Periodontitis (gum disease) affects 47% of adults over 30 in the United States (P. I. Eke, B. A. Dye, L. Wei, G. O. Thornton-Evans, R. J. Genco, et al., J Dent Res 91:914–920, 2012), and it cost between $39 and $396 billion worldwide in 2015 (A. J. Righolt, M. Jevdjevic, W. Marcenes, and S. Listl, J Dent Res, 17 January 2018, https://doi.org/10.1177/0022034517750572). Many bacteria associated with the disease are known only by the DNA sequence of their 16S rRNA gene. In this publication, amplification and sequencing of DNA from single bacterial cells are used to obtain nearly complete genomes of Tannerella sp. BU045, a species of bacteria that is more prevalent in patients with periodontitis than in healthy patients. Comparing the complete genome of this bacterium to genomes of related bacterial species will help to better understand periodontitis and may help to grow this organism in pure culture, which would allow a better understanding of its role in the mouth.
KEYWORDS: Tannerella, oral microbiology, periodontitis, single cell, WGA
ABSTRACT
Despite decades of research into the human oral microbiome, many species remain uncultivated. The technique of single-cell whole-genome amplification and sequencing provides a means of deriving genome sequences for species that can be informative on biological function and suggest pathways to cultivation. Tannerella forsythia has long been known to be highly associated with chronic periodontitis and to cause periodontitis-like symptoms in experimental animals, and Tannerella sp. BU045 (human oral taxon 808) is an uncultivated relative of this organism. In this work, we extend our previous sequencing of the Tannerella sp. BU063 (human oral taxon 286) genome by sequencing amplified genomes from 11 cells of Tannerella sp. BU045, including 3 genomes that are at least 90% complete. Tannerella sp. BU045 is more closely related to Tannerella sp. BU063 than to T. forsythia by gene content and average nucleotide identity. However, two independent data sets of association with periodontitis, one based on 16S rRNA gene abundance and the other based on gene expression in a metatranscriptomic data set, show that Tannerella sp. BU045 is more highly associated with disease than Tannerella sp. BU063. Comparative genomics shows genes and functions that are shared or unique to the different species, which may direct further research of the pathogenesis of chronic periodontitis.
IMPORTANCE Periodontitis (gum disease) affects 47% of adults over 30 in the United States (P. I. Eke, B. A. Dye, L. Wei, G. O. Thornton-Evans, R. J. Genco, et al., J Dent Res 91:914–920, 2012), and it cost between $39 and $396 billion worldwide in 2015 (A. J. Righolt, M. Jevdjevic, W. Marcenes, and S. Listl, J Dent Res, 17 January 2018, https://doi.org/10.1177/0022034517750572). Many bacteria associated with the disease are known only by the DNA sequence of their 16S rRNA gene. In this publication, amplification and sequencing of DNA from single bacterial cells are used to obtain nearly complete genomes of Tannerella sp. BU045, a species of bacteria that is more prevalent in patients with periodontitis than in healthy patients. Comparing the complete genome of this bacterium to genomes of related bacterial species will help to better understand periodontitis and may help to grow this organism in pure culture, which would allow a better understanding of its role in the mouth.
INTRODUCTION
The genus Tannerella comprises a set of bacterial species that have been found in the oral cavities of various mammals, including humans, cats (1, 2), dogs (3), and horses (4). The type species is Tannerella forsythia, (formerly known as Bacteroides forsythus and Tannerella forsythensis [5–7]). Additional related species have been identified by 16S rRNA gene sequencing, including the taxa designated Tannerella sp. BU063 (also known as human oral taxon 286 [HOT 286]) and Tannerella sp. BU045 (HOT 808) (8). We previously reported that several nearly complete genomes for BU063 (HOT 286) have been determined by single-bacterial-cell whole-genome amplification (WGA) and sequencing (9). Those BU063 genomes shared about 50% of their genes with T. forsythia strain 92A2 but had little synteny beyond operon level and were quite different in GC content (9). Recently, BU063 has been cultivated; however, it required proximity to other bacteria for efficient growth (10). The genome for cultivated BU063 (strain W11667) has recently been deposited in sequence databases (e.g., GenBank accession number CP017038).
Tannerella forsythia has long been known as a human periodontal pathogen, due to its increased abundance and prevalence in patients with chronic periodontitis (11, 12) and its ability to cause periodontitis-like symptoms in experimental animals (13, 14). Conversely, it does not appear to be associated with gingivitis or mild periodontitis in cats (1). A previous study from our group examined the prevalence of Tannerella sp. BU063 and T. forsythia by endpoint PCR of the internal transcribed spacer of the ribosomal operon using genus-specific primers (15). This work compared healthy subjects and patients with periodontitis and concluded that Tannerella sp. BU063 was found most often in healthy patients but that T. forsythia was found in patients with periodontitis. The methodology used for that study, however, was qualitative, and more-direct methods are now available to measure the abundance of specific bacteria.
Kistler and coworkers (16) identified an additional phylotype of Tannerella designated CP6_C2 or human oral taxon 916 by its 16S rRNA gene sequence (GenBank accession number KC203065), which is about 98% identical to both Tannerella sp. BU045 and Tannerella sp. BU063. Three additional genomes from gut microbes that are deposited in databases with the label Tannerella (i.e., “Tannerella CAG:118,” accession number CAYC010000000, “Tannerella CAG:51,” accession number CBHX000000000, and “Tannerella 6_1_58FAA_CT1,” GenBank accession number ACWX00000000) seem to be much more closely related to the genus Coprobacter based on BLAST analyses of 16S rRNA and other genes.
An earlier study had indicated that Tannerella sp. BU045 was highly associated with periodontitis (12). Therefore, it is of significant interest to determine the genome sequence of Tannerella sp. BU045 (HOT 808), as it may give insights into the disease process.
RESULTS AND DISCUSSION
Sequencing libraries were prepared and sequenced from 11 bacterial single-cell amplified genomes (SAGs), numbered 101 to 111, and individual de novo assemblies were done (Table 1). SAGs 103, 108, and 110 represent relatively complete genomes (96.94%, 96.97%, and 89.87%, respectively, by CheckM [17]), suggesting that the genome size for Tannerella sp. BU045 is about 2.8 Mbp. All three genomes also contained at least 65 of a set of 66 core housekeeping genes (Table 1). Note that the GC content for Tannerella sp. BU045 is 56 to 58%, which is similar to the 55 to 56% GC content of BU063 (9) but substantially different from that of T. forsythia, at 47% GC.
TABLE 1 .
