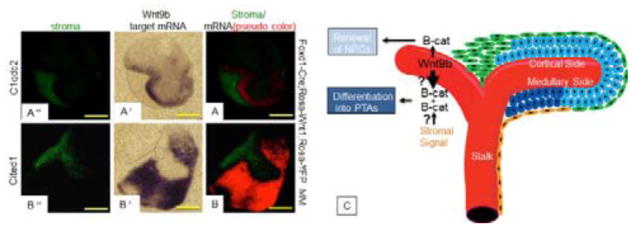
Abstract
Formation of a functional kidney depends on the balance between renewal and differentiation of nephron progenitors. Failure to sustain this balance can lead to kidney failure or stem cell tumors. For nearly 60 years, we have known that signals from an epithelial structure known as the ureteric bud were essential for maintaining this balance. More recently it was discovered that one molecule, Wnt9b, was necessary for both renewal and differentiation of the nephron progenitor cells. How one ligand signaling through one transcription factor promoted two seemingly contradictory cellular processes was unclear. In this study, we show that Wnt9b/beta-catenin signaling alone is sufficient to promote both renewal and differentiation. Moreover, we show that discrete levels of beta-catenin can promote these two disparate fates, with low levels fostering progenitor renewal and high levels driving differentiation. These results provide insight into how Wnt9b regulates distinct target genes that balance nephron progenitor renewal and differentiation.
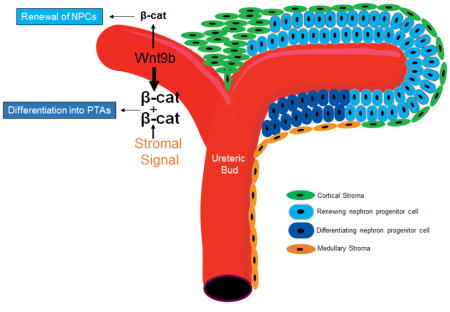
Graphical Abstract
Introduction
Formation of the metanephric kidney of mammals depends on interactions between an epithelial structure known as the ureteric bud (UB) and a heterogeneous population of cells known as the metanephric mesenchyme (MM). During embryonic development, the UB undergoes branching morphogenesis within the MM. As the UB branches, the MM proliferates along with it so that each new tip is encompassed by MM. The MM consists of at least two cell types: a self-renewing, multipotent progenitor cell, known as the nephron progenitor cell (NPC) and a stromal progenitor population. Adjacent to each UB tip, a subset of the NPCs undergoes a mesenchymal to epithelial transition (MET) forming an epithelial vesicle that eventually becomes the nephron. The stromal cells mature into the smooth muscle, mural cells and peritubular fibroblasts of the mature kidney.
The UB is necessary for the survival, renewal and differentiation of the MM. The number of nephrons that form per kidney is determined by the relative rate of NPC renewal and differentiation. As kidney function and susceptibility to certain diseases is believed to be dependent on nephron number, it is essential to understand how NPC renewal and differentiation are balanced. For many years, it was believed that these processes were regulated by distinct signals produced by the UB. However, more recently, we found that Wnt9b, produced by the UB, was necessary for the activation of the renewal and differentiation programs. Each program is characterized by the expression of distinct, beta-catenin dependent target genes. (Carroll, Park, Hayashi, Majumdar, & McMahon, 2005; Karner et al., 2011; Park et al., 2012; Park, Valerius, & McMahon, 2007). How Wnt/beta-catenin signaling promotes these two seemingly contradictory programs within the NPC population has remained unclear.
In this study, we show that distinct levels of beta-catenin activity are sufficient to activate the different classes of target genes and promote the different developmental programs within the NPCs. High levels of activity promote differentiation and low levels promote renewal. Response can be transposed by altering Wnt ligand levels. These findings enhance our understanding of kidney development and will have direct impact on current efforts to bioengineer renal replacement tissue ex vivo.
Results
Uniform activation of Rosa-Wnt1 in NPCs of isolated kidney mesenchymes maintains a progenitor population but does not induce differentiation
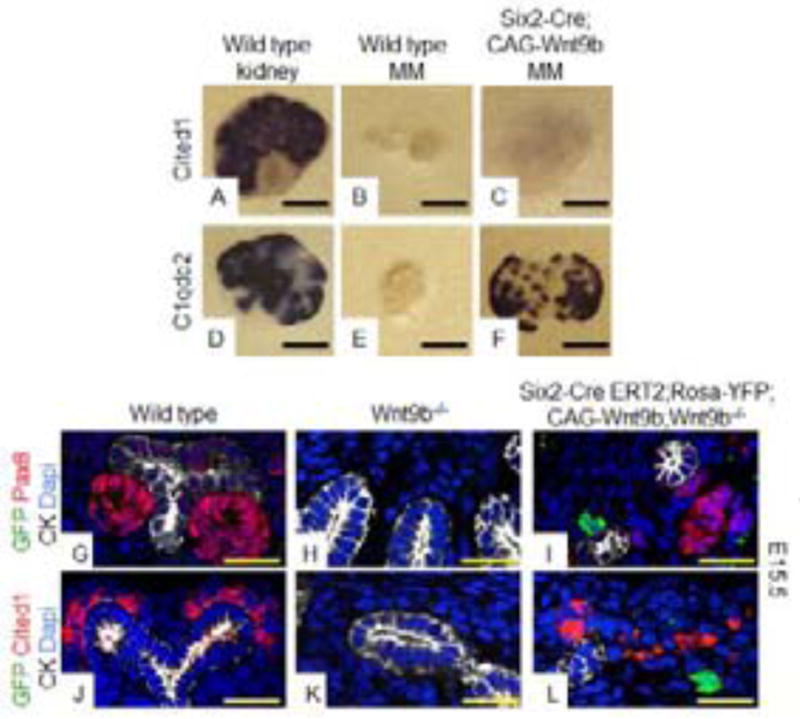
We previously showed that Wnt9b is necessary for the activation of two distinct transcriptional programs within the MM. One program correlates with the formation of pre-tubular aggregates and is represented by the transcription of several target genes including C1qdc2, Pax8 and Wnt4. The other program is active within the renewing NPCs and is represented by the expression of a distinct set of targets including Cited1, Amphiphysin (Amph), Fam19a5 (Tafa5) and Pla2g7. How Wnt9b activates these two distinct programs is unclear.
The first possibility we tested was whether another factor produced by the UB was necessary in addition to Wnt9b to activate one (or both programs). Although, it was previously shown that Wnt ligands (including Wnt1 and 9b) produced by fibroblast cells could induce survival, renewal and differentiation of isolated MM (Carroll et al., 2005; Herzlinger, Qiao, Cohen, Ramakrishna, & Brown, 1994; Kispert, Vainio, & McMahon, 1998), it is possible that fibroblasts produce additional factors that cooperate with the Wnt ligand to promote renewal or differentiation. To test this, we turned to a genetic strategy.
