Abstract
Black soldier fly (Hermetia illucens) larvae (BSFL) are rich in protein and have the potential to be used in animal feed. The aim of the present study was to determine the immunoprophylactic effect of BSFL against Salmonella Gallinarum in broiler chicks as an alternative feed additive. Results showed that BSFL improved body weight gain and increased frequency of CD4+ T lymphocyte, serum lysozyme activity, and spleen lymphocyte proliferation. Moreover, BSFL reinforced bacterial clearance and increased survivability of broiler chicks against S. Gallinarum. These data suggested that BSFL has prophylactic properties with stimulating non-specific immune responses, as well as reduced bacterial burden against S. Gallinarum.
Keywords: black soldier fly, broiler chicks, immune response, Salmonella Gallinarum
Black Soldier fly (BSF; Hermetia illucens (L.) (Diptera, Stratiomydae) is a large wasp-like fly distributed throughout the world. BSF is useful for managing large amounts of animal manure and other organic waste. It is a representative environmental purification insect [4]. BSF lives in places where there are organic wastes such as livestock products and garbages [17, 19]. Various studies have used BSF for food waste disposal and green waste treatment of livestock products [6, 18]. Larvae of BSF have also been used as feed [2, 7, 23].
BSF larvae (BSFL) can provide high-value feedstuff because they are rich in protein (40 to 44%) with better amino acid profile compared to soybean meal [21]. BSFL has high dry matter (DM) content (35 to 45%). They are rich in lysine (6 to 8% of crude protein (CP)), Ca (5 to 8% DM), and P (0.6 to 1.5% DM) [20]. BSFL are also rich in fat which has extreme quantitative (15 to 49%) and qualitative variability depending on the chemical compositions of their rearing substrates [22]. Recently, interesting results have been published about the suitability of different types of insect meal as diet ingredients for pigs and poultry [16, 24]. Moreover, when BSFL meal is used as feed ingredient for poultry diets, BSFL has been found to be excellent source of energy and digestible amino acids for broilers [4]. Another report has found that black soldier fly meal can improve the growth rate of broiler quails as a component of a complete diet [3]
Salmonella enterica serovar Gallinarum (S. Gallinarum) causes fowl typhoid, a severe systemic infection that can lead to anemia, leukocytosis, hepatosplenomegaly, and intestinal tract hemorrhage [21]. Although fowl typhoid has been eradicated from some countries such as Australia, North America, and most European countries, it is still a serious problem in Korean poultry industry [12]. On the basis of the above-mentioned facts, the objective of this study was to evaluate productivity, immunity, and experimental Salmonella infection of broiler chicken fed with BSLF.
Three independent studies, including growth performance, immunological assays, and monitoring of survivability against experimental Salmonella infection described below were conducted with broiler chicks from a single healthy stock. All chicks were housed in separate air-controlled rooms. They were provided free access to tap water and particular diet. All animal procedures were approved by the Institutional Animal Care and Use Committee of Chonnam National University. In each independent study, chicks were randomized into four feeding groups. The control group received a commercial and nutritionally complete antibiotic-free chicken feed (Hanvit Bio., Gwangyang, Korea). Experiment groups received the same chicken feed supplemented with 1% (w/w) BSFL (1% BSFL-fed group), 2% (w/w) BSFL (2% BSFL-fed group), or 3% (w/w) BSFL (3% BSFL-fed group).
For growth performance assay, a total of 80 one-day-old unsexed Ross broiler chicks were randomly distributed to the four dietary treatment groups (20 chicks per group). Performance traits including average daily weight gain (ADWG), feed intake (ADFI), and feed conversion ratio (FCR) were recorded. Dietary replacement of BSFL resulted in increased ADWG throughout the experimental period (data not shown). After examining the length of time to reach the final target weight of 1.3 kg, the control group needed 32 days. However, the 1, 2 and 3% BSFL-fed groups only needed 30 days. This indicates that growth performance of broiler chicks can be enhanced by ingestion of BSFL.