Characteristics of assemblies from Tannerella sp. BU045 single-cell amplified genomes 101 to 111
| Assembly | N50 | Total length | No. of contigs |
Largest contig |
GC content (%) |
CheckM completeness (%) |
No. of core genes (of 66)a |
|---|---|---|---|---|---|---|---|
| SAG 101 | 17,806 | 2,202,921 | 646 | 73,758 | 55.59 | 76.07 | 53 |
| SAG 102 | 8,054 | 691,366 | 491 | 23,434 | 55.85 | 20.01 | ND |
| SAG 103 | 18,376 | 2,779,294 | 921 | 84,200 | 56.77 | 96.94 | 65 |
| SAG 104 | 9,600 | 1,565,841 | 870 | 34,268 | 56.31 | 43.10 | ND |
| SAG 105 | 17,717 | 1,879,977 | 782 | 79,978 | 56.66 | 62.40 | ND |
| SAG 106 | 11,176 | 9,329,179 | 4,175 | 68,043 | 56.05 | 76.11 | ND |
| SAG 107 | 12,123 | 1,726,727 | 957 | 40,033 | 56.16 | 60.33 | 44 |
| SAG 108 | 24,033 | 2,825,906 | 865 | 90,172 | 57.43 | 96.97 | 66 |
| SAG 109 | 11,707 | 1,517,345 | 787 | 45,937 | 56.62 | 56.09 | ND |
| SAG 110 | 23,317 | 2,797,228 | 855 | 82,488 | 56.50 | 89.87 | 65 |
| SAG 111 | 11,725 | 1,869,816 | 712 | 46,230 | 56.99 | 70.03 | 42 |
ND, not determined.
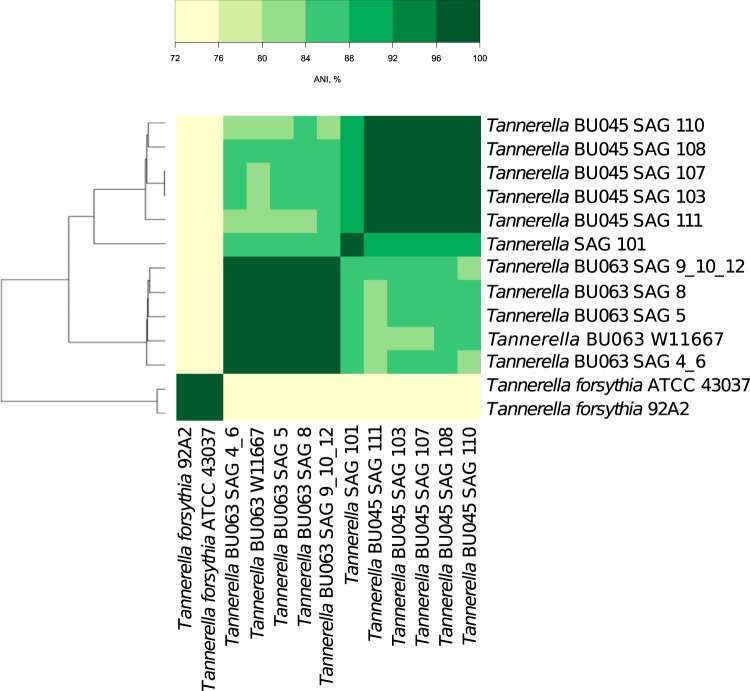
The pairwise genomic-average nucleotide identities (gANIs) and aligned fractions (AFs) for a set of Tannerella genomes (not including the putative Coprobacter genomes mentioned earlier) were determined. It was observed by the developers of the measurements that the usual thresholds for bacterial species are 96.5% for gANIs and 0.6 for AFs (18). Figure 1 shows a heatmap of gANIs between 6 assemblies of single cells from this study, 4 assemblies of Tannerella sp. BU063 cells from our previous study (9), the recently cultivated Tannerella sp. BU063 (W11667), and two Tannerella forsythia genomes. The T. forsythia genomes are for strain 92A2 (previously widely misidentified as strain ATCC 43037) and the true ATCC 43037 strain (19). Of the six newly sequenced BU045 single-cell genomes in Fig. 1, five of them formed a gANI clique that was over the 96.5% threshold for gANI (and the threshold of 0.6 for AFs, when incomplete assemblies were considered). We therefore consider these (SAGs 103, 107, 108, 110, and 111) as provisional conspecifics. A separate analysis using the mummer program (20) found that the five additional assemblies not included in Fig. 1 (SAGs 102, 104, 105, 106, and 109) also belonged to this ANI clique (over 96.5%), with SAGs 103, 104, 105, and 107 forming a nearly identical group (>99% ANI). It is possible that these four SAGs represent the same strain or closely related organisms. It is notable that they were derived from the same experimental subject. The set of nearly complete genomes, SAGs 103, 108, and 110, likely represent three different strains of the species.
FIG 1 .
Heatmap of gANIs between various genome assemblies. Hierarchical clustering by the average method was performed using 100-ANI as the distance. Cells are colored as shown on the scale. Genomes along the vertical axis were the first in the comparison, and those on the horizontal axis were the second.
The incomplete assembly from SAG 101 had gANI to the clique members of only 88 to 89% and may be derived from a different species, although the 16S rRNA gene extracted from the SAG 101 assembly has >99% sequence identity to Tannerella sp. BU045. Meanwhile, the Tannerella sp. BU063 SAGs and the cultivated W11667 strain had pairwise gANIs of over 96.5% within their group but gANIs to the BU045 group of 83 to 85%, and the Tannerella forsythia 92A2 and ATCC 43037 genomes had >96.5% gANI to each other but 72 to 74% gANI to all of the other compared genomes. Overall, the results support the existence of at least three discrete species (Tannerella forsythia, Tannerella sp. BU045, and Tannerella sp. BU063) within the Tannerella genus, and it is possible that SAG 101 represents a fourth species.