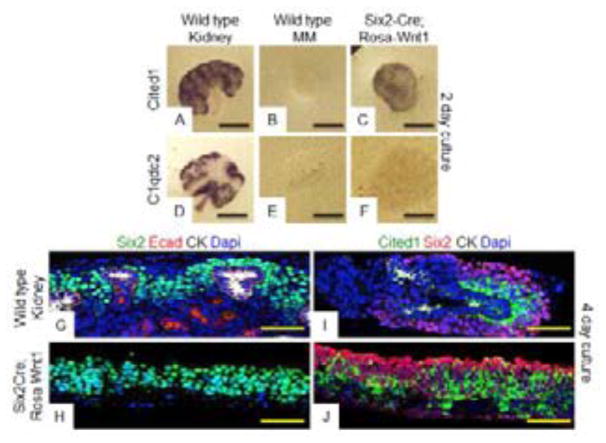
Activation of a Wnt1 transgene (Rosa26-lox-stop-lox-Wnt1 - hereon referred to as Rosa-Wnt1) in the UB rescues kidneys lacking endogenous Wnt9b (Carroll et al., 2005). To test if additional UB-produced factors were necessary in addition to the Wnt ligand, Rosa-Wnt1 was activated in the Wnt responsive NPCs using Six2-Cre (Kobayashi et al., 2008) and MMs were isolated away from the UB at E11.5. Isolated MMs were cultured for 2 days then assayed for molecular and morphological signs of renewal and differentiation. Although the vast majority of wildtype isolated MMs died within 48 hours (N=25/27, Fig. 1B and 1E), indicating that the UB was efficiently removed, all 49 of the Six2-Cre;Rosa-Wnt1 MMs assayed were still alive and expanded significantly in size over a 2–4 day culture period. In situ hybridization analysis revealed that 20/24 of the Six2-Cre;Rosa-Wnt1 MMs assayed expressed renewal target genes (Fig. 1C, Fig. S1F) but only 5/25 expressed differentiation targets (Fig. 1F and S1C). After an additional 2 days of culture, the Six2-Cre;Rosa-Wnt1 MMs still maintained expression of renewal markers Cited1 and Six2 but did not express differentiation markers nor did they form epithelial structures (as indicated by a lack of E-cadherin staining. N=3/3, Fig. 1G–1J).
Figure 1. Uniform activation of Rosa-Wnt1 ligand in NPCs of isolated metanephric mesenchymes promotes renewal.
Wildtype (A, B, D, E, G, I) or Six2-Cre; Rosa-Wnt1 (C, F, H, J) kidneys (A, D, G, I) or isolated metanephric mesenchyme (MM) (B, C, E, F, H, J) were dissected at E11.5 and cultured for 48 (A–F) or 96 hours (G–J). Tissues were fixed and hybridized with antisense probes to the Wnt9b renewal target gene, Cited1 (A–C) or the Wnt9b differentiation target gene, C1qdc2 (D–F). Immunofluorescence (IF) staining with antibodies to the NPC marker Six2, epithelial tubule marker E-cadherin and the UB marker pan-cytokeratin (green, red, white respectively in G, H) or Cited1, Six2, Pan-Cytokeratin (green, red, white respectively in I, J). All IF sections were counterstained with Dapi. Scale bars are 200uM for (A–F) and 100uM for (G–J).
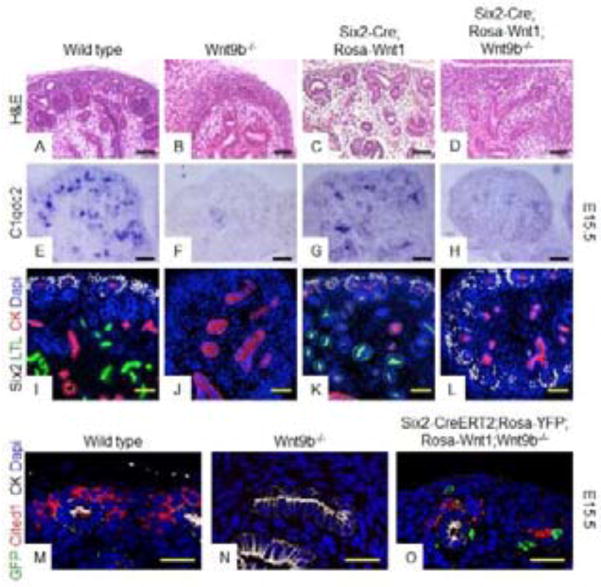
This observation raises the possibility that the UB produces an additional factor that cooperates with Wnt9b to promote differentiation. If this is the case, then addition of a Wnt9b mutant UB to the Six2-Cre;Rosa-Wnt1 MMs should result in activation of the differentiation program. To test this possibility, we analyzed intact (i.e. with the UB) Wnt9b−/− kidneys expressing Rosa-Wnt1 in the NPCs (Six2-Cre;Rosa-Wnt1;Wnt9b−/−). Contrary to what we expected, Six2-Cre;Rosa-Wnt1;Wnt9b−/− kidneys still expressed renewal targets Cited1, Fam19a5 and Pla2g7 and the Wnt independent marker Six2 (Fig. S2D and not shown) but did not express differentiation targets C1qdc2, Wnt4 or Pax8 (Fig. 2H, Fig. S2H and not shown). Consistent with the lack of expression of differentiation targets, Six2-Cre;Rosa-Wnt1;Wnt9b−/− kidneys showed a complete absence of nephrons at E15.5 (Fig. 2L) although they maintained a renewing NPC population (as marked by the expression of Six2 and the renewal targets Cited1, Fam19a5, Pla2g7) throughout embryogenesis (Fig. 2L and not shown). This suggests that the failure of Wnt1 to induce differentiation within isolated MM (Fig. 1) is not simply due to the absence of an additional UB derived signal(s). Importantly, Six2-Cre;Rosa-Wnt1;Wnt9b+/+ kidneys were of normal size and histology and, expressed both renewal genes (Six2, Cited1, Fam19a5, Pla2g7) and differentiation targets, C1qdc2, Wnt4, Pax8 (Fig. 2C, 2G, 2K, S2C, S2G and not shown) ruling out a dominant gain of function activity for Wnt1 in the MM.
Figure 2. Failure of Six2-Cre;Rosa-Wnt1 metanephric mesenchymes to differentiate is not due to lack of UB-derived signals or secretion of functional Wnt protein.
Hematoxylin and Eosin staining (A–D), in-situ hybridization (E–H) or IF staining (I–O) of sections of E15.5 wildtype (A, E, I, M), Wnt9b−/− (B, F, J, N), Six2-Cre;Rosa-Wnt1 (C, G, K),Six2-Cre;Rosa-Wnt1;Wnt9b−/−(D,H,L) or Six2-CreERT2;Rosa-YFP;Rosa-Wnt1;Wnt9b−/−(O) kidneys. Tissues were hybridized with anti-sense probes for the Wnt9b differentiation target gene, C1qdc2 (E–H). Antibodies are to the NPC marker Six2 (gray), the proximal tubule marker LTL (green) and the UB marker pan-cytokeratin (red) in I–L or to GFP (green), renewal target Cited1 (red) and pan-cytokeratin (grey) in M–O. All IF sections were counterstained with DAPI. Scale bars are 50uM for (A–H),100uM for (I–L) or 25uM for (M–O).
Although Rosa-Wnt1 produced by the UB (using Hoxb7-Cre) was previously shown to rescue the Wnt9b−/− phenotype at the histological level, the two ligands may trigger distinct transcriptional programs. To test this possibility, we performed a more detailed analysis of Hoxb7-Cre;Rosa-Wnt1;Wnt9b−/− kidneys. First, we assessed size and morphology of Wnt1 rescued kidneys and found that they were indistinguishable from wildtype kidneys (Fig. S3D). Next, we assessed the expression of Wnt9b specific targets in Hoxb7-Cre;Rosa-Wnt1;Wnt9b−/− kidneys and found that the abundance and spatial expression of all Wnt9b target genes examined were indistinguishable between Hoxb7-Cre;Rosa-Wnt1;Wnt9b−/− and wildtype kidneys (Fig. S3J, S3P). Thus, when produced by the UB, Rosa-Wnt1 is functionally interchangeable with Wnt9b.
