Abstract
We present a case of an intact 14-year-old male dog with a prostate B-cell lymphoma recognized in the contents of an irreducible perineal hernia. The enlarged prostate was replaced after reducing its size by partial excision, and the perineal hernia was repaired using the tunica vaginalis communis. However, the pelvic cavity was largely occupied by the replaced prostate, and urinary retention developed. The prostate was resected on the next day via abdominal median incision, and a bladder-urethra anastomosis was performed. Lymphoma has rarely been demonstrated to develop in the prostate, with the lesion comprising the hernia contents. The present study suggests the necessity of early treatment for perineal hernias and the possibility that lymphoma may be present in the hernia contents.
Keywords: B-cell lymphoma, dog, irreducible, perineal hernia, prostate
Perineal hernia in dogs is a condition in which rupture of the pelvic diaphragm causes prolapse of abdominal contents between the pelvic diaphragm and the rectum; the condition is treated by reconstruction of the pelvic diaphragm [3]. Fat cells, enlarged rectum, and serous fluid are often present in the hernia sac, which may also contain the urinary bladder, small intestine, and prostate [3].
Lymphomas, including B-cell lymphoma and T-cell lymphoma, are hematopoietic tumors that are among the most frequent tumors in dogs [4, 10]. Canine lymphomas are classified as multicentric, digestive system, skin, mediastinum, and other extranodal types, according to the location of origin [5]. In the veterinary setting, lymphomas rarely develop in the prostate. Only two cases of primary prostate lymphomas [6, 13] and two cases of prostate lymphomas that developed secondary to digestive system and skin lymphomas [1, 7] have been reported.
This study presents a rare case of B-cell lymphoma, demonstrated by histological examination, in the extracted prostate of a dog with irreducible perineal hernia that contained the enlarged prostate and the urinary bladder.
A 14-year-old intact male beagle dog (body weight, 8.2 kg) presented to a veterinary practice with a swelling on the perineum; the dog was diagnosed with a perineal hernia. One month later, the dog was diagnosed with hypothyroidism and was administered a thyroid hormone preparation (100 µg, twice daily [b.i.d.]); his perineal hernia was left untreated. One year and three months after the dog’s initial visit, he developed difficulty urinating; the urinary bladder was confirmed in the hernia contents. The presence of a splenic mass was also confirmed. The splenic mass was completely resected at 1 year 5 months after the initial visit, and the dog was diagnosed with nodular lymphoid hyperplasia. Short-term placement of a urethral catheter resulted in improvement in urination. At 1 year 7 months after the initial visit, the dog developed dyschezia and was presented to our facility, the Animal Medical Center, Faculty of Applied Biological Sciences, Gifu University, for perineal hernia repair.
At the first visit, the owner reported that the dog had begun to vomit more frequently than before. Overall protuberance of the anus and the perianal area to the tail side was observed. A hard structure was detected by rectal palpation and was suspected to be the prostate. From these findings, the dog was tentatively diagnosed with a perineal hernia containing the prostate. Hematological tests revealed leukocytosis, anemia, mild hypoalbuminemia, hyperfibrinogenemia, and lymphocytosis (Table 1). Because the dog was an intact male, repair of both sides of the perineal hernia using the tunica vaginalis communis was performed on the day of the examination [8, 11]. The dog vomited once before anesthesia, but was considered operable. The dog was injected intravenously with 4 mg/kg of propofol (PropoFlo, DS Pharma Animal Health Co., Ltd., Osaka, Japan) for induction of anesthesia and was administered inhaled 1.0–1.8% end-tidal isoflurane (Isoflu, DS Pharma Animal Health Co., Ltd.) with a 40% oxygen-air mixture (FiO2) by tracheal intubation for maintenance of anesthesia. Remifentanil (10–20 µg/kg/hr, Ultiva, Janssen Pharmaceutical K.K., Tokyo, Japan), fentanyl (2 µg/kg/hr, 50 µg/ml, Janssen Pharmaceutical K.K.), and ketamine (0.08 mg/kg/hr, 50 mg/ml, Ketalar, Daiichi Sankyo Propharma Co., Ltd., Tokyo, Japan) were administered for continuous analgesia during the operation. Morphine (0.1 mg/kg, 10 mg/ml, Takeda Pharmaceutical Co., Ltd., Osaka, Japan) and bupivacaine (0.5 mg/kg, 5 mg/ml, Marcain, AstraZeneca K.K., Osaka, Japan) were diluted to 0.2 ml/kg with physiological saline and used for lumbosacral epidural anesthesia. Lidocaine (1 mg/kg, 20 mg/ml, Xylocaine, AstraZeneca K.K.) was administered into both testes for intratestis block. A urinary catheter (8Fr Silicon Foley catheter, Create Medic Co., Ltd., Yokohama, Japan) was easily inserted before the start of the operation. Drainage of the urine proceeded without incident.
