greater laterality in older girls. These results suggest there are reliable differences in the lateralization of language as measured by high frequency oscillations. But how this relates to language performance is unclear given no behavioral response were recorded. Importantly, the results seem to corroborate the greater REA occurring in girls than boys in the 10-15 age range as noted above in a study by Hirnstein et al. (2013).
More evidence supporting earlier occurring and greater left laterality for word recognition in girls than boys comes from an EEG study by Molfese (1990). In this study, auditory evoked potentials (AEPs) in the left hemisphere differentiated between known and unknown words in infant girls, whereas AEPs in both hemispheres did so for infant boys. This would suggest more left lateralized representation for word recognition in girls as compared to boys. However, this conclusion should be taken with caution given the very small number of subjects. Likewise, Shucard et al. (1981) reported that in the left hemisphere, girls produced higher auditory evoked potentials to tones played while they were listening to a story. Boys showed additional activation in the right hemisphere. These authors concluded that greater left lateralization for girls may underpin behavioral differences in language ability in early childhood. Molfese and Hess (1978) recorded EEG activity while participants listened to consonant vowel syllables. Using principal components analysis, the authors decomposed the 64.4% of the variance across experimental conditions into four factors. They showed that as voice onset time increased, the contribution of the first factor increased in a non-linear fashion; with respect to this first factor, boys had a larger right hemisphere response than girls, suggesting stronger right lateralization in boys than girls. However, the authors note that the differences they observed were likely due to maturation, since they were not found in adults. Furthermore, the small number of subjects and lack of correction for multiple comparisons in the Molfese and Hess (1978) study means that the study does not provide strong support for greater right lateralization in boys. Although the studies tend to converge in terms of findings, given the small number of children from both sexes, these results await confirmation by future studies that employ larger sample sizes.
Using near-infrared spectroscopy – a neuroimaging technique that enables measurement of hemodynamic activity of brain regions near the scalp – Yamasaki et al. (2013) collected responses in bilateral temporal areas in response to the mother’s voice, an unfamiliar voice, and environmental sounds in 10 younger (3-4.5 years) and 10 older (4.5-6 years) children. Overall, sensitivity to the mother’s voice was higher in the younger group than the older group of children, highlighting the crucial role of mother’s voice throughout early childhood. The only sex difference observed was that older girls exhibited weaker responses to their mother’s voice in left temporal areas as compared to older boys. This result may indicate that girls achieve more mature patterns of activity in the left auditory cortex earlier compared to their male peers, who seem to be still sensitive to their mother’s voice, similar to that seen in younger children during language development. However, as with several other studies mentioned above, this study also used a small number of subjects, thereby reducing the confidence that can be placed in the conclusions. Overall, there is inconsistent evidence of sexual dimorphism involving language comprehension and speech perception. Left-lateralized activity in girls compared to boys, particularly during early childhood, has been reported, however these results are inconsistent and await confirmation by future studies that compare the sexes across narrower age brackets.
3.3.3 Speech production
Using a verb generation task, Yu et al. (2014) examined sex differences in spatiotemporal patterns of language lateralization by recording neuromagnetic activity in the gamma band. This task requires word retrieval, semantic processing, and expressive language (Yu et al., 2014) and is a reliable task to compare sex-related developmental differences in language function. Whereas boys showed left hemisphere lateralization in the frontal and temporal language areas, girls showed a more bilateral pattern, especially in frontal areas. Additionally, these differences were most evident at a younger age but converged in later (preteen) years. The authors again suggested they were associated with different rates of maturation and that they did not persist into adulthood. Interestingly, there was no difference in performance on tests of language ability (Peabody Picture Vocabulary Test (PPVT, Dunn & Dunn 2007) and the Expressive Vocabulary Test (EVT Williams 1997), so while there may be differences in neural activity, there are not corresponding differences in performance on language tests.
Another MEG study of children between 5 and 19 focusing on the frontal lobe reported no sex differences in laterality during an overt verb generation task (Kadis et al., 2011). Corroborating this finding, multiple fMRI studies report that boys and girls do not differ in the degree of lateralization of left frontal regions (e.g. Broca’s) during a verb generation task (Gaillard et al., 2003; Plante et al., 2006; Szflarski et al., 2006; Wood et al., 2004). This consistent result from multiple studies, most of which corrected for multiple comparisons and used large samples, suggest that boys and girls likely do not differ in the degree of lateralization of brain activity associated with speech production. In addition, there appears to be a positive relationship between the number of active voxels in the left frontal regions and performance on an oral reading task regardless of sex (Wood et al., 2004). The lack of sex differences in functional laterality associated with speech production tasks conflict with the results in the previous section, where several studies reported a greater left laterality of function associated with speech/language comprehension in girls than boys. This difference may reflect greater left auditory-motor integration needed for speech production relative to comprehension. Greater left auditory-motor integration for speech production is supported by a left lateralized arcuate/superior longitudinal fasciculus, which was reviewed in a previous section (3.2.4 Association fibers) to be comparable between the sexes in structural laterality.