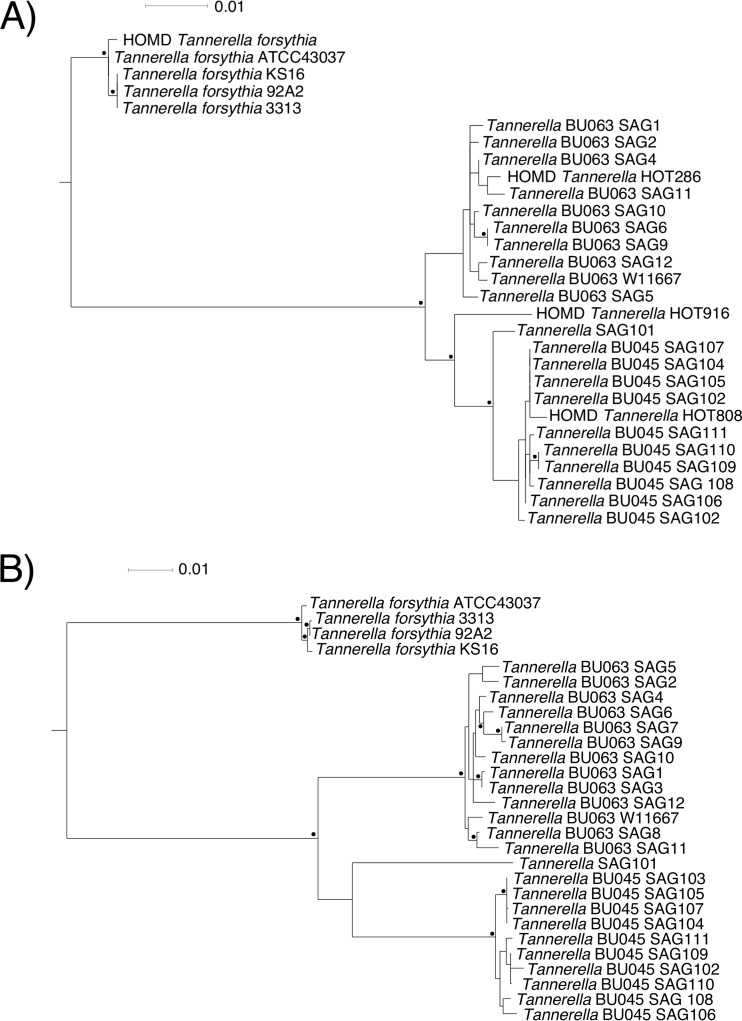
The relationship between genomes was confirmed by calculating maximum-likelihood phylogenetic trees using either 16S rRNA gene sequences or concatenated protein sequences from 37 genes that are highly conserved in bacteria (Fig. 2). These phylogenetic trees reinforce the finding that Tannerella sp. BU063 and BU045 are more related to each other than either is to T. forsythia. The newly cultivated BU063 strain W11667 exists in a well-supported clade with the previously sequenced BU063 SAGs, using either 16S rRNA or concatenated proteins. SAG 101 and Tannerella HOT 916/CP6_C2 appear to be closely related to BU045 but may represent separate species or subspecies. However, it is difficult to be certain about these relationships, since SAG 101 is a partial genome and there is only 16S rRNA gene information for HOT 916/CP6_C2.
FIG 2 .
Maximum-likelihood phylogenetic trees of the Tannerella genus computed with 16S rRNA gene sequences (A) or concatenated protein sequences from 37 conserved genes (B). In both trees, Porphyromonas gingivalis W83 was used as an outgroup but was removed from the tree shown. The black dots represent branch points that are supported by bootstrap values of ≥70%. The scales represent 1% divergence. The nodes labeled “HOMD” in panel A are the representative sequences from that database. Other 16S rRNA gene sequences shown were extracted from the genomic contigs.
A core set of genes for each species was computed by starting with the genes from one strain and selecting the core genome that had homologs in other nearly complete genomes from that species (see Materials and Methods for details). The number of core genes was found to be 2,425 for T. forsythia, 2,214 for BU063, and 1,895 for BU045. The IMG/MER (21) Web tool was used to compare genes present in the core sets. However, it is notable that the numbers of shared genes determined varied slightly depending on the direction in which the analysis was done, i.e., depending on which core gene set was used to search for homologs. This may be due to duplicated or partial genes. Also, the single-cell genomes are likely to have missing regions, and sequencing or gene prediction errors may have occurred. Figure 3 presents a Venn diagram of shared and unique genes, with ranges representing uncertainty in the numbers calculated by different approaches. In Tables S1 to S5 in the supplemental material, we list the accession numbers of the genomes used, the gene identification numbers, and predicted products of the core gene sets from each species, and we indicate whether homologs were found in the genomes from the other two species.
FIG 3 .
Venn diagram of core gene overlap of the Tannerella species. The presence of genes was evaluated using the “Profile and Alignment” tool on the IMG/ER website using thresholds of 50% identity and an E value of 10−5. Genes that are present in all genomes of each species were saved as a set, put into the “gene cart,” and evaluated for their presence in the genomes of the other species. Selected functional pathways that were found in the various categories are shown. NAM, N-acetylmuramic acid; AA, amino acid.
Strain identifications or single-cell amplified genome (SAG) numbers, type of genome assembly, and accession numbers of the genomes used in the current study. Download TABLE S1, XLSX file, 0.01 MB (9.3KB, xlsx) .
Copyright © 2018 Beall et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
About one-half of the genes in each core genome are common to all three, while the distribution of the other categories generally reflects that BU045 contains fewer core genes than the other genomes and that BU045 and BU063 are closely related. There also is some indication of the BU045 assemblies being less complete, as seen by larger numbers of genes that are present in some but not all of the genomes in Table S1.
The pathway analysis tools in IMG/ER were used on the various groups of genes to make some predictions about the functional capabilities of the three species. First, although there is a set of genes unique to BU045, we were unable to ascribe well-defined functions to that group. In fact, 74 of the 122 genes were annotated as “hypothetical protein” or “protein of unknown function,” and many more had functions, such as restriction-modification, that are often found in mobile DNA (Table S4). T. forsythia 92A2, ATCC 43037, and BU063 shared 20 to 45 genes that were not found in BU045; however, none of these had well-defined metabolic functions. With regard to functions that were not ubiquitous in the genus, BU045 often resembled BU063. T. forsythia 92A2 and ATCC 43037 possessed pathways for arginine biosynthesis, glutaminase, and menaquinone biosynthesis that were lacking in both BU063 (9) and BU045. Similarly, several enzymes and pathways are present in both BU063 and BU045 but absent in T. forsythia, including the branched-chain amino acid biosynthesis pathway, cysteine synthase, glutamine synthase, nitrite reductase, and the pathway producing UDP-N-acetylmuramic acid from UDP-N-acetylglucosamine. The lack of the last pathway in T. forsythia is apparently why that organism requires exogenous N-acetylmuramic acid for growth (22). However, in two functions, BU045 resembled T. forsythia more than BU063. Genes predicted to allow the ability to biosynthesize tryptophan from chorismate are present in BU063 but absent in T. forsythia and BU045. Additionally, while all three species carry genes encoding vacuolar/archaeon-type rotary ATPase/ATP synthase subunits, BU045 and T. forsythia also carry genes for the subunits of the F1Fo-type ATPase/ATP synthase, while BU063 lacks such genes (9).
