Abstract
Magnesium’s complete in vivo degradation is appealing for medical implant applications. Rapid corrosion and hydrogen bubble generation along with inflammatory host tissue response have limited its clinical use. Here we electropolymerized a poly (3,4-ethylenedioxythiophene) (PEDOT) and graphene oxide (GO) film directly on Mg surface. GO acted as nano-drug carrier to carry anti-inflammatory drug dexamethasone (Dex). PEDOT/GO/Dex coatings improved Mg corrosion resistance and decreased the rate of hydrogen production. Dex could be released driven by the electrical current generated from Mg corrosion. The anti-inflammatory activity of the released Dex was confirmed in microglia cultures. This PEDOT/GO/Dex film displayed the ability to both control Mg corrosion and act as an on demand release coating that delivers Dex at the corrosion site to minimize detrimental effects of corrosion byproducts. Such multi-functional smart coating will improve the clinical use of Mg implants. Furthermore, the PEDOT/GO/Drug/Mg system may be developed into self-powered implantable drug delivery devices.
Keywords: Magnesium, Conducting polymer, Graphene, Corrosion, On demand release, Dexamethasone
Magnesium (Mg) is a promising material for use in implantable devices largely due to its safe biodegradation.1–4 As the fourth most common cation in the body, Mg2+ takes part in a variety of biologic processes including ATP energy generation and DNA synthesis, and has been shown to promote bone growth and nerve regeneration.5–8 Corrosion of an Mg in the body results in local release of Mg2+, OH−, and hydrogen which can diffuse away provided the corrosion rate is slow.9 While the eventual degradation of Mg is desired, uncontrolled corrosion leads to rapid loss of mechanical properties and hydrogen gas formation and pH change.4,10–12 Excessive gas bubbles and pH change may increase the inflammatory host tissue response and result in poor device tissue integration. Biodegradable polymer coatings that prevent corrosion and release therapeutics to improve tissue integration have been developed.13–15 These coatings may retard corrosion by acting as a barrier layer, but once the polymer starts to degrade, Mg corrosion will proceed unhindered. Furthermore, drug release is passive and does not respond to the local need. To better improve Mg’s clinical potential, a more effective coating that could both control the rate of Mg corrosion and deliver anti-inflammatory therapeutics on demand is desired.
CPs have previously been shown to be corrosion protective on a variety of different metals via mechanisms including barrier function, ennobling, anodic protection, facilitation of a protective oxide formation, shifting of the electrochemical interface and self healing.16–19 On the other hand, 2D nanosheets of graphene may provide corrosion protection due to its excellent barrier function.20,21 Graphene oxide (GO), with additional negative charges from the carboxylic acid groups, can further inhibit the corrosion by preventing aggressive negative ions from reaching the surface.22 GO coatings have been used as barrier layers on Mg alloys following PEO treatment to further improve corrosion resistance.23
Recently, we reported the successful electrodeposition of conducting polymer polyethylenedioxythiophene (PEDOT) and graphene oxide (GO) nanocomposite coating on the surface of Mg. PEDOT/GO coating significantly improved the corrosion resistance of pure Mg and reduced the hydrogel evolution.24 The corrosion protection from PEDOT/GO is attributed to three factors; an initial passive layer preventing solution ingress, buildup of negative charges in the film, and formation of corrosion protective Mg phosphate layer through redox coupling with Mg corrosion. In normal corrosion, the electrons produced by the Mg corrosion are used to reduce water into H2 and OH−. Then the OH− will combine with Mg ions to form Mg OH2, which is unstable and porous. When PEDOT/GO is coated on Mg, the electrons from the Mg oxidation are scavenged by PEDOT to reduce PEDOT instead of water. Without the increase of OH− from water reduction, Mg ions will bind with the more available phosphate to form the corrosion resistant magnesium phosphate passivation layer. We hypothesized that this mechanism of redox coupling can be used for corrosion driven drug release described below.
Drug release from CP through electrochemically controlled redox reactions has been well documented.25–27 During polymerization, the polymer backbone is oxidized requiring negative dopants to provide charge balance. Following polymerization, reduction of the CP removes the positive charge on the backbone releasing the negative dopants. This electrically controlled release provides good temporal control of the drug release rate. Additionally, there are multiple ways in which CP dopant composites can be altered to adjust the release rate.25,28 One mechanism previously reported by our lab is through the addition of GO nanosheets.29 These nanosheets allow for altering both the amount of drug loaded into the film as well as the release rate of the drug. Here, drug loading is accomplished via the π-π stacking of the aromatic ring on the drug molecule and the GO. The amount of drug that is carried into the polymer is dependent on the size of the GO sheets. Additionally, the use of large immobile polyanion such as GO provides the potential to entrap and release neutral and positive species.30,31
Here we report the use of PEDOT/GO coating on Mg to load and release Dexamethasone. Dex is a general steroidal anti-inflammatory that minimizes inflammation around the implant site and has been shown to be effective in a variety of tissues.32–34 We show that Dex can be loaded onto GO sheets and be incorporated into the PEDOT/GO film via electropolymerization. PEDOT/GO/Dex film can control Mg corrosion rate and reduce hydrogen gas formation. Dex release can be controlled via electrical stimulation and that the released drug remains bioactive. Importantly, electrically controlled release systems require a power source and wiring to power the release, which may not be readily available in many implants. We show that by utilizing the redox coupling between Mg and PEDOT, drug release can be driven by the Mg corrosion current, consequently eliminating the need for external power.
Methods
Materials
Single layer graphene oxide sheets were purchased from Cheap Tubes (Cambridgeport, VT). 200 proof ethanol was purchased from Decon Labs Inc. (King of Prussia, PA). Magnesium ribbon ≥99.5% (3 mm wide × 0.2 mm thick), 3,4-ethylenedioxythiphene (EDOT) monomer, and dexamethasone were purchased from Sigma-Aldrich. PBS 10× concentrate (136 mM NaCl, 2.7 mM potassium chloride, 10 mM phosphate buffer) was purchased from EMD Millipore (Billerica, MA) and diluted using de-ionized water from a Millipore Milli-Q system.