Different from results reporting lack of sex differences in laterality, some studies found significant sex differences when performing speech production tasks. Plante et al. (2006) collected behavioral and fMRI data from 225 subjects across multiple language tasks, one of which included a verb generation task where subjects were asked to ‘think’ of a verb related to a given noun. They found a significant, albeit small, interaction between sex and age in a left frontal ROI for the verb generation task such that girls showed greater activation in the left inferior frontal region. They did not find significant sex differences in functional laterality. Notably, any sex differences involved an interaction with age, highlighting that what was described as a sex difference may be more accurately described as maturational changes. More specifically, the “sex differences” reflected the fact that boys and girls mature at slightly different rates and times. Further, interactions between age and sex generally also depended on the language task performed. Overall, the results reported in this study (see also Schmidhorst et al., 2006) underscore that sex differences are subtle and dependent not only on age, but also the specific task performed, and the neuroanatomical regions of interest examined.
3.3.4 Phonology and orthography
Bitan et al. (2010) examined the direction of interhemispheric interaction associated with performing rhyming judgment of spoken words using an effective connectivity analysis of fMRI data. Subjects performed a task involving rhyming judgment of spoken words. The words were either conflicting or non-conflicting in terms of their orthography and phonology. For example, in the two non-conflicting conditions, orthography and phonology were either both similar (dime, lime) or different (staff, gain). In the conflicting conditions, either orthography matched and phonology did not (pint, mint) or phonology matched and orthography did not (e.g. jazz, has). Thirty-nine children aged 9-15 participated in this study, where interesting findings on inter-hemispheric communication, involving connections among primary and association auditory and inferior frontal regions were reported. A generally greater interhemispheric connectivity was found in girls compared to boys. However, greater interhemispheric connectivity from the right STG to the left STG was associated with lower verbal IQ and slower reaction times in girls (Bitan et al., 2010; see also Schmithorst et al., 2007). The authors interpreted these results to indicate that while the heightened inter-hemispheric interaction may provide benefits for some aspects of language processing, it may adversely affect performance in girls with low verbal IQ. For example, the authors suggested that in the rhyming task, some girls may rely too much on less relevant information such as melodic pitch of the speaker’s voice (processed by the right STG) that occurs at the expense of focusing on more relevant phonological information (processed by the left STG). Related to sex differences in laterality, Spironelli et al. (2010) conducted an ERP study using orthographic, phonological, and semantic tasks in 28 school-age children (14 boys). The findings pointed to reduced left language lateralization in girls compared to boys in all tasks during the early period of reading skill development.
Others have reported that despite a lack of difference in behavioral performance on an orthographic judgment task, girls exhibited greater activity of bilateral IFG and left fusiform gyrus across all tasks, and this was correlated with accuracy (Burman et al., 2008). Additionally, activity in the left fusiform gyrus was correlated with Wide Ranging Achievement Test spelling ([WRAT-III]; Wilkinson, 1993), Woodcock-Johnson III Tests of Cognitive Abilities reading ([WJ-III]; Woodcock, Mather, McGrew, & Schrank, 2001), and Test of Word Reading Efficiency phonetic decoding ([TOWRE]; Torgesen, Wagner, & Rashotte, 1999), but only in girls (Burman et al., 2008). Burman et al. (2013) further examined the location of connectivity as it related to orthographic judgement tasks. The orthographic judgement task described here involved visual presentation of two words sequentially on a screen. Subjects were asked to judge whether the letter sequence from the first vowel onwards in the two words was the same or different. Increases in age and verbal IQ were associated with a lateral shift from the primary visual cortex to the left temporal lobe (fusiform gyrus) in girls and a posterior shift in the occipital cortex for boys, indicating that a different strategy might be used between boys and girls to perform orthographic judgment tasks. But despite the relatively large number of subjects (42), these conclusions should be taken with caution given that there was no mention of correction for multiple comparisons. In sum, the findings discussed in this section indicate that boys and girls may differentially engage visual and language regions to support orthographic and phonological processing during development. There appears to be some evidence supporting sex differences in laterality of language area activity during these tasks, with females tending to engage more inter-hemispheric activity compared to males.
4 Conclusions
4.1 Emerging themes from the reviewed literature
There is inconsistent evidence of sex differences in brain structure/function related to language. Although there may be statistically significant differences in brain structure and function between boys and girls, the practical significance of these differences seems to be negligible. On the other hand, it is possible that boys and girls employ different, but equally effective, cognitive strategies for certain tasks that lead to minor differences in performance as evidenced by brain function, but not in the behavioral performance itself.
Sex differences in brain structure/function often interact with a variety of factors. For example, age effects have been frequently reported to interact with any sex differences. This highlights the need to examine narrow age ranges particularly in children. Interestingly, such work has also led multiple authors to conclude that what is often described as a sex difference might be more accurately characterized as a difference in maturation between the sexes. Another important factor is task; sex differences might be dependent on very specific task parameters. For example, the emergence of sex differences may depend on whether the task involves perception, production, or judgements about language stimuli. It might also depend on the complexity of the stimuli (such as whether they are syllables, single words, or entire sentences) and how they are presented. Further, the identification of sex differences seems to depend on what brain structure or function is measured, such as fractional anisotropy, connectivity, area, volume, thickness, amplitude. Taken together, finding sexual dimorphism of brain development relevant to language appears to depend largely on considering the effects of age, brain region(s) examined, and the technique and analyses utilized.
