Abstract
Objective:
Bevacizumab is approved for use in combination with chemotherapy for metastatic/recurrent cervical cancer (CC), with increased survival/response rates. However, use of bevacizumab is not always feasible or safe. The purpose of this study was to identify the percentage of metastatic/recurrent CC patients at our institution who would have been eligible to receive bevacizumab.
Methods:
A retrospective study was conducted to identify metastatic/recurrent CC patients treated at UFHealth between 2006 and 2016. Chart review was performed to determine if the patient met bevacizumab eligibility criteria.
Results:
In total, 79 patients with metastatic/recurrent CC were identified; 85.5% would have been ineligible to receive bevacizumab, and 14.5% would have been eligible. The most common reason for exclusion was active bleeding (68.4%); 94% of which was vaginal. In all, 27.6% would be excluded due to poor renal function, and 23.7% due to poor performance status (PS).
Conclusions:
Despite improved survival, only 14.5% of metastatic/recurrent CC patients treated over a 10-year period would have been eligible to receive bevacizumab. Most patients would have been excluded due to active bleeding, most commonly vaginal bleeding, a common complication from their disease. Identifying novel therapies for metastatic/recurrent CC patients with improved safety profiles that would allow for their use in this challenging population is critical.
Keywords: Bevacizumab, metastatic cervical cancer, recurrent cervical cancer, eligibility, bleeding, retrospective review
Introduction
Cervical cancer is the fourth most common cancer in women worldwide,1 and 12th most common cancer in women in the United States.2 Since the 1950s, the number of new diagnoses and deaths from cervical cancer have fallen dramatically, by over 70%,3 largely due to a concerted effort from multiple organizations emphasizing health maintenance with early screening for cervical cancer with the Papanicolaou test (more commonly known as the “Pap smear” or “Pap test”) and pelvic examination. The Pap test was developed by George Papanicolaou together with Herbert Traut4 and is considered the first screening test to be widely used for cancer. From an epidemiological standpoint, it is considered to be the most successful screening test leading to the detection and prevention of invasive malignancies. Nevertheless, despite vast compliance among physicians and patients regarding appropriate screening, there are still numerous cases of cervical cancer diagnosed annually,2 partly due to inadequate access to healthcare resources and decreased health literacy.5
There are roughly 12,800 new cases of cervical cancer diagnosed in the United States annually,6 with approximately 4200 deaths. Females have a 1 in 147 chance (0.68%) from birth to death to develop metastatic cervical cancer.6 Metastatic cervical cancer is defined as International Federation of Gynecology and Obstetrics (FIGO) stage IVA or IVB, meaning that the cancer has spread from the cervix to the rectum, bladder, para-aortic/inguinal lymph nodes, or distant organs. Through the years, there have been numerous advances in the treatment of cervical cancer. Early stage cervical cancer (up to Ib1) can be cured with surgical resection alone, while locally advanced stages can be cured with concurrent chemotherapy and radiation. The mainstay of treatment for metastatic cervical cancer has long been platinum-based chemotherapy.7 Unfortunately, the 5-year survival rate for patients diagnosed with all stages of cervical cancer is 70%, and for patients with metastatic cervical cancer, the survival rate is significantly lower, at 17%.6 This has led to numerous trials to identify therapies to prolong survival.
The only biologic agent approved for use in cervical cancer is bevacizumab (Avastin™).8 Bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, acts as an anti-angiogenic agent and therefore inhibits blood vessel formation, impeding the growth of tissues (including cancer), and by that avenue causing cell death. Bevacizumab was approved by the Food and Drug Administration (FDA) for use in metastatic or recurrent cervical cancer in 20148 after results of Gynecologic Oncology Group (GOG)-240, a pivotal randomized phase III study, showed that the addition of bevacizumab to chemotherapy (topotecan-paclitaxel and cisplatin-paclitaxel) was associated with statistically significant higher response rates as well as increased overall survival (17.0 months vs 13.3 months, hazard ratio for death 0.71, 95% confidence interval 0.54-0.95).9 Although chemotherapy plus bevacizumab is a first line therapy option for the treatment of metastatic cervical cancer, it comprises three of eight such first line options,10 and it may not be able to be used in many patients due to its contraindications and side effect profile. Bevacizumab is known to lead to an increased risk for hypertension, gastrointestinal perforation, hemorrhage, wound healing complications, thromboembolism, and proteinuria.9 In metastatic cervical cancer, bevacizumab used with chemotherapy was associated with improved length of survival without significantly increased side effects or decrease in quality of life,9 suggesting that it could play a very important role in the current and future treatment of metastatic/recurrent cervical cancer.
