Abstract
Paeoniflorin (PF) is one of the main pharmacodynamic components of Paeonia suffruticosa Andr, which has a significant anti-inflammatory effect on rheumatoid arthritis (RA), with a mechanism related to the tumor necrosis factor α (TNF-α). The aim of the present study was to investigate the role of PF in the apoptosis and expression of NF-κBp65 of L929 fibroblastoma cells induced by TNF-α. Our results showed that different concentrations of PF can significantly reduce the growth inhibition of L929 cells. Moreover, morphological observations, Hoechst 33342 staining, and flow cytometry detection of apoptosis showed that PF can significantly attenuate the TNF-α-induced apoptosis in a dose-dependent manner. Western blot analysis revealed that TNF-α induced the activation of NF-κBp65, whereas PF treatment had a marked dose-dependent suppression on it, which indicates that its action might be associated with inhibiting NF-κB signaling pathway. These results show that PF exerts a beneficial effect on L929 cells to prevent TNF-α-induced apoptosis and expression of NF-κBp65, which would be helpful to clarify its role in the treatment of RA.
Keywords: paeoniflorin, TNF-α, L929 fibroblastoma cells, apoptosis, NF-κBp65
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by abnormal immune function, joint synovial hyperplasia, articular cartilage, and joint destruction, but its details of the underlying mechanism remain unknown.1–2 Tumor necrosis factor α is a pro-inflammatory cytokine associated with RA, which contributes to chronic, low-grade inflammation and plays a pivotal role in the development of RA.3–4 Studies showed that the antibody blocking tumor necrosis factor α (TNF-α) could significantly reduce the expression of TNF-α of patients with RA.5 Therefore, the TNF-α blockade will be conducive to preventing RA inflammation. Tumor necrosis factor α inhibitors, such as etanercept, have shown miraculous curative effect in RA therapy. It not only significantly improved the clinical symptoms of most patients with RA but also significantly reduced the disease activity of RA.6–8
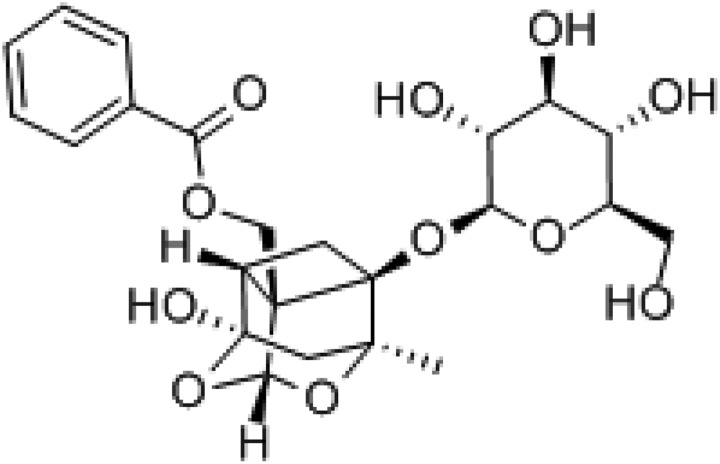
Paeoniflorin (PF, Figure 1) is one of the bioactive monoterpene glucoside in Paeoniae Radix, the roots of Paeonia suffruticosa Andr, which has various pharmacological effects such as anti-inflammation and regulating immunity, and so on.9 Today, it has been widely used in treating patients with RA in traditional Chinese herbal medicine with a good therapeutic effect, but its mechanism for treating RA is unclear. We hypothesized that PF may be adopted as a kind of TNF-α inhibitors to treat RA by antagonizing the TNF-α pathway. The fibroblastoma cell line L929 is highly sensitive to TNF-α-induced cell apoptosis and widely used to explore the mechanisms underlying TNF-α-induced cells apoptosis.10 So, it could be selected as a model for studying the antagonistic action of PF on TNF-α pathway. The present study was designed to investigate the mechanism of PF treating RA, through exploring the protective effect of PF on L929 cell apoptosis induced by TNF-α.