PEDOT/GO/DEX film polymerization
Composite films of PEDOT and GO, as well as PEDOT/GO/Dex were polymerized on Mg (20 mm × 3 mm × 0.2 mm, Sigma) and gold (20 mm × 3 mm × 0.1 mm, Ted Pella) strips. Prior to polymerization, samples were sonicated in ethanol briefly then dabbed dry. The polymerization solution was created in two stages. Firstly, 10 mg/ml single layer GO (Cheap Tubes, Cambridgeport, VT) with and without 3.3 mg/ml Dex were added to 200 proof ethanol and sonicated for 30 min. Sonication facilitated loading of the Dex on the GO for those samples with Dex. 35 µl and 20 µl of water and EDOT monomer respectively were then added per ml of the GO/±Dex/ethanol solution and triturated to make the final polymerization solution.
Polymerization was conducted in this solution using a Gamry potentiostat/femtostat on the Gamry Framework software. A two-electrode system was used with the sample to be coated as the working electrode and a gold counter/reference electrode. Polymerization was conducted at 0.6 V vs. Au until a total charge of 10 mC with samples immersed in the solution 9 mm. Following polymerization, samples were dipped in ethanol and stored at −20 °C for 48 hours followed by 2 °C for 24 h Samples were then stored at room temperature until release.
SEM
SEM analysis was conducted using a Jeol JSM 6330F SEM using an accelerating voltage of 3 kV. Samples were analyzed following above drying procedures in addition to an overnight storage in a vacuum desiccator. Coating integrity and surface morphology of coated and uncoated samples were analyzed.
Impedance spectroscopy
A three-electrode system was used to measure EIS with an Ag/AgCl reference, platinum counter, and Mg working electrode. PEDOT/GO and PEDOT/GO/Dex as well as uncoated Mg ribbon samples were immersed 5 mm into PBS for the scan. This ensured only the coated portion of the sample was exposed to the PBS. Samples were allowed to sit in PBS for 5 minutes prior to running EIS analysis. Following the 5 minutes, OCP was recorded for 20 s or until it reached a stability of 0.05 mV/s. EIS was then taken from 100 kHz to 0.1 Hz with an applied potential of 0 V vs. OCP and a voltage amplitude of 10 mV.
Tafel scan
Following EIS, using the same electrochemical cell, Tafel scans of the samples were conducted. Open circuit potential was recorded for 20 seconds prior to the scan. The scan was then run from −0.25 V to +0.25 V vs. OCP.
Hydrogen evolution
PEDOT/GO/Dex coated Mg as well as uncoated Mg samples were mounted in epoxy on glass microscope slides so that 5 mm of the sample was exposed. Samples were then placed in a 2 L crystallization dish that was filled with PBS. 2 ml plastic pipettes were modified so that the tip was closed and the opposite end was flared out. These pipettes were filled with PBS and placed over the corroding samples. Hydrogen evolved from the samples rose up the pipette and collected at the top allowing for quantification of the total evolved hydrogen.
Powered release
Electrochemical release from PEDOT/GO/Dex on Mg was stimulated using the Gamry system with a three electrode system using a Pt counter electrode and Ag/AgCl reference electrode in 3 ml PBS. Samples, immersed in the solution 5 mm, were subject to pulsatile stimulation of −0.25 V vs. open circuit potential (OCP) for 5 s followed by 0 V vs. OCP for 5 s. This stimulus was repeated 10 times. Following stimulation, the absorbance of the release solution was analyzed using SpectroMax M5 plate reader at the characteristic absorbance of Dex 242 nm and the analyzed solution was returned to the release solution. This 10 stimulation paradigm followed by solution analysis was repeated for a total of 50 simulations. The number of stimulations between solution analyses was then increased. The release solution was analyzed after a total of 100, 200, 400, and 600 simulations. Control samples were placed in PBS and measurements were taken at time intervals mimicking the stimulation paradigm but without the stimulation.
Corrosion triggered release
As described before, only 9 mm of the 20 mm long Mg ribbon and Gold strip samples were coated with PEDOT/GO/Dex leaving 11 mm without coating. For passive release experiments, ‘long’ Mg samples were left un-altered while ‘short’ Mg samples had 10 mm of the uncoated portion removed leaving 1 mm of uncoated regions as displayed in Figure 6. Gold strips were kept long and used as non-corrosion control. Long Mg, short Mg and gold samples were completely immersed in 3 ml PBS. Samples of the release solution were taken at 5 min, 15 min, 30 min, 1 h, 2 h, and 3 h and absorbance was analyzed with SpectroMax M5 plate reader at the wavelength of 242 nm.
Figure 6.
Corrosion driven drug release. Dex release from PEDOT/GO/Dex films on Mg where drug release is powered by Mg corrosion. Mg samples had either long or short exposed area but the same amount of coverage by the PEDOT/GO/Dex coating (see schematic). (* P < 0.05).
Bioactivity assessment
Bioactivity of released dexamethasone was tested using previously reported methods.25 A microglia cell line, highly aggressive proliferating immortalized (HAPI) cells, was challenged with a gram negative bacteria derived lipopolysaccharide (LPS) in combination with interferon gamma (IFNɤ). Dex bioactivity was assessed by the drug’s ability to suppress microglia activation and consequently decrease the nitrite production.