4.2 Limitations of reviewed studies
4.2.1 Relationship between brain and behavior
Most studies failed to directly examine the relationship between brain structure/function and performance on tests of language ability. On one hand, the functional relevance of sex differences in the brain is unclear because it is difficult to determine whether they are a cause or consequence of differences in behavior. On the other hand, sex differences in the brain do not always imply differences in behavior. Instead of producing differences at a behavioral level, sex differences in brain structure and function may arise to minimize them. More generally, there is a disconnect between studies examining brain structure and function and those examining behavioral performance of language tasks. To gain a clearer understanding of the brain and language, it is crucial for these to be integrated.
4.2.2 Challenges of conducting functional imaging studies in young children
Successful collection of brain imaging data from children is a challenging endeavor. The experimental procedure must be carefully designed and implemented to ensure the child is comfortable and any movement is minimized throughout the duration of the experiment, particularly in the case of MRI. Movement artifacts can introduce additional sources of variance and contribute to null results. Similarly, the short scanning time inadvertently generates a high degree of inter-individual variation thereby further diluting any difference between the sexes (Grayson et al., 2017). This is a difficult challenge to overcome because long sessions are typically prohibitive to scanning young children. Further, when a task is involved, it must be simple for the child to understand and perform. Most task-based imaging studies reviewed here tested the perception or (covert) production of single words or syllables. Simple tasks may not be sufficiently demanding to elicit and reveal sex differences in behavior. To capture the true extent of sex, it may be necessary to examine language using tasks that are more ecologically valid. The choice of tasks and specific experimental parameters may have a strong influence on whether sex differences are identified.
4.2.3 Biases in reported results
There are a small number of published studies finding significant differences in brain structure and function and language ability in boys and girls. It is important to consider however, the phenomena of “publication bias” or the tendency of researchers to submit, and journals to publish, only manuscripts reporting significant results (Ferguson & Heene, 2012). More specifically, this raises the question of how many unpublished studies or those rejected by journals failed to find significant differences between the sexes. Publication bias may obscure a far larger number of studies finding no significant differences in brain structure/function and behavior. This can be solved by journals implementing pre-registered reports and publishing studies regardless of significance (c.f. Cortex). Another important consideration is the motivation of researchers to find significant differences. The effect of sex was routinely analyzed even though it was not always the main focus of investigation. Not surprisingly, these studies tended to find no differences between boys and girls. For example, an a priori hypothesis using regions of interest may allow for greater statistical power relative to an exploratory investigation using whole brain analysis.
It is somewhat disconcerting to find that papers that failed to explicitly correct for multiple comparisons seemed to identify sex differences about as frequently as those that did make such corrections. 26 studies examined differences in brain structure. Fifteen of the 26 explicitly reported correcting for multiple comparisons while 11 did not. Of those 15, 13 (87%) reported significant differences and 2 (13%) did not. At least 3 of the 15 studies on brain structure cautioned interpreting the sex differences due to small samples, issues with p values and a lack of correlation with behavioral values. Among the studies that explicitly mentioned correcting for multiple comparisons, 50% mainly reported on sex differences in overall gray or white matter volume that tended to favor boys. The other 50% reported differences in various structures or tracts. Of the 11 studies that did not explicitly correct for multiple comparisons, 9 (82%) reported significant group differences and 2 (18%) did not.
Ten (67%) of the 15 studies of brain structure corrected for multiple comparisons and also reported a significant sex difference, compared to 9 (82%) of the 11 studies reporting significant sex differences in brain structure that did not correct for multiple comparisons.
Twenty studies examined differences in brain function. 60% (12 studies) of these explicitly mentioned correcting for multiple comparisons and 40% (8 studies) did not. Of the 12 studies that corrected for multiple comparisons, 8 (67%) reported significant differences in brain activity and 4 (33%) did not. At least one study among the studies that corrected for multiple comparisons reported no corresponding difference in behavioral measures and another study noted that the differences were very small in magnitude.
The percentage of studies investigating brain function that corrected for multiple comparisons and also reported significant sex differences was 50%. The percentage of studies reporting significant differences in brain function that did not correct for multiple comparisons is 75%. Taken together, this illustrates that the percentage of robustly conducted functional studies reporting significant sex differences is lower than the percentage of less rigorously conducted studies reporting significant differences.
Notably too, the sex of the first author tends to affect whether the paper finds sex differences or not (Hyde & Linn, 1988). Nevertheless, if publication bias is a significant contributing factor, it would only serve to bolster the conclusion that the magnitude of sex differences is smaller than described here.
4.3 Limitations of this review
There are a number of limitations of this review. First, while the authors made every attempt to identify published studies examining sex differences in language and faithfully represent their main findings, there is the possibility some papers have been missed in our search. This may be attributed to a combination of factors such as the databases examined and the specific search terms used. Such limitations are not unique to our review, but inherent to all systematic reviews. Second, the scope of this review was limited. It specifically focused on sex differences in the brain associated with language ability in typically developing children and ipso facto did not address the large body of literature documenting sex differences in children with neurodevelopmental disorders of language. This is an area of considerable interest given the vast discrepancies in the prevalence of stuttering, dyslexia, and specific language impairment among boys and girls. Moreover, the review also did not cover the effects socioeconomic status (Barbu et al., 2015; see also Tarullo et al., 2017), hormones (Schaadt et al., 2015), brain metabolites (Lebel et al., 2016), or diet (Li et al., 2010), which are associated with sex differences in performance on language tasks. Consequently, it is important to consider how these variables might also influence differences in brain structure and function between boys and girls.