Figure 1.
Chemical structure of paeoniflorin (PF).
Materials and Methods
Drugs and Reagents
Paeoniflorin (purity > 98.5%, Sigma-Aldrich, Figure 1) was dissolved in phosphate-buffered saline (PBS) according to the manufacturer’s instructions. RPMI1640 was purchased from HyClone Inc (Logan, Utah, USA); Fetal bovine serum from Gibco Co; Recombinant mouse TNF-α was purchased from Hangzhou biologic biotechnology Limited; anti-NF-κBp65 antibody was purchased from Cell Signaling Technology Inc (Beverly, MA, USA); β-actin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); Annexin V/Propidium Iodide (PI) staining kit (BD Biosciences Pharmingen, California, USA); Hoechst 33342, BCA kit was purchased from Beyotime Biotechnology Inc (Hangzhou, China); sulforhodamine B (SRB) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell Culture and Drug Preparation
L929 cell line was purchased from American Type Culture Collection. L929 cells were grown in RPMI1640 medium (supplemented with 100 U·mL−1 penicillin, 100 U·mL−1 streptomycin, and 10% fetal bovine serum) and cultured in the incubator of 37°C. According to Jin et al,11 PF was dissolved in PBS, and serial dilutions (52, 26, 13, 6.5, 3.25, 1.625, and 0.813 μg·mL−1) were prepared as the drug treatment.
Measurement of Cytotoxicity of PF and TNF-α
L929 cells of the stationary phase were plated to a 96-well plate at a density of 1 × 104 cells/well. According to Vanlangenakke et al,10 L929 cells were treated with serial dilutions of TNF-α (40, 20, 10, 5, 2.5, 1.25, and 0.63 ng·mL−1) and PF (52, 26, 13, 6.5, 3.25, 1.625, and 0.813 μg·mL−1) for 24 hours. Then, the viability of L929 cells was measured by SRB, as described by Bahadori et al.12 According to the absorbance value, Optical Density (OD), we calculated cell viability rate: cell viability rate = 1 − (OD490test − OD490blank)/(OD490control − OD490blank) × 100%.
Cytomorphology
L929 cells of the stationary phase were plated to a 6-well plate at a density of 2 × 105 cells/well, treated with TNF-α (10 ng·mL−1, obtained by SRB) and different concentrations of PF for 24 hours. The morphological changes of L929 cells with different concentrations of PF were observed by an inverted microscope at ×100 magnification.
Hoechst33342 Fluorescent Staining
L929 cells of the stationary phase were plated to a 24-well plate at a density of 1 × 105 cells/well, treated with TNF-α (10 ng·mL−1) and different concentrations of PF solution for 24 hours. Then L929 cells were stained with Hoechst 33342 at 37°C for 20 minutes and then washed 2 times with PBS. Nuclear condensation and DNA fragmentation were visualized by fluorescence microscopy.
Flow Cytometric Analysis
Cell apoptosis of TNF-α-induced L929 cells was analyzed by Annexin-V/PI. Briefly, cells were harvested by trypsin digestion. Dual staining with Fluorescein Isothiocyanate (FITC)-conjugated Annexin V and PI was carried out to detect the cell apoptotic inducted by TNF-α. Cells were washed with PBS and suspended in 100 μL binding buffer (containing 1 μg·mL−1 Annexin V-FITC and 5 μg·mL−1 PI). Following incubated for 15 minutes at room temperature, cells were analyzed by flow cytometry. Annexin V-positive/PI-negative signifies apoptotic cells, whereas Annexin V/PI double positive signifies necrotic cells.