We next tested whether Wnt1 activity in the MM was dependent on beta-catenin. Isolated Six2-Cre;Rosa-Wnt1 MM were cultured in media containing the beta-catenin antagonist IWR1 at 100uM, a concentration we previously showed blocks all beta-catenin activity (Karner et al., 2010). The IWR1 treated Six2-Cre;Rosa-Wnt1 MMs behaved identically to wildtype MMs and died within 2 days (N=8/9, Fig. S7E and S7G). We also assessed beta-catenin dependence by genetically ablating beta-catenin at the same time that we activated Wnt1 (Six2-Cre;Rosa-Wnt1;beta-cateninflox/−). Six2-Cre;Rosa-Wnt1;beta-cateninflox/− MMs did not renew and died within 2 days of culture (N=5/5, Fig. S7D) indicating that Rosa-Wnt1 requires beta-catenin to promote NPC survival/renewal.
It is possible that the MM lacks the molecular machinery necessary to produce and/or secrete a fully active Wnt ligand (e.g. enzymes necessary to post-translationally modify the protein or in some other way secrete an active ligand). To test whether NPC-produced Wnt1 ligand could be secreted and signal in a paracrine fashion, we generated clones of Wnt1 producing cells using a Six2-CreERT2 transgene and a low dose of tamoxifen. To remove the influence of endogenous Wnt9b on NPCs, the analysis was performed on Wnt9b−/− kidneys. E15.5 kidneys were subsequently assessed for the expression of Cited1 relative to the Wnt1 source using Rosa26-lox-stop-lox-YFP (Rosa-YFP). Cells producing the Wnt show cytoplasmic GFP while cells that have received the Wnt signal should have membrane bound GFP (from the GFP tag on the Wnt1 ligand).
Unlike Wnt9b nulls, Six2-CreERT2;Rosa-Wnt1;Rosa-YFP;Wnt9b−/− kidneys maintained a nephrogenic zone and their UBs underwent extensive branching, a process dependent on signals from the NPCs (Fig. S4B, S4C). They expressed Wnt9b target genes including Amphiphysin and Cited1 (Fig. S4 and Fig. 2O). To determine if Wnt1 was produced and secreted properly when expressed in the NPCs, we examined expression of Wnt9b target genes with the hypothesis that if Wnt1 is able to be secreted, targets should be expressed adjacent to the Rosa-YFP+, Wnt1 producing cells (with cytoplasmic YFP) in cells that are either negative for YFP or have membrane bound GFP. However, if a functional Wnt1 protein is not secreted then we will not see any non-cytoplasmic YFP positive cells that express Wnt9b target genes. In the Six2-CreERT2;Rosa-Wnt1;Rosa-YFP;Wnt9b−/− kidneys, we rarely saw cells with membrane GFP only, even in live images viewed with a spinning disk confocal (not shown). The most likely explanation for this observation is that either the Wnt1GFP does not travel far from the producing cell or, if it does, it is rapidly degraded or at levels below detection. Whatever the case, the renewal target gene Cited1 was expressed in YFP/GFP negative cells, suggesting it was activated by the Wnt non-autonomously (Fig. 2O). This finding rules out the possibility that the lack of expression of differentiation targets is caused by an inability of the NPCs to produce/secrete a functional Wnt ligand.
Uniform activation of CAG-Wnt9b in NPCs of isolated mesenchyme induces differentiation without renewal
Although Wnt1 is sufficient to induce MM (Herzlinger et al., 1994) and it can functionally substitute for Wnt9b in vivo, we could not formally rule out the possibility that the phenotype observed upon activation of this factor in the NPCs, was ligand specific. As Wnt9b is the endogenous ligand, we repeated this analysis with a Cre inducible Wnt9b allele (CAG-lox-stop-lox-Wnt9b-IRES-GFP, from here on referred to as CAG-Wnt9b) (Kiefer, Robbins, & Rauchman, 2012; Kiefer et al., 2010). Similar to what was previously described, activation of this transgene in the UB could fully rescue Wnt9b−/− kidneys. Hoxb7-Cre;CAG-Wnt9b;Wnt9b−/− kidneys were indistinguishable from Rosa-Wnt1 rescue (Hoxb7-Cre;Rosa-Wnt1;Wnt9b−/−) and wildtype kidneys in both gross anatomy and expression of Wnt9b target genes (Fig. S3F, S3L, S3R).
We next induced CAG-Wnt9b in the NPCs using Six2-Cre. Proper expression of this transgene was confirmed through expression of GFP protein which is the result of an IRES-GFP cassette 3′ to the Wnt9b cDNA (Fig. S5C). In contrast to what was observed with Rosa-Wnt1, activation of CAG-Wnt9b in the NPCs resulted in expression of differentiation (N=41/42) but not renewal targets (4/15 positive, Compare Fig. 3C and 3F to Fig. 1C and 1F) in MM cultures. After 4 days of culture, the entire Six2-Cre;CAG-Wnt9b NPC population had undergone MET as indicated by the expression of E-cadherin and the absence of Six2 (N=3/3, Compare Fig. S6B to Fig. 1I and 1J). In vivo activation of CAG-Wnt9b in the NPCs of wildtype or Wnt9b−/− intact kidneys showed ectopic differentiation within the NPC domain at E12.5 and E15.5 (Fig. S6E–S6F, S6I–S6J and S6M–S6N).
Figure 3. Homogenous expression of Wnt9b in the NPCs induces differentiation while mosaic expression induces both the renewal and differentiation.
A–F: Whole mount in-situ hybridization with probes to Wnt9b renewal target gene, Cited1 (A–C) or Wnt9b differentiation target gene, C1qdc2 (D–F) was performed on E11.5 wildtype kidneys (A,D), wildtype MMs (B,E) or Six2-Cre;CAG-Wnt9b MM (C, F) after 48 hours of culture. G–L: IF staining on sections of E15.5 wildtype (G,J), Wnt9b−/− (H,K) and Six2-CreERT2;CAG-Wnt9b;Rosa-YFP;Wnt9b−/− (I,L) kidneys. Antibodies are to GFP (green), pan-cytokeratin (white) and renewal target gene Cited1 (red in J–L) or differentiation target gene Pax8 (red in G–I). All sections are counterstained with DAPI. Scale bars are 200uM for A–F and 50uM for G–L.
To determine whether the CAG-Wnt9b allele was processed and secreted by the NPCs, we generated clones of Wnt9b expressing cells using Six2-CreERT2 and a low dose of tamoxifen. At E15.5, mutants had a small kidney (compared to Wnt9b mutants that completely lack a kidney) with a branched UB and apparent nephrogenic zone (Fig. S4). To observe target gene expression relative to the Wnt9b producing cells, we co-stained tissues with antibodies to the differentiation target Pax8 and GFP, which marked the cells expressing Cre (Fig. 3G–I). Although Pax8+ cells were not observed in Wnt9b−/− kidneys (Fig. 3H), numerous Pax8+ cells were observed in the Six2-CreERT2;CAG-Wnt9b;Rosa-YFP;Wnt9b−/− kidneys. Although frequently they were observed in GFP positive cells, frequently they were also observed adjacent to the GFP positive source (Fig. 3I). It is important to note that the CAG-Wnt9b transgene includes an IRES-GFP tag which is strongly expressed and visible with standard immunostaining (Fig. S5C). Thus although we acknowledge that CAG-Wnt9b expression and Rosa-YFP expression may not overlap in 100% of cells, all CAG-Wnt9b cells should express GFP (some YFP expressing cells may be CAG-Wnt9b negative).
