Abstract
The marginal zone (MZ) is largely composed of a unique subpopulation of B cells, the so-called MZ-B cells. At a molecular level, memory B cells are characterized by the presence of somatically mutated IGV genes. The earliest studies in the rat have documented the presence of hapten-specific MZ-B cells after immunization in the MZ. This work later received experimental support demonstrating that the IGHV-Cµ transcripts expressed by phenotypically defined splenic MZ-B cells (defined as CD90negIgMhighIgDlow B cells) can carry somatic hypermutation. However, only a minor fraction (< 10%–20%) of these MZ-B cells is mutated and is considered to represent memory B cells. Memory B cells can either be class-switched (IgG, IgA, IgE), or non–class-switched (IgM) B cells. B cells in the MZ are a heterogeneous population of cells and both naïve MZ-B cells; class switched and unswitched memory MZ-B cells are present at this unique site in the spleen. Naïve MZ-B cells carry unmutated Ig genes, produce low-affinity IgM molecules and constitute a first line of defense against invading pathogens. Memory MZ-B cells express high-affinity Ig molecules, directed to (microbial) antigens that have been encountered. In this review, we report on the memory compartment of splenic MZ-B cells in the rat to provide insights into the origin and function of these memory MZ-B cells.
Keywords: memory B cells, marginal zone (MZ), immunoglobulin heavy (IGH) chain genes, spleen, rat
I. INTRODUCTION
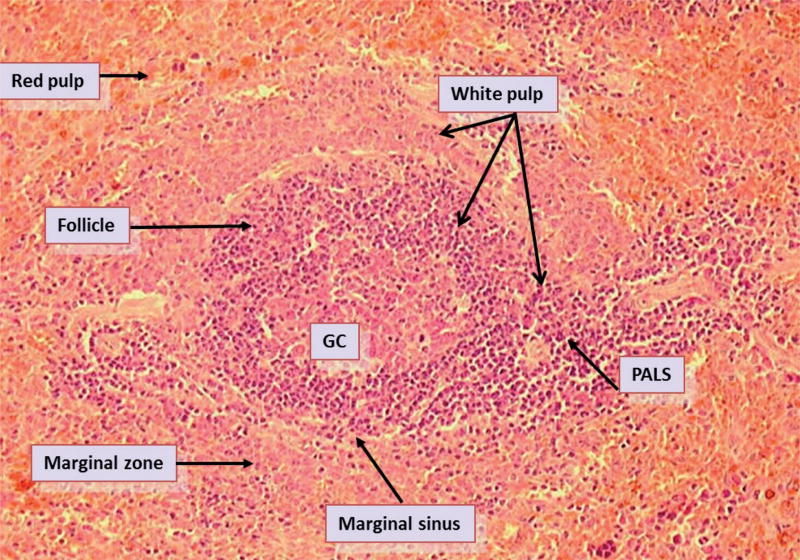
The marginal zone (MZ) is a well-defined anatomical compartment that encloses the follicles and periarteriolar lymphocyte sheaths (PALS) (Fig. 1). Together, these structures form the so-called “the white pulp,” which contains mostly lymphocytes. T and B cells are predominantly, but not exclusively, located in their own distinct compartments, the periarteriolar lymphocyte sheaths (PALS) and follicles, respectively. The follicles contain recirculating B cells that are in search of their appropriate antigen. Most B cells in these follicles are naïve IgM− and IgD−expressing B cells called follicular B (FO-B) cells. Upon antigenic stimulation with protein antigens, areas of proliferating B cells can be found within these follicles, the so-called germinal centers (GCs). The GCs are sites where memory B cells are generated, a process that is associated with class switch recombination (CSR) and somatic hypermutation (SHM) of the immunoglobulin (Ig) genes.1 Although the classical mechanism of CSR involves direct switching from IgM to any other class (isotype) of Ig molecule, CSR can also occur sequentially from IgM via IgG to IgA2 or from IgG to IgA.3 Somatic hypermutation is the process whereby point mutations are introduced in the variable (V) region of Ig genes of B cells. The MZ forms an interface with the red pulp, which is very rich in venous sinuses. In rats and mice (Fig. 1) but not in humans, the MZ is separated from the WP by the marginal sinus. This sinus is very porous due to gaps in the endothelium that lines the sinusoids, which allows the transit of lymphocytes and dendritic cells (DCs) from the circulation to enter the WP. The MZ is largely composed of a unique subpopulation of B cells, the so-called MZ-B cells, in addition to nonlymphoid cells such as MZ macrophages (MZMs), marginal metallophilic macrophages (MMMs), and DCs. Because of their anatomical location at the border of the red pulp, they have a rich supply of capillaries that are widely open.4 MZ-B cells are thought to play an important role in rapid immune responses against bloodborne pathogens. In particular, they are believed to respond rapidly to polysaccharide antigens (TI-2 antigens), which are present on the surface of encapsulated bacteria such as pneumococci and meningococci.5 Thus, MZ and MZ-B cells in particular may play an important role in the prevention of sepsis.
FIG. 1.
Histological structural organization of the rat spleen. The spleen is divided into red pulp (RP) and white pulp (WP) regions. The WP are further divided into B and T lymphocyte regions including the follicles, marginal zone and periarteriole lymphatic sheath (PALS). Visible in the WP are a secondary follicle i.e., a follicle containing a germinal center. The marginal zone (MZ) forms an interface between the RP and the WP. The MZ is further separated from the follicle and PALS by the marginal sinus.
II. MARGINAL ZONE B CELL PHENOTYPES
Marginal zone B cells have been described as a distinct cell type with unique cell-surface phenotype and physiological functions.6 In rodents, MZ-B cells are IgMhighIgDlowCD21highCD23low cells that distinguish them from the majority of naïve, recirculating, FO-B cells in these animals: IgMlowIgDhighCD21intermediate CD23high. In rats, MZ-B cells appear to be derived from (mature) FO-B cells.7 In mice, MZ-B cells also characteristically express high levels of CD1d, a MHC class-I like molecule that is likely involved in presenting lipid molecules to NK cells.8 MZ-B cells in rats can be further discriminated from FO-B cells by their low levels of CD45R, recognized by the HIS24 monoclonal antibody9 and high levels of binding to the monoclonal antibody HIS57 directed to an undefined molecule.7 In rats, CD90 (Thy-1) distinguishes immature B cells from mature B cells10; mature MZ-B cells lack CD90. Human MZ-B cells are IgMhigh and IgDlow.11 Importantly, human MZ-B cells also express CD27.12 CD27 is a member of the TNF-receptor family, and in humans it is expressed by memory B cells.12 Klein et al.12 observed that not only class switched B cells express CD27, but that in human peripheral blood also IgM+IgD+CD27+ B cells are present in large numbers. Studies by Weller et al.13 and Arakawa et al.14 revealed that these IgM+IgD+CD27+ B cells in blood have a gene expression profile similar to splenic MZ-B cells, suggesting that peripheral blood IgM+IgD+CD27+ B cells may represent circulating MZ-B cells.13,14 In humans, these IgM+IgD+CD27+ B cells in the blood are also called natural effector cells.15 In line with the notion that the IgM+IgD+CD27+ B cells are circulating MZ-B cells is the observation that numbers of IgM+IgD+CD27+ B cells in blood are reduced after splenectomy and are almost completely undetectable in children younger than 2 years of age, when mature MZ-B cells are also absent in spleen.16 This absence of MZ-B cells in very young children is associated with a reduction in antibody responses to encapsulated bacteria.