Detection of the Expression of NF-κBp65
Western blotting analysis was performed as described previously.13 Briefly, L929 cells were lysed and harvested with lysis buffer. After centrifugation, the supernatants were subjected to Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), followed by transferring to nitrocellulose membranes. Nitrocellulose membranes were incubated with 5% (wt/vol) dry nonfat milk in washing buffer at room temperature for 2 hours to block the nonspecific protein binding. Anti-NF-κBp65 antibody was diluted in washing buffer containing 5% (wt/vol) nonfat milk and applied to the membranes, overnight at 4°C. After extensive washing, the membranes were incubated with fluorescence antibodies for 2 hours at room temperature and washed 3 times. Target protein and β-actin were analyzed by using software of WCIF Image J.
Statistical Method
The data was analyzed by Graphpad Prism 7.0 and SPASS 22.0 and shown as mean (standard deviation [SD]; n = 3). Student t test was employed to determine significance between 2 groups and 1-way analysis of variance was used to determine significance among several groups. Differences were considered statistically when P < .05.
Results
Effect of PF and TNF-α on the inhibition of L929 cells
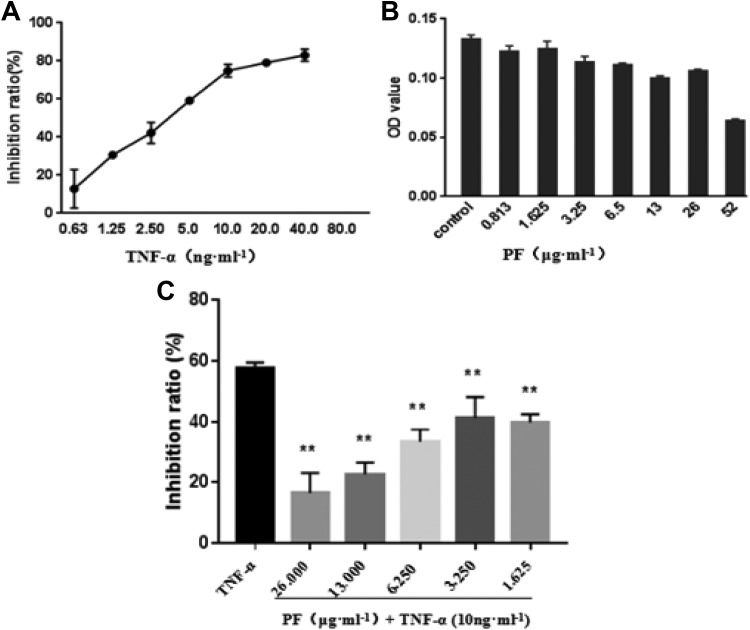
Sulforhodamine B was performed to exclude the possibility of cytotoxicity of PF and TNF-α on L929 cells that could misinterpret results. As shown in Figure 2A, the data obtained from Graphpad Prism 7.0 showed that IC50 (half maximal inhibitory concentration) value of TNF-α for 24 hours was 4.786 ng·mL−1. Moreover, L929 cells treated with PF in the concentration of 0.813 to 26 μg·mL−1 for 24=hours did not show significant cytotoxicity (Figure 2B). Therefore, L929 cells were treated with TNF-α (10 ng·mL−1) and PF (0.813-26 μg·mL−1) incubated for 24 hours. Then, the inhibition rate was calculated and compared to the TNF-α (10 ng·mL−1) control group. We concluded that PF could significantly reduce the inhibitory effect of TNF-α (10 ng·mL−1) on L929 cells in the range of 1.625 to 26 μg·mL−1, which was statistically significant and a certain concentration correlation (Figure 2C). These results indicated that TNF-α could inhibit the proliferation of L929 cells, whereas PF could antagonize this effect.
Figure 2.