4.4 Conclusions and future directions
The existing literature suggests there is inconsistent evidence for sex differences in brain structure and function, and any such differences do not generally result in corresponding differences in behavioral performance on language tasks. Conflicting reports of sex differences in structure and function of language areas of the developing brain suggest that these differences may be negligible. These results are consistent with a systematic review of studies of adults (Wallentin et al., 2009). However, it should be noted that a lack of consistent sex differences in children associated with language does not preclude the existence of sex differences in other cognitive domains. Further research is needed and below we provide some recommendations for this endeavor. Future studies would benefit from using significantly larger samples to have sufficient power to detect differences should they exist. Additionally, given that sex differences found in several studies were age dependent, examining a restricted age range rather than one spanning more than a decade would increase the likelihood of gaining insights into sex differences that occur at each developmental stage. For example, research could focus on investigating whether there are sex differences in brain structure/function at specific milestones in linguistic development (see Pujol et al., 2006) such as when a child can comprehend two-word sentences, produce 10 words, is mostly intelligible, or can produce a story in response to a picture (Luinge et al., 2006). Second, it would be necessary for such studies to relate measures of brain structure/function to performance on language tasks to establish functional relevance of any differences. This is likely a feasible goal, given that most studies already collect data on language function. Third, utilizing gross statistical measures may obscure subtle sex differences. Therefore, future studies could utilize more sophisticated analysis techniques or scanning protocols. For example, this may involve measures of connectivity and conducting multimodal imaging. In the case of electrophysiological methods, time frequency analysis could be used rather than assessing amplitude and latency. Fourth, future research should examine the influence of genes and hormones on language development as well as how they interact with neurodevelopmental disorders. To date, detailed investigations on genetic and hormonal influences on sexual dimorphism of brain and language development have been sparse. Such studies would provide clearer insights into the biological bases of brain structural and functional differences that have been reported in previous studies such as those reviewed here. Overall, further investigation of sexual dimorphism of brain differences associated with language function is needed, to establish if indeed they exist, how and when they arise and their relationship to behavior. Such work will have important implications not only for typical development, but also for those with neurodevelopmental disorders.
Supplementary Material
Acknowledgments
This study was supported by Award Number R01DC011277 from the National Institute on Deafness and other Communication Disorders (NIDCD) to SC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health.
References
- American Psychiatric Association. DSM 5. American Psychiatric Association; 2013. [Google Scholar]
- American Psychological Association. Guidelines for psychological practice with transgender and gender nonconforming people. American Psychologist. 2015;70(9):832–864. doi: 10.1037/a0039906. [DOI] [PubMed] [Google Scholar]
- Apgar Virginia. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg. 1953;32(4):260–267. [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna BS. White matter development in adolescence: a DTI study. Cerebral Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbu S, Nardy A, Chevrot JP, Guellaï B, Glas L, Juhel J, Lemasson A. Sex differences in language across early childhood: Family socioeconomic status does not impact boys and girls equally. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P, Burger M, Kummer P, Lohscheller J, Eysholdt U, Doellinger M. Correlation between psychometric tests and mismatch negativity in preschool children. Folia Phoniatrica et Logopaedica. 2009;61(4):206–216. doi: 10.1159/000227998. [DOI] [PubMed] [Google Scholar]
- Bava S, Boucquey V, Goldenberg D, Thayer RE, Ward M, Jacobus J, Tapert SF. Sex differences in adolescent white matter architecture. Brain Research. 2011;1375:41–48. doi: 10.1016/j.brainres.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LC, Saykin AJ, Flashman LA, Johnson S, Guerin SJ, Babcock D, Wishart HA. Sex differences in semantic language processing: a functional mri study. Brain and Language. 2003;84(2):264–272. doi: 10.1016/s0093-934x(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2nd. Psychological Coporation; 1993. (Bayley-II) [Google Scholar]
- Bitan T, Lifshitz A, Breznitz Z, Booth JR. Bidirectional connectivity between hemispheres occurs at multiple levels in language processing but depends on sex. The Journal of Neuroscience. 2010;30(35):11576–11585. doi: 10.1523/JNEUROSCI.1245-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton RE, Levitt JG, Peterson JR, Fadale D, Sporty ML, Lee M, To D, Mormino EC, Thompson PM, McCracken JT, et al. Gender differences in the left inferior frontal gyrus in normal children. NeuroImage. 2004;22(2):626–636. doi: 10.1016/j.neuroimage.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Bullock D, Guenther FH. Neural representations and mechanisms for the performance of simple speech sequences. Journal of Cognitive Neuroscience. 2010;22(7):1504–1529. doi: 10.1162/jocn.2009.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles DB. A large-sample study of sex differences in functional cerebral lateralization. Journal of Clinical and Experimental Neuropsychology. 2005;27(6):759–768. doi: 10.1081/13803390590954263. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain research. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J, et al. Dorsal and ventral pathways in language development. Dorsal and ventral pathways in language development. 2013;127(2):289–295. doi: 10.1016/j.bandl.2013.03.001. http://doi.org/10.1016/j.bandl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Brouwer RM, van Soelen IL, Swagerman SC, Schnack HG, Ehli EA, Kahn RS, Boomsma DI. Genetic associations between intelligence and cortical thickness emerge at the start of puberty. Human Brain Mapping. 2014;35(8):3760–3773. doi: 10.1002/hbm.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. Handbook of nonverbal assessment. Springer; Boston, MA: 2003. Test of nonverbal intelligence; pp. 191–221. [Google Scholar]