III. DEVELOPMENTAL ORIGIN OF MARGINAL ZONE B CELLS
Naïve MZ-B cells are antigen-inexperienced cells that express germline (unmutated)-encoded immunoglobulin variable (IGV) region genes, whereas memory B cells are antigen-experienced cells that carry somatically mutated IGV region genes that are selected during the humoral immune responses by the immunizing antigen. Both naïve and memory B cells are present in the MZ, but their relative frequencies appear to be different dependent upon the species. In rodents, the vast majority (> 80%) of B cells located in the MZ express IgM encoded by unmutated IGHV region genes, whereas in humans only a small percentage of B cells present in the MZ express unmutated IGHV genes.17,18 Thus, these cells likely represent naïve, IgM+ MZ-B cells. In rats, MZ-B cells appear to be derived from (mature) FO-B cells.19,20 In the mouse, newly generated immature B cells are called early transitional B cells. Three populations, designated T1, T2, and T3 cells, have been identified in this species,21 and T2 cells can subsequently become FO-B cells or MZ-B cells.22 Not all FO-B cells (rats) or T2 B cells (mice) develop into MZ-B cells. Naïve MZ-B cells have a somewhat shorter H-CDR3 region compared to FO-B cells in rats17 and mice.18 Apparently, there is some form of selection of B cells that may enter the MZ-B cell pool. Dammers et al.17 have speculated that in rats more tonic signaling of the BCR of MZ-B cell precursor cells would result in positive selection of these B cells into the MZ-B cell compartment. They also reasoned that this enhanced tonic signaling might be the consequence of shorter H-CDR3 region, which leads to multireactivity and, hence, to more (low-affinity) binding of autoantigens to the BCR.23 As mentioned above, in mice there is a checkpoint for cells to become either FO-B cells or MZ-B cells at the T2 cell stage. Also, Pillai et al.24 argue that signaling strength determines which B cell subset the cells develop into, but in contrast to rats, they reasoned that reduced tonic signaling results in MZ-B cells. Different signaling proteins such as Btk (Bruton’s tyrosine kinase), Aiolos (a zinc finger protein of the Ikaros family), MINT (MSH-homeoboxhomologue 2-interacting nuclear target), neurogenic locus notch homolog protein 2 (Notch2) and protein tyrosine kinase (Pyk-2) contribute to BCR signaling in the development of either FO-B cells or naïve MZ-B cells in mice.25,26 Sufficient stimulation by (self) antigens allows BCR signaling via activation of the Btk pathway to induce FO-B cell development, whereas lower stimulation induces MZ-B cell development.24 Aiolos is a negative regulator of Btk; therefore, the absence of Aiolos will enhance BCR signaling. Consequently, precursor B cells preferentially develop into FO-B cells at the expense of MZ-B cells, which are significantly reduced in Aiolos−/− mice.27 Notch-2 signaling has been shown to be indispensable for development of MZ-B cells. In mice with a conditionally targeted deletion of Notch-2 mice, their T2 precursor cells and MZ-B cells do not develop.28 The work of Saito et al.28 has also shown that Notch-2 are preferentially expressed on mature B cells, whereas Descatoire et al.29 have shown that Notch-2 ligand Delta-Like1 expression on precursor MZ-B cells results in the development of MZ-B cells. MINT acts as a negative regulator of Notch-2, and MINT-deficient B cells differentiate more effectively into MZ-B cells.30 Studies by Astier et al.31 revealed in both transformed and normal human B cells that Pyk-2 phosphorylation is induced by integrins and BCR ligation. Pyk-2–deficient mice fail to develop into MZ-B cells; therefore, Pyk-2 is also considered to be important in the development of MZ-B cells.32 Together, these data support the hypothesis that variable levels of BCR-mediated activation are required for either FO-B or MZ-B cell development. Because MZ-B cells from neonatal and germfree animals already express shorter H-CDR3 regions, we also speculate that endogenous (auto) antigens are involved as ligand in this (positive) selection of naïve MZ-B cells.23
IV. FUNCTION AND ACTIVATION OF MARGINAL ZONE B CELLS
MZ-B cells can respond to both T cell-independent (TI) antigens and T cell-dependent (TD) antigens.33 MacLennan et al. were the first to propose that MZ-B cells in rats are involved in immune responses directed against Type 2, T cell-independent (TI-2) antigens.34 Upon the engagement of antigens through their B cell receptor (BCR) and/or in cooperation with either the CR CD2135 or toll-like receptors (TLRs),36 MZ-B cells can become activated. The activation of MZ-B cells can be enhanced by macrophages and dendritic cells in the MZ through the secretion of cytokines such as BAFF (B cell activating factor) and APRIL (a proliferation inducing ligand).37 Human splenic MZ also contain IgG+ cells.38 Uniquely, these IgG+ MZ-B cells can be synergistically stimulated by IL-21 and BAFF, a member of the TNF family, to induce B-lymphocyte–induced maturation protein-1 (BLIMP-1) in the absence of any further costimulation.38 BLIMP-1 is an essential transcription factor for the differentiation of B cells to plasma cells. In vitro assays have shown that IL-21 and BAFF are secreted respectively by CD4+ T cells39 and dendritic cells (DCs).40 Thus, Ettinger et al.38 speculated that IgG+ MZ-B cells contribute to serological memory in an antigen-independent fashion. Studies by Balazs et al.41 showed that blood-derived neutrophils and DC carrying bacterial cargo can interact with splenic MZ-B cells. Puga et al.42 implicated the involvement of neutrophils to assist B cells in the clearance of TI-2 antigens. These authors observed that neutrophils exclusively present in the spleen stimulate IgM production to TI-2 antigens, such as LPS, and even do so better than MZM or DCs and are as effective as CD4+ helper T cells. Furthermore, they showed that neutrophils stimulate MZ-B cells to upregulate the expression of activation-induced deaminase (AID), a different class (isotype) of switched transcripts, and they showed that in the presence of neutrophils, MZ-B cells accumulate SHM. In conclusion, neutrophils activate MZ-B cells via BAFF, APRIL, and IL-21 to make antibody responses to LPS.42 A newly defined subset of ILCs has been identified in the splenic MZ by Magri et al.43 Several subsets of innate lymphoid cells (ILC) can be discriminated based on their cytokine secretion profiles.44 Magri et al. showed that these MZ-related ICLs activate MZ-B cells through BAFF, the ligand of the costimulatory factor CD40 (CD40L) and notch-2 ligand Delta-Like 1 (DLL1) molecule. They further showed that these ICLs amplified the response of MZ-B cells by activating neutrophils through granulocyte macrophage-colony stimulating factor (GM-CSF). Importantly, the depletion of ICLs results in the impairment of TI antibody responses and reflects the involvement of ILCs in MZ-B cell responses against TI bloodborne particulate antigens. IL-7 is required for the development of ILCs.45 Importantly, work by Willems et al.46 using IL-7 deficient mice has demonstrated that IL-7 signaling is required in the development of the intrinsic MZ-B cell function to rapidly induce IgM production against polysaccharide antigens, providing additional evidence that ILCs are involved in MZ-B cell responses. Activation of MZ-B cells induces their migration from the MZ. Either they shuttle between the MZ and follicular areas,47 or they proliferate and differentiate to plasmablasts, leading to the generation of extrafollicular foci.48 It is possible that the type of antigens (i.e., TI antigens or TD antigens) might be responsible for diverting the development of activated MZ-B cells into either the follicular or the extrafollicular pathway.48 Antigens can stimulate the exit of MZ-B cells from MZ by inducing the downregulation of SIP1 and SIP3 and by the upregulation of chemokine receptor CXCR5.47,49 The expression of