Previously, it was examined whether genes that are associated with virulence in T. forsythia 92A2 were present in BU063 (9). A similar analysis was performed on BU045 genomes in the present study, using genes as predicted by IMG/ER and following up with tblastn analysis of the assembled contigs to find genes that might have been missed during annotation (Table 2). The distributions of such genes were identical between BU063 and BU045 except with the wecC gene, encoding UDP-N-acetyl-d-mannosaminuronic acid dehydrogenase. Homologs of wecC were found in some of the BU063 SAGs but not in the finished genome of the cultivated W11667 strain. This gene was also not found in any of the BU045 SAGs, although a homolog was found in SAG 101 (which, as mentioned earlier, may represent a different species). The wecC gene is involved in glycosylation of the S-layer and other extracellular proteins of T. forsythia (23), and a mutation in the gene affects biofilm formation (24) and T-helper-type 17 cell induction (25, 26). As seen in Table 2 (and reported previously for BU063 [9]), BU045 contained S-layer protein genes that were detectable by tblastn, although the regions of identity were sometimes fragmented in assemblies, possibly due to their lengths (3.5 to 4.3 kb).
TABLE 2 .
Virulence genes in Tannerella forsythia and their presence or absence in uncultivated genomes
| Gene | Function | Present in BU063? |
Present in BU045? |
|---|---|---|---|
| bspA | Surface protein | No | No |
| kly | Metalloprotease (karilysin) | No | No |
| mir | Metalloprotease (mirolysin) | No | No |
| nanH | Sialidase | No | No |
| tfsA, tfsB | S-layer proteins | Yes | Yes |
| prtH | Protease | No | No |
| wecC | UDP-mannosaminuric acid dehydrogenase (glycosylation, biofilm) |
Yes | No (except in SAG 101) |
| mgsA | Methylglyoxal production | Yes | Yes |
Both BU063 and BU045 appear to lack homologs of the protease karilysin or mirolysin (Table 2). These metalloproteases have been shown to inactivate complement, resulting in the protection of T. forsythia ATCC 43037 (27, 28). Recently, they have been discussed as part of a group of encoded proteins termed KLIKK proteases (29). The tblastn program was used to search for similar proteins, and it was found that both BU063 and BU045 have genes about 60% similar to the KLIKK serine protease miropsin-1 and other genes about 30% similar to both miropsin-1 and miropsin-2. They also had some sequences about 30% similar to mirolysin, but these sequences were incomplete and represented either pseudogenes, misassemblies, or whole-genome amplification artifacts. The level of 30% identity is substantially lower than that seen for other orthologous genes between these species, so it appears that these may not be functionally identical, though they probably encode secreted proteases.
To examine the possible role of the Tannerella species T. forsythia, BU063, and BU045 in periodontitis, we reexamined two published data sets using our new genome sequences. One is 16S rRNA gene abundance data from our laboratory (12). This study used 29 healthy subjects and 29 patients with periodontitis, with the shallow and deep pockets of periodontitis patients sampled separately. Additionally, the newly derived genome assemblies were used to analyze metatranscriptomic data from a recent publication (30). That study used 10 healthy subjects and 6 patients with periodontitis. Those data were analyzed as discussed in Materials and Methods. The strains of the genomes used as mapping templates are shown in Fig. 4 and in Materials and Methods, although the metatranscriptomic reads that are quantitated by mapping likely derive from novel strains. Figure 4 shows the results of the two analyses. As previously determined (12, 30), Tannerella forsythia is highly associated with periodontitis. Tannerella sp. BU045 is also significantly associated with disease, although both its measured 16S gene abundance (which is not an absolute quantification) and its gene expression are lower than those of T. forsythia. Tannerella sp. BU063 has an intermediate measured abundance and less association with disease, with the metatranscriptomic data not showing a significant difference and the 16S data having borderline significance. BU063 was not identified as periodontitis associated in the earlier study because of false-discovery rate correction (12). The current observations are somewhat in contrast to those of a previous study seeming to show that BU063 was strictly health related (15). This might be due to differences in the patient populations or methodology.
FIG 4 .

16S rRNA gene abundance and total gene expression of Tannerella species in healthy patient and periodontitis patient samples. (A) Fractional 16S rRNA gene abundances versus disease and periodontal pocket state for the 3 species. Data are from reference 12. Horizontal lines show the mean values. Wilcoxon rank sum test between healthy subjects and periodontitis patients (whose deep pockets were sampled). T. forsythia P = 1.01 × 10–8; Tannerella sp. BU045 P = 0.00039; Tannerella sp. BU063 P = 0.028. (B) Fraction of metatranscriptomic bacterial protein-coding gene expression for the species in samples from periodontitis patients and healthy controls. Raw data are from reference 30. Horizontal line segments indicate mean values. Wilcoxon rank sum tests: T. forsythia P = 0.0420; Tannerella sp. BU045 P = 0.0312; Tannerella sp. BU063 P = 0.875.
Overall, the presence or absence of known genes involved in pathogenic processes do not give great insight into mechanisms driving the greater association of BU045 than BU063 with periodontitis, as seen in Fig. 4. It is possible that the common association of BU045 and T. forsythia with infected pockets is due to ecological differences in inflamed sites rather than to direct virulence determinants. Speculatively, if tryptophan is limited in healthy sites but more plentiful in disease sites with proteolytic bacteria, that might give BU063 an advantage in healthy sites relative to BU045 and T. forsythia. Another possibility is that the driving force is the presence of the F1Fo ATP synthase, one of the few well-defined functional proteins that is common to T. forsythia and BU045 but absent in BU063. Intriguingly, a recent transposon-sequencing (Tn-Seq) study using a mouse abscess model of Aggregatibacter actinomycetemcomitans found that mutations of the F1Fo ATP synthase in this organism affect its fitness in a monoinfection or a coinfection with Streptococcus gordonii (31). Although it is possible that similar mechanisms are at work with the Tannerella species, it may be worth noting that A. actinomycetemcomitans does not have genes for the V/A-type ATP synthase, which are present in all the Tannerella species.
MATERIALS AND METHODS
Sampling and DNA amplification.