Table 1. Results of hematology and blood chemistry panels.
| Before first surgery | Before second surgerya) | At discharge | At reexamination | |
|---|---|---|---|---|
| RBC (×104/µl) | 438 | - | 389 | 310 |
| Ht (%) | 30.2 | - | 26.9 | 23 |
| Hb (g/dl) | 10.2 | - | 9.3 | 7.3 |
| WBC (×102/µl) | 250 | - | 115 | 350 |
| Neu (%) | 21.5 | - | 81.2 | 26.1 |
| Lym (%) | 74.1 | - | 13.1 | 70.6 |
| Mon (%) | 3.4 | - | 2.9 | 2.7 |
| Eos (%) | 1 | - | 2.8 | 0.6 |
| FIB (mg/dl) | 557.6 | 468.2 | - | 586 |
| PLT (×104/µl) | 32.4 | - | 11 | 23.6 |
| TP (g/dl) | 6.6 | - | 5.8 | 6 |
| ALB (g/dl) | 2.5 | 2.1 | - | 2.4 |
| AST (IU/l) | 22 | 225 | 139 | 26 |
| ALT (IU/l) | 11 | 109 | 5 | 9 |
| ALP (IU/l) | 71 | 290 | 441 | 105 |
| BUN (mg/dl) | 15 | 48 | 23 | 21 |
| Cre (mg/dl) | 0.6 | 2.3 | 0.9 | 0.7 |
| BS (mg/dl) | 92 | - | 85 | 110 |
| T-CHO (mg/dl) | 193 | - | - | 193 |
| iP (mg/dl) | 3.1 | 7.3 | - | 4.3 |
| T-Bil (mg/dl) | 0.1 | - | - | 0.1 |
| Na (mEq/l) | 144 | 148 | 160 | 149 |
| Cl (mEq/l) | 110 | 117 | 135 | 112 |
| K (mEq/l) | 4.3 | 6 | 4.3 | 4.6 |
| Ca (mEq/l) | 3.1 | - | - | 9.5 |
RBC: red blood cell; Ht: hematocrit; Hb: hemoglobin; WBC: white blood cell; Neu: neutrophil; Lym: lymphocyte; Mon: monocyte; Eos: eosinophil; FIB: fibrinogen; PLT: platelet; TP: total protein; ALB: albumin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline aminotransferase; BUN: blood urea nitrogen; Cre: creatine; BS: blood sugar; T-CHO: total cholesterol; iP: inorganic phosphorus; T-Bil: total bilirubin. a) Tests were performed between the first and second surgery.
Following catheter insertion, the dog was first retained in the dorsal position, and the skin near the inguinal region was incised at both sides of the penis. Opening-style castration was performed, and the tunica vaginalis communis was held. The dorsal skin on the left and right sides of the intersection of the ischial tuberosity and the ischial arch was perforated using a surgical knife. A Kelly clamp was carefully inserted into the pelvic cavity through the perforated skin, and the clamp tip was directed outside the external inguinal ring to grasp the tunica vaginalis communis. The tunica vaginalis communis was introduced into the perineum by pulling the Kelly clamp and then held in place. The incised wounds at both sides of the penis were closed according to protocol. The dog was retained in the abdominal position, and the right perineum was incised to redress the hernia. There was a hernia foramen between the external sphincter of the anus and the levator muscle of the anus, and the enlarged prostate and urinary bladder were detected. Based on these observations, the dog was definitively diagnosed with a perineal hernia (Fig. 1A). The exposed prostate was very large (7 × 5.5 × 4 cm) and firm (Fig. 1A). Because these organs were too large to replace into the abdominal cavity through the hernia foramen, the enlarged prostate was partially excised, and the reduced prostate and the urinary bladder were replaced into the abdominal cavity. The excised prostate was sent for histopathological examination. The tunica vaginalis communis was incised longitudinally, pushed open in a fan shape, and sutured to the sacrotuberous ligament, internal obturator muscle, levator muscle of the anus, and the external sphincter of the anus around the hernia foramen using a 2–0 absorptive monofilament suture and a sharply curved needle (ETHICON PDS-II®, Johnson & Johnson, Co., Ltd., Tokyo, Japan) to close the hernia foramen (Fig. 1B). Protruding retroperitoneal adipose tissue was detected at the hernia foramen upon incision of the left perineum. The adipose tissue was replaced into the abdominal cavity, and the hernia foramen was closed in a similar manner. Both sides of the skin, which was relaxed due to the swelling of the hernia, were incised in a crescent shape. Both sides of the subcutaneous tissue were sutured to both sides of the skin according to protocol, and the operation