CXCR5 allows MZ-B cells to be attracted along a gradient induced by chemokine CXCL13 produced by follicular dendritic cells (FDCs) in the follicles. When MZ-B cells bind either to TD antigens50 or to TI antigens51 with their BCR in combination with crosslinking to the complement receptor CD21 (as part of the BCR coreceptor), they become permissive to a cognate interaction with CD4+ T cells at the T–B cell border (outer PALS) in the spleen. Thereafter, they can proliferate and produce an antibody response,50 forming extracellular foci, or they can further proliferate inside the follicles to form germinal centers (GCs). Possibly, TI antigens stimulate MZ-B cells to proliferate and differentiate to become plasmablasts at extracellular foci, whereas TD antigens most likely cause the migration of MZ-B cells into the follicles to generate GCs. Although a role of MZ-B cells in the generation of plasmablasts or cells is well known, their capacity to generate GCs is less well understood. The work of Song and Cerny52 shed some light on this aspect. They provide experimental evidence showing that MZ-B cells are capable of forming GCs, albeit with a delay in comparison to FO-B cells. However, the signals that determine either divergence into the GC independent (i.e., extrafollicular foci) or GC dependent pathway remain unclear.
V. MEMORY B CELLS
Both naïve and memory B cells are present in the MZ, but their relative frequencies appear to be different dependent upon the species. In rodents, the majority (> 80%) of the B cells located in the MZ express IgM encoded by unmutated IGHV region genes, whereas in humans, only a small percentage of B cells present in the MZ express unmutated IGHV genes.53,54 Thus, these cells likely represent naïve, IgM+ MZ-B cells. In contrast to naïve B cells, memory B cells are antigen experienced cells, which frequently express non-IgM isotypes on their membrane, which are encoded by mutated IGV genes. The presence of somatic hypermutations (SHM) in the IGV regions of Ig genes is a the primary hallmark of memory B cells. Mutations are introduced both in the Ig heavy-chain (IGH) region genes and in the Ig light-chain V region genes during humoral immune responses when antigen-activated B cells expand in a T cell-dependent (TD) fashion in the GCs. Memory B cells provide the host with long-term protection against pathogens and launch rapid humoral immune responses upon secondary stimulation. In rats and mice, a minor fraction (10%–20%) of the MZ-B cells carries SHM IGV genes, and therefore, these cells are considered to represent memory B cells.53,54 Memory B cells can either be class-switched or non–class-switched B cells. Early experiments by Liu et al.50 in rodents have provided evidence that GC-derived memory antigen-specific B cells can colonize the MZ. This finding was further supported by the work of Gatto et al.55 who observed QB-specific IgG expressing B cells after immunization with viral QB capsid that remain associated with the MZ in mice.
In contrast to rodents, in humans, the proportion of mutated B cells in the MZ is much larger; more than 90% of the B cells carry mutated Igs.56 Furthermore, in adults, as mentioned before, nearly all splenic MZ-B cells express CD27,57 which is a marker for mutated (memory) B cells.12 The origin of these CD27+ MZ-B cells in humans is hotly debated in the literature. On one hand, Seifert et al.58 consider these cells as bona fide GC-derived memory B cells, whereas Weill et al.11 proposed that these cells mutate their Ig receptor outside the GC during their generation. This conclusion was largely based upon the observation that the IGHV genes of MZ-B cells are also mutated in patients with hyper IgM type I syndrome.59 Hyper IgM type I patients are characterized by a mutation in the CD40L gene and therefore do not express functional CD40L protein. These patients cannot generate GC or produce class-switched Ig molecules. Because these patients lack GCs but still have MZ-B cells, the authors proposed that CD27+ MZ-B cells are apparently not generated inside GCs and therefore are distinct from the classical GC-derived memory B cells. The authors postulate that mutations are introduced during their generation, outside the GCs in a T cell-and antigen-independent fashion and that the reason for these mutations is to diversify their primary Ig repertoire. Although GCs are certainly the main site for generation of memory B cells,60 there is also evidence from studies in mice that memory B cells can develop outside GCs.61 Toyama et al.62 showed that memory B cells can be generated in Bcl-6–deficient mice. Bcl-6 is a transcriptional repressor that regulates lymphocyte differentiation during immune responses into memory B cells and is particularly highly expressed in GC B cells.63 Although these mice completely lack GCs, their B cells were able to differentiate into IgM and IgG1 memory B cells. In addition, CD40-deficient mice that lack GC formation revealed normal IgG and IgM responses to TI antigens.64 These findings have led to the proposal that some memory B cells might be formed independently of GCs. Also, memory toward TD antigens can be GC independent, as shown by Kaji et al.65 However, these IgG memory B cells having hallmarks of memory cells (i.e., antigenic experienced B cells that express a class-switched isotype) are unmutated, most likely indicate that mutated memory B cells are primarily generated in GCs.65
VI. IGM EXPRESSING MARGINAL ZONE MEMORY B CELLS
Previous experiments provide evidence for the existence of mutated, IgM expressing, memory MZ-B cells in rat.66 Dammers et al. demonstrated that less than 20% of the MZ-B cells isolated from spleens of PVG rats carried mutated IGHV genes. These findings contrasted markedly with findings in humans, where > 95% of the splenic MZ-B cells are mutated.56,57,67 Two possible explanations for this difference are that only one particular IGHV gene family (viz., the IGHV5 family, the homologue of PC7183 in the mouse) has been analyzed in the (PVG) rat and that this IGHV gene family is not representative for other IGHV genes, or IGHV gene families. By establishing the genomic germline IGHV gene repertoire of the BN rat68 as illustrated in Fig. 2, it became possible to accurately analyze other IGHV gene families as well. In addition, it has helped to avoid possible strain differences, as previous analysis was done on the PVG rat strain.66 The analysis was confined on the frequency of mutated sequences in rearranged IGHV-Cµ transcripts derived from FACS sorted MZ-B cells (IgMhighIgDlow) in comparison with FO-B cells (IgMlowIgDhigh) from three different IGHV gene families, which differ in size: IGHV3, IGHV4, and IGHV5.69 These three IGHV gene families have 4, 2, and 26 functional IGHV genes, respectively.68 The IGHV3 and IGHV4 genes families were chosen to determine whether there is a difference in mutation frequencies among members of IGHV gene families that are relatively small and to compare this frequency to the second-largest IGHV gene family (IGHV5) in the rat, which had also been analyzed previously in the PVG rat.53 The BN rat strain contains 26 functional IGHV5 (germline) genes compared to the 28 germline genes in the PVG rat. As expected, splenic MZ-B cells express a significantly higher percentage of mutated sequences than FO-B cells, and all three analyzed IGHV gene families contributed to this difference. In BN rats, a slightly higher proportion (27%)69 of the MZ-B cells expressed mutated IgM molecules encoded by IGHV5 family genes, compared to this proportion in the PVG rat (10%–20%).53 This difference in mutation frequency might be due to the strain differences that exist between the PVG and BN rat strain (e.g., BN rats have fewer IGHV genes) or might be caused by different environmental conditions (e.g., microbial environment or different microbiota) of the two rat strains. Analysis of the IGHV3 gene family showed a similar proportion (~ 30%).
