Abstract
The IGF-1 signaling pathway has emerged as a major regulator of the aging process, from rodents to humans. However, given the pleiotropic actions of IGF-1, its role in the aging brain remains complex and controversial. While IGF-1 is clearly essential for normal development of the central nervous system, conflicting evidence has emerged from preclinical and human studies regarding its relationship to cognitive function, as well as cerebrovascular and neurodegenerative disorders. This review delves into the current state of the evidence examining the role of IGF-1 in the aging brain, encompassing preclinical and clinical studies. A broad examination of the data indicates that IGF-1 may indeed play opposing roles in the aging brain, depending on the underlying pathology and context. Some evidence suggests that in the setting of neurodegenerative diseases that manifest with abnormal protein deposition in the brain, such as Alzheimer’s disease, reducing IGF-1 signaling may serve a protective role by slowing disease progression and augmenting clearance of pathologic proteins to maintain cellular homeostasis. In contrast, inducing IGF-1 deficiency has also been implicated in dysregulated function of cognition and the neurovascular system, suggesting that some IGF-1 signaling may be necessary for normal brain function. Furthermore, states of acute neuronal injury, which necessitate growth, repair and survival signals to persevere, typically demonstrate salutary effects of IGF-1 in that context. Appreciating the dual, at times opposing “Dr. Jekyll” and “Mr. Hyde” characteristics of IGF-1 in the aging brain, will bring us closer to understanding its impact and devising more targeted IGF-1-related interventions.
Keywords: Alzheimer’s, cognition, Parkinson’s, CNS
Introduction
The growth hormone/insulin-like growth factor-1 (GH/IGF1) signaling pathway, also referred to as the somatotropic axis, has been extensively implicated in the aging process (Bartke, et al. 2003; Barzilai, et al. 2012; Brown-Borg and Bartke 2012; Kenyon 2010). Attenuation of this conserved signaling pathway has been demonstrated to reduce the incidence of age-related diseases and extend survival in numerous rodent models (Brown-Borg, et al. 1996; Ikeno, et al. 2003; Kinney, et al. 2001a; Kinney, et al. 2001b). Remarkably, this pathway also appears to be relevant to human aging, as functional mutations in the IGF-1R resulting in attenuated IGF-1 signaling are enriched in centenarians (Suh, et al. 2008; Tazearslan, et al. 2011), while stratifying nonagenarian females by IGF-1 levels revealed a survival advantage in those with low IGF-1 (Milman, et al. 2014). Likewise, lower serum IGF-1 levels were associated with protection from cognitive impairment late into the tenth decade among women (Perice, et al. 2016). The role of IGF-1 in the central nervous system (CNS) has been a particularly intriguing, albeit controversial area of investigation, with evidence linking IGF-1 to favorable, detrimental, and indifferent effects on CNS function and disease risk. In this review we will delve into the current state of the evidence examining the role of IGF-1 in the aging brain, with a particular focus on the consistencies and controversies that have emerged from preclinical and clinical studies.
IGF-1 and the brain
Endocrine vs. autocrine IGF-1
Similar to many other tissues, the brain is exposed to two sources of IGF-1, endocrine and autocrine. The regulation of endocrine production of IGF-1, which is primarily under the control of GH pulses secreted from the pituitary in response to growth hormone releasing hormone (GHRH) and ghrelin, consists of a classic negative feedback loop and has been extensively described elsewhere (Berelowitz, et al. 1981). In brief, GH binds to the GH receptor to stimulate IGF-1 production mainly in the liver and the rise in circulating IGF-1 levels results in enhanced binding to IGF-1 receptors (IGF-1R) in the pituitary and the hypothalamus to inhibit GH and GHRH secretion, respectively. Hepatic-derived IGF-1 comprises ~70% of total circulating IGF-1 (Ohlsson, et al. 2009) levels and can provide input to the CNS by crossing the blood brain barrier (BBB) at the choroid plexus into the cerebrospinal fluid (CSF) via the IGF-1R and low-density lipoprotein receptor-related protein 2 (LRP2) transport protein (also known as megalin) (Carro, et al. 2005). During early life, IGF-1 is abundantly expressed throughout the CNS and is essential for normal brain development. The critical role of autocrine IGF-1 for neuronal development is evidenced by findings that mutations resulting in global IGF-1 loss or insensitivity manifest as microcephaly and cognitive deficiencies in children (Abuzzahab, et al. 2003; Netchine, et al. 2011; Woods, et al. 1996). In contrast, mutations that result in GH deficiency or resistance frequently present with normal cognitive ability (Kranzler, et al. 1998) suggesting that autocrine brain IGF-1 production may be preserved in these individuals (Joseph D’Ercole and Ye 2008).” In the circulation, most IGF-1 is bound to IGF binding proteins (IGFBP), with IGFBP-3 being most abundant, and therefore is inactive (Baxter 2000; Holly and Perks 2012; Rajaram, et al. 1997). IGF-1 exerts its action in tissues by binding to the high-affinity IGF-1R on the cell surface (Holly and Perks 2012); notably, IGF-1 also binds to the insulin receptor, but with much lower affinity (Novosyadlyy and Leroith 2012). IGF-1 binding to the IGF-1R initiates a complex intracellular signaling cascade that includes phosphorylation of the insulin receptor substrate (IRS) molecules and subsequent activation of phosphoinositide 3-kinase-Protein kinase B (PI3K-Akt) and the mitogen-activated protein kinase (MAPK) pathways that regulate, among several downstream effectors, mechanistic target of rapamycin (mTOR) activity and Forkhead box O (FoxO) translocation (Taniguchi, et al. 2006). Signaling through these pathways influences autophagy, growth, stress resistance, oxidative stress, and lifespan (Barzilai et al. 2012).
IGF-1 and the aging brain
In the brain, autocrine IGF-1 production is thought to peak during development (Joseph D’Ercole and Ye 2008). Meanwhile, endocrine production of IGF-1, and presumably IGF-1 input into the CNS, remains high during the early years, peaking during puberty, a period that coincides with rapid cell proliferation and linear growth (Yamamoto, et al. 1991). By the third decade, IGF1 production abruptly drops off and then continues steadily declining with aging, raising questions regarding the potential role of reduced IGF-1 levels in the manifestations of brain aging (Yamamoto et al. 1991). The endocrine decline in IGF-1 has been attributed to diminished GH pulse amplitude and frequency that is observed in aging, at least in part due to decreased ghrelin binding to the growth hormone secretagogue receptor (GHSR) (Sun, et al. 2004); thus, resulting in steady, but low-level secretion of GH in older individuals (Carlson, et al. 1972; Finkelstein, et al. 1972). Limited evidence suggests that concomitant with the decline in systemic IGF-1 levels, local production, as assessed by CSF and brain tissues levels, also declines with age, in spite of the fact that brain IGF-1 production is believed to act independent of GH regulation (Ashpole, et al. 2015). In fact, observations from aged rodent models show that IGF-1R transcription increases with age in the hippocampal and cortical regions of the brain, possibly in a compensatory attempt to preserve brain IGF-1 signaling in the setting of less IGF-1 availability; however, this response is inadequate to restore IGF-1 back to youthful levels and IGF-1 levels in the brain of aged animals remain lower compared to young animals (Ashpole et al. 2015). Beyond the decline in local and systemic IGF-1 levels with aging, there is some evidence that the aged brain may be resistant to IGF-1 signaling (Muller, et al. 2012). Observations of declining autocrine and endocrine IGF-1 levels with age, paired with knowledge that aging is associated with increased risk of cognitive decline and diseases affecting the brain, beg the question of whether IGF-1 is involved in brain aging.
IGF-1 and cognition
Epidemiologic and clinical studies
Age-related cognitive decline, which in its more progressive forms, may be characterized by mild cognitive impairment (MCI) or dementia, secondary to causes such as cerebrovascular ischemia or Alzheimer’s disease (AD), poses a significant burden for an increasingly aging population, raising the urgency to identify underlying contributors to these conditions in order to delay or prevent their onset (Langa, et al. 2017; Prince, et al. 2016; Wu, et al. 2017). Studies in humans and rodents have been conducted into the possible relationship between IGF-1 and the cognitive domain, and have uncovered interesting, albeit at times conflicting associations (Frater, et al. 2017). A number of cross-sectional studies in middle and older age adults have revealed a positive correlation between IGF-1 levels and cognition (Al-Delaimy, et al. 2009; Doi, et al. 2015; Wennberg, et al. 2018). However, this observation was not always consistent between sexes, with significant findings limited only to males or females across different studies (Al-Delaimy et al. 2009; Wennberg et al. 2018). On the other hand, another cross-sectional analysis found an inverse relationship between IGF-1 and cognitive processing capacity in men age 60 and older (Tumati, et al. 2016). Likewise, in a cohort of exceptional longevity, females with IGF-1 levels in the lowest tertile had a nearly 50% lower prevalence of cognitive impairment compared to females with IGF-1 levels in the upper two tertiles (Perice et al. 2016). Similarly, prospective studies have done little to settle this controversy. One study demonstrated a positive association between baseline serum IGF-1 levels and future cognitive performance among older women (Okereke, et al. 2007), whereas another found no association in men (Green, et al. 2014). Yet, a different prospective analysis found lower cognitive scores among men with highest baseline IGF-1 levels after 8 years of follow-up (Tumati et al. 2016). Higher serum IGF-1 levels were also correlated with greater total brain volume on MRI in older and middle-aged adults without dementia, but no relationship with hippocampal volume was noted (Westwood, et al. 2014). Clinical trials have further fueled the uncertainty regarding the role of IGF-1 on cognition. A 20-week intervention study showed beneficial effects of GHRH administration on cognitive function in older adults who were healthy or had MCI (Baker, et al. 2012). However, a year-long experimental replacement of peripheral IGF-1 in older females failed to achieve discernable benefits in cognitive outcomes (Friedlander, et al. 2001). Although the results from human studies do not offer a conclusive resolution about the role of IGF-1 on cognitive function in aging, they should be interpreted in the context of substantial heterogeneity between cohorts, cognitive assessment tools, and definitions of cognitive function. Extended longitudinal follow-up paired with more precise characterization may help clarify this uncertainly in the future.