was completed. The dog recovered from anesthesia without any complications. Very little urinary excretion was observed in the urethral catheter and syringe suction of urine was impossible. On the following day, hematological tests revealed increased liver enzyme activities, hyperkalemia, azotemia, and hypercreatininemia (Table 1). Ultrasound revealed retention of urine in the bladder and showed that the enlarged prostate was compressing the bladder. These findings suggested the possibility that urine was retained in the urinary bladder due to compression of the urethra by the prostate, which had been replaced into the abdominal cavity. The dog underwent surgery the same day to remove the prostate. The abdominal midline of the dog was incised for laparotomy from below the umbilicus to the leading edge of the pubis. The locations of the prostate, urinary bladder, and urethra were confirmed (Fig. 2A), and the prostate was removed, leaving the catheter inside the urethra. The urethra and urinary bladder were anastomosed (Fig. 2B) using an absorptive monofilament suture and needle (5–0 PDS-II®), and the abdomen was closed according to protocol. The dog was administered analgesics and anesthetizing agents, as in the previous operation. Postoperative examination revealed normal urinary volume, and the hyperkalemia, azotemia, and hypercreatininemia had disappeared (Table 1). The dog was discharged from the hospital two days later. Examination prior to discharge showed a decrease in lymphocyte counts.
Fig. 1.
Operation for perineal hernia repair in a dog. A: The urinary bladder (b) and enlarged prostate (p) are shown in the right hernia contents. B: The hernia foramen was closed with the tunica vaginalis communis (t).
Fig. 2.
Excision of the prostate. A: The urinary bladder (b) and reduced prostate (p) are shown in the abdominal cavity. B: The prostate was removed and the urinary bladder (b) was anastomosed (Δ) with the ureter (u) and urethra (*).
Histopathological examination of the extracted prostate showed diffuse proliferation of medium-sized lymphoid cells with intensely stained round-to-oval-shaped nuclei and small amounts of cytoplasm surrounding the characteristic structures of the prostate. The nucleoli of the cells were obscured. Histopathological examinations of the partially excised and the totally removed prostate revealed similar findings. Based on these findings, the dog was diagnosed with a case of terminally differentiated lymphoma (Fig. 3).
Fig. 3.
Histopathology of the excised prostate. Diffuse proliferation of medium-size lymphoid cells with intensely stained round-to-oval-shaped nuclei and small amounts of cytoplasm surrounding the characteristic structures of the prostate. Nucleoli of the cells are obscured.
The dog revisited 17 days after the first visit for careful examination of the lymphoma and treatment. The owner reported the development of frequent vomiting, and the dog exhibited significant emaciation and wasting (body weight, 7.2 kg). Palpation did not confirm enlargement of the body surface lymph nodes. Hematological examination indicated advanced anemia and increased lymphocytes (Table 1). Because of the frequent vomiting and significant emaciation, ultrasound examination of the liver was performed. Ultrasonography showed diffuse areas of high echogenicity (Fig. 4A); enlargement of the medial subiliac lymph node (16.3 × 7.1 mm), jejunal lymph node (16.9 × 8.6 mm), and gastric lymph node (12.5 × 8.3 mm); and hyperplasia of the stomach walls (11.1 mm). Ultrasound-guided fine-needle aspiration (FNA) of the liver and stomach walls was performed, and the specimens were sent for cytological examination. The dog was prescribed prednisolone (2 mg/kg, once per day [s.i.d.] orally [p.o.]), which was to be administered until the next visit. However, the dog died 10 days later. The owner did not grant permission for necropsy of the animal.
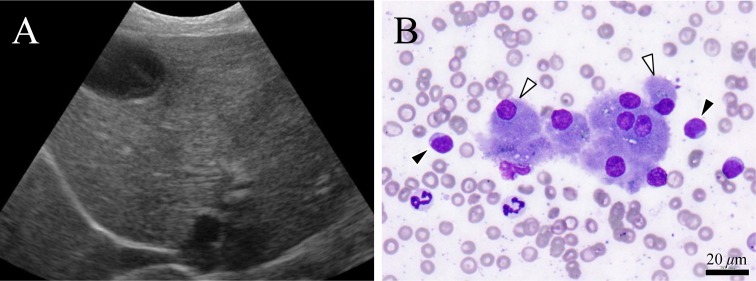
Fig. 4.