Unexpectedly, we also observed expression of the renewal targets Cited1 and Amphiphysin in the Six2-CreERT2;CAG-Wnt9b;Wnt9b−/− kidneys (Fig. 3L and S4H). These cells apparently represented a functional, renewing NPC population as evidenced by more extensive branching of the UB than in a Wnt9b−/− mutant and sustained maintenance of a Six2-positive progenitor population which is not present beyond E13.5 in straight Wnt9b−/− mutants (Fig. S4D) and (Carroll et al., 2005). The fact that mosaic expression of CAG-Wnt9b in the NPCs results in non-autonomous activation of both renewal and differentiation genes (molecularly and functionally) in Wnt9b mutants refutes the possibility that an active ligand cannot be secreted from NPCs.
Low level beta-catenin activity maintains renewal targets while high level activity induces differentiation targets in isolated MM
The data presented to this point produce a paradox. Why does uniform activation of Wnt9b promote a different response than uniform activation of Wnt1? One possibility is that the two ligands trigger differential levels of beta-catenin activation and different levels of beta-catenin could be promoting different cell fates within the NPCs. Such a model has been proposed in several other progenitor cell types (Hirata et al., 2013; Hoffman, Wu, & Merrill, 2013; ten Berge et al., 2011). This could also explain why mosaic activation of Wnt9b within the NPCs promotes both renewal and differentiation.
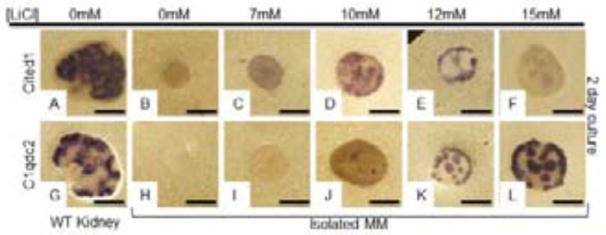
To determine if different levels of beta-catenin activation promote distinct responses in NPCs, isolated MMs were treated with various doses of the Gsk3 inhibitor LiCl (0, 7, 10, 12 and 15mM) and assayed by in situ hybridization. Although LiCl can have pleiotropic effects, previous studies have shown that within the NPCs, it induces Wnt9b target gene expression in a beta-catenin dependent manner (Karner et al., 2010). As expected, untreated wild type MM did not express Wnt9b renewal target Cited1 (N=0/8) or differentiation target C1qdc2 (0/2) (Fig. 4B and 4H). In contrast, MMs treated with a 7mM concentration of LiCl survived, rarely expressed the renewal target Cited1 (N=2/8) but never expressed the differentiation target C1qdc2 (N= 0/5, Fig. 4C and 4I). 19/26 MMs treated with 10mM LiCl showed strong expression of the renewal target Cited1 (Fig. 4D) while 12/25 showed expression of the differentiation target C1qdc2 (Fig. 4J). Importantly, in 10mM treated MMs, C1qdc2 expression was usually confined to 2 or fewer PTAs where its levels appeared similar to what is observed in wildtype PTAs (Fig. 4D, 4J and S9K). Treatment with 12mM LiCl also resulted in survival in all instances (18/18). 8/8 MMs assayed expressed renewal marker Cited1 and 10/10 expressed differentiation target C1qdc2 (Fig. 4E, 4K). In contrast to what was observed with 10mM, the majority of 12mM treated MMs had greater than 3 PTAs per MM (S9K). MMs treated with 15mM LiCl survived and 18/18 assayed expressed differentiation target Clqdc2 (Fig. 4L). In comparison to 10 and 12mM treated, 17/18 15mM treated MMs had greater than 3 PTAs and the majority had more than 5 (Fig. S9K). Fewer than half (16/35) of 15mM treated MMs expressed detectable levels of Cited1 mRNA and relative to other treatments, expression was extremely low and appeared epithelial (Fig. 4F). To determine the identity of these cited expressing cells, we sectioned the tissue and performed immunostaining but were unable to detect any cells that expressed Cited1 protein in these tissues suggesting the detected Cited1 expression was either artefactual or represented an mRNA that was not translated into protein (Fig. S9J).
Figure 4. Distinct thresholds of beta-catenin activity determine NPC fate.
Wildtype E11.5 kidneys (A, G) or isolated MM (B–F, H–L) were cultured in media containing 0mM (A, B, G, H), 7mM (C, I), 10mM (D, J), 12mM (E, K) or 15mM (F, L) of the Gsk3 antagonist LiCl for 48 hours (A–L). Cultured tissues were hybridized with anti-sense probes for the Wnt9b renewal target gene Cited1 (A–F) or the Wnt9b differentiation target gene, C1qdc2 (G–L).
To assure that the effects of different doses of Li+ were not limited to C1qdc2 and Cited1, we analyzed the expression of additional differentiation and renewal targets. First, we generated a Bac transgenic mouse line expressing YFP under the control of the Pax8 regulatory elements to serve as a live indicator of the differentiation state of the isolated MMs. This line is a faithful reporter of Pax8YFP expression (Fig. S8). MMs from Pax8-YFP E11.5 kidneys were isolated, placed in culture and assessed for YFP expression. Similar to what was observed with C1qdc2, treatment with 15mM LiCl resulted in robust Pax8-YFP expression (N=4/5 MMs had greater than 7 PTAs. Fig. S9A). In contrast, the majority (12/17) of MMs treated with the 10mM concentration were Pax8YFP negative and the ones that showed expression had 2 or fewer Pax8YFP + structures (Fig. S9A and not shown).
Cited1 is the only fully characterized Wnt9b target that is expressed specifically in the renewing NPCs. Additional characterized renewal targets are expressed in both the NPCs and the PTAs and show expression in the presence of both high and low LiCl (data not shown). To determine whether these gene products mark different cell types under different concentrations of lithium, we stained sections of MMs treated with 10 or 15mM LiCl with antibodies to Six2 and Cited1 or Six2 and Amph, a Wnt9b/beta-catenin target expressed in both the renewing NPCs and the renal vesicles (Karner et al., 2011 and Fig. S9). 3/3 MMs treated for 48 hours with 10mM LiCl showed extensive Six2/Amph and Six2/Cited1 costaining (Fig. S9F and S9I) suggesting that most, if not all, of the Amph+ cells found under these conditions are marking the renewing NPCs. In contrast, we were unable to detect Six2+/Amph+ or Six2+/Cited1+ cells in MMs treated with 15mM LiCl (N=3, Fig. S9G and S9J) although we did detect Amph+/Six2− cells, which likely represent differentiated cells (Fig. S9G).
Although the MMs cultured at 10mM express markers indicative of an NPC, expression alone does not prove that they are competent to differentiate. To functionally test these cells, MMs from E11.5 Pax8-YFP embryos were treated with 10mM LiCl for 2 days then switched to 15mM for an additional 3 days. While the majority of MMs treated with 10mM LiCl did not show reporter activity over the first two days of culture, they did express high levels of Pax8YFP after being switched to 15mM (N=6/7, Fig. S9D).