HAPI cells (courtesy of Dr. Xiaoping Hu’s lab, University Pittsburgh) were plated at 105 cells/well in a 24 well plate. After about 24 hours cells reached 80% confluence. Culture media was then removed and replaced with media of different conditions. The control media contained 194 µl PBS and 818 µl DMEM F12 and the remaining groups additionally contained (1) released Dex (10.7 µg/ml) released from PEDOT/GO/Dex coatings on Mg, (2) control Dex: as purchased Dex (10.7 µg/ml), (3) LPS (10 µg/ml) + IFNɤ (1 µl/ml), (4) LPS + IFNɤ + released Dex, (5) LPS + IFNɤ + control Dex.
Cells were incubated in the treatment media for 24 h at 37 °C following which culture media supernatant was extracted and added to Griess reagent kit following instructions. Nitrite production was quantified by analyzing this solution with Spectromax M5 Spectrophotometer at an absorbance wavelength of 540 nm. Cells were fixed in with 4% paraformaldehyde for 30 min, stained with DAPI and imaged using a Leica DMI 4000 B inverted fluorescent microscope. The cell number from each well was counted in ImageJ and used to normalize the reading from the Griess assay.
Results
SEM
SEM imaging of the PEDOT/GO/Dex samples revealed a uniform coating on the Mg ribbon sample (Figure 1, A & B). Higher magnification images (Figure 1, C) show a visibly rough surface morphology resulting from the edges of the GO sheets being exposed at the surface.
Figure 1.
PEDOT/GO/Dex Mg morphology. (A) 20× image of PEDOT/GO/Dex coated Mg showing the uniform coating. (B) 100× image of PEDOT/GO/Dex coated Mg. (C) 500× image of PEDOT/GO/Dex coated Mg where the morphology provided by the GO sheets can be seen.
Impedance spectroscopy
Immediately after OCP readings, EIS was recorded to provide additional assessment of corrosion protection afforded by the coating. Nyquist plots for uncoated and coated samples, with and without drug, following the 24 h OCP recording can be seen in Figure 2. The radius of the Nyquist plot is attributed to the corrosion resistance with larger radii indicating higher corrosion resistance.35 PEDOT/GO/Dex films show an increase in the arc radius when compared to uncoated Mg (Figure 2, B). PEDOT/GO only films resulted in a nyquist curves with a 90% greater arc radii that PEDOT/GO/Dex films suggesting that the incorporation of the Dex in PEDOT/GO reduced the barrier function of the coating and provided less effective corrosion prevention (Figure 2, A).
Figure 2.
Electrochemical impedance spectroscopy (EIS). (A) Nyquist plots from PEDOT/GO coatings on Mg. (B) Nyquist plots from PEDOT/GO/Dex coated Mg and Uncoated Mg.
Tafel scan
Tafel scans represent the balance of anodic and cathodic half reactions of the corrosion reaction. Lower corrosion current and positive corrosion potential are both indicatives of increased corrosion resistance. As can be seen in Figure 3, Both PEDOT/GO and PEDOT/GO/Dex films show a positive shift in their corrosion potential. In addition, both coatings show a decrease in corrosion current when compared to the Mg only sample. Similar to the result in Figure 3.2, the PEDOT/GO/Dex films do not show as significant of a positive corrosion potential shift or as dramatic of a decrease in corrosion current when compared to PEDOT/GO coatings. The lower corrosion resistance is most likely due to the addition of the Dex into the polymer creating an imperfect barrier layer.
Figure 3.
Tafel scan. Current output from voltages applied during Tafel scan for PEDOT/GO on Mg, PEDOT/GO/Dex on Mg, and uncoated Mg.
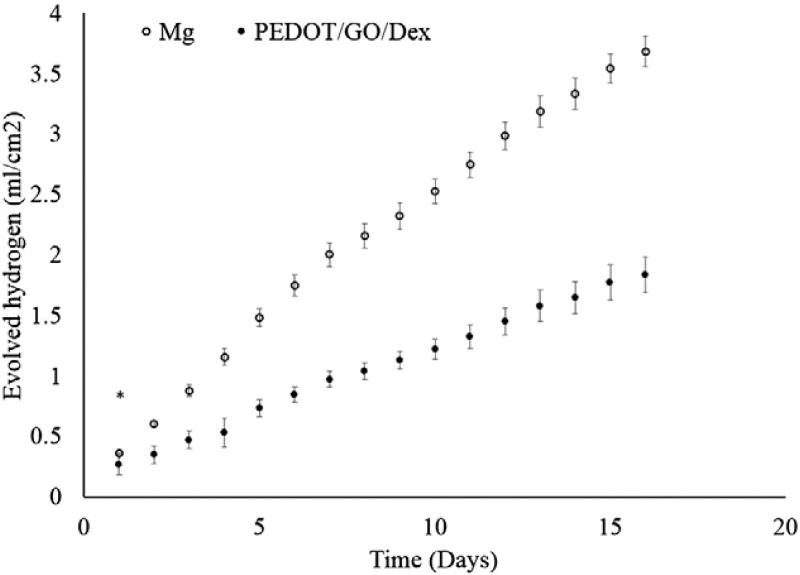
Hydrogen evolution
Evolved hydrogen is the byproduct of Mg corrosion resulting from water reduction. The PEDOT/GO/Dex films resulted in a 51% decrease in the rate of hydrogen evolution (Figure 4). It must be noted that visible delamination of the film was observed on 75% of the PEDOT/GO/Dex samples within the first 24 h. Despite this delamination, the decrease in rate of hydrogen evolution was still significantly lower on the PEDOT/GO/Dex.
Figure 4.
Hydrogen evolution. Evolved hydrogen from PEDOT/GO/Dex coated Mg and uncoated Mg. (* p > 0.05, all other p < 0.05).
Mg corrosion often involves two major redox reactions shown below.
| (1) |
| (2) |
With the presence of electroactive polymer PEDOT, another cathodic reaction is available shown in Eq. (3).
| (3) |
Therefore the electrons produced by oxidation of the exposed Mg (corrosion) may be readily scavenged by the overlaying PEDOT film to cause PEDOT reduction, as opposed to water reduction, which generates H2. This mechanism largely contributes to the reduced hydrogen evolution, and also serves as the fundamental basis of corrosion driven release discussed later.