We hypothesize that, despite the potential for improvement in survival associated with the use of bevacizumab in metastatic/recurrent cervical cancer, only a limited number of these women are actually eligible to receive this drug if the strict exclusion criteria from the pivotal GOG-240 trial are followed for appropriate patient selection. Oftentimes, strict exclusionary criteria in trials leave out many patients compared to what is seen in clinical practice, for example, using XRT to control bleeding and then potentially giving bevacizumab. In the GOG-240 trial, patients were eligible if they presented with measurable metastatic, persistent, or recurrent cervical cancer, performance status score of 0 or 1, and adequate renal, hepatic, and bone marrow function. Patients were excluded if they were candidates for curative therapy by means of pelvic exenteration, patients with nonhealing wounds, active bleeding conditions, or inadequately anticoagulated thromboembolism.9 To that end, we performed a retrospective review of patient medical charts at University of Florida Health (UFHealth), a tertiary cancer referral center in Gainesville, Florida. Our aim was to determine what percentage of patients with metastatic/recurrent cervical cancer would have been eligible to receive bevacizumab-based strictly on the GOG-240 exclusion criteria.
Methods
A retrospective study was conducted to identify all patients in the last decade (2006-2016 inclusive) who had a diagnosis of cervical cancer who were either diagnosed or received treatment at UFHealth. This study was reviewed and approved by the University of Florida Institutional Review Board. Patients were identified though the UFHealth Tumor Registry. This information included name, date of birth, medical record number, year of diagnosis, and age of diagnosis. Detailed chart review was performed on these charts to determine that the selected patients did in fact have invasive cervical cancer as well as to determine the stage of the cervical cancer. Patients with cervical interstitial neoplasia (CIN) were excluded.
For those patients who were determined to have stage IV cervical cancer (or those patients who had recurrent cervical cancer), detailed chart review was performed to determine their kidney, liver, and bone marrow function, GOG performance status, presence of an active bleeding condition or nonhealing wound, or inadequately treated venous thromboembolism. For purposes of analysis, the aforementioned data points were determined based on when metastatic cervical cancer was diagnosed or when the cervical cancer recurred (e.g. if a patient had normal renal function when diagnosed with metastatic cervical cancer, but months later developed renal insufficiency, they would not be excluded as their renal function was normal at time of diagnosis of metastatic disease). Data were then analyzed to determine if patients would have been eligible to receive bevacizumab based on the exclusion criteria used in the Tewari et al publication. Specifically, these exclusion criteria included the following: renal impairment (estimated glomerular filtration rate [eGFR] < 45 mL/min), hepatic impairment, bone marrow dysfunction, active bleeding conditions, nonhealing wounds, inadequately anticoagulated thromboembolism, or poor performance status (GOG 2 or higher).9
Results
There were 265 patients with a diagnosis of cervical cancer seen at UFHealth between 2006 and 2016. Patient demographic and clinical data are presented in Table 1. Of those, 62 patients (23.4%) were determined to have stage IV cervical cancer (Table 1). In all, 17 patients initially presented with a lower stage disease but ultimately developed recurrent disease. The most frequent site of metastasis was the lung (25/62 patients, 40.3%). This was followed by the liver (14/62 patients, 22.6%), bone (14/62 patients, 22.6%), and para-aortic lymph nodes (11/62 patients, 17.7%). Sixteen of 62 patients (25.8%) had multiple sites of metastasis, the most common of which was lung and liver (7 patients, 11.3%) (Table 2).
Table 1.