Molecular and animal studies
In rodents, few studies have been conducted that address cognition in the context of aging. However, one study exploring the potential therapeutic benefit of administering IGF-1 by intracerebroventricular (ICV) infusion to old male Fisher-Brown Norway (FBN) rats over a one month period demonstrated improved cognitive function, in terms of spatial reference memory and working memory (Markowska, et al. 1998; Pardo, et al. 2018). It has also been shown that old male FBN rats have a decreased number of newly generated cells in the hippocampus, a brain region important for memory domain acquisition, as well as a significant reduction of newborn cells differentiating into neurons (Lichtenwalner, et al. 2001). However, ICV administration of IGF-1 significantly restored hippocampal neurogenesis, without an effect on progenitor differentiation or newborn cell survival, which could be related to the observed improvement in cognitive function noted previously in this strain of rats (Lichtenwalner et al. 2001). Furthermore, old female Sprague-Dawley rats injected ICV with an IGF-1 expressing adenovirus resulted in increased IGF-1 levels in CSF, and restoration of neurogenesis and spatial memory assessed by the Barnes maze (Pardo et al. 2018; Pardo, et al. 2016). In this model, transcription of genes related to synaptic function and neurogenesis was upregulated (Pardo et al. 2018). Astrocyte specific knock out of IGF1R gene at 3-4 months of age resulted in impairments in working memory in mice; however, it is not known what effect this intervention may have at an older age (Logan, et al. 2018). Interestingly, both male and female long-lived Ames dwarf mice, which are characterized by circulating GH and IGF-1 deficiency, have normal cognitive function that is better maintained with age, based on performance in memory tests, when compared to age-matched controls (Kinney et al. 2001b). Although there is some evidence that local IGF-1 production in these animals is enhanced (Sun, et al. 2005), suggesting that autocrine IGF-1 production may be an important contributor to cognitive health, subsequent studies did not confirm these results, finding lower IGF-1 levels in the cortex and hippocampus of these mice compared to wild-type controls(Puig, et al. 2016). On the contrary, liver-specific IGF-1 deficient (LID) mice, which have an approximately 70% reduction in systemic IGF-1 levels, manifest early hippocampal-dependent cognitive deficits (Trejo, et al. 2007), including a reduction in spatial learning and memory (assessed by Water Maze) ((Svensson, et al. 2006; Trejo et al. 2007) despite maintained autocrine IGF-1 production. However, these mice also present with markedly elevated GH levels, due to lack of feedback inhibition by IGF-1, resulting in insulin resistance (Haluzik, et al. 2003) and these perturbations in GH and insulin may also directly contribute to the neuronal and vascular dysfunction observed in this model (Bailey-Downs, et al. 2012; Talbot, et al. 2012).
IGF-1 and Alzheimer’s disease
Epidemiologic and clinical studies
Alzheimer’s disease (AD), characterized by a progressive decline in memory and loss of independent functioning, has become a major burden for older adults, their families and the health care system (Callahan 2017). Although IGF-1 has been extensively investigated in AD patients, most of the studies have been observational and substantial controversy continues to surround this topic in epidemiologic literature. A meta-analysis of 9 case-control studies did not find an association between IGF-1 levels and AD, with individual studies showing higher, similar and lower serum IGF-1 levels in AD patients compared to controls (Ostrowski, et al. 2016). On the other hand, a prospective study demonstrated an inverse association between baseline serum IGF-1 levels and AD risk (Westwood et al. 2014), while an analysis of AD patients reported that lower baseline serum IGF-1 was associated with faster progression in cognitive decline over 2 years (Vidal, et al. 2016). Additionally, a clinical trial that evaluated the effect of a GH secretagogue on progression of AD revealed a lack of efficacy, despite achieving higher IGF-1 levels (Sevigny, et al. 2008). Interestingly, higher measured stimulating activity of the IGF-1R using a specialized assay was related to greater prevalence and incidence of dementia (de Bruijn, et al. 2014). Acknowledging that IGF-1 bioavailability is tightly regulated by IGFBPs and that genetic or acquired conditions may predispose to IGF-1 resistance (Suh et al. 2008; Tazearslan et al. 2011) this latter study raises the important consideration that IGF-1 levels may not always be indicative of action, especially in aging and diseased brains, where IGF-1 resistance has been reported to occur (Muller et al. 2012; Talbot et al. 2012).
Molecular and animal studies
Molecular and animal studies attempting to shed light on the role of somatotropic signaling in AD have also not been free of controversy. A number of rodent studies have suggested that relative IGF-1 deficiency and/or reduced IGF-1 signaling confers protection against progression of AD pathology. For instance, Ames Dwarf mice expressing human mutant amyloid precursor protein (APP) and presenillin-1 (PS1) demonstrated lower brain IGF-1 levels and reduced amyloid plaque deposition than controls (Puig et al. 2016). Likewise, heterozygous deletion of the IGF1R in a similar AD model protected from AD-like symptoms, neuroinflammation, neuronal loss, and delayed proteotoxicity in 12-13 month old mice compared to age-matched controls (Cohen, et al. 2009). Deletion of neuronal IGF1R or IRS2, which signals downstream of IGF-1R, demonstrated reduced amyloid plaque accumulation, reduced neuroinflammation, improved spatial memory and delayed death in APP and APP/PS1 models (Freude, et al. 2009; Gontier, et al. 2015). However, there was no benefit to reducing IGF-1 signaling in an advanced model of AD (George, et al. 2017), suggesting that there may be a limited window for intervention. Adult-onset deletion of IGF1R specifically in neurons also reduced neuronal size through changes to the soma and dendrites (George et al. 2017; Gontier et al. 2015), suggesting that larger volume does not necessarily equate with better function (Westwood et al. 2014). Of note, subjecting 3xTg-AD mice to protein restriction cycles, which reduces circulating IGF-1 levels by 30-70% (Parrella, et al. 2013), alleviated symptoms of working memory and short-term spatial memory deficits, as well as reduced hippocampal Tau phosphorylation (Parrella et al. 2013) that is associated with cognitive deficits in humans with AD (de Leon, et al. 2006). In contrast to evidence implicating IGF-1 as a detrimental player in AD pathology, age-related decline in IGF-1 has been linked with brain metabolic deficiencies in AD mouse models (Carro, et al. 2002; Trueba-Saiz, et al. 2013) and treatment with IGF-1 has been shown to confer protection in hippocampal neurons from the toxic effects of amyloid peptides (Dore, et al. 1997). However, results that showed that peripheral administration of IGF-1 promoted clearance of Aβ amyloid in aged rats and mutant mice (Carro, et al. 2006), (Carro et al. 2002) have not been replicated in later studies (Lanz, et al. 2008).
Interestingly, a study in human AD brains documented insulin and IGF-1 resistance via decreased activation of downstream signaling of insulin receptor substrate-1 (IRS-1) and IGF-1R/IRS-2, respectively (Talbot et al. 2012). Similar findings have been observed in rodent models (Muller et al. 2012; Trueba-Saiz et al. 2013). Whether brain resistance to IGF-1 is a pathologic feature or a protective adaption remains uncertain. However, a recent genome-wide microarray analysis that compared neurons in early-stage AD with IGF1R knock out neurons demonstrated very similar transcriptomic signatures, suggesting that IGF-1 resistance in AD neurons may be an adaptive response intended to protect neurons from further damage (George et al. 2017). In contrast, several investigators have proposed that reduced CNS input of a related hormone, insulin, may underlie cognitive impairment, and data have demonstrated that intranasal delivery of insulin improves cognition in individuals with MCI or early-stage AD (Claxton, et al. 2015). While this latter observation seems somewhat contradictory to those results obtained from AD models, it is important to note that data from the intervention trial were obtained from individuals with MCI or early stage disease. Therefore, the relationship of insulin and IGF-1 signaling to cognition could vary based upon disease susceptibility and severity, a possibility that will require further study to confirm.
Mechanistically, many potential pathways and processes are impacted by IGF-1, which could have both beneficial and detrimental effects on the CNS to influence brain aging, cognitive decline and AD. Certainly, IGF-1 has been linked to neurogenesis, axonal and dendrite growth, synaptogenesis, myelination, and neuronal cell survival (Liang, et al. 2007; Nieto-Estevez, et al. 2016). On the other hand, increased IGF-1 signaling could impair macroautophagy in neurons, which is a cellular process shown to confer protection from AD. Macroautophagy is the process that eliminates cellular components through sequestration in autophagosomes followed by degradation upon fusion with lysosomes and plays an important role in eliminating misfolded or aggregated proteins that can be damaging to cells. This process has been shown to be dysfunctional not only in aging, but also in age-related neurodegenerative diseases that include Parkinson’s disease, as well as sporadic and familial AD (Lee, et al. 2010; Martinez-Vicente and Cuervo 2007). Indeed, reduced somatotropic signaling has been implicated in increased macroautophagy. In nematodes, loss of IGF-1 signaling was associated with improved autophagy, a pathway required for lifespan extension observed in this model (Melendez, et al. 2003). Knockout of IGF1R in neurons resulted in better autophagy and clearance of Aβ plaques (Gontier et al. 2015), whereas prolonged exposure to IGF-1 resulted in decreased autophagy in human fibroblasts (Bitto, et al. 2010). Autophagy was also induced by inhibition of mTOR or AMPK, molecules that signal downstream of IGF-1R (Samari and Seglen 1998; Schmelzle and Hall 2000). In summary, most of the experimental evidence suggests that IGF-1 acts as “Mr. Hyde” in the progression of AD. However, what character IGF-1 signaling plays preceding disease onset remains to be definitively determined.