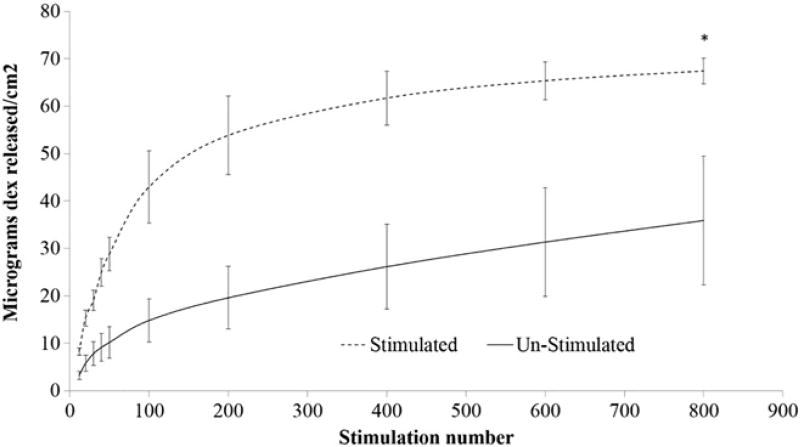
Externally powered drug release
Electrical voltage stimulations were used to release Dex from the PEDOT/GO/Dex films on Mg substrate. As can be seen in Figure 5, the electrical stimulation resulted in Dex release. The release rate is greater for the first 100 stimulations, following which the amount of drug release per 100 stimulations decreased, likely because the initial stimulations lead to drug from superficial layer of the film to release, while the stimulations later had to drive drugs out from deeper layer of the films. Interestingly, there is also a significant release of Dex from the non-stimulated samples that had just been soaked in saline for the same amount of time as their stimulation counterpart. This passive release cannot be simply explained by the diffusion of loosely bound Dex, as the release persisted and did not plateau throughout the experiment. We hypothesize that part of the release especially towards the later time points is driven by corrosion current, which caused electrochemical reduction of the PEDOT/GO/Dex film. Even though the Mg sample was fully coated with the PEDOT/GO/Dex film, corrosion could still occur through pores and imperfections of the film, which allowed Mg to be in contact with water. This corrosion driven drug release is further tested and verified in the section below.
Figure 5.
Powered drug release. Dex release resulting from applied reducing potential on PEDOT/GO/Dex Mg and drug release from PEDOT/GO/Dex Mg without stimulation. (* P > 0.05, all other data points P < 0.05).
Corrosion triggered drug release
To verify that the drug release from PEDOT/GO/Dex on Mg without external electrical stimulation was driven by corrosion, we designed the samples to have same area of Mg coated with the PEDOT/GO/Dex but altered the length of the uncoated region (schematically shown in Figure 6). The samples with greater exposed Mg had a greater release rate than those with only a small portion of Mg exposed. Coated Mg strips with long exposure had an average drug release rate of 3.36 µg/cm2·min for the first 5 min while samples with short exposure had a release rate of 1.5 µg/cm2·min. For the following 10 min long exposed samples had an average drug release rate of 3.19 µg/cm2·min while short exposed samples had a rate of 1.3 µg/cm2 min. At the end of the experiment, the long exposed samples maintained a higher release rate at 1.16 µg/cm2·min while the short exposed samples had a release rate of 0.73 µg/cm2·min. The total release of the long exposed samples was significantly greater than that of the short exposed samples for the initial 30 min of the release experiment. The remaining 2.5 h of the release experiment showed that the long exposed samples trended to release more although not statistically significant. This is firstly due to the stabilization of the corrosion reaction on the exposed Mg regions. Initial time points following immersion see a rapid corrosion of the exposed Mg, while after 1 h the corrosion has begun to stabilize. Additionally at these later time points it is likely that some of the media has diffused through the PEDOT/GO/Dex coating and also begun driving release. Both the long and short exposed Mg has the same total coated area, and therefore the total possible drug release from the two samples would be the same. At later time points the corrosion under the PEDOT/GO/Dex film is likely driving increased release of the short exposed Mg. PEDOT/GO/Dex on gold samples showed virtually no release of Dex.
Bioactivity of released Dex
To confirm the bioactivity of Dex released from the PEDOT/GO coatings, microglia derived HAPI cells were stimulated with LPS and IFNɤ.36 Microglia stimulation with pro-inflammatory LPS and IFNɤ results in the release of a variety of inflammatory products including NO and pro-inflammatory cytokines. Dex quenches the HAPI cell activation and decreases the release of NO through inhibiting expression of inflammatory mediators.37 Microglia stimulated with LPS and IFNɤ are activated to produce a five folds increase in NO production as can be seen in Figure 7 (“LPS IFN Stimulated” group). Treatment with both released and as-purchased Dex at 5 µM suppresses microglia activation to a similar degree (“released Dex + LPS IFN” and “control Dex + LPS IFN”). This indicates that entrapment of Dex in the PEDOT/GO film on Mg and subsequent release did not damage the drug. Additionally, there was no significant difference between HAPI cells exposed to released or as-purchased Dex without stimulation with LPS and IFNɤ (“released Dex” and “control Dex”). This indicates that the PEDOT/GO/Dex samples are not releasing appreciable monomers, macromoers, or GO during the stimulation that could generate confounding biological effect.
Figure 7.
Dex bioactivity. Assessment of Dex bioactivity through quantification of NO generated by HAPI cells due to LPS + IFNɤ stimulation using Griess Assay. n = 4 for each group (* P < 0.05).