Clinical sampling was approved by the Institutional Review Board of the Ohio State University and Oak Ridge site-wide Institutional Review Board (ORSIRB) for the National Laboratory. SAGs 101 to 108 were derived from periodontitis patients at the Ohio State College of Dentistry, and SAGs 109 to 111 were from volunteers from Oak Ridge, TN.
Subgingival plaque was collected using paper points, single cells were isolated with flow cytometry, and genomic DNA was amplified with Phi29 DNA polymerase. Single-cell amplified genomes (SAGs) corresponding to Tannerella sp. BU045 were identified by PCR and direct sequencing of a fragment of the 16S rRNA gene. Detailed procedures are given in previous publications (32, 33).
DNA sequencing.
Sequencing libraries were prepared from 100 ng of amplified DNA with the NEBNext Ultra library kit (New England Biolabs, Ipswich, MA) and sequenced with 150-bp paired-end reads on the HiSeq 2500 sequencer (Illumina, San Diego, CA), producing 13.5 to 25.8 million reads per sample.
Bioinformatics assembly and annotation.
The sequence reads were trimmed with Trimmomatic version 0.35 (34) using settings Illuminaclip 2:30:10:1:true, sliding window 4:15, and minlen 50. They were then assembled with SPAdes version 3.5 (35) in single-cell mode with otherwise-default parameters. The assemblies were evaluated with Quast 3.0 (36) and CheckM 1.0.5 (17).
Multiple approaches were used to search for and remove possible contamination of the genomes. RNAmmer (37) was employed to identify assembled contigs containing rRNA genes. The rRNA-containing contigs were used for a BLAST (38) search of the NCBI refseq_genomic database, restricted to bacterial sequences. If the 16S rRNA gene matched with greater than 99% identity the known Tannerella BU045 sequence, the contigs were assigned to a white list to ensure their inclusion in the final assembly. If they corresponded to unrelated bacteria, representative genomes were downloaded to act as BLAST databases to identify additional contaminating contigs. By this procedure, we identified a number of potential contaminants. rRNA genes similar to those from RefSeq accession number NZ_ACYI00000000.1 were found in SAGs 102 and 106. This genome currently is listed as “Enhydrobacter aerosaccus” in the NCBI, but the rRNAs appear to be extremely similar to those of Moraxella osloensis, a known oral community member only distantly related to the true Enhydrobacter bacterium (39). The assembled genome from SAG 110 contained contigs with ribosomal genes related to RefSeq accession number NZ_AOTF00000000.1, SR1 bacterium MGEHA (33). These genomes were used as search databases for the corresponding assemblies. The identified SAG assemblies were used as queries against BLAST databases of those genomes, while all assembled SAGs were used as queries against human genomic DNA, Saccharomyces cerevisiae (nuclear and mitochondrial genomes), Escherichia coli, phage PhiX 174, and the UniVec vector database. The search conditions used were “-task megablast -evalue.01 -max_target_seqs 1.” We also searched for simple repeat sequences with the dustmasker command and parameter “-level 50.” We removed contigs that were not white-listed rRNA-containing fragments and either matched the genomic sequences of over half the contig length (or over 500 bp) or matched UniVec sequences of over 48 bp. Since UniVec is not an exhaustive catalog of vector sequences, even short matches are likely to be problematic. Contigs that had greater than 75% of their length marked by dustmasker were likewise discarded.
The 3 assemblies that were close to full length, SAGs 103, 108, and 110, were uploaded to the IMG/MER website (21), and annotation was carried out by the default pipeline. Further screening for contamination was performed by examining contigs that contained unusual GC contents (<40%, visualized in the chromosome viewer of the IMG/MER website), unusual kmer content as visualized by principal-component analysis (PCoA) (Scaffold consistency [kmer frequency tool on the IMG/MER website]), or sequence similarity of >90% to genes from organisms outside the Bacteroidetes phylum (phylogenetic profiler on the IMG/MER website). As had been found for Tannerella sp. BU063, the gene annotation pipeline in IMG/MER misannotated a number of ribosomal protein genes that could be identified based on genome arrangement and protein similarity, so these annotations were manually corrected.
Bioinformatics metatranscriptomics.
Metatranscriptomic raw data files were downloaded from MG-RAST (30, 40) for 10 samples from control subjects and 6 samples from patients with periodontitis. The bowtie2-build program was used to build a database from the combined genomes of Tannerella forsythia 92A2 (GCF_000238215.1) Tannerella sp. BU063 (GCF_000510385.1), and Tannerella sp. BU045 SAG 110 from this study. Reads were mapped to the genomes with bowtie2 2.2.6 using the parameters --very-sensitive-local, --no-unal, -X 1000, --score-min G,20,28, and --no-mixed. We used samtools 0.1.19 to convert the output to bam, sort, and index and samtools with gawk to remove reads that mapped in the ribosomal rRNA operons, as these had potential cross-reaction with other species. Finally, samtools idxstats was used to count the number of reads mapping to each genome.
To estimate the total number of bacterial-protein-coding reads from each sample, the raw reads were trimmed with Trimmomatic and reservoir sampling was used to select 50,000 random trimmed reads from each sample. We then mapped the protein-coding sequences of the sampled forward reads against the NCBI nr database (20 June 2015 version) using the Diamond program, version 0.8.14.76. (41) The alignments were then processed with the Meganize DAA File option of MEGAN community edition version 6.4.15 (42), and the total number of bacterial reads was estimated as the sum of bacterial reads in MEGAN divided by the sample size, 50,000, multiplied by the total number of reads per sample.
Bioinformatics genome comparisons.
The genomic average nucleotide identity (gANI) was calculated by the method derived by Varghese and coworkers (18) as implemented on the IMG/ER website (Compare Genomes/Avg Nucleotide Ident./Pairwise ANI) (21). In this method, all protein-coding genes over 70% identical between two genomes are aligned and used to compute an average identity. Additionally, the method computes the aligned fraction (AF) of the two genomes. The numbers vary slightly depending on which genome is given first and which second. For the heatmap, genomes were clustered based on the percent nucleotide difference from this measurement using the R function hclust and the average method, also known as unweighted pair group using average linkages (UPGMA).
16S rRNA gene sequences were extracted from genome assemblies using RNAmmer (37) and aligned with SSU-ALIGN 0.1 (43), and masked alignments were converted from Stockholm format to Phylip with BioPython AlignIO (44). Concatenated protein alignments were generated using phylosift 1.0.1 with the command phylosift all-isolate -besthit (45, 46). Alignments were inspected, trimmed (to the equivalent of E. coli positions 29 and 1389 for 16S rRNA), and adjusted if necessary, and formats were changed using Mesquite 3.02 (47). Trees were calculated using RAxML (48) on the CIPRES computing cluster with Porphyromonas gingivalis W83 as an outgroup.