- Bryden MP. An overview of the dichotic listening procedure and its relation to cerebral organization 1988 [Google Scholar]
- Buckhalt JA. Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) Diagnostique. 1990;15:254–63. [Google Scholar]
- Burman DD, Bitan T, Booth JR. Sex differences in neural processing of language among children. Neuropsychologia. 2008;46(5):1349–1362. doi: 10.1016/j.neuropsychologia.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman DD, Minas T, Bolger DJ, Booth JR. Age, sex, and verbal abilities affect location of linguistic connectivity in ventral visual pathway. Brain and Language. 2013;124(2):184–193. doi: 10.1016/j.bandl.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Science. 2001;25(5):663–678. [Google Scholar]
- Caplan R, Levitt J, Siddarth P, Wu KN, Gurbani S, Sankar R, Shields WD. Frontal and temporal volumes in childhood absence epilepsy. Epilepsia. 2009;50(11):2466–2472. doi: 10.1111/j.1528-1167.2009.02198.x. [DOI] [PubMed] [Google Scholar]
- Carrow-Woolfolk E. OWLS, Oral and Written Language Scales: Written Expression Scale. American Guidance Service; 1996. [Google Scholar]
- Caviness VS, Kennedy DN, Richelme C, Rademacher JFPA, Filipek PA. The human brain age 7-11 years: a volumetric analysis based on magnetic resonance images. Cerebral Cortex. 1996;6(5):726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Choe MS, Ortiz-Mantilla S, Makris N, Gregas M, Bacic J, Haehn D, Grant PE. Regional infant brain development: an MRI-based morphometric analysis in 3 to 13 month olds. Cerebral Cortex. 2012;23(9):2100–2117. doi: 10.1093/cercor/bhs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornoldi C, Colpo G. Prove di lettura MT per la scuola elementare—2 [Italian MT reading tests for primary school—2](Organizzazioni speciali) 1998 [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11(6):552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Dean DC, O’Muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Walker L, Han M, Deoni SC. Modeling healthy male white matter and myelin development through 60 months of age. NeuroImage. 2014;84:742–752. doi: 10.1016/j.neuroimage.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, O’Muircheartaigh J, Dirks H, Waskiewicz N, Walker L, Doernberg E, Piryatinsky I, Deoni SC. Characterizing longitudinal white matter development during early childhood. Brain Structure and Function. 2015;220(4):1921–1933. doi: 10.1007/s00429-014-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Dean DC, Remer J, Dirks H, O’Muircheartaigh J. Cortical maturation and myelination in healthy toddlers and young children. NeuroImage. 2015;115:147–161. doi: 10.1016/j.neuroimage.2015.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn Lloyd M. PPVT-III: Peabody picture vocabulary test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Dunn LM, Dunn DM. Peabody picture vocabulary test. Bloomington: NCS Pearson Inc; 2007. [Google Scholar]
- Ferguson CJ, Heene M. A vast graveyard of undead theories: Publication bias and psychological science’s aversion to the null. Perspectives on Psychological Science. 2012;7(6):555–561. doi: 10.1177/1745691612459059. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Cindass R, Mock JR, Corey DM. Atypical caudate anatomy in children who stutter. Perceptual and Motor Skills. 2013;116(2):528–543. doi: 10.2466/15.10.PMS.116.2.528-543. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Gierhan SME. The language network. 2013;23(2):250–254. doi: 10.1016/j.conb.2012.10.002. http://doi.org/10.1016/j.conb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Fujita K, Maekawa H, Dairoku H, Yamanaka K. Japanese Wechsler Adult Intelligence Scale. Tokyo: Nihon Bunka Kagakusha; 2006. [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Grandin CB. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Human Brain Mapping. 2003;18(3):176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal mri study. Progress in Neuro- Psychopharmacology and Biological Psychiatry. 1999;23(4):571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal mri study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1997;21(8):1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(5):465. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural mri of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YSK, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. The Journal of Neuroscience. 2007;27(6):1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH. Dynamic development of regional cortical thickness and surface area in early childhood. Cerebral Cortex. 2015;25(8):2204–2212. doi: 10.1093/cercor/bhu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. NeuroImage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, Fair DA. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe T, Zwiers MP, Wittfeld K, Teumer A, Vasquez AA, Hoogman M, Hegenscheid K. Asymmetry within and around the human planum temporale is sexually dimorphic and influenced by genes involved in steroid hormone receptor activity. Cortex. 2015;62:41–55. doi: 10.1016/j.cortex.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Gummadavelli A, Wang Y, Guo X, Pardos M, Chu H, Liu Y, Horn P, Zhang F, Xiang J. Spatiotemporal and frequency signatures of word recognition in the developing brain: a magnetoencephalographic study. Brain Research. 