Discussion
Here we utilize a PEDOT/GO/Dex composite coating to both control Mg corrosion as well as release bioactive cues in a smart self-powered way. Mg corrosion, while being ideally safe, has shown local tissue damage through increases in pH and gas pockets resulting from hydrogen evolution if corrosion is too fast or if the byproducts cannot be cleared easily. The PEDOT/GO/Dex coating developed here controls corrosion and reduces the production of harmful corrosion products such as H2 gas, which forms pockets preventing tissue integration, as well as OH−, which decreases the local pH. In addition to corrosion protection, when corrosion inevitably does occur, anti-inflammatory drugs can be locally released to minimize detrimental effects on the surrounding tissue. This single layer dual function coating will serve to bring Mg based implants closer to clinical applications.
Polymerizing the PEDOT/GO/Dex film directly on the Mg allows it to act as a corrosion inhibitor. The PEDOT/GO/Dex coating improved corrosion resistance over uncoated Mg as indicated by EIS measurements (Figure 2) and Tafel scan data (Figure 3), albeit not as significant as PEDOT/GO alone. The lower corrosion protection provided by PEDOT/GO/Dex is also noted in the hydrogen evolution experiments (Figure 4) where 75% the PEDOT/GO/Dex films showed film delamination within the first 24 h of immersion in PBS. Despite this delamination, over the following 17 days, the PEDOT/GO/Dex films maintained a decreased rate of hydrogen evolution (Figure 4). This indicates that the electrochemical coupling of the PEDOT/GO/Dex films and the Mg was maintained. This electrochemical coupling provides improved corrosion protection even if some areas of the film begin to delaminate.
The combination of expedited film delamination observed during hydrogen evolution experiments as well as decreased corrosion protection assessed through EIS and Tafel scans indicates that the PEDOT/GO/Dex coating is not as effective at preventing corrosion as the PEDOT/GO coatings without Dex. The Dex is incorporated into the film both by being trapped in the polymer matrix during polymerization, as well as being carried into the film by GO. The incorporation of Dex disrupts the packing of the PEDOT/GO film and leaving pinholes for media to diffuse through. The barrier properties of the film are likely further decreased as the Dex leaves the film, driven out by the external electrical or internal corrosion current.
Corrosion triggered drug release from CPs has been shown previously with active metals wired to the CP acting as a power source. In one study, CP doped with negatively charged phenol red was electrically connected to a zinc anode and both were placed in aqueous media.38 Oxidation of the zinc in water generated negative current that reduced the CP and drove drug release. In another study, external coupling of Mg corrosion for drug release was shown when the corroding Mg was wired to a polypyrrole/Dex coating and both were placed in an aqueous media.39 None of these studies directly adhere CP on the corroding metal, instead the corroding metal was wire connected to the CP film that was deposited on an inert and stable substrate. Controlled release of adenosine triphosphate from CP has been shown where Mg is sputtered onto the polymer film and the corrosion of the thin Mg layer drives the release.40,41 Here however, the Mg was purely sacrificial and served only to power release from the CP.
Different from the above studies, PEDOT/GO/Dex was directly deposited on active Mg using an optimized ethanol based polymerization solution. Conventional aqueous solutions used for electropolymerization of CP cause Mg to rapidly corrode making it impossible to form an adherent coating. After successful deposition of the PEDOT/GO/Dex on Mg, self-powered drug release was achieved. Common CP drug releasing systems often utilize a negatively charged drug that is incorporated during electropolymerization to be associated with the positively charged backbone of the CP. Upon reduction, the CP backbone loses the positive charge allowing the drug to diffuse out of the film. Initially the charged salt form of Dex, dexamethasone 21-phosphate disodium salt was tested as the negative drug as has been done in our previous studies.25,29 It was found that salt Dex induced aggregation of the GO sheets due to shrinking of the electric double layer the keeps the GO sheets suspended.42 To prevent aggregation, polymerization of the PEDOT/GO/Dex films was accomplished using a non-salt form of Dex in ethanol based PEDOT/GO solution.
Here, incorporation of the Dex into the polymer is no longer through electrostatic interaction, but facilitated both by entrapment during the polymerization as well as bonding of Dex with the GO sheets. The binding is possibly facilitated by hydrophobic π-π stacking between the aromatic rings on the Dex and GO as well as hydrogen bonding between hydroxyl groups of Dex and GO. Once attached, the Dex can be carried into the PEDOT film with the negatively charged GO that acts as the dopant.43–46
Application of external voltage stimuli resulted in well controlled Dex release (Figure 5). This externally powered reduction of the PEDOT/GO/Dex film may release the entrapped uncharged Dex through multiple mechanisms. Firstly, reduction can interfere with the π-π stacking of the Dex and GO.47 Here the reduction interferes with the quadrapole established by the aromatic rings which drive π-π stacking.48 Also, reduction may also occur on some of the GO resulting in less hydroxyl groups expressed. This could minimize hydrogen boding between the Dex and GO.44 Both the interference of the π-π stacking and loss of hydrogen bonds eliminate the forces holding the Dex in the film causing it to be released. Additionally, reduction and oxidation of CP films cause significant density changes as ions and solvent are transported in and out of the film.49 Repeated volume change could lead to release drug from CP films,50,51 and may be another mechanism for the release of non-charged Dex from the PEDOT/GO/Dex film.