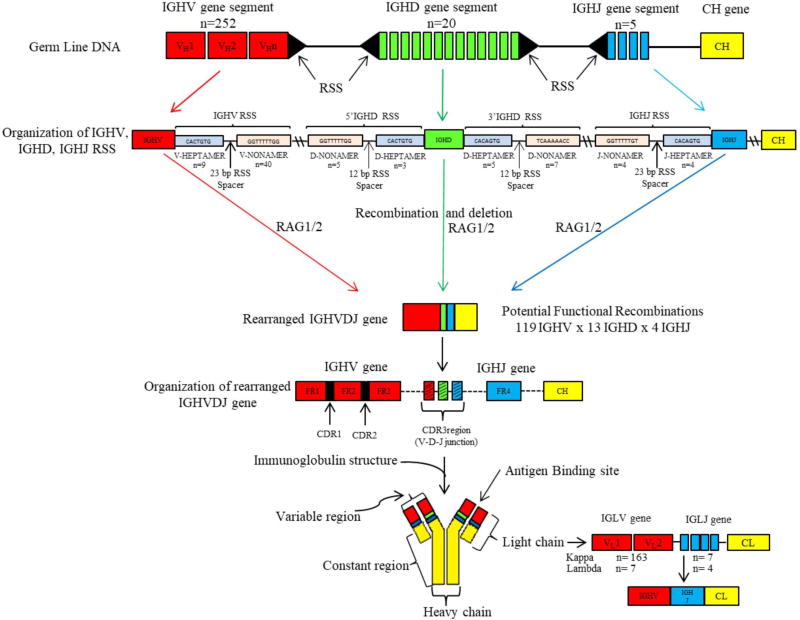
FIG. 2.
Structural organization of IGH and IGL in the rat. The above diagram demonstrates the organization of IGH gene segments position on chromosome 6q32-33 on the IGH locus of the Brown Norway (BN) rat. The IGH locus consists of an IGH variable region and heavy constant (CH) region. IGH variable region is grouped into three germline elements: IGHV, IGHD, and IGHJ gene segments respectively. Also depicted in the above figure are the recognition signal sequenced (RSS) flanking each of IGHV, IGHJ on one side, and IGHD on both sides. RSS consist of conserved heptamer and nonamer sequences, separated from each other by either a 12 or a 23 base-pair (bp) RSS spacer. Recombination of germline IGHV, IGHD, IGHJ (VDJ rearrangement) gene segments follow a 12/23 rule, i.e., VDJ rearrangement occurs only between a 12 bp RSS spacer and a 23 bp RSS spacer. Recombination activating Genes (RAG1/2) bind to RSS’s initiating VDJ rearrangement that results in the sequential random joining of a single gene segment of IGHD to IGHJ and IGHV to IGHDJ forming a rearranged IGHVDJ gene that encodes for the variable part of the immunoglobulin molecule. The IGHV gene is further arranged into CDR1 and CDR2 that are separated from each other by conserved framework regions (FR1, FR2, FR3). FR4 is completely encoded by the IGHJ gene. The CDR3 (junctional) region is the product of VDJ rearrangement. CH chain genes are separately joined by splicing to the rearranged IGHVDJ gene. The total number of gene segments, heptamer and nonamer sequences are designated by “n.”
In marked contrast to these two IGHV gene families, a very high proportion (66%) of the IGHV4 sequences from purified MZ-B cells was mutated.69 This family consists of only two potential functional IGHV genes, but only one of these members appeared to be functionally expressed. These findings show that the proportion of mutated sequences derived from MZ-B cells varies between the different IGHV gene families in the BN rat and that, in total, a higher proportion (27%–66%) of IGHV genes was mutated compared to the (10%–20%) of mutated sequences found previously for the IGHV5 gene family in PVG rats.53,54 The observation that the highest percentage of mutated frequencies occurred in the single functional member IGVH4 gene family suggests that there could be more antigen selection pressure on this particular IGHV4 gene in expanding its available repertoire by SHM. Although a higher average number of mutated sequences among rat MZ-B cells was observed overall than previously, the frequency of mutated sequences among human MZ-B cells is still much higher. In humans, nearly all MZ-B cells are mutated.56,57,67 The variation in frequency of mutated IGHV genes between the different IGHV families in rats may also contribute to the difference in mutated MZ-B cells between humans and rats because in humans the analysis of mutated IGHV genes has been restricted to a restrictive set of IGHV genes. Dunn-Walters et al.56 analyzed only two particular IGHV genes: the IGHV6 gene and IGHV4.21 gene. It is possible that these IGHV genes are more mutated than other genes. However, the analysis by Tangye et al.57 proved that Ig genes isolated from IgM+ memory B cells among IGHV5 and IGHV6 gene families were all mutated. They also showed that the high frequency of mutations is not only due to individual IGHV genes. Furthermore, Colombo et al.67 investigated and compared the presence of mutations in human IGHV1, IGHV3, and IGHV4 gene families among spleen-derived MZ-B cells (IgMhighCD27+), GC B cells, and class switched B cells. In addition, they found that most of the MZ-B cells were mutated, albeit with a lower average number of mutations than both GCs and class-switched B cells. However, the average number of mutations in human MZ-B cells (11.8)67 is higher for both rat IgM+ MZ-B cells (8.8) and rat IgG+ MZ-B cells (7).70 This might be because humans have fewer functional IGHV genes than rats. We postulate that the higher number of germline IGHV genes in rodents consequently require fewer mutations to diversify their antibody repertoire after immunization than humans because rats can encode for a larger pool of different antibodies for their primary repertoire. In addition, it is possible that differences in life span and environmental conditions also contribute to differences in average mutation frequency per IGHV gene. During their (long) lives, humans may encounter much more different antigens than laboratory rats that live in well-controlled laboratory conditions.