Characteristics of patients with metastatic and recurrent cervical cancer.
| Characteristic | Age < 50 (n = 40) | Age > 50 (n = 39) |
|---|---|---|
| Age at diagnosis | ||
| Mean (standard deviation) | 40.5 (6.2) | 59.7 (6.9) |
| Median | 42 | 57 |
| Race | ||
| White (%) | 33 (82.5%) | 26 (66.7%) |
| Black (%) | 4 (10.0%) | 13 (33.3%) |
| Other (%) | 3 (7.5%) | 0 (0.0%) |
| BMI (kg/m2) (standard deviation) | 27.4 (7.4) | 25.7 (7.5) |
| Histology | ||
| Squamous | 30 (75.0%) | 29 (74.4%) |
| Adenocarcinoma | 5 (12.5%) | 5 (12.8%) |
| Other | 5a (12.5%) | 5b (12.8%) |
| Surgery (%) | 13 (32.5%) | 13 (33.3%) |
| Radiation (%) | 39 (97.5%) | 33 (84.6%) |
| Chemotherapy (%) | 34 (85.0%) | 24 (61.5%) |
| Platinum-based | 29 (72.5%) | 22 (56.4%) |
2 adenosquamous, 1 glassy cell, 1 small cell, 1 unknown.
1 adenosquamous, 1 serous, 1 poorly differentiated, 2 unknown.
Table 2.
Sites of metastasis in patients with metastatic cervical cancer.
| Lung | 13 (21.0%) |
| Para-aortic lymph nodes | 10 (16.1%) |
| Bone | 7 (11.3%) |
| Bladder | 5 (8.1%) |
| Liver | 4 (6.5%) |
| Rectum | 2 (3.2%) |
| Other | 5 (8.1%) |
| Two sites of metastasis | 14 (22.6%) |
| Lung + liver | 5 |
| Lung + brain | 3 |
| Liver + bone | 3 |
| Lung + bone | 2 |
| Rectum + bone | 1 |
| Three sites of metastasis | 2 (3.2%) |
Of the 79 patients with metastatic cervical cancer, there was sufficient data in the medical records to analyze for the eligibility of bevacizumab in 76 of 79 cases (96.2%). Of those 76 patients, only 11 (14.5%) would have been eligible to receive bevacizumab based on the exclusion criteria. Sixty-five of 76 patients (85.5%) would have been ineligible to receive bevacizumab due to exclusion criteria (Figure 1).
Figure 1.
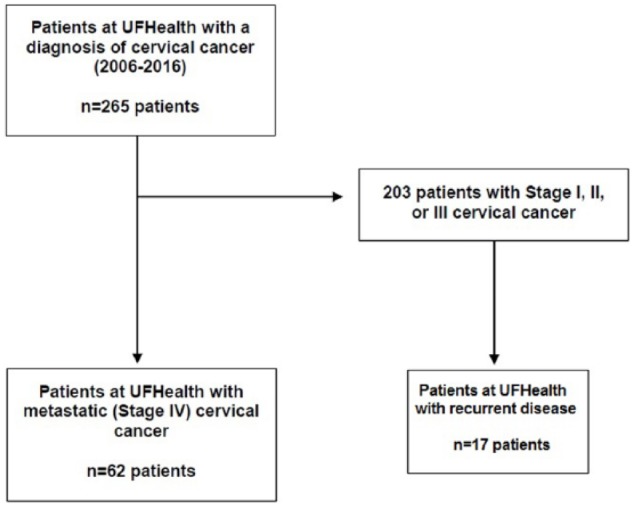
CONSORT Diagram for eligibility of bevacizumab.
The most common reason patients would have been excluded from bevacizumab use was due to active bleeding (52/76 patients, 68.4%), and of these, 49 of the 52 with active bleeding had vaginal bleeding (94.2%). The second most common reason for exclusion was inadequate renal function (21/76 patients, 27.6%), followed by poor performance status (18/76 patients, 23.7%) and inadequately anticoagulated thromboembolism (14/76 patients, 18.4%). Twenty-seven patients (35.5%) were excluded due to one excluding factor present, 27 patients (35.5%) were excluded due to two factors, 9 patients (11.8%) were excluded due to three factors, and 2 patients (2.6%) were excluded due to four factors. Of the 27 patients who would have been excluded due to one factor, 22 (81.5%) of them were due to vaginal bleeding.