Drug release was also accomplished without the application of external power, but through the coupling of the PEDOT/GO/Dex coatings and current generated by Mg oxidation. As can be seen in Figure 6, samples with greater area of exposed Mg showed increased total drug release as well as increased drug release rates. Greater amount of exposed Mg providing more surface area for corrosion, and more electrons are therefore generated to reduce the polymer resulting in greater drug release. Self-powered release of anti-inflammatory Dex in the area of Mg corrosion could help combat local tissue response to increased pH and gas pocket formation that can prevent proper healing and tissue interfacing with the device. Since the release amount is coupled with the degree of corrosion, which in turn is correlated to the degree of tissue inflammation, the release here is a truly on demand delivery system. The increase in drug release with increasing exposed Mg did not however linearly correlate with the total exposed surface area. As can be seen, those samples with 1mmexposedMg did not have 11 times less drug release than those samples with 11 mm exposed Mg. This is likely due to the poor barrier properties of the PEDOT/GO once the Dex is added. Consequently, while corrosion is occurring at the exposed Mg, there is also corrosion occurring underneath the PEDOT/GO/Dex film. This corrosion drives drug release and this local corrosion may be more efficient at driving release from the polymer as opposed to the more distant corrosion of the exposed Mg.
Of paramount importance with drug releasing systems is the viability of the drug following release.25 During electropolymerization, Dex is exposed to ethanol and oxidizing potentials that could potentially damage the bioactivity of the Dex released from the PEDOT/GO/Dex coating. The effective quenching of the HAPI cell activation in response to pro-inflammatory stimuli by the released Dex confirms that the bioactivity of Dex in the PEDOT/GO/Dex coating is maintained. This self-powered drug releasing paradigm has the potential to also be used for other drugs such as bisphosphonate molecules for orthopedic implants52 and Sirolimus and Paclitaxel for vascular stents.53 In addition, the drug release rate could be tuned to provide optimal release paradigm for each specific application through sonication of the GO to alter the amount of drug that is loaded into the film as well as the release kinetics.29 Additionally as demonstrated with the long and short exposure samples, it is entirely possible to design patterns of coated and uncoated areas as a mean to adjust the corrosion as well as the drug release rate.
In this study, characterization of the corrosion and drug release was only performed in vitro. It is perceivable that many factors could influence the corrosion and drug release profile when applying the technology in vivo. For example, Mg corrosion can be accelerated or decelerated based on the local perfusion, the water content and biological composition of the tissue and the static and dynamic loads applied to the implants.54 Additionally, protein adsorption on the implant surface and fibrotic encapsulation55 also significantly alter the corrosion rate, which affect the corrosion driven drug release, or directly affect drug release due to barrier function. Future studies must be performed in vivo to assess the efficacy of corrosion protection and drug release in different implant situations.
Finally, it should be pointed out that the novel results presented here could promote the development of stand-alone degradable self-powered drug release device based on conductive polymers grown on Mg. Implantable drug delivery devices have a wide variety of potential applications from cancer treatments to ingestible devices that deliver drug to the complex geometry of the small intestine.56,57 These devices have initially taken advantage of microelectromechanical systems (MEMS) using silicon technology developed for the electronics industry.58,59 However, these silicon based devices suffer from poor biocompatibility and require removal once the therapeutic reservoir is exhausted, or no longer needed.60 The degradability of Mg eliminates the need for removal and drug release rate can be easily tuned by micropatterning the coated and uncoated areas.
In summary, PEDOT/GO/Dex can be successfully deposited on Mg substrates using an ethanol based electropolymerization solution. GO acted as the negatively charged dopant to dope the PEDOT film as well as a drug carrier to load anti-inflammatory drug dexamethasone. The PEDOT/GO/Dex coating provides improved corrosion resistance as well as reduced H2 evolution through both a barrier function and redox coupling. The Dex can be released by electrical stimulation applied to the film. Alternatively, corrosion current was also found to cause drug release without external stimulation. The released Dex maintained the anti-inflammatory property. This dual function smart coating is poised to improve the biocompatibility and functionality of Mg based medical implants.
Acknowledgments
The project described was supported by the National Science Foundation Grants ERC-0812348 and National Institute of Health R01NS062019. Funding source: National Science Foundation Grants ERC-0812348 and National Institute of Health R01NS062019.
Footnotes
Conflict of interest: None.
References
- 1.Atrens A, Song GL, Cao F, Shi Z, Bowen P. Advances in Mg corrosion and research suggestions. J Magnes Alloys. 2013;1:177–200. [Google Scholar]
- 2.Kirkland NT, Birbilis N, Staiger MP. Assessing the corrosion of biodegradable magnesium implants: a critical review of current methodologies and their limitations. Acta Biomater. 2012;8:925–36. doi: 10.1016/j.actbio.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Erbel R, Di Mario C, Bartunek J, Bonnier J, De Bruyne B, Erne F, et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomized multicenter trial. Lancet. 2007;369:1869–75. doi: 10.1016/S0140-6736(07)60853-8. [DOI] [PubMed] [Google Scholar]