Ultrasonogram of the liver and smear cytology. A: Diffuse areas of high echogenicity in the liver. B: Hepatocytes (Δ) and lymphocytes (▲) with medium to large nuclei scattered around the hepatocytes are visible. The same types of cells were found in the stomach walls.
Histopathological examinations of the specimens collected at the revisit showed a number of atypical lymphocytes with medium to large nuclei in the smears of blood, liver, and stomach walls (Fig. 4B); lymphoma was confirmed in those organs. Immunohistochemistry of the extracted prostate was negative for cluster of differentiation 3 (CD3) and positive for CD20 and CD79a. From these results, the dog was diagnosed with B-cell lymphoma (Fig. 5).
Fig. 5.
Immunohistochemistry of the prostate was negative for cluster of differentiation 3 (CD3) (A) and positive for CD20 (B) and CD79a (C).
The dog presented in this report was diagnosed with prostate B-cell lymphoma in the content of an irreducible perineal hernia. Generally, most canine lymphomas are B-cell lymphomas [10], as was this one. However, lymphoma rarely develops in the prostate in dogs, and only four cases—including two dogs with primary prostate lymphoma [6, 13] and two dogs with prostate lymphomas that developed secondary to digestive organ lymphoma and skin lymphoma [1, 7]—have been reported. Among these, one lymphoma case [7] was confirmed to have a phenotype of T-cell lymphoma that developed secondary to skin lymphoma of the oral mucosa. This dog died 17 months after the first visit. Necropsy of the dog showed metastases of the lymphoma in multiple organs, but enlargement of the prostate was not reported [7]. The present paper is the first to report B-cell lymphoma in a dog with an enlarged prostate.
Far longer remission phases and survival times are reported in dogs with B-cell lymphomas than in those with T-cell lymphomas [10]. The dog presented in this report died about one month after the diagnosis of B-cell lymphoma, but the disease was considered to have developed long before the diagnosis and continued to progress following the diagnosis. In cases of prostate lymphoma, development of clinical symptoms due to enlargement of the prostate has been reported [1, 6, 13]. Difficulty in urination had been observed for more than 1 year before the first visit in the present case. However, a perineal hernia was considered to be the cause of this symptom, and the presence of lymphoma in the prostate was not considered. Although the causes of anemia, increased white blood cell count, and vomiting should have been investigated at the first visit, the perineal hernia repair was preferentially performed. Furthermore, because increased lymphocyte counts were mitigated at the final examination prior to discharge, a detailed examination was not conducted. Diagnosis of lymphoma may have been delayed because the perineal hernia was left untreated for 19 months.
In this case, a low-grade prostatic lymphoma was diagnosed by histopathological examination. Generally, better prognoses are reported in low-grade lymphomas than in high-grade lymphomas [12]. However, the present case was considered an advanced case at the time of the first visit for the following reasons [9]: the dog died one month after the first visit; the dog had already presented with clinical symptoms such as vomiting; and lymphoma was indicated shortly after diagnosis by examinations of smear specimens from the blood, liver, and stomach walls. In human cases, it has been reported that diagnoses of lymphoma in the liver, spleen, lymph nodes, or peripheral blood within one month of detection of prostate lesions suggests the presence of primary sites other than the prostate [2]. In the present case, the prostatic lymphoma was considered to have developed secondary to lymphoma that had emerged in other organs, but the primary site could not be confirmed.
The hernia contents, enlarged prostate and urinary bladder, could not be replaced at first; therefore, a portion of the prostate was partially excised for size reduction. There is a possibility that this partial excision disseminated the lymphoma in the abdominal cavity. However, the ureter and urethra were present in the hernia foramen, and total resection of the prostate was impossible before repositioning them into the abdominal cavity. Perineal hernia repair seemed unachievable without reducing the size of the prostate. In this case, we did not conduct preoperative imaging of the lower abdomen or the contents of the hernia. This is a point for consideration, although it was difficult to determine the possibility of repositioning the hernia contents before surgery. Even in cases in which the hernia contents cannot be replaced during the examination prior to surgery, the contents can sometimes be replaced after exposure. It was presumed that the catheter bent while repositioning the bladder and the prostate during the first surgery. The enlarged size of the prostate was considered the reason the catheter did not resume its original function, even in the pelvic cavity. Therefore, to circumvent the development of disease progression, perineal hernia repair should be performed as early as possible, before the prostate becomes too large to replace.