The average hemoglobin/hematocrit of patients with metastatic cervical cancer was 10.4 g/dL (32.0%). For those patients with active bleeding, their average hemoglobin/hematocrit was 9.9 g/dL (30.8%) compared to those without active bleeding, whose average hemoglobin/hematocrit was 11.3 g/dL (34.6%). For those patients with active bleeding, 11/49 (22.4%) of patients had a hemoglobin above 12 g/dL, 22/49 (44.9%) had a hemoglobin above 10 g/dL, and 44/49 (89.8%) had a hemoglobin above 7 g/dL.
Discussion
It is clear that despite the advances in screening and diagnosis of cervical cancer, there are still numerous cases yearly of cervical cancer, with a substantial fraction of those metastatic. The development of novel and targeted therapies have shown promise, particularly bevacizumab, which first showed an increase in progression free survival in the phase II GOG-227 C trial.11 This was followed by the phase III GOG-240 trial, which showed that the addition of bevacizumab resulted in increased overall survival and higher response rates.9 Major side effects associated with bevacizumab included hypertension, thromboembolic events, and gastrointestinal (GI) fistulas,9 while in clinical practice, the major side effects were fistula formation, anemia, thrombocytopenia, and bleeding.12 This study established that combination chemotherapy with bevacizumab was a first-line treatment for patients with metastatic cervical cancer who were not excluded based on the aforementioned seven exclusion criteria, representing a very select population.
Despite the survival benefits and higher response rates of patients treated with bevacizumab in combination with chemotherapy, only a fraction (14.5%) of metastatic or recurrent cervical cancer patients treated at our institution over a 10-year period would have been eligible to receive bevacizumab. Most patients would have been excluded due to a bleeding condition (68.4%), the most common site of which was vaginal (94.2% of bleeding cases). It should be noted that many of these patients with active bleeding at time of diagnosis of their metastatic cervical cancer had vaginal bleeding with mild anemia (not requiring blood transfusions), and oftentimes, vaginal bleeding was their chief complaint that led them to seek medical attention, thereby leading to their diagnosis (often presenting with advanced disease). However, despite this being the most common presentation of patients with metastatic and recurrent cervical cancer, the use of bevacizumab would not be recommended for use in this population of women due to its side effect profile and risk for hemorrhage. It is possible that patients enrolled in trials with bevacizumab likely represent a selected group of women with advanced cervical cancer, not excluded by the aforementioned criteria. It is also possible (and likely) that investigators used palliative pelvic radiation to control bleeding prior to enrollment in these trials, in order to increase likelihood of patient eligibility.
With strict inclusion criteria, it is a challenge to find the ideal therapy in this patient population, especially at our tertiary care center in an area of under-served counties. Health disparities of poor socioeconomic status and decreased income also play a role. The topic of broadening eligibility and inclusionary criteria to clinical trials has recently generated a great deal of discussion.13,14 Specifically, there is a concern that restrictive eligibility criteria for cancer trials causes the results to be less generalizable,14 as many patients who ultimately receive a medication in real world practice may not meet the strict inclusionary criteria of the patient cohort in the trial. In our patient population in particular, loosening restrictive eligibility criteria is important because it would expand the number of patients eligible to receive a medication. Many patients with metastatic cervical cancer received radiation therapy to help control bleeding, and especially in patients with recurrent cervical cancer, some patients got repeat radiation (i.e. after definitive chemoradiation from their initial presentation) to help control bleeding as a palliative measure. The incorporation of real-world practice (e.g. prior radiation therapy) rather than strictly following the inclusionary criteria on the approved trial often happens as many clinicians are comfortable giving medications; however, this is not fully generalizable and therefore some patients may not receive therapies based on the comfort level of the particular clinician. With expanding strict eligibility criteria to patients who may likely get the medication regardless, improved access can be seen with hopefully improved survival outcomes.
Of patients excluded due to one factor, 22 of 27 (81.5%) would be excluded due to vaginal bleeding. Of that subset, six patients had a hemoglobin over 12 g/dL and 11 patients had a hemoglobin over 10 g/dL. Only one patient had a hemoglobin under 7 g/dL, the threshold at which a blood transfusion may be considered acceptable. In the pivotal trial, establishing bevacizumab with chemotherapy as a new option for women with metastatic cervical cancer, women were excluded from participation if there was evidence of active bleeding.9 This included vaginal bleeding. Anemia due to bleeding or need for blood transfusions due to bleeding were not used in quantifying or clarifying this exclusion criterion. It is unknown whether use of bevacizumab in the setting of mild bleeding, perhaps quantified by hemoglobin level, may be safe.