- 4.Staiger M, Pietak A, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27(9):1728–34. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Elin R. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010;23(4):s194–8. doi: 10.1684/mrh.2010.0213. [DOI] [PubMed] [Google Scholar]
- 6.Witte F, Kase V, Haferkamp H, Switzer E, Myer-Lindenberg A, Wirth CJ, et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26:3557–63. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 7.Pan Hc, Sheu Ml, Hl Su, Chen Yj, Chen Cj, Yang Dy, et al. Magnesium supplement promotes sciatic nerve regeneration and down-regulates inflammatory response. Magnes Res. 2011;24(2):54–70. doi: 10.1684/mrh.2011.0280. [DOI] [PubMed] [Google Scholar]
- 8.Muir K. Magnesium for neuroprotection in ischaemic stroke. CNS Drugs. 2001;15(12):921–30. doi: 10.2165/00023210-200115120-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kuhlmann J, Bartsch I, Willbold E, Schuchardt S, Holz O, Hort N, et al. Fast escape of hydrogen from gas cavities around corroding magnesium implants. Acta Biomater. 2013;9:8714–21. doi: 10.1016/j.actbio.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Song Gl, Song Sz. A possible biodegradable magnesium implant material. Adv Eng Mater. 2007;9:298–302. [Google Scholar]
- 11.Witte Frank. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6:1680–92. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Witte F, Fischer J, Nellesen J, Crostack HA, Kaese V, Pisch A. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials. 2006;27:1013–8. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Wittchow E, Adden N, Riedmuller J, Savard C, Waksman R, Braune M. Bioresorbable drug-eluting magnesium-alloy scaffold: design and feasibility in a porcine coronary model. EuroIntervention. 2013;8(12) doi: 10.4244/EIJV8I12A218. [DOI] [PubMed] [Google Scholar]
- 14.Campos C, Muramatsu T, Iqbal J, Zhang YJ, Onuma Y, Garcia-Garcia H, et al. Bioresorbable drug-eluting magnesium-alloy scaffold for treatment of coronary artery disease. Mol Sci. 2013;14(12):24492–500. doi: 10.3390/ijms141224492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borck A, Adden N. In: Biocorrodible Implant with a Coating Containing Drug Eluting Polymer Matrix. USPTO, editor. USA: A Borck, N Adden; 2010. [Google Scholar]
- 16.Tallman Dennis, Spinks Geoff, Dominis Anton, Wallace Gordon. Electroactive conducting polymers for corrosion control Part 1: general introduction. J Solid State Electrochem. 2002;6:73–84. [Google Scholar]
- 17.Tallman Dennis, Spinks Geoff, Dominis Anton, Wallace Gordon. Electroactive conducting polymers for corrosion control Part 2. Ferrous metals. J Solid State Electrochem. 2002;6:85–100. [Google Scholar]
- 18.Ohtsuka Toshiaki. Corrosion protection of steels by conducting polymer coating. Corrosion. 2012;2012 [Google Scholar]
- 19.Hien NTL, Garcia B, Pailleret A, Deslouis C. Role of doping, ions in the corrosion protection of iron by polypyrrole films. Electrochim Acta. 2005;50(7–8):1747–55. [Google Scholar]
- 20.Prasai Dhiraj, Tuberquia Juan Carlos, Harl Robert R, Jennings G Kane, Bolotin Kirill I. Graphene: corrosion-inhibiting coating. ACS Nano. 2012;6(2):1102–8. doi: 10.1021/nn203507y. [DOI] [PubMed] [Google Scholar]
- 21.Lih Ellie Teo Yi, Rubaiyi Bt, Zaid Mat, Ling Tan Ling, Chong Kwok Feng. Facile corrosion protection coating from graphene. Chem Eng Appl. 2012;3(6):453–5. [Google Scholar]
- 22.Prabakar Rchard, Hwang Yun-Hwa, Bae Eun Gyoung, Lee Dong Kyu, Pyo Myoung. Graphene oxide as a corrosion inhibitor for the aluminum current collector in lithium ion batteries. Carbon. 2013;52:128–36. [Google Scholar]
- 23.Qiu Zhaozhong, Wang Rui, Wu Jinzhu, Zhang Yushen, Yunfei Qu, Wu Xiaohong. Graphene oxide as a corrosion-inhibitive coating on magnesium alloys. RSC Adv. 2015;5(55):44149–59. [Google Scholar]
- 24.Catt K, Li H, Tracy Cui X. Poly (3,4-ethylenedioxythiophene) graphene oxide composite coatings for controlling magnesium implant corrosion. Acta Biomater. 2016 doi: 10.1016/j.actbio.2016.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Xiliang, Matranga Christopher, Tan Susheng, Alba Nicolas, Cui Xinyan Tracy. Carbon nanotube nanoreservoir for controlled release of anti-inflammatory dexamethasone. Biomaterials. 2011;32(26):6316–23. doi: 10.1016/j.biomaterials.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadhwa Reecha, Lagenaur Carl F, Cui Tracy. Electrochemically controlled release of dexamethasone from conducting polmyer polypyrrole coated electrode. J Control Release. 2006;110:531–41. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Kolarcik C, Catt K, Rost E, Albrecht I, Bourbeau D, Du Z, et al. Evaluation of poly(3,4-ethylenedioxythiophene)/carbon nanotube neural electrode coatings for stimulation in the dorsal root ganglion. J Neural Eng. 2015;12(1):016008. doi: 10.1088/1741-2560/12/1/016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo Xiliang L, Cui Xinyan T. Sponge-like nanostructured conducting polymers for electrically controlled drug release. Electrochem Commun. 2009;11(10):1956–9. doi: 10.1016/j.elecom.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver Cassandra, Larosa Jaclyn, Luo Xiliang, Cui Xinyan Tracy. Electrically controlled drug delivery from graphene oxide nanocomposite films. ACS Nano. 2014;8(2):1834–43. doi: 10.1021/nn406223e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bidan G, Lopez C, Mendes-Viegas F, Vieil E. Incorporation of sulphonated cyclodextrins into polypyrrole: and approach for the electro-controlled delivering of neutral drugs. Biosens Bioelectron. 1995;10(1–2):219–29. doi: 10.1016/0956-5663(95)96808-c. [DOI] [PubMed] [Google Scholar]
- 31.Lien M, Smyrl W, Morita M. Cation and anion insertion in separate processes in poly(pyrrole) composite films. J Electroanal Chem Interfacial Electrochem. 1991;309(1–2):333–40. [Google Scholar]