IGF-1 and Parkinson’s disease
Epidemiologic and clinical studies
Parkinson’s disease (PD) is a progressive neurodegenerative disorder distinctively characterized clinically by rest tremor, rigidity and bradykinesia, and pathologically by the destruction of dopaminergic (DA) neurons in the substantia nigra (SN) (Forno 1996) in the basal ganglia. However, PD is often accompanied by several other cognitive and neuropsychiatric dysfunctions, as well as accumulation of Lewy bodies throughout the brain, that include alpha-synuclein among other proteins, suggesting that the neurodegenerative process also targets other brain areas (Mu, et al. 2017; Schapira, et al. 2017). Circulating IGF-1 has been proposed as a potential biomarker for PD. Significantly sustained elevations of IGF-1 levels, without concomitant elevations in GH levels, were noted among patients with drug-treated, stable PD compared to healthy controls (Godau, et al. 2010). Similarly, a small group of drug naïve patients demonstrated elevations in serum IGF-1 levels compared to controls (Godau, et al. 2011). Higher IGF-1 levels were also found among individuals with shorter duration of PD, with levels falling with longer disease duration (Numao, et al. 2014), sometimes even reaching near-control levels (Godau et al. 2010). This was confirmed by other studies that noted that elevations in IGF-1, GH and IGFBP-3 were less pronounced among patients with longer disease duration and more advanced PD (Tuncel, et al. 2009). Interestingly, even individuals who did not meet criteria for PD diagnosis, but with some movement impairment and SN abnormalities on transcranial ultrasound, had higher IGF-1 levels compared to controls without any abnormalities (Godau et al. 2011), suggesting that elevated IGF-1 may be a potential risk factor for progression to PD. Indeed, a prospective study that followed early-stage PD patients for up to 2 years found that individuals with baseline IGF-1 levels in the top quartile demonstrated worse motor impairment and Parkinsonian symptoms throughout the duration of the study, compared to those with baseline IGF-1 in the lower quartiles (Picillo, et al. 2013), with a caveat that the symptoms had not significantly progressed in either group due to effective medical treatment. It was also noted that patients with PD did not only manifest elevations in serum IGF-1 and IGFBP levels compared to controls, but also had elevated levels of these proteins in the CSF (Mashayekhi, et al. 2010), suggesting that circulating IGF-1 crosses the BBB in PD. However, it still remains unresolved whether PD is associated with elevations of systemic or autocrine IGF-1 levels, or both.
Serum IGF-1 levels have also been studied in association with other symptoms of PD. Patients with PD have an approximate 6-fold increased risk for developing dementia, compared to the general population (Aarsland, et al. 2001). In contrast to those reports described above, PD patients with higher baseline IGF-1 levels demonstrated better attention and executive functions after 2 years of follow-up (Pellecchia, et al. 2014). In addition, a recent study of a large cohort of early, drug naïve PD patients found worse executive function, attention and verbal memory among individuals with IGF-1 levels in the lowest quartile (Picillo, et al. 2017). These observations may indicate that higher IGF-1 levels are in fact protective against PD-associated cognitive decline, although reverse causality cannot be ruled out, such that patients with lower IGF-1 may actually have more advanced and sustained PD that is associated with more severe cognitive impairments, as suggested by studies above.
Molecular and animal studies
Some of the earlier evidence suggesting the relevance of IGF-1 to DA neurons and PD was derived from autoradiographic studies demonstrating the presence of IGF-1R in moderate densities in the SN of middle and older age adults (De Keyser, et al. 1994).. Experiments involving human and animal cell cultures have demonstrated neuroprotective actions of IGF-1 on DA neurons (Offen, et al. 2001; Sun, et al. 2010; Zawada, et al. 1996). Treatment of cell cultures obtained from E15 rat ventral mesencephalon with IGF-1 resulted in a two-fold increased preservation the number of DA neurons. Follow up studies further demonstrated that administration of IGF-1 to rat cerebellar granular neurons increased protein expression of B-cell lymphoma-2 (Bcl-2), placing this potent cell survival factor downstream of IGF-1 signaling to provide protection against DA apoptosis (Offen et al. 2001).
The role of IGF-1 signaling has also been investigated in several models of PD. One such model uses 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a prodrug to the neurotoxin 1-Methyl-4-phenyl pyridinium (MPP+), to induce DA cell injury that is characteristic of PD. MPTP intoxication resulted in upregulated IGF-1R levels that was not accompanied by changes in Akt phosphorylation levels (D’Astous, et al. 2006). Using the same PD model, a different study found that intraperitoneal injection of MPTP resulted in more severe lesions in IGF-1R haploinsufficient mice, as compared to controls (Nadjar, et al. 2009), and further suggested that IGF-1 signaling may oppose the anti-inflammatory and pro-oxidative effects provoked by this insult (Nadjar et al. 2009). Another PD model utilizing 6-hydroxydopamine (6-OHDA) induced unilateral nigrostriatal lesions in rats also showed reduced neuronal loss and sustained motor function with peripherally or centrally administered IGF-1 (Quesada and Micevych 2004). Interestingly, in this study peripherally administered estrogen was also protective, but an IGF-1R antagonist blocked the neuroprotective effects of both estrogen and IGF-1, suggesting that the neuroprotective effect of estrogen may be mediated via IGF-1 signaling.
In summary, both central and peripheral administrations of IGF-1 have resulted in neuroprotection in animal PD models. However, it is important to note that the above referenced studies used PD models that introduced SN lesions using acute injury strategies, which is distinct from the etiology of the human disease that is characterized by slow, progressive neurodegenerative changes accompanied by abnormal deposition of proteins, such as alpha-synuclein. For instance, in Drosophila and C. elegans, reduced IGF-1/insulin-like signaling (IIS) resulted in reduced neurotoxicity and alpha-synuclein misfolding, a feature of PD (Knight, et al. 2014). Additionally, impaired autophagic function, a process that is inhibited by IGF-1, has been implicated in PD pathogenesis (Menzies, et al. 2017). Thus, the role of IGF-1 needs to be further investigated in PD models that better represent the human disease.
IGF-1 and Cerebrovascular disease
Epidemiologic and clinical studies
Whether circulating levels of IGF-1 are associated with the risk of cerebrovascular events remains somewhat equivocal. A recent prospective study of older adults from the Framingham cohort demonstrated a 2.3-fold increase in incident stroke among individuals with baseline IGF-1 in the lowest quintile (Saber, et al. 2017). Free IGF-1 was also inversely related to carotid artery intima-media thickness (Schwab, et al. 1997; Van den Beld, et al. 2003); however, no association was identified between IGF-1, IGFBP-3, and IGF-1:IGFBP-3 molar ratios and the risk of stroke among older participants in the Cardiovascular Health Study (Kaplan, et al. 2007). On the other hand, a nested case-control analysis found an increase in stroke risk among individuals in the bottom quartile IGFBP-3, but the association between IGF-1 and stroke was non-significant after adjustment for IGFBP-3 levels (Johnsen, et al. 2005).
A number of studies have investigated IGF-1 levels in patients who sustained strokes and its prognostic role in neurologic outcomes. One of the earlier studies found that levels of IGF-1 and IGFBP-3 were lower in patients within 24 hours of an acute ischemic stroke, as compared to age-matched subjects with non-ischemic neurological illness, and this difference persisted for at least 10 days after the event (Schwab et al. 1997). Several other studies similarly found reduced serum IGF-1 and IGFBP-3 levels in patients with acute ischemic (Denti, et al. 2004) and hemorrhagic strokes (Fu, et al. 2002; Iso, et al. 2012), while post-stroke serum IGF-1 and IGFBP-3 levels were also inversely associated with infarct volume (Schwab et al. 1997; Tang, et al. 2014). However, not all results have been consistent. One study described higher IGF-1 levels within the first 10 days of ischemic stroke that remained elevated 3 months out from the event (Åberg, et al. 2011). Another study did not find an association between IGF-1 levels obtained within 6 hours of stroke onset and stroke severity (De Smedt, et al. 2011). However, in the same study, higher IGF-1 levels during the acute stroke period and during the rehabilitation period were associated with better cognitive and functional recovery after the stroke. Åberg et al. showed that higher IGF1 levels acutely and at 3 months post stroke positively correlated with better functional recovery between 3 and 24 months (Åberg et al. 2011). Higher serum IGF1 levels among individuals during their rehabilitation after stroke were associated with better cognitive and functional recovery (Bondanelli, et al. 2006). On the other hand, lower serum IGF-1 levels on admission has been associated with unfavorable functional outcomes following acute ischemic stroke (Klionsky, et al. 2016). Similarly, serum samples obtained from individuals with persistent chronic hemiparesis of ≥6 months duration showed lower IGF-1 and IGFBP-3 serum levels (Silva-Couto, et al. 2014).