Memory cells are generally believed to be generated in GCs. However, whether mutated (memory) IgM+ MZ-B cells are derived from GCs or whether they represent a GC-independent B cell population remains controversial. In humans, Colombo et al.67 also observed a small number of clonally related sequences that were shared between MZ-B cells and GC B cells, which indicates that mutated IgM+ MZ-B cells can be derived from GCs. In contrast, Weill et al.11 and Weller et al.71 suggested that the (mutated) IgM+ MZ-B cells are not GC-derived memory B cells. Instead, these authors postulated that the mutations in human MZ-B cells are acquired during their development to diversify their primary repertoire in a GC- (and T cell-) independent fashion. To test this hypothesis in rats, the possible presence of mutated IgM+ MZ-B cells was investigated in neonatal rats.69 Neonatal rats do not develop GC in the first weeks of their life.72 Thus, MZ-B cells that are unmutated in neonatal rats strongly argue against the hypothesis of Weill et al.11 and Weller et al.,71 in which SHM is part of the developmental program of MZ-B cells.11,71 However, no mutations were found in any of the neonatal sequences, not even in IGHV4 gene family genes with the highest number of mutated sequences (66%) in the adult rat. These results support the notion that, at least in rats, mutated IgM+ MZ-B cells seen in adult animals are bona fide memory cells that are most probably generated under the influence of external antigenic stimuli in GCs.
VII. IgG EXPRESSING MARGINAL ZONE MEMORY B CELLS
In addition to unswitched (IgM+) MZ-B cells, also class switched B cells can be found within the human73 and rodent MZ.74 The phenotype of these cells is not clear, nor is it clear whether their IGHV genes exhibit SHM, a hallmark of memory B cells. IgG+ expressing B cells with a MZ-B cell phenotype were analyzed considering the fact that rat MZ-B cells express low levels of CD45R, defined by monoclonal antibody HIS24, and high levels of a surface molecule defined by the monoclonal antibody HIS57. Thus, mature (CD90−) MZ-B cells can be defined as CD90−HIS24lowHIS57high cells and FO-B cells CD90−HIS24highHIS57neg/low cells. Furthermore, from purified (FACS-sorted) MZ-B cells (CD90−HIS24lowHIS57high) and FO-B cells (CD90−HIS24highHIS57neg/lo), amplified rearranged IGHV-Cγ transcripts specific for the IGHV5 gene family were compared to amplified IGHV-Cγ transcripts from a fraction of cells that should include classical class-switched memory B cells, i.e., IgM−IgD−B cells. The presence of IGHV5-Cγ transcripts was detected in all B cell subsets analyzed, which implies that IgG expressing cells can apparently exhibit at least two different phenotypes: cells with a MZ-B cell phenotype and cells with a FO-B cell phenotype. Analysis of the individual IGHV5 genes used by these IgG transcripts revealed that almost all IGHV5 genes were mutated, as is typical for memory B cells. In addition, the results reveal that IgG-expressing MZ-B cells had a lower number of mutations than IgG-expressing FO-B cells. There were no differences in the use of IGHV, IGHD, and IGHJ genes between the IgG+ MZ-B cells and IgG+ FO-B cell subsets, and the H-CDR3 lengths were comparable between these two subsets. Notably, the identification of sets of clonally related IgG-encoding sequences (sequences with identical H-CDR3 regions, and usage of the same IGHV genes) derived from cells with members both in MZ-B cell (CD90−HIS24low-HIS57high) and FO-B cell (CD90−HIS24highHIS57neg/low) fractions. Such sets of clonally related sequences were also found among the mutated IgM+ MZ-B cells and IgM+ FO-B cells (unpublished data). These observations strongly indicate that (mutated) memory B cells with a MZ-B cell phenotype and a FO-B cell phenotype have a common origin. The origin of these cells is not clear. Classical memory B cells (i.e., mutated class-switched B cells) are usually generated in the GCs. At these sites, the mutated B cells undergo some form of selection for the antigen that drives the GC reaction. This antigen selection is crucial to increasing the affinity of the Ig molecules that recognize the antigen, and it is reflected in the fact that the IGHV genes that encode for the Ig molecules exhibit nonrandom mutations. Some of the mutation patterns of IgG-encoded sequences from both the MZ-B cell fraction and the FO-B cell fraction have shown signs of antigen selection, which favors the hypothesis that they are both generated in the GCs. The finding that mutated IgM+ memory B cells are absent in neonatal animals, in which GCs cannot be formed yet, supports the hypothesis that IgM+ memory MZ-B cells are also generated in the GCs. The common origin of IgM+ and IgG+ memory MZ-B cells and FO-B cells, illustrated by the presence of clonally related mutated sequences between MZ-B and FO-B cells, suggest that at least some GC-derived memory cells can acquire either a FO-B cell phenotype or a MZ-B cell phenotype. The factors that drive this differentiation toward these two phenotypes are not known.