Effect of paeoniflorin (PF) on L929 cell proliferation. A, According to the OD value, we calculated cell viability rate: cell viability rate = 1 − (OD490test − OD490blank)/(OD490control − OD490blank) × 100%. Graphpad Prism 7.0 was employed to calculate IC50 for further experiment. B, The cytotoxicity of PF on L929 cells. L929 cells were treated with increasing concentrations of PF (from 0.813 to 52 μg·mL−1 for 24 hours, the cell viability was then determined by sulforhodamine B (SRB). C, Dose-dependent inhibition changes of L929 cells with PF incubation of 24 hours (10 ng·mL−1 tumor necrosis factor α [TNF-α] as positive control). Compared to TNF-α (10 ng·mL−1) group, *P < .05, **P < .01 (n = 3).
Morphological Observation
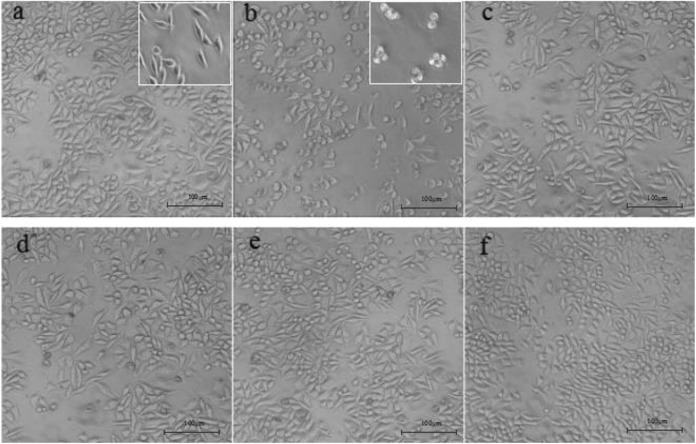
L929 cells were incubated with different doses of PF and TNF-α (10 ng·mL−1). Control group and TNF-α (10 ng·mL−1) group were set up. After being cultured for 24 hours, results showed that the cell density of TNF-α (10 ng·mL−1)-treated group (Figure 3B) decreased significantly, compared to the control group (Figure 3A), and morphology changes (shrink, round, poor refractive index, and irregular cell edge) were observed by an inverted light microscope. The morphology of L929 cells changed greatly and the number of fusiform cells increased, while shrinkage cells decreased with the increase of PF concentrations. These results indicated that PF could effectively improve the TNF-α-induced adverse changes in L929 cells.
Figure 3.
(×100) Effect of paeoniflorin (PF) on the morphologic changes in L929 cells. A, Control; B, tumor necrosis factor α (TNF-α; 10 ng·mL−1); C-F, TNF-α (10 ng·mL−1) + PF (3.25, 6.5, 13, and 26 μg·ml−1).
Observation of Hoechst 33342 Staining
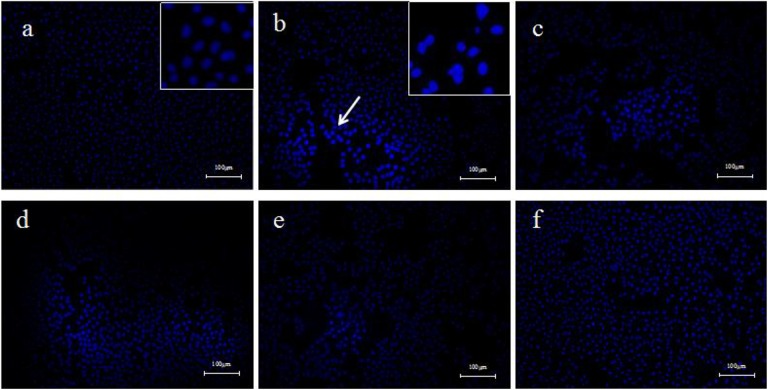
L929 cells were incubated with TNF-α (10 ng·mL−1) and different doses of PF. Solvent control group and TNF-α (10 ng·mL−1) group were set up. The results of hoechst33342 staining and fluorescence microscopy showed that the L929 cells in the control group were homogeneous and clear, and the nucleus were homogeneous blue. However, a large number of apoptotic cells were observed in the TNF-α (10 ng·mL−1) group, and the nucleus was condensed into dense and concentrated staining state. After being incubated with different doses of PF, apoptotic cells were significantly reduced and cellular state were improved with the increase of PF concentrations (Figure 4).