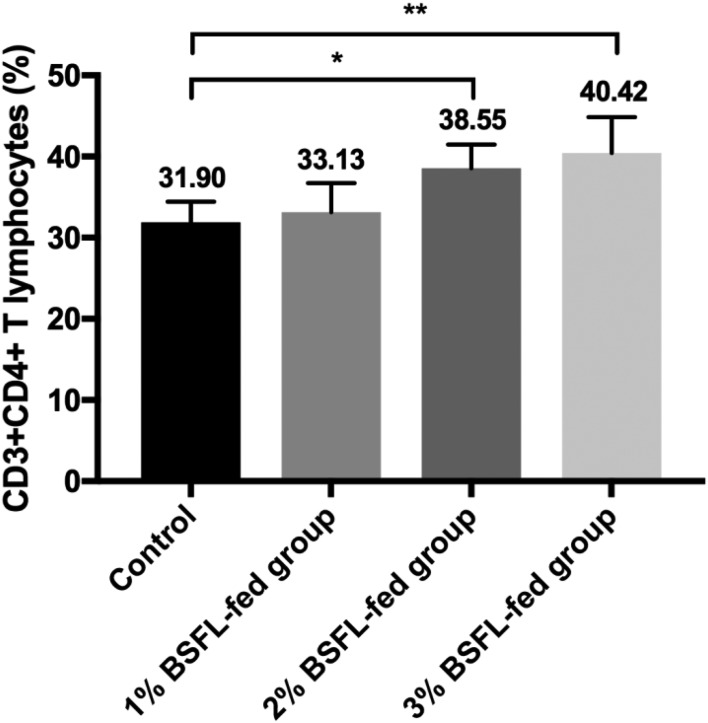
For immunological assay, chicks (n=10 in each group) were fed particular diet for 20 days. Animal experiment procedure was the same as described above. Chicks were subjected to immunological assays including the assay for determining spleen T lymphocyte subpopulations, lysozyme activity, and spleen cell proliferation assay. The spleen was obtained from each chick and single-cell suspension was prepared by pushing the tissue through a 40-µm nylon mesh (BD Biosciences, Franklin Lakes, NJ, U.S.A.). Isolated cells were stained with both fluorescein isothiocyanate (FITC)-conjugated mouse anti-chicken CD3 (BD Biosciences.) and phycoerythrin (PE)-conjugated mouse anti-chicken CD4 (Southern Biotech) to determine the component ratio of T helper cells (CD3+CD4+) as described previously [1, 9]. To determine T cytotoxic cells, cells were stained with both FITC-conjugated mouse anti-chicken CD3 (Southern Biotech) and PE-conjugated mouse anti-chicken CD8 (BD Biosciences). Lymphocyte subpopulations were analyzed using a FAC Sort flow cytometer (BD Biosciences). Percentages of CD3+CD4+ T lymphocytes in spleens of BSFL-fed groups (1, 2 and 3% BSFL: 33.13, 38.55 and 40.42%, respectively) were significantly higher compared to those of the control group (31.90%) in a dose dependent manner (control vs. 2% BSFL-fed group, P<0.05; control vs. 3% BSFL-fed group, P<0.01) (Fig. 1). In the present study, percentages of spleen CD3+CD4+ T lymphocytes in BSFL-fed groups were significantly increased in a dose-dependent manner compared to that of the control group. These results imply that BSFL can confer benefit to immune function in broiler chicks.
Fig. 1.
Effect of BSFL feeding on spleen T lymphocyte subpopulations in broiler chicks. Percentages of CD3+CD4+ T lymphocyte in spleens of BSF-fed groups were significantly higher compared to those of the control group (*, P<0.05; **, P<0.01). For each group, data are presented as mean ± SD (n=10).