VIII. CONCLUDING REMARKS
B cells in the MZ comprise a heterogeneous population of cells and both naïve MZ-B cells; class-switched and -unswitched memory MZ-B cells are present at this unique site in the spleen. Naïve MZ-B cells carry unmutated Ig genes, produce low-affinity IgM molecules, and constitute a first line of defense against invading pathogens. The antibody repertoire expressed by these B cells has been suggested to be selected to bind to carbohydrate, carried by microorganisms, for example.75 These antigens do not require T cell help for their responses. Furthermore, the heavy chains of the IgM molecules expressed by naïve MZ-B cells contain shorter H-CDR3 regions compared to FO-B cells in rats53 and mice.76 Shorter H-CDR3 is associated with polyreactive antibody responses such as the binding of an antibody to several different structural antigenic elements.77 Memory MZ-B cells express high-affinity Ig molecules, directed to (microbial) antigens that have been encountered during life. Thus, the presence of naïve MZ-B cells and memory MZ-B cells allows the MZ to make rapid innate-like and adaptive antibody responses to microbial antigens, both TI and TD antigens. A novel role for neutrophils in the response of MZ-B cells has been proposed by Puga et al.42 Neutrophils present in the spleen induce IgM+ production by activating MZ-B cells via BAFF, APRIL, and IL-21 to make antibody responses to TI-2 antigens, such as LPS, after induction of BLIMP-1 in the activated MZ-B cells.42 BAFF and IL-21 can also stimulate IgG+ MZ-B cells in a T cell-independent fashion, to become antibody-secreting plasma cells.38
MZ-B cells are in some form of preactivated state, and they express high levels of complement receptor (CR) CD2178 and toll-like receptors (TLRs),79 which underlie the fact that MZ-B cells are equipped for rapid and easy activation in (primary) immune responses. Furthermore, Garraud et al.6 suggest that shuttling of MZ-B cells between the MZ and follicles and transport of antigens (immune complexes) to follicular dendritic cells (FDCs) decipher the role of MZ-B cells as antigen-presenting cells to participate in immune responses that generate high-affinity antibodies. They observed that type 1 interferon produced in response to bloodborne pathogens inactivates the sphingosin-1-phosphate receptor 1 (S1P1) and S1P3, allowing MZ-B cells to migrate from the MZ in response to CXCL13, which is highly expressed in follicles. Downregulation of CXCR5 allows MZ-B cells to exit from the follicles and return to the MZ. Herewith, MZ-B cells transport immune complexes toward the follicles, where the immune complexes are subsequently captured by FDCs in a complement-dependent manner. The immune complexes trapped by the FDCs are involved in the selection of mutated B cells that express Ig with higher affinities with the help of follicular helper T (Tfh) cells. First, FDCs present antigen to the B cells that undergo SHM and class-switch recombination (CSR). These B cells then go on to present antigens to Tfh cells, which deliver survival signals to these high-affinity GC B cells, leading to selection. The selected GC B cells then differentiate either into memory B cells or into plasma cells.
MZ-B cells express the inhibitory IgG-binding Fc-like receptor FcRL5.80 After binding IgG, this receptor can inhibit the BCR. Like FcRL4, which binds IgA and inhibits the BCR, it is possible that occupation of FcRL5 by IgG similarly acts as an adaptive innate molecular switch dampening the BCR signaling and enhancing the TLR signaling.81 Herewith, MZ-B cells become more innate-like B cells that no longer rely as much on their BCR for their activation but more on TLRs. These cells can respond rapidly to microbiological antigens present in the blood in a BCR-independent fashion. Also primary HIV infection results in B cell dysfunction and the loss of particular IgM memory B cells.82 In addition, theses researchers also found that, like T cells, the upregulation of PD-L1/PDL2 complexes acts as a regulator of BCR and TLR9 signaling on B cells. The characterization of the PD-1/PD-Ls inhibitory pathways in B cells of HIV-infected patients could reveal targets for improving the antibody response in HIV. Also, primary HIV infection results in B cell dysfunction and the loss of particular IgM memory B cells.83 B cells from HIV patients are also poorly responsive to stimulation and express inhibitory receptors like PD-L1 and FCRL4, probably contributing to the delayed development of neutralizing antibodies; data showed that inadequate T follicular helper (Tfh) cells help impair B cell immunity during HIV infection.84 Based on this observation, it would be interesting to explore whether PD-1/PDLs complexes have any pivotal role to play in memory B cell exhaustion during primary HIV infection and whether this role is distinguishable to MZ-like B cells. Because MZ-B cells have a broad repertoire, contain memory B cells (IgM and IgG), and are in a kind of preactivated state, these cells are ideally suited to respond rapidly to a broad range of bloodborne antigens to prevent sepsis. Thus, targeting these B cells by vaccination in the future will be crucial for efficient protection against life-threatening infections.
Acknowledgments
J.H. is a recipient of an Ubbo Emmius scholarship. The research reported in this publication was supported by the Fogarty International Center (FIC), NIH Common Fund, Office of Strategic Coordination, Office of the Director (OD/OSC/CF/NIH), Office of AIDS Research, Office of the Director (OAR/NIH), National Institute of Mental Health (NIMH/NIH) of the National Institutes of Health under Award Number D43TW010131. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- FO-B
follicular B cells
- GC
germinal center
- IGHV
immunoglobulin variable region
- H-CDR3
heavy-chain complementarity regions 3
- SHM
somatic hypermutation
- MZ-B
marginal zone B cell
- TI-2
T cell-independent type 2 antigens
References
- 1.Victora GD, Mesin L. Clonal and cellular dynamics in germinal centers. Curr Opin Immunol. 2014;28:90–6. doi: 10.1016/j.coi.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Zelm MC. B cells take their time: sequential IgG class switching over the course of an immune response[quest] Immunol Cell Biol. 2014;92(8):645–6. doi: 10.1038/icb.2014.48. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi M, Tsukada T, Kojima M, Koide T, Koike T, Takahashi H, Sakai C, Kashimura M, Shibata A. Immunoglobulin class switch from IgG to IgA in a patient with smoldering multiple myeloma. Blood. 1986;67(6):1710–3. [PubMed] [Google Scholar]
- 4.Kusumi S, Koga D, Kanda T, Ushiki T. Three-dimensional reconstruction of serial sections for analysis of the microvasculature of the white pulp and the marginal zone in the human spleen. Biomed Res. 2015;36(3):195–203. doi: 10.2220/biomedres.36.195. [DOI] [PubMed] [Google Scholar]
- 5.Vinuesa CG, Sze DM, Cook MC, Toellner KM, Klaus GG, Ball J, MacLennan IC. Recirculating and germinal center B cells differentiate into cells responsive to polysaccharide antigens. Eur J Immunol. 2003;33(2):297–305. doi: 10.1002/immu.200310003. [DOI] [PubMed] [Google Scholar]
- 6.Garraud O, Borhis G, Badr G, Degrelle S, Pozzetto B, Cognasse F, Richard Y. Revisiting the B cell compartment in mouse and humans: more than one B cell subset exists in the marginal zone and beyond. BMC Immunol. 2012;13:63. doi: 10.1186/1471-2172-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dammers PM, de Boer NK, Deenen GJ, Nieuwenhuis P, Kroese FG. The origin of marginal zone B cells in the rat. Eur J Immunol. 1999;29(5):1522–31. doi: 10.1002/(SICI)1521-4141(199905)29:05<1522::AID-IMMU1522>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12(2):211–21. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 9.Kroese FG, Wubbena AS, Opstelten D, Deenen GJ, Schwander EH, De LL, Vos H, Poppema S, Volberda J, Nieuwenhuis P. B lymphocyte differentiation in the rat: production and characterization of monoclonal antibodies to B lineage-associated antigens. Eur J Immunol. 1987;17(7):921–8. doi: 10.1002/eji.1830170705. [DOI] [PubMed] [Google Scholar]