Figure 4.
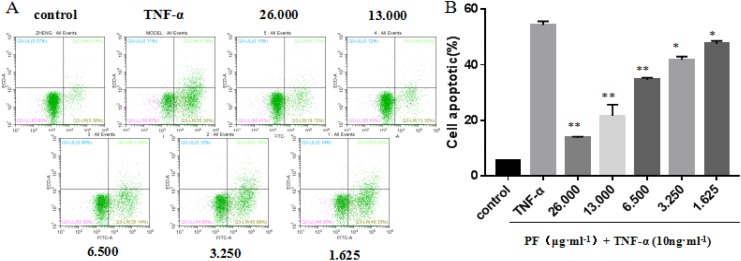
Paeoniflorin (PF) suppress tumor necrosis factor α (TNF-α)-induced cell apoptotic in L929 cells. A, L929 cells were treated with 10 ng·mL−1 TNF-α and various doses of PF for 24 hours. Cell apoptotic was measured by Annexin-V/PI double staining. B, The percentages of cell death are shown in the lower panel as mean (standard deviation [SD]; n = 3). Compared to TNF-α (10 ng·mL−1) group, **P < 0.01, *P < 0.05.
Paeoniflorin Suppress TNF-α-Induced Cell Apoptotic in L929 Cells
It remains unknown whether PF can promote TNF-α-induced cell apoptosis in L929 cells.14 For this purpose, L929 cells were treated with different doses of PF for 24 hours, after stimulating with 10 ng·mL−1 TNF-α. Surprisingly, cell apoptosis assay with Annexin-V/PI double staining revealed that PF could significantly suppress TNF-α-induced cell apoptosis in L929 cells, which is dose-dependent (Figure 5).
Figure 5.
(×100) Effect of paeoniflorin (PF) on the apoptosis of L929 cells. A, Control; B, tumor necrosis factor α (TNF-α; 10 ng·mL−1); C-F, TNF-α (10 ng·mL−1) + PF (3.250, 6.5, 13, and 26 μg·mL−1).
Paeoniflorin Suppresses TNF-α-Induced Apoptosis in L929 Cells via Inhibition of NF-κBp65
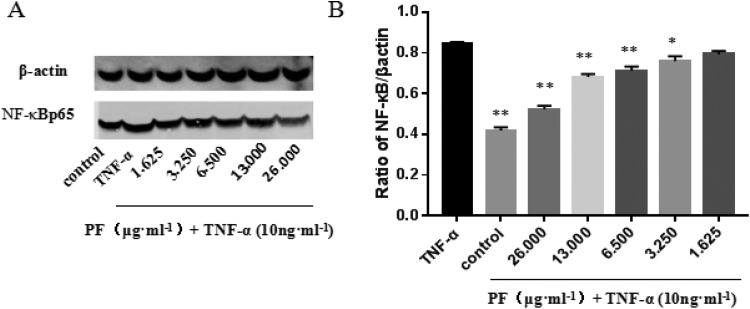
Western blotting was explored to analyze the expression of NF-κBp65 in L929 cells with different doses of PF. It was found that L929 cells coincubated with different doses of PF and TNF-α (10 ng·mL−1) for 24 hours. With the increase in PF concentration, high expression of NF-κBp65 induced by TNF-α was significantly inhibited, showing a certain concentration correlation between 1.625 and 26 μg·mL−1 (Figure 6).
Figure 6.
Effect of paeoniflorin (PF) on activation of NF-κBp65 of L929 cells. A, Western blotting was explored to analyze the expression of NF-κBp65 in L929 cells with different concentrations of PF. B, Western blotting shows expression of NF-κBp65 is increased by tumor necrosis factor α (TNF-α) and reduced by PF. Representative blots from 3 independent experiments are shown (**P < .01).