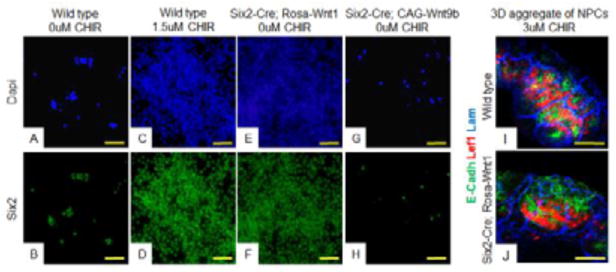
The MM is a heterogenous population of cells containing both NPCs and stroma. We know from previous work that signals produced by the stroma can influence NPC fate (Das et al., 2013; Mao, Francis-West, & Irvine, 2015; McNeill & Reginensi, 2017). As the stroma also responds to Wnt/beta-catenin signaling, it is possible that antagonism of Gsk3 is affecting the stroma which is secondarily impacting the fate of the NPCs. To determine if a stromal population was required to elicit different responses in NPCs, we cultured purified NPCs in different levels of GSK3 inhibitors (Brown, Muthukrishnan, & Oxburgh, 2015). As previously shown, wildtype NPCs did not renew unless the media was supplemented with low levels of the GSK-3 antagonist/beta-catenin agonist CHIR (Fig. 5A–5D). In contrast, supplementation with higher dose CHIR (3uM) induced expression of the differentiation marker Lef1 in 2-D culture and MET in 3-D culture (Fig. S10C–S10D, 5I). To determine whether this result could also be achieved using the different Wnt transgenics, NPCs were purified from Six2-Cre;Rosa-Wnt1 or Six2-Cre;CAG-Wnt9b kidneys cultured. Similar to what was observed with intact MMs, purified Six2-Cre;Rosa-Wnt1 NPCs could be maintained and passaged in a Six2+/Lef1-progenitor state without supplementation with GSK3 inhibitor (Fig. 5E–5F, S10E–S10F). In contrast, NPCs purified from Six2-Cre;CAG-Wnt9b kidneys could not be passaged even if the media was supplemented with 1.5 uM CHIR and they expressed the differentiation marker Lef1 without CHIR supplementation (Fig. 5G–5H, S10I–S10J and not shown). These data suggest that in the absence of stroma, altering Wnt/beta-catenin activity alone is sufficient to determine NPC fate.
Figure 5. Distinct beta-catenin activity levels induce distinct cell fates on isolated, pure NPC cultures.
Wildtype (A–D, I), Six2-Cre;Rosa-Wnt1 (E–F, J,) or Six2-Cre;CAG-Wnt9b (G–H,) purified NPCs were cultured in media with 1.5uM CHIR (C–D,), 0uM CHIR (A–B, E–H,) or 3uM CHIR (I–J) and stained with antibodies to the NPC marker Six2 (green) (B–H), differentiation marker Lef1 (red in I,J), epithelial tubule marker E-cadherin (green in I,J), or basement membrane marker Laminin (blue in I,J). In I and J, cells were aggregated and grown at the air media interface for two days. All images are counterstained with DAPI (blue). Scale bars are 100uM.
As uniform activation of Rosa-Wnt1 maintains NPCs in an undifferentiated state while uniform CAG-Wnt9b promotes differentiation, we hypothesized that Rosa-Wnt1 induces low level beta-catenin activation and CAG-Wnt9b induces high level beta-catenin activity. To test this, we supplemented Six2-Cre;Rosa-Wnt1 MMs with a low dose of LiCl (10mM) and then assessed expression of both renewal and differentiation target genes. Although MMs expressing either Six2-Cre;Rosa-Wnt1 (Fig. 1C and 1F) or 10mM LiCl treated wild type MMs (Fig. 4D and 4J) form few if any PTAs (but show strong expression of renewal marker Cited1), the 10mM LiCl treated Six2-Cre;Rosa-Wnt1 MMs form high numbers of PTAs as assessed by the expression of differentiation target C1qdc2 (N=3/3, Fig. 6M–6N). Similarly, supplementation of purified Six2-Cre;Rosa-Wnt1 NPCs with CHIR stimulated expression of the differentiation marker Lef1 in 2D culture and induced MET in 3D organoid culture (Fig. S10G–S10H, 5J).
Figure 6. Response to Wnt transgenes can be interchanged by altering ligand or beta-catenin levels.
Wildtype intact kidneys (A, H), wildtype isolated MM (B, I), Six2-Cre;CAG-Wnt9b isolated MM (C–E and J–L) or Six2-Cre;Rosa-Wnt1 isolated MM (F–G, M–N) were analyzed by whole mount in-situ hybridization with probes to Wnt9b renewal target gene, Cited1 (A–G) or Wnt9b differentiation target gene, C1qdc2 (H–N). Analysis was performed after 48 hours of culture in media supplemented with DMSO (A–C, F, H–J, M), a low dose (500nM) of the Porcupine inhibitor IWP2 (D, K), a low dose (1uM) of the tankyrase inhibitor IWR1 (E, L) or low dose (10mM) of LiCl (G, N). Scale bars are 200uM.
We next tested if we could promote NPC renewal in Six2Cre;CAG-Wnt9b MMs by attenuating either Wnt or beta-catenin levels. We first treated MMs with inhibitors of the o-acyl-transferase Porcupine. Porcupine is necessary for production of an active ligand and we previously showed that treatment of wildtype kidneys with a high dose (5 uM of IWP2 or 50 nM of IWPL6) resulted in complete loss of both differentiation and renewal targets (Karner et al., 2010; Wang et al., 2013). Although treatment of Six2-Cre;CAG-Wnt9b MMs with a high dose of IWP2 or IWPL6 (5uM or 15nM, respectively) was sufficient to block all Wnt/beta-catenin activity, treatment with lower doses (500nM or 10nM, respectively) rescued Cited1 expression (Fig. 6D and Fig. S7H–N).
We next asked whether partial attenuation of beta-catenin would have the same effect. Six2-Cre;CAG-Wnt9b MMs were treated with different doses of the beta-catenin antagonist IWR1. High dose treatment with this drug completely blocked Wnt response (Karner et al., 2010). However, similar to what was observed with the Porcupine inhibitors, treatment of Six2-Cre;CAG-Wnt9b MMs with a low dose of IWR1 (1uM) rescued Cited1 expression (Fig. 6E) although differentiation targets were also expressed (Fig. 6K–L). We were unable to perform similar experiments with NPCs purified from E17.5 Six2-Cre;CAG-Wnt9b kidneys as the progenitor population was lost in these organs by E15.5 (figure S6).
In sum, while uniform expression of Wnt1 in NPCs results in renewal target expression, this response can be shifted to differentiation by low level GSK3 inhibition. Reciprocally, while uniform expression of a Wnt9b transgene in the NPCs results in differentiation, this response can be shifted to renewal by lowering the levels of either the Wnt ligand or beta-catenin. These data further support the hypothesis that NPCs respond to different Wnt/beta-catenin activity levels with low levels promoting renewal and high levels promoting differentiation.
Stromal production of Wnt ligand elicits a graded response in NPCs
The data presented to this point suggest that NPC fate can be dictated by different levels of Wnt/beta-catenin signaling. We next asked how differential beta-catenin activity is established in the NPCs? As Wnts are secreted factors, the simplest hypothesis is that a gradient of the ligand exists across the NPC field with different levels of ligand producing distinct responses. However, it is difficult to envision how this could be the mechanism in the developing kidney as the expression domains of the renewal and differentiation targets of Wnt9b do not correlate with the relative distance of the receiving cells to the Wnt9b source, the UB (Fig. 7C).
Figure 7. Proximity to the stroma affects NPC fate.