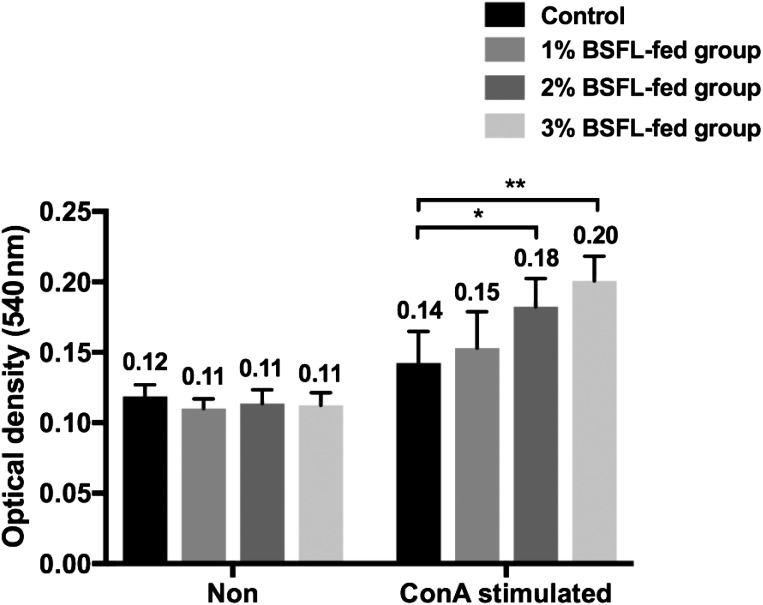
To perform spleen lymphocyte proliferation assay, spleen cells were washed with PBS three times prior to resuspension in 2 ml RPMI-1640 medium (Lonza, Basel, Switzerland) supplemented with 2% (v/v) antibiotic-antimycotics (Invitrogen, Valencia, CA, U.S.A.). Cell suspensions were diluted to a final density of 1 × 107 cells/ml in RPMI-1640 medium. One milliliter of cell suspension and 100 µg/ml concanavalin a (ConA) (Sigma-Aldrich, St. Louis, MO, U.S.A.) were added to wells in 24-well cell culture plate (Iwaki, Tokyo, Japan). After 24 hr of incubation in 5% CO2 incubator at 41°C, 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium salt (MTT) (Sigma-Aldrich) was added to cell culture to a final concentration of 500 µg/ml. Cells were incubated for a further of 4 hr. Then 300 µl of dimethyl sulfoxide (DMSO)(Sigma-Aldrich) was added to cell culture. The absorbance of each sample was read using an enzyme-linked immunosorbent assay (ELISA) plate reader (Thermo Labsystems, Helsinki, Finland) at wavelength of 540 nm to obtain the optical density (OD540nm) as described previously [14]. Spleen lymphocyte proliferation of BSFL-fed groups (OD540nm values for 1, 2 and 3% BSFL: 0.15, 0.18 and 0.20, respectively) was significantly enhanced compared to that of the control group (OD540nm value: 0.14) in a dose-dependent manner group (control vs. 2% BSFL-fed group, P<0.05; control vs. 3% BSFL-fed group, P<0.01) (Fig. 2). These results indicate that mitogenicity of lymphocytes in broiler chicks is enhanced by ingestion of BSFL.
Fig. 2.
Effect of BSFL feeding on spleen cell proliferation in broiler chicks. Spleen cells were co-incubated with or without mitogen (ConA). Spleen cell proliferation was then determined as OD540nm value of colored material after incubation with MTT. Spleen cell proliferation in BSFL-fed group was significantly enhanced compared to that of the control group (*, P<0.05; **, P<0.01). Proliferation of unstimulated cells did not show difference between groups. For each group, data are presented as mean OD540nm ± SD (n=10).