In addition to functional outcomes, IGF-1 levels have been investigated in relationship to survival and hospital discharge after a cerebrovascular event. Lower IGF-1 serum levels have been associated with death at 90 days following ischemic stroke (Tang et al. 2014). IGF-1 level of less than 60 ng/mL measured 24 hours after an ischemic stroke was associated with higher 6-month mortality, but not with the severity of neurologic impairment (Denti et al. 2004); yet, the studied cohort was substantially older, therefore the low IGF-1, as well as the high mortality may have been attributable to older age. In contrast, another report found reduced serum IGF-1 levels and IGF-1/IGFBP ratios measured within 72 hours and at 1 week following a stroke to be associated with a shorter hospital length of stay. However, patients with higher serum IGF-1 had larger infarcts, and the higher levels may have been reflective of a more robust compensatory protective response (Mattlage, et al. 2016).
Molecular and animal studies
In the setting of ischemic neural injury, IGF-1 has been demonstrated to have neurotrophic and neuroprotective activities (Chung, et al. 2007; Gluckman, et al. 1992; Hu, et al. 2009; Knusel, et al. 1990). Circulating IGF-1 that can cross the BBB (De Geyter, et al. 2016) and local IGF-1 that is produced by proliferating microglia have been implicated in promoting neurological recovery after a stroke (Lalancette-Hébert, et al. 2007; Ploughman, et al. 2005; Thored, et al. 2009). Following a middle cerebral artery occlusion (MCAO) in rats, IGF-1 expression was found to be upregulated in the astrocytes surrounding the ischemic penumbra and neuronal progenitors (Yan, et al. 2006); whereas the administration of an IGF1-neutralizing antibody significantly reduced progenitor proliferation, suggesting that IGF-1 may be an important growth factor for neuronal recovery after a stroke. Evidence also indicates that neurological and functional recovery in the context of physical therapy is associated with marked activation of IGF-1 and downstream Akt signaling in the peri-infarct region (Zheng, et al. 2014). On the other hand, higher serum IGF-1 levels prior to MCAO procedure in mice correlated with a larger infarct size (Endres, et al. 2007).
Impaired cerebrovascular function has also been demonstrated in several GH and/or IGF-1 deficient models. One such model is the Lewis dwarf rat, which has a genetic GH deficiency that, among other outcomes, results in an increased incidence of late-life stroke (Sonntag, et al. 2005). Similarly, studies in a mouse model of post-developmental liver knockdown of Igf-1 show that IGF-1 deficiency has a negative effect on cerebrovascular adaptation to hypertension and this dysfunction is most likely associated with BBB disruption, as well as neuroinflammation, mimicking the aging phenotype (Toth, et al. 2014). Moreover, neurovascular coupling, which is a process that adjusts local cerebral blood flow to the energy requirements of activated neurons, is known to decrease with age (Toth, et al. 2015). Using the same IGF-1 deficient mouse model, Toth et al. showed that circulating IGF-1 deficiency led to neurovascular dysregulation and a concomitant decline in cognitive function, akin to what is seen with aging (Toth et al. 2015). Therefore, these studies support the contention that circulating IGF-1 has supportive and protective effects on cerebromicrovascular function.
Several therapeutic interventions involving GH/IGF-1 have been investigated in ischemic stroke models. In hippocampal cell cultures deprived of oxygen and nutrients, IGF-1 and IGFBP-ligand inhibitor prevented cell death (Mackay, et al. 2003). Likewise, ICV administration of IGF-1 resulted in reduced neuronal loss and improved neurological outcomes in several rodent models of hypoxic ischemic brain injury induced by arterial ligation (Guan, et al. 2001; Guan, et al. 1993; Mackay et al. 2003; Schäbitz, et al. 2001) and IGF-1 was more effective than insulin in protecting from neuronal loss (Guan et al. 1993). Similar results were reported in fetal sheep subjected to cerebral ischemia (Johnston, et al. 1996). GH treatment via ICV administration improved motor function in endothelin induced stroke in rats (Pathipati, et al. 2009). Additional routes of IGF-1 administration post neural ischemic injury, including intranasal (Fletcher, et al. 2009; Lin, et al. 2009; Liu, et al. 2001), subcutaneous (Schäbitz et al. 2001), intramuscular (Chang, et al. 2010), and intravenous (Rizk, et al. 2007), have also been shown to be effective. Interestingly, administration of human marrow stromal cells improved functional recovery in rats with MCAO induced ischemic infarcts and was associated with an increase in IGF-1 mRNA expression and IGF-1R immunoreactivity in cells at the ischemic boundary and subventricular zones (Zhang, et al. 2004). Administration of IGF-1 also ameliorated the negative effect of estrogen on ischemic stroke in middle-aged rats (Selvamani and Sohrabji 2010). ICV injection of adeno-associated viral vectors containing human IGF-1 has been shown to promote prolonged functional recovery and enhanced neurogenesis after ischemic injury in mice (Liu, et al. 2017; Zhu, et al. 2008). In another study, antagonists targeted at IGF-1 signaling related miRNAs promoted neuroprotection (Selvamani, et al. 2012). Although the evidence examining the links between IGF-1 and cerebrovascular disease has been somewhat ambiguous in humans, when paired together with molecular and animal studies, the results indicate that IGF-1 is likely a beneficial factor for cerebral vasculature and aids in neuronal recovery after an ischemic injury.
The “strange case” of IGF-1 in the aging brain: Dr. Jekyll or Mr. Hyde?
When taking into account the full breadth and depth of the evidence examining the role of IGF-1 on brain aging and its related diseases, as summarized in Figure 1, substantial uncertainty continues to surround many aspects related to this pleiotropic hormone in the CNS. While it is not yet entirely clear how to reconcile many of these conflicting findings reported across animal and human studies, a closer look at the subtle, but important, nuances and inconsistencies could begin to shed some light on the subject.
Figure 1. IGF-1 playing the roles of Dr. Jekyll and Mr. Hyde in the brain.
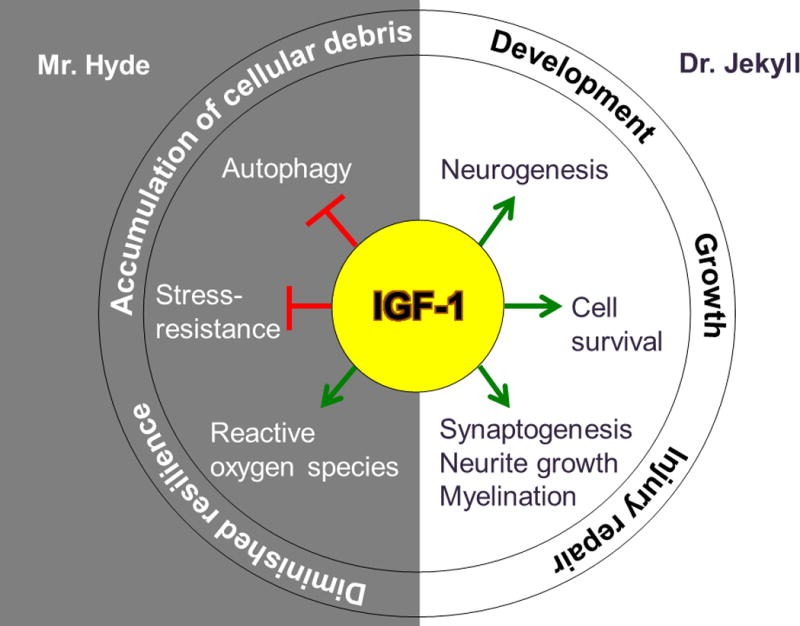
IGF-1 exerts its beneficial effects on the brain by stimulating neurogenesis, synaptogenesis, neurite growth, myelination, and promoting cell-survival. These processes are important during early life for proper brain development and growth; whereas during aging, they contribute to repair of injured neural tissue, as may result from a stroke. On the other hand, the adverse effects of IGF-1 on the brain include generation of reactive oxygen species, and inhibition of both autophagy and stress responses. Inhibition of these functions results in diminished cell resilience and accumulation of cellular debris, which are characteristic of age-related neurodegenerative conditions such as AD and PD.
The effect of aging on the brain
Whereas it has been well-established that IGF-1 is required for normal brain development (Abuzzahab et al. 2003; Kranzler et al. 1998; Netchine et al. 2011; Woods et al. 1996), its role in the aging brain has been less clear. Although most evidence from rodent models suggests that interventions that raise IGF-1 level in the brain are beneficial to an otherwise healthy aging brain, studies in humans or disease models have not been consistently confirmatory. After early life development, the CNS shifts priorities from growth and expansion to preservation. Thus, maintaining the same robustness of IGF-1 signaling throughout the lifespan, as during development, may actually be counterproductive or even harmful, as IGF-1 signaling is known to attenuate pathways that promote cell-preservation through stress-resistance, reduction of oxidative stress, and proteostasis (Barzilai et al. 2012). Since aging is the greatest risk factor for neurodegenerative disorders that are accompanied by abnormal protein deposition, such as AD and PD (Kaushik and Cuervo 2015; Martinez-Vicente and Cuervo 2007), the physiologic decline in IGF-1 that accompanies aging may serve a protective role against these disorders by promoting an environment favoring clearance of dysfunctional proteins and cell-maintenance. On the other hand, older adults may benefit from temporary elevations of IGF-1 in settings of acute neuronal injury, such as a stroke or traumatic brain injury (Bianchi, et al. 2017), where neuroregeneration regains priority over maintenance. This hypothesis is supported by findings that most models of AD benefit from reduced IGF-1 signaling. On the contrary, in models of acute ischemic injury, higher IGF-1 improves recovery (Åberg et al. 2011; Bondanelli et al. 2006). However, PD models need to be interpreted with caution. While IGF-1 appears to be protective against DA neuron loss in models that induce substantia nigra injury, this approach is more reminiscent of acute neuronal injury that occurs with sudden ischemic or traumatic injury rather than the progressive, neuronal degeneration that is characteristic of PD in humans. Interestingly, decreased signaling via the IIS in invertebrates conferred protection from abnormal α-synuclein accumulation (Knight et al. 2014). Thus, the role of IGF-1 in PD should be further investigated in more representative models of the disease.