Discussion
Tumor necrosis factor α is an important regulator of RA, which plays an important role in synovial lesions and cartilage matrix degradation.15 Inhibiting the expression of TNF-α could significantly reduce the incidence of RA.16–17 In this work, results showed that PF at the doses of 1.625 to 26 μg·mL−1 can reduce the inhibitory effect of TNF-α on L929 cell growth in a dose-dependent manner (Figure 2). Morphological observations showed that the cells treated by PF were significantly improved, whereas TNF-α-treated cells showed a decrease in cell density, morphological changes, refractive degeneration, and the proliferation of crinkled cells (Figure 3). Studies also showed that PF has a significant anti-inflammatory effect, and its mechanism is related to the inhibition of the production of inflammatory mediators.18
L929 cells are highly sensitive to TNF-α-induced apoptosis, and the abnormal increase in TNF-α may play a leading role in the pathogenesis of RA.16,19 Therefore, it is helpful to investigate the apoptosis effect of PF on TNF-α-induced L929 cells for a comprehensive understanding of the mechanisms of PF on RA. Our results showed that apoptotic cells were significantly reduced after being incubated with different doses of PF, whereas the homogeneous blue nuclei and a large number of apoptotic cells were observed in the TNF-α (10 ng·mL−1)-treated cells (Figure 5). Cell apoptosis assay with Annexin-V/PI double staining revealed that PF could significantly suppress TNF-α-induced L929 cell apoptosis in a dose-dependent manner (Figure 4).
In addition, TNF-α is an important cytokine that induces the activation of NF-κBp65 in vivo. Studies have found that the expression of NF-κB in the synovial membrane of patients with RA is increased and the activity is significantly higher in patients with RA than osteoarthritis.20–21 The classical NF-κB pathway plays an important role in the inflammatory process of RA, through up expression of p65.22 According to these mechanisms, the anti-inflammatory properties of PF, such as attenuation of cell apoptosis, may attribute to the suppression of NF-κB activation in L929 cells. Our results showed that PF could inhibit activation of NF-κBp65, whereas NF-κBp65 may contribute to inflammatory-related complex cellular pathways, and it is also one of the mechanisms of its therapeutic effect on RA (Figure 6). Moreover, Jia et al23 also showed that inhibiting NF-κBp65 signaling pathway could play a suppressive role in RA inflammatory response, which supports the effect of PF on suppression of NF-κBp65 in TNF-α-induced L929 cells.
Taken together, our study reveals a mechanism underlying TNF-α-induced cell apoptosis: PF could inhibit the activity of NF-κBp65 and apoptosis of L929 cells induced by TNF-α, suggesting that PF may play a role in the treatment of RA through the antagonism of TNF-α pathway.
Footnotes
Authors’ Note: All experimental protocols used in this work were approved by the institutional review board of the Institute of Basic Medical Sciences. The methods were carried out in accordance with the approved guidelines.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Public Welfare Technology Application Research Project of Zhejiang Province, China (NO. 2016C33086), Natural Science Foundation of China (NO. 81774179), and the Project of Zhejiang Provincial Department of Education (NO. Y201019151).