2013;1498:20–32. doi: 10.1016/j.brainres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gur RE. Complementarity of sex differences in brain and behavior: From laterality to multimodal neuroimaging. Journal of Neuroscience Research. 2017;95(1–2):189–199. doi: 10.1002/jnr.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in mr images. Human Brain Mapping. 2002;15(4):247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon HW, Thatcher RW, Cline MJ. Gender differences in the development of EEG coherence in normal children. Developmental Neuropsychology. 1999;16(3):479–506. [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, Sowell ER. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural mri study. Human Brain Mapping. 2014;35(11):5633–5645. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Sowell ER. Puberty and structural brain development in humans. Frontiers in neuroendocrinology. 2017;44:122–137. doi: 10.1016/j.yfrne.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley LB, Marco EJ, Brown EG, Bukshpun P, Gold J, Hill S, Mukherjee P. The contribution of the corpus callosum to language lateralization. Journal of Neuroscience. 2016;36(16):4522–4533. doi: 10.1523/JNEUROSCI.3850-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirnstein M, Westerhausen R, Korsnes MS, Hugdahl K. Sex differences in language asymmetry are age-dependent and small: A large-scale, consonant–vowel dichotic listening study with behavioral and fMRI data. Cortex. 2013;49(7):1910–1921. doi: 10.1016/j.cortex.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited— comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Holland D, Chang L, Ernst TM, Curran M, Buchthal SD, Alicata D, Skranes J, Johansen H, Hernandez A, Yamakawa R, et al. Structural growth trajectories and rates of change in the first 3 months of infant brain development. JAMA Neurology. 2014;71(10):1266–1274. doi: 10.1001/jamaneurol.2014.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nature Neuroscience. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Hyde JS. The gender similarities hypothesis. American Psychologist. 2005;60(6):581. doi: 10.1037/0003-066X.60.6.581. [DOI] [PubMed] [Google Scholar]
- Hyde JS. Sex and cognition: gender and cognitive functions. Current Opinion Neurobiology. 2016;38:53–56. doi: 10.1016/j.conb.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Linn MC. Gender differences in verbal ability: A meta-analysis. Psychological Bulletin. 1988;104(1):53. [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Berman Z, Tavor I, Wexler N, Gaber O, Stein Y, Shefi N, Pool J, Urchs S, Margulies DS, et al. Sex beyond the genitalia: The human brain mosaic. Proceedings of the National Academy of Sciences. 2015;112(50):15468–15473. doi: 10.1073/pnas.1509654112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Fausto-Sterling A. Beyond sex differences: new approaches for thinking about variation in brain structure and function. Philosophical Transactions Royal Society B: Biological Sciences. 2016;371(1688):20150451. doi: 10.1098/rstb.2015.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadis DS, Pang EW, Mills T, Taylor MJ, McAndrews MP, Smith ML. Characterizing the normal developmental trajectory of expressive language lateralization using magnetoencephalography. Journal of the International Neuropsychological Society. 2011;17(5):896–904. doi: 10.1017/S1355617711000932. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY: A developmental neuropsychological assessment. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- Kotz SA, Schwartze M, Schmidt-Kassow M. Non-motor basal ganglia functions: A review and proposal for a model of sensory predictability in auditory language perception. Cortex. 2009;45(8):982–990. doi: 10.1016/j.cortex.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Lange N, Giedd JN, Castellanos FX, Vaituzis AC, Rapoport JL. Variability of human brain structure size: ages 4–20 years. Psychiatry Research: Neuroimaging. 1997;74(1):1–12. doi: 10.1016/s0925-4927(96)03054-5. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human Brain Mapping. 2009;30(11):3563–3573. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Nordahl CW, Amaral DG, Lee A, Solomon M, Ghetti S. Assessing hippocampal development and language in early childhood: Evidence from a new application of the automatic segmentation adapter tool. Human Brain Mapping. 2015;36(11):4483–4496. doi: 10.1002/hbm.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F, Cai Q, Bogart SL, Dubois J, Coulon O, Monzalvo K, Fischer C, Glasel H, Van der Haegen L, B́eńezit A, et al. New human-specific brain landmark: the depth asymmetry of superior temporal sulcus. Proceedings of the National Academy of Sciences. 2015;112(4):1208–1213. doi: 10.1073/pnas.1412389112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Dykman RA, Jing H, Gilchrist JM, Badger TM, Pivik RT. Cortical responses to speech sounds in 3-and 6-month-old infants fed breast milk, milk formula, or soy formula. Developmental Neuropsychology. 2010;35(6):762–784. doi: 10.1080/87565641.2010.508547. [DOI] [PubMed] [Google Scholar]
- Lindberg SM, Hyde JS, Petersen JL, Linn MC. New trends in gender and mathematics performance: a meta-analysis. Psychological Bulletin. 2010;136(6):1123. doi: 10.1037/a0021276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luinge MR, Post WJ, Wit HP, Goorhuis-Brouwer SM. The ordering of milestones in language development for children from 1 to 6 years of age. Journal of Speech, Language, and Hearing Research. 2006;49(5):923–940. doi: 10.1044/1092-4388(2006/067). [DOI] [PubMed] [Google Scholar]
- Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, Gilmore JH. Dynamic development of regional cortical thickness and surface area in early childhood. Cerebral Cortex. 2015;25(8):2204–2212. doi: 10.1093/cercor/bhu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccoby EE, D’Andrade RG, et al. The development of sex differences. Stanford University Press; 1966. p. 5. [Google Scholar]