- 10.Kroese FG, de Boer NK, de Boer T, Nieuwenhuis P, Kantor AB, Deenen GJ. Identification and kinetics of two recently bone marrow-derived B cell populations in peripheral lymphoid tissues. Cell Immunol. 1995;162(2):185–93. doi: 10.1006/cimm.1995.1068. [DOI] [PubMed] [Google Scholar]
- 11.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–85. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 12.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188(9):1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova JL, Reynaud CA, Weill JC. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104(12):3647–54. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arakawa H, Hauschild J, Buerstedde JM. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. 2002;295(5558):1301–6. doi: 10.1126/science.1067308. [DOI] [PubMed] [Google Scholar]
- 15.Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or “memory” B cells? J Immunol. 2007;179(1):13–9. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, Quinti I, Carsetti R. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197(7):939–45. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FG. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J Immunol. 2000;165(11):6156–69. doi: 10.4049/jimmunol.165.11.6156. [DOI] [PubMed] [Google Scholar]
- 18.Makowska A, Faizunnessa NN, Anderson P, Midtvedt T, Cardell S. CD1high B cells: a population of mixed origin. Eur J Immunol. 1999;29(10):3285–94. doi: 10.1002/(SICI)1521-4141(199910)29:10<3285::AID-IMMU3285>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Dammers PM, de Boer NK, Deenen GJ, Nieuwenhuis P, Kroese FG. The origin of marginal zone B cells in the rat. Eur J Immunol. 1999;29(5):1522–31. doi: 10.1002/(SICI)1521-4141(199905)29:05<1522::AID-IMMU1522>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Kumararatne DS, MacLennan IC. Cells of the marginal zone of the spleen are lymphocytes derived from recirculating precursors. Eur J Immunol. 1981;11(11):865–9. doi: 10.1002/eji.1830111104. [DOI] [PubMed] [Google Scholar]
- 21.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167(12):6834–40. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava B, Quinn WJ, 3rd, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202(9):1225–34. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dammers PM, Kroese FG. Recruitment and selection of marginal zone B cells is independent of exogenous antigens. Eur J Immunol. 2005;35(7):2089–99. doi: 10.1002/eji.200526118. [DOI] [PubMed] [Google Scholar]
- 24.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14(5):603–15. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 25.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 26.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9(11):767–77. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 27.Wang JH, Avitahl N, Cariappa A, Friedrich C, Ikeda T, Renold A, Andrikopoulos K, Liang L, Pillai S, Morgan BA, Georgopoulos K. Aiolos regulates B cell activation and maturation to effector state. Immunity. 1998;9(4):543–53. doi: 10.1016/s1074-7613(00)80637-8. [DOI] [PubMed] [Google Scholar]
- 28.Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K, Yamaguchi T, Yamamoto G, Seo S, Kumano K, Nakagami-Yamaguchi E, Hamada Y, Aizawa S, Hirai H. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18(5):675–85. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 29.Descatoire M, Weller S, Irtan S, Sarnacki S, Feuillard J, Storck S, Guiochon-Mantel A, Bouligand J, Morali A, Cohen J, Jacquemin E, Iascone M, Bole-Feysot C, Cagnard N, Weill JC, Reynaud CA. Identification of a human splenic marginal zone B cell precursor with NOTCH2-dependent differentiation properties. J Exp Med. 2014;211(5):987–1000. doi: 10.1084/jem.20132203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuroda K, Han H, Tani S, Tanigaki K, Tun T, Furukawa T, Taniguchi Y, Kurooka H, Hamada Y, Toyokuni S, Honjo T. Regulation of marginal zone B cell development by MINT, a suppressor of Notch/RBP-J signaling pathway. Immunity. 2003;18(2):301–12. doi: 10.1016/s1074-7613(03)00029-3. [DOI] [PubMed] [Google Scholar]
- 31.Astier A, Avraham H, Manie SN, Groopman J, Canty T, Avraham S, Freedman AS. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272(1):228–32. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 32.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1(1):31–6. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 33.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21(12):2951–62. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 34.MacLennan IC, Bazin H, Chassoux D, Gray D, Lortan J. Comparative analysis of the development of B cells in marginal zones and follicles. Adv Exp Med Biol. 1985;186:139–44. doi: 10.1007/978-1-4613-2463-8_17. [DOI] [PubMed] [Google Scholar]
- 35.Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci U S A. 1996;93(8):3357–61. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pone EJ, Zhang J, Mai T, White CA, Li G, Sakakura JK, Patel PJ, Al-Qahtani A, Zan H, Xu Z, Casali P. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-kappaB pathway. Nat Commun. 2012;3:767. doi: 10.1038/ncomms1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3(9):822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ettinger R, Sims GP, Robbins R, Withers D, Fischer RT, Grammer AC, Kuchen S, Lipsky PE. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J Immunol. 2007;178(5):2872–82. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi S, Haruo N, Sugane K, Ochs HD, Agematsu K. Interleukin-21 stimulates B cell immunoglobulin E synthesis in human beings concomitantly with activation-induced cytidine deaminase expression and differentiation into plasma cells. Hum Immunol. 2009;70(1):35–40. doi: 10.1016/j.humimm.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Fayette J, Durand I, Bridon JM, Arpin C, Dubois B, Caux C, Liu YJ, Banchereau J, Briere F. Dendritic cells enhance the differentiation of naïve B cells into plasma cells in vitro. Scand J Immunol. 1998;48(6):563–70. doi: 10.1046/j.1365-3083.1998.00471.x. [DOI] [PubMed] [Google Scholar]
- 41.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17(3):341–52. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 42.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13(2):170–80. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L, Barra CM, Comerma L, Chudnovskiy A, Gentile M, Llige D, Cols M, Serrano S, Arostegui JI, Juan M, Yague J, Merad M, Fagarasan S, Cerutti A. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol. 2014;15(4):354–64. doi: 10.1038/ni.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells–how did we miss them? Nat Rev Immunol. 2013;13(2):75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 45.Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207(2):273–80. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willems L, Li S, Rutgeerts O, Lenaerts C, Waer M, Billiau AD. IL-7 is required for the development of the intrinsic function of marginal zone B cells and the marginal zone microenvironment. J Immunol. 2011;187(7):3587–94. doi: 10.4049/jimmunol.1004012. [DOI] [PubMed] [Google Scholar]