References
- 1. John M, Davis I, Matteson EL. My treatment approach to rheumatoid arthritis. Mayo Clin Proc. 2012;87(7):659–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang SH, Ping LF, Sun FY, et al. Protective effect of taraxasterol against rheumatoid arthritis by the modulation of inflammatory responses in mice. Exp Ther Med. 2016;12(6):4035–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khansai M, Phitak T, Klangjorhor J, et al. Effects of sesamin on primary human synovial fibroblasts and SW982 cell line induced by tumor necrosis factor-alpha as a synovitis-like model. BMC Complement Altern Med. 2017;17(1):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Umar S, Hedaya O, Singh AK, Ahmed S. Thymoquinone inhibits TNF-α-induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation. Toxicol Appl Pharmacol. 2015;287(3):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rendas-Baum R, Wallenstein GV, Koncz T, et al. Evaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-α inhibitors. Arthritis Res Ther. 2011;13(1):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kolls J, Peppel K, Silva M, Beutler B. . Prolonged and effective blockade of tumor necrosis factor activity through adenovirus-mediated gene transfer. Proc Natl Acad Sci. 1994;91(1):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navarro-Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumor-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford). 2010;49(7):1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang W, Dai SM. Mechanisms involved in the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid arthritis. Int Immunopharmacol. 2012;14(1):27–31. [DOI] [PubMed] [Google Scholar]
- 10. Vanlangenakke N, Bertrand MJM, Bogaert P, et al. TNF-α-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2011;2:e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin L, Zhang LM, Xie KQ, Ye Y, Feng L. PF suppresses the expression of intercellular adhesion molecule-1 (ICAM-1) in endotoxin treated human monocytic cells. Br J Pharmacol. 2011;164(2b):694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bahadori F, Kocyigit A, Onyuksel H, Vandenabeele P, Vanden Berghe T. Cytotoxic, apoptotic and genotoxic effects of lipid-based and polymeric nano micelles, an in vitro evaluation. Toxics. 2017;6(1). pii: E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shelke GV, Jagtap JC, Kim DK, et al. TNF-α and IFN-γ together up-regulates par-4 expression and induce apoptosis in human neuroblastomas. Biomedicines. 2017;6(1). pii: E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kong P, Chi RX, Zhang LL, et al. Effects of paeoniflorin on tumor necrosis factor-α-induced insulin resistance and changes of adipokines in 3T3-L1 adipocytes. Fitoterapia. 2013;91:44–50. [DOI] [PubMed] [Google Scholar]
- 15. Luo XJ, Zuo XX, Zhou YU, et al. Extracellular heat shock protein 70 inhibits tumour necrosis factor-α induced proinflammatory mediator production in fibroblast-like synoviocytes. Arthritis Res Ther. 2008;10(2):R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshida K, Nakai A, Kaneshiro K, et al. TNF-α induces expression of the circadian clock gene Bmal1 via dual calcium-dependent pathways in rheumatoid synovial cells. Biochem Biophys Res Commun. 2018;495(2):1675–1680. [DOI] [PubMed] [Google Scholar]
- 17. Darwish SF, El-Bakly WM, Arafa HM, El-Demerdash E. Targeting TNF-α and NF-κB activation by bee venom: role in suppressing adjuvant induced arthritis and methotrexate hepatotoxicity in rats. PLoS One. 2013;8(11):e79284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo RB, Wang GF, Zhao AP, Gu J, Sun XL, Hu G. PF protects against ischemia-induced brain damage in rats via inhibiting MAPKs/NFKB-mediated inflammatory responses. PLoS One, 2012;7(11):e49701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao YY, Chen ZW, Gao YH, et al. Exenatide reduces tumor necrosis factor-α-induced apoptosis in cardiomyocytes by alleviating mitochondrial dysfunction. Chin Med J (Engl). 2015;128(23):3211–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheinman R. NF-κB and rheumatoid arthritis: will understanding genetic risk lead to a therapeutic reward. For Immunopathol Dis Therap. 2013;4(2):93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loo GV, Beyaert R. Negative regulation of NF-κB and its involvement in rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sehnert B, Burkhardt H, Wessels JT, et al. NF-κB inhibitor targeted to activated endothelium demonstrates a critical role of endothelial NF-κB in immune-mediated diseases. Proc Natl Acad Sci USA. 2013;110(41):16556–16561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jia Q, Cheng W, Yue Y, et al. Cucurbitacin E inhibits TNF-α-induced inflammatory cytokine production in human synoviocyte MH7A cells via suppression of PI3K/Akt/NF-κB pathways. Int Immunopharmacol. 2015;29(2):884–890. [DOI] [PubMed] [Google Scholar]