- Maccoby EE, Jacklin CN. The psychology of sex differences. Stanford University Press 1974 [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of disease in childhood. 1969;44:235–291. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of disease in childhood. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohades SG, Van Schuerbeek P, Rosseel Y, Van De Craen P, Luypaert R, Baeken C. White-matter development is different in bilingual and monolingual children: a longitudinal DTI study. PloS One. 2015;10(2):e0117968. doi: 10.1371/journal.pone.0117968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese DL. Auditory evoked responses recorded from 16-month-old human infants to words they did and did not know. Brain and Language. 1990;38(3):345–363. doi: 10.1016/0093-934x(90)90120-6. [DOI] [PubMed] [Google Scholar]
- Molfese DL, Hess TM. Hemispheric specialization for VOT perception in the preschool child. Journal of Experimental Child Psychology. 1978;26(1):71–84. doi: 10.1016/0022-0965(78)90110-8. [DOI] [PubMed] [Google Scholar]
- Moro A, Tettamanti M, Perani D, Donati C, Cappa SF, Fazio F. Syntax and the brain: disentangling grammar by selective anomalies. NeuroImage. 2001;13(1):110–118. doi: 10.1006/nimg.2000.0668. [DOI] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, Schissel AM, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. NeuroImage. 2008;39:1918–1925. doi: 10.1016/j.NeuroImage.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JA, Zielinski BA, Ferguson MA, Lainhart JE, Anderson JS. An evaluation of the left-brain vs. right-brain hypothesis with resting state functional connectivity magnetic resonance imaging. PloS One. 2013;8(8):e71275. doi: 10.1371/journal.pone.0071275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, Byars AW. Sex differences in the activation of language cortex during childhood. Neuropsychologia. 2006a;44(7):1210–1221. doi: 10.1016/j.neuropsychologia.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Preis S, Jancke L, Schmitz-Hillebrecht J, Steinmetz H. Child age and planum temporale asymmetry. Brain and Cognition. 1999;40(3):441–452. doi: 10.1006/brcg.1998.1072. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fmri studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191(1):62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20years of pet and fmri studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junqué C, Martí‐Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of Neurology. 1993;34(1):71–75. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Ortiz H, Sebastian-Galles N, Losilla JM, Deus J. Myelination of language-related areas in the developing brain. Neurology. 2006;66(3):339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Siok WT, Zhou K, Khong PL. Lateralization of the arcuate fasciculus and its differential correlation with reading ability between young learners and experienced readers: A diffusion tensor tractography study in a chinese cohort. Human Brain Mapping. 2011;32(12):2054–2063. doi: 10.1002/hbm.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. Brain. 1996;119(5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Ringli M, Kurth S, Huber R, Jenni OG. The sleep eeg topography in children and adolescents shows sex differences in language areas. International Journal of Psychophysiology. 2013;89(2):241–245. doi: 10.1016/j.ijpsycho.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Sartori G, Job R, Tressoldi PE. Batteria per la valutazione della Dislessia e della Disortografia Evolutiva. Firenze, Italy: Organizzazioni Speciali; 1995. [Italian battery for the evaluation of Dyslexia and Dysorthographya] [Google Scholar]
- Schaadt G, Hesse V, Friederici AD. Sex hormones in early infancy seem to predict aspects of later language development. Brain and Language. 2015;141:70–76. doi: 10.1016/j.bandl.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Human Brain Mapping. 2008;29(6):696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Sex differences in the development of neuroanatomical functional connectivity underlying intelligence found using Bayesian connectivity analysis. Neuroimage. 2007;35(1):406–419. doi: 10.1016/j.neuroimage.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seunarine KK, Clayden JD, Jentschke S, Munoz M, Cooper JM, Chadwick MJ, Banks T, Vargha-Khadem F, Clark CA. Sexual dimorphism in white matter developmental trajectories using tract-based spatial statistics. Brain Connectivity. 2016;6(1):37–47. doi: 10.1089/brain.2015.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L, et al. Sex differences in the functional organization of the brain for language. Nature. 1995;373(6515):607. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fletcher JM, Escobar MD. Prevalence of reading disability in boys and girls: Results of the Connecticut longitudinal study. Journal of the American Medical Association. 1990;264(8):998–1002. [PubMed] [Google Scholar]
- Shucard JL, Shucard DW, Cummins K, Campos JJ. Auditory evoked potentials and sex-related differences in brain development. Brain and Language. 1981;13(1):91–102. doi: 10.1016/0093-934x(81)90131-0. [DOI] [PubMed] [Google Scholar]
- Skeide MA, Friederici AD. The ontogeny of the cortical language network. The ontogeny of the cortical language network. 2016;17(5):323–332. doi: 10.1038/nrn.2016.23. http://doi.org/10.1038/nrn.2016.23. [DOI] [PubMed] [Google Scholar]
- Solé-Padullés C, Castro-Fornieles J, de la Serna E, Calvo R, Baeza I, Moya J, Sugranyes G. Intrinsic connectivity networks from childhood to late adolescence: effects of age and sex. Developmental Cognitive Neuroscience. 2016;17:35–44. doi: 10.1016/j.dcn.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowman PF, Ryan M, Johnson BW, Savage G, Crain S, Harrison E, Martin E, Burianová H. Grey matter volume differences in the left caudate nucleus of people who stutter. Brain and Language. 2017;164:9–15. doi: 10.1016/j.bandl.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine & Child Neurology. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spironelli C, Penolazzi B, Angrilli A. Gender differences in reading in school-aged children: An early ERP study. Developmental Neuropsychology. 2010;35(4):357–375. doi: 10.1080/87565641.2010.480913. [DOI] [PubMed] [Google Scholar]