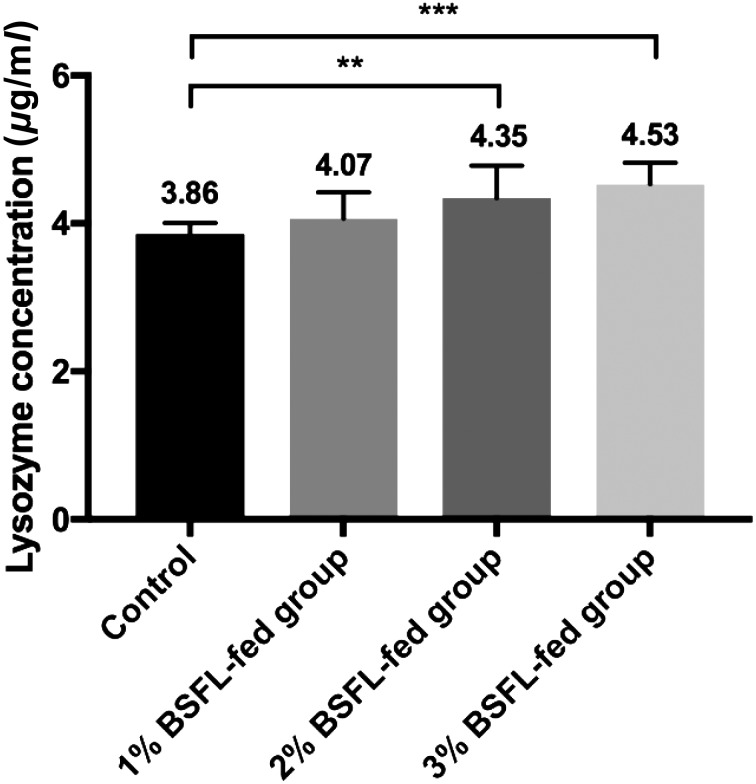
To determine lysozyme activity in serum, blood was collected from the wing vein of each chick on day 20. Serum was obtained by centrifugation at 2,000 × g for 10 min at 4°C. Lysozyme activity was determined using a previously described method [11]. Serum lysozyme concentration in BSFL-fed groups (1, 2 and 3% BSFL: 4.07, 4.46 and 4.70, respectively) was significantly higher than that in the control group (3.76) in a dose-dependent manner (control vs. 2% BSFL-fed group, P<0.05; control vs. 3% BSFL-fed group, P<0.01) (Fig. 3). Lysozyme is secreted by some phagocytes such as macrophages and polymorphonuclear leukocytes. After internalization of antigens, lysozyme can destroy glucosidic bonds in cell walls of bacteria as a result of their phagocytic activity [18]. In this study, serum lysozyme activities of 2 and 3% BSFL-fed groups were significantly higher than those of the control group. Increased lysozyme activity in the serum is associated with increased destructive activity of phagocytes [11]. These results indicate that continuous ingestion of BSFL is able to stimulate the activation of phagocytes.
Fig. 3.
Effect of BSFL feeding on serum lysozyme activity in broiler chicks. Serum lysozyme concentration in BSFL-fed group was significantly higher than that of the control group (*, P<0.05; **, P<0.01). For each group, data are presented as mean ± SD (n=10).
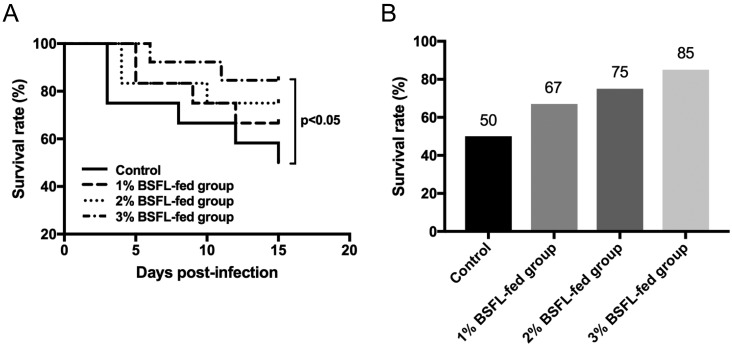
To determine the immunomodulation effect of BSFL on broiler chicks, prophylactic effect of BSFL against Salmonella Gallinarum, the most serious problem in Korean poultry industry [15], was determined in this study. Salmonella Gallinarum (SG3001) used in the present study was originally isolated from a chick with naturally occurring fowl typhoid (National Veterinary Research & Quarantine Service). All chicks (n=20 in each group) were acclimatized to their particular diets for 3 weeks before experimental bacterial infection. Prior to the experiment, chicks were confirmed to be Salmonella-free by bacteriological culture of fecal samples obtained by cloacal swabs [10]. S. Gallinarum (SG3001) was prepared as described previously [25]. Each chick was orally challenged with 5 × 1010 cfu, the optimal dose determined in our previous study [8, 10]. Chicks were then observed for 15 days after bacterial challenge. Their survivability was recorded. Fecal samples from cloacal swabs were collected at 1, 5, 10 and 15 days post-infection (DPI). Liver, spleen, cecum, and bursa of Fabricus samples were also collected from all remaining chicks at the end of the experiment. Viable bacteria counts in fecal samples and each tissue were determined as described previously [2]. Mortality was first observed at 3 DPI in the control group. In BSFL-fed groups, mortality was delayed for 2–3 days compared to that in the control group. Final survival rates in 1, 2 and 3% BSFL-fed groups were 67, 75 and 85%, respectively. However, the survival rate of the control group was only 50% (Fig. 4). Strikingly, 3% BSFL-fed group showed significant increase of survival rate compared to that of control group. These results indicate that ingestion of BSFL can increase survival rates of broiler chicks experimentally infected by Salmonella Gallinarum. This might be due to the general immune enhancing effect of BSFL.