The prostate is frequently found in the contents of perineal hernias [3]. Development of lymphoma in the prostate is unusual, but possible [1, 6, 7, 13]. These two conditions developed at the same time in the present case, and the dog’s condition was ultimately fatal because of a delay in diagnosis and appropriate treatment. It should be recognized that the development of such a disease state is possible. As many aspects regarding the pathology of prostatic lymphoma remain unknown, accumulation of more case information is expected in the future.
REFERENCES
- 1.Assin R., Baldi A., Citro G., Spugnini E. P.2008. Prostate as sole unusual recurrence site of lymphoma in a dog. In Vivo 22: 755–757. [PubMed] [Google Scholar]
- 2.Bostwick D. G., Mann R. B.1985. Malignant lymphomas involving the prostate. A study of 13 cases. Cancer 56: 2932–2938. doi: [DOI] [PubMed] [Google Scholar]
- 3.Christopher R. B., Rhondda B. C.2002. Perineal hernia. pp. 487–497. In: Text Book of Small Animal Surgery, 3rd ed. (Slatter, D. ed.), Saunders, Philadelphia. [Google Scholar]
- 4.Greenlee P. G., Filippa D. A., Quimby F. W., Patnaik A. K., Calvano S. E., Matus R. E., Kimmel M., Hurvitz A. I., Lieberman P. H.1990. Lymphomas in dogs. A morphologic, immunologic, and clinical study. Cancer 66: 480–490. doi: [DOI] [PubMed] [Google Scholar]
- 5.Madewell B. R., Theilen G. H.1987. Hematopoietic neoplasms, sarcomas and related conditions: canine. Part IV. pp. 392–407. In: Veterinary Cancer Medicine, 2nd ed. (Theilen, G. H. and Madewell, B. R. eds.), Lea & Febiger, Philadelphia. [Google Scholar]
- 6.Mainwaring C. J.1990. Primary lymphoma of the prostate in a dog. J. Small Anim. Pract. 31: 617–619. doi: 10.1111/j.1748-5827.1990.tb00709.x [DOI] [Google Scholar]
- 7.Mineshige T., Kawarai S., Yauchi T., Segawa K., Neo S., Sugahara G., Kamiie J., Hisasue M., Shirota K.2016. Cutaneous epitheliotropic T-cell lymphoma with systemic dissemination in a dog. J. Vet. Diagn. Invest. 28: 327–331. doi: 10.1177/1040638716637642 [DOI] [PubMed] [Google Scholar]
- 8.Miyawaki S., Watanabe K., Ohba Y., Murakami M., Fujiwara H., Yamazoe K.2011. Paraprostatic cyst within the perineal region of a perineal hemia in a dog. Nihon Jui Masui Gekagaku Zasshi 42: 25–28. [Google Scholar]
- 9.Owen L. N.1980. TNM classification of tumours in domestic animals. Geneva: World Health Organization. 1st. [Google Scholar]
- 10.Ruslander D. A., Gebhard D. H., Tompkins M. B., Grindem C. B., Page R. L.1997. Immunophenotypic characterization of canine lymphoproliferative disorders. In Vivo 11: 169–172. [PubMed] [Google Scholar]
- 11.Tanaka S., Asano K., Yamaya Y., Sato T., Tsumagari S., Nagaoka K.2004. Reconstructive surgery of the pelvic diaphragm using the tunica vaginalis communis in a dog with perineal hernia. Jpn. J. Vet. Med. Assoc. 57: 451–454. doi: 10.12935/jvma1951.57.451 [DOI] [Google Scholar]
- 12.Teske E., van Heerde P., Rutteman G. R., Kurzman I. D., Moore P. F., MacEwen E. G.1994. Prognostic factors for treatment of malignant lymphoma in dogs. J. Am. Vet. Med. Assoc. 205: 1722–1728. [PubMed] [Google Scholar]
- 13.Winter M. D., Locke J. E., Penninck D. G.2006. Imaging diagnosis—urinary obstruction secondary to prostaticlymphoma in a young dog. Vet. Radiol. Ultrasound 47: 597–601. doi: 10.1111/j.1740-8261.2006.00193.x [DOI] [PubMed] [Google Scholar]