- 32.Herrero-Vanrella R, Cardillo J, Kuppermann B. Clinical applications of the sustained release dexamethasone implant for treatment of macular edema. Clin Ophthalmol. 2011;5:139–46. doi: 10.2147/OPTH.S15783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickey T, Kreutzer D, Burgess D, Moussy F. In vivo evaluation of dexamethasone/PLGA microsphere system to supress te inflammatory tissue response to implantable medical devices. J Biomed Mater Res. 2002;61(2):180–7. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- 34.Zhong Y, Bellamkonda R. Dexamethasone coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007;1148:15–27. doi: 10.1016/j.brainres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rammelt U, Nguyen P, Plieth W. Protection of mild steel by modification with thin films of polymethylthiophene. Elecrochim Acta. 2001;46:4251–7. [Google Scholar]
- 36.Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor Jr. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- 37.Abraham S, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, et al. Antiinflammatory effects of dexamethasone are partly demendent on induction of dual specificity phosphatase. J Exp Med. 2006;203(8):1883–9. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Whitten P, Too C, Wallace G. A galvanic cell driven controlled release system based on conducting polymers. Sens Actuators B. 2008;129:605–11. [Google Scholar]
- 39.Moulton A, Imisides M, Sheperd R, Wallace G. Galvanic coupling conducting polymers to biodegradable Mg initiates autonomously powered drug release. J Mater Chem. 2008;18:3608–13. [Google Scholar]
- 40.Ge D, Ru X, Hong S, Jiang S, Tu J, Wang J, et al. Coating metals on cellulose-polypyrrole composites: a new route to self-powered drug delibery system. Electrochem Commun. 2010;12:1367–70. [Google Scholar]
- 41.Ru X, Shi W, Huang X, Cui X, Ren B, Ge D. Synthesis of polypyrrole nanowire network with high adenosine triphosphate release efficency. Electrochim Acta. 2011:9887–92. [Google Scholar]
- 42.Gudarzi Mohsen Moazzami. Colloidal stability of graphene oxide: aggregation in two dimensions. Langmuir. 2016;32(20):5058–68. doi: 10.1021/acs.langmuir.6b01012. [DOI] [PubMed] [Google Scholar]
- 43.Bao H, Pan Y, Ping Y, Sahoo Ng, Wu T, Li L, et al. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small. 2011;7(11):1569–78. doi: 10.1002/smll.201100191. [DOI] [PubMed] [Google Scholar]
- 44.Liu Hw, Hu Sh, Chen Yw, Chen Sy. Characterization and drug release behavior of highly responsive electrically modulated reduced graphene oxide-poly(vinyl alcohol) membranes. J Mater Chem. 2012;22:17311–20. [Google Scholar]
- 45.Sun X, Liu Z, Welsher K, Robinson Jt, Goodwin A, Zaric S, et al. Nanographene oxide for cellular imaging and drug delivery. Nano Res. 2008;1(3):203–12. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao C, Wang J, Qin S, Lv Y, Long Y, Zhu H, et al. Fabrication of pH-sensitive graphene oxide-drug supramolecular hydrogels as contorlled release systems. J Mater Chem. 2012;22:24856–61. [Google Scholar]
- 47.Schlosser F, Moos M, Lambert C, Wurthner F. Redox-switchable intramolecular pi-pi stacking of perylene bismide dyes in a cyclophane. Adv Mater. 2013;25:410–4. doi: 10.1002/adma.201201266. [DOI] [PubMed] [Google Scholar]
- 48.Sinnokrot Mutasem Omar, Valeev Edward F, Sherrill C David, et al. Estimates of the ab initio limit for π−π interactions: the benzene dimer. J Am Chem Soc. 2002;124(36):10887–93. doi: 10.1021/ja025896h. [DOI] [PubMed] [Google Scholar]
- 49.Suárez Marco F, Compton Richard G. In situ atomic force microscopy study of polypyrrole synthesis and the volume changes induced by oxidation and reduction of the polymer. J Electroanal Chem. 1999;462(2):211–21. [Google Scholar]
- 50.Chen X, Xing Kz, Inganas O. Electrochemically induced volume changes in poly(3,4-ethylenedioxythiophene) Chem Mater. 1996;8:2439–43. [Google Scholar]
- 51.Abidian M, Kim D-H, Martin D. Conducting-polymer nanotubes for controlled drug release. Adv Mater. 2006;18(4):405–9. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cattalini Jp, Pharm M, Boccaccini Ar, Habil I, Lucangioli S, Mourino V. Bisphosphonate-based strategies for bone tissue engineering and orthopedic implants. Tissue Eng Part B. 2012;18(5):323–40. doi: 10.1089/ten.teb.2011.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burt H, Hunter W. Drug-eluting stents: a multidisciplinary sucess story. Adv Drug Deliv Rev. 2006;58:350–7. doi: 10.1016/j.addr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Witte F, Fischer J, Nellesen J, Crostack HA, Kaese V, Pisch A, et al. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials. 2006;27(7):1013–8. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 55.Witte F, Ulrich H, Rudert M, Willbold E. Biodegradable magnesium scaffolds: Part 1: appropriate inflammatory response. J Biomed Mater Res A. 2007;81(3):748–56. doi: 10.1002/jbm.a.31170. [DOI] [PubMed] [Google Scholar]
- 56.Bettinger C. Materials advances for next-generation ingestible electronic medical devices. Trends Biotechnol. 2015;33(10):575–85. doi: 10.1016/j.tibtech.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 57.Mahnama A, Nourbakhsh A, Ghorbaniasl G. A survey on the applications of implantable micropump systems in drug delivery. Curr Drug Deliv. 2014;11:123–31. doi: 10.2174/156720181101140212165729. [DOI] [PubMed] [Google Scholar]
- 58.Nisar A, Afzulpurkar N, Mahaisavariya B, Tuantranont A. MEMS-based micropumps in drug delivery and biomedical applications. Sens Actuators B. 2008;130:917–42. [Google Scholar]
- 59.Shawgo R, Grayson A Richards, Li Y, Cima M. BioMEMS for drug delivery. Curr Opin Solid State Mater Sci. 2002;6(4):329–34. [Google Scholar]
- 60.Park H, Park K. Biocompatibility issues of implantable drug delivery systems. Pharm Res. 1996;13(12):1770–6. doi: 10.1023/a:1016012520276. [DOI] [PubMed] [Google Scholar]