Fig. 4.
Trend of survival rate of broiler chicks experimentally infected by Salmonella Gallinarum. (A) 20 chicks were experimentally challenged with Salmonella Gallinarum and monitored up to 15 days after the infection. Survival rate of BSFL-fed group, especially in 3% BSFL-fed group, was significantly higher compared to that of the control group (*, P<0.05). (B) Final survival rate of each group at the end of the experiment. For each group, data are presented as mean ± SD.
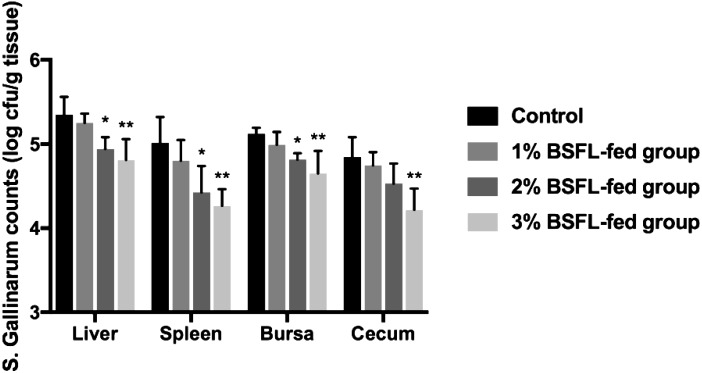
Viable bacteria cell counts in liver, spleen, bursa of Fabricius, and cecum samples collected from each sacrificed chick at 16 DPI were determined. Each tissue sample was homogenized using a Precellys®24 (Bertin Technologies, Montigny-le-Bretonneux, France). Homogenate was then serially diluted 10-fold in PBS and 100 µl of each dilution was spread onto xylose lysine deoxycholate agar plate followed by incubation at 37°C for 24 hr. Characteristic black-colored colonies were counted and expressed as log cfu/g tissue as described previously [14]. In addition, representative colonies were subjected to Gram-staining and biochemical tests for identification [10]. Each sample was tested in duplicates. The number of viable bacterial cells in tissues of BSFL-fed groups tended to decrease during the entire experimental infection period compared to that of the control group. Differences between viable bacterial cell counts in tissues of 2 and 3% BSFL-fed groups and those of the control group were significant (P<0.05) (Fig. 5).
Fig. 5.
Viable bacteria cell was counted in the liver, spleen, bursa, and cecum. Viable bacteria cells count in tissues of the BSFL-fed group were significantly lower compared to those of the control group at the end of the experiment (*, P<0.05; **, P<0.01). For each group, data are presented as mean ± SD.
Taken together, these findings suggest that BSFL feeding can stimulate nonspecific immune responses in broiler chicks and increase their survivability against S. Gallinarum experimental infection. Previous studies suggested that application of immune stimulating agent might result in maximum growth performance since animals is more prone to be influenced by the health and immune status so that a stressed or weak immune system with a load of infectious diseases causes decreased growth performance [5, 13]. Therefore, increasing growth performance induced by ingestion of BSFL in this present study might related in stimulated non-specific immune status. However, the present study did not investigate the exact mechanism involved in the protection of BSFL against S. Gallinarum in broiler chicks. In addition, precise knowledge about the major component (s) of BSFL responsible for its immune enhancing effect is needed since BSFL contains a complex array of compounds. Such studies are currently in progress.
All data are expressed as means ± standard deviation (SD) of means. Student’s t-test was used for statistical analysis of data. All statistical analyses were performed using SigmaPlot® version 10.0 software (Systat Software, San Jose, CA, U.S.A.). Statistical significance was considered when P value was less than 0.05 (*, P<0.05, **, P<0.01).