The set of core genes for each species was calculated with the phylogenetic Profiler Tool on IMG/ER (Find Genes/Phylogenetic Profilers/Single Genes) set to find genes in a template genome, plus 100% of the remaining genomes with a parameter E value of 10−5 and 50% identity. The gene sets used were (i) Tannerella forsythia 92A2 compared against Tannerella forsythia ATCC 43037, (ii) Tannerella sp. BU063 W11667 compared against Tannerella sp. BU063 SAG 2 and SAG 5, and (iii) Tannerella sp. BU045 SAG 108 compared against SAGs 103 and 110. We then compared the three core genome gene sets against the set of remaining genomes using the profile and alignment tool available in the gene cart area, again with the following settings: an E value of 10−5 and 50% identity. The resulting list was parsed to predict the number of genes that are shared between species. The tables in the supplemental material contain listings of the genomes used (Table S1), genes of core sets with indications of homologs in other species (Tables S2 to S4), and summary numbers (Table S5).
Presence of core genes from Tannerella forsythia in the genomes of the other Tannerella species. The genes are identified by their IMG/MER gene identifiers for the 92A2 genome and had homologs in the ATCC 43037 genome. The presence of homologs to these core genes is shown for the following genomes: Tannerella sp. BU045 (SAG 103), Tannerella sp. BU045 (SAG 108), Tannerella sp. BU045 (SAG 110), Tannerella sp. BU063 (SAG 2), Tannerella sp. BU063 (SAG 5), and Tannerella sp. BU063 (W11667). “1” indicates that a homolog was present and “0” that it was absent. Summary columns indicate the numbers of genomes with homologs from each species and totals. Download TABLE S2, XLSX file, 0.1 MB (156KB, xlsx) .
Copyright © 2018 Beall et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Presence of core genes from Tannerella sp. BU063 in the genomes of the other Tannerella species. The genes are identified by their IMG/MER gene identifiers for the W11667 genome and had homologs in the SAG 2 and SAG 5 genomes. The presence of homologs to these core genes is shown for the following genomes: Tannerella sp. BU045 (SAG 103), Tannerella sp. BU045 (SAG 108), Tannerella sp. BU045 (SAG 110), Tannerella forsythia 92A2, and Tannerella forsythia ATCC 43037. “1” indicates that a homolog was present and “0” that it was absent. Summary columns indicate the numbers of genomes with homologs from each species and totals. Download TABLE S3, XLSX file, 0.1 MB (135.1KB, xlsx) .
Copyright © 2018 Beall et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Presence of core genes from Tannerella sp. BU045 in the genomes of the other Tannerella species. The genes are identified by their IMG/MER gene identifiers for the SAG 108 genome and had homologs in the SAG 103 and SAG 110 genomes. The presence of homologs to these core genes is shown for the following genomes: Tannerella forsythia ATCC 43037, Tannerella forsythia 92A2, Tannerella sp. BU063 (SAG 2), Tannerella sp. BU063 (SAG 5), and Tannerella sp. BU063 (W11667). “1” indicates that a homolog was present and “0” that it was absent. Summary columns indicate the numbers of genomes with homologs from each species and totals. Download TABLE S4, XLSX file, 0.1 MB (118KB, xlsx) .
Copyright © 2018 Beall et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Summary of core gene presence in other genomes. For each of the three core genome gene sets, the numbers of genes with homologs in different numbers of genomes of the other Tannerella species are tabulated. For instance, 981 Tannerella forsythia core genes had homologs in zero BU063 genomes and zero BU045 genomes, while 1,136 genes had homologs in all three BU063 and all three BU045 genomes. Download TABLE S5, XLSX file, 0.01 MB (9.6KB, xlsx) .
Copyright © 2018 Beall et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data availability.
The data for SAGs 103, 108, and 110 have been deposited under NCBI BioProject number PRJNA342492, including SRA experiments SRX2157181, SRX2157182, and SRX2157183, and WGS accession numbers MIQB00000000, MIQC00000000, and MIQD00000000.
Software scripts used can be accessed at the OSU code repository with the URL https://code.osu.edu/beall-3/Single_Cell_Genomics.
ACKNOWLEDGMENTS
We thank Pearlly Yan of the Nucleic Acid Shared Resource, OSU Comprehensive Cancer Center, for performing Illumina sequencing. We thank Steve Allman and Sarah Kauffman for laboratory work.
Oak Ridge National Laboratory is managed by UT-Battelle, LLC, for the U.S. Department of Energy under contract DE-AC05-00OR22725. Support was provided by NIDCR grants DE010467 and DE024463.
