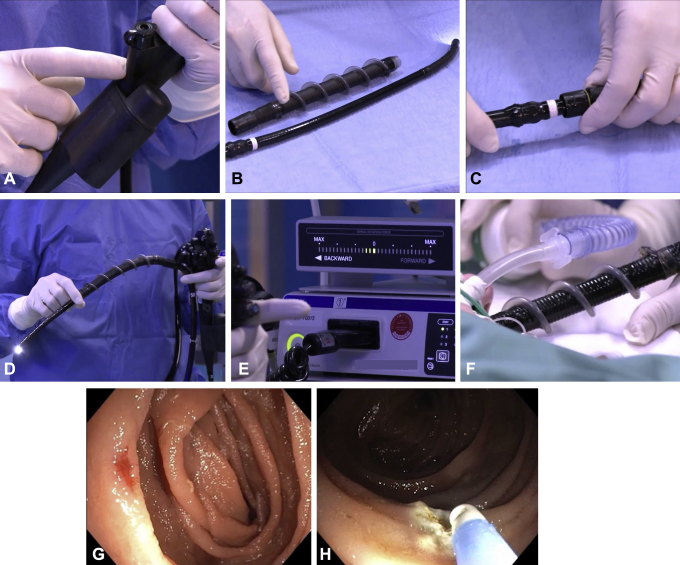
A novel reusable endoscope (Olympus Corp, Tokyo, Japan) with an integrated motor (Fig. 1A) was developed for rotating a disposable short spiral overtube mounted on the insertion tube portion. The drive motor located in the endoscope handle is activated by foot pedals and controls the direction and speed of rotation of a coupler located in the middle of the endoscope’s insertion tube (Figs. 1B and C). The rotation coupler is the only portion of the endoscope that rotates; the distal and proximal portions of the insertion tube do not rotate. Other than the presence of the drive motor and the rotation coupler, the design of the endoscope is the same as that of conventional flexible endoscopes (Fig. 1D). The single-use spiral assembly is composed of corrugated tubing with an atraumatic plastic spiral bonded to its exterior. It relies on rotation of the spiral component to “pleat” or “unpleat” the bowel either on or off the insertion tube as the spiral thread rotates in a clockwise or counterclockwise direction, respectively. The operator can monitor how much torque is being applied to the small bowel by observing the system’s visual force gauge. This display provides the operator with a visual indication of the direction of rotation and the force of rotation throughout the examination (Fig. 1E). This clinical case represents the first use of this device in humans. Enteroscopy was indicated for treatment of angiodysplasias in the jejunum identified by small-bowel capsule endoscopy in a 48-year-old patient with iron-deficiency anemia. The novel motorized enteroscope could be smoothly inserted approximately 250 cm distal to the ligament of Treitz within 20 minutes (Fig. 1F). The technique allowed controlled movement of the tip of the endoscope, and it provided excellent visualization of the intubated small bowel. An angiodysplastic lesion was detected (Fig. 1G) and treated with argon plasma coagulation (Fig. 1H; Video 1, available online at www.VideoGIE.org). Careful removal of the endoscope with counterclockwise rotation of the spiral revealed no iatrogenic mucosal trauma. No adverse events were registered.
Figure 1.
A, Integrated electric motor of the novel enteroscope. B, Disposable short spiral overtube and rotation coupler of the insertion tube. C, The spiral overtube is connected to the rotation coupler. D, Prototype spiral enteroscope ready for use. E, Spiral rotation force indicator. F, Insertion of spiral portion of the endoscope. G, Detection of arteriovenous malformation of the jejunum during spiral enteroscopy. H, Treatment of arteriovenous malformation by argon plasma coagulation.
Disclosure
Dr Neuhaus and Dr Devière are consultants for Olympus Corp. All other authors disclosed no financial relationships relevant to this publication.
Footnotes
Written transcript of the video audio is available online at www.VideoGIE.org.
Dr Neuhaus and Dr Beyna contributed equally to this work.
Supplementary data
Presentation of the prototype novel motorized spiral enteroscope. First clinical use in a human patient with obscure gastrointestinal bleeding and iron-deficiency anemia. Diagnosis of an arteriovenous malformation of the small bowel and treatment with argon plasma coagulation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Presentation of the prototype novel motorized spiral enteroscope. First clinical use in a human patient with obscure gastrointestinal bleeding and iron-deficiency anemia. Diagnosis of an arteriovenous malformation of the small bowel and treatment with argon plasma coagulation.