- Sroka MC, Vannest J, Maloney TC, Horowitz-Kraus T, Byars AW, Holland SK, Consortium CA et al. Relationship between receptive vocabulary and the neural substrates for story processing in preschoolers. Brain Imaging and Behavior. 2015;9(1):43–55. doi: 10.1007/s11682-014-9342-8. [DOI] [PubMed] [Google Scholar]
- Su P, Kuan CC, Kaga K, Sano M, Mima K. Myelination progression in language-correlated regions in brain of normal children determined by quantitative MRI assessment. International Journal of Pediatric Otorhinolaryngology. 2008;72(12):1751–1763. doi: 10.1016/j.ijporl.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people fMRI data. Neurology. 2002;59(2):238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Human brain mapping. 2006;27(3):202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Arakawa MM, Matsui M, Tanaka C, Uematsu A, Uda S, Miura K, Sakai T, Noguchi K. Developmental changes in the corpus callosum from infancy to early adulthood: A structural magnetic resonance imaging study. PloS One. 2015;10(3):e0118760. doi: 10.1371/journal.pone.0118760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti M, Moro A, Messa C, Moresco RM, Rizzo G, Carpinelli A, Perani D. Basal ganglia and language: phonology modulates dopaminergic release. Neuroreport. 2005;16(4):397–401. doi: 10.1097/00001756-200503150-00018. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Rashotte CA, Wagner RK. TOWRE: Test of word reading efficiency. Austin, TX: Pro-ed; 1999. [Google Scholar]
- Vadlamudi L, Hatton R, Byth K, Harasty J, Vogrin S, Cook M, Bleasel A. Volumetric analysis of a specific language region–the planum temporale. Journal of Clinical Neuroscience. 2006;13(2):206–213. doi: 10.1016/j.jocn.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Comprehensive test of phonological processing. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Wallentin M. Putative sex differences in verbal abilities and language cortex: A critical review. Brain and Language. 2009;108(3):175–183. doi: 10.1016/j.bandl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Adamson C, Yuan W, Altaye M, Rajagopal A, Byars AW, Holland SK. Sex differences in white matter development during adolescence: a DTI study. Brain research. 2012;1478:1–15. doi: 10.1016/j.brainres.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler intelligence scale for children, revised. Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. Scala di intelligenza Wechsler per bambini-riveduta. Firenze, Italy: Organizzazioni Speciali; 1986. [Wechsler Intelligence Scale for Children–Revised (Italian version)] [Google Scholar]
- Wechsler D. Manual for the Wechsler preschool and primary scale of intelligence, rev. San Antonio, TX: Psychological Corporation; 1989. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children-Third Edition. San Antonio, TX: Psycho-logical Corporation; 1991. [Google Scholar]
- Wechsler D. WAIS-III, Wechsler adult intelligence scale: Administration and scoring manual. Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. WISC-IV: Wechsler Intelligence Scale for Children: Technical and Interpretive Manual. Psychological Corporation; 2004. [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. NeuroImage. 2003;20(1):202–215. doi: 10.1016/s1053-8119(03)00199-x. [DOI] [PubMed] [Google Scholar]
- Wilke M, Krägeloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Experimental Brain Research. 2007;178(3):296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test 3—Administration Manual. Wilmington, DE: Jastak Associates; 1993. [Google Scholar]
- Williams KT. Expressive vocabulary test second edition (EVT™ 2) J Am Acad Child Adolesc Psychiatry. 1997;42:864–872. [Google Scholar]
- Wood AG, Harvey AS, Wellard RM, Abbott DF, Anderson V, Kean M, Saling MM, Jackson GD. Language cortex activation in normal children. Neurology. 2004;63(6):1035–1044. doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- Woodcock RW. Woodcock reading mastery tests, revised, examiner’s manual. American Guidance Service; 1998. [Google Scholar]
- Woodcock RW. Woodcock reading mastery tests—revised/normative update. Circle Pines, MN: American Guidance Service; 1998. [Google Scholar]
- Woodcock RW, Mather N, McGrew KS, Schrank FA. Woodcock-Johnson III Normative Update: Tests of Achievement Form A. Riverside Publishing; 2001. [Google Scholar]
- Wu K, Taki Y, Sato K, Hashizume H, Sassa Y, Takeuchi H, Thyreau B, He Y, Evans AC, Li X, et al. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PloS One. 2013;8(2):e55347. doi: 10.1371/journal.pone.0055347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiojuan G, Jin Z, Chen K, Peng D, Yao L. Medical Imaging. International Society for Optics and Photonics; 2008. Mar, Gender differences in brain development in Chinese children and adolescents: a structural MRI study; pp. 69160A–69160A. [Google Scholar]
- Yamasaki T, Ogata K, Maekawa T, Ijichi I, Katagiri M, Mitsudo T, Kamio Y, Tobimatsu S. Rapid maturation of voice and linguistic processing systems in preschool children: A near-infrared spectroscopic study. Experimental Neurology. 2013;250:313–320. doi: 10.1016/j.expneurol.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Yu VY, MacDonald MJ, Oh A, Hua GN, De Nil LF, Pang EW. Age- related sex differences in language lateralization: A magnetoencephalography study in children. Developmental Psychology. 2014;50(9):2276. doi: 10.1037/a0037470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zell E, Krizan Z, Teeter SR. Evaluating gender similarities and differences using metasynthesis. American Psychologist. 2015;70(1):10. doi: 10.1037/a0038208. [DOI] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proceedings of the National Academy of Sciences. 2010;107:18191–18196. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