Limitations of human observational studies
Endocrine pathways, including the somatotropic axis, are dynamic, adaptive and complex. Therefore, hormone secretion and bioavailability may vary widely in response to physiologic stressors in an effort to maintain desired homeostasis. This was highlighted by a study in which trajectories of IGF-1 levels predicted mortality better than absolute levels (Sanders, et al. 2017). Stress and illness can attenuate somatotropic signaling via changes in GH pulsatility and GH resistance (Van den Berghe 2001). Alternatively, a low IGF-1 level may be a consequence of AD or PD and serve as a marker of more severe disease, as there is evidence that amyloid plaques and neurofibrillary tangles accumulate in the hypothalamus in patients with neurodegenerative diseases and disrupt endocrine function (Ishii and Iadecola 2015). Yet another theory, for which there is some supportive evidence, is that the reduction in IGF-1 may be a compensatory mechanism employed by the body to shift resources away from proliferation and toward cell-maintenance, in an effort to preserve neuronal function (George et al. 2017), and thereby limit the accumulation of cellular debris and damage during the aging process. On the other hand, a low IGF-1 level may reflect lifelong low IGF-1 due to presence of genetic variants. Similarly, an elevated IGF-1 level may be inherited (Suh et al. 2008). Furthermore, peripheral levels may have no association with CNS signaling due to adaptive responses induced to protect vulnerable cells in the brain (George et al. 2017; Talbot et al. 2012). In contrast, IGF-1 levels may rise in response to neuronal injury in an effort to repair damaged neural tissue. These biological scenarios demonstrate that the same measured IGF-1 level may reflect different physiologic processes that may be distinguished only through long-term longitudinal follow-up and genetic studies.
IGF-1 levels versus function
Most clinical observational studies focus on a single molecular phenotype, such as level of IGF-1, as a surrogate of the somatotropic axis signaling (Al-Delaimy et al. 2009; Doi et al. 2015; Ostrowski et al. 2016; Tumati et al. 2016; Westwood et al. 2014). However, given the complexity of the pathway and its many interacting components, it is apparent that the function of the pathway cannot be reliably interpreted by merely measuring IGF-1 levels. For example, such an approach could be misleading in individuals harboring functional IGF1R mutations that result in elevated IGF-1 levels due to IGF-1R resistance, thereby misclassifying them as having enhanced IGF-1 signaling rather than reduced (Suh et al. 2008; Tazearslan et al. 2011). Similarly, IGF-1 resistance at the level of the IGF-1R and IRS-2 in the brain that accompanies certain disease states could potentially explain the compensatory elevations observed in serum IGF-1(Talbot et al. 2012; Trueba-Saiz et al. 2013). More accurate characterization of IGF-1 signaling in human studies can be achieved by accounting for the functional genetic variants in the genes that code for key intermediates of the somatotropic pathway and through functional studies.
Conclusion
Undeniably, genetic disruptions of endocrine GH/IGF-1 signaling in experimental models have extended health-span and lifespan. Although the models of brain aging are more complex, substantial advancements have been made in understanding the role of IGF-1 in the aging brain. What ultimately determines the effect of IGF-1 on the aging brain is the process that occurs in every dynamic biologic system: It is the ability of cells to modulate IGF-1 signaling in an adaptation to the changing physiologic environment. Thus, the function of IGF-1 in the CNS likely differs across the lifespan and different pathologic conditions. The results summarized above suggest that long-term maintenance of aging neurons, which are prone to the accumulation of cellular debris and damage that result in age-related neurodegenerative disorders such as AD and PD, benefits from reduced IGF-1 signaling. On the other hand, recovery from an acute neuronal insult, such as a stroke, is augmented in the setting of higher IGF-1. This evidence points to the fact that IGF-1 indeed plays a double role in the aging brain, sometimes that of a good actor and at other times that of a bad actor, depending on the circumstance. Embracing this complexity may ultimately lead to better-targeted therapies for conditions that could benefit the aging brain.
However, before such strategies could be considered there still remain a number of important questions that need answers. These include: 1. What is the relative contribution of autocrine versus endocrine-derived IGF-1 in the aged CNS?; 2. What is the effect of IGF-1 on neurons versus on other cells found in the CNS, such as microglia, smooth muscle cells, endothelial cells, and astrocytes?; 3. What are the effects of acute versus chronic elevations of IGF-1 in the aging brain?; 4. Is low IGF-1 a sign of accelerated aging or a genetically-encoded protective mechanism in aging?; 5. Is there an interaction between sex and IGF-1 in the aging brain, as some studies suggest?; 6. Are current experimental models of AD and PD good representations of human pathophysiology?; 7. What is the effect of IGF-1 signaling through the insulin receptor?; 8. Are current genetic models of life-long disruptions of GH/IGF-1 signaling representative of age-related IGF-1 decline?; 9. What are the differential effects of GH and IGF-1?; 10. Is low or high IGF-1 a cause for, an adaptation to, or a consequence of disease? Some of these questions can be investigated in humans using longitudinal follow-up studies that thoroughly characterize participants phenotypically and genetically. However, many other questions will need to rely for answers on animal models. Therefore, the attempt to understand the relationship of IGF-1 to brain aging and CNS diseases presents important challenges and opportunities to gain greater insight into how to invoke Dr. Jekyll, rather than Mr. Hyde qualities of IGF-1 in the aging brain.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (grant numbers P30AG038072, P30DK250541, P01AG021654, R37AG18381, R01AG044829, R01AG046949, R01AG057909, R00AG037574, K23AG051148); the Paul Glenn Foundation for Medical Research; and the American Federation for Aging Research.
References
- Aarsland D, Andersen K, Larsen J, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson’s disease A community-based, prospective study. Neurology. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- Åberg D, Jood K, Blomstrand C, Jern C, Nilsson M, Isgaard J, Åberg ND. Serum IGF-I levels correlate to improvement of functional outcome after ischemic stroke. The Journal of Clinical Endocrinology & Metabolism. 2011;96:E1055–E1064. doi: 10.1210/jc.2010-2802. [DOI] [PubMed] [Google Scholar]
- Abuzzahab MJ, Schneider A, Goddard A, Grigorescu F, Lautier C, Keller E, Kiess W, Klammt J, Kratzsch J, Osgood D, et al. IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. The New England journal of medicine. 2003;349:2211–2222. doi: 10.1056/NEJMoa010107. [DOI] [PubMed] [Google Scholar]
- Al-Delaimy WK, von Muhlen D, Barrett-Connor E. Insulinlike growth factor-1, insulinlike growth factor binding protein-1, and cognitive function in older men and women. Journal of the American Geriatrics Society. 2009;57:1441–1446. doi: 10.1111/j.1532-5415.2009.02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging brain. Experimental gerontology. 2015;68:76–81. doi: 10.1016/j.exger.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, Barsness SM, Borson S, Merriam GR, Friedman SD, Craft S, Vitiello MV. Effects of growth hormone-releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial. Arch Neurol. 2012;69:1420–1429. doi: 10.1001/archneurol.2012.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Chandrashekar V, Dominici F, Turyn D, Kinney B, Steger R, Kopchick JJ. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4:1–8. doi: 10.1023/a:1022448532248. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. American journal of physiology Endocrinology and metabolism. 2000;278:E967–976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- Berelowitz M, Szabo M, Frohman LA, Firestone S, Chu L, Hintz RL. Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitary. Science. 1981;212:1279–1281. doi: 10.1126/science.6262917. [DOI] [PubMed] [Google Scholar]
- Bianchi VE, Locatelli V, Rizzi L. Neurotrophic and Neuroregenerative Effects of GH/IGF1. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18112441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto A, Lerner C, Torres C, Roell M, Malaguti M, Perez V, Lorenzini A, Hrelia S, Ikeno Y, Matzko ME, et al. Long-term IGF-I exposure decreases autophagy and cell viability. PloS one. 2010;5:e12592. doi: 10.1371/journal.pone.0012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondanelli M, Ambrosio MR, Onofri A, Bergonzoni A, Lavezzi S, Zatelli MC, Valle D, Basaglia N, degli Uberti EC. Predictive value of circulating insulin-like growth factor I levels in ischemic stroke outcome. The Journal of Clinical Endocrinology & Metabolism. 2006;91:3928–3934. doi: 10.1210/jc.2006-1040. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012;67:652–660. doi: 10.1093/gerona/gls086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Callahan CM. Alzheimer’s Disease: Individuals, Dyads, Communities, and Costs. Journal of the American Geriatrics Society. 2017;65:892–895. doi: 10.1111/jgs.14808. [DOI] [PubMed] [Google Scholar]