Acknowledgments
This research was financially supported by Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea and Chonnam National University, 2016. The authors have no conflicts of interests to declare.
REFERENCES
- 1.Abdukalykova S. T., Zhao X., Ruiz-Feria C. A.2008. Arginine and vitamin E modulate the subpopulations of T lymphocytes in broiler chickens. Poult. Sci. 87: 50–55. doi: 10.3382/ps.2007-00315 [DOI] [PubMed] [Google Scholar]
- 2.Bondari K., Sheppard D. C.1987. Soldier fly, Hermetia illucens L., larvae as feed for channel catfish, Ictalurus puctatus Rafinesque, and blue tilapia, Oreochromis aureus (Steindachner). Aquacult. Fish. Manage. 18: 209–220. [Google Scholar]
- 3.Cullere M., Tasoniero G., Giaccone V., Miotti-Scapin R., Claeys E., De Smet S., Dalle Zotte A.2016. Black soldier fly as dietary protein source for broiler quails: apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 10: 1923–1930. doi: 10.1017/S1751731116001270 [DOI] [PubMed] [Google Scholar]
- 4.De Marco M., Martínez S., Hernandez F., Madrid J., Gai F., Rotolo R., Belforti M., Bergero D., Katz H., Dabbou S., Kovitvadhi A., Zoccarato I., Gasco L., Schiavone A.2015. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 209: 211–218. doi: 10.1016/j.anifeedsci.2015.08.006 [DOI] [Google Scholar]
- 5.Faluyi O. B., Agbede J. O., Adebayo I. A.2015. Growth performance and immunological response to Newcastle disease vaccinations of broiler chickens fed lysine supplemented diets. J. Vet. Med. Anim. Health 7: 77–84. doi: 10.5897/JVMAH2014.0328 [DOI] [Google Scholar]
- 6.Furman D. P., Young R. D., Catts E. P.1959. Hermetia illucens Latr. Rev. Soc. Entomol. Argent. 1: 23–27. [Google Scholar]
- 7.Hale O. M.1973. Dried Hermetia illucens larvae (Diptera: Stratiomyidae) as feed additive for poultry. J. Ga. Entomol. 8: 16–20. [Google Scholar]
- 8.Huberman Y. D., Terzolo H. R.2008. Fowl typhoid: assessment of a disinfectant oral dose to reduce horizontal spread and mortality. Avian Dis. 52: 320–323. doi: 10.1637/8105-090307-ResNote.1 [DOI] [PubMed] [Google Scholar]
- 9.Jung B. G., Ko J. H., Lee B. J.2010. Dietary supplementation with a probiotic fermented four-herb combination enhances immune activity in broiler chicks and increases survivability against Salmonella Gallinarum in experimentally infected broiler chicks. J. Vet. Med. Sci. 72: 1565–1573. doi: 10.1292/jvms.10-0152 [DOI] [PubMed] [Google Scholar]
- 10.Jung B. G., Lee J. A., Park S. B., Hyun P. M., Park J. K., Suh G. H., Lee B. J.2013. Immunoprophylactic effects of administering honeybee (Apis melifera) venom spray against Salmonella gallinarum in broiler chicks. J. Vet. Med. Sci. 75: 1287–1295. doi: 10.1292/jvms.13-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreukniet M. B., Nieuwland M. G., van der Zijpp A. J.1995. Phagocytic activity of two lines of chickens divergently selected for antibody production. Vet. Immunol. Immunopathol. 44: 377–387. doi: 10.1016/0165-2427(94)05304-B [DOI] [PubMed] [Google Scholar]
- 12.Kwon Y. K., Kim A., Kang M. S., Her M., Jung B. Y., Lee K. M., Jeong W., An B. K., Kwon J. H.2010. Prevalence and characterization of Salmonella Gallinarum in the chicken in Korea during 2000 to 2008. Poult. Sci. 89: 236–242. doi: 10.3382/ps.2009-00420 [DOI] [PubMed] [Google Scholar]