It is important to note that in clinical practice, eligibility for any particular therapy is a dynamic process. Patients presenting with vaginal bleeding, especially if such bleeding is low-grade, could potentially be managed with palliative radiation to control or stop bleeding15 in order to allow more women to receive bevacizumab as part of their therapy. This is left to the discretion of the practitioner, anticipating control of tumor-associated bleeding. An approach utilizing bevacizumab after palliative pelvic radiation for bleeding control may be appropriate in a selected patient population. Further investigation is necessary to determine whether the amount of bleeding, possibly quantified by the hemoglobin level, affects the risk of hemorrhage in patients receiving bevacizumab.
The strengths of our study is that our institution is a referral center with a multidisciplinary approach to cancer treat-ment, and our patient population has similar demographic characteristics to the GOG-240 trial patients, including age, white race, and most the common histology as squamous cell carcinoma. The limitation of our study is inherent to our retrospective study design, and single institution experience.
Conclusion
Despite the documented improved survival and higher response rates associated with bevacizumab use in combination with chemotherapy, only 14.5% of metastatic cervical cancer patients treated at our tertiary referral cancer center over a 10-year period would have been eligible to receive bevacizumab. Most patients would have been excluded due to active bleeding, most commonly vaginal bleeding, often the presenting symptom of advanced cervical cancer. Though clinical trial data supports the use of bevacizumab with chemotherapy, many patients are simply not eligible due to complications from their disease. Identifying novel therapies for metastatic and recurrent cervical cancer patients with improved safety profiles that would allow for their use in this challenging population, as well as potentially broadening eligibility criteria for clinical trials of new therapies are critical.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Publication of this article was funded in part by the University of Florida Open Access Publishing Fund.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions: All authors participated in the conceptualization, methodology, investigation, analysis, and writing of the manuscript.
ORCID iD: William Paul Skelton IV  https://orcid.org/0000-0003-4923-8178
https://orcid.org/0000-0003-4923-8178
References
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.1, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon: International Agency for Research on Cancer; 2014. http://globocan.iarc.fr. [Google Scholar]
- 2. American Cancer Society. Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 3. Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer. 2003;97:3133–3275. [DOI] [PubMed] [Google Scholar]
- 4. Traut HF, Papanicolaou GN. Cancer of the uterus: the vaginal smear in its diagnosis. Cal West Med. 1943;59:121–122. [PMC free article] [PubMed] [Google Scholar]
- 5. Barnholtz-Sloan JL, Patel N, Rollison D, Kortepeter K, MacKinnon J, Giuliano A. Incidence trends of invasive cervical cancer in the United States by combined race and ethnicity. Cancer Causes Control. 2009;20:1129–1138. [DOI] [PubMed] [Google Scholar]
- 6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 7. Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bevacizumab BLA 125085 approval letter, 14 August 2014. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2004/125085ltr.pdf
- 9. Tewari KS, Sill MW, Long HJ, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Cervical Cancer (version 1.2017). Fort Washington, PA: National Comprehensive Cancer Network; 2016. [Google Scholar]
- 11. Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Godoy-Ortiz A, Plata Y, Alcaide J, et al. Bevacizumab for recurrent, persistent or advanced cervical cancer: reproducibility of GOG 240 study results in “real world” patients [published online ahead of print December 8, 2017]. Clin Transl Oncol. doi: 10.1007/s12094-017-1808-x. [DOI] [PubMed] [Google Scholar]
- 13. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and friends of cancer research joint research statement. J Clin Oncol. 2017;35:3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin S, Pazdur R, Sridhara R. Re-evaluating eligibility criteria for oncology clinical trials: analysis of investigational new drug applications in 2015. J Clin Oncol. 2017;35:3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnstone C, Rich SE. Bleeding in cancer patients and its treatment: a review. Ann Palliat Med 2018;7:265–273. [DOI] [PubMed] [Google Scholar]