- Carlson HE, Gillin JC, Gorden P, Snyder F. Abscence of sleep-related growth hormone peaks in aged normal subjects and in acromegaly. The Journal of clinical endocrinology and metabolism. 1972;34:1102–1105. doi: 10.1210/jcem-34-6-1102. [DOI] [PubMed] [Google Scholar]
- Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci. 2005;25:10884–10893. doi: 10.1523/JNEUROSCI.2909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Gerber A, Loetscher H, Torrado J, Metzger F, Torres-Aleman I. Therapeutic actions of insulin-like growth factor I on APP/PS2 mice with severe brain amyloidosis. Neurobiol Aging. 2006;27:1250–1257. doi: 10.1016/j.neurobiolaging.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- Chang H-C, Yang Y-R, Wang PS, Kuo C-H, Wang R-Y. Effects of insulinlike growth factor 1 on muscle atrophy and motor function in rats with brain ischemia. Chin J Physiol. 2010;53:337–348. doi: 10.4077/cjp.2010.amk080. [DOI] [PubMed] [Google Scholar]
- Chung H, Seo S, Moon M, Park S. IGF-I inhibition of apoptosis is associated with decreased expression of prostate apoptosis response-4. Journal of endocrinology. 2007;194:77–85. doi: 10.1677/JOE-07-0073. [DOI] [PubMed] [Google Scholar]
- Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo T. Implication of the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in the neuroprotective effect of estradiol in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. Mol Pharmacol. 2006;69:1492–1498. doi: 10.1124/mol.105.018671. [DOI] [PubMed] [Google Scholar]
- de Bruijn RF, Janssen JA, Brugts MP, van Duijn CM, Hofman A, Koudstaal PJ, Ikram MA. Insulin-like growth factor-I receptor stimulating activity is associated with dementia. J Alzheimers Dis. 2014;42:137–142. doi: 10.3233/JAD-140186. [DOI] [PubMed] [Google Scholar]
- De Geyter D, De Smedt A, Stoop W, De Keyser J, Kooijman R. Central IGF-I Receptors in the Brain are Instrumental to Neuroprotection by Systemically Injected IGF-I in a Rat Model for Ischemic Stroke. CNS neuroscience & therapeutics. 2016;22:611–616. doi: 10.1111/cns.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keyser J, Wilczak N, De Backer JP, Herroelen L, Vauquelin G. Insulin-like growth factor-I receptors in human brain and pituitary gland: An autoradiographic study. Synapse. 1994;17:196–202. doi: 10.1002/syn.890170309. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, Rusinek H, Li J, Tsui W, Saint Louis LA, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27:394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- De Smedt A, Brouns R, Uyttenboogaart M, De Raedt S, Moens M, Wilczak N, Luijckx G-J, De Keyser J. Insulin-like growth factor I serum levels influence ischemic stroke outcome. Stroke. 2011;42:2180–2185. doi: 10.1161/STROKEAHA.110.600783. [DOI] [PubMed] [Google Scholar]
- Denti L, Annoni V, Cattadori E, Salvagnini MA, Visioli S, Merli MF, Corradi F, Ceresini G, Valenti G, Hoffman AR. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. The American journal of medicine. 2004;117:312–317. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Doi T, Shimada H, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Suzuki T. Association of insulin-like growth factor-1 with mild cognitive impairment and slow gait speed. Neurobiol Aging. 2015;36:942–947. doi: 10.1016/j.neurobiolaging.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A. 1997;94:4772–4777. doi: 10.1073/pnas.94.9.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Piriz J, Gertz K, Harms C, Meisel A, Kronenberg G, Torres-Aleman I. Serum insulin-like growth factor I and ischemic brain injury. Brain research. 2007;1185:328–335. doi: 10.1016/j.brainres.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Finkelstein JW, Roffwarg HP, Boyar RM, Kream J, Hellman L. Age-related change in the twenty-four-hour spontaneous secretion of growth hormone. The Journal of clinical endocrinology and metabolism. 1972;35:665–670. doi: 10.1210/jcem-35-5-665. [DOI] [PubMed] [Google Scholar]
- Fletcher L, Kohli S, Sprague SM, Scranton RA, Lipton SA, Parra A, Jimenez DF, Digicaylioglu M. Intranasal delivery of erythropoietin plus insulin-like growth factor–I for acute neuroprotection in stroke. Journal of neurosurgery. 2009;111:164–170. doi: 10.3171/2009.2.JNS081199. [DOI] [PubMed] [Google Scholar]
- Forno LS. Neuropathology of Parkinson’s disease. Journal of Neuropathology & Experimental Neurology. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Frater J, Lie D, Bartlett P, McGrath JJ. Insulin-like Growth Factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: A review. Ageing Res Rev. 2017;42:14–27. doi: 10.1016/j.arr.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Freude S, Hettich MM, Schumann C, Stohr O, Koch L, Kohler C, Udelhoven M, Leeser U, Muller M, Kubota N, et al. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:3315–3324. doi: 10.1096/fj.09-132043. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, Butterfield GE, Moynihan S, Grillo J, Pollack M, Holloway L, Friedman L, Yesavage J, Matthias D, Lee S, et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. The Journal of clinical endocrinology and metabolism. 2001;86:1496–1503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- Lu Fu X, Lu YK, Liao J. Relation between insulin-like growth factor-1 and insulin resistance in patients with acute stroke. Zhonghua yi xue za zhi. 2002;82:410–412. [PubMed] [Google Scholar]
- George C, Gontier G, Lacube P, Francois JC, Holzenberger M, Aid S. The Alzheimer’s disease transcriptome mimics the neuroprotective signature of IGF-1 receptor-deficient neurons. Brain. 2017;140:2012–2027. doi: 10.1093/brain/awx132. [DOI] [PubMed] [Google Scholar]
- Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochemical and biophysical research communications. 1992;182:593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- Godau J, Herfurth M, Kattner B, Gasser T, Berg D. Increased serum insulin-like growth factor 1 in early idiopathic Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 2010;81:536–538. doi: 10.1136/jnnp.2009.175752. [DOI] [PubMed] [Google Scholar]
- Godau J, Knauel K, Weber K, Brockmann K, Maetzler W, Binder G, Berg D. Serum insulinlike growth factor 1 as possible marker for risk and early diagnosis of Parkinson disease. Archives of neurology. 2011;68:925–931. doi: 10.1001/archneurol.2011.129. [DOI] [PubMed] [Google Scholar]
- Gontier G, George C, Chaker Z, Holzenberger M, Aid S. Blocking IGF Signaling in Adult Neurons Alleviates Alzheimer’s Disease Pathology through Amyloid-beta Clearance. J Neurosci. 2015;35:11500–11513. doi: 10.1523/JNEUROSCI.0343-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CJ, Holly JM, Bayer A, Fish M, Ebrahim S, Gallacher J, Ben-Shlomo Y. The role of IGF-I, IGF-II, and IGFBP-3 in male cognitive aging and dementia risk: the Caerphilly Prospective Study. J Alzheimers Dis. 2014;41:867–875. doi: 10.3233/JAD-132183. [DOI] [PubMed] [Google Scholar]
- Guan J, Miller O, Waugh K, McCarthy D, Gluckman P. Insulin-like growth factor-1 improves somatosensory function and reduces the extent of cortical infarction and ongoing neuronal loss after hypoxia–ischemia in rats. Neuroscience. 2001;105:299–306. doi: 10.1016/s0306-4522(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Guan J, Williams C, Gunning M, Mallard C, Gluckman P. The effects of IGF-1 treatment after hypoxic-ischemic brain injury in adult rats. Journal of Cerebral Blood Flow & Metabolism. 1993;13:609–616. doi: 10.1038/jcbfm.1993.79. [DOI] [PubMed] [Google Scholar]
- Haluzik M, Yakar S, Gavrilova O, Setser J, Boisclair Y, LeRoith D. Insulin resistance in the liver-specific IGF-1 gene-deleted mouse is abrogated by deletion of the acid-labile subunit of the IGF-binding protein-3 complex: relative roles of growth hormone and IGF-1 in insulin resistance. Diabetes. 2003;52:2483–2489. doi: 10.2337/diabetes.52.10.2483. [DOI] [PubMed] [Google Scholar]
- Holly JM, Perks CM. Insulin-like growth factor physiology: what we have learned from human studies. Endocrinology and metabolism clinics of North America. 2012;41:249–263. doi: 10.1016/j.ecl.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Hu M, Zhang X, Liu W, Cui H, Di N. Longitudinal changes of defensive and offensive factors in focal cerebral ischemia-reperfusion in rats. Brain research bulletin. 2009;79:371–375. doi: 10.1016/j.brainresbull.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- Ishii M, Iadecola C. Metabolic and Non-Cognitive Manifestations of Alzheimer’s Disease: The Hypothalamus as Both Culprit and Target of Pathology. Cell metabolism. 2015;22:761–776. doi: 10.1016/j.cmet.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso H, Maruyama K, Ikehara S, Yamagishi K, Tamakoshi A. Cellular growth factors in relation to mortality from cardiovascular disease in middle-aged Japanese: the JACC study. Atherosclerosis. 2012;224:154–160. doi: 10.1016/j.atherosclerosis.2012.05.026. [DOI] [PubMed] [Google Scholar]
- Johnsen SP, Hundborg HH, Sørensen HT, Ørskov H, Tjønneland A, Overvad K, Jørgensen JOL. Insulin-like growth factor (IGF) I,-II, and IGF binding protein-3 and risk of ischemic stroke. The Journal of Clinical Endocrinology & Metabolism. 2005;90:5937–5941. doi: 10.1210/jc.2004-2088. [DOI] [PubMed] [Google Scholar]