(A,B) E11.5 Foxd1-Cre;Rosa-Wnt1; Rosa-YFP isolated MM were cultured for 2 days and subjected to in-situ hybridization for Wnt9b Wnt9b differentiation target gene C1qdc2 (A′) or renewal target gene Cited1 (B′) followed by IF stain for GFP (A″, B″). In A and B, the fluorescent image and a pseudo-colored version of the in-situ image were merged. Note that the differentiation target C1qdc2 is expressed immediately adjacent to the Wnt source while the renewal target is expressed at a distance from the source of the Wnt. Scale bars are 200uM. C: Model for Wnt/beta-catenin signaling in NPCs. Schematic representation of the embryonic kidney indicating the Wnt9b-expressing UB epithelium in red, the renewing NPCs that express Wnt9b renewal targets in light blue, the differentiating NPCs or pre-tubular aggregates (PTAs) that express Wnt9b differentiation targets in dark blue, the cortical stromal cells in green and the medullary stromal cells in orange. Wnt9b ligand is produced by the UB and secreted to the surrounding MM where it stimulates a low/baseline level of beta-catenin activity, which promotes renewal. High levels of beta-catenin activity which promote differentiation are a result of unknown beta-catenin amplifying signal(s), perhaps emanating from the medullary stroma and/or higher active Wnt ligand in the medullary NPCs.
During kidney development, the differentiating cells sit at the concave cleft of the Wnt9b expressing UB tip/stalk while the NPCs surround the convex tip of the UB. Previous modeling studies predict that secreted molecules will be present at higher levels in the cleft region of the branched structures, which corresponds exactly with the location of differentiation target gene expression (Nelson, Vanduijn, Inman, Fletcher, & Bissell, 2006). Thus, it seemed plausible that the shape of the UB could affect the levels of Wnt ligand that the NPCs were exposed to. We first examined kidneys from Hoxb7Cre;RosaWnt1-GFP kidneys to determine if we could detect spatial differences in the concentration of GFP in NPCs. Using a variety of techniques including frozen sections of unfixed tissues that were not treated with detergents (Fig. S11) and live imaging (not shown), we were able to detect GFP protein in on the basolateral side of the UB (Fig. S11). However, GFP was never detected beyond the UB epithelium in adjacent NPCs (Fig. S11). Thid did not rule out the possibility that the shape of the UB could affect local concentrations of membrane bound Wnt1. To test if shape and morphology of the Wnt source can influence cell fate decision, we activated Wnt expression within the stromal cells of isolated MMs and analyzed target gene expression relative to the morphology of the Wnt source.
Isolated Foxd1-Cre;Rosa-Wnt1;Rosa-YFP MMs were cultured and assessed for the expression of C1qdc2 (differentiation gene) and Cited1 (renewal gene). In contrast to isolated wildtype MMs which died within 48 hours, the Foxd1-Cre;Rosa-Wnt1 MMs survived and expressed both renewal and differentiation targets (N=26/26, Fig. 7A–7B). Somewhat unexpectedly, irrespective of the morphology of the Wnt source relative to the receiving NPCs (concave or convex), the Foxd1-Cre;Rosa-Wnt1 MMs expressed the differentiation target gene C1qdc2 directly abutting the source of Wnt1 (N=11/11) and the renewal target Cited1 at a distance from the source (N=8/9, Fig. 7A′, A″, B′ B″). This finding rules out the hypothesis that morphology of the source dictates ligand levels and response. However, it is further support that the relative strength of the Wnt/beta-catenin signal dictates NPC fate.
Discussion
We previously showed that Wnt9b was necessary for both renewal and differentiation of NPCs in the embryonic kidney however the mechanism underlying this pleiotropic role was unknown (Karner et al., 2011). Here we demonstrate that the disparate responses of NPCs to Wnt9b can be mimicked by modulating Wnt/beta-catenin levels. Treatment of isolated MMs with different doses of Gsk3 antagonist triggers different responses within isolated MMs at both a cellular and molecular level. Further, two different Wnt transgenes can elicit opposite responses when expressed homogeneously in the NPC population (in vivo and in isolated cultured MMs) but these responses can be transposed by altering Wnt/beta-catenin levels suggesting that they stimulate different levels of beta-catenin activation. It is not clear whether the ability of these transgenes to elicit different responses is due to differences in mRNA levels driven by the different promoters, the levels/stability of the two different ligands or the strength of receptor ligand interactions. But together, our data show that distinct levels of Wnt/beta-catenin activity are sufficient to direct the decision of a progenitor to engage in self renewal versus differentiation.
We acknowledge that we have used a relatively small number of beta-catenin target genes in this analysis. This may raise concerns that the results we have observed are specific to the targets being shown and not representative of the cell state. We feel this is extremely unlikely. First, it is important to note that we have examined multiple markers and, although we have not examined all markers under all conditions, in all cases (with the exception of isolated NPCs which do not appear to represent either a clear PTA cell or NPC but instead show molecular characteristics of both) the data is supportive (not shown). But most importantly, in all conditions tested, we also have functional data showing either progenitor expansion/renewal or differentiation/MET. Our model is further supported by recent findings showing that low levels of Gsk3 inhibition can promote renewal of isolated NPCs while high levels are known to induce differentiation in vitro (Brown et al., 2015; Davies & Garrod, 1995; Tanigawa, Sharma, Hall, Nishinakamura, & Perantoni, 2015).
Work done in multiple tissues including the kidney, epiblast, hair follicles and the gut (Hirata et al., 2013; Hoffman et al., 2013; Lien et al., 2014; Lindstrom et al., 2015; Sumi, Oki, Kitajima, & Meno, 2013; ten Berge et al., 2011) show pleiotropic roles for Wnt/beta-catenin. Frequently, gradients for the Wnt ligand are invoked in establishing pleiotropic response (Aulehla et al., 2008; Neumann & Cohen, 1997). Such a model for Wnt signaling has recently come under disfavor, as studies have shown that the ligands are tightly associated with the membranes of both the producing and receiving cells. Indeed, using the GFP tag on the Hoxb7-Cre;Rosa-Wnt1 allele, we are unable to detect tagged Wnt protein beyond the UB ECM (Fig. S11). Further, it is important to note that we do not detect differences in the levels of target gene expression in our experimental conditions or in vivo. Instead our data suggest more of a digital response with cells falling below a minimum threshold of beta-catenin activity turning on progenitor targets while those above this threshold turning on the differentiation targets. This is also inconsistent with the idea of a true gradient of Wnt protein across the receiving cells. Interestingly, none of the so-called universal targets of beta-catenin (such as Axin2, Nkd1/2 or Lef/TCF reporter genes) are robustly expressed in the NPCs, which perhaps supports the notion of a unique mechanism of the pleiotrophic response to beta-catenin in the NPCs(Pan, Karner, & Carroll, 2017). This observation of threshold activation further emphasizes the importance of using in situ hybridization and/or antibody staining as more unbiased techniques that do not provide spatial information could give misleading results.
Most recently, a model where the Wnt gradient is created by dilution of the ligand as the source and receiving cells divide, was proposed for the intestinal stem cell niche (Farin et al., 2016). The situation in the embryonic kidney is also inconsistent with this model as the oldest (differentiating) NPCs require the highest levels of beta-catenin, not the lowest as would exist in the dilution model. Our studies are also inconsistent with the possibility that the shape of the Wnt source can impact response as has previously been proposed (Farin et al., 2016; Nelson et al., 2006) as ectopic expression of Wnt1 or Wnt9b from the stroma activates different classes of target genes based on proximity to the stroma and independent of the morphology of the source. We can also discount the possibility that activation of Wnt4 within the responding cells is the amplifying mechanism as Wnt4 does not activate expression of any of the target genes tested in this study (Karner et al., 2011) and recent evidence suggests that the role for Wnt4 in kidney development is beta-catenin independent and our data suggest that both responses require beta-catenin (Tanigawa et al., 2011).