- 47.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9(1):54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 49.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297(5580):409–12. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 50.Liu YJ, Oldfield S, MacLennan IC. Memory B cells in T cell-dependent antibody responses colonize the splenic marginal zones. Eur J Immunol. 1988;18(3):355–62. doi: 10.1002/eji.1830180306. [DOI] [PubMed] [Google Scholar]
- 51.Vinuesa CG, Sunners Y, Pongracz J, Ball J, Toellner KM, Taylor D, MacLennan IC, Cook MC. Tracking the response of Xid B cells in vivo: TI-2 antigen induces migration and proliferation but Btk is essential for terminal differentiation. Eur J Immunol. 2001;31(5):1340–50. doi: 10.1002/1521-4141(200105)31:5<1340::AID-IMMU1340>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 52.Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J Exp Med. 2003;198(12):1923–35. doi: 10.1084/jem.20031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FG. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J Immunol. 2000;165(11):6156–69. doi: 10.4049/jimmunol.165.11.6156. [DOI] [PubMed] [Google Scholar]
- 54.Makowska A, Faizunnessa NN, Anderson P, Midtvedt T, Cardell S. CD1high B cells: a population of mixed origin. Eur J Immunol. 1999;29(10):3285–94. doi: 10.1002/(SICI)1521-4141(199910)29:10<3285::AID-IMMU3285>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 55.Gatto D, Bauer M, Martin SW, Bachmann MF. Heterogeneous antibody repertoire of marginal zone B cells specific for virus-like particles. Microbe Infect. 2007;9(3):391–9. doi: 10.1016/j.micinf.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 56.Dunn-Walters DK, Isaacson PG, Spencer J. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J Exp Med. 1995;182(2):559–66. doi: 10.1084/jem.182.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188(9):1691–703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seifert M, Kuppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J Exp Med. 2009;206(12):2659–69. doi: 10.1084/jem.20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weller S, Faili A, Garcia C, Braun MC, Le Deist FF, de Saint Basile GG, Hermine O, Fischer A, Reynaud CA, Weill JC. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci U S A. 2001;98(3):1166–70. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247(1):52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 61.Takemori T, Kaji T, Takahashi Y, Shimoda M, Rajewsky K. Generation of memory B cells inside and outside germinal centers. Eur J Immunol. 2014;44(5):1258–64. doi: 10.1002/eji.201343716. [DOI] [PubMed] [Google Scholar]
- 62.Toyama H, Okada S, Hatano M, Takahashi Y, Takeda N, Ichii H, Takemori T, Kuroda Y, Tokuhisa T. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity. 2002;17(3):329–39. doi: 10.1016/s1074-7613(02)00387-4. [DOI] [PubMed] [Google Scholar]
- 63.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13(2):199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 64.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1(3):167–78. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 65.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, Nagashima T, Takahashi Y, Kurosaki T, Okada M, Ohara O, Rajewsky K, Takemori T. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med. 2012;209(11):2079–97. doi: 10.1084/jem.20120127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FG. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J Immunol. 2000;165(11):6156–69. doi: 10.4049/jimmunol.165.11.6156. [DOI] [PubMed] [Google Scholar]
- 67.Colombo M, Cutrona G, Reverberi D, Bruno S, Ghiotto F, Tenca C, Stamatopoulos K, Hadzidimitriou A, Ceccarelli J, Salvi S, Boccardo S, Calevo MG, De Santanna A, Truini M, Fais F, Ferrarini M. Expression of immunoglobulin receptors with distinctive features indicating antigen selection by marginal zone B cells from human spleen. Mol Med. 2013;19:294–302. doi: 10.2119/molmed.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hendricks J, Terpstra P, Dammers PM, Somasundaram R, Visser A, Stoel M, Bos NA, Kroese FG. Organization of the variable region of the immunoglobulin heavy-chain gene locus of the rat. Immunogen. 2010;62(7):479–86. doi: 10.1007/s00251-010-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hendricks J. Heterogeneity of memory marginal zone B cells in the rat [dissertation] Groningen (The Netherlands): University of Groningen Medical Center; 2015. [Google Scholar]
- 70.Hendricks J, Visser A, Dammers PM, Burgerhof JG, Bos NA, Kroese FG. Class-switched marginal zone B cells in spleen have relatively low numbers of somatic mutations. Mol Immunol. 2011;48(6–7):874–82. doi: 10.1016/j.molimm.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 71.Weller S, Mamani-Matsuda M, Picard C, Cordier C, Lecoeuche D, Gauthier F, Weill JC, Reynaud CA. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med. 2008;205(6):1331–42. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kroese FG, Wubbena AS, Kuijpers KC, Nieuwenhuis P. The ontogeny of germinal centre forming capacity of neonatal rat spleen. Immunol. 1987;60(4):597–602. [PMC free article] [PubMed] [Google Scholar]
- 73.Ettinger R, Sims GP, Robbins R, Withers D, Fischer RT, Grammer AC, Kuchen S, Lipsky PE. IL-21 and BAFF/ BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J Immunol. 2007;178(5):2872–82. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
- 74.Gatto D, Ruedl C, Odermatt B, Bachmann MF. Rapid response of marginal zone B cells to viral particles. J Immunol. 2004;173(7):4308–16. doi: 10.4049/jimmunol.173.7.4308. [DOI] [PubMed] [Google Scholar]
- 75.Galson JD, Clutterbuck EA, Truck J, Ramasamy MN, Munz M, Fowler A, Cerundolo V, Pollard AJ, Lunter G, Kelly DF. BCR repertoire sequencing: different patterns of B cell activation after two Meningococcal vaccines. Immunol Cell Biol. 2015 doi: 10.1038/icb.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. J Exp Med. 2008;205(9):2043–52. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schroeder HW, Jr, Mortari F, Shiokawa S, Kirkham PM, Elgavish RA, Bertrand FE., 3rd Developmental regulation of the human antibody repertoire. Ann N Y Acad Sci. 1995;764:242–60. doi: 10.1111/j.1749-6632.1995.tb55834.x. [DOI] [PubMed] [Google Scholar]
- 78.Timens W, Boes A, Poppema S. Human marginal zone B cells are not an activated B cell subset: strong expression of CD21 as a putative mediator for rapid B cell activation. Eur J Immunol. 1989;19(11):2163–6. doi: 10.1002/eji.1830191129. [DOI] [PubMed] [Google Scholar]
- 79.Gunn KE, Brewer JW. Evidence that marginal zone B cells possess an enhanced secretory apparatus and exhibit superior secretory activity. J Immunol. 2006;177(6):3791–8. doi: 10.4049/jimmunol.177.6.3791. [DOI] [PubMed] [Google Scholar]
- 80.Wilson TJ, Fuchs A, Colonna M. Cutting edge: human FcRL4 and FcRL5 are receptors for IgA and IgG. J Immunol. 2012;188(10):4741–5. doi: 10.4049/jimmunol.1102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sohn HW, Krueger PD, Davis RS, Pierce SK. FcRL4 acts as an adaptive to innate molecular switch dampening BCR signaling and enhancing TLR signaling. Blood. 2011;118(24):6332–41. doi: 10.1182/blood-2011-05-353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thibult ML, Mamessier E, Gertner-Dardenne J, Pastor S, Just-Landi S, Xerri L, Chetaille B, Olive D. PD-1 is a novel regulator of human B cell activation. Int Immunol. 2013;25(2):129–37. doi: 10.1093/intimm/dxs098. [DOI] [PubMed] [Google Scholar]
- 83.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9(4):235–45. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr, Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19(4):494–9. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]