- Johnston BM, Mallard EC, Williams CE, Gluckman PD. Insulin-like growth factor-1 is a potent neuronal rescue agent after hypoxic-ischemic injury in fetal lambs. The Journal of clinical investigation. 1996;97:300–308. doi: 10.1172/JCI118416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D’Ercole A, Ye P. Expanding the mind: insulin-like growth factor I and brain development. Endocrinology. 2008;149:5958–5962. doi: 10.1210/en.2008-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RC, McGinn AP, Pollak MN, Kuller LH, Strickler HD, Rohan TE, Cappola AR, Xue X, Psaty BM. Association of total insulin-like growth factor-I, insulin-like growth factor binding protein-1 (IGFBP-1), and IGFBP-3 levels with incident coronary events and ischemic stroke. The Journal of clinical endocrinology and metabolism. 2007;92:1319–1325. doi: 10.1210/jc.2006-1631. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21:1406–1415. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001a;72:653–660. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001b;39:277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AL, Yan X, Hamamichi S, Ajjuri RR, Mazzulli JR, Zhang MW, Daigle JG, Zhang S, Borom AR, Roberts LR. The glycolytic enzyme, GPI, is a functionally conserved modifier of dopaminergic neurodegeneration in Parkinson’s models. Cell metabolism. 2014;20:145–157. doi: 10.1016/j.cmet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knusel B, Michel P, Schwaber J, Hefti F. Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. Journal of Neuroscience. 1990;10:558–570. doi: 10.1523/JNEUROSCI.10-02-00558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler JH, Rosenbloom AL, Martinez V, Guevara-Aguirre J. Normal intelligence with severe insulin-like growth factor I deficiency due to growth hormone receptor deficiency: a controlled study in a genetically homogeneous population. The Journal of clinical endocrinology and metabolism. 1998;83:1953–1958. doi: 10.1210/jcem.83.6.4863. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. Journal of Neuroscience. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Internal Medicine. 2017;177:51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz TA, Salatto CT, Semproni AR, Marconi M, Brown TM, Richter KE, Schmidt K, Nelson FR, Schachter JB. Peripheral elevation of IGF-1 fails to alter Abeta clearance in multiple in vivo models. Biochem Pharmacol. 2008;75:1093–1103. doi: 10.1016/j.bcp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Cline GW, Macica CM. IGF-1 stimulates de novo fatty acid biosynthesis by Schwann cells during myelination. Glia. 2007;55:632–641. doi: 10.1002/glia.20496. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lin S, Fan L-W, Rhodes PG, Cai Z. Intranasal administration of IGF-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Experimental neurology. 2009;217:361–370. doi: 10.1016/j.expneurol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-F, Fawcett JR, Thorne RG, DeFor TA, Frey WH. Intranasal administration of insulin-like growth factor-I bypasses the blood–brain barrier and protects against focal cerebral ischemic damage. Journal of the neurological sciences. 2001;187:91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang X, Li W, Zhang Q, Li Y, Zhang Z, Zhu J, Chen B, Williams PR, Zhang Y. A sensitized IGF1 treatment restores corticospinal axon-dependent functions. Neuron. 2017;95:817–833.e814. doi: 10.1016/j.neuron.2017.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S, Pharaoh GA, Marlin MC, Masser DR, Matsuzaki S, Wronowski B, Yeganeh A, Parks EE, Premkumar P, Farley JA, et al. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-beta uptake in astrocytes. Mol Metab. 2018;9:141–155. doi: 10.1016/j.molmet.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay KB, Loddick SA, Naeve GS, Vana AM, Verge GM, Foster AC. Neuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivo. Journal of Cerebral Blood Flow & Metabolism. 2003;23:1160–1167. doi: 10.1097/01.WCB.0000087091.01171.AE. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M, Cuervo AM. Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol. 2007;6:352–361. doi: 10.1016/S1474-4422(07)70076-5. [DOI] [PubMed] [Google Scholar]
- Mashayekhi F, Mirzajani E, Naji M, Azari M. Expression of insulin-like growth factor-1 and insulin-like growth factor binding proteins in the serum and cerebrospinal fluid of patients with Parkinson’s disease. Journal of Clinical Neuroscience. 2010;17:623–627. doi: 10.1016/j.jocn.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Mattlage AE, Rippee MA, Sandt J, Billinger SA. Decrease in insulin-like growth factor-1 and insulin-like growth factor-1 ratio in the first week of stroke is related to positive outcomes. Journal of Stroke and Cerebrovascular Diseases. 2016;25:1800–1806. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Fullgrabe J, Jackson A, Jimenez Sanchez M, Karabiyik C, et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron. 2017;93:1015–1034. doi: 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, Barzilai N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13:769–771. doi: 10.1111/acel.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Chaudhuri KR, Bielza C, de Pedro-Cuesta J, Larranaga P, Martinez-Martin P. Parkinson’s Disease Subtypes Identified from Cluster Analysis of Motor and Non-motor Symptoms. Front Aging Neurosci. 2017;9:301. doi: 10.3389/fnagi.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AP, Fernandez AM, Haas C, Zimmer E, Portela LV, Torres-Aleman I. Reduced brain insulin-like growth factor I function during aging. Mol Cell Neurosci. 2012;49:9–12. doi: 10.1016/j.mcn.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Berton O, Guo S, Leneuve P, Dovero S, Diguet E, Tison F, Zhao B, Holzenberger M, Bezard E. IGF-1 signaling reduces neuro-inflammatory response and sensitivity of neurons to MPTP. Neurobiol Aging. 2009;30:2021–2030. doi: 10.1016/j.neurobiolaging.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Netchine I, Azzi S, Le Bouc Y, Savage MO. IGF1 molecular anomalies demonstrate its critical role in fetal, postnatal growth and brain development. Best Pract Res Clin Endocrinol Metab. 2011;25:181–190. doi: 10.1016/j.beem.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Nieto-Estevez V, Defterali C, Vicario-Abejon C. IGF-I: A Key Growth Factor that Regulates Neurogenesis and Synaptogenesis from Embryonic to Adult Stages of the Brain. Front Neurosci. 2016;10:52. doi: 10.3389/fnins.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novosyadlyy R, Leroith D. Insulin-like growth factors and insulin: at the crossroad between tumor development and longevity. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67:640–651. doi: 10.1093/gerona/gls065. [DOI] [PubMed] [Google Scholar]
- Numao A, Suzuki K, Miyamoto M, Miyamoto T, Hirata K. Clinical correlates of serum insulin-like growth factor-1 in patients with Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. Parkinsonism & related disorders. 2014;20:212–216. doi: 10.1016/j.parkreldis.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Offen D, Shtaif B, Hadad D, Weizman A, Melamed E, Gil-Ad I. Protective effect of insulin-like-growth-factor-1 against dopamine-induced neurotoxicity in human and rodent neuronal cultures: possible implications for Parkinson’s disease. Neuroscience letters. 2001;316:129–132. doi: 10.1016/s0304-3940(01)02344-8. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Mohan S, Sjogren K, Tivesten A, Isgaard J, Isaksson O, Jansson JO, Svensson J. The role of liver-derived insulin-like growth factor-I. Endocrine reviews. 2009;30:494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke O, Kang JH, Ma J, Hankinson SE, Pollak MN, Grodstein F. Plasma IGF-I levels and cognitive performance in older women. Neurobiol Aging. 2007;28:135–142. doi: 10.1016/j.neurobiolaging.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Ostrowski PP, Barszczyk A, Forstenpointner J, Zheng W, Feng ZP. Meta-Analysis of Serum Insulin-Like Growth Factor 1 in Alzheimer’s Disease. PloS one. 2016;11:e0155733. doi: 10.1371/journal.pone.0155733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J, Abba MC, Lacunza E, Ogundele OM, Paiva I, Morel GR, Outeiro TF, Goya RG. IGF-I Gene Therapy in Aging Rats Modulates Hippocampal Genes Relevant to Memory Function. The journals of gerontology Series A, Biological sciences and medical sciences. 2018;73:459–467. doi: 10.1093/gerona/glx125. [DOI] [PubMed] [Google Scholar]
- Pardo J, Uriarte M, Console GM, Reggiani PC, Outeiro TF, Morel GR, Goya RG. Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur J Neurosci. 2016;44:2120–2128. doi: 10.1111/ejn.13278. [DOI] [PubMed] [Google Scholar]
- Parrella E, Maxim T, Maialetti F, Zhang L, Wan J, Wei M, Cohen P, Fontana L, Longo VD. Protein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer’s disease mouse model. Aging Cell. 2013;12:257–268. doi: 10.1111/acel.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathipati P, Surus A, Williams CE, Scheepens A. Delayed and chronic treatment with growth hormone after endothelin-induced stroke in the adult rat. Behavioural brain research. 2009;204:93–101. doi: 10.1016/j.bbr.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Pellecchia M, Santangelo G, Picillo M, Pivonello R, Longo K, Pivonello C, Vitale C, Amboni M, Rosa A, Moccia M. Insulin-like growth factor-1 predicts cognitive functions at 2-year follow-up in early, drug-naïve Parkinson’s disease. European journal of neurology. 2014;21:802–807. doi: 10.1111/ene.12137. [DOI] [PubMed] [Google Scholar]