Based on our data and previous work by others, we favor a model wherein signals from the NPC niche non-autonomously regulate beta-catenin levels in the responding cells independent of Wnt9b ligand concentration (Figure 7C). Specifically, we propose that all cells of the NPC population are exposed to similar levels of UB-produced Wnt9b that is tightly associated with the UB membrane or extracellular matrix which keeps them in a state of renewal. Once these cells come in apposition to the more medullary stroma, they are exposed to a signal that amplifies beta-catenin activity and triggers differentiation. Although a model where a cortical stromal signal antagonizes beta-catenin activity is also consistent with the data produced in this study, previous studies showed that ablation of the stroma by expression of diphtheria toxin resulted in NPC renewal without differentiation (Das et al., 2013; Hum, Rymer, Schaefer, Bushnell, & Sims-Lucas, 2014; Mao et al., 2015; McNeill & Reginensi, 2017; Reginensi et al., 2013). This suggests that the stroma produces a Wnt/beta-catenin agonizing signal that promotes differentiation. The identity of the beta-catenin agonizing signal is still unknown. Although the stroma expresses several Wnts (including Wnt4, Wnt5a, Wnt11), all are expressed at later stages of development and in the deeper medullary stroma which is inconsistent with them playing a role. Further, in the absence of the UB or Wnt9b, neither class of beta-catenin target genes is expressed. If stroma simply produced another Wnt that acted additively with Wnt9b to amplify beta-catenin levels, then upon removal of the UB, NPCs should renew. This is not the case.
Identification of the stromal agonist will be of great interest in future studies focused on nephron endowment, repair, engineering and diseases that affect beta-catenin activity within the NPCs such as Wilms’ tumors. Given that depletion of several genes in the stroma results in a vast array of defects within the NPCs and developing nephrons, we think it is possible if not probable that the stroma produces multiple factors that impact development of the adjacent epithelia and endothelia (Boivin et al., 2015; Das et al., 2013; Hatini, Huh, Herzlinger, Soares, & Lai, 1996; Hum et al., 2014; Levinson et al., 2005; Mao et al., 2015; McNeill & Reginensi, 2017; Ohmori, Tanigawa, Kaku, Fujimura, & Nishinakamura, 2015; Reginensi et al., 2013; Yang et al., 2002).
Methods
Mouse strains
The mouse alleles Wnt9b+/−, Hoxb7-Cre, Rosa-Wnt1 (Carroll et al., 2005), Six2-CreERT2, Six2-Cre, Foxd1-Cre (Kobayashi et al., 2008), CAG-Wnt9b (Kiefer et al., 2012) and Rosa-YFP are all previously described. With the exception of the CAG-Wnt9b line, all have been deposited at the Jackson labs for distribution.
Pax8-YFP Transgenic generation
To generate the Pax8-YFP Bac Transgenic (Pax8-YFPtg), a 166 kb C57BL/6J mouse bacterial artificial chromosome (BAC) from the RPCI23 library was identified that encompassed the entire Pax8 locus. This BAC clone was modified by targeting a Yellow Fluorescent Protein (YFP) coding sequence into the ATG start site of the Pax8 locus. This inserted a YFP fusion protein coding sequence, SV40 polyA signal, and a FRT-flanked Kanamycin resistance gene cassette. Once correctly targeted clones were identified and the selection cassette was removed using FLP recombination. This final modified BAC clone was linearized and prepared for microinjection into CD-1 zygotes. Transgenic founders were obtained and bred to C57BL/6 mice to establish the Pax8-YFP-tg colony. The BAC clone backbone vector (pBACe3.6) was not removed prior to microinjection and thus is retained in the transgenic animals. The Chloramphenicol Resistance (CmR) gene present in the vector is used for genotyping mice carrying the Pax8YFP transgene.
In-vivo mosaic expression analysis
Pregnant mothers carrying potential Six2-CreERT2; Rosa-Wnt1; Wnt9b−/− or Six2-CreERT2; CAG-Wnt9b; Rosa-YFP; Wnt9b−/− were gavaged with 1.5mg/40gm body weight tamoxifen at E10.5 of pregnancy. At E15.5, kidneys of these embryos were dissected, sectioned at 10uM each and performed H&E or immunostained with antibodies against Cited1, GFP, Pan-Cytokeratin or Pax8.
LiCl treatment and PTA Quantification
In the LiCl treatment on wildtype MMs experiment, the two kidneys from a wildtype embryo were cultured either with media or with media containing 7mM, 10mM, 12mM or 15mM concentration of LiCl for 48 hours.
To obtain quantification of PTAs in LiCl treated MMs, each continuous C1qdc2 expressing structure was considered a single PTA. Statistical significance between PTA counts of 10mM and 12mM treatment and 10mM and 15mM treatment was obtained by running Student’s t-test. Two tailed distribution and two samples with unequal variances were chosen as parameters.
Antibody information for immunofluorescence stain
Antibodies against Six2, Cited1, E-Cadherin, Pan-cytokeratin, GFP, Lotus tetragonolobus lectin (LTA), Lef1, Laminin and Amphiphysin were used as previously described (Das et al., 2013). Pax8 antibody (Proteintech, 10336-1-AP for Fig. 3 and Abcam Ab189249 for Fig. S4) was used at 1:700 dilution.
Immunofluorescence on NPCs
Cells were fixed in 4% PFA for 10 min at RT. After fixation, cells were washed 3 times with PBST (PBS without calcium magnesium salts + 0.1% TritonX 100) for 5 min each and blocked for an hour in PBST+10%FBS block. Cells were incubated with primary antibodies at room temperature (Six2 at 1:100, Lef1 at 1:200) for 2 hours then washed 3 times with PBST, blocked again and incubated for an hour with Alexa fluor secondary antibodies. 3 washes with PBST. Incubation of Dapi at 1:1000 for 10 min. 3 washes with PBST.
Ex-vivo culture
Organ culture, metanephric mesenchyme isolation at E11.5 and small molecule treatments (LiCl, CHIR, IWR1 and Iwp2, IwpL6) were performed as previously described (Carroll et al., 2005, Karner et al., 2011). IWR1 containing media was changed every 12 hours and while all other media was changed every 24 hours. All assays were repeated 5 or more times with kidneys/isolated mesenchymes from at least two different litters. After culture, the tissue with the transmembrane filter still attached was fixed in 4% PFA overnight at 4 degrees and washed in PBS 3X5 minutes before proceeding with further analysis.
Histology/Immunofluorescence on tissue section
Hematoxylin and Eosin staining and immunofluorescence on all tissue were performed as described(Das et al., 2013). Antibody information is in SI.
In-situ Hybridization
Whole-mount and section in-situ hybridization were performed as previously described (Das et al., 2013; Karner et al., 2011).
NPC isolation, & culture
NPCs from E17.5 kidneys of wildtype, Six2-Cre;Rosa-Wnt1 or Six2-Cre;CAG-Wnt9b kidneys were isolated and cultured as per published protocol (Brown et al., 2015). Cells were cultured in 24 well plates at 100K/well seeding density and passaged or set up for differentiation at 70% confluency.
Supplementary Material
Highlights.
Wnt/β-catenin is sufficient for nephron progenitor cell renewal and differentiation.
Low levels of Wnt/β-catenin activity promote nephron progenitor renewal.
High levels of Wnt/β-catenin activity promote nephron progenitor differentiation.