REFERENCES
- 1.Harris S, Croft J, O’Flynn C, Deusch O, Colyer A, Allsopp J, Milella L, Davis IJ. 2015. A pyrosequencing investigation of differences in the feline subgingival microbiota in health, gingivitis and mild periodontitis. PLoS One 10:e0136986. doi: 10.1371/journal.pone.0136986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booij-Vrieling HE, van der Reijden WA, Houwers DJ, de Wit WEAJ, Bosch-Tijhof CJ, Penning LC, van Winkelhoff AJ, Hazewinkel HAW. 2010. Comparison of periodontal pathogens between cats and their owners. Vet Microbiol 144:147–152. doi: 10.1016/j.vetmic.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 3.Oh C, Lee K, Cheong Y, Lee SW, Park SY, Song CS, Choi IS, Lee JB. 2015. Comparison of the oral microbiomes of canines and their owners using next-generation sequencing. PLoS One 10:e0131468. doi: 10.1371/journal.pone.0131468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao W, Chan Y, You M, Lacap-Bugler DC, Leung WK, Watt RM. 2016. In-depth snapshot of the equine subgingival microbiome. Microb Pathog 94:76–89. doi: 10.1016/j.micpath.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Sakamoto M, Suzuki M, Umeda M, Ishikawa I, Benno Y. 2002. Reclassification of Bacteroides forsythus (Tanner et al. 1986) as Tannerella forsythensis corrig., gen. nov., comb. nov. Int J Syst Evol Microbiol 52:841–849. doi: 10.1099/00207713-52-3-841. [DOI] [PubMed] [Google Scholar]
- 6.Maiden MFJ, Cohee P, Tanner ACR. 2003. Proposal to conserve the adjectival form of the specific epithet in the reclassification of Bacteroides forsythus Tanner et al. 1986 to the genus Tannerella Sakamoto et al. 2002 as Tannerella forsythia corrig., gen. nov., comb. nov. Request for an opinion. Int J Syst Evol Microbiol 53:2111–2112. doi: 10.1099/ijs.0.02641-0. [DOI] [PubMed] [Google Scholar]
- 7.Judicial Commission of the International Committee on Systematics of Prokaryotes 2008. The adjectival form of the epithet in Tannerella forsythensis Sakamoto et al. 2002 is to be retained and the name is to be corrected to Tannerella forsythia Sakamoto et al. 2002. Opinion 85. Int J Syst Evol Microbiol 58:1974–1974. doi: 10.1099/ijs.0.2008/006007-0. [DOI] [PubMed] [Google Scholar]
- 8.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol 192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beall CJ, Campbell AG, Dayeh DM, Griffen AL, Podar M, Leys EJ. 2014. Single cell genomics of uncultured, health-associated Tannerella BU063 (Oral Taxon 286) and comparison to the closely related pathogen Tannerella forsythia. PLoS One 9:e89398. doi: 10.1371/journal.pone.0089398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vartoukian SR, Moazzez RV, Paster BJ, Dewhirst FE, Wade WG. 2016. First cultivation of health-associated Tannerella sp. HOT-286 (BU063). J Dent Res 95:1308–1313. doi: 10.1177/0022034516651078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr.. 1998. Microbial complexes in subgingival plaque. J Clin Periodontol 25:134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 12.Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, Podar M, Leys EJ. 2012. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J 6:1176–1185. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesavalu L, Sathishkumar S, Bakthavatchalu V, Matthews C, Dawson D, Steffen M, Ebersole JL. 2007. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun 75:1704–1712. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma A, Inagaki S, Honma K, Sfintescu C, Baker PJ, Evans RT. 2005. Tannerella forsythia-induced alveolar bone loss in mice involves leucine-rich-repeat BspA protein. J Dent Res 84:462–467. doi: 10.1177/154405910508400512. [DOI] [PubMed] [Google Scholar]
- 15.Leys EJ, Lyons SR, Moeschberger ML, Rumpf RW, Griffen AL. 2002. Association of Bacteroides forsythus and a novel Bacteroides phylotype with periodontitis. J Clin Microbiol 40:821–825. doi: 10.1128/JCM.40.3.821-825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kistler JO, Booth V, Bradshaw DJ, Wade WG. 2013. Bacterial community development in experimental gingivitis. PLoS One 8:e71227. doi: 10.1371/journal.pone.0071227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varghese NJ, Mukherjee S, Ivanova N, Konstantinidis KT, Mavrommatis K, Kyrpides NC, Pati A. 2015. Microbial species delineation using whole genome sequences. Nucleic Acids Res 43:6761–6771. doi: 10.1093/nar/gkv657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrich V, Pabinger S, Chen T, Messner P, Dewhirst FE, Schäffer C. 2015. Draft genome sequence of Tannerella forsythia type strain ATCC 43037. Genome Announc 3:e00660-15. doi: 10.1128/genomeA.00660-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol 5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markowitz VM, Mavromatis K, Ivanova NN, Chen IM, Chu K, Kyrpides NC. 2009. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 25:2271–2278. doi: 10.1093/bioinformatics/btp393. [DOI] [PubMed] [Google Scholar]
- 22.Wyss C. 1989. Dependence of proliferation of Bacteroides forsythus on exogenous N-acetylmuramic acid. Infect Immun 57:1757–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posch G, Pabst M, Brecker L, Altmann F, Messner P, Schäffer C. 2011. Characterization and scope of S-layer protein O-glycosylation in Tannerella forsythia. J Biol Chem 286:38714–38724. doi: 10.1074/jbc.M111.284893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honma K, Inagaki S, Okuda K, Kuramitsu HK, Sharma A. 2007. Role of a Tannerella forsythia exopolysaccharide synthesis operon in biofilm development. Microb Pathog 42:156–166. doi: 10.1016/j.micpath.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Settem RP, Honma K, Nakajima T, Phansopa C, Roy S, Stafford GP, Sharma A. 2013. A bacterial glycan core linked to surface (S)-layer proteins modulates host immunity through Th17 suppression. Mucosal Immunol 6:415–426. doi: 10.1038/mi.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Settem RP, Honma K, Sharma A. 2014. Neutrophil mobilization by surface-glycan altered Th17-skewing bacteria mitigates periodontal pathogen persistence and associated alveolar bone loss. PLoS One 9:e108030. doi: 10.1371/journal.pone.0108030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karim AY, Kulczycka M, Kantyka T, Dubin G, Jabaiah A, Daugherty PS, Thogersen IB, Enghild JJ, Nguyen KA, Potempa J. 2010. A novel matrix metalloprotease-like enzyme (karilysin) of the periodontal pathogen Tannerella forsythia ATCC 43037. Biol Chem 391:105–117. doi: 10.1515/BC.2010.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jusko M, Potempa J, Mizgalska D, Bielecka E, Ksiazek M, Riesbeck K, Garred P, Eick S, Blom AM. 2015. A metalloproteinase mirolysin of Tannerella forsythia inhibits all pathways of the complement system. J Immunol 195:2231–2240. doi: 10.4049/jimmunol.1402892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ksiazek M, Mizgalska D, Eick S, Thøgersen IB, Enghild JJ, Potempa J. 2015. KLIKK proteases of Tannerella forsythia: putative virulence factors with a unique domain structure. Front Microbiol 6:312. doi: 10.3389/fmicb.2015.