- Perice L, Barzilai N, Verghese J, Weiss EF, Holtzer R, Cohen P, Milman S. Lower circulating insulin-like growth factor-I is associated with better cognition in females with exceptional longevity without compromise to muscle mass and function. Aging. 2016;8:2414–2424. doi: 10.18632/aging.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picillo M, Erro R, Santangelo G, Pivonello R, Longo K, Pivonello C, Vitale C, Amboni M, Moccia M, Colao A. Insulin-like growth factor-1 and progression of motor symptoms in early, drug-naïve Parkinson’s disease. Journal of neurology. 2013;260:1724–1730. doi: 10.1007/s00415-013-6851-0. [DOI] [PubMed] [Google Scholar]
- Picillo M, Pivonello R, Santangelo G, Pivonello C, Savastano R, Auriemma R, Amboni M, Scannapieco S, Pierro A, Colao A. Serum IGF-1 is associated with cognitive functions in early, drug-naïve Parkinson’s disease. PloS one. 2017;12:e0186508. doi: 10.1371/journal.pone.0186508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Tucker B, Mearow K, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience. 2005;136:991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Prince M, Ali G-C, Guerchet M, Prina AM, Albanese E, Wu Y-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s research & therapy. 2016;8:23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig KL, Kulas JA, Franklin W, Rakoczy SG, Taglialatela G, Brown-Borg HM, Combs CK. The Ames dwarf mutation attenuates Alzheimer’s disease phenotype of APP/PS1 mice. Neurobiol Aging. 2016;40:22–40. doi: 10.1016/j.neurobiolaging.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. Journal of neuroscience research. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocrine reviews. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- Rizk NN, Myatt-Jones J, Rafols J, Dunbar JC. Insulin like growth factor-1 (IGF-1) decreases ischemia-reperfusion induced apoptosis and necrosis in diabetic rats. Endocrine. 2007;31:66–71. doi: 10.1007/s12020-007-0012-0. [DOI] [PubMed] [Google Scholar]
- Saber H, Himali JJ, Beiser AS, Shoamanesh A, Pikula A, Roubenoff R, Romero JR, Kase CS, Vasan RS, Seshadri S. Serum Insulin-Like Growth Factor 1 and the Risk of Ischemic Stroke: The Framingham Study. Stroke STROKEAHA. 2017;116:016563. doi: 10.1161/STROKEAHA.116.016563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samari HR, Seglen PO. Inhibition of hepatocytic autophagy by adenosine, aminoimidazole-4-carboxamide riboside, and N6-mercaptopurine riboside. Evidence for involvement of amp-activated protein kinase. J Biol Chem. 1998;273:23758–23763. doi: 10.1074/jbc.273.37.23758. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Guo W, O’Meara ES, Kaplan RC, Pollak MN, Bartz TM, Newman AB, Fried LP, Cappola AR. Trajectories of IGF-I Predict Mortality in Older Adults: The Cardiovascular Health Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2017 doi: 10.1093/gerona/glx143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäbitz W-R, Hoffmann TT, Heiland S, Kollmar R, Bardutzky J, Sommer C, Schwab S. Delayed neuroprotective effect of insulin-like growth factor-I after experimental transient focal cerebral ischemia monitored with MRI. Stroke. 2001;32:1226–1233. doi: 10.1161/01.str.32.5.1226. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Schwab S, Spranger M, Krempien S, Hacke W, Bettendorf M. Plasma insulin-like growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke. 1997;28:1744–1748. doi: 10.1161/01.str.28.9.1744. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PloS one. 2012;7:e32662. doi: 10.1371/journal.pone.0032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. Journal of Neuroscience. 2010;30:6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny JJ, Ryan JM, van Dyck CH, Peng Y, Lines CR, Nessly ML, Group MKPS Growth hormone secretagogue MK-677: no clinical effect on AD progression in a randomized trial. Neurology. 2008;71:1702–1708. doi: 10.1212/01.wnl.0000335163.88054.e7. [DOI] [PubMed] [Google Scholar]
- Silva-Couto MdA, Prado-Medeiros CL, Oliveira AB, Alcântara CC, Guimaraes AT, Salvini TdF, Mattioli R, Russo TLd. Muscle atrophy, voluntary activation disturbances, and low serum concentrations of IGF-1 and IGFBP-3 are associated with weakness in people with chronic stroke. Physical therapy. 2014;94:957–967. doi: 10.2522/ptj.20130322. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Sun X, Huang L, Zhang M, Sun S, Wu Y. Insulin like growth factor-1 prevents 1-mentyl-4-phenylphyridinium-induced apoptosis in PC12 cells through activation of glycogen synthase kinase-3beta. Toxicology. 2010;271:5–12. doi: 10.1016/j.tox.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson J, Diez M, Engel J, Wass C, Tivesten A, Jansson JO, Isaksson O, Archer T, Hokfelt T, Ohlsson C. Endocrine, liver-derived IGF-I is of importance for spatial learning and memory in old mice. J Endocrinol. 2006;189:617–627. doi: 10.1677/joe.1.06631. [DOI] [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. The Journal of clinical investigation. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J-H, Ma L-L, Yu T-X, Zheng J, Zhang H-J, Liang H, Shao P. Insulin-like growth factor-1 as a prognostic marker in patients with acute ischemic stroke. PloS one. 2014;9:e99186. doi: 10.1371/journal.pone.0099186. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Tazearslan C, Huang J, Barzilai N, Suh Y. Impaired IGF1R signaling in cells expressing longevity-associated human IGF1R alleles. Aging cell. 2011;10:551–554. doi: 10.1111/j.1474-9726.2011.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SEW, Ekdahl CT, Kokaia Z. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, et al. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Piriz J, Llorens-Martin MV, Fernandez AM, Bolos M, LeRoith D, Nunez A, Torres-Aleman I. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry. 2007;12:1118–1128. doi: 10.1038/sj.mp.4002076. [DOI] [PubMed] [Google Scholar]
- Trueba-Saiz A, Cavada C, Fernandez AM, Leon T, Gonzalez DA, Fortea Ormaechea J, Lleo A, Del Ser T, Nunez A, Torres-Aleman I. Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Transl Psychiatry. 2013;3:e330. doi: 10.1038/tp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumati S, Burger H, Martens S, van der Schouw YT, Aleman A. Association between Cognition and Serum Insulin-Like Growth Factor-1 in Middle-Aged & Older Men: An 8 Year Follow-Up Study. PloS one. 2016;11:e0154450. doi: 10.1371/journal.pone.0154450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncel D, Inanc Tolun F, Toru I. Serum insulin-like growth factor-1 and nitric oxide levels in Parkinson’s disease. Mediators of inflammation. 2009 doi: 10.1155/2009/132464. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Beld A, Bots M, Janssen J, Pols H, Lamberts S, Grobbee D. Endogenous hormones and carotid atherosclerosis in elderly men. American journal of epidemiology. 2003;157:25–31. doi: 10.1093/aje/kwf160. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G. The neuroendocrine response to stress is a dynamic process. Best Pract Res Clin Endocrinol Metab. 2001;15:405–419. doi: 10.1053/beem.2001.0160. [DOI] [PubMed] [Google Scholar]
- Vidal JS, Hanon O, Funalot B, Brunel N, Viollet C, Rigaud AS, Seux ML, le-Bouc Y, Epelbaum J, Duron E. Low Serum Insulin-Like Growth Factor-I Predicts Cognitive Decline in Alzheimer’s Disease. J Alzheimers Dis. 2016;52:641–649. doi: 10.3233/JAD-151162. [DOI] [PubMed] [Google Scholar]
- Wennberg AMV, Hagen CE, Machulda MM, Hollman JH, Roberts RO, Knopman DS, Petersen RC, Mielke MM. The association between peripheral total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 and functional and cognitive outcomes in the Mayo Clinic Study of Aging. Neurobiol Aging. 2018;66:68–74. doi: 10.1016/j.neurobiolaging.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood AJ, Beiser A, Decarli C, Harris TB, Chen TC, He XM, Roubenoff R, Pikula A, Au R, Braverman LE, et al. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology. 2014;82:1613–1619. doi: 10.1212/WNL.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. The New England journal of medicine. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- Wu Y-T, Beiser AS, Breteler MM, Fratiglioni L, Helmer C, Hendrie HC, Honda H, Ikram MA, Langa KM, Lobo A. The changing prevalence and incidence of dementia over time—current evidence. Nature Reviews Neurology. 2017;13:327. doi: 10.1038/nrneurol.2017.63. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sohmiya M, Oka N, Kato Y. Effects of aging and sex on plasma insulin-like growth factor I (IGF-I) levels in normal adults. Acta endocrinologica. 1991;124:497–500. doi: 10.1530/acta.0.1240497. [DOI] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. European Journal of Neuroscience. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- Zawada WM, Kirschman DL, Cohen JJ, Heidenreich KA, Freed CR. Growth factors rescue embryonic dopamine neurons from programmed cell death. Experimental neurology. 1996;140:60–67. doi: 10.1006/exnr.1996.0115. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M, Chopp M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain research. 2004;1030:19–27. doi: 10.1016/j.brainres.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Zheng H-Q, Zhang L-Y, Luo J, Li L-L, Li M, Zhang Q, Hu X-Q. Physical exercise promotes recovery of neurological function after ischemic stroke in rats. International journal of molecular sciences. 2014;15:10974–10988. doi: 10.3390/ijms150610974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Fan Y, Frenzel T, Gasmi M, Bartus RT, Young WL, Yang G-Y, Chen Y. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke. 2008;39:1254–1261. doi: 10.1161/STROKEAHA.107.500801. [DOI] [PMC free article] [PubMed] [Google Scholar]