Differential β-catenin activity occurs independent of a clear ligand gradient.
Signals from the interstitial microenvironment amplify β-catenin activity.
Acknowledgments
We thank Carroll lab members, HR’s thesis committee and the Thelium Interest Group for useful comments. We thank Mike Buszczak, Ondine Cleaver and Denise Marciano for reading and commenting on this manuscript. This work was supported by a fellowship from CRSM at UTSW to HR and, NIH – DK080004, DK095057, DK106743, DK090127 to TJC, DK054364 to APM, 5F32DK060319 to MTD, DK078161, DK106743 to LO, DK098563 and March of Dimes #6-FY13-127 to MR.
Footnotes
Author Contributions
MTD, TJC and APM generated the Pax8YFP reporter mouse line. JB, LR and MR generated the CAG-Wnt9b mouse line. AC and LO developed the NPC in-vitro culture system and taught us the technique. HR and TJC designed and performed experiments, interpreted the results and wrote the paper. ARF helped with NPC isolations and the related immunofluorescence stains. AD helped with designing and performing experiments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aulehla A, Wiegraebe W, Baubet V, Wahl MB, Deng C, Taketo M, … Pourquie O. A beta-catenin gradient links the clock and wavefront systems in mouse embryo segmentation. Nat Cell Biol. 2008;10(2):186–193. doi: 10.1038/ncb1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin FJ, Sarin S, Lim J, Javidan A, Svajger B, Khalili H, Bridgewater D. Stromally expressed beta-catenin modulates Wnt9b signaling in the ureteric epithelium. PLoS ONE. 2015;10(3):e0120347. doi: 10.1371/journal.pone.0120347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Muthukrishnan SD, Oxburgh L. A synthetic niche for nephron progenitor cells. Dev Cell. 2015;34(2):229–241. doi: 10.1016/j.devcel.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9(2):283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Das A, Tanigawa S, Karner CM, Xin M, Lum L, Chen C, … Carroll TJ. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol. 2013;15(9):1035–1044. doi: 10.1038/ncb2828. ncb2828 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JA, Garrod DR. Induction of early stages of kidney tubule differentiation by lithium ions. Dev Biol. 1995;167(1):50–60. doi: 10.1006/dbio.1995.1006. [DOI] [PubMed] [Google Scholar]
- Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV, … Clevers H. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530(7590):340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10(12):1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- Herzlinger D, Qiao J, Cohen D, Ramakrishna N, Brown AM. Induction of kidney epithelial morphogenesis by cells expressing Wnt-1. Dev Biol. 1994;166(2):815–818. doi: 10.1006/dbio.1994.1360. [DOI] [PubMed] [Google Scholar]
- Hirata A, Utikal J, Yamashita S, Aoki H, Watanabe A, Yamamoto T, … Yamada Y. Dose-dependent roles for canonical Wnt signalling in de novo crypt formation and cell cycle properties of the colonic epithelium. Development. 2013;140(1):66–75. doi: 10.1242/dev.084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JA, Wu CI, Merrill BJ. Tcf7l1 prepares epiblast cells in the gastrulating mouse embryo for lineage specification. Development. 2013;140(8):1665–1675. doi: 10.1242/dev.087387. dev.087387 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hum S, Rymer C, Schaefer C, Bushnell D, Sims-Lucas S. Ablation of the renal stroma defines its critical role in nephron progenitor and vasculature patterning. PLoS ONE. 2014;9(2):e88400. doi: 10.1371/journal.pone.0088400. PONE-D-13-49152 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, … Carroll TJ. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138(7):1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner CM, Merkel CE, Dodge M, Ma Z, Lu J, Chen C, … Carroll TJ. Tankyrase is necessary for canonical Wnt signaling during kidney development. Dev Dyn. 2010;239(7):2014–2023. doi: 10.1002/dvdy.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer SM, Robbins L, Rauchman M. Conditional expression of Wnt9b in Six2-positive cells disrupts stomach and kidney function. PLoS ONE. 2012;7(8):e43098. doi: 10.1371/journal.pone.0043098. PONE-D-12-05949 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer SM, Robbins L, Stumpff KM, Lin C, Ma L, Rauchman M. Sall1-dependent signals affect Wnt signaling and ureter tip fate to initiate kidney development. Development. 2010;137(18):3099–3106. doi: 10.1242/dev.037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125(21):4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3(2):169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132(3):529–539. doi: 10.1242/dev.01604. dev.01604 [pii] [DOI] [PubMed] [Google Scholar]
- Lien WH, Polak L, Lin M, Lay K, Zheng D, Fuchs E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat Cell Biol. 2014;16(2):179–190. doi: 10.1038/ncb2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom NO, Lawrence ML, Burn SF, Johansson JA, Bakker ER, Ridgway RA, … Hohenstein P. Integrated beta-catenin, BMP, PTEN, and Notch signalling patterns the nephron. Elife. 2015;3:e04000. doi: 10.7554/eLife.04000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Francis-West P, Irvine KD. Fat4/Dchs1 signaling between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching. Development. 2015;142(15):2574–2585. doi: 10.1242/dev.122630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H, Reginensi A. Lats1/2 Regulate Yap/Taz to Control Nephron Progenitor Epithelialization and Inhibit Myofibroblast Formation. J Am Soc Nephrol. 2017;28(3):852–861. doi: 10.1681/ASN.2016060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314(5797):298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development. 1997;124(4):871–880. doi: 10.1242/dev.124.4.871. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Tanigawa S, Kaku Y, Fujimura S, Nishinakamura R. Sall1 in renal stromal progenitors non-cell autonomously restricts the excessive expansion of nephron progenitors. Sci Rep. 2015;5:15676. doi: 10.1038/srep15676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Karner CM, Carroll TJ. Myc cooperates with beta-catenin to drive gene expression in nephron progenitor cells. Development. 2017;144(22):4173–4182. doi: 10.1242/dev.153700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, … McMahon AP. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell. 2012;23(3):637–651. doi: 10.1016/j.devcel.2012.07.008. S1534-5807(12)00327-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134(13):2533–2539. doi: 10.1242/dev.006155. dev.006155 [pii] [DOI] [PubMed] [Google Scholar]
- Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, … McNeill H. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9(3):e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi T, Oki S, Kitajima K, Meno C. Epiblast ground state is controlled by canonical Wnt/beta-catenin signaling in the postimplantation mouse embryo and epiblast stem cells. PLoS One. 2013;8(5):e63378. doi: 10.1371/journal.pone.0063378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa S, Sharma N, Hall MD, Nishinakamura R, Perantoni AO. Preferential Propagation of Competent SIX2+ Nephronic Progenitors by LIF/ROCKi Treatment of the Metanephric Mesenchyme. Stem Cell Reports. 2015;5(3):435–447. doi: 10.1016/j.stemcr.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa S, Wang H, Yang Y, Sharma N, Tarasova N, Ajima R, … Perantoni AO. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev Biol. 2011;352(1):58–69. doi: 10.1016/j.ydbio.2011.01.012. S0012-1606(11)00036-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, … Nusse R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13(9):1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moon J, Dodge ME, Pan X, Zhang L, Hanson JM, … Chen C. The development of highly potent inhibitors for porcupine. J Med Chem. 2013;56(6):2700–2704. doi: 10.1021/jm400159c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Blum A, Novak T, Levinson R, Lai E, Barasch J. An epithelial precursor is regulated by the ureteric bud and by the renal stroma. Dev Biol. 2002;246(2):296–310. doi: 10.1006/dbio.2002.0646. S0012160602906469 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.