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szafrański SP, Deng ZL, Tomasch J, Jarek M, Bhuju S, Meisinger C, Kühnisch J, Sztajer H, Wagner-Döbler I. 2015. Functional biomarkers for chronic periodontitis and insights into the roles of Prevotella nigrescens and Fusobacterium nucleatum; a metatranscriptome analysis. NP J Biofilms Microbiomes 1:15017. doi: 10.1038/npjbiofilms.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stacy A, Fleming D, Lamont RJ, Rumbaugh KP, Whiteley M. 2016. A commensal bacterium promotes virulence of an opportunistic pathogen via cross-respiration. mBio 7:e00782-16. doi: 10.1128/mBio.00782-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell AG, Campbell JH, Schwientek P, Woyke T, Sczyrba A, Allman S, Beall CJ, Griffen A, Leys E, Podar M. 2013. Multiple single-cell genomes provide insight into functions of uncultured deltaproteobacteria in the human oral cavity. PLoS One 8:e59361. doi: 10.1371/journal.pone.0059361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell JH, O’Donoghue P, Campbell AG, Schwientek P, Sczyrba A, Woyke T, Söll D, Podar M. 2013. UGA is an additional glycine codon in uncultured SR1 bacteria from the human microbiota. Proc Natl Acad Sci U S A 110:5540–5545. doi: 10.1073/pnas.1303090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurevich A, Saveliev V, Vyahhi N, Tesler G. 2013. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lagesen K, Hallin P, Rødland EA, Staerfeldt HH, Rognes T, Ussery DW. 2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 39.Kawamura Y, Fujiwara N, Naka T, Mitani A, Kubota H, Tomida J, Morita Y, Hitomi J. 2012. Genus Enhydrobacter Staley et al. 1987 should be recognized as a member of the family Rhodospirillaceae within the class Alphaproteobacteria. Microbiol Immunol 56:21–26. doi: 10.1111/j.1348-0421.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 40.Wilke A, Bischof J, Harrison T, Brettin T, D’Souza M, Gerlach W, Matthews H, Paczian T, Wilkening J, Glass EM, Desai N, Meyer F. 2015. A RESTful API for accessing microbial community data for MG-RAST. PLoS Comput Biol 11:e1004008. doi: 10.1371/journal.pcbi.1004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchfink B, Xie C, Huson DH. 2015. Fast and sensitive protein alignment using DIAMOND. Nat Methods 12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 42.Huson DH, Beier S, Flade I, Górska A, El-Hadidi M, Mitra S, Ruscheweyh HJ, Tappu R. 2016. MEGAN community edition—interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput Biol 12:e1004957. doi: 10.1371/journal.pcbi.1004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nawrocki EP. 2009. Structural RNA homology search and alignment using covariance models. Washington University in St Louis, ; St Louis, MO. [Google Scholar]
- 44.Cock PJ, Antao T, Chang JT, Chapman BA, Cox CJ, Dalke A, Friedberg I, Hamelryck T, Kauff F, Wilczynski B, de Hoon MJ. 2009. Biopython: freely available python tools for computational molecular biology and bioinformatics. Bioinformatics 25:1422–1423. doi: 10.1093/bioinformatics/btp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darling AE, Jospin G, Lowe E, Matsen FA, Bik HM, Eisen JA. 2014. PhyloSift: phylogenetic analysis of genomes and metagenomes. PeerJ 2:e243. doi: 10.7717/peerj.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rinke C, Schwientek P, Sczyrba A, Ivanova NN, Anderson IJ, Cheng JF, Darling A, Malfatti S, Swan BK, Gies EA, Dodsworth JA, Hedlund BP, Tsiamis G, Sievert SM, Liu WT, Eisen JA, Hallam SJ, Kyrpides NC, Stepanauskas R, Rubin EM, Hugenholtz P, Woyke T. 2013. Insights into the phylogeny and coding potential of microbial dark matter. Nature 499:431–437. doi: 10.1038/nature12352. [DOI] [PubMed] [Google Scholar]
- 47.Maddison WP, Maddison DR. 2009. Mesquite: a modular system for evolutionary analysis, version 2.6. http://www.mesquiteproject.org.
- 48.Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol 57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strain identifications or single-cell amplified genome (SAG) numbers, type of genome assembly, and accession numbers of the genomes used in the current study. Download TABLE S1, XLSX file, 0.01 MB (9.3KB, xlsx) .
Copyright © 2018 Beall et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Presence of core genes from Tannerella forsythia in the genomes of the other Tannerella species. The genes are identified by their IMG/MER gene identifiers for the 92A2 genome and had homologs in the ATCC 43037 genome. The presence of homologs to these core genes is shown for the following genomes: Tannerella sp. BU045 (SAG 103), Tannerella sp. BU045 (SAG 108), Tannerella sp. BU045 (SAG 110), Tannerella sp. BU063 (SAG 2), Tannerella sp. BU063 (SAG 5), and Tannerella sp. BU063 (W11667). “1” indicates that a homolog was present and “0” that it was absent. Summary columns indicate the numbers of genomes with homologs from each species and totals. Download TABLE S2, XLSX file, 0.1 MB (156KB, xlsx) .
Copyright © 2018 Beall et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Presence of core genes from Tannerella sp. BU063 in the genomes of the other Tannerella species. The genes are identified by their IMG/MER gene identifiers for the W11667 genome and had homologs in the SAG 2 and SAG 5 genomes. The presence of homologs to these core genes is shown for the following genomes: Tannerella sp. BU045 (SAG 103), Tannerella sp. BU045 (SAG 108), Tannerella sp. BU045 (SAG 110), Tannerella forsythia 92A2, and Tannerella forsythia ATCC 43037. “1” indicates that a homolog was present and “0” that it was absent. Summary columns indicate the numbers of genomes with homologs from each species and totals. Download TABLE S3, XLSX file, 0.1 MB (135.1KB, xlsx) .
Copyright © 2018 Beall et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Presence of core genes from Tannerella sp. BU045 in the genomes of the other Tannerella species. The genes are identified by their IMG/MER gene identifiers for the SAG 108 genome and had homologs in the SAG 103 and SAG 110 genomes. The presence of homologs to these core genes is shown for the following genomes: Tannerella forsythia ATCC 43037, Tannerella forsythia 92A2, Tannerella sp. BU063 (SAG 2), Tannerella sp. BU063 (SAG 5), and Tannerella sp. BU063 (W11667). “1” indicates that a homolog was present and “0” that it was absent. Summary columns indicate the numbers of genomes with homologs from each species and totals. Download TABLE S4, XLSX file, 0.1 MB (118KB, xlsx) .
Copyright © 2018 Beall et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Summary of core gene presence in other genomes. For each of the three core genome gene sets, the numbers of genes with homologs in different numbers of genomes of the other Tannerella species are tabulated. For instance, 981 Tannerella forsythia core genes had homologs in zero BU063 genomes and zero BU045 genomes, while 1,136 genes had homologs in all three BU063 and all three BU045 genomes. Download TABLE S5, XLSX file, 0.01 MB (9.6KB, xlsx) .
Copyright © 2018 Beall et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
The data for SAGs 103, 108, and 110 have been deposited under NCBI BioProject number PRJNA342492, including SRA experiments SRX2157181, SRX2157182, and SRX2157183, and WGS accession numbers MIQB00000000, MIQC00000000, and MIQD00000000.
Software scripts used can be accessed at the OSU code repository with the URL https://code.osu.edu/beall-3/Single_Cell_Genomics.