- 13.Landy N., Ghalamkari G. h., Toghyani M., Moattar F.2011. The effects of Echinacea purpurea L. (purple coneflower) as an antibiotic growth promoter substitution on performance, carcass characteristics and humoral immune response in broiler chickens. J. Med. Plants Res. 5: 2332–2338. [Google Scholar]
- 14.Lee J. A., Jung B. G., Kim T. H., Kim Y. M., Park M. H., Hyun P. M., Jeon J. W., Park J. K., Cho C. W., Suh G. H., Lee B. J.2014. Poly D,L-lactide-co-glycolide (PLGA) nanoparticle-encapsulated honeybee (Apis melifera) venom promotes clearance of Salmonella enterica serovar Typhimurium infection in experimentally challenged pigs through the up-regulation of T helper type 1 specific immune responses. Vet. Immunol. Immunopathol. 161: 193–204. doi: 10.1016/j.vetimm.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 15.Lee Y. J., Mo I. P., Kang M. S.2007. Protective efficacy of live Salmonella gallinarum 9R vaccine in commercial layer flocks. Avian Pathol. 36: 495–498. doi: 10.1080/03079450701691278 [DOI] [PubMed] [Google Scholar]
- 16.Makkar H. P. S., Tran G., Heuze V., Ankers P.2014. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 197: 1–33. doi: 10.1016/j.anifeedsci.2014.07.008 [DOI] [Google Scholar]
- 17.McCallan E.1974. Hermetia illucens (L.) (Diptera: Stratiomyidae), a cosmopolitan American species long established in Australia and New Zealand. Entomol. Mo. Mag. 109: 232–234. [Google Scholar]
- 18.Melnick D. A., Nauseef W. M., Markowitz S. D., Gardner J. P., Malech H. L.1985. Biochemical analysis and subcellular localization of a neutrophil-specific antigen, PMN-7, involved in the respiratory burst. J. Immunol. 134: 3346–3355. [PubMed] [Google Scholar]
- 19.Sheppard D. C., Tomberlin J. K., Joyce J. A., Kiser B. C., Sumner S. M.2002. Rearing methods for the black soldier fly (Diptera: Stratiomyidae). J. Med. Entomol. 39: 695–698. doi: 10.1603/0022-2585-39.4.695 [DOI] [PubMed] [Google Scholar]
- 20.Sheppard D. C., Newton G. L., Thompson S., Davis J., Gascho G., Bramwell K.1998. Using soldier flies as a manur management tool for volume reduction, house fly control and reduction, house fly control and feed stuff production, pp. 51–52. In: Sustainable Agriculture Research and Education, Southern Region, Annual Report. Sustainable Agriculture Research and Education, Southern Region (Gwen, R. ed.), Georgia Station, Griffin. [Google Scholar]
- 21.Shivaprasad H. L.2000. Fowl typhoid and pullorum disease. Rev. - Off. Int. Epizoot. 19: 405–424. doi: 10.20506/rst.19.2.1222 [DOI] [PubMed] [Google Scholar]
- 22.St-Hilaire S., Sheppard C., Tomberlin J. K., Irving S., Newton L., McGuire M. A., Mosely E. E., Hardy R., Sealey W.2007. Fly prepupae as a feedstuff for rainbow trout, Onchorynchus mykiss. J. World Aquacult. Soc. 38: 59–67. doi: 10.1111/j.1749-7345.2006.00073.x [DOI] [Google Scholar]
- 23.Tran G., Heuzé V., Makkar H. P. S.2015. Insects in fish diets. Anim. Front. 5: 37–44. [Google Scholar]
- 24.Veldkamp T., van Duinkerken G., van Huis A., Lakemond C. M. M., Ottevanger E., Bosch G., van Boekel M. A. J. S.2012. Insects as a sustainable feed ingredient in pig and poultry diets: a feasibility study. Wageningen UR Livestock Production, Wageningen, the Netherlands, report no. 638: 1–48. [Google Scholar]
- 25.Waihenya R. K., Mtambo M. M., Nkwengulila G., Minga U. M.2002. Efficacy of crude extract of Aloe secundiflora against Salmonella gallinarum in experimentally infected free-range chickens in Tanzania. J. Ethnopharmacol. 79: 317–323. doi: 10.1016/S0378-8741(01)00397-X [DOI] [PubMed] [Google Scholar]